Abstract
Ophthalmology is one of the most enriched fields, allowing the domain of artificial intelligence to be part of its point of interest in scientific research. The requirement of specialized microscopes and visualization systems presents a challenge to adapting robotics in ocular surgery. Cyber-surgery has been used in other surgical specialties aided by Da Vinci robotic system. This study focuses on the current perspective of using robotics and cyber-surgery in ophthalmology and highlights factors limiting their progression. A review of literature was performed with the aid of Google Scholar, Pubmed, CINAHL, MEDLINE (N.H.S. Evidence), Cochrane, AMed, EMBASE, PsychINFO, SCOPUS, and Web of Science. Keywords: Cybersurgery, Telesurgery, ophthalmology robotics, Da Vinci robotic system, artificial intelligence in ophthalmology, training on robotic surgery, ethics of the use of robots in medicine, legal aspects, and economics of cybersurgery and robotics. 150 abstracts were reviewed for inclusion, and 68 articles focusing on ophthalmology were included for full-text review. Da Vinci Surgical System has been used to perform a pterygium repair in humans and was successful in ex vivo corneal, strabismus, amniotic membrane, and cataract surgery. Gamma Knife enabled effective treatment of uveal melanoma. Robotics used in ophthalmology were: Da Vinci Surgical System, Intraocular Robotic Interventional Surgical System (IRISS), Johns Hopkins Steady-Hand Eye Robot and smart instruments, and Preceyes’ B.V. Cybersurgery is an alternative to overcome distance and the shortage of surgeons. However, cost, availability, legislation, and ethics are factors limiting the progression of these fields. Robotic and cybersurgery in ophthalmology are still in their niche. Cost-effective studies are needed to overcome the delay. Technologies, such as 5G and Tactile Internet, are required to help reduce resource scheduling problems in cybersurgery. In addition, prototype development and the integration of artificial intelligence applications could further enhance the safety and precision of ocular surgery.
Keywords: Cybersurgery, Telesurgery, Ophthalmology robotics, Da Vinci robotic system, Artificial intelligence, Telerobotic technology
Introduction
Ophthalmology is a field with rapid progression. This field includes medical and surgical specialties with distinct demands. Ocular procedures can be divided into an extraocular, intraocular anterior segment, or intraocular posterior segment surgery. Surgical microscopes are needed in intraocular surgeries. In addition, ocular surgery necessitates visualization systems and specific parameters, which make integrating robotics in ocular surgery difficult.
Artificial intelligence (A.I.) has emerged recently in medical and surgical fields. Ophthalmology is one of the most enriched fields that allowed the A.I. domain to be part of its point of interest in scientific research [1–6].
Many applications with the aid of A.I. helped diagnose many pathologies through image recognition and deep learning (DL)1. A.I., Machine Learning (ML), and DL have been used in an ophthalmic setting to validate the diagnosis of diseases, read images, and perform corneal topographic mapping and intraocular lens calculations. Diabetic retinopathy (D.R.), age-related macular degeneration (AMD), and glaucoma are the three most common causes of irreversible blindness on a global scale [7].
COVID-19 has affected healthcare systems. A.I. applications have emerged in ophthalmology and will be used more in clinical research, education, and patient healthcare [8].
When it comes to A.I., the surgical field in ophthalmology is in its infancy.
Ophthalmic surgery requires high precision and high degrees of magnification. Surgical microscopes are the main tools used. Assistance facilitated by surgical robots improves movement control, cancels tremors, and enhances visualization and distance sensing. Robotic technology is only in its initial stages in ocular surgery [9].
Cybersurgery, also referred to as Telesurgery, is most commonly defined as a surgical technique that allows a surgeon to operate on a patient remotely, either from a different location or nearby, through a telecommunications channel attached to a robotic operating machine [10]. This technology not only benefits the shortage of surgeons and the sanitary crisis of COVID-19, but it also eliminates geographical barriers that prevent timely and high-quality surgical intervention, financial burden, complications, and often risky long-distance travel.
This study aimed to focus on the current perspectives on the development of Robotic and Cybersurgery in Ophthalmology, evolution, innovation, and reasons for the delay.
Methods
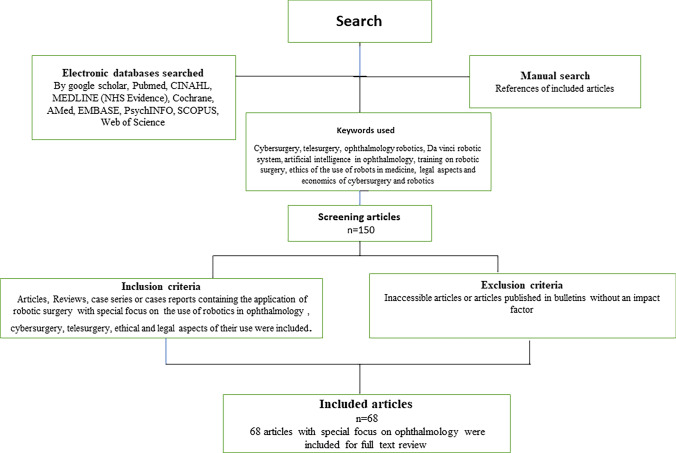
A review of the literature with the aid of Google Scholar, Pubmed, CINAHL, MEDLINE (N.H.S. Evidence), Cochrane, AMed, EMBASE, PsychINFO, SCOPUS, and Web of Science was performed to gather information from articles. Keywords used: Cybersurgery, Telesurgery, ophthalmology robotics, Da Vinci robotic system, artificial intelligence in ophthalmology, training on robotic surgery, ethics of the use of robots in medicine, legal aspects, and economics of cybersurgery and robotics. 150 abstracts were reviewed for inclusion, and 68 articles focusing on ophthalmology were included for full-text review. (Flowchart Fig. 1).
Fig. 1.
Study flowchart
Inclusion criteria
Articles or case series containing the application of robotic surgery with a particular focus on using robotics in ophthalmology, cybersurgery, Telesurgery, and ethical and legal aspects of their service were included.
Exclusion criteria
Inaccessible articles or articles published in bulletins without an impact factor were excluded.
Ethical approval
The study is conducted according to the French data protection law. No submission to IRB/ethical committee was needed. The study adheres to the tenets of the Declaration of Helsinki.
Results
Robotic surgery in ophthalmology
Robotics history
The term robotics derives from “robota” Czech word meaning “servant” or “worker” [11, 12]. It is known that the word was coined by Karel Capek in the theatrical spectacle R.U.R. (Rossum’s Universal Robots). However, the term was popularized only years later, through the works of Russian Isaac Asimov, responsible for making the “Three Laws of Robotics” [4], which, in fiction, standardize the robot’s behavior [11].
The application of robots started in the industry by replacing workers in dangerous functions, such as car assembly lines, to prevent injuries [11].
The use of robots in surgeries could help improve the gesture of tasks, decrease tremors, better visualization, and distance control. Robotics has been used in different medical fields for more than 20 years and assisted physicians in surgical rooms. The first robotic surgery was conducted in 1985 with the help of a robotic arm called Puma 560, which was used for non-laparoscopic neurosurgical biopsies [13]. The first robot, Probot, was designed primarily to aid the medical team in the transurethral resection of the prostate in 1991 [14]. In 1992, the U.S. Food and Drug Administration (F.D.A.) approved the first medical use of a robot [15].
Uses in surgical fields
Recent publications proved superior functional outcomes with equal oncologic safety compared to conventional open surgery. Its field of application may extend to nasopharynx and skull base surgery. The preliminary results encourage the role of trans-oral robotic surgery in head and neck cancer [16].
Other surgical fields use robotic surgery for minimally invasive surgery, such as cardiac, digestive, gynecology, plastic reconstructive surgery, throat surgery, neurosurgery, vascular surgery, hand surgery, and peripheral nerve surgery [17–20].
Role of robotic surgery in ophthalmology
Analysis of previous ocular robotic assisted surgery studies summerized in (Table 1). Definition of main ocular surgical procedures:
Phacoemulsification: Removal of the intraocular lens with an ultrasound machine and a manual arm.
Keratoplasty: Performing corneal grafts with donor corneas to be sutured or implemented to a host recipient.
Vitrectomy: The procedure of removing the vitreous from the posterior chamber of the eye just before the retina using instruments called vitrectomy attached to specified machines.
Intravitreal injection: The instillation of drugs in the intravitreal cavity using needles/syringes.
Table 1.
Analysis of previous ocular assisted robotic surgery studies
| Authors/date | Subject | Robot | Result/outcome |
|---|---|---|---|
| Tsirbas et al. [21] | Sutures at the corneal level (porcine model) | Da Vinci surgical robot | Successfully performed. The robotic system provided excellent visualization and controlled and delicate placement of the sutures at the corneal level |
| Bourges et al. [22] | Confirmed the feasibility of Robot-assisted Penetrating Keratoplasty | Da Vinci surgical robot | Successfully performed on both porcine eyes and human eyes |
| Ueta et al. [45] | Vitreoretinal surgery (porcine model) | Experimental Prototype robotic system | Surgical outcomes were equally successful in the robotic-surgery and manual-surgery groups |
| Belyea et al. [58] | Confirmed the feasibility to remotely photocoagulate the ciliary body for the treatment of glaucoma with the diode laser in fresh unoperated enucleated human eyes | Robotic Slave Micromanipulator Unit (RSMU) | Therapeutic tissue disruption of the ciliary body was achieved |
| Bourcier et al. [24] | Pterygium surgeries in non-living biological pterygium models | DaVinci Si HD | Successful with no intraoperative complications |
| Bourcier et al. [26] | Amniotic membrane transplantation on three patients | Da Vinci surgical robot | Successful with no intraoperative complications |
| Chammas et al. [25] | Penetrating Keratoplasty was also successfully performed on human donor | Da Vinci surgical robot | Successfully performed on 12 corneas. The Da Vinci Xi Surgical System provided the necessary dexterity to perform the different surgery steps. The mean duration of the procedures was 43.4 ± 8.9 min (range 28.5–61.1 min)—no unexpected intraoperative events |
| Bourcier et al. [27] | Cataract surgery was successfully performed on a Kitaro cataract wet-lab training system | Da Vinci Xi | The procedure was successfully performed procedures on 25 lens nuclei. The feasibility of robot-assisted simulated cataract surgery was confirmed. The mean operative time was 26.44 min ± 5.15 (SD) |
| Edwards et al. [43] | Retinal internal limiting membrane peeling | Robotics- Preceyes’ BV research platform | Surgical outcomes were equally successful in the robotic surgery and manual-surgery groups. Duration time with robotic surgery (median time, 4 min 5 s) than with manual surgery (1 min 20 s) |
| Bourcier et al. [29] | Simulated strabismus surgery | Da Vinci Xi | With appropriate dexterity, the mean duration to complete the procedure was 27 min (range, 22–35): no complications or unexpected intraoperative events |
| Jasmina Cehajic-Kapetanovic et al. [32] | Subretinal drug delivery under local anesthesia | Robot-assisted | The time is taken to complete the injection and retinal microtrauma were not clinically significant |
| Koen Willekens et al. [33] | Robot-assisted retinal vein cannulation with ocriplasmin infusion for central retinal vein occlusion | Robot-assisted | Robot-assisted retinal vein cannulation is feasible and safe. In addition, local, intravenous infusion with Ocriplasmin led to improved retinal circulation |
| Mads Forslund Jacobsen et al. [68] |
Manual and robot-assisted vitreoretinal surgery using a virtual-reality surgical simulator (porcine eyes) |
Robot-assisted | Robot-assisted surgery was slower than manual surgery Robot-assisted surgery allowed for greater precision in novices and vitreoretinal surgeons |
| Franziska Ullrich et al. [34] | Intravitreal therapy | Assistive injection system | Successful, safe and cost effective |
Ocular microsurgery was successfully performed using the Da Vinci surgical robot in the porcine model. The robotic system provided excellent visualization and controlled and delicate placement of the sutures at corneal level [21].
Back in 2009, Bourges et al. performed Robot-assisted Penetrating Keratoplasty [22]. Three arms of the Da Vinci surgical robot were loaded with a dual-channel video and two 360°-rotating, 8 mm, wrested-end effector instruments and placed over porcine eyes or a human cadaver head. Trephination of corneal grafts, cardinal sutures, continuous 10.0 nylon sutures and adjustments on both eyes were performed remotely on both porcine and human eyes facilitated by the wrested-end forceps. No limitation of surgical motion was noted [22].
Micro-hands of 4 mm in length were developed pneumatically with microelectromechanical systems (MEMS) technology to mimic a human hand for small object manipulation needed in retinal manipulation [23].
Robotically assisted pterygium surgeries in non-living biological pterygium models were performed using the DaVinci Si H.D. robotic surgical system. Twelve models were prepared, and 12 pterygium excision and conjunctival autografts were performed [24].
Robot-assisted Penetrating Keratoplasty was also successfully performed on human donor 12 corneas with low endothelial cell count mounter on the artificial anterior chamber. The mean duration of the procedures was 43.4 6 8.9 min (range 28.5–61.1 min). There were no unexpected intraoperative events [25].
Amniotic membrane transplantation on corneal pathologies including (Neurotrophic keratitis, graft failure and post-radiation keratoconjunctivitis sicca) has been successfully performed on three human patients [26].
Robot-assisted cataract phacoemulsification surgery was successfully performed on 25 lens nuclei with a mean operative time of 26.44 min ± 5.15 (S.D.). Intraocular dexterity and operative field visualization are necessary for achieving the main steps of the phacoemulsification procedure [27].
There are current uses and developments of cataract surgeries aided by Femtosecond lasers. It is a partially performed cataract operation where many steps of the procedure are done in another setting. The rest is left for the surgeon’s intervention.
Femtosecond lasers are used in corneal and almost all types of refractive surgery, such as laser in situ keratomileusis (LASIK), small incision lenticule extraction (SMILE), penetrating keratoplasty (P.K.P.), insertion of intra-corneal ring segments, anterior and posterior lamellar keratoplasty Deep anterior lamellar keratoplasty (DALK), and Descemet's stripping endothelial Keratoplasty (DSEK). In addition, femtosecond lasers provide more accurate and safe procedures [28].
Robot-assisted strabismus procedures were successfully performed on six eyes. The feasibility of robot-assisted simulated strabismus surgery is confirmed [29].
Classic microsurgery of the eye is performed using an operating microscope. The structures of the eye anterior to the vitreous are operated on under direct vision, whereas posterior regions, such as the retina and vitreous, use a specialized lens and viewing systems.
Robotic-assisted uses in the posterior region of the Retina and Vitreous include Retinal surgery, Gene therapy, Retinal implantation, drug therapy, Retinal Vein Cannulation and intravitreal injections [30–34].
Using devices designed by PRECEYES, a Dutch medical robotics firm, the procedure involved removing a membrane from the back of the eye. Successful human intraocular surgery performed using the Preceyes surgical system [35]. Apart from Preceyes’ B.V. research platform, none of the currently eye-specific systems has reached a commercial stage [35].
The robotic system was used to carry out micro-cannulation experiments on a pig’s eye. As a result, a surgeon was able to perform micro-cannulation [36] successfully.
The Gamma Knife, designed by Lars Leksell in the early 1950s gave rise to a new discipline of medicine-stereotactic radiosurgery. The gamma-ray beam concentration can be used to treat uveal melanoma, choroidal hemangioma, orbital tumors or even choroidal neovascularization [37].
Robotics types used in ophthalmology
The robotic Da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) and the ARES (Auris Surgical Endoscopy System) robot (Auris Surgical Robotics, San Carlos, CA) are the only two surgical robots approved by the U.S. Food and Drug Administration for human surgery not being specifically designed for microsurgical specialties such as ocular surgery.
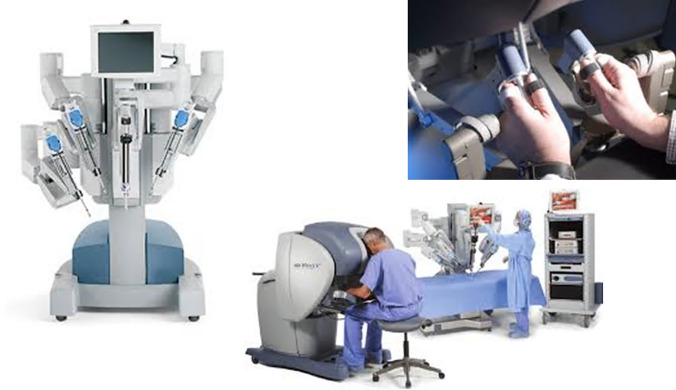
1. The Da Vinci Robot (Fig. 2): It is the most widespread platform used in human surgery. Since 2000, the indications for operations assisted by robotics systems are emerging progressively. They rose from 1500 in 2000 to more than 20,000 in 2004 [38]. It includes three‐dimensional stereoscopic vision with three robotic slave arms that can be equipped with instruments with 7 degrees of freedom and wrist‐like motions. Four models have been launched since they received U.S. Food and Drug Administration approval in 2000: S, Si, Si H.D., and Xi. Surgeons can control the tools and camera from a remote workstation. However, limitations have been documented including the artificial wrist movements that differ from the human range of motion and endoscopic vision, as a result, difficulties in performing microsurgical steps such as sclerotomies could be encountered [39].
Fig. 2.
The Da Vinci Robot
2. Intraocular robotic interventional surgical system (IRISS): This ophthalmic platform was proposed by Jules Stein Eye Institute and the UCLA Department of Mechanical and Aerospace Engineering. It is composed of master controller with two joysticks and a slave manipulator. The manipulator has two independent arms that each hold surgical instruments. The arms have an independent pivot point and 7 degrees of freedom necessary for surgical maneuvers. This system has been used in anterior and posterior ocular procedures, such as capsulorhexis, lens cortex removal, core vitrectomies, and retinal vein micro-cannulation in porcine eyes [40].
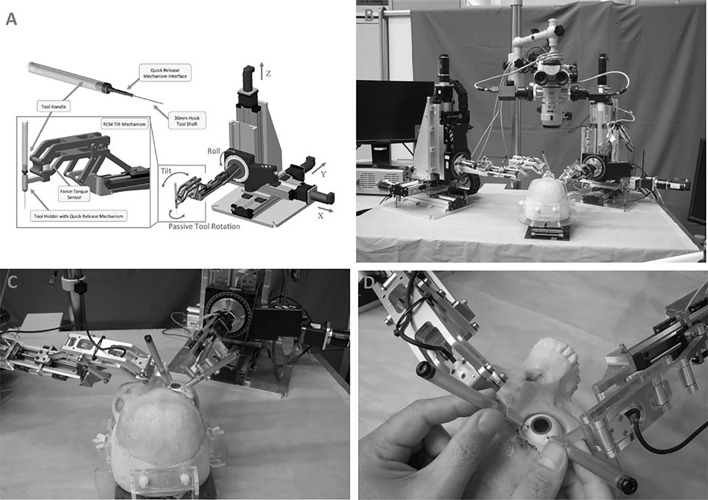
3. Johns Hopkins steady-hand eye robot (Fig. 3): This robot is designed to share the control of surgical instruments, mainly during posterior segment surgeries. It consists of three major components: the X.Y.Z. system, the rolling mechanism, and the tilt mechanism. The X.Y.Z. system allows movement of the surgical tool in all directions. The roll mechanism consists of a rotating table designed to optimize the access of the surgical device to the patient’s eye. The tilt mechanism is attached to the tool holder at one end and the rolling mechanism at the other, allowing the instrument to be at any angle. As a result, the robot improves the effectiveness of each movement. This instrument can be used free-hand or incorporated into the Steady-Hand-Eye robot [41].
Fig. 3.
Johns Hopkins Steady Hand Robot
The latest model has improved the range of motion, stiffness, and speed of holder release.
4. “Smart” instruments: Additional systems and “smart” instruments have been developed to improve technical performance [30]. For example, sensors detect the force applied to the eye; this could be transferred directly to the surgeon via an auditory feedback system [42]. In addition, they can notice tactile sensations lower than the human threshold, which could minimize the risk of possible surgical complications.
Retinal membrane removal was successfully performed with the aid of PRECEYES surgical system robotic assistant, which serves as an instrument holder for over six patients as part of a trial at Oxford's John Radcliffe hospital [35, 43].
The median time was longer (four minutes and 55 s) than the traditional method (one minute and 20 s) [35].
Assistive devices for Intravitreal Injection have been demonstrated through ex vivo experiments with porcine eyes. It used an automatic fine positioning and intravitreal injection through the pars plana. In addition, several safety features, such as continuous eye-tracking and iris recognition, have been implemented [34].
Prototypes development and innovation in ophthalmology
Guerrouad and Vidal in 1989 created a robotic ocular system composed of a Stereotaxical Microtelemanipulator (SMOS), a spherical micromanipulator mounted on an x, y, and z stage, which allowed 6 degrees of freedom. No development was made after this stage.
Robot-Assisted Microsurgery (RAMS) tele-robotic platform emerged in 1997 (Charles S 1997). It comprises a slave robot arm (2.5 cm in diameter and 25 cm long) and a primary device supported by cables and encoders facilitating the operator's arm movement guided by computers [44]. In the same year, another prototype used the Stewart-based platform (Jensen, Grace et al. 1997). This was developed to measure intraluminal (20–130 microns) retinal vessel pressure and to extract blood from these vessels for research purposes.
In 2009, Ueta et al. [45] developed a newer prototype with more accuracy adapted to assist in vitreoretinal surgeries.
Cybersurgery
History and background
Electrocardiogram was first introduced in 1906, the first step in telemedicine.
Cybersurgery, also referred to as Telesurgery, from the Greek tele, “far off”, also called “remote surgery”, is defined as a surgical technique which allows for a surgeon to operate on a patient remotely, either from a different location or at proximity, through a telecommunications channel attached to a robotic operating machine.
Tele-surgery is a surgical system that utilizes wireless networking and robotic technology to connect surgeons and patients distantly. It can be divided into three main components: Telesurgery, telementoring, and teleconsultation [10]. The telerobotic Zeus and Da Vinci surgical systems allow surgeons to operate remotely. These telerobots hold the camera, replace the surgeon’s two hands with robotic instruments, and serve in a master–slave relationship for the surgeon. They are characterized by their capabilities to simulate the motions of the surgeon’s wrist and different surgeon positions [46].
In 1988: Minimally invasive surgery enabled surgical procedures to be guided by introducing a camera without requiring an opening of the abdomen or thorax. In 1996: Computer-assisted surgery was introduced, which enabled to transmit surgeons’ actions remotely to manipulation devices. September 7, 2001: Telesurgery: The world's first Telesurgery was performed by a surgical team in New York, U.S.A. using the ZEUS robotic system (Intuitive Surgical, Sunnyvale, CA, U.S.A.). This project produced a successful two-hour-long laparoscopic cholecystectomy performed on a female patient at a hospital in Strasbourg, France [47]. The patient had an uneventful recovery [48]. In 2003, a surgical system was set up in Canada between two hospitals 400 kms away [49].
Robotics could be helpful in surgical tele-mentoring by expert surgeons to supervise younger surgeons remotely, given its endoscopic optics and mechanized movement. However, the maturity of these modalities depends on financial factors, legislation and collaboration with cybersecurity experts to ensure safety and cost-effectiveness [46, 50].
Current applications of cybersurgery
Current applications of cybersurgery include tele-education, tele-training, telementoring, tele-proctoring, and tele-accreditation. Different projects have been developed; different site videoconferences used images and data transmission at the European Institute of TeleSurgery of Strasbourg via The TESUS project through the realization of international multi-site video conferences between surgeons. The WEBSurg project created the first virtual university by placing surgical techniques at the surgeon’s disposal through the Internet. It is a comprehensive source of knowledge in minimally invasive surgery. It promotes technological advances in its fields, such as general and digestive surgery, urology, gynecology, pediatric surgery, endoscopic surgery, skull base surgery, arthroscopy, and upper limb surgery [51].
The HESSOS project (Hepatic Surgery Simulation and Operative Strategy) uses virtual reality as a surgical simulation system, allowing the development of the concept of distant tele-manipulation. It serves as an operative system available for clinical application in liver surgery. In addition, it allows worldwide surgical teaching [51].
Tele-cystoscopy was tested suitable for diagnosis. The trade-offs between cost and tele-cystoscopy system component quality were compared with efficiency frontiers to elucidate the optimal system [52].
Tele-oncology covers diagnosis, treatment, supportive care of cancers, education, and medical training. Modern strategies were addressed to ensure global access to essential cancer care services (Telemedicine and Telesurgery in Cancer Care (TTCC) conference) [53].
To overcome the shortage of surgeons, the “Virtual Interactive Presence” (V.I.P.) platform allows remote participants to simultaneously view each other’s visual field, creating a shared field of view for real-time surgical tele-collaboration [54].
Video analysis yielded a mean compositing delay of 760 ± 606 ms (when compared with the audio signal). Image resolution adequately visualizes neurosurgery’s complex intracranial anatomy and provides interactive guidance [54].
Based on preclinical work, trans-oral robotic surgery (TORS) was performed in February 2007 on a patient with a para-pharyngeal to infratemporal fossa cystic neoplasm as part of a large prospective human trial. The robotic procedure allowed adequate and safe identification of the internal carotid artery and cranial nerves, and excellent hemostasis was achieved with no complications during or after surgery [55].
Later, the Telelap Alf-x, telesurgical system was introduced. It composed of individual arms, which enabled free access to the patient throughout surgery, an extensive range of reusable surgical instruments, an open console with an eye-tracking system, where the camera followed the eye and head movements of the surgeon. The existing force feedback enables for the first time to feel the consistency of the tissues and avoid tearing the stitches while suturing. The system combines the benefits of open surgery and endoscopy [56].
The first clinical application, which involved 146 operations at the gynecological department of the Gemelli University Hospital in Rome, proved the safety and the surgical team’s quick adaptation to the system [56].
In 1992, the National Aeronautics and Space Administration and the Department of Defense supported Telesurgery to rescue wounded soldiers. The Defense Advanced Research Projects Agency invested in tele-medical technologies to help operate injured soldiers remotely [15].
Applications of cybersurgery or telesurgery in ophthalmology
There is no current application of Cybersurgery or Telesurgery in Ophthalmology. However, the feasibility of telerobotic microsurgical repair of corneal lacerations has been evaluated [57]. Five mm central full-thickness corneal wounds were fashioned in five enucleated rabbit eyes and repaired remotely using the telerobotic system [57].
The feasibility of using the Robotic Slave Micromanipulator Unit (RSMU) in photocoagulating the ciliary body remotely to treat glaucoma with the diode laser was tested in fresh un-operated, enucleated human eyes. Histology examination of remote robotic contact trans-scleral cyclophotocoagulation and “by hand” technique produced similar degrees of ciliary body tissue disruption [58].
Various projects have recently been launched at academic and corporate levels to develop lightweight, miniaturized surgical robotic prototypes [59]. This delay could be explained by the delicacy of this field which deals with the sense of vision and the small anatomical size.
Advanced virtualization and augmented-reality techniques should help human operators to adapt better to special conditions [60]. To meet safety standards and requirements in space, a three-layered architecture is recommended to provide the highest quality of telepresence technically achievable for provisional exploration missions [60].
Discussion
Ophthalmology is one of the most enriched fields that allowed artificial intelligence to be part of its point of interest in scientific research. Robotic surgery in ocular surgeries is not well established and still in the experimental process in ophthalmology [25, 27, 29].
Although the current study is limited by the number of published studies discussing robotic and cybersurgeries in ophthalmology, being mainly in experimental stages, a review of current aspects of robotic and cybersurgery in ophthalmology could help in the progression of these disciplines.
The advantages of robots in surgery originate from the need to achieve two goals: telepresence and the performance of repetitive and accurate tasks which are the gold standards of ocular surgery. An accelerometer can cancel the operator's physiologic tremor in real-time. Robotic arms minimize the natural limits of human wrists, favoring more precise and efficient movements.
The risk of human errors combined with mechanical failure as electrical current and misapplication to surrounding tissues coupled with a longer duration of surgery are disadvantages of this technology.
Robotics platforms and prototypes specializing in ophthalmology surgeries are not yet met. Speed and velocity are required during ocular surgery. The time delay threshold must be acceptable by adopting strategies that preserve path-tracking accuracy.
The latest model of Johns Hopkins Steady-Hand Eye Robot has improved the range of motion, stiffness, and speed of holder release. These criteria would be helpful in emergencies requiring rapid actions. In addition, smart instruments coupled with robotics could minimize the risk of possible surgical complications. However, apart from Preceyes’ B.V. research platform, none of the currently eye-specific systems has reached a commercial stage.
Anterior segment surgeries, such as cataract, strabismus, pterygium, keratoplasty, and amniotic membrane suturing, were successfully performed on porcine and human donors. Subretinal drug delivery to treat submacular hemorrhage aided by robots, demonstrated its feasibility and safety; this could be useful in gene or cell therapy [32].
Further development in the instruments used in intravitreal injections could enhance the technique [61].
Cybersurgery benefits today’s shortage of surgeons and eliminates geographical barriers, financial burdens, complications, and often risky long-distance travel. Fibreoptic A.T.M. lines to minimize latency and optimize connectivity and computer motion are elements to consider when planning Telesurgery. In addition, Telesurgery allows for international surgical collaboration and helps in improving surgical education. Different models have been used, such as virtual simulators (DV-Trainer®, Robotic Mentor®, DVSS®), mechanical simulators, microsurgery and wet lab using ex vivo animal organs, anaesthetized animals, and cadavers [62, 63].
There was no significant difference between the lengths of the learning curves for robot-assisted vitreoretinal surgery compared to manual surgery. However, robot-assisted vitreoretinal surgery was more precise, associated with less tissue damage, and slower [64].
Surgical robots are rarely found in healthcare systems and are provided for other surgical specialties, where evidence-based medicine confirms their feasibility. Ophthalmology specialized hospitals are more commonly separated, making access to surgical robots difficult and time-consuming. This factor could contribute to the low number of studies and trials on ocular surgery after the high cost of these robots.
Data regarding costs and litigation of robotics and cyber-surgery versus conventional techniques in ophthalmology are limited. This may be responsible for the delay in this field [65, 66]. Another drawback is the low number of qualified, trained surgeons in robotic surgery.
The technical specifications of robotics used in microsurgery are highly challenging [67]. Future perspectives include technologies, such as 5G, Tactile Internet, and A.I., to help reduce resource scheduling problems in cybersurgery. In addition, specific prototypes to be implemented for robotic ocular surgery to increase the accuracy, as seen in previous studies [68] are crucial for developing this field.
Increasing the number of short- and long-term clinical training programs in robotic surgery could facilitate this field's progression.
Conclusion
The robotic Da Vinci Surgical System and the ARES (Auris Surgical Endoscopy System) robot are the only two surgical robots approved for human surgery; however, it is not designed for microsurgical specialties. Robotic technology has only recently been integrated into ophthalmology; hence, the progression is only in its initial stages.
The cybernetic revolution in surgery supported by artificial intelligence could enable surgeons to perform surgeries remotely. Tele-surgery can provide urgent medical services and allows highly skilled doctors to operate globally.
Technologies, such as 5G and Tactile Internet, are required to help reduce resource scheduling problems in cybersurgery. In addition, prototype development and the integration of artificial intelligence applications could further enhance the safety and precision of ocular surgery.
Surgeons must embrace these technologies to render these technologies available; however, further studies to overcome these challenges limiting the progression of these fields in terms of cost, availability, legislation, and ethics are crucial.
Acknowledgements
Mr Yacine ASLIMI, Professor, Expert R—Lead Data scientist at the Artificial intelligence school—France for his supervision of the Master thesis.
Author contributions
MA: was responsible for conducting the entire research.
Funding
None.
Data availability
Not applicable.
Declarations
Conflict of interest
The author declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ting DSW, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–175. doi: 10.1136/bjophthalmol-2018-313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid JE, Eaton E. Artificial intelligence for pediatric ophthalmology. Curr Opin Ophthalmol. 2019;30(5):337–346. doi: 10.1097/ICU.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1–29. doi: 10.1016/j.preteyeres.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Gunasekeran DV, Ting DSW, Tan GSW, Wong TY. Artificial intelligence for diabetic retinopathy screening, prediction and management. Curr Opin Ophthalmol. 2020;31(5):357–365. doi: 10.1097/ICU.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 5.Devalla SK, et al. Glaucoma management in the era of artificial intelligence. Br J Ophthalmol. 2020;104(3):301–311. doi: 10.1136/bjophthalmol-2019-315016. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor R, Whigham BT, Al-Aswad LA. Artificial intelligence and optical coherence tomography imaging. Asia Pac J Ophthalmol (Phila) 2019;8(2):187–194. doi: 10.22608/APO.201904. [DOI] [PubMed] [Google Scholar]
- 7.Balyen L, Peto T. Promising artificial intelligence-machine learning-deep learning algorithms in ophthalmology. Asia Pac J Ophthalmol (Phila) 2019;8(3):264–272. doi: 10.22608/APO.2018479. [DOI] [PubMed] [Google Scholar]
- 8.Hallak JA, Scanzera AC, Azar DT, Chan RVP. Artificial intelligence in ophthalmology during COVID-19 and in the post COVID-19 era. Curr Opin Ophthalmol. 2020;31(5):447–453. doi: 10.1097/ICU.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey SK, Sharma V. Robotics and ophthalmology: are we there yet? Indian J Ophthalmol. 2019;67(7):988–994. doi: 10.4103/ijo.IJO_1131_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raison N, Khan MS, Challacombe B. Telemedicine in surgery: what are the opportunities and hurdles to realising the potential? Curr Urol Rep. 2015;16(7):43. doi: 10.1007/s11934-015-0522-x. [DOI] [PubMed] [Google Scholar]
- 11.Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113–118. doi: 10.1007/s11701-007-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishihara K, Fukushi T. Introduction: roboethics as an emerging field of ethics of technology. Account Res. 2010;17(6):273–277. doi: 10.1080/08989621.2010.523672. [DOI] [PubMed] [Google Scholar]
- 13.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239(1):14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris SJ, et al. The Probot–an active robot for prostate resection. Proc Inst Mech Eng H. 1997;211(4):317–325. doi: 10.1243/0954411971534449. [DOI] [PubMed] [Google Scholar]
- 15.Newman JG, Kuppersmith RB, O’Malley BW. Robotics and telesurgery in otolaryngology. Otolaryngol Clin North Am. 2011;44(6):1317–1331. doi: 10.1016/j.otc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Morisod B, Simon C. The role of transoral robotic surgery in head and neck cancer. Rev Med Suisse. 2013;9(400):1765–1769. [PubMed] [Google Scholar]
- 17.Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc. 2002;16(10):1389–1402. doi: 10.1007/s00464-001-8283-7. [DOI] [PubMed] [Google Scholar]
- 18.Hockstein NG, Nolan JP, O’Malley BW, Woo YJ. Robotic microlaryngeal surgery: a technical feasibility study using the daVinci surgical robot and an airway mannequin. Laryngoscope. 2005;115(5):780–785. doi: 10.1097/01.MLG.0000159202.04941.67. [DOI] [PubMed] [Google Scholar]
- 19.Broeders IAMJ, Ruurda JP. Robotics in laparoscopic surgery: current status and future perspectives. Scand J Gastroenterol Suppl. 2002;236:76–80. doi: 10.1080/003655202320621508. [DOI] [PubMed] [Google Scholar]
- 20.Nicolau S, Soler L, Mutter D, Marescaux J. Augmented reality in laparoscopic surgical oncology. Surg Oncol. 2011;20(3):189–201. doi: 10.1016/j.suronc.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Tsirbas A, Mango C, Dutson E. Robotic ocular surgery. Br J Ophthalmol. 2007;91(1):18–21. doi: 10.1136/bjo.2006.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourges J-L, Hubschman J-P, Burt B, Culjat M, Schwartz SD. Robotic microsurgery: corneal transplantation. Br J Ophthalmol. 2009;93(12):1672–1675. doi: 10.1136/bjo.2009.157594. [DOI] [PubMed] [Google Scholar]
- 23.Hubschman J-P, et al. “The Microhand”: a new concept of micro-forceps for ocular robotic surgery. Eye (Lond) 2010;24(2):364–367. doi: 10.1038/eye.2009.47. [DOI] [PubMed] [Google Scholar]
- 24.Bourcier T, et al. Robot-assisted pterygium surgery: feasibility study in a nonliving porcine model. Transl Vis Sci Technol. 2015;4(1):9. doi: 10.1167/tvst.4.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chammas J, et al. Da Vinci Xi robot-assisted penetrating keratoplasty. Transl Vis Sci Technol. 2017;6(3):21. doi: 10.1167/tvst.6.3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourcier T, Becmeur P-H, Mutter D. Robotically assisted amniotic membrane transplant surgery. JAMA Ophthalmol. 2015;133(2):213–214. doi: 10.1001/jamaophthalmol.2014.4453. [DOI] [PubMed] [Google Scholar]
- 27.Bourcier T, et al. Robot-assisted simulated cataract surgery. J Cataract Refract Surg. 2017;43(4):552–557. doi: 10.1016/j.jcrs.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Aristeidou A, et al. The evolution of corneal and refractive surgery with the femtosecond laser. Eye Vis (Lond) 2015;2:12. doi: 10.1186/s40662-015-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourcier T, et al. Robot-assisted simulated strabismus surgery. Transl Vis Sci Technol. 2019;8(3):26. doi: 10.1167/tvst.8.3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Channa R, Iordachita I, Handa JT. Robotic vitreoretinal surgery. Retina. 2017;37(7):1220–1228. doi: 10.1097/IAE.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molaei A, et al. Toward the art of robotic-assisted vitreoretinal surgery. J Ophthalmic Vis Res. 2017;12(2):212–218. doi: 10.4103/jovr.jovr_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cehajic-Kapetanovic J, et al. First-in-human robot-assisted subretinal drug delivery under local anesthesia. Am J Ophthalmol. 2021;237:104–113. doi: 10.1016/j.ajo.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Willekens K, et al. Phase I trial on robot assisted retinal vein cannulation with ocriplasmin infusion for central retinal vein occlusion. Acta Ophthalmol. 2021;99(1):90–96. doi: 10.1111/aos.14480. [DOI] [PubMed] [Google Scholar]
- 34.Ullrich F, Michels S, Lehmann D, Pieters RS, Becker M, Nelson BJ. Assistive device for efficient intravitreal injections. Ophthalmic Surg Lasers Imaging Retina. 2016;47(8):752–762. doi: 10.3928/23258160-20160808-09. [DOI] [PubMed] [Google Scholar]
- 35.de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol. 2018;29(3):248–253. doi: 10.1097/ICU.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 36.Ida Y, Sugita N, Ueta T, Tamaki Y, Tanimoto K, Mitsuishi M. Microsurgical robotic system for vitreoretinal surgery. Int J Comput Assist Radiol Surg. 2012;7(1):27–34. doi: 10.1007/s11548-011-0602-4. [DOI] [PubMed] [Google Scholar]
- 37.Wygledowska-Promieńska D, Jurys M, Wilczyński T, Drzyzga Ł. The gamma knife in ophthalmology. Part one-uveal melanoma. Klin Oczna. 2014;116(2):130–134. [PubMed] [Google Scholar]
- 38.Kumar R, Hemal AK. Emerging role of robotics in urology. J Minim Access Surg. 2005;1(4):202–210. doi: 10.4103/0972-9941.19268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourla DH, Hubschman JP, Culjat M, Tsirbas A, Gupta A, Schwartz SD. Feasibility study of intraocular robotic surgery with the da Vinci surgical system. Retina. 2008;28(1):154–158. doi: 10.1097/IAE.0b013e318068de46. [DOI] [PubMed] [Google Scholar]
- 40.Rahimy E, Wilson J, Tsao T-C, Schwartz S, Hubschman J-P. Robot-assisted intraocular surgery: development of the IRISS and feasibility studies in an animal model. Eye (Lond) 2013;27(8):972–978. doi: 10.1038/eye.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He X, Handa J, Gehlbach P, Taylor R, Iordachita I. A submillimetric 3-DOF force sensing instrument with integrated fiber Bragg grating for retinal microsurgery. IEEE Trans Biomed Eng. 2014;61(2):522–534. doi: 10.1109/TBME.2013.2283501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cutler N, et al. Auditory force feedback substitution improves surgical precision during simulated ophthalmic surgery. Invest Ophthalmol Vis Sci. 2013;54(2):1316–1324. doi: 10.1167/iovs.12-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards TL, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng. 2018;2:649–656. doi: 10.1038/s41551-018-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsui I, Tsirbas A, Charles W, Stevan D, Hubschm J-P. Robotic surgery in ophthalmology. In: Hyuk S, editor. Robot surgery. InTech; 2010. [Google Scholar]
- 45.Ueta T, Yamaguchi Y, Shirakawa Y, Nakano T, Ideta R, Noda Y, Morita A, Mochizuki R, Sugita N, Mitsuishi M, Tamaki Y. Robot-assisted vitreoretinal surgery: development of a prototype and feasibility studies in an animal model. Ophthalmology. 2009;116(8):1538–1543.e2. doi: 10.1016/j.ophtha.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Surg Endosc. 2002;16(10):1389–1402. doi: 10.1007/s00464-001-8283-7. [DOI] [PubMed] [Google Scholar]
- 47.Korte C, Sudhakaran Nair S, Nistor V, Low TP, Doarn CR, Schaffner G. Determining the threshold of time-delay for teleoperation accuracy and efficiency in relation to telesurgery. Telemed e-Health. 2014;20(12):1078–1086. doi: 10.1089/tmj.2013.0367. [DOI] [PubMed] [Google Scholar]
- 48.Cazac C, Radu G. Telesurgery–an efficient interdisciplinary approach used to improve the health care system. J Med Life. 2014;7(Spec No. 3):137–141. [PMC free article] [PubMed] [Google Scholar]
- 49.Shahzad N, Chawla T, Gala T. Telesurgery prospects in delivering healthcare in remote areas. J Pak Med Assoc. 2019;69(Suppl 1):S69–S71. [PubMed] [Google Scholar]
- 50.Hung AJ, Chen J, Shah A, Gill IS. Telementoring and telesurgery for minimally invasive procedures. J Urol. 2018;199(2):355–369. doi: 10.1016/j.juro.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 51.Marescaux J, Mutter D, Soler L, Vix M, Leroy J. The virtual university applied to telesurgery: from tele-education to telemanipulation. Chirurgie. 1999;124(3):232–239. doi: 10.1016/s0001-4001(99)80088-9. [DOI] [PubMed] [Google Scholar]
- 52.Hougen HY, et al. Optimizing and validating the technical infrastructure of a novel tele-cystoscopy system. J Telemed Telecare. 2016;22(7):397–404. doi: 10.1177/1357633X15610040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satcher RL, et al. Telemedicine and telesurgery in cancer care: inaugural conference at MD Anderson cancer center: telemedicine and telesurgery in cancer care. J Surg Oncol. 2014;110(4):353–359. doi: 10.1002/jso.23652. [DOI] [PubMed] [Google Scholar]
- 54.Shenai MB, Tubbs RS, Guthrie BL, Cohen-Gadol AA. Virtual interactive presence for real-time, long-distance surgical collaboration during complex microsurgical procedures: technical note. JNS. 2014;121(2):277–284. doi: 10.3171/2014.4.JNS131805. [DOI] [PubMed] [Google Scholar]
- 55.O’Malley BW, Weinstein GS. Robotic skull base surgery: preclinical investigations to human clinical application. Arch Otolaryngol Head Neck Surg. 2007;133(12):1215. doi: 10.1001/archotol.133.12.1215. [DOI] [PubMed] [Google Scholar]
- 56.Stark M, Pomati S, D’Ambrosio A, Giraudi F, Gidaro S. A new telesurgical platform–preliminary clinical results. Minim Invasive Ther Allied Technol. 2015;24(1):31–36. doi: 10.3109/13645706.2014.1003945. [DOI] [PubMed] [Google Scholar]
- 57.Mines MJ, et al. Feasibility of telerobotic microsurgical repair of corneal lacerations in an animal eye model. J Telemed Telecare. 2007;13(2):95–99. doi: 10.1258/135763307780096177. [DOI] [PubMed] [Google Scholar]
- 58.Belyea DA, Mines MJ, Yao W-J, Dan JA, Bower KS. Telerobotic contact transscleral cyclophotocoagulation of the ciliary body with the diode laser. J Robot Surg. 2014;8(1):49–55. doi: 10.1007/s11701-013-0424-1. [DOI] [PubMed] [Google Scholar]
- 59.Diana M, Marescaux J. Robotic surgery. Br J Surg. 2015;102(2):e15–28. doi: 10.1002/bjs.9711. [DOI] [PubMed] [Google Scholar]
- 60.Haidegger T, Sándor J, Benyó Z. Surgery in space: the future of robotic telesurgery. Surg Endosc. 2011;25(3):681–690. doi: 10.1007/s00464-010-1243-3. [DOI] [PubMed] [Google Scholar]
- 61.Melo GB, et al. Critical analysis of techniques and materials used in devices, syringes, and needles used for intravitreal injections. Prog Retin Eye Res. 2021;80:100862. doi: 10.1016/j.preteyeres.2020.100862. [DOI] [PubMed] [Google Scholar]
- 62.Bresler L, Perez M, Hubert J, Henry JP, Perrenot C. Residency training in robotic surgery: the role of simulation. J Visc Surg. 2020;157(3 Suppl 2):S123–S129. doi: 10.1016/j.jviscsurg.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 63.MacCraith E, Forde JC, Davis NF. Robotic simulation training for urological trainees: a comprehensive review on cost, merits and challenges. J Robot Surg. 2019;13(3):371–377. doi: 10.1007/s11701-019-00934-1. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsen MF, Konge L, la Cour M, Sørensen RB, Park YS, Thomsen ASS. The learning curve of robot-assisted vitreoretinal surgery - a randomized trial in a simulated setting. Acta Ophthalmol. 2021;99(8):e1509–e1516. doi: 10.1111/aos.14822. [DOI] [PubMed] [Google Scholar]
- 65.McLean TR, Torrance AW. Are the Brookhill-Wilk patents impediments to market growth in cybersurgery? Int J Med Robot. 2008;4(1):3–9. doi: 10.1002/rcs.176. [DOI] [PubMed] [Google Scholar]
- 66.McLean TR. The legal and economic forces that will shape the international market for cybersurgery. Int J Med Robot. 2006;2(4):293–298. doi: 10.1002/rcs.109. [DOI] [PubMed] [Google Scholar]
- 67.Cleary K, Nguyen C. State of the art in surgical robotics: clinical applications and technology challenges. Comput Aided Surg. 2001;6(6):312–328. doi: 10.1002/igs.10019. [DOI] [PubMed] [Google Scholar]
- 68.Forslund Jacobsen M, Konge L, Alberti M, la Cour M, Park YS, Thomsen ASS. Robot-assisted vitreoretinal surgery improves surgical accuracy compared with manual surgery: a randomized trial in a simulated setting. Retina. 2020;40(11):2091–2098. doi: 10.1097/IAE.0000000000002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.