Abstract
Non-invasive brain stimulation has been increasingly recognized for its potential as an investigational, diagnostic and therapeutic tool across the clinical neurosciences. Transcranial magnetic stimulation (TMS) is a non-invasive method of focal neuromodulation. Diagnostically, TMS can be used to probe cortical excitability and plasticity, as well as for functional mapping. Therapeutically, depending on the pattern employed, TMS can either facilitate or inhibit stimulated cortex potentially modulating maladaptive physiology through its effects on neuroplasticity. Despite this potential, applications of TMS in neurology have only been approved for diagnostic clinical neurophysiology, pre-surgical mapping of motor and language cortex, and the treatment of migraines. In this article, we discuss the principles of TMS and its clinical applications in neurology, including experimental applications in stroke rehabilitation, seizures, autism spectrum disorder, neurodegenerative disorders, movement disorders, tinnitus, chronic pain and functional neurological disorder. To promote increased cross-talk across neurology and psychiatry, we also succinctly review the TMS literature for the treatment of major depression and obsessive compulsive disorder. Overall, we argue that larger clinical trials that are better informed by circuit-level biomarkers and pathophysiological models will lead to an expansion of the application of TMS for patients cared for by neurologists.
Keywords: neuromodulation, transcranial magnetic stimulation, neuropsychiatry
Over the past two decades, interest in non-invasive brain stimulation as a therapeutic tool has increased dramatically. These modalities are believed to exert their therapeutic effects by altering cortical excitability locally (at the site of stimulation) as well as across neural networks (through trans-synaptic spread from the stimulation site). Translationally, these techniques have mostly been applied in psychiatric disorders. However, there have been several studies investigating the putative utility of these modalities in neurological disease.
This review will focus on neurological applications of transcranial magnetic stimulation (TMS), a common form of non-invasive brain stimulation. First, we will provide a brief overview of the principles underlying TMS and how TMS is differentially applied to alter neurophysiology. Second, we will review putative applications of TMS across several neurological and psychiatric diseases including migraine, stroke rehabilitation, neurodegenerative disorders, epilepsy, tinnitus, autism spectrum disorders, functional neurological disorder, major depressive disorder, and obsessive-compulsive disorder. In each case, we offer an overview of studies examining the clinical utility of TMS in each condition, including the TMS parameters and targets employed.
Principles and Mechanisms of TMS
First introduced by Barker and colleagues in 1985,1 TMS is based on Faraday’s principle of electromagnetic induction. Specifically, a TMS stimulator (consisting of a capacitor and charging unit) passes a high-intensity, rapidly changing current through looped windings encased in a plastic case (the TMS coil). This coil is placed on the scalp, and the current passing through it generates a rapidly fluctuating magnetic field with its primary vector perpendicular to the direction of current flow, i.e., toward the intracranial space (Fig. 1). Magnetic fields move through space unimpeded as long as they do not interact with materials able to conduct electricity. Therefore, the magnetic field generated by the TMS coil diffuses through the scalp, skull, and dura fundamentally unchanged (though it becomes weaker as it moves away from the source), until it reaches the cortex (which is conductive). There, cortical neurons act as “pick-up coils” and the magnetic field is transformed into a secondary electrical field. This results in neuronal depolarization and action potentials which then spread trans-synaptically through a circuit of interconnected neurons.2,3
Fig. 1.
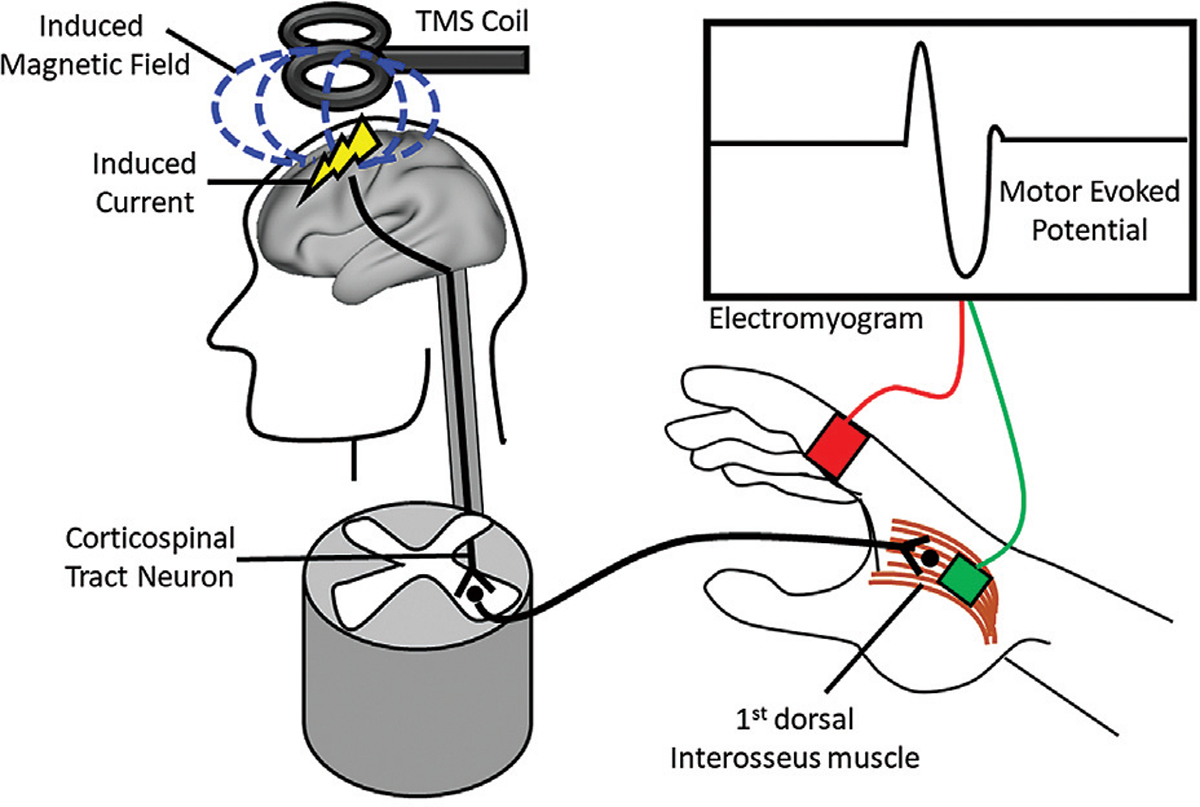
Schematic of how a motor evoked potential (MEP) is evoked by single-pulse TMS (Left). A high-intensity, rapidly changing current is passed into a TMS coil. This coil is placed on the scalp, and the current passing through it generates a rapidly fluctuating magnetic field (blue dashed lines) with its primary vector perpendicular to the direction of current flow. The magnetic field diffuses through the skull until it reaches the hand region of primary motor cortex. There, the magnetic field is transformed into a secondary electrical field, which causes neuronal depolarizations which are spread trans-synaptically from the descending corticospinal tract neuron to the exiting lower motor neuron in the ventral spinal cord. (Right) The lower motor neuron synapses on the 1st dorsal interosseous muscle of the contralateral hand causing the index finger to abduct. An electromyogram (EMG) records the motor evoked potential (MEP) through placement of electrodes on the muscle belly (green).
It is important for TMS researchers and clinicians to be well acquainted with the parameter space, as any clinical, cognitive, or physiological effects will depend on specific parameter choices. One can consider five primary parameters of relevance: location, focality, frequency, pulse intensity, and duration. The first two are primarily anatomical while the last three are primarily physiological.
The location of stimulation is critical as it will determine what cortical neurons and circuits are modulated, and hence will influence what brain functions are affected, or what pathophysiological mechanisms are therapeutically engaged. TMS has the advantage of being non-invasive, allowing it to modulate cortical neurons safely and painlessly in health and disease. However, a disadvantage of being non-invasive is the fact that it can be difficult to determine the precise location of the cortical regions being targeted. Nonetheless, given the focality of the technique, it is imperative to estimate the functional neuroanatomy of the region being stimulated with the best possible precision. When stimulating eloquent cortex, particularly the primary motor and primary visual cortices, functional mapping approaches can be used: a single pulse of TMS over M1 will induce a muscle activation, and over V1 the perception of a phosphene. Therefore, even if we cannot see the motor or visual cortex, when TMS pulses lead to these behavioral and perceptual effects, we know we are targeting the corresponding anatomical targets. Localization is more complex when aiming for stimulation targets outside of sensorimotor regions. For that, population-based approaches have been established, including the use of the 10 to 20 international EEG system that establishes broad relationships between surface skull landmarks and the underlying neuroanatomical regions. While these approaches are reasonably helpful, non-expensive, fast and easy to apply, they are inherently inaccurate and unable to address functional and structural individual differences. For greater accuracy and individualization, stereotactic neuronavigation technologies exist that allow the co-registration of the subject’s head to a previously acquired brain image (typically an MRI), allowing the dynamic precise visualization of the patient’s brain region underlying the TMS coil as we move it around the patient’s head. While structural neuroimaging has traditionally been used to guide stimulation, the use of functional MRI has been popularized in recent years.4 Although the potential of using individual functional maps is certainly attractive for basic and therapeutic applications, technical issues related to the inherent individual variability of functional MRI need to be addressed to improve the reproducibility of functional targets, which is important for therapeutic efficacy.5 Stereotactic neuronavigation systems are ubiquitous in TMS research laboratories, as the advantage of using neuronavigation has been well established for scientific applications, facilitating greater effect sizes, and reducing variance by improving the anatomical precision of the stimulation.6 Although the use of neuronavigation is a critical component of certain clinical diagnostic applications (e.g., pre-surgical cortical mapping), its use in therapeutic TMS clinics (particularly in non-academic settings, where the majority of TMS treatments take place) is still uncommon. Moreover, whereas the theoretical benefits of individualized specificity are clear, and smaller studies have demonstrated the therapeutic advantage of using individualized targeting,7,8 large studies confirming the greater efficacy and safety of neuronavigated TMS versus standard protocols based on fiducial markers will probably be required to justify the additional cost (and insurance reimbursement) of neuronavigated therapeutic TMS.
Beyond the location, the determination of the depth and focality of stimulation has critical functional implications. Depth and focality are always linked to one another in TMS, as any choice that increases depth will reduce focality, and vice-versa. Depth and focality are partially determined by the intensity of the stimulation (e.g., the greater the pulse intensity or energy applied, the greater the depth, and the lesser the focality), as well as by the anatomy of an individual’s head and the coil orientation.9 Still, the architecture and design of the TMS coil are the primary variables defining depth and focality. TMS coils exist in many configurations, but they are typically versions of two main models: circular coils or “figure-of-eight” coils. Circular coils are the simplest and consist of a single loop leading to diffuse non-focal stimulation. These were the original coils but they are rarely used presently with the exception of some neurophysiological applications. The most common coil design is the “figure-of-eight” or “butterfly” coil, characterized by two intersecting loops of conductive material (e.g., copper wires) that allow for the production of highly focal electrical fields at the intersection point.2,10 Newer coils, e.g., H-coils, purport to penetrate to deeper cortical structures. However, these coils tend to produce less focal and more diffuse electrical fields.11 In addition to modulating activity focally, the effects of TMS can also propagate trans-synaptically to regions that are anatomically and functionally connected to the stimulation site. This has been used to modulate activity at the network level with TMS (Fig. 1).4,12
Once these anatomical parameters have been worked out, additional variables of predominant physiological relevance must be determined. TMS paradigms vary with respect to the patterns of stimulation that are administered (Table 1). TMS can be applied as single pulse of stimulation, as paired pulses, or as trains of repetitive stimulation (repetitive TMS, rTMS). Each of these induces different effects upon the direction and duration of cortical excitability and each has different investigational, diagnostic, and therapeutic applications.13 Perhaps the most common application of single pulse TMS is in the determination of the motor threshold (MT) (Fig. 1). This is assessed as the lowest percentage of maximum stimulator output, following stimulation of primary motor cortex, needed to elicit a motor evoked potential (MEP) in a contralateral muscle on 50% of trials.14 The MT is used in physiology as a measure of cortical excitability, and commonly used as an individualized metric of stimulation intensity in research and clinical paradigms. For example, a TMS study or clinical protocol may involve stimulation at 80 to 120% of a person’s MT. Single pulses of TMS have also been used to disrupt local cortical activity, generating a so-called “virtual lesion.”15 Early TMS studies often used this virtual lesion approach to probe brain–symptom relationships by impairing cortical function in a pre-determined region and surveying the behavioral impact of this perturbation. As the name implies, paired pulse TMS involves the application of two pulses in rapid succession: a conditioning stimulus, followed milliseconds later by a test stimulus. Paired pulse TMS is typically used in basic and clinical neurophysiology to measure inhibitory and excitatory central mechanisms of mechanistic and diagnostic relevance.16,17 Additionally, translational and clinical research has explored the role of paired pulse TMS as a diagnostic, prognostic, and response biomarker.18,19 Specific paired-pulse measures include short-interval cortical inhibition (SICI), long-interval cortical inhibition, short-latency afferent inhibition, and intracortical facilitation. Interhemispheric inhibition is used to assess motor excitability across the corpus callosum.20 Finally, repetitive TMS (rTMS) is commonly used to induce long-lasting neuroplastic changes, including as part of therapeutic applications. Repetitive TMS is often divided into low-frequency (i.e., 1 Hz) and high-frequency (i.e., >5 Hz) stimulation paradigms. These patterns are believed to have different effects upon cortical excitability—with low-frequency rTMS being inhibitory and high-frequency being excitatory.20,21 Another form of rTMS, theta burst stimulation (TBS) uses more complex patterns of stimulation that combine different frequencies: commonly delivered as triplet pulses at 50 Hz every 200 ms (e.g., at 5 Hz).22 Similar to traditional rTMS, a variety of different TBS protocols exist that are capable of exciting or inhibiting the target cortical region in a parameter-dependent manner: continuous TBS is inhibitory while intermittent TBS (iTBS) is excitatory.23,24 TBS is particularly interesting given that its mechanism of action is thought to be the induction of long-lasting neuroplasticity: a course of 40 to 180 seconds (compared with 20 to 40 minutes of traditional rTMS) can lead to measurable neuroplastic physiological changes lasting more than 1 hour, much longer than the effects of traditional rTMS.
Table 1.
Transcranial magnetic stimulation (TMS) parameters
| TMS pattern | Frequency | Sustained effect upon motor excitability |
|---|---|---|
| Single pulse | N/A | None |
| Low-frequency rTMS | 1 Hz | Cortical inhibition |
| High-frequency rTMS | ≤5Hz | Cortical excitation |
| Continuous theta-burst stimulation | Single pulse triplets delivered at 50 Hz every 200 ms | Cortical inhibition |
| Intermittent theta-burst stimulation | TBS 2 second on– 8 second off |
Cortical excitation |
The concept of TMS dose is complex and dependent on several factors.25 Generally speaking, the main variables determining the quantity of stimulation are the pulse intensity and the stimulation duration. The pulse intensity is the amount of energy delivered with every pulse. This can be measured using physical units (e.g., Tesla) but is generally measured as a given percentage of the maximum output of a machine (in absolute terms), or as a percentage of a subject’s MT (in relative terms). While pulse intensity measures how much energy is applied by the TMS coil, several factors, including brain and non-brain head anatomy, coil position and angle (among others) also determine how much energy actually reaches the brain. Electric field modeling techniques allow an estimation of the actual dose and its topographic distribution in the brain.9,26 Beyond the energy of a single pulse, the number or pulses in a given TMS session is also relevant in defining the dose. Similarly, in a therapeutic context, the number of sessions in a given course of treatment is relevant.
A careful understanding of the mechanisms of TMS and the biological impact of the parameter space is critical for the appropriate clinical application of this noninvasive neuromodulation technology. In the next section, we will review the diagnostic and therapeutic applications of TMS in neurology and in neuropsychiatry.
Diagnostic Applications
Clinical Neurophysiology
TMS has most commonly been used to probe the integrity of the motor system. Cortical excitability is generally measured using the motor threshold as described above, and more dynamically using input–output curves which map the amplitude of the induced MEPs as a function of the pulse intensity. Paired pulse protocols, as described above, can be used clinically to identify inhibitory and excitatory dynamics and cortico-cortical connectivity.13,16 Corticospinal tract integrity can be assessed by measuring the central motor conduction time (CMCT). This is done by assessing the MEP following stimulation of primary motor cortex and following stimulation of cervical spinal roots (through placement of the coil over these roots). The difference in MEP onset latency between these two is then calculated, and this represents the CMCT.10,27 Potential uses of the CMCT include the detection of a myelopathic process (e.g., from multiple sclerosis or cord compression) and the determination of the spinal level at which this is occurring. The International Federation of Clinical Neurophysiology has published guidelines with detailed descriptions of technical considerations and diagnostic applications.17
TMS metrics of motor excitability can also be used as biomarkers of drug efficacy. For example, a recent study examined whether the drug ezogabine, when compared with placebo, decreased pathological cortical hyperexcitability in patients with amyotrophic lateral sclerosis. Ezogabine was found to increase both the SICI and the resting MT in these patients, supporting the hypothesis that the drug reduces this putative pathophysiologic mechanism of the disease.18
Pre-surgical Mapping
As mentioned above, single pulse TMS can be used to modulate neural excitability in a highly focal manner, and in so doing transiently alter cortical function. This has been leveraged to interrogate motor and language function in cortical regions so as to avoid resection of these regions during neuro-oncological and epilepsy surgery.28–30 More specifically, neuronavigation is employed to map TMS targets near an excision site in a patient’s brain (e.g., around a glioma). These targets are then transiently “functionally lesioned” through TMS to assess whether they meaningfully contribute to a patient’s language or motor function, and if they do, these regions are not resected. Notably, neuronavigated TMS has demonstrated accuracy profiles in detecting “eloquent” motor and language cortex which are similar to that of more invasive techniques.27
Therapeutic Applications
Migraine
Currently, the only U.S. Food and Drug Administration (FDA) 31 that cleared therapeutic application of TMS in neurology is for migraine with aura. This is supported by at least five randomized controlled studies.32 Specifically, TMS has been approved as an abortive therapy in which patients self-administer single TMS pulses to their occiput using a hand-held device: the Cerena device—later renamed the SpringTMS device (eNeura, Sunnyvale, California). TMS has been shown to disrupt cortical spreading depression associated with migraine in rats.33 In humans,34 a sham-controlled study randomized 201 individuals to receive either active or sham single pulse TMS. The primary outcome measure was being pain-free at intervals of 2, 24, and 48 hours after stimulation. At each of these intervals, active TMS was superior to sham.35
Chronic (Non-migrainous) Pain
TMS has been explored to alleviate chronic neuropathic pain. A 2014 review found 19 controlled studies in which rTMS was used for chronic neuropathic pain.36 In many of these studies, primary motor cortex (contralateral to the side where pain is experienced in the case of unilateral pain) has served as the target. This is partially based on the premise that stimulation of sensorimotor cortex will affect descending pathways to the sensory thalamus and brain-stem.37 High-frequency rTMS has proved the most effective with low-frequency rTMS appearing to be ineffective.36,38 Evidence for the utility of TMS in other chronic pain syndromes (e.g., fibromyalgia, complex regional pain syndrome, and chronic visceral pain) has been less compelling.31
Stroke Recovery and Rehabilitation
There is a long history of using TMS as a means of improving and/or accelerating recovery following stroke. The use of TMS in stroke recovery is predicated on the existence of ipsilesional inhibition and contralesional excitation following stroke. TMS has therefore been used either to activate ipsilesional motor cortex with high-frequency rTMS or iTBS, or to inhibit contralesional motor cortex with low-frequency rTMS or cTBS (thus preventing maladaptive inter-hemispheric inhibition). A large pivotal study (the NICHE trial) recently assessed the efficacy of neuronavigated rTMS using the Nexstim Navigated Brain Therapy device (Helsinki, Finland). Neuronavigated 1 Hz rTMS was delivered to the contralesional hemisphere and this was compared with sham stimulation. However, despite several smaller open-label trials showing benefits of this approach, 1 Hz rTMS proved to be no better than sham on motor recovery metrics in the first 3 months following stroke.39
TMS has also been used for improvement of aphasia and hemi-spatial neglect after stroke. Paralleling work done in motor strokes, aphasia has been treated with low-frequency (inhibitory) stimulation of the contralateral (right) inferior frontal gyrus (near Broca’s area), and neglect has been treated with low-frequency (inhibitory) stimulation of contralateral (left) parietal cortex. However, many of these studies have explored the use of TMS in the chronic stage of stroke recovery, with fewer protocols investigating the utility of TMS in acute and subacute recovery phases.40 This fact, coupled with small and heterogeneous patient samples and a lack of randomized controlled studies, has made the adoption of neuromodulation in stroke recovery limited.41
Epilepsy
TMS protocols in epilepsy can be divided into those that seek to abort an ongoing seizure, e.g., during status epilepticus or epilepsia partialis continua,42 and those which aim to decrease seizure frequency by stimulating patients in the interictal period. These protocols have in common the use of “inhibitory” paradigms, such as 1 Hz rTMS or cTBS. This is based off the premise of suppressing cortical hyperexcitability. Success in these protocols has been linked to the degree to which a seizure focus on the cortical surface can be directly stimulated with TMS, or whether a cortically accessible target can be stimulated that is anatomically connected to the seizure focus.27,43 In contrast, when foci are deep, or when multiple epileptogenic foci are present, success rates are far lower.43
Suppression of ongoing seizures with TMS has shown promise, but this has only been demonstrated through eight case reports and case series,42 and randomized controlled trials are currently lacking. In contrast, a handful of sham-controlled trials have investigated the use of inhibitory TMS paradigms in the interictal period between seizures. These trials have thus far yielded conflicting results, in part because of heterogeneity with respect to whether seizure foci were accessible to the TMS coil.43
Movement Disorders
TMS has been extensively explored as a treatment for motor symptoms in Parkinson’s disease (PD), with over a 100 papers dedicated to this topic.36 The most common targets for this have included primary motor cortex and supplementary motor cortex,44 with the best efficacy obtained following high-frequency rTMS to bilateral primary motor cortex.36 Targeted symptoms have included bradykinesia and freezing.27 While various paradigms have been tried, effect sizes of reduction in UPDRS scores appear to be greatest when stimulation is given in the off-medication state and when TMS is delivered over multiple sessions.44,45 The use of TMS as a treatment for levodopa-induced dyskinesias has been explored, e.g., with low-frequency rTMS.27 However, there have not been large, controlled studies to support its use for this indication.36
Similar cortical targets, i.e., primary motor cortex and supplementary motor cortex, have been assessed in focal dystonia. In these cases, however, TMS is almost always delivered as inhibitory stimulation (i.e., low-frequency rTMS).46 Overall, studies using TMS to treat dystonia have been underwhelming, in part because of the heterogeneity of these trial designs.
Neurodegenerative Disease
There has been a considerable interest in using non-invasive brain stimulation for Alzheimer’s disease (AD) and related disorders. Due to the relatively superficial penetration of TMS, this modality is unlikely to be able to reach limbic structures commonly associated with AD (e.g., hippocampus, parahippocampal gyrus, entorhinal cortex).47 Therefore, heteromodal association cortex is often targeted. A particularly appealing target is the angular gyrus, as this region is structurally and functionally connected to the hippocampal formation.12 In fact, stimulation of this region with high-frequency rTMS has been shown to improve memory performance in cognitively normal older adults.48
Recent reviews have identified approximately 10 to 15 trials studying the utility of rTMS in improving cognition in AD.47,49 Outcome measures for these have included language metrics (e.g., action naming) as well as global cognition metrics such as the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog).47,49 For example, one large study, the NeuroAD trial (Neuronix, Yoqneam, Israel), involved 10 Hz rTMS delivered to six different cortical sites. At each site, cognitive training was provided together with rTMS.50 However, despite some positive effects, an FDA advisory panel voted against recommending this system for the treatment of AD in 2019.
Similar to its use in aphasia recovery following stroke, TMS has been studied as a means of improving expressive speech in primary progressive aphasia (PPA). Common target sites include the left inferior frontal gyrus or caudal middle frontal gyrus (again, near Broca’s area). In a recent study, 20 PPA patients showed improvement in spontaneous speech after repeated sessions of rTMS. However, target sites varied widely across these patients. Also, patients with non-expressive deficits were included (semantic variant PPA). Lastly, only a subset of patients underwent a control condition as part of a crossover design.51 A recent meta-analysis supported the efficacy of TMS in improving naming in PPA patients.52
Autism Spectrum Disorder
Several studies have used TMS to examine neurobiological abnormalities in autism spectrum disorder (ASD). These studies have identified abnormalities in cortical plasticity and excitation/inhibition imbalance.53 A 2016 review identified 29 studies that probed the therapeutic potential of TMS in ASD.53 Therapeutic studies have focused on treating specific symptoms associated with this condition. The majority of these trials have demonstrated some efficacy using low-frequency, inhibitory rTMS (0.5–1 Hz) to dorsolateral prefrontal cortex (DLPFC) as a treatment for irritability and repetitive behaviors.54,55 Fewer studies have employed excitatory (i.e., high-frequency) rTMS paradigms to activate medial prefrontal cortex in ASD patients. This approach is based on the evidence that this region plays a significant role in mentalizing and social perspective taking (core impairments in ASD).56 A major limitation of TMS studies in ASD is the variability of the experimental methodology (e.g., the severity of the patient population studied).55 As such, it has been difficult to draw generalizations regarding findings.
Traumatic Brain Injury
The majority of TMS applications in traumatic brain injury (TBI) have been diagnostic. For example, some studies have found altered cortical excitability and altered intra-cortical inhibition and facilitation.57 Therapeutic studies for cognitive recovery in TBI have yet to demonstrate significant effects, and there have been a paucity of studies examining this. For example, one study found no improvement in executive function (i.e., performance on Trail-Making Test part B) in patients with chronic severe TBI following 10 sessions of high-frequency rTMS to the left DLPFC.58
Tinnitus
While paradigms for treating tinnitus with TMS have varied, the majority have used inhibitory stimulation delivered to primary auditory cortex. Furthermore, this is typically delivered to the contralateral hemisphere from which the tinnitus is experienced when the symptom is unilateral.36 Alternative strategies have attempted stimulation of top-down inhibitory centers (e.g., DLPFC or pre-supplementary motor cortex, pSMA) taking a circuit-based approach to trans-synaptically modulate the maladaptive sensory computations and symptoms of tinnitus, as well as core cognitive and affective processes.59 While symptom alleviation has been observed, tinnitus reduction tends to be transient and partial, with reports of complete resolution being mixed.36 Variability in responses may be due to patient-specific factors (e.g., symptom duration or the presence of hearing loss).27 In addition, a recent review examined the efficacy of cTBS in tinnitus—7 controlled trials were identified, with 4/7 being sham-controlled. Of these four, two demonstrated a significant effect of active cTBS.60
Functional Neurological Disorder
TMS has also been investigated as a putative treatment for functional neurological disorder (FND). These studies have mostly explored the utility of TMS in treating functional motor symptoms, e.g., functional limb weakness or functional movement disorders. Relatedly, the target for TMS in these studies is often primary motor cortex.61–64 In these studies, therapeutic effects of TMS are theorized to occur either through its neuromodulatory impact or through psychological factors—e.g., a patient with a functionally paretic limb witnessing that this limb can be moved through exogenous stimulation with TMS.63 However, such studies are significantly limited by the heterogeneity of the population being studied, the specific TMS parameters applied, and the FND outcome measures used.62,63 Moreover, while many of these protocols demonstrated some positive effects, very few were sham-controlled,62 making claims about neuromodulatory effects dubious.
Major Depressive Disorder
Perhaps the most established clinical indication for TMS is in the treatment of Major Depressive Disorder (MDD). A seminal multisite, randomized, sham-controlled trial by O’Reardon et al65 led to the FDA clearing TMS for MDD in 2008. Following this, the so called “deep” TMS using H-coils (H1 model in particular) was also cleared for MDD.66 The efficacy of TMS in treatment-resistant MDD has since been supported by several sham-controlled trials and meta-analyses,67 and, as of the writing of this article, there are several devices which are FDA-approved for the treatment of MDD. In MDD, TMS is administered in a repetitive fashion, either as traditional rTMS or as TBS, to the DLPFC. Notably, a recent trial established that TBS was not inferior to traditional rTMS,68 which is significant as TBS can be delivered in briefer sessions (i.e., 3 vs. 38 minutes for the original FDA cleared 10 Hz protocol) as noted above. Treatment duration in MDD is typically 36 sessions, with 30 sessions administered every weekday (for 6 weeks) followed by a six session taper over two additional weeks. Clinicians will sometimes choose targets and stimulation paradigms based on a patient’s clinical presentation. For example, patients presenting with predominantly anhedonic depression are treated with high-frequency (e.g., 10 Hz) rTMS or iTBS in the left DLPFC, while those with predominantly anxio-somatic presentations or Cluster B character pathology are treated with low-frequency (e.g., 1 Hz or cTBS) in the right DLPFC.
Obsessive Compulsive Disorder
As expected given the differences in circuit pathophysiology between MDD and obsessive compulsive disorder (OCD), TMS treatment targets for OCD differ from those used in MDD. The FDA cleared protocol involves excitatory stimulation to the dorsomedial prefrontal cortex/anterior cingulate cortex using deep coils. including H-coils and also figure-of-eight configurations designed to reach deeper structures. Treatment duration is comparable to MDD, and clinical progress is often monitored through the administration of the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). The TMS protocol for OCD is accompanied by a behavioral intervention (i.e., symptom provocation,69 e.g., asking patients with contamination symptoms to picture unsanitary items during treatment) before each TMS session. This is a strategy to modulate the state of the network and have this modulate the effects of TMS enhancing its therapeutic efficacy.
Finally, given the proven efficacy of TMS in MDD, it has been explored as a treatment for depressive symptoms occurring in the context of other neurological disorders. For example, TMS has been explored as a means of treating depression in the setting of PD70 and in post-stroke depression.71 Despite these preliminary findings, no specialized protocols have been adopted for treating depression or OCD in neurological conditions, and default parameters used in for treating these in non-neurological populations are often used.
Conclusion
TMS is currently FDA cleared for therapeutic applications in acute migraine management, in addition to MDD, OCD and for smoking cessation. It is also approved as a neurophysiological diagnostic tool for multiple pathologies affecting the motor system and for mapping motor and language function prior to neurosurgery. Expansion of the diagnostic and therapeutic applications of TMS to other neurological disorders has thus far been hampered by a lack of randomized controlled studies or by studies with heterogeneous or small patient populations. Importantly, studies have also been limited by an incomplete understanding of the neurobiological mechanisms underlying neurological and neuropsychiatric conditions, particularly at the circuit level (e.g., excitation/inhibition imbalance, aberrant neuroplasticity, neurotransmitter abnormalities and aberrant functional network architecture). Furthermore, TMS paradigms are currently not optimized with respect to where stimulation is applied and with what pattern. The number of successful TMS applications is likely to increase with the use of more tailored paradigms in larger clinical trials informed by circuit-level biomarkers and pathophysiological models.
Funding
M.C.E. reports grants or contracts from the the National Institutes of Health (NIH). J.A.C. reports grants or contracts from NIH, Harvard Brain Initiative, AE foundation, Gerstner Foundation, and Solinsky Foundation; Royalties or licenses from Springer; Consulting fees from Neuronetics; Patents planned, issued or pending from system and method for simultaneous electric field simulation and neuronavigation for TMS; Participation on a Data Safety Monitoring Board or Scientific Advisory Board from Hyka Therapeutics (SAB) and Feelmore Labs (SAB); Leadership or fiduciary role in other board, society, committee, or advocacy group from Program Committee ACNP, SOBP, ANPA; and stock or stock options from Hyka Therapeutics and Feelmore Labs. Participation on a Data Safety Monitoring Board or Advisory Board for Merck, Lilly; and Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for Alzheimer’s Association (Association for Frontotemporal Degeneration).
Footnotes
Conflict of Interest
M.C.E. has no financial interests to disclose. B.C.D. has been a consultant for Acadia, Alector, Arkuda, Biogen, Denali, Lilly, Merck, Novartis, Takeda, Wave Lifesciences. J. A.C. is a member of the Scientific Advisory Board of Hyka Therapeutics and Feelmore Labs, and has been a consultant for Neuronetics.
References
- 1.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1(8437):1106–1107 [DOI] [PubMed] [Google Scholar]
- 2.Burke MJ, Fried PJ, Pascual-Leone A. Transcranial magnetic stimulation: neurophysiological and clinical applications. Handb Clin Neurol 2019;163:73–92 [DOI] [PubMed] [Google Scholar]
- 3.Camprodon JA. Transcranial Magnetic Stimulation. In: Camprodon JA, Rauch SL, Greenberg BD, Dougherty DD, eds. Psychiatric Neurotherapeutics: Contemporary Surgical & Device-Based Treatments in Psychiatry. New York, NY: Humana Press (Springer); 2016:165–186 [Google Scholar]
- 4.Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 2012;62(04):2232–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ning L, Makris N, Camprodon JA, Rathi Y. Limits and reproducibility of resting-state functional MRI definition of DLPFC targets for neuromodulation. Brain Stimul 2019;12(01):129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sack AT, Cohen Kadosh R, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci 2009;21(02):207–221 [DOI] [PubMed] [Google Scholar]
- 7.Schönfeldt-Lecuona C, Lefaucheur JP, Cardenas-Morales L, Wolf RC, Kammer T, Herwig U. The value of neuronavigated rTMS for the treatment of depression. Neurophysiol Clin 2010;40(01):37–43 [DOI] [PubMed] [Google Scholar]
- 8.Kim WJ, Min YS, Yang EJ, Paik NJ. Neuronavigated vs. conventional repetitive transcranial magnetic stimulation method for virtual lesioning on the Broca’s area. Neuromodulation 2014;17(01):16–21, discussion 21 [DOI] [PubMed] [Google Scholar]
- 9.Lee EG, Rastogi P, Hadimani RL, Jiles DC, Camprodon JA. Impact of non-brain anatomy and coil orientation on inter- and intra-subject variability in TMS at midline. Clin Neurophysiol 2018;129(09):1873–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallett M Transcranial magnetic stimulation: a primer. Neuron 2007;55(02):187–199 [DOI] [PubMed] [Google Scholar]
- 11.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 2013;6(01):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A 2011;108(52):21229–21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camprodon JA, Pascual-Leone A. Multimodal applications of transcranial magnetic stimulation for circuit-based psychiatry. JAMA Psychiatry 2016;73(04):407–408 [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Hallett M, Rossini PM, Pascual-Leone ASafety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120(12):2008–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol 2000;10(02):232–237 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol 2003;2(03):145–156 [DOI] [PubMed] [Google Scholar]
- 17.Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2012;123(05):858–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wainger BJ, Macklin EA, Vucic S, et al. Effect of ezogabine on cortical and spinal motor neuron excitability in amyotrophic lateral sclerosis: a randomized clinical trial. JAMA Neurol 2021;78(02):186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunse T, Wobrock T, Strube W, et al. Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimul 2014;7(02):158–169 [DOI] [PubMed] [Google Scholar]
- 20.Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev 2017;83:381–404 [DOI] [PubMed] [Google Scholar]
- 21.Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994;117(Pt 4):847–858 [DOI] [PubMed] [Google Scholar]
- 22.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45(02):201–206 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y-Z, Sommer M, Thickbroom G, et al. Consensus: new methodologies for brain stimulation. Brain Stimul 2009;2(01):2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol 2007;118(05):1028–1032 [DOI] [PubMed] [Google Scholar]
- 25.Peterchev AV, Wagner TA, Miranda PC, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul 2012;5(04):435–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thielscher A, Antunes A, Saturnino GB. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? Annu Int Conf IEEE Eng Med Biol Soc 2015;2015:222–225 [DOI] [PubMed] [Google Scholar]
- 27.Eldaief MC, Press DZ, Pascual-Leone A. Transcranial magnetic stimulation in neurology: a review of established and prospective applications. Neurol Clin Pract 2013;3(06):519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefaucheur JP, Picht T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol Clin 2016;46(02):125–133 [DOI] [PubMed] [Google Scholar]
- 29.Umana GE, Scalia G, Graziano F, et al. Navigated transcranial magnetic stimulation motor mapping usefulness in the surgical management of patients affected by brain tumors in eloquent areas: a systematic review and meta-analysis. Front Neurol 2021;12:644198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babajani-Feremi A, Holder CM, Narayana S, et al. Predicting postoperative language outcome using presurgical fMRI, MEG, TMS, and high gamma ECoG. Clin Neurophysiol 2018;129(03):560–571 [DOI] [PubMed] [Google Scholar]
- 31.Young NA, Sharma M, Deogaonkar M. Transcranial magnetic stimulation for chronic pain. Neurosurg Clin N Am 2014;25(04):819–832 [DOI] [PubMed] [Google Scholar]
- 32.Lan L, Zhang X, Li X, Rong X, Peng Y. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J Headache Pain 2017;18(01):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain 2016;139(Pt 7):2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 2010;9(04):373–380 [DOI] [PubMed] [Google Scholar]
- 35.Barker AT, Shields K. Transcranial magnetic stimulation: basic principles and clinical applications in migraine. Headache 2017;57(03):517–524 [DOI] [PubMed] [Google Scholar]
- 36.Lefaucheur JP, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125(11):2150–2206 [DOI] [PubMed] [Google Scholar]
- 37.Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A. Transcranial brain stimulation: clinical applications and future directions. Neurosurg Clin N Am 2011;22(02):233–251, ix ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosomi K, Shimokawa T, Ikoma K, et al. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. Pain 2013;154(07):1065–1072 [DOI] [PubMed] [Google Scholar]
- 39.Harvey RL, Edwards D, Dunning K, et al. ; NICHE Trial Investigators * Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke 2018;49(09):2138–2146 [DOI] [PubMed] [Google Scholar]
- 40.Smith MC, Stinear CM. Transcranial magnetic stimulation (TMS) in stroke: ready for clinical practice? J Clin Neurosci 2016;31:10–14 [DOI] [PubMed] [Google Scholar]
- 41.Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013;(05):CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuboyama M, Kaye HL, Rotenberg A. Review of transcranial magnetic stimulation in epilepsy. Clin Ther 2020;42(07):1155–1168 [DOI] [PubMed] [Google Scholar]
- 43.VanHaerents S, Chang BS, Rotenberg A, Pascual-Leone A, Shafi MM. Noninvasive brain stimulation in epilepsy. J Clin Neurophysiol 2020;37(02):118–130 [DOI] [PubMed] [Google Scholar]
- 44.Chen KS, Chen R. Invasive and noninvasive brain stimulation in Parkinson’s disease: clinical effects and future perspectives. Clin Pharmacol Ther 2019;106(04):763–775 [DOI] [PubMed] [Google Scholar]
- 45.Chung CL, Mak MK. Effect of repetitive transcranial magnetic stimulation on physical function and motor signs in Parkinson’s disease: a systematic review and meta-analysis. Brain Stimul 2016;9(04):475–487 [DOI] [PubMed] [Google Scholar]
- 46.Erro R, Tinazzi M, Morgante F, Bhatia KP. Non-invasive brain stimulation for dystonia: therapeutic implications. Eur J Neurol 2017;24(10):1228–e64 [DOI] [PubMed] [Google Scholar]
- 47.Buss SS, Fried PJ, Pascual-Leone A. Therapeutic noninvasive brain stimulation in Alzheimer’s disease and related dementias. Curr Opin Neurol 2019;32(02):292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilakantan AS, Mesulam MM, Weintraub S, Karp EL, VanHaerents S, Voss JL. Network-targeted stimulation engages neurobehavioral hallmarks of age-related memory decline. Neurology 2019;92(20):e2349–e2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonsalvez I, Baror R, Fried P, Santarnecchi E, Pascual-Leone A. Therapeutic noninvasive brain stimulation in Alzheimer’s disease. Curr Alzheimer Res 2017;14(04):362–376 [DOI] [PubMed] [Google Scholar]
- 50.Andrade SM, de Oliveira EA, Alves NT, et al. Neurostimulation combined with cognitive intervention in Alzheimer’s disease (NeuroAD): study protocol of double-blind, randomized, factorial clinical trial. Front Aging Neurosci 2018;10:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pytel V, Cabrera-Martín MN, Delgado-Álvarez A, et al. Personalized repetitive transcranial magnetic stimulation for primary progressive aphasia. J Alzheimers Dis 2021;84(01):151–167 [DOI] [PubMed] [Google Scholar]
- 52.Nissim NR, Moberg PJ, Hamilton RH. Efficacy of noninvasive brain stimulation (tDCS or TMS) paired with language therapy in the treatment of primary progressive aphasia: an exploratory meta-analysis. Brain Sci 2020;10(09):E597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oberman LM, Enticott PG, Casanova MF, Rotenberg A, Pascual-Leone A, McCracken JTTMS in ASD Consensus Group. Transcranial magnetic stimulation in autism spectrum disorder: challenges, promise, and roadmap for future research. Autism Res 2016;9(02):184–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci 2014;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khaleghi A, Zarafshan H, Vand SR, Mohammadi MR. Effects of non-invasive neurostimulation on autism spectrum disorder: a systematic review. Clin Psychopharmacol Neurosci 2020;18(04):527–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enticott PG, Fitzgibbon BM, Kennedy HA, et al. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul 2014;7(02):206–211 [DOI] [PubMed] [Google Scholar]
- 57.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil 2012;27(04):274–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neville IS, Zaninotto AL, Hayashi CY, et al. Repetitive TMS does not improve cognition in patients with TBI: a randomized double-blind trial. Neurology 2019;93(02):e190–e199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minen MT, Camprodon J, Nehme R, Chemali Z. The neuropsychiatry of tinnitus: a circuit-based approach to the causes and treatments available. J Neurol Neurosurg Psychiatry 2014;85(10):1138–1144 [DOI] [PubMed] [Google Scholar]
- 60.Schwippel T, Schroeder PA, Fallgatter AJ, Plewnia C. Clinical review: the therapeutic use of theta-burst stimulation in mental disorders and tinnitus. Prog Neuropsychopharmacol Biol Psychiatry 2019;92:285–300 [DOI] [PubMed] [Google Scholar]
- 61.Pick S, Hodsoll J, Stanton B, et al. Trial Of Neurostimulation In Conversion Symptoms (TONICS): a feasibility randomised controlled trial of transcranial magnetic stimulation for functional limb weakness. BMJ Open 2020;10(10):e037198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollak TA, Nicholson TR, Edwards MJ, David AS. A systematic review of transcranial magnetic stimulation in the treatment of functional (conversion) neurological symptoms. J Neurol Neurosurg Psychiatry 2014;85(02):191–197 [DOI] [PubMed] [Google Scholar]
- 63.Schönfeldt-Lecuona C, Lefaucheur JP, Lepping P, et al. Non-invasive brain stimulation in conversion (functional) weakness and paralysis: a systematic review and future perspectives. Front Neurosci 2016;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McWhirter L, Ludwig L, Carson A, McIntosh RD, Stone J. Transcranial magnetic stimulation as a treatment for functional (psychogenic) upper limb weakness. J Psychosom Res 2016;89:102–106 [DOI] [PubMed] [Google Scholar]
- 65.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007;62(11):1208–1216 [DOI] [PubMed] [Google Scholar]
- 66.Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 2015;14(01):64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul 2016;9(03):336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 2018;391(10131):1683–1692 [DOI] [PubMed] [Google Scholar]
- 69.Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul 2018;11(01):158–165 [DOI] [PubMed] [Google Scholar]
- 70.Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology 2016;87(18):1907–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frey J, Najib U, Lilly C, Adcock A. Novel TMS for Stroke and Depression (NoTSAD): accelerated repetitive transcranial magnetic stimulation as a safe and effective treatment for post-stroke depression. Front Neurol 2020;11:788. [DOI] [PMC free article] [PubMed] [Google Scholar]


