Abstract
Several lipoxygenase enzymes and cyclooxygenase-2 stereoselectively convert the polyunsaturated fatty acids arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, and n-3 docosapentaenoic acid into numerous oxygenated products. Biosynthetic pathway studies have shown, during the resolution phase of acute inflammation, that distinct families of endogenous products are formed. These products were named specialized pro-resolving mediators, given their specialized functions in the inflammation-resolution circuit, enhancing the return of inflamed and injured tissue to homeostasis. The lipoxins, resolvins, protectins and maresins, together with the sulfido-conjugates of the resolvins, protectins and maresins, constitute the four individual families of these local mediators. When administrated in vivo in a wide range of human disease models, the specialized pro-resolving mediators display potent bioactions. The detailed and individual biosynthetic steps constituting the biochemical pathways, the metabolism, recent reports on structure-function studies and pharmacodynamic data of the protectins, are presented herein. Emphasis are on the structure-function results on the recent members of the sulfido conjugated protectins and further metabolism of protectin D1. Moreover, the members of the individual families of specialized pro-resolving mediators and their biosynthetic precursor is presented. Today 43 specialized pro-resolving mediators possessing pro-resolution and anti-inflammatory bioactions are reported and confirmed, constituting a basis for resolution pharmacology. This emerging biomedical field provides a new approach for drug discovery, that is also discussed.
Keywords: protectins, specialized pro-resolving mediators, lipoxygenases, cyclooxygenase-2, biosynthesis, resolution pharmacology
1. Introduction
The polyunsaturated fatty acids (PUFAs) arachidonic acid (AA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and n-3 docosapentaenoic acid (n-3 DPA) are each substrates in the biosynthesis of numerous oxygenated and hydroxy products involved in the physiology and local acute inflammatory response of living organisms [1]. When this response is unresolved or fails to resolve, a low-level, harmful and ongoing inflammatory condition is associated with and amplifies many widely occurring human diseases, where the oxygenated PUFA biosynthesis products, e.g. prostaglandins and leukotrienes, are elevated within disease tissues compared to healthy tissues. Using an unbiased lipidomics and system approach, Serhan and co-workers reported in 2002 the biosynthetic formation, isolation and structural elucidations of several novel DHA oxygenated products, including a dihydroxy and E,E,Z-conjugated triene [2]. The EPA derived products, present in inflammatory exudates, were identified some years earlier [3], which opened this new line of research [4]. The overall strategy involved the isolation of potent bioactive molecules from self-limited acute inflammatory exudates in the resolution phase, determine their biosynthesis and proposed structures, followed by confirming their potent pro-resolving actions using materials prepared via stereoselective total synthesis and matching studies to establish their stereochemical assignment. These endeavors were performed for each of the new mediators. Matching studies are needed since the endogenous mediators were produced in too low quantities to obtain direct NMR evidence [3]. Thus, the detailed biochemical pathways or their complete chemical structures, namely stereochemistry, were not disclosed in these original studies that focused on their potent novel bioactions [2], [5], [6]. In 2005 this dihydroxy and E,E,Z-conjugated product was named protectin D1 (PD1) [6]. The name neuroprotectin D1 (NPD1) was also introduced and used in order to denote its presence in neural cells [7]. Of note, the biochemical pathway and the chemical structure are identical for PD1 and NPD1. PD1 activates inflammation-resolution programs [8] and belongs to a novel class of lipid mediators termed specialized pro-resolving mediators (SPMs) [9]. As of today, 33 members of the oxygenated polyunsaturated SPMs have been reported that are biosynthetically formed by different lipid oxygenase (LOX) enzymes (Figure 1). In addition, aspirin acetylated cyclooxygenase-2 (COX-2) forms six R-configured epimers, while native COX-2 gives rise to four resolvins of the T-series of SPMs [9] (Figure 1). While the number of pro-resolving mediators appears high, 43 is still far less than the many pro-inflammatory mediators that orchestrate the initiation phase of the acute inflammatory response in vivo, which reaches into the hundreds of molecules, e.g. prostaglandins, leukotrienes, gases such as nitric oxides and carbon monoxide, as well as the many chemokines, cytokines and complement components. Of interest, the SPMs are more potent on the tissue and cellular level than the many initiator peptides which require micromolar range to effect leukocyte trafficking in vivo. SPMs each stop leukocyte migration, trans-migration (epithelial and endothelial) as well as chemotaxis in the pico- to nanograms range as assessed using microfluidic system to obtain direct evidence with human leukocytes [10], [11], [12].
Figure 1.
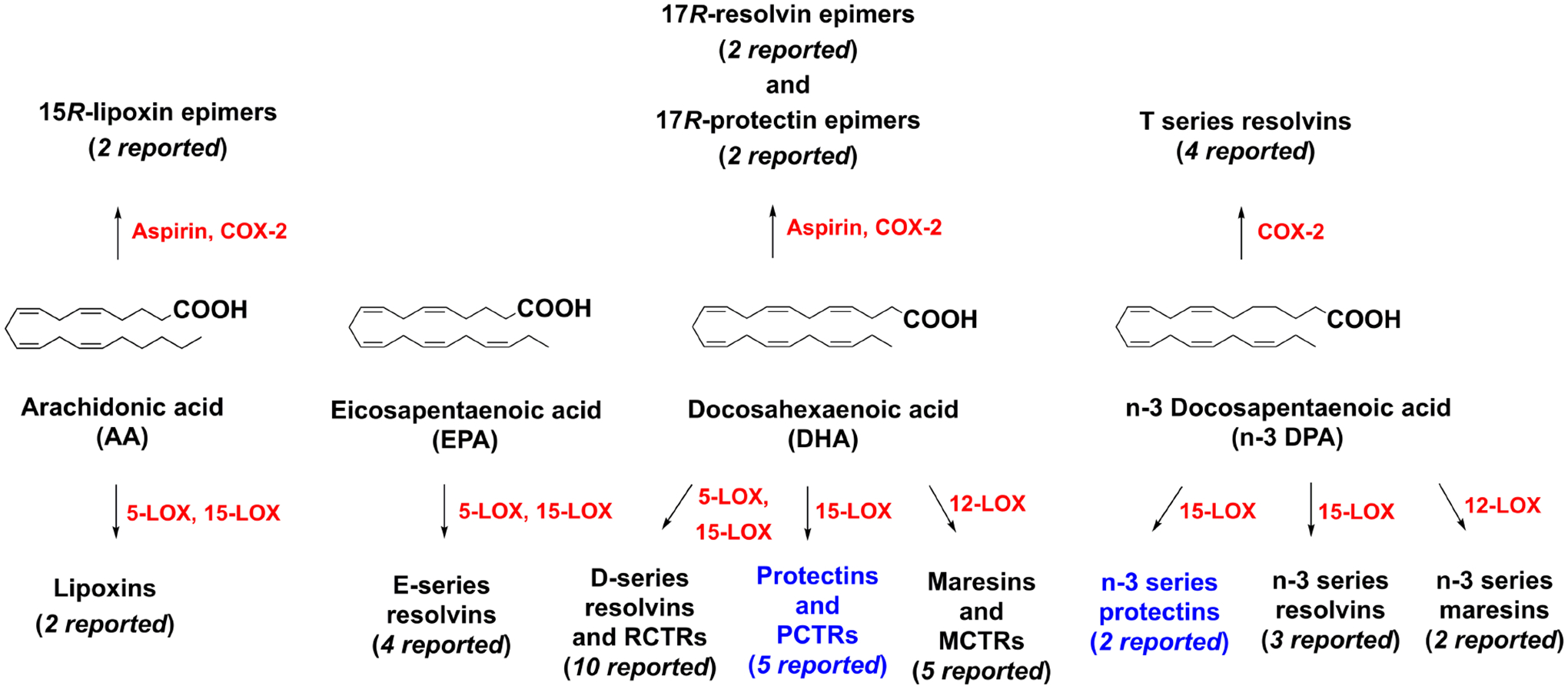
An outline of the individual families of SPMs biosynthesized from PUFAs. The protectins, marked in blue, are the focus of this review. RCTRs: resolvin conjugates in tissue regeneration 1–3; PCTRs: protectin conjugates in tissue regeneration 1–3; MCTRs: maresin conjugates in tissue regeneration 1–3.
The biosynthetic pathways of SPMs, including the protectins, often involve the activation of polymorphonuclear leukocytes (PMNs) and transcellular cooperation where the first oxygenation product is transferred to a neighboring cell site for a second oxygenation step [5], [9]. The SPMs, including the protectins, have attracted a great interest in the biomedical field, since their function in inflammation is to limit the magnitude and duration of the acute inflammatory response and govern tissue regeneration [8]. Moreover, SPMs are resolution agonists towards individual G-protein coupled receptors (GPCRs), with low nanomolar EC50- and KD-values. Altogether, these bioactions constitute an organizational principle in medicine and biology [9]. Due to these bioactions, as well as the non-toxic and non-immune suppressive properties of these endogenously produced mediators, SPMs are interesting leads for developing drugs related to resolution pharmacology [13], [14].
The protectins defend the host from bacterial [15] and viral infections [16], [17] by enhancing the killing and clearance of microbes. In addition, PD1 also display potent and interesting neuroprotective effects in neuronal systems [7], and this SPM has entered clinical trial development programs [18]. The established biochemical pathways of the protectins will be discussed in detail vide infra, but also the metabolites and structure-functions focusing on novel protectins and synthetic analogs, as well as pharmacodynamic data, will be presented.
2. Biosynthesis of the protectins
Among the several LOX enzymes known, the biosynthesis of the SPMs uses arachidonic acid 5-lipoxygenase (5-LOX/ALOX5/5-LO) and different types of arachidonic acid 12- and 15-lipoxygenating paralogues (15-LOX1/ALOX15/15-LO1; 15-LOX2/ALOX15B/15-LO2; 12-LOX/ALOX12, 12-LO). The term 15-LOX will be used. The LOXs belong to a family of non-heme iron-containing dioxygenase enzymes that insert molecular oxygen (oxygenate) into a PUFA that contain one or more Z,Z-1,4-pentadiene moieties, via consecutive and distinct, stereoselective biosynthetic steps that predominately insert molecular oxygen in the S-configuration, due to a concomitant antarafacial abstraction of hydrogen [19]. The 15-LOX types 1 and 2, where the number 15 is derived from the carbon position of the first oxygenation of AA, catalyze the formation of the hydroperoxide coined 17(S)-HpDHA in human whole blood, human leukocytes, human glial cells, and mouse brain [20] with DHA as substrate. This highly reactive hydroperoxide is formed after a hydrogen abstraction, that occurs in an antarafacial manner at C15 in DHA, producing a carbon based radical that enables simultaneous insertion of molecular oxygen at C17. This gives a peroxyl radical that is reduced to the secondary hydroperoxide named 17S-HpDHA, see Figure 3. Of note, a conjugated E,Z-diene is formed [19] that is thermodynamically more stable than a skipped Z,Z-diene and also UV-active [21]. In the following biosynthetic step, the reactive and short-lived 17S-HpDHA intermediate undergoes a second hydrogen abstraction at C12, followed by an intramolecular attack at C16 by the oxygen atom at C17, resulting in the formation of an epoxide under the resulting loss of water (Figure 3). The hydrogen abstraction at C12 also forms a conjugated E,Z-diene moiety, constituting the C10-C13 carbon atoms in the epoxide intermediate, that was named 16S,17S-epoxyprotectin [22]. This biosynthetic intermediate contains an UV-active triene with the 10Z,12E,14E geometry, counting from C1 (Figure 3). The bond angles of an epoxide deviate largely from the tetrahedral angle of 109.5 degrees, present in other sp3-hybridized carbon atoms, resulting in a high amount of ring-strain for the epoxide in 16S,17S-epoxyprotectin, where the bond angles are closer to 60 degrees. This provides a rational for the high chemical reactivity in saline buffered solution at pH 7.45 where a T50-value of ~19 seconds was determined [22]. A stereoselective nucleophilic addition of water then occurs at C10, catalyzed by an unidentified hydrolase. This biosynthetic opening of the 16S,17S-epoxide produces PD1. This process must be catalyzed and controlled by an enzyme, since the triene geometry in PD1 is altered from a 10Z,12E,14E triene to 11E,13E,15Z, and the chiral center at C-10 is formed in a stereospecific manner with the R-configuration, as evidenced by performing matching experiments with synthetic material of PD1 [23], [24], [25]. The configuration at C17 is kept with the S-configuration during these biosynthetic steps. Addition of water to activated double bonds, such as the C10-C11 double bond in 16S,17S-epoxyprotectin is well-established in biochemistry [19], [26] and organic chemistry. These steps are envisioned to occur via a highly conjugated and stabilized double allylic carbocation intermediate (Figure 3). Of note, the biochemical mediated addition of oxygen by 15-LOX at C-10, produces the double-dioxygenation product [2], [5] that was later named PDX [27]. PDX contains the 11E,13Z,15E-triene and 10S-configuration, and is therefore a stereoisomer (diastereomer) of PD1. However, non-enzymatic opening of 16S,17S-epoxyprotectin yields two diastereomers of PD1, named 10-epi-S-15-Δ-trans PD1 and 15-Δ-trans PD1, respectively (Figure 3). These two stereoisomers of PD1 contain an 11E,13E,15E-triene. Such E,E,E-conjugated trienes are thermodynamically more stable than the trienes present in PD1 and in PDX. Of biological importance, the 15-Δ-trans-PD1 isomer is inactive in human neutrophil transmigration and did not show any anti-inflammatory activities [25]. Similar results were reported for the 10S-epimer, named 10-epi-S-15-Δ-trans-PD1. The E,E,E-substituted stereoisomers of PD1 were biosynthesized in minute amounts compared to PD1, but the 15-Δ-trans-PD1 isomer was isolated using HPLC-chromatography [25]. The unnatural 10S-epimer of PD1 was synthesized in a highly stereoselective manner and subjected to in vivo studies using a peritonitis mouse model [25]. These in vivo studies, comparing PD1 against PDX, 10S-epimer and 15-Δ-trans-PD1, showed that 1 ng/mouse of PD1 inhibited PMN infiltration within the mice exudates. At 100 ng/mouse, PDX was active, but display significantly lower PMN inhibition than PD1 at this dose. The 15-Δ-trans-PD1 was essential inactive at 100 ng/mouse. Hence, these results support that PD1 shows potent stereoselective anti-inflammatory activities.
Figure 3.
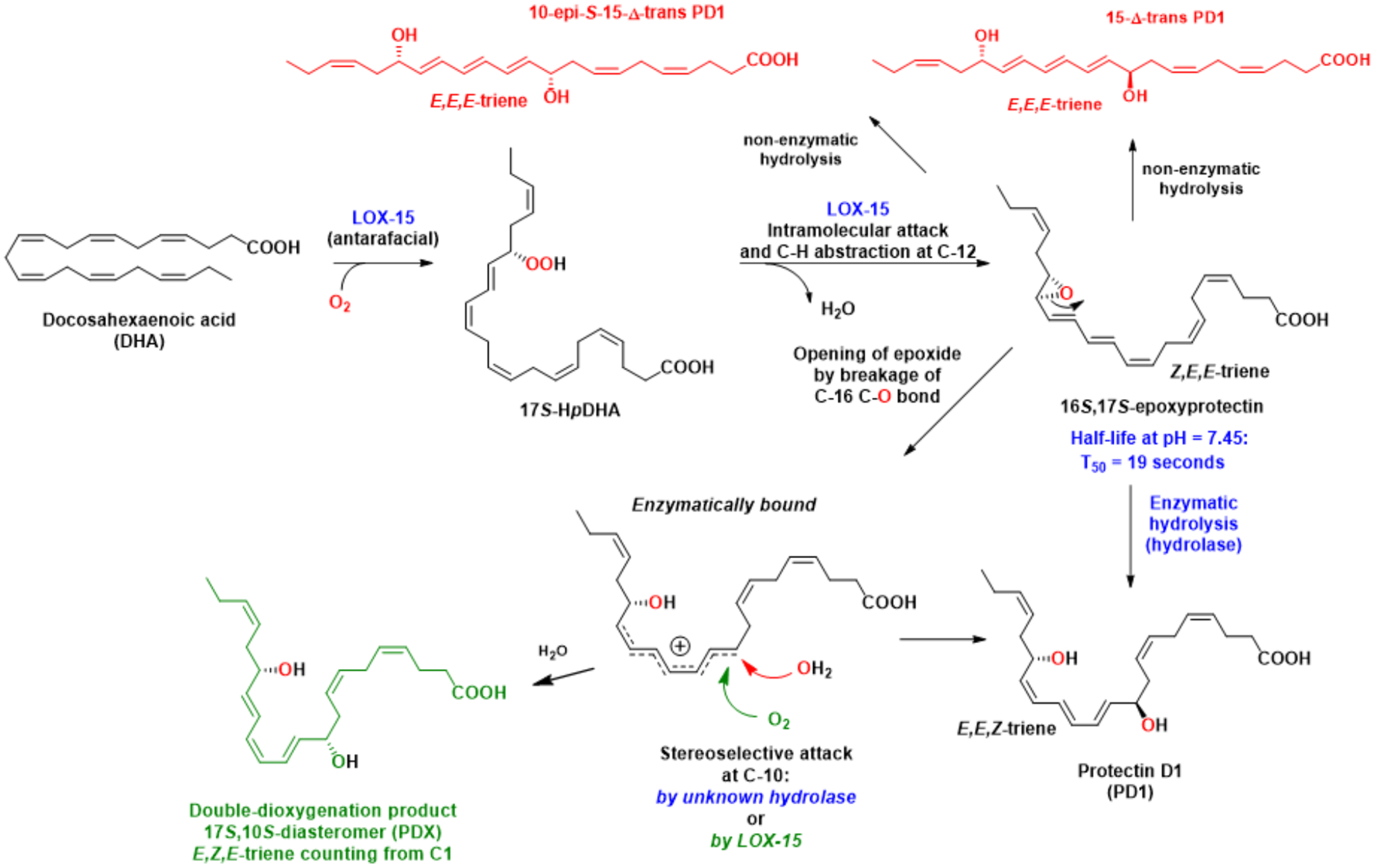
The detailed biosynthetic pathways of PD1 and PDX from the common intermediate 16S,17S-epoxyprotectin. The non-enzymatically formed stereoisomers are also shown.
Moreover, the enzymatically controlled stereo- and regioselective nucleophilic addition of water at C16, produces PD2 (Figure 4). This biosynthetic step occurs in a SN2-type reaction at the more activated allylic C16 atom in 16S,17S-epoxyprotectin [22], see Figure 4. Also, for this biosynthetic step the hydrolase is unknown at this time, but again enzymatic reactions must occur due to two reasons: i) stereochemical control is observed regarding the newly formed R-configured carbon atom at C16; and ii) the 10Z,12E,14E triene geometry found in PD2 is preserved from its epoxide precursor.
Figure 4.
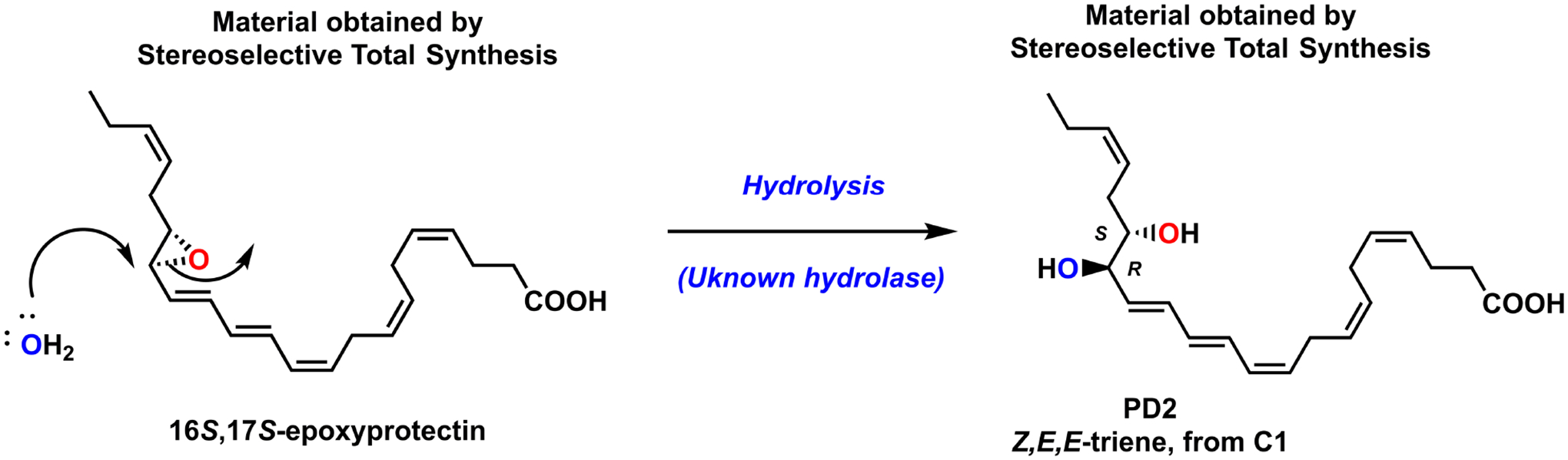
Biosynthesis of PD2 occurs stereoselectively in a SN2-type reaction.
In 2014, Dalli, Chiang and Serhan reported the identification of several potent sulfido-conjugated SPMs, biosynthesized during self-limited infections and in human milk [28]. The following year, these 17-series sulfido-conjugates of the protectins were named protectin conjugates in tissue regeneration 1–3 and abbreviated PCTR1, PCTR2 and PCTR3 [29], respectively, see Figure 5 for chemical structures.
Figure 5.
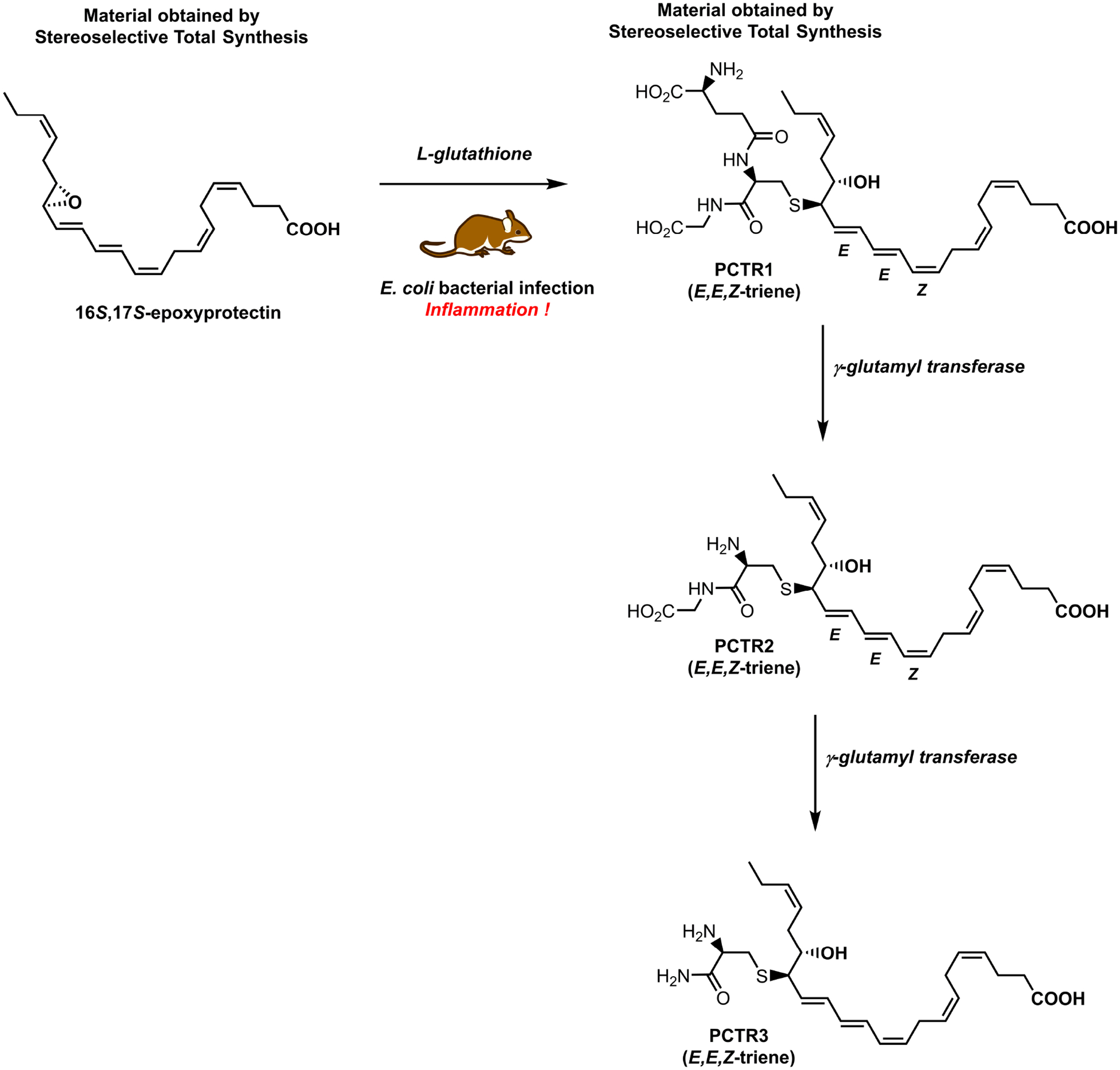
Biosynthesis, via 16S,17S-epoxyprotectin, of the sulfido protectin conjugate PCTR1 and its enzymatic conversion into PCTR2 and PCTR3.
3. Structure-functions of the protectins
There are several review articles on the early reports on the bioactions of PD1 that can be consulted [9], [13], [23]. In this section, the bioactions of the more recently reported sulfido protectin conjugates and some synthetic protectin analogs, will be presented. However, some examples of prominent bioactions of the protectins are listed in Table 1.
Table 1.
Examples of pro-resolving mechanisms of protectins. Please see text for key references and reviews [9], [13] and [23] for further details.
| Mechanism | Cellular Function | Protectin |
|---|---|---|
| Efferocytosis | Upregulate | PD1, PD2, PCTR1-3, PD1n-3 DPA |
| Phagocytosis | Upregulate | PD1, PD2, PCTR1-3, PD1n-3 DPA |
| Bacterial clearance | Enhance | PD1, PCTR1-3 |
| Neuroprotection | Enhance | PD1 |
| Leukocyte infiltration | Limit | PD1, PD2, PCTR1-3, PD1n-3 DPA |
| NF-KB signaling | Downregulate | PD1 |
| COX-2 activation | Inhibition | PD1 |
| Pro-inflammatory chemokines and cytokines | Downregulate | PD1, PD1n-3 DPA |
| Inflammasome formation | Downregulate | PD1 |
| T-cell migration | Regulate | PD1 |
The protectins and other SPMs have been studied for their resolution actions in several in human disease models, see Table 2 for some highlight studies on the protectins.
Table 2.
Protectins and their pro-resolution effects in human disease models, see reviews [9], [13] and [23] for details.
| Disease | Resolution Actions |
|---|---|
| Infection | Levels of PD1 increased with enhanced E. coli killing and clearance in vivo in mouse peritonitis |
| Obesity | PD1 levels were lower in obese patients compared to healthy controls |
| Atherosclerosis | Biosynthesis of PD1 enhanced in macrophages by activation of 15-LOX |
| Neuroblastoma | PD1 decreased in DHA-treated human neuroblastoma cells compared to control |
| Multiple sclerosis | PD1 levels decreased in patients compared to healthy controls |
| Infection | PD1 levels decreased in SARS-COV-2 infected patients compared to control |
| Sepsis | Level of 17R-PD1 (epimer) increased in plasma from survivors compared to non-survivors |
The novel conjugated protectins promote clearance of bacteria and enhance tissue regeneration [9]. Among these, PCTR1 showed organ protection exposed to inflammatory conditions and upregulation of proteins involved in tissue repair [28]. The sulfido-conjugates of the resolvin and maresin families, as for the three novel protectins PCTR1, PCTR2 and PCTR3, proved to be 13-glutathionyl,14-hydroxy-docosahexaenoic acid, 13-cysteinylglycinyl, 14-hydroxy-docosahexaenoic acid and 13-glycinyl, 14-hydroxy-docosahexaenoic acid, respectively [28], [29]. Further studies showed that PCTR1 enabled the rescue of Escherichia coli (E. coli) infection mediated delay in tissue regeneration intervals in planaria, lowered to ~3.7 days, while control experiments without PCTR1 required ~4.2 days [28]. Moreover, these novel SPMs protected mice from second-organ reflow injury and promoted injury repair via limiting neutrophil infiltration. Furthermore, in the nanomolar range, these protectin conjugates also limited the neutrophil infiltration and stimulated bacterial phagocytosis clearance and efferocytosis of apoptotic cells [28]. Additional studies have shown that PCTR1 is temporally regulated during self-resolving infection, enhance macrophage recruitment and phagocytosis of E. coli, but also decrease PMN infiltration [29]. These bioactions counter the formation and regulation of inflammation-initiating lipid mediators, e.g., prostaglandins and leukotrienes. In addition, biologically produced PCTR1 promoted human monocyte and macrophage migration in a concentration-dependent manner (0.001–10.0 nM) [28]. PCTR1 has been prepared by stereoselective total synthesis, via synthetic 16S,17S-epoxyprotectin [30], [31], that contributed to its exact structural elucidation and configuration assignment, see Figure 5. Synthetic PCTR1 showed increased macrophage and monocyte migration, enhanced macrophage efferocytosis and accelerated tissue regeneration in planaria [30]. Moreover, it was reported that PCTR1 levels were significantly higher in M2 macrophages than in the M1 phenotype [30]. Altogether, these findings show that PCTR1 is a potent monocyte and macrophage agonist regulating key anti-inflammatory and pro-resolving processes during bacterial infections. Recently, it was reported that enhancement of skin wound repair associated with bacterial clearance was mediated by PCTR1 [32]. Stimulation of keratinocyte migration, essential in re-establishment of disrupted epithelial layer in skin wounds, was observed in the presence of 10 nM of PCTR1. Also, the level of cyclic adenosine monophosphate was raised. However, these effects were not observed when cells were pretreated with the protein kinase A inhibitor H89 [32]. These results render support that PCTR1 is an agonist of human keratinocyte migration. Furthermore, topical application of PCTR1 (100 ng/day) to mice with full-thickness dorsal skin wounds, showed enhanced wound closure in the early stages of wounding, in accordance with accelerated keratinocyte migration [32]. Recently Levy and co-workers, using a mice model of respiratory syncytial virus (RSV) pneumonia, reported that PCTR1 and PD1 each reduced the viral load in mice, dampening lung inflammation significantly [33]. Moreover, when PCTR1 was administrated post-infection, the levels of eosinophils, neutrophils and NK cells were all reduced; the same was observed for the interferon-gamma production by lung CD4+ T cells [33]. Of interest, PCTR1 and PD1 each enhanced interferon-lambda expression in human bronchial epithelial cells. In vivo studies, combined with LC/MS-MS analyses of lung exudates, showed that the resolution of RSV infected mice was associated with increased levels of both PCTR1 and PD1, while the levels of cysteinyl-leukotrienes decreased, compared to pre-infection levels [33]. Hence, PCTR1 and PD1 are each regulated during RSV pneumonia infections. Taken together, these results clearly demonstrate that these two protectins display distinct, as well as overlying, pro-resolution mechanisms in RSV pneumonia infections [33].
In order to advance structure-function relationships of each SPMs, including the protectins, stereoselective total synthesis are in demand [23]. PD1 [25], [34], [35], [36] and its C-17R epimer named 17R-PD1 [37], the PCTRs1–3 [31] as well as PDX [38], have each been targets for stereoselective total synthesis. These efforts have produced stereochemically defined materials essential for configurational assignments and biosynthetic studies [35]. These protectins are now commercially available from Cayman Chemicals, enabling many other groups to investigate the exciting biology and pharmacology of the protectins. In particular useful for biosynthetic investigations and structural elucidations, have been the use of data from matching studies using multiple reaction monitoring (MRM) LC/MS-MS experiments, combined with UV-data [3]. Comparing data from the UV- and the LC/MS-MS experiments of biogenetic materials of the protectins with their synthetic materials, have provided direct evidence for the biosynthetic pathways and chemical structures presented in Figures 3–5. Moreover, isotopic oxygen incorporation experiments with 18-labelled water and acidic alcohol solutions for the investigations of formation of products from an epoxide intermediate, were performed during early biosynthetic investigations on PD1 [34]. Recently, Holman and co-workers reported in vitro biosynthetic experiments confirming the existence of 16S,17S-epoxyprotectin and its formation by human recombinant 15-LOX1 and 12-LOX enzymes, with the former being far more efficient [39]. These authors also reported the biosynthetic formation of PD1 and PDX from 15-LOX1 from the 16S,17S-epoxyprotectin intermediate [22], providing additional support for the in vivo biosynthetic pathways presented in Figure 3 [39], with 16S,17S-epoxyprotectin as a common intermediate. The double dioxygenation product named PDX has also been the subject of biological studies as reviewed in [27]. Here we highlight that this oxygenated lipid, when administrated perioperative to mice, reduced leukocyte infiltration into surgically manipulated muscularis externa and improved gastrointestinal motility. Of interest, 12/15-lipoxygenase-deficient mice showed increased postoperative leukocyte levels, while intravenous administration of an enriched docosahexaenoic acid lipid emulsion reduced postoperative leukocyte infiltration in wild-type mice [40]. Moreover, in 12/15-lipoxygenase-deficient mice, PDX reduced leukocyte influx and rescued 12/15-lipoxygenase-deficient mice from postoperative ileus, demonstrating the pro-resolving properties of this protectin [40]. Imai and co-workers used PDX and found that its biosynthesis was suppressed during severe influenza caused by H5N1 viruses [16]. The biosynthetic formation of PDX was genetically correlated to the 12/15-lipoxygenase activity, in accord with the biosynthesis presented in Figure 3. It was observed that mice suffering severe influenza induced by H5N1 viruses, the survival level was significantly raised when 1 μg/mouse/day of PDX was administrated intravenously [16]. The roles bioactive lipid mediators, biosynthesized within the host, play in virus infections have been reviewed [41]. Of note, Levy and Serhan reported already in 2007 that PD1 lessen airway inflammation [42], a condition patients with viral lung infections often suffer.
4. Biochemical further local metabolism of protectin D1
In their seminal 2002 article, Serhan and co-workers also disclosed that a trihydroxy C22 compound was present in inflammatory exudates [2], with a similar UV-spectrum as PD1. The LC/MS-MS data revealed that this compound was the ω-C22 hydroxylated metabolite of PD1 that was named 22-OH-PD1, see Figure 6.
Figure 6.
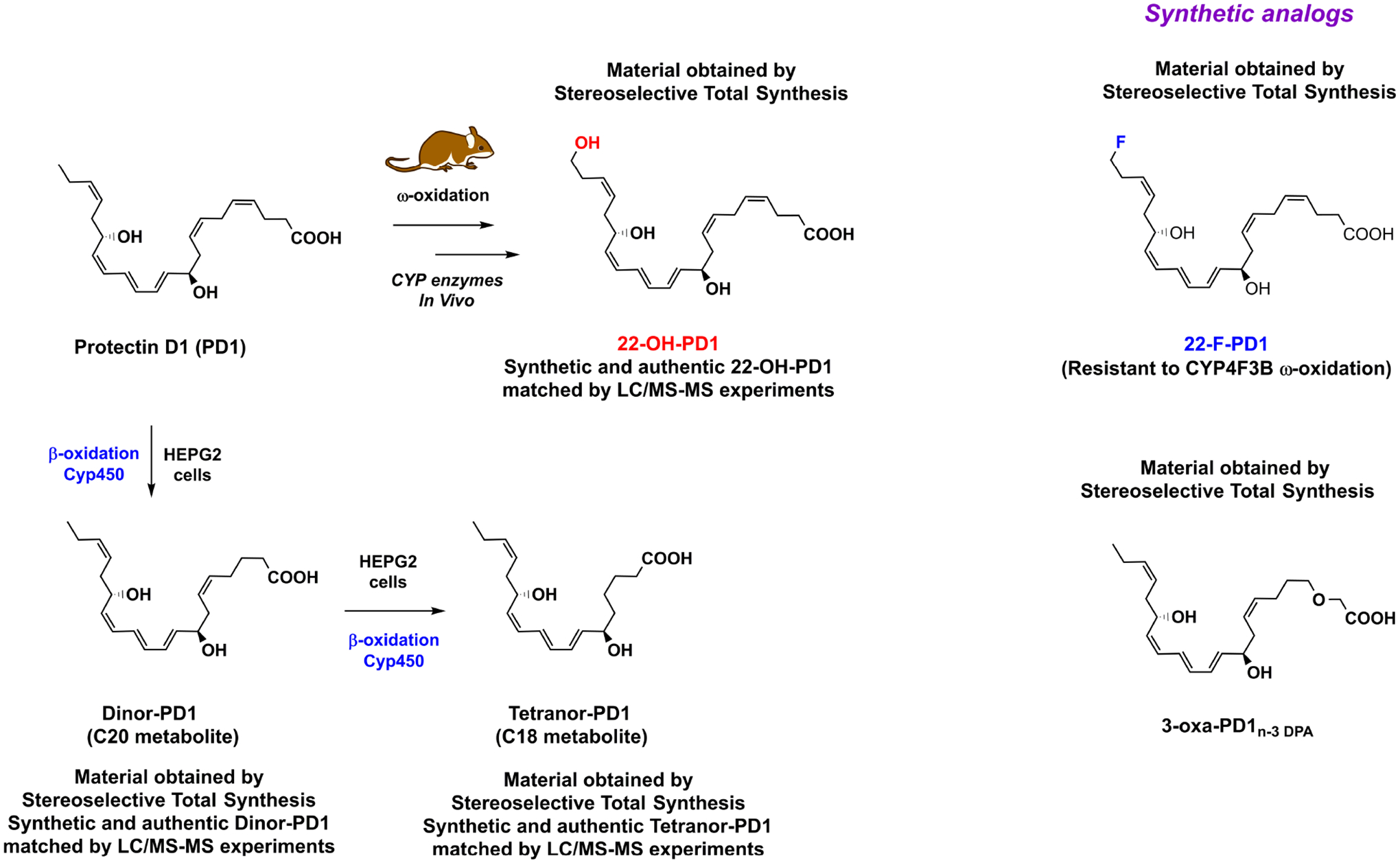
Outline of the enzymatic conversion of PD1 into the metabolites 22-OH-PD1, dinor-PD1 and tetranor-PD1. The synthetic analogs 22-F-PD1 and 3-oxa-PD1n-3 DPA are also shown.
In 2014, a stereoselective synthesis of 22-OH-PD1 was reported [43], that allowed the complete structural assignment and biological evaluations of this metabolite. Of interest, and in contrast to the ω-C20 hydroxylated and the ω-C20 carboxylic acid metabolites of leukotriene B4 that are inactive [44], the metabolite 22-OH-PD1 displays biological activity. Nanomolar pro-resolving actions by inhibiting PMN chemotaxis in vivo and in vitro, comparable to the SPM PD1, were observed [43]. Also, in inflammatory exudates, the chemotaxis of pro-inflammatory interleukin-8 and leukotriene B4 were significantly reduced by low nanomolar (0.01–10 nM) concentrations of 22-OH-PD1. These results inspired the stereoselective preparation of the synthetic analog coined 22-F-PD1 [45], where the fluorine atom has replaced one of the three hydrogen atoms at C22 in PD1, or the primary alcohol in 22-OH-PD1 at C22, see Figure 6. The synthetic 22-F-PD1 analog showed potent pro-resolving and anti-inflammatory properties. Using E. coli infected mice, both PD1 and 22-F-PD1 (100 ng/mouse of each), when administrated via intraperitoneal injection, revealed a significant reduction of neutrophil recruitment [45]. Furthermore, 22-F-PD1, in the range of 0.001 nM to 10 nM, enhanced human macrophage efferocytosis of apoptotic PMN [45]. Of note, 22-F-PD1 showed reduced activity in a CYP4F3B assay. Recently, Sala and co-workers reported studies on the further metabolism of PD1 [46] in HEPG2 cells expressed with Cyp450 enzymes. These studies demonstrated the formation of the C20 and the C18 metabolites of PD1, named dinor-PD1 and tetranor-PD1, respectively. The C20 metabolite dinor-PD1 is the result of one cycle of β-oxidation metabolism, while an additional one gives tetranor-PD1. These authors were not able to identify the presence of PD1 or any of the two β-oxidation products in urine [46]. However, this should not come as a surprise, since the total amount of all SPMs detected in urine have been reported to be in the pico- to nanomolar range in a study from Japan [47], where n-3 PUFAs intake is known to be higher than in Western diets [47]. Moreover, PD1 is a local acting mediator at the site of inflammation. Again, stereoselective synthesis was applied, making the C20 dinor-PD1 and the C18 tetranor-PD1 metabolites available for further biological and biochemical investigations [46].
5. Synthetic protectin analogs and structure-function studies
Based on our biosynthetic studies reported in 2015 and in order to advance structure-function relations further, it was decided to prepare a β-oxidation resistant analog of n-3 DPA, as well as a protectin analog based on PD1 and its congener PD1n-3 DPA; the latter also displays potent hallmark bioactions of protectins [48], [49], [50]. These two analogs were named 3-oxa n-3 DPA, where the methylene group at C3 in n-3 DPA was replaced with an oxygen atom [51], [52], and 3-oxa PD1n-3 DPA [53], respectively. The synthetic PUFA analog 3-oxa n-3 DPA was subjected to enzymatic studies that showed the formation of mono-hydroxylated products in the presence of 5-LOX, 12-LOX, 15-LOX and COX-2 [52]. The synthetic protectin analog 3-oxa PD1n-3 DPA, together with PD1 and PD1n-3 DPA, were used for in vivo studies in a model of T-cell lymphoma induced chronic itch [53]. These studies revealed that a notable reduction of itch was observed for all protectins after intrathecal administration in the presence of 30, 100 or 300 pmol of each protectin. The effect was sustained after three and five hours for PD1 and the 3-oxa analog, but was lost for PD1n-3 DPA after five hours, most likely due to a faster metabolism of this protectin, lacking the Z double bond at the C4-C5-position. These observations are in line with the fast β-oxidative pathway reported by Sala and co-workers for PD1 [46]. Moreover, these three compounds were subjected to a mouse model of Streptocozin induced neuropathic pain, that showed potent pain relief for all three protectins, again in the 30 to 300 pmol range [53]. The synthetic analog 3-oxa PD1n-3 DPA is a useful pharmacological tool in metabolic and structure functions studies of the protectins. Over the last decade, several examples of SPMs, including the two protectins PD1n-3 DPA and PD2n-3 DPA, have been reported biosynthesized from the PUFA n-3 DPA, outlined in Figure 1. The readers should consult the reviews listed as reference [50] and [54], regarding bioactions and biosynthetic pathway studies of PD1n-3 DPA, respectively. This SPM is a congener of PD1 and shares many of the hallmarks bioactivities associated with PD1 and other n-3 DPA derived SPMs [48], [49], as well at the biochemical pathway [55] and metabolism [56]. The biosynthetic formation of PD1n-3 DPA and PD2n-3 DPA was found to be important in the differentiation of monocytes to macrophages [55]. Of note, deficiencies in this pathway during macrophage differentiation results in impaired monocyte-derived macrophage responses [57]. Moreover, Perretti and co-workers reported that PD1n-3 DPA potently provided tissue protective effects in the nanomolar range in intestinal inflammation using experimental colitis and intestinal ischemia/reperfusion-induced inflammation mice models [58]. Moreover, this protectin has been reported to detain epileptogenesis and potently promote resolution of neuroinflammation, again using experimental mice models [59].
6. Resolution pharmacology and drug discovery
The results listed in Tables 1 and 2 in Section 3 above provide a solid foundation for drug discovery and drug development efforts. As of today, several companies use SPM and protectins as leads for such efforts. For example, Anida Pharma is a biopharmaceutical company aiming to develop new treatments for amyotrophic lateral sclerosis, Parkinson’s disease as well as other disorders of the central nervous system, using PD1 as a lead compound. As the company state, Anida Pharma “is a preclinical-stage biopharmaceutical company leveraging the knowledge around the body’s own homeostatic and protective mechanisms to address human neurodegenerative diseases with proresolving drugs based on PD1/NPD1 structure and bioaction in reducing neural inflammation” [60]. Thetis Pharmaceuticals LLC base their program on resolvin analogues for treating inflammatory bowel disease (IBD).
A vital advancement regarding the resolution program arrived with the discovery of new mechanisms in local SPM biosynthesis via microparticles [61], [62], that paved the way for the introduction of the concept named nanoproresolving medicine. This approach has shown to successfully deliver 17S-hydroxy-DHA, the reduced form of 17S-HpDHA, the first biosynthetic intermediate in the biosynthesis of the protectins [22], [25], [29], [30]. The nanoproresolving medicine approach has demonstrated its potential in human pre-clinical models of sepsis, where prolonged pro-resolution effects were observed [63]. The protectins PD2 and PD2 n-3 DPA, see Figure 2 for chemical structures, have been at this point investigated much less, because these were uncovered fairly recently. The two protectins, namely PD1 and PD2 are constitutional isomers, biosynthesized from the same epoxide intermediate (Figure 3). PD2 [37] and PD2 n-3 DPA [64] have both been stereoselectively synthesized and are available for future biological evaluations, enabling drug discovery studies.
Figure 2.
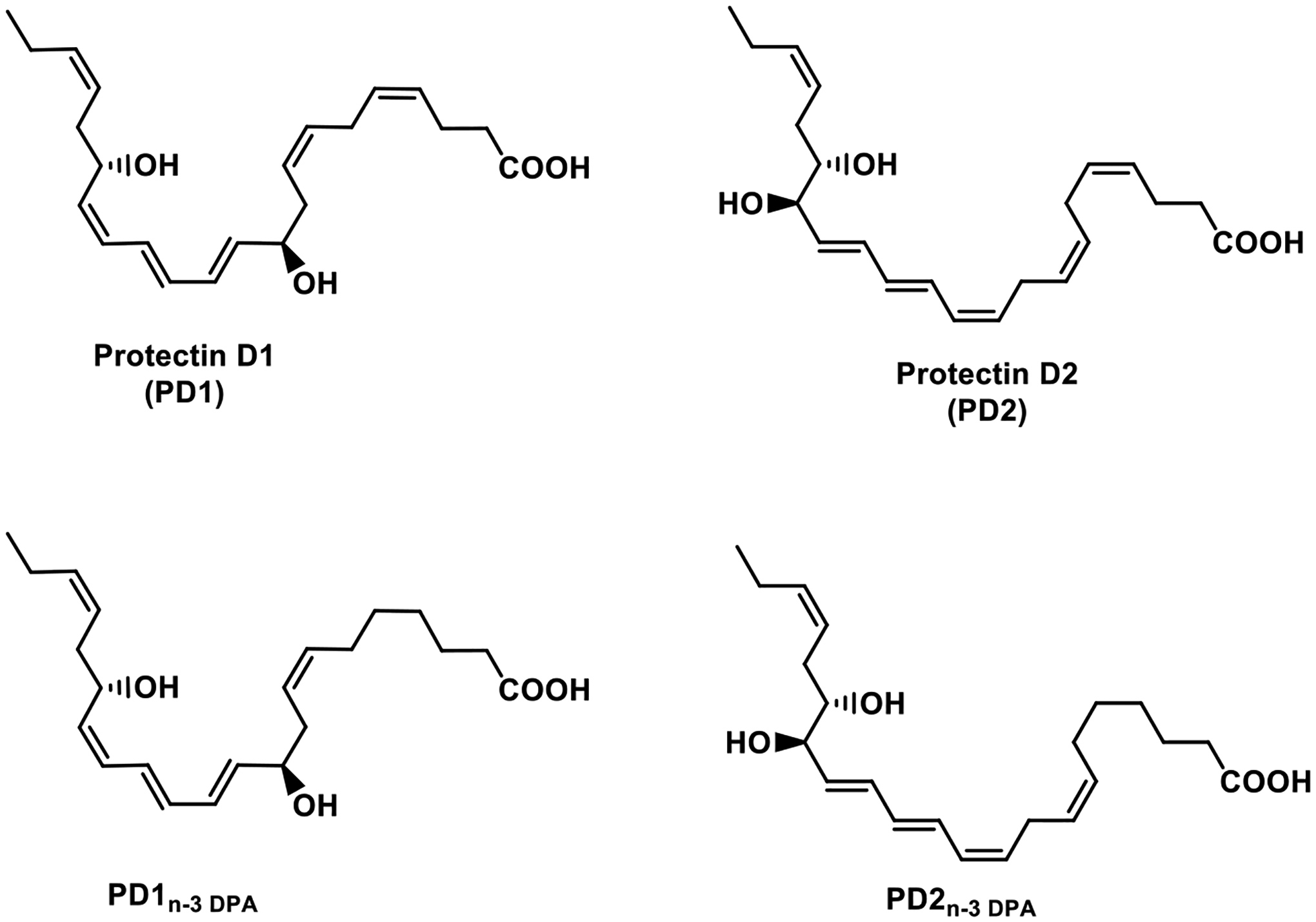
Chemical structures of protectin D1 (PD1) and protectin D2 (PD2), biosynthesized from DHA, and the more recent members PD1n-3 DPA and PD2n-3 DPA, biosynthesized from n-3 DPA. PD1n-3 DPA and PD2n-3 DPA are congeners of PD1 and PD2, respectively, due to the lack of the Z-configured C4-C5 double bond in their n-3 DPA precursor.
7. Pharmacodynamic data
Initial investigations of PD1-receptor binding interactions using synthetic tritium-labeled PD1 at carbon atoms C11 and C12 of PD1, provided evidence for cell type specific binding of this SPM to human retinal pigment epithelial cells and leukocytes [65]. High affinity and stereoselective binding were observed with a KD-value of 31 nM [65]. Since the radiolabeled material of PD1 did not compete with resolvin E1 or lipoxin A4 towards their individual receptors, these data were the first evidence for specific binding towards human PMNs by PD1, and that PD1 exhibits high and selective affinity towards an individual receptor [65]. In 2018 Ji and co-workers reported that PD1 shows selective signaling and binding to the G-protein coupled receptor 37 (GPR37), a receptor highly expressed in the brain [66]. Moreover, activation of GPR37 by PD1 in human macrophages resulted in marked enhancement of macrophage phagocytosis of fluorescent-labelled particles [66]. Furthermore, the PD1-GPR37 interactions in these cell cultures suppressed the production of the pro-inflammatory cytokine interleukin-1β (IL-1β) and increased the expression of the anti-inflammatory cytokine interleukin-10 (IL-10). Of great interest and merit, Ji and co-workers confirmed, by using mouse models, the role of GPR37 in regulating macrophage phagocytosis and resolving inflammatory induced pain [66]. Overall, these findings revealed that PD1 interacts with the GPR37 receptor. As GPCR agonists, the SPMs play essential roles in the return to homeostasis, where the molecular, biochemical and cellular events involved have been coined catabasis [67]. In Table 3, an overview of the SPM-receptors and the low nanomolar EC50-values reported to date, are presented [68], [69], [70]. Of note, the ALX/FPR2 receptor structure was obtained, using lipidic cubic phase crystallization technique, in recent elegant studies from the group of Ye [71].Fluorescence resonance energy transfer (FRET) functional assays demonstrated the potent actions of the receptor agonist LXA4 and 15-epi-LXA4 [72]. Such studies advancing the understanding on how SPM receptors are activated and how their downstream signaling functions, as well as determining the structures of GPCRs activated by SPMs, will altogether aid efforts towards drug discovery and drug development based on resolution pharmacology.
Table 3.
Specialized pro-resolving mediators and reported receptors.
| SPM | Receptor | EC50 (nM) | Reference |
|---|---|---|---|
| Lipoxin A4 | ALX/FRP2; GPR32/DRV1 | ~1; ~10–25 | 68,72 |
| Resolvin E1 | BLT1; ChemR23 | ~45; ~11 | 68 |
| Resolvin D1 | ALX/FRP2; GPR32/DRV1 | ~10; ~0.2 | 68 |
| Resolvin D2 | GPR18/DRV2 | ~10 | 68 |
| Maresin 1 | LGR6 | ~0.7 | 68 |
| Protectin D1 | GPR37 | ~10, 31* | 66, 65 |
| RvD1n-3 DPA | ALX/FRP2; GPR32/DRV1 | ~15, ~10 | 69 |
| RvD5n-3 DPA | GPR101 | ~0.005,~6.9# | 70 |
KD-value measured against pmol/mg of cell protein, see reference 65.
KD-value.
Conclusion
Inflammation and its natural resolution are host-protective responses triggered by infection and/or injury. The resolution phase of inflammation is regulated by enzymatically produced SPMs, as discussed in detail herein for the protectins. The protectin family of SPMs display high structural complexity compared to many other lipids, due to the presence of several stereogenic centers, both in the form of chiral, secondary alcohols and conjugated E- and Z-double bonds, reflecting their biochemical origins and functions. Moreover, stereoselective agonism towards individual GPCRs have also been reported. Elucidating the biosynthetic pathways and receptor effects are important in order to understand and appreciate the bioactivities of the protectins, but are also highly relevant for the resolution pharmacology field [73]. Today this an attractive and rapidly maturing field of biomedical research, exemplified by recent findings [74], [75], [76]. For example, Nguyen-Chi and co-workers discovered that during wound healing in Zebra fish, PD1 mediated a macrophage phenotype switch to the M2 phenotype that is required for wound healing [74]. This process was governed by 15-LOX, the key enzyme in the biosynthesis of PD1 [74]. Earlier Yao and co-workers reported that PDX increased the survival rates of mice suffering sepsis and that the percentage of M2 macrophages in peritoneum of septic mice was elevated [75]. Data from these studies revealed that PDX promoted macrophage M2 polarization and increased phagocytosis in macrophages, resulting in resolution of inflammation and thereby contributing to higher survival rate of septic mice [75]. Also recently, Hu et al. showed that PDX stimulated the resolution of inflammation in rat, using a model of acute respiratory distress syndrome, a disease associated with inflammatory conditions [76]. These authors also reported that PDX activated the prostaglandin DP1 receptor, via biosynthesis of prostaglandin D1 during resolution processes, rendering evidence for a new mechanism for resolution of inflammation mediated by PDX.
The protectins are exciting, potent, non-toxic resolution agonists that activates individual GPCRs. Such bioactions are of interest in drug discovery programs towards developing new drugs without immunosuppressive properties for treatment of inflammatory driven diseases [13], [14], [73]. Reports from the nanoproresolving medicine studies discussed above are very encouraging and constitute a solid platform for further advances, also due to improved quantitative profiling of SPMs in human samples [77]. Last year it was 50 years since Bang and Dyerberg’s published their first landmark article on the levels of ω-3 PUFAs present in the Greenland Inuit population [78]. Based on the impact these results have had on the PUFA field, it is interesting to look another 30 years ahead, when the “Golden Anniversary” occurs on the first report of a protectin SPM. Based on the early impact the protectins have had on the biomedical field and resolution pharmacology, we envision further advances in basic science enabling translational projects within drug discovery, as has been observed for other oxygenated PUFA products [79].
Acknowledgement
We want to thank current and past co-workers for fruitful collaborations, experimental and methodological efforts. The Norwegian Research Council is gratefully acknowledged for generous funding to T.V.H (BIOTEK2021 224811, FRIPRO-FRINATEK 230470). T.V.H. is also thankful for a Leiv Eriksson travel grant from The Norwegian Research Council, enabling a sabbatical period with Professor Charles N. Serhan in 2013. C.N.S. is supported by the National Institutes of Health GM Grant R35 GM139430-02.
Footnotes
Declaration of interest
C.N.S. has filed patents on protectins and related compounds composition. C.N.S.’s interests are reviewed and are managed by BWH and Partners HealthCare in accordance with their conflict-of-interest policies.
References
- [1].Calder PC, Willemsen LEM, Immunopharmacology of fatty acids, Eur. J. Pharmacol 785 (2016) 1, 10.1016/j.ejphar.2016.07.022. [DOI] [PubMed] [Google Scholar]
- [2].Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L, Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals, J. Exp. Med 196 (2002) 1025–1037, 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Serhan CN, Petasis NA, Resolvins and protectins in inflammation resolution, Chem. Rev 111 (2011) 5922–5943, 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms, FASEB J 31 (2017) 1273–1288, 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN, Novel Docosatrienes and 17S-Resolvins Generated from Docosahexaenoic Acid in Murine Brain, Human Blood, and Glial Cells, J. Biol. Chem 278 (2003) 14677–14687, 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- [6].Ariel A, Pin-Lan L, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN, J. Biol. Chem 280 (2005) 43079–43086, doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- [7].Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG, Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress, Proc. Natl. Acad. Sci. USA 101 (2004) 8491–8496, 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schwab JM, Chiang N, Arita M, Serhan CN, Resolvin E1 and Protectin D1 activate inflammation-resolution programmes, Nature 447 (2007) 869–874, 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Panigrahy D, Gilligan MM, Serhan CN, Khasfi K, Resolution of inflammation: An organizing principle in biology and medicine, Pharm. Ther 227 (2021) 107879, 10.1016/j.pharmthera.2021.107879. [DOI] [PubMed] [Google Scholar]
- [10].Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M, Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis, JCI Insight 1 (2016) e85922, 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oh SF, Pillai PS, Recchiuti A, Yang R Serhan CN, Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation, J. Clin. Invest 121 (2011) 569–581, 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN, Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution, J. Immunol 181 (2008) 8677–8687, 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510 (2014) 92–101, doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Perretti M, The resolution of inflammation: New mechanisms in patho-physiology open opportunities for pharmacology, Semin. Immunol 27 (2015) 145–148, 10.1016/j.smim.2015.06.001. [DOI] [PubMed] [Google Scholar]
- [15].Chiang N, Fredman G, Baeckhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN, Infection regulates pro-resolving mediators that lower antibiotic requirements, Nature 484 (2012) 524–528, 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, et al. , Y. Cell 153 (2013) 112–125, 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- [17].Serhan CN, Levy BD, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J. Clin. Invest 128 (2018) 2657–2669, 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dalli J, Serhan CN, Br. J. Pharmacol 176 (2019) 1024–1037, 10.1111/bph.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Haeggström JZ, Funk CD, Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease, Chem. Rev 111 (2011) 5866–5898, 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- [20].Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN, Molecular circuits of resolution: formation and actions of resolvins and protectins, J. Immunol 174 (2005) 4345–4355, 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- [21].Balas L, Durand T, Dihydroxylated E,E,Z-docosatrienes, An overview of their synthesis and biological significance, 61 (2016) 1–18, 10.1016/j.plipres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- [22].Aursnes M, Tungen JE, Colas RA, Vlasakov I, Dalli J, Serhan CN, et al. Synthesis of the 16S,17S-epoxyprotectin intermediate in the biosynthesis of protectins by human macrophages, J. Nat. Prod 78 (2015) 2924–2931, 10.1021/acs.jnatprod.5b00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N, Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome, Biochim. Biophys. Acta 1851 (2015) 397, 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aursnes M, Tungen JE, Vik A, Dalli J, Hansen TV, Stereoselective synthesis of protectin D1: a potent anti-inflammatory and proresolving lipid mediator, Org. Biomol. Chem 12 (2014) 432–437, 10.1039/C3OB41902A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. , Anti-Inflammatory Actions of Neuroprotectin D1/Protectin D1 and Its Natural Stereoisomers: Assignments of Dihydroxy-Containing Docosatrienes, J. Immunol 176 (2006) 1848–1859, 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- [26].Demming RM, Fischer M-P, Schmid J, Hauer B, (De)hydratases - recent developments and future perspectives, Curr. Opin. Chem. Biol 43 (2018) 43–50, 10.1016/j.cbpa.2017.10.030. [DOI] [PubMed] [Google Scholar]
- [27].Lagarde M, Guichardant M, Bernoud-Hubac N, Anti-inflammatory and anti-virus potential of poxytrins, especially protectin DX, Biochimie 281 (2020) 179–284, 10.1016/j.biochi.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dalli J, Chiang N, Serhan CN, Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. USA 111 (2014) E4753–E4761, 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN, Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways, FASEB J 29 (2015) 2120–2136, 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ramon S, Dalli J, Sanger JM, Winkler JW, Aursnes M, Tungen JE, et al. , The Protectin PCTR1 Is Produced by Human M2 Macrophages and Enhances Resolution of Infectious Inflammation, Am. J. Pathol 186 (2016) 962–973, 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].R Rodriguez A, Spur BW Total synthesis of pro-resolving and tissue-regenerative Protectin sulfido conjugates, Tet. Lett 56 (2015) 5811–5815 10.1016/j.tetlet.2015.09.020. [DOI] [Google Scholar]
- [32].Sansbury BE, Li X, Wong B, Riley CO, Shaw AE, Nshimiymana R, Petasis NA, Serhan CN, Spite M, PCTR1 Enhances Repair and Bacterial Clearance in Skin Wounds, Am. J. Pathol 191 (2021) P1049–1063, 10.1016/j.ajpath.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Walker KH, Nshimiymana R, Bruggermann NT, Shaw AE, Serhan CN, Protectins PCTR1 and PD1 Reduce Viral Load and Lung Inflammation During Respiratory Syncytial Virus Infection in Mice, Front Immunol 704427 (2021) 12, 10.3389/fimmu.2021.704427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hansen TV, Vik A, Serhan CN, The Protectin Family of Specialized Pro-Resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Frontiers in Pharmacology, 9 (2019) 10.3389/fphar.2018.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Petasis NA, Yang R, Winkler JW, Zhu M, Uddin J, Bazan NG, et al. , Stereocontrolled total synthesis of Neuroprotectin D1/Protectin D1 and its aspirin-triggered stereoisomer, Tet. Lett 53 2012. 1695–1698, https://doi: 10.1016/j.tetlet.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hamidzadeh K, Westcott J, Wourms N, Shay AE Panigrahy A, Martin MJ, et al. , Biochem Pharmacol. A newly synthesized 17-epi-NeuroProtectin D1/17-epi-Protectin D1: Authentication and functional regulation of Inflammation-Resolution, 203 (2022) 115181, 10.1016/j.bcp.2022.115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rodriguez AR, Spur BW First total synthesis of the anti-inflammatory and pro-resolving lipid mediator 16(R),17(S)-diHDHA, Tet. Lett 59 (2018) 1143–1146, 10.1016/j.tetlet.2018.02.029. [DOI] [Google Scholar]
- [38].Sancéau J-Y, Maltais R, Poirier D, Marette A, Total Synthesis of the Antidiabetic (Type 2) Lipid Mediator Protectin DX/PDX, J. Org. Chem 84 (2019) 495–505, 10.1021/acs.joc.8b01973. [DOI] [PubMed] [Google Scholar]
- [39].Tsai WC, Kalyanaraman C, Ymaguchi A, Holinstat M, Jacobson MP, Holman TR, In vitro Biosynthetic Pathway Investigations of Neuroprotectin D1 (NPD1) and Biosynthetic DX (PDX) by Human 12-Lipoxygenase, 15-Lipoxygenase-1, and 15-Lipoxygenase-2, Biochemistry 60 (2021) 1741–1754, 10.1021/acs.biochem.0c00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stein K, Stoffels M, Lysson M, Schneiker B, Dewald O, Krönke G, et al. , A role for 12/15-lipoxygenase-derived proresolving mediators in postoperative ileus: protectin DX-regulated neutrophil extravasation, J. Leukoc. Biol 99 (2016) 23, 10.1189/jlb.3HI0515-189R. [DOI] [PubMed] [Google Scholar]
- [41].Russell CD, Schwarze J, The role of pro-resolution lipid mediators in infectious disease. Immunology, 141 (2014) 166–173, 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. , Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness, J. Immunol 178 (2007) 496–502, 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tungen JE, Aursnes M, Vik A, Ramon S, Colas RA, Dalli J, et al. , Synthesis and Anti-inflammatory and Pro-resolving Activities of 22-OH-PD1, a Monohydroxylated Metabolite of Protectin D1, J. Nat. Prod 77 (2014) 2241–2247, 10.1021/np500498j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sumimoto H, Minakami S, Oxidation of 20-hydroxyleukotriene B4 to 20-carboxyleukotriene B4 by human neutrophil microsomes. Role of aldehyde dehydrogenase and leukotriene B4 omega-hydroxylase (cytochrome P-450LTB omega) in leukotriene B4 omega-oxidation, J. Biol. Chem 265 (1990) 4348–4353, 10.1016/S0021-9258(19)39570-5. [DOI] [PubMed] [Google Scholar]
- [45].Tungen JE, Aursnes M, Ramon S, Colas RA, Serhan CN, Olberg DE, et al. , Synthesis of Protectin D1 Analogs: Novel Pro-resolution and Radiotracer Agents, Org. Biomol. Chem 16 (2018) 6818–6823, 10.1039/C8OB01232F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Balas L, Risé P, Gandrath D, Rovati G, Bolego C, Stellari F, et al. , Rapid Metabolization of Protectin D1 by β-Oxidation of Its Polar Head Chain, J. Med. Chem 62 (2019) 9961–9975, 10.1021/acs.jmedchem.9b01463. [DOI] [PubMed] [Google Scholar]
- [47].Sasaki A, Fukuda H, Shiida N, Tanaka N, Furugen A, Ogura J,et al. , Determination of ω-6 and ω-3 PUFA metabolites in human urine samples using UPLC/MS/MS, Anal. Bioanal. Chem 407 (2015) 1625–1639, 10.1007/s00216-014-8412-5. [DOI] [PubMed] [Google Scholar]
- [48].Dalli J, Colas RA, Serhan CN,Novel n-3 immunoresolvents: structures and actions, Sci. Rep 3 (2013) 1940, https://doi.org/doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Aursnes M, Tungen JE, Vik A, Collas RA, Cheng C-Y, Dalli J, et al. , Total Synthesis of the Lipid Mediator PD1n-3 DPA: Configurational Assignments and Anti-inflammatory and Pro-resolving Actions, J. Nat. Prod 77 (2014) 910–916, 10.1021/np4009865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vik A, Dalli J, Hansen TV. Recent advances in the chemistry and biology of anti-inflammatory and specialized pro-resolving mediators biosynthesized from n-3 docosapentaenoic acid, Bioorg. Med. Chem. Lett 27 (2017) 2259–2266, 10.1016/j.bmcl.2017.03.079. [DOI] [PubMed] [Google Scholar]
- [51].Tian Y, Aursnes M, Hansen TV, Tungen JE, Galpin JD, Leisle L, C.A. et al. Atomic determinants of BK channel activation by polyunsaturated fatty acids, Proc. Natl. Acad. Sci. USA 113 (2016) 13905–13910, 10.1073/pnas.1615562113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pangopoulos MK, Nolsøe JMN, Antonsen SG, Colas RA, Dalli J, Aursnes M, et al. , Enzymatic studies with 3-oxa n-3 DPA, Bioorg. Chem 96 (2020) 103653, 10.1016/j.bioorg.2020.103653. [DOI] [PubMed] [Google Scholar]
- [53].Nesman JI, Chen O, Luo X, Ji R-R, Serhan CN, Hansen TV, A new synthetic protectin D1 analog 3-oxa-PD1n-3 DPA reduces neuropathic pain and chronic itch in mice, Org. Biomol. Chem 19 (2021) 2744–2752, 10.1039/D0OB02136A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hansen TV, Dalli J, C.N., Serhan, The Novel Lipid Mediator PD1n-3 DPA: An Overview of the Structural Elucidation, Synthesis, Biosynthesis and Bioactions, Prostaglandins Other Lipid Mediat 133 (2017) 103–110, 10.1016/j.prostaglandins.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Primdahl K, Tungen JE, De Souza PRS, Colas RA, Dalli J, Hansen TV, et al. , Stereocontrolled Synthesis and Investigation of the biosynthetic transformations of 16(S),17(S)-epoxy-PDn-3 DPA, Org. Biomol. Chem 15 (2017) 8606–8613, 10.1039/C7OB02113E [DOI] [PubMed] [Google Scholar]
- [56].Nesman JI, Primdahl KG, Tungen JE, Palmas F, Dalli J, Hansen TV, Synthesis, Structural Confirmation, and Biosynthesis of 22-OH-PD1n-3 DPA, Molecules 24 (2019) 3328, 10.3390/molecules24183228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pistorius K, R Souza P, De Matteis R, Austin-Williams S, Primdahl KG, Vik A et al. , PD n-3 DPA Pathway Regulates Human Monocyte Differentiation and Macrophage Function, Cell Chem. Biol 25 (2018) 749–760, 10.1016/j.chembiol.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gobbetti T, Dalli J, Colas RA, Canova DF, Aursnes M, Bonnet D, et al. , Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection, Proc. Natl. Acad. Sci. USA, 114 (2017) 3963–3968, 10.1073/pnas.1617290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Frigerio F, Pasqualini G Craparotta I, Marchini S, van Vliet EA, Foerch P, et al. , n-3 Docosapentaenoic acid-derived protectin D1 promotes resolution of neuroinflammation and arrests epileptogenesis, Brain 141 (2018) 3130–3143, doi: 10.1093/brain/awy247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].See www.anidapharma.com. Accessed August 10.th 2022.
- [61].Dalli J, Serhan CN, Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators, Blood, 120 (2012) e60–e72, 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN, Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing, J. Immunol 186 (2011) 5543–5547, 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dalli J, Norling LV, Montero-Melendez T, Canova DF, Lashin H, Pavlov AM, et al. , Microparticle alpha-2-macroglobulin enhances pro-resolving responses and promotes survival in sepsis, EMBO Mol. Med 6 (2014) 27–42, 10.1002/emmm.201303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tungen JE, Primdahl KG, Hansen TV, The First Total Synthesis of the Lipid Mediator PD2n-3 DPA, J. Nat. Prod 83 (2020) 2255–2260, 10.1021/acs.jnatprod.0c00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, et al. , Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils, Prostaglandins Leukot. Essent. Fatty Acids, 82 (2010) 27–34, 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji R-R, GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain, J. Clin. Invest 128 (2018) 3568–3582, 10.1172/JCI99888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Serhan CN, Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways, Annu. Rev. Immunol 25 (2007) 101–137, 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- [68].Dyall SC, Balas L, Bazan NG, Brenna JT, Chiang N, Souza FC, et al. , Prog. Lipid Res (2022) 101165, 10.1016/j.plipres.2022.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tungen JE, Gerstmann L, Vik A, De Mateis R, Colas RA, Dalli J, Resolving inflammation - Synthesis, Configurational Assignment and Biological Evaluations of RvD1n-3 DPA, Chem. Eur. J 25 (2019) 1476–1480, 10.1002/chem.201806029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Flak MB, Koenis DS, Sobrino A, Smith J, Pistorius K, Palmas F, et al. , GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections, J. Clin. Invest 130 (2020) 359–373, 10.1172/JCI131609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chen T, Xiong M, Zong X, Ge Y, Zhang H, Wang M, et al. , Structural basis of ligand binding modes at the human formyl peptide receptor 2, Nature Com 11 (2020) 1208, 10.1038/s41467-020-15009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ge Y, Zhang S, Wang J, Xia F, Wan J-B-, Lu J, et al. , Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity, FASEB J, 34 (2020) 6920–6933, doi: 10.1096/fj.201903206R. [DOI] [PubMed] [Google Scholar]
- [73].Perretti M, Leroy X, Bland EJ, Montero-Melendez T, Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation, Trends. Pharmacol. Sci 36 (2015) 737–755, 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [74].Sipka T, Park SA, Ozbilgic R, Balas L, Durand T, Mikula K, Macrophages undergo a behavioural switch during wound healing in zebrafish. Free Rad. Bio. Med 192 (2022) 200–212, 10.1016/j.freeradbiomed.2022.09.021. et al. [DOI] [PubMed] [Google Scholar]
- [75].Hu X, Zhang YA, Chen B, Jen Z, Lin M-L, Li M, et al. , Protectin DX promotes the inflammatory resolution via activating COX-2/L-PGDS-PGD2 and DP1 receptor in acute respiratory distress syndrome, Int. Immunpharmacol 102 (2022) 108348, 10.1016/j.intimp.2021.108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xia H, Chen L, Liu H, Sun Z, Yang W, Yang Y, et al. , Protectin DX increases survival in a mouse model of sepsis by ameliorating inflammation and modulating macrophage phenotype, Sci. Rep 7 (2017) 99, https://doi.org/doi: 10.1038/s41598-017-00103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hartling I, Cremonesi A, Osuna E, Lou PH, Lucchinetti E, Zaugg M, et al. , Quantitative profiling of inflammatory and pro-resolving lipid mediators in human adolescents and mouse plasma using UHPLC-MS/MS, Clin. Chem. Lab. Med 59 (2021) 1811–1823, 10.1515/cclm-2021-0644. [DOI] [PubMed] [Google Scholar]
- [78].Harris WS, Calder CP, Mozaffarian D, Serhan CN, Bang and Dyerberg’s omega-3 discovery turns fifty, Nature Food 2 (2021) 303–305, 10.1038/s43016-021-00289-7. [DOI] [PubMed] [Google Scholar]
- [79].Samuelsson B, Role of Basic Science in the Development of New Medicines: Examples from the Eicosanoid Field, J. Biol. Chem 287 (2012) 10070–10080, 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]


