Highlights
-
•
Counseling and 12-Step attendance were associated with later reduced opioid use.
-
•
Associations between psychosocial support and use grew over weeks of the trial.
-
•
12-Step had a larger association with reduced opioid use for those on naltrexone.
-
•
Group counseling had larger association with reduced use for buprenorphine-naloxone.
-
•
Relationship between psychosocial support and use are not causal; may be reciprocal.
Keywords: Opioid use disorder, Psychosocial counseling, 12-Step, Medication for opioid use disorder (MOUD)
Introduction
Psychosocial support is recommended in conjunction with medication for opioid use disorder (MOUD), although optimal “dose,” modality, and timing of participation is not established. This study comprised a secondary analysis of counseling and 12-Step attendance and subsequent opioid use in a MOUD randomized clinical trial. Methods: The parent study randomly assigned 570 participants to receive buprenorphine-naloxone (BUP-NX, n=287) or extended-release injectable naltrexone (XR-NTX, n=283). Mixed-effects logistic regression models were fit with opioid use as the response variable, and a counseling/12-Step attendance predictor. Differences by treatment assignment were examined. Results: Any counseling or 12-Step attendance was associated with reduced odds of opioid use at the subsequent visit, whether considered individually or aggregated across type. A continuous relationship was observed for 12-Step attendance (F(1,5083)=5.01, p=.025); with each additional hour associated with 13% (95% CI: 0.83, 0.90) reduction in odds of opioid use. The strength of this association grew over time. In the BUP-NX arm, group counseling was associated with a greater reduction in odds of opioid use than for XR-NTX, (OR=0.32 (95% CI: .22, 0.48) vs. OR=0.69 (95% CI: 0.43, 1.08)). For XR-NTX, 12-Step was associated with a greater reduction in odds of opioid use (OR=0.35 (95% CI: 0.22, 0.54) vs. OR=0.65 (95% CI: 0.47, 0.89) for BUP-NX)). Conclusions: Psychosocial engagement has a proximal association with opioid use, the strength of that association may grow with dose and time. Alternatively, more motivated individuals may both attend more counseling/12-Step and have better treatment outcomes, or the relationship may be reciprocal.
1. Introduction
The opioid epidemic continues to plague the United States, with rates of opioid use and associated overdose and mortality climbing significantly and remaining high, resulting in over 450,000 deaths from 1999 to 2018 (CDC Injury Center, 2020). Although there exist several effective medications for opioid use disorder (MOUD) (i.e., methadone, buprenorphine, and injectable extended-release naltrexone), (Kampman and Jarvis, 2015), treatment utilization remains low (Brady et al., 2016) and is complicated by ongoing drug use and short durations of treatment (Carroll and Weiss, 2017; LaRochelle et al., 2018).
A seminal study examining the efficacy of methadone treatment found that patients randomized to receive basic counseling in addition to standard methadone treatment experienced more frequent, faster, and greater improvements than those receiving methadone maintenance alone, although a more intensive psychosocial treatment regimen produced minimum additional benefit (McLellan et al., 1993). Recent systematic reviews indicate that concurrent psychosocial treatment in clinical trials for OUD improves treatment outcomes including opioid use and retention compared to pharmacological treatment alone (Amato et al., 2011) and is beneficial for OUD generally (Dugosh et al., 2016). Accordingly, the American Society of Addiction Medicine's (ASAM) National Practice Guideline and medication-first models of OUD treatment recommend psychosocial treatment be provided in conjunction with any pharmacological treatment for OUD (Kampman and Jarvis, 2015; Winograd et al., 2019). In contrast, one study involving individuals with prescription OUD randomized to receive a manualized treatment for OUD vs. standard medication management alone identified no benefit associated with the psychosocial treatment in terms of opioid use outcomes (Weiss et al., 2011). A recent review found a limited number of clinical trials assessing the addition of counseling to buprenorphine with a mixture of negative and positive studies, the strongest support being for Cognitive Behavioral Therapy plus Contingency Management (Carroll and Weiss, 2017).
These latter findings suggesting limited or inconsistent benefit (e.g., (Carroll and Weiss, 2017; Weiss et al., 2011)) are seemingly contradictory with existing guidelines; however, there are numerous limitations of existing research that make it difficult to interpret discrepancies observed across different lines of research. For example, little is known regarding what form(s) of psychosocial engagement are necessary and/or sufficient in order to effectively supplement MOUD (e.g., group, individual, 12-Step programs such as Alcoholics Anonymous (AA) and Narcotics Anonymous (NA)), and whether the relative importance of psychosocial engagement changes across different stages along the treatment continuum of MOUD initiation and maintenance (Dugosh et al., 2016), or across different forms of MOUD. Specifically, while findings specifically support the efficacy of group counseling in conjunction with methadone treatment (e.g., (Scherbaum et al., 2005)), the evidence for group counseling in support of office-based buprenorphine treatment is less clear (Sokol et al., 2017), and data on psychosocial support for extended-release naltrexone are scarce. In addition, research is lacking that evaluates temporal relationships between psychosocial treatment attendance and indicators of clinical status like relapse or smaller milestones like “slips” that may not be conceptualized as a relapse. In particular, while research suggests that 12-Step group attendance is associated with reduced drug and alcohol use in clinical trials (Humphreys et al., 2020), scant research to date has specifically examined 12-Step program attendance in the context of MOUD, perhaps in part due to philosophical conflict and stigma surrounding the use of partial opioid agonists like buprenorphine and the specific definition of what constitutes abstinence among12-Step adherents (Klein and Seppala, 2019; Monico et al., 2015).
The present investigation aimed to address these gaps by examining the relationship between psychosocial counseling and 12-Step attendance on subsequent opioid use over several months of MOUD treatment. This study is a secondary analysis of a national multisite randomized clinical trial comparing buprenorphine-naloxone (BUP-NX) to extended-release naltrexone (XR-NTX) for OUD (Lee et al., 2017, 2016; Nunes et al., 2016). Of note, a previously published secondary analysis of this dataset examined psychosocial treatment attendance and the association with opioid use at weeks 24 (end of treatment) and 36 (follow-up), concluding that increased hours of 12-Step and individual counseling treatment attendance were associated with reductions in opioid use at these visits (Harvey et al., 2020). The present study aimed to extend this analysis of the relationship between psychosocial treatment or 12-Step attendance and opioid use over time in the trial. Specifically, using a time-lagged model, we assessed relationships between attendance at individual or group psychosocial treatment or 12-Step, respectively, in the week prior to subsequent opioid use as reported at the next weekly study visit (as assessed via Timeline Followback and urine drug screen), with the first visit being considered the “index visit”. We examined both (1) attendance of any counseling or 12-Step, as well as (2) potential additive contributions of hours of counseling/12-Step prior to the index visit. Finally, we assessed potential differences in the association between counseling/12-Step attendance and subsequent opioid use by randomly assigned treatment arm (BUP-NX vs. XR-NTX).
2. Method
2.1. Participants and parent study design
The current study is a secondary data analysis of CTN-0051, a multi-site, open-label, randomized controlled trial sponsored by the National Institute on Drug Abuse through its National Drug Abuse Treatment Clinical Trials Network (NDAT CTN), which examined the comparative effectiveness of extended-release naltrexone vs. buprenorphine-naloxone for opioid relapse prevention (Lee et al., 2017, 2016; Nunes et al., 2016). Details of the study design and methods (Lee et al., 2016; Nunes et al., 2016) and primary outcome (Lee et al., 2017) have been reported previously.
A total of 570 participants with OUD were enrolled in the study and randomly assigned to receive either extended-release naltrexone (XR-NTX, n=283) or buprenorphine-naloxone (BUP-NX, n=287). Medication management occurred at each study visit, weekly during the first month, then every two weeks, and finally every four weeks (weeks 16, 20, and 24) and included a focus on provider–patient rapport, medication adherence and side-effects, as well as promoting other psychosocial treatment (e.g., counseling, 12-Step involvement). Additional ancillary (non-study) counseling and/or 12-Step was recommended at all eight participating research sites, and while variable across sites, each site offered at least a minimum level of non-study outpatient psychosocial care consisting of at least one group and/or individual and group counseling session per week for up to 24 weeks.
As in the parent study, Visit 3 (Day 21) was the first study visit at which opioid use outcomes were considered, to account for the fact that recently detoxified participants were likely to “test” for blockade, or have residual positive urine samples for long-acting opioids prescribed as part of the detoxication regimen (Lee et al., 2016), even if they were abstinent from non-study opioids. In the parent study, study medications were discontinued following a relapse event (regular use of non-study opioids any time after day 20 post-randomization: either 4 consecutive opioid use weeks with at least 1 day of non-study self-reported opioid use, positive urine drug screen, or missing urine drug screen; or 7 consecutive days of self-reported opioid use). Otherwise, participants remained on study medications until either the end of the 24-week trial, or discontinuation in response to safety concerns or participant preference, after which participants were referred to community treatment support and services for MOUD and psychosocial treatment, as appropriate (Nunes et al., 2016). Follow up research visits occurred post-treatment at weeks 28 and 36. Outreach procedures were used to contact as many participants as possible to encourage attendance at these follow-up visits.
2.2. Measures
2.2.1. Demographic information
Demographic characteristics of the participants, including gender, date of birth, ethnicity, race, education, employment status, and marital status, were collected via self-report at baseline.
Psychiatric status
Psychiatric severity was assessed at baseline via the Psychiatric Composite Score from the ASI-Lite, derived from the Fifth Edition of the ASI (McLellan et al., 1992).
2.2.2. Urine drug screen (UDS)
Urine drug screens were collected at screening, as part of XR-NTX induction, weekly throughout the 24-week active treatment phase, and at the follow-up visits. A urine drug test was coded positive for opioids if the toxicology sample was positive for non-study opioids (buprenorphine [for XR-NTX group only], methadone, morphine [heroin, codeine, morphine] or oxycodone), or the participant did not provide a urine sample (missed visits or refusals).
2.2.3. Timeline Follow-Back (TLFB)
The Timeline Follow-Back (Fals-Stewart et al., 2000; Sobell and Sobell, 1992) was administered at each study visit throughout the active treatment phase and through the end of the follow-up period to document the participants’ self-reported use of opioids (heroin or prescription opioids) and other substances for each day since the previous assessment.
2.2.4. Opioid use
Use of opioids at a given study visit was defined by at least 1 day of non-study opioids as reported on TLFB or UDS, as described above, with missing data treated as positive for opioid use (if only UDS was missing, we used TLFB and vice versa). As in the parent study, Visit 3 (Day 21) was the first study visit at which opioid use outcomes were counted toward a relapse endpoint (Lee et al., 2016).
2.2.5. Psychosocial counseling/12-Step participation
At each weekly visit (weeks 1-24), participants were asked to report on their participation in psychosocial counseling and 12-Step (AA/NA) during the previous week. The Psychosocial Counseling Participation Log assessed whether any counseling (individual, group) or 12-Step occurred (No, Yes, or Unknown), and if so, for how many hours during that week. If counseling/12-Step attendance was missing due to a missed visit, at the next visit attended, the researchers attempted to gather information on counseling/12-Step attendance since the participant was last seen. Otherwise, missing counseling/12-Step attendance was treated as missing.
2.3. Data analytic plan
Descriptive statistics were used to characterize the sample in terms of demographic variables including gender, date of birth, ethnicity, race, education, employment status, and marital status. Descriptive statistics including frequencies, means, and standard deviations were also used to summarize the overall psychosocial counseling data.
Counseling and 12-Step attendance variables were computed: Total number of hours of counseling (Any Type of Counseling/12-Step, as well as Individual, Group, 12-Step hours) attended per week. In addition, dichotomous predictors were computed for each counseling/12-Step category: Any Type of Counseling/12-Step (Yes/No), Any Individual Counseling (Yes/No), Any Group Counseling (Yes/No), and Any 12-Step (Yes/No) for each week of the study. Variables were computed both for all weeks and for all weeks before relapse.
Opioid Use outcome for each week was computed as a dichotomous indicator of either opioid use self-reported via TLFB or detected in UDS. The outcome was missing only if both TLFB and UDS were unavailable for a particular subject at a given visit. Mixed-effects logistic regression models were fit with weekly Opioid Use (Yes vs. No) as the response variable, and a counseling/12-Step variable as a time varying predictor. Counseling/12-Step variables were computed with respect to the number of hours or any hours of attendance since the prior assessment visit. Models also featured a random intercept for subject, included parent trial site as a fixed, random effect, and psychiatric severity as a fixed-effect covariate. Models first tested the interaction of the counseling predictor with (linear) time. Observations following relapse were not included in the model. Separate models were fit for each counseling predictor. The models also tested interactions of Counseling/12-Step attendance (for both Any (dichotomous predictor; Yes vs. No) as well as continuous hours) with treatment group (XR-NTX or BUP-NX). If the interaction was found to be significant, then the main effect of the counseling predictor was estimated within each treatment group, along with the comparison of the treatment effects between the two groups. Time by Treatment by Counseling/12-Step predictor interactions were also examined.
3. Results
3.1. Participants
Among the 570 randomized participants, mean [SD] age was 33.9 [9.6] years; 401 [70.4%] were men; 471 [82.6%] were not Hispanic or Latino; 421 [73.9%] were white; 157 [27.5%] had some college, no degree; 132 [23.3%] had less than high school diploma; 109 [19.1%] were high school graduates; 81 [14.2% had a GED; 51 [8.9%] had an associate's degree,; 40 [7.0%] had a bachelors’ or masters’ degree; 376 [66.0%] were never married; 360 [63.2%] were looking for work, unemployed.
3.2. Summary of counseling and 12-Step attendance, UDS availability and treatment duration
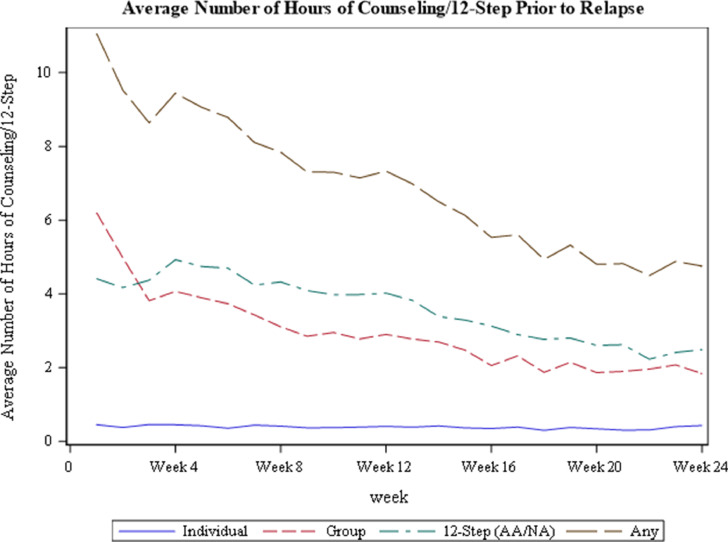
Of the 570 participants, 315 (55.3%) received individual counseling, 344 (60.4%) received group counseling, 427 (74.9%) attended 12-Step, and 174 (30.5%) reported “other counseling” for at least one hour during the 24 weeks of the study. Prior to relapse, participants attended a cumulative mean of 0.36 h of individual counseling per week, 3.40 h of group counseling per week, 3.55 h of 12-Step, and 0.57 h of other counseling per week. Mean hourly attendance of counseling and 12-Step, by week in the trial (prior to meeting relapse criteria), is presented in Fig. 1. On average, UDS records were available for 96% of pre-relapse weeks, with 97% of such records available for BUP-NX subject and 94% available for XR-NTX subjects. Participants randomized to XR-NTX remained on study medications a median of 59 days, while those randomized to BUP-NX remained on study medications a median of 97 days.1
Fig. 1.
Mean hours of counseling/12-Step among participants throughout the parent trial.
4.3. Associations of counseling or 12-step attendance with opioid use over time
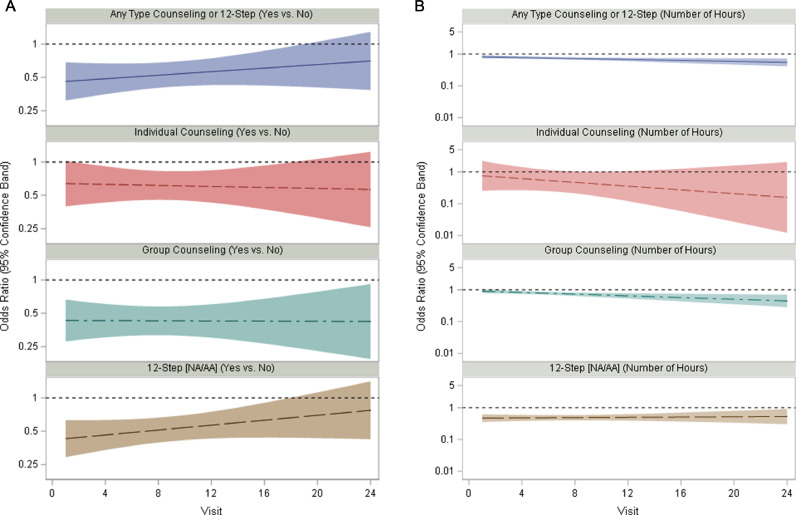
Fig. 2a and b plot the odds ratios for the associations between attendance at the different types of counseling or 12-Step in an index week with opioid use the next week over the 24 weeks of the trial, with Any Type of Counseling or 12-Step (Yes vs. No) odds ratios displayed in Fig. 2a, and continuous hours displayed in Fig. 1b. Odds ratios of less than 1 reflect reduction in opioid use at the weekly study visit, associated with increased number of hours attending counseling/12-Step reported at the index visit (prior weekly study visit). Time by continuous hours of attendance variable interactions were found to be significant for hours of any counseling or 12-Step, F(1,5083)=5.01, p=.025), and for hours of group counseling specifically, (F(1,5083)=6.75, p=.009). For hours of Any Type of Counseling/12-Step and for hours of 12-Step attendance, the interactions with time were significant, such that hours of participation were associated with a greater reduction in opioid use over time in the trial. One additional hour of Any Type of Counseling /12-Step reported in the index visit was associated with 5% lower odds of opioid use at Visit 3 (OR (95% CI): 0.95 (0.94, 0.97)) and with 11% lower odds of opioid use at Visit 24 (OR (95% CI): 0.89 (0.84, 0.94)) (Fig. 1b). One additional hour of group counseling reported in the index visit was associated with 3% lower odds of opioid use at Visit 3 (OR (95% CI): 0.97 (0.95, 0.99) and with 15% lower odds of opioid use at Visit 24 (OR (95% CI): 0.85 (0.78, 0.93). No time by attendance association was noted for hours of individual counseling or 12-Step (See Table 1), or for Any (Yes vs. No) across types of counseling/12-Step, although the pattern of stronger association between attendance and reduced opioid use over time appear similar.
Fig. 2.
a. Odds ratios for the effect of dichotomous (any counseling/12-Step) variables since index visit on opioid use over time. 2b. Odds ratios for the effect of continuous (number of hours) index visit counseling/12-step variables on opioid use over time. Note: Values displayed as Odds Ratios (ORs) for opioid use reported at visit. Y-axis values are displayed as logarithmic. Indicates that Number of Hours Attendance by Time effect was significant (for any type of counseling/12-Step and for group counseling).
Table 1.
Time by counseling/12-step attendance interactions with subsequent opioid use.
| Time by Predictor Interaction | ||||
|---|---|---|---|---|
| Predictor | NumDF | DenDF | F-value | p-value |
| Additional hour of Any Type Counseling/12-Step | 1 | 5083 | 5.01 | 0.0252* |
| Additional hour of Individual Counseling | 1 | 5083 | 0.92 | 0.3383 |
| Additional hour of Group Counseling | 1 | 5083 | 6.75 | 0.0094* |
| Additional hour of 12-Step (NA/AA) | 1 | 5083 | 0.09 | 0.7588 |
denotes that interaction between counseling/12-Step attendance and time is significant
3.4. Associations between counseling or 12-Step attendance with opioid use by treatment group
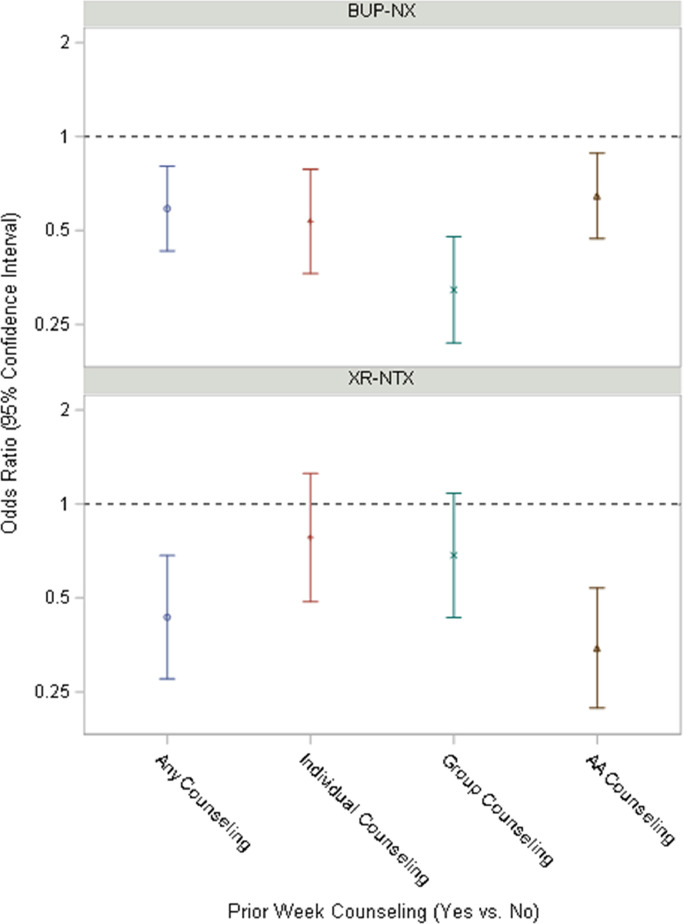
Fig. 3 displays point estimates of the weekly odds ratios and the surrounding shaded areas show the 95% confidence limits for each type of counseling/12-Step attendance by Treatment Group (BUP-NX vs. XR-NTX). Point estimates are predominantly less than 1, suggesting overall that Counseling/12-Step attendance was associated with reductions in opioid use. However, treatment groups significantly differed in association of any group counseling reported at the index visit, with subsequent Opioid Use (F(1,5083)=6.06, p=.014). Any group counseling was associated with 68% (OR (95% CI): 0.32 (0.22, 0.48) lower odds of opioid use in the BUP-NX group, but only 31% (OR (95% CI): 0.69 (0.43, 1.08) in the XR-NTX group (See Table 2).
Fig. 3.
Odds ratio of opioid use based on counseling/12-Step attendance type, interaction with treatment assignment BUP-NX = buprenorphine-naloxone XR-NTX = extended release naltrexone.
Table 2.
Treatment group by counseling/12-Step attendance interactions with opioid use.
| Predictor by Treatment Arm Interaction |
Effect of Predictor(Within BUP-NX) |
Effect of Predictor(Within XR-NTX) |
Interaction Effect |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | NumDF | DenDF | F-value | p-value | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| Any Type of Any Counseling/12-Step | 1 | 5083 | 1.18 | 0.2779 | |||||||||
| Any Individual Counseling | 1 | 5083 | 1.47 | 0.2251 | |||||||||
| Any Group Counseling | 1 | 5083 | 6.06 | 0.0139* | 0.32 | 0.22 | 0.48 | 0.69 | 0.43 | 1.08 | 0.47 | 0.26 | 0.86 |
| Any 12-Step (NA/AA) | 1 | 5083 | 5.11 | 0.0238* | 0.65 | 0.47 | 0.89 | 0.35 | 0.22 | 0.54 | 1.87 | 1.09 | 3.22 |
| Additional hour of Any Type of Counseling/12-Step | 1 | 5083 | 3.61 | 0.0575 | |||||||||
| Additional hour of Individual Counseling | 1 | 5083 | 1.02 | 0.3137 | |||||||||
| Additional hour of Group Counseling | 1 | 5083 | 10.34 | 0.0013* | 0.92 | 0.89 | 0.95 | 0.99 | 0.96 | 1.02 | 0.93 | 0.89 | 0.97 |
| Additional hour of 12-Step Support (NA/AA) | 1 | 5083 | 1.57 | 0.2098 | |||||||||
Treatment groups also significantly differed in association of index visit-reported attendance of any 12-Step and subsequent opioid use F(1,5083)=5.11, p=.024, with 12-Step associated with 65% (OR (95% CI): 0.35 (0.22, 0.54) lower odds of opioid use in the XR-NTX group, but only 35% (OR (95% CI): 0.65 (0.47, 0.89) in the BUP-NX group. Finally, treatment groups also significantly differed in effect of past week hours of group counseling on opioid use, F(1,5083)=10.34, p=.001, with each additional hour of group counseling associated with 8% (OR (95% CI): 0.92 (0.89, 0.95) lower odds of opioid use in the BUP-NX group, but only 1% (OR (95% CI): 0.99 (0.96, 1.02) in the XR-NTX group. Time by treatment group by attendance predictor interactions were not significant.
For those attendance predictors that were not found to be significantly moderated in their effects on opioid use by Treatment Arm or time, main effects across time and treatment arm are reported in Table 3. Any type and any amount of counseling or 12-Step attendance reported in the index visit was associated with 46% (OR= 0.54, 95% CI: 0.41,0.69) reduced odds of opioid use at the subsequent visit. Any amount of individual counseling reported in the index visit was associated with 39% (OR=0.61, 95% CI: 0.45, 0.82) lower odds of opioid use. Each additional hour of 12-Step reported in the index visit was also associated with 13% (OR=0.87, 95% CI: 0.83, 0.90) lower odds of opioid use. Each hour of additional individual counseling was the only non-significant predictor on subsequent opioid use (main effect or interaction).
Table 3.
Main effects of counseling/12-Step attendance with subsequent opioid use.
| Main Effect of Predictor | |||
|---|---|---|---|
| (Without Interaction) | |||
| Predictor | OR | 95% CI | |
| Any Type of Any Psychosocial Counseling or 12-Step | 0.54* | 0.41 | 0.69 |
| Any Individual Counseling | 0.61* | 0.45 | 0.82 |
| Any Group Counseling | 0.43b | 0.32 | 0.57 |
| Any 12-Step (NA/AA) | 0.52b | 0.41 | 0.68 |
| Additional hour of Any Type of Counseling/12-Step | 0.94a | 0.93 | 0.96 |
| Additional hour of Individual Counseling | 0.87 | 0.75 | 1.02 |
| Additional hour of Group Counseling | 0.95a,b | 0.93 | 0.97 |
| Additional hour of 12-Step (NA/AA) | 0.87* | 0.83 | 0.90 |
4. Discussion
This secondary analysis of the CTN-0051 data examined associations between psychosocial treatment and 12-Step (AA/NA) attendance in the previous week with opioid use in the subsequent week, and compared associations between attendance and subsequent use over time throughout the trial, and across randomly assigned treatment conditions. Any amount and any type of psychosocial treatment or 12-Step attendance reported at the index visit was associated with around half the odds of opioid use in the subsequent week, whether examined separately as any individual counseling, any group counseling, any 12-Step participation, or a composite of these. Participation in psychosocial counseling and 12-Step diminished over the course of the trial. However, the strength of the association between both (1) hours of 12-Step and (2) hours of group counseling with subsequent opioid use, grew over time in the trial. While both group counseling and 12-Step attendance were associated with reduced odds of subsequent opioid use across treatments, group counseling attendance was associated with reduced odds of opioid use more substantially for BUP-NX, while 12-Step was associated with reduced odds of opioid use more substantially for XR-NTX (by approximately two-thirds in each case).
The primary finding of the present study that psychosocial counseling or 12-Step attendance one week is associated with reduced opioid use the following week is novel, and advances understanding of proximal relationships between psychosocial support and treatment progress. Such findings improve upon the reliance on summary measures of cumulative attendance across the life of randomized controlled trials as has typically been reported previously (e.g., Amato et al., 2011). These findings also corroborate that of other recent studies demonstrating that apparent benefits of psychosocial supports in combination with MOUD grow over time (Hammond et al., 2020; Montoya et al., 2020). Taken together, such findings lend further support to the conventional clinical wisdom that psychotherapy and 12-Step effects may be cumulative, and mirror catchphrases used in 12-Step groups such as “keep coming back.” Moreover, cognitive behavioral and other skills-based therapies require time to build skills in areas supporting behavioral relapse prevention, emotion regulation, and cognitive control that are key for supporting recovery. While such findings may not be surprising, they should be interpreted with caution, however, as they are not causal, and are likely reciprocal in nature. Individuals who are more motivated at the beginning of a trial (or treatment episode) may have better prognosis to begin with and may also be more apt to participate in counseling/12-Step. Then, attendance at counseling/12-Step may in turn contribute to improved opioid use-related outcomes.
4.1. Differences by random treatment group assignment
Although group counseling and 12-Step attendance were each associated with subsequent reduced opioid use across both treatment groups, this relationship was greater for 12-Step attendance among participants randomized to receive XR-NTX as compared to BUP-NX. Conversely, the opposite pattern was observed for group counseling; the reduction in odds of opioid use associated with group counseling attendance was stronger for BUP-NX participants than for those assigned to XR-NTX. These findings may reflect stigma associated with opioid agonist therapy such as buprenorphine (and methadone) and preference or bias towards an “abstinence based” recovery in some 12-Step groups. It should also be noted that in the parent trial, participants randomized to XR-NTX were overall less likely than BUP-NX participants to successfully initiate MOUD (72% vs 94%), and this could have impacted findings of the present study as failure to initiate treatment may influence both subsequent psychosocial engagement and return to use. Notwithstanding, the findings of the present study join other recent research supporting the notion that MOUD is feasible in conjunction with 12-Step program attendance (Klein and Seppala, 2019) and may support abstinence months (Monico et al., 2015) to years (Gossop et al., 2007) later. Thus, there is accumulating evidence to support the recommendation of 12-Step attendance to patients on MOUD (see also, Weiss et al., 2019); however, this may be even more beneficial for those who are not on opioid agonist therapies such as buprenorphine (versus naltrexone) for the reasons mentioned above.
4.2. Strengths and limitations
The main strength of the present investigation is that the parent study was a multi-site, randomized-controlled trial for MOUD in which opioid use and psychosocial counseling/12-Step attendance was assessed at each weekly visit, using a time-lagged analysis to examine proximal relationships between attendance of group and individual counseling and 12-Step. However, it is important to note that associations of attendance at counseling/12-Step programs with reduced opioid use may simply reflect greater motivation amongst those who attended more counseling and 12-Step. If true, those participants who experienced a slip (that did not meet relapse criteria) may have been expected to attend less psychosocial counseling and 12-Step, related to pre-existing factors.
On the other hand, those who met study-defined relapse criteria were not included in analysis at later weekly visits, which reflects a strength of the current analytic approach. Data following relapse was excluded from the analysis due to the fact that its inclusion would make it more difficult to interpret associations between attendance and opioid use for several reasons: (1) study medications would have been discontinued following relapse (Lee et al., 2016); (2) relapse is typically considered a treatment failure, which would likely lead to modified treatment plans, including recommendations to attend additional psychosocial therapy and 12-Step; and (3) because those who had relapsed would presumably remain at increased likelihood of continuing to use opioids. Thus, while it is not possible to eliminate the possibility of indirect effects of factors such as motivation, the present study provides an analysis of associations between psychosocial counseling and 12-Step attendance with MOUD treatment outcomes, as compared to a previous report on a similar analysis of the parent study (Harvey et al., 2020). As an alternative to motivation, the findings of this secondary analysis could suggest that psychosocial and or 12-Step attendance could be a marker of eventual success, for those who continue to attend are more likely to be abstinent at later time points.
Another potential explanation for the observed findings is that in the analysis, the attendance variables could have been more influential at week 24 as compared to week 3 because there were simply fewer participants included in the analyses at later time points. For example, those who had relapsed were removed from the model at subsequent weeks, resulting in a reduced number of participants, whose data may become more influential. However, this is not inconsistent with the explanation that for those individuals who manage to maintain engagement with the trial, counseling and/or 12-Step may have a larger impact in supporting their recovery.
In addition to differences in MOUD initiation rates reflecting the XR-NTX “induction hurdle” which we have previously noted, XR-NTX and XR-BUP differ in frequency and mode of administration (XR-NTX monthly by injection, XR-BUP daily sublingually), which in turn, could lead to differences in adherence to medication; likewise, it is possible that adherence to medication might affect (or be associated with) adherence to psychosocial counseling/12-Step. The present paper focuses on the relationship between psychosocial counseling/12-Step attendance (any vs. none, and how much) and opioid use during the following week. There are other relationships that might be equally important, such as between adherence to psychosocial counseling and adherence to medication, or between adherence to medication and subsequent opioid use – however, the exploration of these complex relationships is beyond the scope of the current study, and this should be acknowledged as a limitation worthy of future research.
4.3. Summary and future directions
Although psychosocial supports are typically offered in conjunction with MOUD, research indicates that poor adherence lowers delivered doses below recommended levels. Specifically, patients with OUD typically miss more than half of prescribed counseling sessions, whether it be individual, group or other types (Brooner et al., 2004). While the results of the present study suggest that when it comes to psychosocial support, “more is better,” the present findings also suggest that “any is better than none.” Moreover, that we found that any counseling or 12-Step attendance was associated with better outcomes (reduced opioid use at subsequent timepoints), is encouraging for a variety of reasons. For instance, this may indicate that a specific modality may not be necessary for MOUD treatment augmentation or support. A patient-centered care perspective on the present findings may suggest that whatever modalities patients feel motivated to attend and/or feel is working for them could be encouraged. Each kind of psychosocial treatment and/or 12-Step was associated with nearly half the odds of subsequent opioid use. Future research could expand upon the present study by examining the contributions of different psychosocial treatment modalities to opioid use and relapse. Moreover, future research could further explore cumulative effects of psychosocial and 12-Step attendance as time-variant predictors of relapse. In addition, future work could expand upon the findings of temporal associations between attendance and opioid use by examining substance use trajectories across different patterns of psychosocial and/or 12-Step engagement.
Acknowledgments
Acknowledgments
None additional to declare.
Author disclosures
Role of the funding source:
This work was supported by HHSN271201400028C, U10DA013046, UG1/U10DA013035, and C75N95020D00012 / N01DA-20-2251).
Authors contributions
Dr. Hefner developed the concept for the secondary analysis and worked with the team to analyze and interpret the data, developed and revised the manuscript.
Dr. Choo conducted the analysis for the manuscript, contributed to the methods and analysis sections, and developed figures.
Dr. Shmueli-Blumberg supported the original parent trial (CTN-0051), contributed to the concept of the current manuscript, contributed to manuscript preparation and editing.
Dr. Pavlicova contributed to the data analysis and interpretation and provided review and editing of the manuscript.
Ms. King contributed to the original concept of the secondary analysis, ran initial analysis models, generated descriptive statistics, and contributed to manuscript development. She also supported the original parent study statistical analysis.
Dr. Fishman contributed to the concept of the secondary analysis and interpretation of the results.
Dr. Shulman contributed to the concept of the secondary analysis and interpretation of the results.
Dr. Campbell contributed to the concept of the secondary analysis, interpretation of the results, and contributed to manuscript revisions.
Ms. Scodes contributed to the analytic model development of the secondary analysis and provided statistical support.
Ms. Meyers-Ohki contributed to the daily oversight and management of the parent trial and provided manuscript review and editing.
Ms. Novo contributed to the daily oversight and management of the parent trial and provided feedback on initial concept for the secondary analysis, and provided review on subsequent versions of the analysis and conclusions.
Dr. Nunes served as Co-Investigator on the original parent trial, and provided substantial feedback on the analysis, interpretation, conclusions, and manuscript as a whole.
Dr. Rotrosen served as Principal Investigator on the parent trial, and provided substantial feedback on the concept, analysis, interpretation, conclusions, and manuscript as a whole.
All authors approved the final submission of the manuscript.
Declaration of Competing Interest
Dr. Hefner has no conflict of interest to declare.
Dr. Shmueli-Blumberg has no conflict of interest to declare.
Dr. Campbell has no conflict of interest to declare.
Dr. Pavlicova has no conflict of interest to declare.
Dr. Fishman serves as a consultant for Alkermes, a consultant for Drug Delivery LLC, holds a research grant from Alkermes and a research grant from Medicasafe.
Ms. Meyers-Ohki has no conflicts of interest to declare.
Dr. Shulman has no conflicts of interest to declare.
Dr. Greiner has no conflicts of interest to declare.
Ms. Novo has no conflicts of interest to declare.
Dr. Rotrosen has served as a Principal Investigator or a co-Investigator on studies for which support in the form of donated medication and/or funds has been, or is, provided by Indivior, Alkermes, and Braeburn, and apps provided by Pear Therapeutics, CHESS Health, and Data Cubed. Dr. Rotrosen recently served in a non-paid capacity as a member of an Alkermes study Steering Committee.
Dr. Nunes has served as a Principal Investigator or co-Investigator on studies for which support in the form of donated medication and/or funds has been, or is, provided by Indivior, Alkermes, and Braeburn-Camurus, and digital therapeutics provided by Pear Therapeutics and CHESS Health, and has served as an unpaid consultant to Alkermes, Braeburn-Camurus and Pear Therapeutics.
Footnotes
Proportion of participants remaining on study medications by treatment arm, over time in the study, is presented in Supplemental Fig. 1.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100100.
Appendix. Supplementary materials
References
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst. Rev. 2011;9 doi: 10.1002/14651858.CD005031.pub4. Article No: CD005031. DOI. [DOI] [PubMed] [Google Scholar]
- Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am. J. Psychiatry. 2016;173(1):18–26. doi: 10.1176/appi.ajp.2015.15020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner RF, Kidorf MS, King VL, Stoller KB, Peirce JM, Bigelow GE, Kolodner K. Behavioral contingencies improve counseling attendance in an adaptive treatment model. J. Subst. Abuse Treat. 2004;27:223–232. doi: 10.1016/j.jsat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Weiss RD. The role of behavioral interventions in buprenorphine treatment: a review. Am. J. Psychiatry. 2017;174(8):738–747. doi: 10.1176/appi.ajp.2016.16070792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Injury Center (2020). Understanding the epidemic | drug overdose. Available from: https://www.cdc.gov/drugoverdose/epidemic/index.html. [cited 2020 Oct 7].
- Dugosh K, Abraham A, Seymour B, McLoud K, Chalk M, Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J. Addict. Med. 2016;10(2):91–101. doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J. Consult. Clin. Psychol. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Marsden J. Attendance at narcotics anonymous and alcoholics anonymous meetings, frequency of attendance and substance use outcomes after residential treatment from drug dependence: a 5-year follow-up study. Addiction. 2007;103:119–125. doi: 10.1111/j.1360-0443.2007.02050.x. [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Kady A, Park G, Vidal C, Wenzel K, Fishman M. Therapy dose mediates the relationship between buprenorphine/naloxone and opioid treatment outcomes in youth receiving medication for opioid use disorder treatment. J. Addict. Med. 2020;16(2):e97–e104. doi: 10.1097/ADM.0000000000000861. [DOI] [PubMed] [Google Scholar]
- Harvey LM, Fan W, Cano MA, Vaughan EL, Arbona C, Essa S, Sanchez H, de Dios MA. Psychosocial intervention utilization and substance abuse treatment outcomes in a multisite sample of individuals who use opioids. J. Subst. Abuse Treat. 2020;112:68–75. doi: 10.1016/j.jsat.2020.01.016. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Barreto NB, Alessi SM, Carroll KM, Crits-Christoph P, Donovan DM, Kelly JF, Schottenfeld RS, Timko C, Wagner TH. Impact of 12 step mutual help groups on drug use disorder patients across six clinical trials. Drug Alcohol Depend. 2020;215 doi: 10.1016/j.drugalcdep.2020.108213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman K, Jarvis M. American society of addiction medicine (ASAM) national practice guideline for the treatment of addiction involving opioid use. J. Addict. Med. 2015;9:358–367. doi: 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AA, Seppala MD. Medication-assisted treatment for opioid use disorder within a 12-Step based treatment center: feasibility and initial results. J. Subst. Abuse Treat. 2019;104:51–63. doi: 10.1016/j.jsat.2019.06.009. [DOI] [PubMed] [Google Scholar]
- LaRochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann. Intern. Med. 2018;169(3):137–145. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, Rotrosen J. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X-BOT): a randomized, open-label, controlled trial. Lancet N. Am. Ed. 2017;39(10118):309–318. doi: 10.1016/S0140-6736(17)32812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Brigham GS, Cohen AJ., Rotrosen J. NIDA clinical trials network CTN-0051, extended-release naltrexone vs. buprenorphine for opioid treatment (X-BOT). study design and rationale. Contemp. Clin. Trials. 2016;50:253–264. doi: 10.1016/j.cct.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O'Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269(15):1953–1959. [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J. Subst. Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Monico LB, Gryczynski J, Gwin Mitchell S, Schwartz RP, O’Grady KE, Jaffe JH. Buprenorphine treatment and 12-Step meeting attendance: conflicts, compatibilities, and patient outcomes. J. Subst. Abuse Treat. 2015;57:89–95. doi: 10.1016/j.jsat.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ID, Schroeder JR, Preston KL, Covi L, Umbricht A, Contoreggi C, Fudala PJ, Johnson RE, Gorelick DA. Influence of psychotherapy attendance on buprenorphine treatment outcome. J. Subst. Abuse Treat. 2020;28:247–254. doi: 10.1016/j.jsat.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EV, Lee JD, Sisti D, Segal A, Caplan A, Fishman M, Bailey G, Brigham G, Novo P, Farkas S, Rotrosen J. Ethical and safety considerations in the design of an effectiveness trial: a comparison of buprenorphine versus naltrexone for opioid dependence. Contemp. Clin. Trials. 2016;51:34–43. doi: 10.1016/j.cct.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbaum N, Kluwig J, Specka M, Krause D, Merget B, Finkbeiner T, Gastpar M. Group psychotherapy for opiate addicts in methadone maintenance treatment – a controlled trial. Eur. Addict. Res. 2005;11:163–171. doi: 10.1159/000086397. [DOI] [PubMed] [Google Scholar]
- Sobell JL, Sobell MB. Measuring Alcohol Consumption. The Human Press, Inc.; 1992. Timeline follow-back: a technique for assessing self-reported alcohol consumption. Litten R, Allen J, eds. [Google Scholar]
- Sokol R, LaVertu AE, Morrill D, Albanese C, Schuman-Oliver Z. Group-based treatment for opioid use disorder: a systematic review. J. Subst. Abuse Treat. 2017;84:78–87. doi: 10.1016/j.jsat.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Marcovitz DE, Hilton BT, Fitzmaurice GM, McHugh K, Carroll KM. Correlates of opioid abstinence in a 42-month posttreatment naturalistic follow-up study of prescription opioid dependence. J. Clin. Psychiatry. 2019;80(2) doi: 10.4088/JCP.18m12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch. Gen. Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [PubMed: 22065255] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd RP, Presnall N, Stringfellow E, Wood C, Horn P, Duello A, Green L, Rudder T. The case for a medication first approach to the treatment of opioid use disorder. Am. J. Drug Alcohol Abuse. 2019;45(3):333–340. doi: 10.1080/00952990.2019.1605372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.