Abstract
Alveolar macrophages (AM) provide one of the first lines of defense against microbial invasion in the lower airways. The role of AM in the clearance of Pseudomonas aeruginosa in mice after intrapulmonary challenge was evaluated. AM were depleted by intranasal administration of liposome-encapsulated dichloromethylene diphosphonate. At 24 h following the instillation of liposomes, a sublethal dose of P. aeruginosa was inoculated intranasally. Spleen, liver, and lung tissue was then evaluated for viable bacteria and for histopathology. AM depletion of 78 to 88% did not affect the survival rate of infected mice or clearance of P. aeruginosa from the spleen, liver, or lung, compared to the control group, but the mice's susceptibility to Klebsiella pneumoniae was greatly enhanced. The recruitment of neutrophils to the lung was also not affected. Freshly explanted AM were not competent to phagocytose unopsonized P. aeruginosa but were able to phagocytose zymosan particles. Further studies were conducted to assess the in situ phagocytic activities of AM. Three hours after the intranasal instillation of P. aeruginosa or other particles, bronchoalveolar lavage was performed. AM phagocytosis of zymosan particles and latex beads exceeded that of P. aeruginosa. Neutrophils were recruited to the lung in response to a high-dose bacterial challenge. These results suggest that AM do not play an important role in defense of the lung against P. aeruginosa.
Pseudomonas aeruginosa is a gram-negative, opportunistic pathogen that causes chronic infections in cystic fibrosis (CF) patients, immunocompromised individuals, and burn patients. Infection of the lung is the leading cause of morbidity and mortality in CF patients. Once acquired, this bacterium is difficult to treat and is rarely eradicated. Most strains of P. aeruginosa show substantial degrees of resistance to a wide variety of antimicrobial agents (12, 21).
Alveolar macrophages (AM), located at the interphase between air and lung tissue, provide the first line of phagocytic defense against microbial invasion in the lower respiratory tract. Besides their phagocytic and microbicidal functions, AM also secrete numerous chemical mediators upon stimulation, thereby playing a role in regulating inflammatory reactions in the lung (14). The role of AM in defending the lung against various pathogens and in regulating inflammatory reactions has been assessed in studies in which AM were depleted. In rodent models, AM can be depleted to 5 to 30% of normal numbers by liposome-encapsulated dichloromethylene diphosphonate (LDMDP) delivered to the pulmonary tract (2, 4, 13, 15–17). The usual fate of liposomes is ingestion and digestion by macrophages. Due to its strong hydrophilic properties, DMDP is not able to escape from the cell when released intracellularly. Therefore, macrophages are selectively depleted without damaging other tissues or cells (27).
AM are the primary phagocytic cells in the uninflamed lower airways. Phagocytosis is categorized into opsonic and nonopsonic mechanisms. Opsonic phagocytosis is mediated by Fc and complement receptors. However, human and mouse AM show minimal expression of receptors for C3b/iC3b (CR1/CR3/CR4). The receptors for immunoglobulin G, Fcγ receptors, are expressed in human AM but negligibly in mouse AM (3, 24). Furthermore, the concentrations of complement and immunoglobulin in the lower airway are minimal; therefore, it is likely that AM utilize nonopsonic, instead of Fc or complement receptor-mediated, phagocytosis to eliminate pathogens such as P. aeruginosa from the lung. We and others have evaluated the in vitro response of macrophages to challenge with P. aeruginosa and other particles (1, 3, 8, 22, 29).
The main objective of this study was to further elucidate the in vivo role of AM in defense of the lung against P. aeruginosa challenge. AM were depleted by the intranasal (i.n.) instillation of LDMDP in mice. AM-depleted mice received a sublethal dose of P. aeruginosa or Klebsiella pneumoniae (as a positive control) by i.n. inoculation. The effects of AM depletion on bacterial clearance in spleen, liver, and lung and on polymorphonuclear leukocyte (PMN) recruitment to the lung were assessed. Secondly, the in vitro phagocytosis of unopsonized P. aeruginosa, zymosan, and latex beads by freshly explanted murine AM was compared to that by peritoneal macrophages (PM). Finally, assessment of the in situ phagocytic activities of AM was performed by challenging mice i.n. with unopsonized P. aeruginosa, zymosan particles, or latex beads and subsequently obtaining bronchoalveolar lavage (BAL) fluid to evaluate BAL cell numbers and the number of ingested particles per cell in the BAL-derived population.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. aeruginosa strain P1 is a nonmucoid, lipopolysaccharide (LPS)-rough derivative of a mucoid isolate from a patient with CF. Strain c2908c was the initial colonizing isolate obtained from a CF patient. Strain PAK (LPS smooth) was obtained from W. Paranchych (Edmonton, Alberta, Canada), PA01 (LPS smooth) was from B. Holloway (Victoria, Australia), and FRD1 was from R. E. W. Hancock (Vancouver, British Columbia, Canada). K. pneumoniae (ATCC 43816) was purchased from the American Type Culture Collection (Manassas, Va.). Bacteria were kept frozen at −70°C in Mueller-Hinton broth with 8.0% dimethyl sulfoxide and grown fresh for each experiment. They were grown with shaking overnight in L broth at 37°C.
Animals.
Specific-pathogen-free (SPF) male CD-1 mice weighing 35 to 37 g were obtained from Charles River Laboratories Canada (St. Constant, Quebec, Canada), and SPF female BALB/c mice weighing 20 to 22 g were obtained from the University of British Columbia Animal Care Centre, Vancouver, British Columbia, Canada. All mice were housed under Canadian Council on Animal Care-approved SPF conditions in the animal care facility at the British Columbia Research Institute for Children's and Women's Health, Vancouver, British Columbia, Canada. The animal procedures were approved by the University of British Columbia Committee on Animal Care, Vancouver, British Columbia, Canada (animal protocol no. A97-0197).
Reagents.
Phagocytosis medium was prepared as described previously (1). Gel-Hanks' balanced salt solution (gHBSS) contained 1× HBSS without sodium bicarbonate and phenol red (Gibco BRL, Grand Island, N.Y.) and 1% gelatin (Gibco BRL).
Preparation of LPBS and LDMDP.
Liposomes were prepared as previously described (28). Briefly, 86 mg of egg phosphatidylcholine (Sigma Canada Ltd., Oakville, Ontario, Canada) and 8 mg of cholesterol (Sigma Canada Ltd.) were dissolved in 10 ml of chloroform in a 500-ml round-bottom flask. The chloroform was evaporated by rotation under vacuum, and a thin phospholipid film was formed around the flask. At room temperature, the lipid was dispersed in 10 ml of phosphate-buffered saline (LPBS) or 0.6 M DMDP (2.5 g in 10 ml of PBS). DMDP was generously provided by Boehringer Mannheim GmbH (Mannheim, Germany). The suspension was kept at room temperature for 2 h under nitrogen gas and then sonicated for 3 min in a Bransonic 12 water bath sonicator (Bransonic Equipment Co., Shelton, Conn.) and kept overnight at 4°C. LPBS and LDMDP were centrifuged and washed three times with sterile PBS at 10,000 × g for 15 min at 4.0°C to remove free DMDP. The final pellet was resuspended in 4 ml of sterile PBS and used immediately or stored at 4°C under nitrogen gas. The liposomes were used within 7 days of preparation.
i.n. instillation of liposomes.
Mice were anesthetized using a dose of 35 mg of ketamine hydrochloride (MTC Pharmaceuticals, Cambridge, Ontario, Canada)/kg of body weight administered intraperitoneally. They were inoculated with 2-μl aliquots of LPBS or LDMDP repeatedly via the external nares for a total volume of 50 μl (25). The entire procedure required 15 to 20 min per mouse. To assess the efficiency of LDMDP in depleting AM, BALs were performed on mice before i.n. challenge with bacteria. The number of AM in BAL fluid from LDMDP-treated mice was then compared to those from the control mice exposed to LPBS. For histologic examination, sections of the lung were excised and fixed in 10% buffered, neutral Formalin (BDH Inc., Toronto, Ontario, Canada), processed in a Fisher histomatic 266 tissue processor, and embedded in paraffin wax blocks. Sections were cut into 2-μm sections and stained with hematoxylin and eosin.
i.n. administration of P. aeruginosa, K. pneumoniae, latex beads, and zymosan.
LPBS and LDMDP were delivered 24 h prior to bacterial infections. An aliquot of the overnight culture of P. aeruginosa was harvested by centrifugation at 13,000 × g for 30 s and resuspended in the same volume of gHBSS. K. pneumoniae was grown overnight to late log phase and then harvested by centrifugation and washed twice with gHBSS for 10 min at 2,075 × g at room temperature. The bacteria were then diluted with gHBSS to the desired dose (in CFU per milliliter). The zymosan particles were prepared as previously described (21). Anesthetized mice were repeatedly inoculated with 2-μl aliquots of the bacterial culture dose (106 to 1010 CFU/ml), latex beads (3 μm), or zymosan; the particles were applied to the external nares in a total volume of 20 μl (25). The K. pneumoniae dose (100 CFU/mouse) was administered as described above in a total volume of 40 μl. The bacteria were kept on ice prior to challenge. The entire procedure required 15 to 20 min per mouse. Serial 1:10 dilutions of the infection inoculum were made in gHBSS, spread on Trypticase soy agar plates (Becton Dickinson, Cockeysville, Md.), and incubated for 18 h at 37°C. The inoculation dose was then evaluated from the viable bacterial CFU.
BAL.
BAL was performed according to procedures described previously (8). The total BAL cell count, excluding erythrocytes, was determined with the aid of a hemocytometer (American Optical, Buffalo, N.Y.) and trypan blue dye exclusion. The differential counts of AM and PMNs were performed on cytocentrifuged preparations using a Cytospin 2 (Shandon Southern Instruments, Sewickley, Pa.) and stained with Diff-Quik (EM Industries Inc., Gibbstown, N.J.).
Analysis of spleen, liver, and lung bacterial CFU and histopathology.
Mice were anesthetized with sodium pentobarbital and killed by cervical dislocation at sequential time points, including 3 h after the i.n. bacterial challenge. Their spleens, livers (gallbladder removed), and lungs were excised and weighed aseptically. A section from each tissue was fixed in 10% buffered, neutral Formalin for histopathology, and the remainder was homogenized for up to 30 s in sterile 10-ml glass tubes with Teflon pestles (Glas-col, Terre Haute, Ind.). Serial 1:10 dilutions of the homogenates were spread on Trypticase soy agar plates and incubated for 18 h at 37°C. Viable bacterial CFU counts were then analyzed. The data are expressed as the means ± standard errors of the means (SEM) CFU (log10) per gram of tissue. For histologic examination, the fixed tissue sections were processed, sectioned, and stained with hematoxylin and eosin.
Preparation of murine resident PM.
Resident PM were obtained from the peritoneal cavities of 6- to 8-week-old SPF female BALB/c mice as previously described (22). Explanted macrophages were kept in complete medium. The number of viable cells was determined by trypan blue exclusion.
In vitro effects of LPBS, LDMDP, and DMDP on murine AM and PM.
AM and PM were plated at 105 cells per acid-washed glass coverslip in Falcon 3047 24-well plastic tissue culture trays (Becton Dickinson, Lincoln Park, N.J.). After 30 min of incubation at 37°C in 5% CO2, 400 μl of complete medium was added to the wells. After the addition of 5, 10, and 15 μl of either LPBS, LDMDP, or DMDP, macrophages were incubated at 37°C in 5% CO2 for 24, 48, and 72 h. At timed intervals, macrophages were washed twice with PBS, fixed with methanol, stained with Giemsa stain (BDH Inc.), and mounted on microscope slides with Entellan mounting medium (Merck, Darmstadt, Germany). The morphology of untreated and treated AM and PM was assessed microscopically. Macrophages with intact cytoplasm and nuclei were considered healthy, while those with no cytoplasm or shriveled cytoplasm and nuclei were considered unhealthy. At least 60 macrophages per coverslip were scored. Each sample was assayed in duplicate, and the experiments were performed two or three times with different macrophage preparations. Data were expressed as means ± SEM.
In vitro phagocytosis assays.
Phagocytosis assays were performed as described previously (1) with two modifications. (i) Two hours after adherence to the coverslips, or 24, 48 and 72 h after incubation with 15 μl of LPBS, LDMDP, and DMDP, macrophages were washed with PBS twice to remove nonadherent cells. (ii) d-Glucose (10 mM) was added at the same time as the bacteria (approximately 107 CFU of unopsonized strain P1 that were shaken overnight). PM were incubated for 60 min with the P1 culture. At least 60 macrophages per coverslip were scored microscopically. The experiments were repeated two or three times with each sample in duplicate. Data were expressed as means ± SEM.
In vitro effects of LPBS, LDMDP, and DMDP on epithelial cell line A549.
Epithelial cell line A549, maintained in F12K (Gibco BRL)–10% fetal calf serum (FCS) (Cansera, Rexdale, Ontario, Canada) was treated with 1× trypsin-EDTA (Gibco BRL) for 15 min. The cell suspension was centrifuged at 454 × g for 10 min at 4.0°C, and resuspended in F12K–10% FCS. Viable cell counts were determined with a hemocytometer by trypan blue exclusion. Then 105 cells (in approximately 100 μl) were plated on acid-washed glass coverslips in 24-well plastic tissue culture trays. After 30 min of incubation at 37°C in 5% CO2, 400 μl of F12K–10% FCS was added to the wells. After the addition of 15 μl of LPBS, LDMDP, or DMDP (600, 50, 5, and 0.5 mM), A549 cells were incubated at 37°C in 5% CO2 for 24 and 48 h. At timed intervals, cells were washed twice with PBS, fixed with methanol, stained with Giemsa, and mounted on microscope slides. The morphology of untreated and treated epithelial cells was assessed microscopically.
In situ phagocytosis assays.
Anesthetized mice were challenged i.n. with 20 μl of P. aeruginosa (FRD1, P1, PAK, PA01, or c2908c), latex beads, or zymosan as described above. Three hours after instillation, BAL was performed. BAL-derived cells were centrifuged at 395 × g for 15 min at 4°C, resuspended, and treated with ice-cold lysozyme to lyse uningested bacteria, as previously described (22). The BAL suspension was centrifuged again and resuspended in 1 ml of PBS. The differential counts of AM and PMNs were performed on Diff-Quik-stained cytocentrifuge preparations of BAL cells. One hundred cells were counted, and the results were expressed as percentages of the total. Data were expressed as means ± SEM.
RESULTS
In vitro effects of LPBS, LDMDP, and DMDP on the morphology and phagocytic capacity of murine PM.
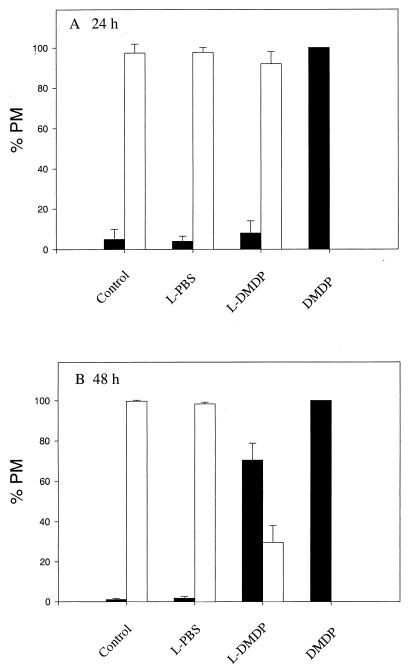
Figure 1 shows that the majority (94 to 99%) of untreated (control) or LPBS-treated PM appeared to be healthy after 48 h. The effects of LDMDP on PM were evident and dose dependent at 48 h (Fig. 1B). Incubation for 48 h with LDMDP (5, 10, and 15 μl) resulted in a substantial percentage (32, 53, and 71%, respectively) of PM with shriveled cytoplasm and nuclei compared to the untreated (1%) and LPBS-treated (0 to 3%) macrophages. As the dose of LDMDP increased, the percentage of unhealthy-looking macrophages increased. At 24 h (Fig. 1A) and 48 h (Fig. 1B), all macrophages incubated with free DMDP appeared to be unhealthy, with no visible cytoplasm.
FIG. 1.
Effects of LPBS, LDMDP, and DMDP on murine PM in vitro. PM, plated on coverslips, were incubated with 15 μl of LPBS, LDMDP, or DMDP (0.6 M stock) for 24 h (A) or 48 h (B). Controls received no treatment. At timed intervals, the morphology of Giemsa-stained PM was assessed microscopically. The percentages of healthy macrophages (□) and unhealthy macrophages (■) were compared. One hundred macrophages per coverslip were scored. The experiments were repeated twice, with each sample in duplicate. Results are expressed as means plus SEM.
Table 1 shows that substantially fewer bacteria were ingested by PM subjected to LDMDP than by control and LPBS-treated PM. The percentage of LDMDP-treated PM that ingested P. aeruginosa was lower than those of untreated and LPBS-treated PM.
TABLE 1.
In vitro phagocytosis of unopsonized P. aeruginosa by murine PM incubated with LPBS, LDMDP, and DMDP for 24 and 48 ha
| Liposome | Avg no. of ingested P. aeruginosa cells per macrophage after:
|
% of PM that ingested P. aeruginosa after:
|
||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Control | 19.7 ± 0.3b | 26.4 ± 6.5 | 100.0 ± 0.0 | 98.5 ± 0.5 |
| LPBS | 18.8 ± 1.5 | 27.4 ± 10.6 | 95.5 ± 0.5 | 96.5 ± 3.5 |
| LDMDP | 15.4 ± 0.4 | 8.2 ± 2.0 | 86.5 ± 10.5 | 55.5 ± 15.5 |
| DMDP | 0 | 0 | 0 | 0 |
Assessment of nonopsonic phagocytosis of P. aeruginosa by PM was performed as described in the text. The experiments were repeated two or three times, with each sample in duplicate. Data are means ± SEM.
Data are for P. aeruginosa P1 incubated with 10 mM d-glucose. Phagocytosis of P1 was negligible in the absence of d-glucose.
In vitro effects of LPBS, LDMDP, and DMDP on the morphology and phagocytic capacity of murine AM.
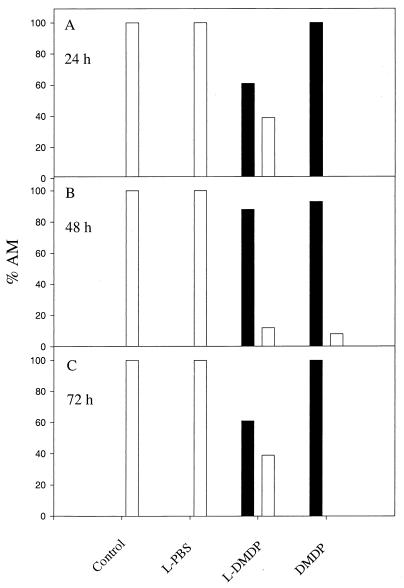
These experiments were repeated three times, and the trend was identical for all. Due to large day-to-day biological variability, one representative experiment is shown (Fig. 2). In it, 100% of untreated (control) and LPBS-treated macrophages appeared to be healthy, whereas 61, 88, and 61% of AM showed unhealthy characteristics after incubation with LDMDP for 24, 48, and 72 h, respectively. All AM incubated with free DMDP for 24, 48, and 72 h appeared to be unhealthy, with no cytoplasm visible.
FIG. 2.
Effects of LPBS, LDMDP, and DMDP on murine AM in vitro. AM, plated on coverslips, were incubated with 15 μl of LPBS, LDMDP, or DMDP (0.6 M stock) for 24 h (A), 48 h (B), and 72 h (C). Controls received no treatment. The morphology of the treated AM was assessed microscopically. One hundred macrophages per coverslip were scored. The experiments were repeated at least three times. The percentages of healthy macrophages (□) and unhealthy macrophages (■) were compared. Results of one representative experiment are shown.
LPBS-treated AM ingested numbers of P. aeruginosa organisms similar to those ingested by untreated cells, whereas LDMDP- and DMDP-treated AM ingested negligible numbers of bacteria. The percentage of LDMDP- and DMDP-treated AM that ingested bacteria was substantially lower than that of control and LPBS-treated AM. AM incubated with DMDP had no visible cytoplasm; therefore, the ingestion of P. aeruginosa was not observed (data not shown).
In vitro effects of LPBS, LDMDP, and DMDP on respiratory epithelial cell line A549.
Changes in the morphology of the respiratory epithelial cell line were not observed after 24 and 48 h of incubation with LPBS or LDMDP. However, unincorporated DMDP was toxic. At 48 h, the percentage of A549 cells with unhealthy characteristics—shriveled nuclei and cytoplasm—increased with increasing concentrations of DMDP (8 to 15% of cells incubated with 0.5 or 5 mM DMDP, 23 to 33% of cells incubated with 50 mM DMDP, and 80 to 85% of A549 cells incubated with 0.6 M DMDP).
In vivo depletion of AM by i.n. instillation of LDMDP.
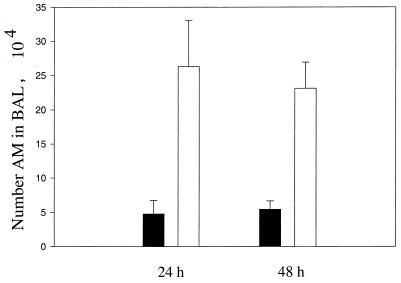
Figure 3 shows that 24 and 48 h after the i.n. instillation of 50 μl of LPBS or LDMDP, the number of AM recovered from LDMDP-treated mice was substantially lower than that recovered from LPBS-treated mice. At 72 h, the number of AM in the BAL from the LDMDP-treated group was slightly increased (data not shown). The histologic appearance of the lungs from LPBS- and LDMDP-treated mice was similar (data not shown).
FIG. 3.
Depletion of AM in vivo by i.n. instillation of DMDP. Fifty microliters of LPBS or LDMDP was delivered to mice by i.n. instillation; 24 and 48 h after the instillation of liposomes, BAL was performed and the number of AM in the BAL fluid was determined from hemocytometer counts and cytocentrifuged preparations. The number of AM in BAL fluid from LPBS-treated (□) and LDMDP-treated (■) mice at 24 and 48 h postinstillation is shown. Experiments were repeated three times. Data are expressed as means plus SEM.
Effects of AM depletion on P. aeruginosa clearance in vivo.
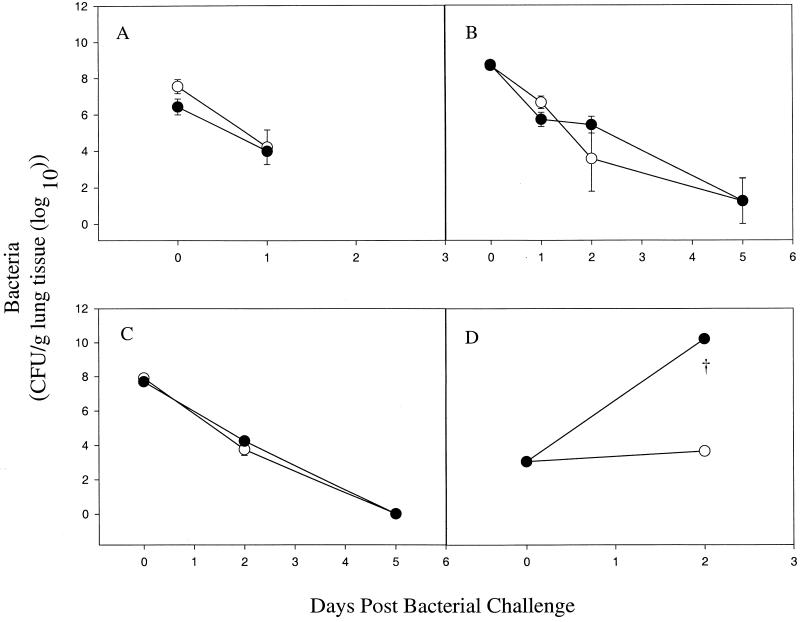
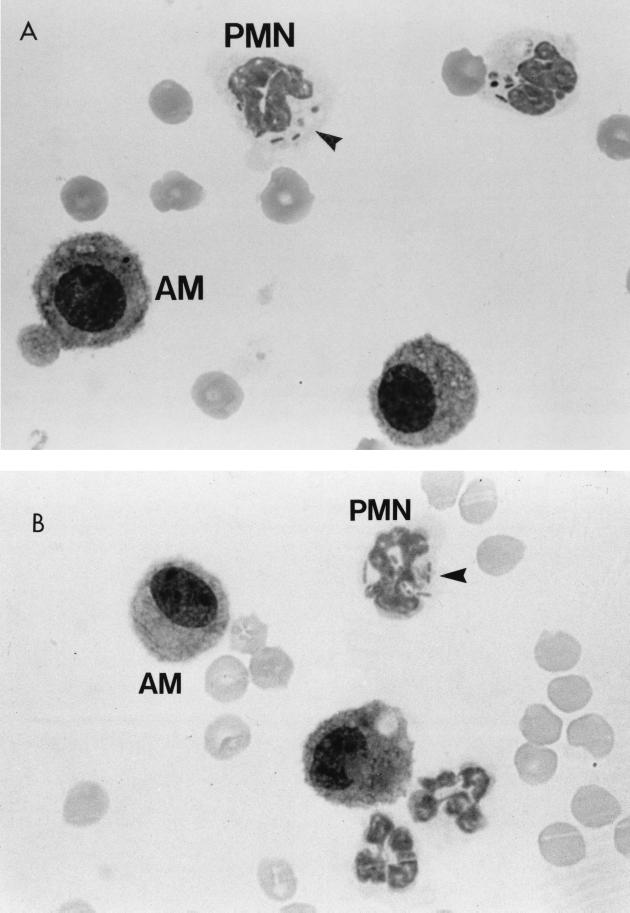
There was no difference between AM-depleted and nondepleted BALB/c and CD-1 mice infected with P. aeruginosa in the clearance of viable bacteria (Fig. 4A through C). No viable bacteria were isolated from the spleens or livers of the infected mice at these time points (data not shown). The number of PMNs isolated from AM-depleted mice was not substantially different from that obtained from nondepleted mice for 5 days postinfection (data not shown). A histologic examination of the lungs of AM-depleted and control infected mice revealed increased numbers of PMNs compared to those of AM-depleted and control uninfected mice (data not shown). Cytospin preparations of BAL cells from LPBS- and LDMDP-treated mice showed that recruited PMNs were competent to phagocytose P. aeruginosa. However, AM appeared not to have phagocytosed the bacteria (Fig. 5). Further studies with LPBS- and LDMDP-treated mice challenged i.n. with P. aeruginosa strain PA01 at various doses—1.32 × 107, 6.5 × 105, and 9 × 103 CFU—did not show any difference between the two groups of mice in bacterial clearance (data not shown).
FIG. 4.
Effects of AM depletion in BALB/c and CD-1 mice on P. aeruginosa and K. pneumoniae clearance in vivo. BALB/c mice, 24 h after the i.n. instillation of LPBS (○) or LDMDP (●), were challenged i.n. with P. aeruginosa FRD1 (6.0 × 106 CFU) (A) and PA01 (2.7 × 107 CFU) (B). CD-1 mice were challenged with PA01 (6.9 × 106 CFU) (C), and BALB/c mice were challenged with K. pneumoniae (1.0 × 102 CFU) (D). Depletion of AM was confirmed by BAL counts and cytospin preparations on the day of infection. At 3 h (day 0), 24 h (day 1), 48 h (day 2), and 120 h (day 5) postinfection, bacterial counts in lung homogenates were determined. Data are expressed as means ± SEM of CFU (log10) per gram of lung tissue from three mice at each time point. †, mice were euthanized due to signs of disease.
FIG. 5.
Differential determination of leukocytes from BAL performed on P. aeruginosa-challenged mice pretreated with LPBS or LDMDP. BAL differentials were calculated for LPBS-treated mice (A) and LDMDP-treated mice (B) 3 h after their i.n. inoculation with P. aeruginosa strain PA01 (6.9 × 106 CFU). Magnification, ×1,125. Arrowheads indicate bacteria within PMN.
Effects of AM depletion on K. pneumoniae clearance in vivo.
Figure 4D shows a striking difference between AM-depleted and nondepleted BALB/c mice in the number of viable bacteria. Signs of disease were evident 48 h after the i.n. instillation of K. pneumoniae (100 CFU/mouse) in the LDMDP-depleted mice, while LPBS-treated mice appeared to be healthy. The spleens and livers of LDMDP-treated mice harbored high numbers of K. pneumoniae cells, but the same tissues from control animals were sterile.
In vitro phagocytosis of freshly explanted AM.
Unopsonized P. aeruginosa was poorly ingested by freshly explanted murine AM from BALB/c and CD-1 mice in the presence or absence of d-glucose. Freshly explanted murine resident PM ingested unopsonized P. aeruginosa strain P1 only in the presence of d-glucose but ingested strain PAK with or without glucose. AM and PM phagocytosed similar numbers of zymosan particles (data not shown).
In situ phagocytic activities of AM.
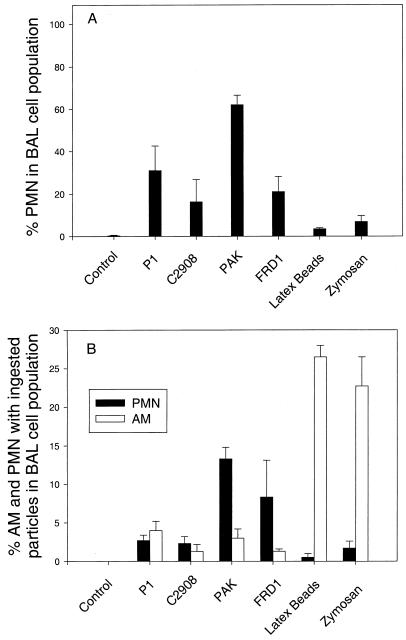
In untreated and uninfected control mice, mononuclear cells comprised 99 to 100% of BAL cells and PMNs comprised less than 1% (Fig. 6A). Recruitment of neutrophils to the lung was substantially enhanced after infection with high doses of P. aeruginosa—PAK at 108 CFU and P1, c2908c, and FRD1 at 107 CFU. PMN recruitment to the lung was minimal in mice inoculated with zymosan particles and latex beads. Figure 6B shows the percentage of AM and PMNs with ingested particles in the BAL population. AM phagocytosis of P. aeruginosa (0 to 5%) was much lower than that of zymosan particles (23%) and latex beads (27%).
FIG. 6.
In situ phagocytic activities of AM. Three hours after the i.n. instillation of P. aeruginosa, zymosan particles, or latex beads, BAL was performed. Differential counts were performed on cytospin preparations, and the percentage of neutrophils was assessed. Each sample was performed in triplicate. One hundred BAL cells were counted. Results are expressed as means plus SEM. (A) Percentages of AM and PMNs in the BAL cell population; (B) percentages of AM and PMNs with ingested particles in the BAL cell population.
DISCUSSION
AM depletion did not compromise the capacity of BALB/c or CD-1 mice to clear P. aeruginosa from the lung. However, AM depletion was noted by others to profoundly compromise pulmonary host defenses against P. aeruginosa and other respiratory pathogens (4, 15, 16). AM depletion results in reduced killing of Mycoplasma pulmonis in C57BL/6 mice (15). Forty-eight hours after K. pneumoniae inoculation, mice with 65% AM depletion show increased bacterial counts in lungs compared to control infected mice (4). However, K. pneumoniae is more virulent than P. aeruginosa; 100 CFU of the former was used to infect mice (4), whereas a much higher dose (106 CFU) of P. aeruginosa can be cleared from the lungs of normal mice. The discrepancy between our results with P. aeruginosa-infected AM-depleted mice and those with Klebsiella (4) and M. pulmonis (15) may be due to differences in infecting agents. This difference was confirmed by the experiments that we performed with K. pneumoniae. On the other hand, decreased bacterial clearance has been observed in CD-1 mice depleted of AM and infected with P. aeruginosa (16). The discrepancy between our results and those of Kooguchi et al. (16) may be due to the method of LDMDP administration, the infecting dose of bacteria, and the strain of P. aeruginosa used. Liposomes can be disrupted by nebulization (9), thereby releasing their contents into the endobronchial space. We chose to deliver LDMDP by i.n. inoculation to avoid the potentially toxic effects of free DMDP. The enhanced susceptibility of LDMDP-treated mice to pulmonary P. aeruginosa infection observed by Kooguchi et al. (16) may have resulted from the nonspecific effect of DMDP on multiple components of the respiratory tract. Our i.n. instillation likely conserved the integrity of the liposomes and delivered them specifically to AM with subsequent selective depletion. This effect was supported by our studies with K. pneumoniae.
Previous in vitro studies have shown that AM actively phagocytose liposomes; AM display the morphological traits characteristic of ongoing phagocytic activity, such as grossly irregular perimeters and extensive pseudopod formation (20). The effects of LDMDP on the phagocytic competence of PM and AM were more apparent than those exerted by LPBS, suggesting that a significant decrease in the phagocytosis of P. aeruginosa by macrophages was not due to liposomes alone. The results of these in vitro experiments assessing the effects of LPBS, LDMDP, and DMDP on AM and PM correlated with those of an in vitro study of rat AM incubated with LPBS, LDMDP, and DMDP (2). LDMDP-treated AM and PM had shriveled nuclei and cytoplasm, suggesting that they had undergone apoptosis (4, 18, 27). AM and PM treated with free DMDP were lysed, resulting in a loss of cytoplasm, indicating that the mechanisms by which DMDP and LDMDP affect macrophages are not the same. A549 respiratory epithelial cells incubated with high concentrations of free DMDP showed the same change in morphology as the macrophages. However, the concentration of DMDP used in in vitro assays was higher than that incorporated into LDMDP. During liposomal preparation, only 1% of DMDP is encapsulated into LDMDP (28). No change in the morphology of epithelial cells incubated with LPBS and LDMDP was observed, suggesting that these liposomes administered in vivo in mice would not likely affect the respiratory epithelium in mice; their effects appear to be limited to macrophages.
Recruitment of PMNs to the lung was not impaired in LDMDP-treated mice. PMNs did not appear to be affected by LDMDP or LPBS, consistent with the results of in vitro and in vivo experiments demonstrating that neutrophils are not affected by LDMDP (4). PMNs, but not AM, actively phagocytosed P. aeruginosa, as shown in BAL preparations from both groups of mice, indicating that PMNs actively participated in bacterial clearance. In the uninfected or untreated lung, PMNs comprise a small proportion of cells in the lower respiratory tract and macrophages constitute 85 to 98% of cells (15, 26, 29). However, the number of PMNs increases in lungs challenged with P. aeruginosa by intratracheal inoculation (19).
Poor nonopsonic phagocytosis of P. aeruginosa by freshly explanted murine AM correlated well with previously published data on freshly explanted and cultivated murine AM, where the former, but not the latter, showed negligible phagocytosis of unopsonized P. aeruginosa (8, 29). However, the ingestion of zymosan particles and latex beads by freshly explanted AM demonstrated that they were phagocytically competent. The phagocytic activities of freshly explanted AM from BALB/c and CD-1 mice were similar, suggesting that the poor phagocytic capacity of uncultivated AM is not mouse strain specific. The difference in phagocytic competency between freshly explanted AM and PM may be due to their differential glycolytic activities. AM reside in the oxygen-rich environment of lung airways (13% O2), whereas PM are in the relatively anaerobic peritoneum (6.5% O2); therefore, their metabolic capacity and dependence upon oxidative phosphorylation versus glycolysis are quite dissimilar, as would be their capacity to transport glucose and to ingest bacteria in a glucose-dependent fashion (6, 8, 22). The association of glucose transport and nonopsonic phagocytosis of P. aeruginosa has been previously reported (1, 8, 29). In addition to the implicated role of glycolysis, signal transduction also plays a role in macrophage phagocytic activities (10, 11). Taken together, the differences in AM and PM phagocytic competency may be due to their differential abilities to transport and utilize glucose and initiate the signalling pathways.
In situ results indicated that AM were phagocytically competent for control particles but were unable to ingest unopsonized P. aeruginosa, consistent with data from in vitro assays showing poor nonopsonic phagocytosis by freshly explanted AM. Buret et al. (5) have demonstrated that the phagocytosis of P. aeruginosa by AM in rats infected with P. aeruginosa decreases substantially at 4 h postinfection from that at 30 min after infection, despite an increase in AM numbers. However, the number of PMNs increases dramatically at 4 h postinfection from that at 30 min postinfection (5). The decreased phagocytic activity of AM in rats at 4 h postinfection reported by Buret et al. may be due to the recruitment of other immune cells, such as PMNs, which migrate to the infected sites and are involved in bacterial clearance, or to suppression by gamma interferon (23). Bactericidal activity is higher in neutrophils than in unactivated macrophages, due to the robust capacity of the former to produce reactive oxygen radicals (7). The number of PMNs elicited in response to latex bead and zymosan challenge was minimal. These results suggest that the recruitment of PMNs is specific and correlates with an orchestrated response.
In summary, depletion of the majority of AM in mice did not result in decreased clearance of P. aeruginosa or impairment of PMN recruitment. AM were phagocytically competent but inefficient in ingesting unopsonized P. aeruginosa in vitro and in situ, whereas PMNs recruited upon bacterial challenge phagocytosed unopsonized P. aeruginosa. These results suggest that the role of AM in defense of the lung against P. aeruginosa challenge may rely upon their capacity to recruit other phagocytic cells rather than on their intrinsic phagocytic function.
ACKNOWLEDGMENTS
This project was supported by a studentship (to D.O.Y.C.) and operating grants (to D.P.S.) from the Canadian Cystic Fibrosis Foundation and the Medical Research Council of Canada.
We thank Robert E. W. Hancock for assistance in liposomal preparations.
REFERENCES
- 1.Barghouthi S, Everett K D E, Speert D P. Nonopsonic phagocytosis of Pseudomonas aeruginosa requires facilitated transport of d-glucose by macrophages. J Immunol. 1995;154:3420–3428. [PubMed] [Google Scholar]
- 2.Berg J T, Lee S T, Thepen T, Lee C Y, Tsan M F. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol. 1993;74:2812–2819. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- 3.Berger M, Norvell T M, Tosi M F, Emancipator S N, Konstan M W, Schreiber J R. Tissue-specific Fcγ and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Pediatr Res. 1994;35:68–77. doi: 10.1203/00006450-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine III R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1α. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterick C J, Williams D A, Boxer L A, Jersild R A, Jr, Mantich N, Higgins C, Baehner R L. Changes in energy metabolism, structure and function in alveolar macrophages under anaerobic conditions. Br J Haematol. 1981;48:523–532. doi: 10.1111/j.1365-2141.1981.tb02749.x. [DOI] [PubMed] [Google Scholar]
- 7.Campa M, Bendinelli M, Friedman H. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 8.Everett K D E, Barghouthi S, Speert D P. In vitro culture of murine peritoneal and alveolar macrophages modulates phagocytosis of Pseudomonas aeruginosa and glucose transport. J Leukoc Biol. 1996;59:539–544. doi: 10.1002/jlb.59.4.539. [DOI] [PubMed] [Google Scholar]
- 9.Finlay W H, Wong J P. Regional lung deposition of nebulized liposome-encapsulated ciprofloxacin. Int J Pharm. 1998;167:121–127. [Google Scholar]
- 10.Goodall G J, Bagley C J, Vadas M A, Lopez A F. A model for the interaction of the GM-CSF, IL-3 and IL-5 receptors with their ligands. Growth Factors. 1993;8:87–97. doi: 10.3109/08977199309046929. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg S. Signal transduction of phagocytosis. Trends Cell Biol. 1995;5:93–99. doi: 10.1016/s0962-8924(00)88957-6. [DOI] [PubMed] [Google Scholar]
- 12.Hancock R E W, Speert D P. Antibiotics for Pseudomonas and related infections. In: Dodge J A, Brock D J H, Widdicombe J H, editors. Cystic fibrosis—current topics. Vol. 3. Toronto, Canada: John Wiley and Sons; 1996. pp. 245–266. [Google Scholar]
- 13.Hashimoto S, Pittet J F, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsurufujia S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–L824. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 14.Hauschildt S, Kleine B. Bacterial stimulators of macrophages. Int Rev Cytol. 1995;161:263–331. doi: 10.1016/s0074-7696(08)62499-7. [DOI] [PubMed] [Google Scholar]
- 15.Hickman-Davis J M, Michalek S M, Gibbs-Erwin J, Lindsey J R. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-susceptible C3H mice. Infect Immun. 1997;65:2278–2282. doi: 10.1128/iai.65.6.2278-2282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limper A H, Hoyte J S, Standing J E. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Investig. 1997;99:2110–2117. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naito M, Nagai H, Kawano S, Umezu H, Zhu H, Moriyama H, Yamamoto T, Takatsuka H, Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol. 1996;60:337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki R, Maeda M, Hayashi H, Nakamura Y, Moriguchi H, Kamei T, Yasuoka S, Ogura T. Role of alveolar macrophages in the neutrophil-dependent defense system against Pseudomonas aeruginosa infection in the lower respiratory tract. Am Rev Respir Dis. 1989;140:1595–1601. doi: 10.1164/ajrccm/140.6.1595. [DOI] [PubMed] [Google Scholar]
- 20.Perry D G, Martin W J., II Fluorescent liposomes as quantitative markers of phagocytosis by alveolar macrophages. J Immunol Methods. 1995;181:269–285. doi: 10.1016/0022-1759(95)00011-x. [DOI] [PubMed] [Google Scholar]
- 21.Speert D P. Pseudomonas aeruginosa infections in patients with cystic fibrosis. In: Baltch A L, Smith R P, editors. Pseudomonas aeruginosa: infections and treatment. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 183–236. [Google Scholar]
- 22.Speert D P, Gordon S. Phagocytosis of unopsonized Pseudomonas aeruginosa by murine macrophages is a two-step process requiring glucose. J Clin Investig. 1992;90:1085–1095. doi: 10.1172/JCI115924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speert D P, Thorson L. Suppression by human recombinant gamma interferon of in vitro macrophage nonopsonic and opsonic phagocytosis and killing. Infect Immun. 1991;59:1893–1898. doi: 10.1128/iai.59.6.1893-1898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes R W, Thorson L M, Speert D P. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 25.Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji C, Minhaz M U, Shioya S, Fukahori M, Tanigaki T, Nakazawa H. The importance of polymorphonuclear leukocytes in lipopolysaccharide-induced superoxide anion production and lung injury: ex vivo observation in rat lungs. Lung. 1998;176:1–13. doi: 10.1007/pl00007587. [DOI] [PubMed] [Google Scholar]
- 27.Van Rooijen N, Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? J Leukoc Biol. 1997;62:702–709. doi: 10.1002/jlb.62.6.702. [DOI] [PubMed] [Google Scholar]
- 28.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 29.Wong S Y C, Guerdoud L M, Cantin A, Speert D P. Glucose stimulates phagocytosis of unopsonized Pseudomonas aeruginosa by cultivated human alveolar macrophages. Infect Immun. 1999;67:16–21. doi: 10.1128/iai.67.1.16-21.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]