Dear Editors,
Introduction
Progressive multifocal leukoencephalopathy (PML) is a rare, opportunistic, and severe infectious disease of the central nervous system (CNS) caused by the John Cunningham virus (JVC) [1]. Antibodies against the virus are possessed by 30–70% of healthy adults [1]. No recognizable clinical event is associated with JCV primary infection [1]. On the contrary, reactivated JCV infection in glial cells, namely oligodendrocytes and astrocytes, causes severe demyelination and presents with various symptoms like weakness, sensory deficits, hemianopsia, aphasia, cognitive dysfunction, balance, and gait disturbances [2]. Most cases of PML occur in immunocompromized patients, especially in those with deficiency of cell-mediated immunity [1]. The most common causes of immunosuppression that can lead to PML are malignancies, human immunodeficiency virus (HIV) infection, conditions which require immunosuppressive treatments like systemic inflammatory diseases or organ transplants, and use of immunomodulatory therapies [3]. Especially, the incidence of PML has raised in the recent years due to the widespread use of immunosuppressive/immunomodulatory therapies for autoimmune diseases [4].
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disorder of the CNS, resulting in demyelination, axonal injury, and neuronal loss [5]. Some debate remains about MS as a primarily autoimmune disease (i.e., disease resulting from peripheral immune attack of the CNS) or as a CNS-intrinsic disease (i.e., disease that begins in the CNS and leads to subsequent peripheral immune activation) [6–8]. Even if there is still no curative treatment for MS, several disease-modifying treatments (DMTs) are currently used for its management. The first cases of PML among MS patients were reported in 2005 following natalizumab and interferon-β treatment [9, 10]. Since then, several other cases have been described, even among MS patients treated with fingolimod, dimethyl-fumarate, ocrelizumab, and alemtuzumab [3, 11–13]. PML diagnosis is based on neurological symptoms, a MRI scan indicating a CNS infection and the presence of JCV DNA in the cerebrospinal fluid (CSF) [14].
In this paper, we highlight how differentiating severe MS relapses from PML based on clinic-radiological findings may be sometimes difficult. Yet, a rapid and correct differential diagnosis is mandatory, in order to provide the best treatment options.
Case report
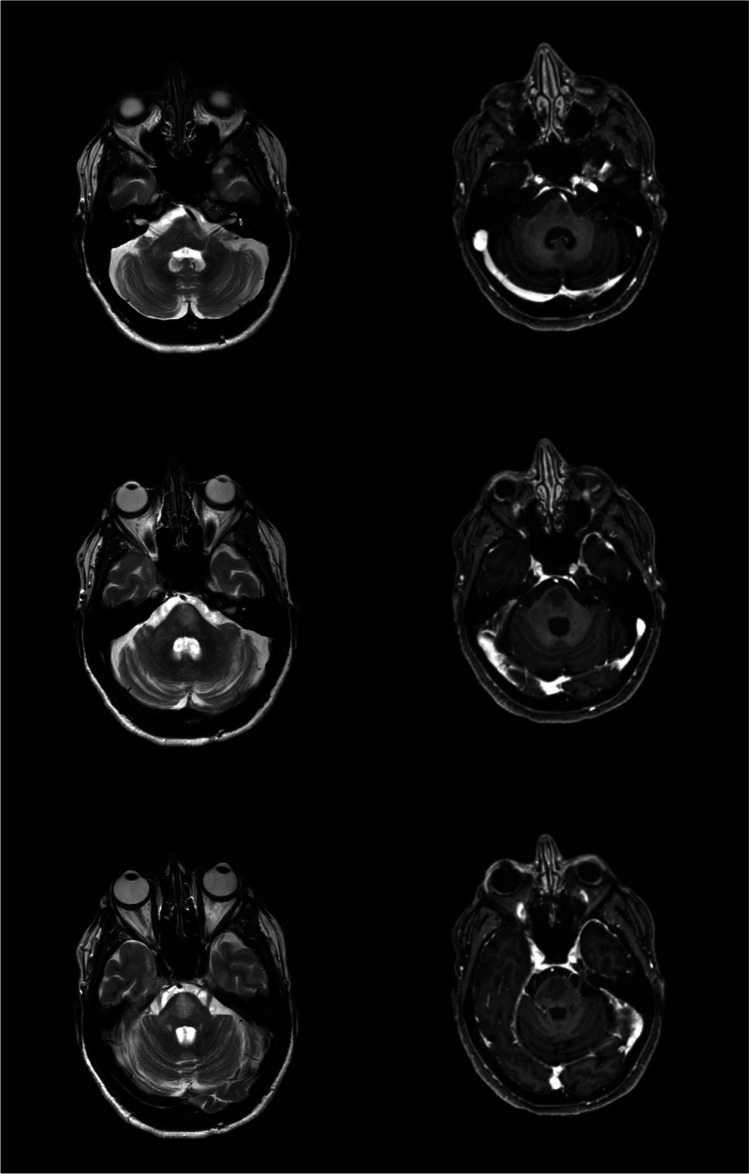
A 55-year-old woman was diagnosed with remittent-relapsing MS in 1989 when she was 22 years old and experienced persistent disease activity on both interferon-β and teriflunomide. Over the years, she developed a depressive disorder and mild cognitive impairment. She transitioned to fingolimod in June 2017 to stabilize disease activity. After 4 years of clinic-radiological stability, she complained reduced visual acuity strongly suspected for optic neuritis and was treated with high-dose steroids for 5 days. She received the first dose of Pfizer-BioNTech COVID-19 vaccine 8 weeks earlier and postponed the second dose for personal reasons. Ten days after ending up steroids, she got the second dose of the Pfizer-BioNTech COVID-19 vaccine despite medical suggestion to postpone the dose due to recent steroid therapy. Four days after vaccine administration, she was admitted to the hospital because of sudden worsening of imbalance and speech disturbances. She underwent brain and spinal cord MRI which revealed a new T2 hyperintense lesion in the ventral pons, with restricted diffusion and patchy, peripheral contrast enhancement (Fig. 1). These findings were suspected for PML, so fingolimod was discontinued. Another brain MRI, performed 6 days later, revealed a caudal extension of the lesion in the ventral pons with persistent, peripheral contrast enhancement (Fig. 2). Rapid evolution and clinic-radiological features suggested an immune reconstitution inflammatory syndrome (IRIS). So, the patient underwent lumbar puncture which resulted negative for JCV-DNA by quantitative polymerase chain reaction. The remaining cyto-chemical and microbiological analysis of the CSF and serum was unremarkable. PML with IRIS was reasonably excluded and disease relapse was then hypothesized, so the patient started high-dose steroids with progressive improvement. At discharge, she was able to walk unaided and her speech, though still dysarthric, became intelligible.
Fig. 1.
Brain MRI revealed a new lesion in the ventral pons, which appeared hyperintense in T2-weighted sequences (a) and presented patchy, peripheral contrast enhancement (b), as well as restricted diffusion on diffusion-weighted imaging (c)
Fig. 2.
Caudal extension of the pontine lesion on T2-weighted sequences (a) with persistent, peripheral contrast enhancement (b)
Three weeks later, a follow-up brain and spinal cord MRI demonstrated a stable lesion load with no contrast-enhancing lesions (Fig. 3). Her neurologic condition remained stable.
Fig. 3.
A follow-up MRI after 3 weeks from the onset of symptoms, with the stable lesion load with no contrast-enhancing lesions
Discussion
We report the first case of a severe MS relapse occurring after COVID-19 vaccine and mimicking PML. The rapid worsening of clinical symptoms and the peculiar radiological aspects were consistent with the suspicion of PML. However, the absence of JVC-DNA in the CSF, the absence of serum anti-JCV antibodies, and the rapid improvement of symptoms after a high-dose steroid course led to reasonably exclude PML and suggested an alternative diagnosis of MS relapse, possibly triggered by COVID-19 vaccine [15]. Indeed, mRNA-based COVID-19 vaccines might induce a strong B and T cell response [16], which in turn may underlie the reactivation of an autoimmune process.
The differential diagnosis between PML and severe MS relapses may be very difficult, especially when clinic-radiological presentations are atypical [17]. Limb weakness, gait disturbance, ataxia, and behavioral and cognitive changes are the most common described symptoms in PML cases, depending on the cerebral areas involved by the lesions [14]. An involvement of the optic nerve or the spinal cord is rare in PML, occurring more frequently in MS relapses [14]. Typical MRI features of PML include large hyperintense T2-weighted lesions (often > 3 cm), a “ground glass” aspect on T2-weighted sequences, monofocal lesions, and involvement of the gray–white matter junction, especially in the frontal lobes [14, 17]. Moreover, PML lesions usually have ill-defined and mixed lesion borders and sometimes present gadolinium enhancement [14]. On the contrary, MS lesions are smaller (often 3–5 mm), periventricular, usually oriented perpendicularly to the ventricular surface and often show contrast enhancement [14]. Beyond clinic-radiological features, the search for JVC-DNA in the CSF is the only tool to clarify the diagnosis [14].
The effect of COVID-19 vaccine on disease activity in MS patients was analyzed in a cross-sectional study of 393 MS patients, a prospective multicentric observational study of 324 MS patients, and a retrospective study of 555 MS patients. According to these studies, the relapse rate in vaccinated patients is similar to that of non-vaccinated patients during the corresponding period, supporting the importance and safety of COVID-19 vaccine in MS patients [18–20]. On the contrary, some recent case series and case reports described disease reactivation in MS patients receiving COVID-19 vaccine [15, 21–24]. The role of vaccine on the risk of developing MS relapses remains to be fully elucidated. The exact mechanism through which a vaccine can trigger MS reactivation probably varies according to the type of vaccine and individual genetic susceptibility [25, 26]. Currently, there are no sufficient data to support or refuse an association between COVID-19 vaccine and MS reactivation, due to the lack of large prospective controlled studies, the short follow-up period [18, 27], and the risk of publication bias [15].
Conclusion
A rapid and severe worsening of clinic-radiologic features in fingolimod-treated MS patients, especially after a long period of disease stability, must be rapidly assessed in order to exclude PML. It is important to achieve an early diagnosis in order to give the patient the best chances to recover. The temporal association of a relapse after COVID-19 vaccine allows the clinician to hypothesize a possible triggering role of the vaccine on disease reactivation. Currently available evidence does not support a role for COVID-19 vaccine in triggering MS relapses, so the vaccine should be recommended to MS patients.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approval
Not applicable.
Patient consent for publication
Obtained.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria D’Apolito, Email: maria.dapolito93@gmail.com.
Marianna G. Rispoli, Email: mariannarispoli92@gmail.com
Paola Ajdinaj, Email: Paola.ajdinaj@gmail.com.
Anna Digiovanni, Email: Annadigiovanni93@gmail.com.
Valentina Tomassini, Email: valentina.tomassini@unich.it.
Luigia Gentile, Email: luigia.gentile@gmail.com.
Giovanna De Luca, Email: gio.deluca05@yahoo.com.
References
- 1.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai S, Brew BJ. Progressive multifocal leukoencephalopathy. Handb Clin Neurol. 2018;152:123–137. doi: 10.1016/B978-0-444-63849-6.00010-4. [DOI] [PubMed] [Google Scholar]
- 3.Kartau M, et al. Progressive multifocal leukoencephalopathy: current insights. Degener Neurol Neuromuscul Dis. 2019;9:109–121. doi: 10.2147/DNND.S203405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger JR. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf. 2010;33(11):969–983. doi: 10.2165/11537510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafler DA, et al. Multiple sclerosis. Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Or A, et al. Clinical perspectives on the molecular and pharmacological attributes of anti-CD20 therapies for multiple sclerosis. CNS Drugs. 2021;35(9):985–997. doi: 10.1007/s40263-021-00843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 10.Langer-Gould A, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 11.Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. doi: 10.1016/j.msard.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Vola EA, et al. Possible progressive multifocal leukoencephalopathy and active multiple sclerosis under dimethyl fumarate: the central role of MRI in informing therapeutic decisions. BMC Neurol. 2021;21(1):146. doi: 10.1186/s12883-021-02165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sriwastava S, et al. Progressive multifocal leukoencephalopathy and sphingosine 1-phosphate receptor modulators used in multiple sclerosis: an updated review of literature. J Neurol. 2022;269(3):1678–1687. doi: 10.1007/s00415-021-10910-1. [DOI] [PubMed] [Google Scholar]
- 14.Berger JR, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nistri R, et al. Case Report: Multiple Sclerosis Relapses After Vaccination Against SARS-CoV2: A Series of Clinical Cases. Front Neurol. 2021;12:765954. doi: 10.3389/fneur.2021.765954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 17.De Mercanti SF, et al. Atypical multiple sclerosis lesions or progressive multifocal leukoencephalopathy lesions: that is the question. J Investig Med High Impact Case Rep. 2020;8:2324709620939802. doi: 10.1177/2324709620939802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achiron A, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Filippo M, Cordioli C, Malucchi S et al (2021) mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry 93(4):448–450. 10.1136/jnnp-2021-327200 [DOI] [PubMed]
- 20.Alonso R, et al. Evaluation of short-term safety of COVID-19 vaccines in patients with multiple sclerosis from Latin America. Mult Scler J Exp Transl Clin. 2021;4:20552173211061543. doi: 10.1177/20552173211061543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etemadifar M, et al. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccin Immunother. 2021;17(10):3481–3483. doi: 10.1080/21645515.2021.1928463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maniscalco GT, et al. Severe Multiple Sclerosis Relapse After COVID-19 Vaccination: A Case Report. Front Neurol. 2021;12:721502. doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fragoso YD, et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult Scler Relat Disord. 2022;57:103321. doi: 10.1016/j.msard.2021.103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Midfai Y, et al. Acute multiple sclerosis exacerbation after vaccination with the Johnson & Johnson COVID-19 vaccine: novel presentation and first documented case report. Cureus. 2022;14(4):e24017. doi: 10.7759/cureus.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen RT, Pless R, Destefano F. Epidemiology of autoimmune reactions induced by vaccination. J Autoimmun. 2001;16(3):309–318. doi: 10.1006/jaut.2000.0491. [DOI] [PubMed] [Google Scholar]
- 26.Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022;362:577765. doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintanilla-Bordás C, et al. Case report: exacerbation of relapses following mRNA COVID-19 vaccination in multiple sclerosis: a case series. Front Neurol. 2022;13:897275. doi: 10.3389/fneur.2022.897275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.