Abstract
By the last third of life, most mammals, including humans, exhibit a decline in immune cell numbers, immune organ structure, and immune defense of the organism, commonly known as immunosenescence. This decline leads to clinical manifestations of increased susceptibility to infections, particularly those caused by emerging and reemerging microorganisms, which can reach staggering levels—infection with SARS-CoV-2 has been 270-fold more lethal to older adults over 80 years of age, compared to their 18–39-year-old counterparts. However, while this would be expected to be beneficial to situations where hyporeactivity of the immune system may be desirable, this is not always the case. Here, we discuss the cellular and molecular underpinnings of immunosenescence as they pertain to outcomes of solid organ and hematopoietic transplantation.
Keywords: Aging, Immunosenescence, Immune response, Transplantation
Introduction
It is well established that the structure of molecules, cells, and tissues of the immune system, as well as their function in immune defense and organismal homeostasis, undergo changes with aging [1]. Such changes are highly variable, affect individuals differently, and are commonly referred to as immune senescence. Their spectrum ranges from manifest qualitative immune response defects via temporal delays to imperfect coordination of molecular and cellular responses. Some of these changes are primary in nature and are due to the process of aging, whereas others may be precipitated by other external stressors, and yet others could be compensatory and reactive to the primary age-related changes. With regard to the outcome for the host, clinically, the most pronounced outcome involves increased vulnerability to acute microbial infections, particularly those caused by emerging and reemerging microbial pathogens [2]. It is believed that the age-related increase in cancer incidence also in part derives from immune senescence because similar immune defects have been observed in response to tumor antigens [3]; however, the efficacy of cancer immunotherapies has not been proven to uniformly decline with age in all studies so far [4, 5], and in some cases, older animals can exhibit increased cancer resistance [6]. The relationship between immune aging and transplantation has been similarly more complex than expected. Clinically, despite reduced immune responses, dosing of immunosuppressive regimens needed to maintain transplant tolerance is not reduced in older adults [7], suggesting robust alloreactivity with aging. Below, we discuss immune aging in light of known defects and put this in the context of existing data as well as likely speculations on their relationship to transplant tolerance and rejection.
Innate immune cells
While much of the research in transplantation immunology has historically focused on the role of adaptive immune responses in the success, or failure, of transplant procedures, the wide array of cell types which comprise the innate arm of the immune response plays a critical role in graft survival via their pro- and anti-inflammatory activities. The major functions of the innate arm of the immune response are to (1) orchestrate the resolution of sterile injury, (2) act as the vanguard of immunity against pathogens, and (3) prime and calibrate the ensuing adaptive immune response. The key players in these activities include monocytes, macrophages, neutrophils, natural killer (NK) cells, NKT cells, and γδ T cells, all of which are affected by the process of aging. It is therefore important to consider how these defects in innate immune function can influence outcomes in transplantation.
In the context of solid organ transplantation, monocytes and macrophages are the major cell types which infiltrate the allograft and surrounding tissues [8]. These populations of cells are critical in the resolution of sterile inflammation resulting from surgical trauma during the transplantation process and ischemia–reperfusion injury (IRI) in a manner largely dependent upon damage-associated molecular pattern (DAMP) signaling (reviewed in [9]). With age, there is a general decrease in the sensitivity of the pattern recognition receptors which recognize these DAMP ligands. For example, in both mice and man, the decreased activation potential of toll-like receptor (TLR) 4, which serves as a receptor for fibronectin, heat shock proteins, and DAMPs (as well as for microbial pathogen-associated molecular patterns, (PAMPs), such as lipopolysaccharides), leads to decreases or delays in cytokine production and phagocytosis [10–12]. This age-linked dysregulation of cytokine production may in part underly diminished wound healing and delayed re-establishment of homeostasis in the surgical setting. This is an especially important consideration when older grafts are utilized, due to their increased susceptibility to IRI [13]. Indeed, IRI has been shown to induce oxidative damage [14], and decreased ability of older cells to deal with this type of insult [15] is well documented. This is further potentially relevant when considering that cytomegalovirus (CMV) reactivation readily occurs under IRI conditions due to oxidative damage [16], which would be expected to have additional deleterious effects on transplant outcomes.
Moreover, upon tissue infiltration, monocytes can differentiate into Ly6Chi and Ly6Clo populations of macrophages. Experiments in mice have demonstrated that Ly6Chi macrophages can contribute to graft injury and rejection through pro-inflammatory cytokine production and alloantigen presentation. Conversely, differentiated DC-SIGN+ (Ly6Clo) macrophages can join tissue-resident populations and promote allograft tolerance through interleukin (IL)-10 signaling [17]. While it is known that aging skews macrophage differentiation toward a pro-inflammatory and away from a reparative phenotype, how this impacts the outcome of transplantation with aging remains an understudied area [18].
Dendritic cells (DCs) are antigen-presenting cells (APCs) that capture and process antigens in tissues and present them to naïve T cells in secondary lymphoid organs, and thus, serve as a critical bridge between the innate and adaptive immune systems. In the setting of transplantation, DCs are responsible for stimulating (or, less commonly, tolerizing) alloreactive T cells via (1) presentation of intact donor major histocompatibility complex (MHC) molecules by donor DCs (direct allopresentation) or (2) recipient DC presentation of processed peptides from donor allogeneic proteins (including presentation of processed peptides from allo-MHC molecules themselves). The importance of DCs in both rejection and tolerance is thus self-evident. In older individuals, DCs exhibit impaired antigen uptake reduced maturation and consequently reduced migratory capacity and costimulatory function [19, 20]. Experiments in old mice have demonstrated decreased expression of MHC-II, CD40L, and CD86 upon infection—molecules critical in priming T cell responses [21]. These changes may in part explain the increased proportion and activation of inducible regulatory T cells (Tregs) in older individuals, which are crucial in immune tolerance [22]. However, studies in older recipients have shown in bone-marrow transplants a surprisingly enhanced allostimulartory capacity by old host DCs, leading to increased activation of donor T cells and exacerbated inflammation and disease [23]. The precise basis for this observation has not been elucidated, thus further study is warranted to help develop targeted anti-rejection therapies for older transplant recipients.
Lymphoid stromal cells and aging
Secondary lymphoid organs (SLOs) provide an optimal microenvironment for the induction of effector immune response during immunity, alloimmunity, and autoimmunity. Particularly, lymph nodes (LNs) serve a critical role in naïve T cell (TN) maintenance, but also are active sites to maintain peripheral tolerance by targeting auto-reactive T cells which have escaped central tolerance in the thymus (Fig. 1) [24]. The LNs are equipped with a wide variety of tolerizing mechanisms including generation of induced regulatory T cells (iTregs), inducing anergy or deletion of auto-reactive T cells, and constraining T cell responses. The majority of such mechanisms are regulated by LN stromal cells. Stromal cells are non-hematopoietic cells that provide an intricate structural network for cellular compartmentalization, organization, and access to survival factors and tonic signals. They play a critical role in orchestrating the cellular interactions needed during various phases of the immune and tolerogenic responses [25]. Based on origin, phenotypic expression, and function, the LN stromal cells are subdivided into four major cell types, fibroblastic reticular cells (FRCs), lymphatic endothelial cells (LECs), blood-endothelial cells (BECs), and double-negative cells, a heterogenous subset believed to contain precursors of other populations. Under the steady-state conditions, the LN stromal cells provide trophic and survival factors, including IL-7, IL-15, chemokine (C–C motif) ligand (CCL) 19, CCL21, B cell activating factor (BAFF), and chemokine (C-X-C motif) ligand (CXCL)13 to T cells and B cells and maintain lymphocyte homeostasis throughout the lifespan [26].
Fig. 1.
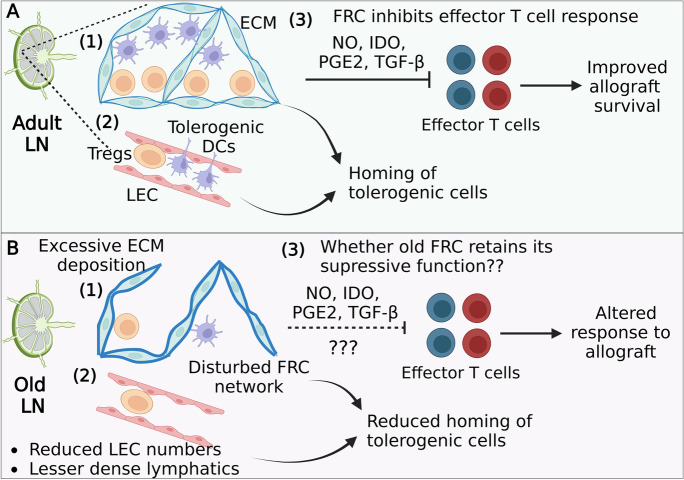
Potential role of lymph node stromal cell aging in transplantation. A Structurally intact (1) FRC reticular network, (2) lymphatic vasculature, and HEV (not shown) in the adult LN support the homing and retention of tolerogenic DCs and Tregs [180, 181]. Such a lymph node near the allograft transplant in mice promotes the survival of grafted tissue by a variety of mechanisms that involve the induction of tolerance to the antigens expressed in the grafted tissue 54. Further, (3) FRC-mediated diverse immunosuppressive pathways constrain effector T cell activation, proliferation, and differentiation leading to the generation of an environment favorable to support allograft survival 44. B However, in old lymph nodes, numerical loss of LECs and structural deterioration of lymphatics and FRC networks [182] in focal areas in the paracortex (T-cell zones) and/or interfollicular areas (T cell/B cell interphase) might negatively affect the mechanisms that support tolerogenic DCs and Tregs. Further, age-related fibrotic changes (excessive extracellular matrix deposition along reticular network) in the old lymph nodes.32 might obscure the DCs’ and Tregs’ access to signals required to maintain their immunosuppressive function. However, whether FRCs retain their capacity to inhibit polyclonal effector T cell responses is not known, but age-related inflammatory changes in the lymph nodes are capable of influencing FRC function and needs systematic investigation. (Created with BioRender.com)
FRCs are myofibroblastic cells that comprise the majority of stromal cells in the SLOs, including the LNs. FRCs produce a meshwork of extracellular matrix (ECM) components that help in the generation and maintenance of three-dimensional conduits for rapid transport of antigens and soluble molecules within the SLOs. FRC processes form the “superhighways” along which T, DC, and other cell types migrate to maximize contact and provide strength and flexibility for the expansion or contraction of SLOs during activation and resolution of immune responses. The deposition of ECM, survival (IL-7, IL-15, and BAFF), and migratory signals (CCL19, CCL21, and CXCL13) on the reticular network sheathing of the conduits facilitate APC-T cell interactions, FRC-T/B cell cross-talk, and leukocyte migration [27, 28].
The BECs and LECs form the blood and lymphatic endovasculature, respectively, and help in antigen and leukocyte trafficking during immunosurveillance, immunity, and alloimmunity. They further provide important factors to maintain FRCs, although the exact spectrum of these interactions is still under investigation. High endothelial venule cells (HEVs) are highly specialized BECs that regulate the entry of circulating leukocytes, soluble antigens, and immunological mediators to the LN. The LECs are specialized endothelial cells that form lymphatic vessels throughout the body and drain tissues into the SLOs via afferent lymphatics, allowing rapid transport of soluble mediators, antigens, pathogens, and immune cells [29]. Several studies have suggested that the dynamic response of these stromal cells to immune activation and regulatory signals regulates effector immunity and tolerance [30, 31]. However, LNs and in particular stromal cells experience age-related dysregulation in their structure and function [32–35], which may affect the response in transplantation settings, ultimately affecting the survival of grafted tissue. Most notably, LECs decline numerically with aging, whereas FRC networks retract and disintegrate, sometimes unexpectedly early depending on the LN location and, likely, its exposure to environmental microorganisms [36].
Role of stromal cells in immune tolerance and transplantation
Many studies have indicated that FRCs and LECs possess the immunoregulatory properties and play an important role in maintaining immune tolerance in the periphery, leveraging some overlapping and distinct mechanisms [37, 38]. It has been shown that peripheral LN FRCs and LECs express transcriptional activators of tissue antigens, such as deformed epidermal autoregulatory factor 1 [39], that allows them to express a wide variety of endogenous peripheral tissue antigens (PTAs) to induce antigen-dependent tolerance [40, 41]. It remains to be determined if FRCs or LECs maintain their capacity to express PTAs and induce deletional tolerance or support tolerogenic DC and iTreg generation with aging. Although LECs lack the expression of functional MHC-II, it has been reported that LECs can capture antigen-loaded MHC-II from DCs [42]. Similarly, FRCs are also known to acquire self-peptide-MHC II complexes from DCs that induce CD4+ T cell anergy or deletion [42]. Moreover, LECs archive antigens from the lymph flow and transfer them to DCs during steady-state conditions, as well as during infection and inflammation [43], thereby facilitating clonal deletion, anergy, or activation of T cells depending on the inflammatory context. It is currently unknown whether these functions of LEC are affected by aging, although the fact that LEC themselves are drastically reduced with aging [32] suggests that this function would be expected to be impaired.
FRCs induce antigen-independent suppression of T cell responses via different mechanisms, including nitric oxide, indoleamine-2,3-dioxygenase, adenosine 2A receptor, prostaglandin E2, and transforming growth factor (TGF)-β receptor pathways [44]. Other mechanisms include the generation of tolerogenic DCs, induction and migration of Tregs, expression of low levels of co-stimulatory molecules to induce T cell anergy, and expression of higher levels of co-inhibitory molecules, such as programmed death-ligand 1 (PD-L1) [44, 45]. LECs also support the maintenance of iTregs in the LN, and their ability to express PTAs allows the generation of antigen-specific iTregs, thus facilitating indirect antigen-specific tolerance in the LN [46]. Moreover, LECs in the afferent lymphatics support the migration of Tregs from tissue to the LN, which has been found to be a critically important step in the survival of allograft [47]. Further, it has been reported that HEVs induce the apoptosis of FasL-expressing lymphocytes thereby contributing to the maintenance of peripheral tolerance [48]. HEVs also support the entry of APCs such as DCs, which captured the antigen from the allograft. Through elevated expression of CCL19/21, DCs enter the host LN, facilitate the generation or maintenance of tolerance to alloantigens, and support the survival of the allograft. The CCL19/21 gradient-mediated entry of tolerogenic APCs to the donor LN seems a critical step, as a tolerance-inducing regimen with anti-CD40L did not induce tolerance in C–C motif chemokine receptor (CCR) 7−/− mice [49]. The expression of different laminin isoforms on the reticular network has been shown to support immunity versus tolerance in a contextual manner by modulating CD4+ T cell differentiation. Laminin 411 inhibits the differentiation of effector T helper type (Th) 1, Th2, and Th17 cells but supports the differentiation of iTregs, while laminin 511 acts in an opposite manner [50, 51]. Recently, it has been shown that expression of laminin 4 in FRCs is critical in maintaining a tolerogenic niche in murine lymph nodes and its deficiency leads to the generation of hyper-active effector alloreactive T cells and humoral responses and reduced Tregs, resulting in the failure of tolerance and ultimately the rejection of cardiac and lung transplant in the mice [52]. Collectively, the evidence suggests that LN stromal cells participate in the maintenance of immune tolerance in the periphery and may play a decisive role in graft survival or rejection.
Recently, the allogeneic donor-specific splenocyte transfusion plus anti-CD40L mAb has been successfully used as a regimen to improve allograft survival, and a part of its mechanism may be via modulation of FRC-T cell interaction. The FRC-T cell interaction via CD40L-CD40 signaling has been shown to induce alloimmune responses and blockade of this pathway using anti-CD40L mAbs significantly improved cardiac transplant survival in mice [51, 53]. The reticular network of FRCs supports the migration of Tregs in the LN, and perturbations of this reticular structure have been shown to adversely affect Treg trafficking and facilitate the rejection of allogeneic cardiac transplant in mice [54]. Similar disruption in the reticular network and cellular organization has been described in the aged LN [33, 35, 55], which may be sufficient to disturb the trafficking of iTregs into and within the aged LN and has the potential to adversely influence allograft survival. In support of this, an experimental perturbation of FRCs and HEV networks has recently shown a negative affect the survival of allografts [47, 54, 56]. The exact spectrum of age-related changes in the above features of stromal cells remains to be established but may have profound implications for the outcome of transplantation.
Stromal cells in graft-versus-host disease
Evidence suggests that LN stromal cells, including FRCs and HEVs, are involved in graft-versus-host disease (GVHD) induced by allogeneic transplant. In murine allogeneic stem cell transplant models, transplanted stem cells have been shown to mount a response against recipient LN FRC structure, affecting the reticular network and HEV structure via Fas-FasL signaling, resulting in the immunological scarring of the recipient LN [57]. The FRCs also contribute to GVHD through activating delta-like ligands (Dll)-1 and Dll4-mediated Notch signaling in alloreactive T cells. Deleting these Notch ligands selectively in FRCs and follicular DCs has been shown to control GVHD [58]. Allogeneic donor graft-derived mast cells have been shown to target host FRCs in the nearest LN. The donor mast cells induce FRC expression of herpes virus entry mediator (HVEM), and stimulation of FRCs by the HVEM-LIGHT axis forced FRCs to acquire a senescence-like phenotype (as marked by expression of the p16INK4a, p21, Trp53, and p57KIP2 genes), secrete increased levels of collagen I, and made the LN fibrotic long after allograft rejection [59]. Countering these issues with transplantation of ex vivo expanded FRCs mitigated fibrosis in the LN and improved the ability of anti-CD40L to increase the survival of allogeneic cardiac transplant [59]. Therefore, therapeutically targeting the non-hematopoietic cells of the SLOs, especially in aged recipients is a viable avenue of investigation to increase transplantation success.
Possible effect of age-related changes on tolerogenic function of LN stromal cells
Recent studies from us and others have suggested that age-related changes affect the LN reticular structure, alter the T and B cell localization, and perturb the overall architecture and organization of SLOs, leading to poor antibody and T cell responses to infection and vaccination [32, 55, 60, 61]. Reduced priming of T cells is a characteristic of old DCs, limiting the ability of old T cells to mount an appropriate response to foreign antigens and alloantigens [12]. Aged peripheral lymph nodes (pLNs) show signs of fibrosis [32], and T cells in the proximity of accumulated collagen slow their migration within the LN [35]. Aged LNs also fail to support the homeostatic proliferation of TN 33. A recent report has shown that homeostatic aging of the mouse spleen is characterized by the erosion of the podoplanin + networks, corresponding to a reduction in T cell zone FRC numbers 55. Interestingly, it has been recently shown that aged pLNs lose their ability to undergo remodeling and expansion in response to viral infections such as West Nile virus and Chikungunya virus [34, 61]. In response to infection, aged FRCs respond poorly, exhibit delayed and slower proliferation, and fail to optimally stretch and elongate, resulting in poor expansion of the LN and a weak immune response [62]. Such age-related defects at the level of LN stromal cells may have the ability to influence the tolerance to alloantigens and grafts. Nonetheless, quantitative and direct studies are needed to address the exact role of aged stromal cells and the mechanisms involved in the transplantation setting where either donor, recipient, or both are experiencing age-related changes. This is particularly necessary to incisively dissect the relative contributions of decreased induction of T cell priming as opposed to reduced induction of transplant tolerance.
T cell aging and transplantation
The function of T cells as the cellular immunity mediators of the adaptive arm of the immune system depends on the structural and functional integrity of the lymphoid organs. Following positive and negative selection in the thymus, TN cells migrate to the SLOs where they are activated by their first encounter with antigen. T cells then proliferate and differentiate into several types of effector T cells: cytotoxic T cells (which kill cells infected with intracellular pathogens), helper T cells (which provide signals to support the functions of other cells like macrophages and B cells), and Tregs (which help dampen immune responses). In addition to affecting the extrinsic factors that T cells depend on for optimal function and survival, aging affects intrinsic T cell functions in several ways. Age-related thymic involution is the earliest dramatic change in our immune system, resulting in a 90% drop in TN output by the time of late puberty [63] and another drop of 90% between the ages of 40 and 50 in humans [64].
Lymph node atrophy (as described above) contributes to T cell defects over time. Overall, aging in the T cell compartment is characterized by reduced numbers and increased turnover of TN [64], an increasing proportion of TN cells converting to virtual memory T cells (Tvm) [65], reduced proliferation of Tvm [66], and reduced TCR repertoire [64, 67]. While there is an unquestionable and reproducible impaired response to new pathogens with aging, that includes reduced numbers and frequencies of responding T cells, as well as the reduced magnitude and polyfunctionality of effector T cell responses [68, 69], it is less clear which of the features of T cell aging directly contribute to impaired immunity. It is now likely that initial innate sensing of microbial infection [70–73] as well as defective SLO environments [34, 36, 61] may be exceptionally important. This is further highlighted by our data showing that on a cell-by-cell basis, old Tn cells are at least equivalent to adult Tn cells in responding to L. monocytogenes when adoptively transferred into young recipients, whereas adult Tn cells cannot respond well in the old environment [74]. It is therefore most likely that the main problem with Tn cells in old age lies in their numerical loss (so that only ~ 25–33% remain), a defect that can be corrected by transfers of antigen-specific T cell precursors [75]. By contrast, memory responses produced in youth and young adulthood appear to be well preserved during aging [69, 76].
All of this, then, needs to be reconciled with the clinical observations and protocols suggesting that in aging, transplant rejection occurs as vigorously (if not more vigorously) as it does in younger organisms. There are several changes in the older immune system that would heighten the likelihood of stronger alloreactivity. First, several lines of the investigation show that with aging, the T cell repertoire undergoes peripheral selection so that CD8+ Tvm, which arise from TN due to competition for trophic factors in the lymph nodes, exhibit both stronger affinity towards self-MHC [65] and a propensity to make cytokines rapidly after stimulation (i.e., appeared partially primed) [66], whereas CD4+ T cells exhibit broader crossreactivity [77]. Second, 70–95% of older individuals are infected with cytomegalovirus (CMV), which leads to a life-long absolute expansion of fully differentiated and highly cytotoxic T effector memory (Tem) and T effector memory re-expressing CD45RA (Temra) cells [78]. Tem and Temra cells likely keep their alloreactive potential and are poised for rapid allograft destruction. Third, deterioration of lymph node structure, and with it the erosion of the LN stromal cell tolerogenic function (see above), also likely potentiates the propensity for strong alloreactivity.
A major complication of bone marrow transplantation is T cell-mediated graft GVHD. An especially devastating consequence of GVHD is the damage done to the GI tract, which naturally harbors and abundance of T cells that can be both protective and pathogenic. Although immune-mediated damage as a result of GVHD has long been observed [79], recent mouse studies have revealed the crypt base stem cell compartment to be the primary target of infiltrating donor T cells [80]. It has been found that the extent of antigenic disparity directly correlates with the number of T cells that infiltrate the intestinal tissue, and T cell infiltration increases over time, but the stem cell compartment always remains the primary target [80]. This finding points to the necessity for targeted therapeutics for the treatment of GVHD, perhaps in addition to general immunosuppressive treatments to ensure the success of the graft. The known age-related dysregulations in T cell biology, discussed at the end of the previous paragraph, should stimulate specific studies to address the roles of each of the changes in GVHD, with the goal to modulate immunosuppressive therapies based on the immunobiology of the older recipient.
A major goal in the field of transplantation is to effectively target Tregs to modulate T-cell-mediated and antibody-mediated graft rejection [81]. Tregs dampen immune responses by producing IL-10 [82, 83], modulating the amount of available IL-2 [84], and even by inducing apoptosis in effector T cells [85, 86]. Trials of Treg therapies in solid organ transplants have only recently begun; thus, no long-term data exists on its efficacy or safety [87, 88]. However, there are aspects of Treg biology that will need to be considered in future trials. The first is bystander suppression, where Tregs suppress responses in a non-antigen-specific manner following Treg activation. This phenomenon has already been demonstrated in mice [89]. The second is Treg plasticity and their ability to take on Th-17 effector functions, which may contribute to graft rejection. Conflicting murine data exist on whether conversion to a Th-17-like phenotype and function can be prevented in humans [90, 91]. In short, Treg therapy has the potential to transform post-transplant patient care so long as specificity for regulating the immune responses within the graft can be achieved without further suppressing immune responses against malignancies or infections.
Aging of B cells
The quality and quantity of newly generated B cells in the bone marrow are impacted by age. While the mechanisms responsible remain to be fully characterized, cell intrinsic and microenvironment changes have been described with aging in mice. Myeloid-biased hemopoietic stem cells accumulate with age in the bone marrow of both mice and humans, promoting the age-related decline in B lymphopoiesis [92]. In the bone marrow of old mice, there is a lower frequency of common lymphoid progenitors and reduced numbers of pro-B, pre-B, and immature B cell subsets when compared to young mice [93, 94]. Bone marrow stromal cells regulate B lymphopoiesis by controlling access to essential growth factors such as IL-7 to progenitor cells. In the aging microenvironment, studies have shown that stromal cells are impaired in IL-7 secretion, with a consequent decrease in pre-B cell numbers [95]. Additionally, alterations in the aged microenvironment are responsible for less efficient V(D)J recombination in pro-B cells due to reduced rag2 gene expression 96. Evidence also exists that key B cell maintenance factors, including BAFF/APRIL/BLyS, are also altered with aging [97]. Therefore, reduced B cell generation and maintenance lead to reduced naïve B (and sometimes total B) cell numbers with aging. Cell-intrinsic factors also contribute to the age-related deficit in B cell generation. Studies show that old common lymphoid progenitor cells express less of the transcription factor, EBF, necessary for B cell commitment and differentiation [98]. Moreover, mature B cells from old mice express less of the transcription factor, PAX5, required for the maintenance of B cell fate [99].
Humoral immune responses are also impaired in old mice and humans. It is well established that antibodies produced by aged individuals provide less protection against bacterial and viral infections when compared to their young adult counterparts. The ability of B cells to undergo class switch recombination (CSR) and switch immunoglobulin classes is vital for an effective and appropriate antibody response. Splenic B cells isolated from old mice undergo limited CSR, produce fewer class-switched antibodies, and express less of the E2A-encoded transcription factor E47 [100]. E47 induces activation-induced cytidine deaminase (AID) which is essential for CSR and somatic hypermutation. Therefore, under-induction of AID leads to antibodies of inferior quality in aged mice. There is evidence that the age-related decline of E47 expression in activated B cells is due to the downregulation of the p38 MAPK signal transduction cascade resulting in elevated degradation of E47 mRNA [101, 102].
The germinal center (GC) reaction is critical for the secretion of high-affinity antibodies. Within the GC, antigen-specific B cells receive crucial signals from CD4+ Th cells. CD4+ T cells undergo differentiation into various functional subsets, including Th1, Th2, Th9, Th17, T follicular helper (Tfh), T follicular regulatory (Tfr), and Tregs which allow the immune response to be tailored to the specific threat encountered. Of these, Tfh cells are essential to the GC reaction; these cells localize to B cell follicles and GCs to provide help to B cells for the efficient production of antibodies [103]. In old mice, there is a defect in the differentiation of CD4+ T cells into Tfh cells which results in fewer GCs [104]. Additionally, reduced levels of CXCL13, a chemokine important for Tfh cell trafficking to the B cell follicle, leads to reduced recruitment of T cells to GCs and impaired B cell help provided by T cells during aged immune responses [105]. Tfr cells have an opposing effect on humoral responses by limiting available T cell help and GC formation [106]. The ratio of Tfr and Tfh cells determines the robustness of the antibody response [104]. Increases in Tfh and Tfr cells are observed in aged mice but there is a greater proportion of Tfr cells, which may contribute to the suppression of the B cell response with aging [107]. Importantly, the suppressive capacity of Tfr cells is not different between young and adult animals, suggesting that the ratio of Tfh and Tfr, and not the quality of suppression, plays a key role in determining the magnitude of the antibody response [107]. Evidence suggests that elevated TGF-β in the aged environment induces expression of FOXP3 in Tfh cells, driving their differentiation and contributing to impaired humoral responses in aged mice [108].
With all the above changes leading to dysregulation and general reduction of antigen-specific humoral immune responses, there are also changes in B cells that may favor hyper-reactivity and increased inflammation, with the potential to maintain or enhance allograft rejection. Specifically, age-associated B cells (ABCs), that accumulate with aging in both mice and humans [109], react to innate receptor ligands and secrete large amounts of cytokines to promote inflammatory responses. Thus, it comes as no surprise that B cells are gaining increasing recognition for their complex effects on the outcomes of transplantation in aged individuals [110].
Aging B cells and transplantation
In the context of transplantation, acute and chronic rejection can be mediated by alloreactive antibodies produced by B cells. HLA incompatibility between donors and recipients is a major cause of solid organ rejection mostly due to antibody-mediated rejection (ABMR) in which antibodies against donor HLA molecules and other non-HLA donor antigens attack allografts and impair their survival. Donor-specific HLA antibodies can be present before transplantation or de novo donor-specific HLA antibodies (dnDSA) can appear late posttransplantation often due to insufficient immunosuppression [111, 112]. A study by Moos et al. found that the risk of developing dnDSA is lower in older adult recipients when compared to pediatric recipients [113]. This finding may be explained by the impaired ability of aged adults to generate effective humoral responses against novel antigens; however, the mechanism responsible for this observation has yet to be established.
In humans, anti-CD20 monoclonal antibody treatments such as Rituximab are used to deplete B cells to improve graft survival in HLA antibody incompatible transplantation [114]. However, there are few studies that describe how B cell depletion affects transplantation in the context of aging. A study by Mori et al. demonstrated that B cell depletion in mice undergoing skin transplantation has disparate effects on allograft survival depending on the age of the recipient mouse [115]. Using a skin allograft model, B cell depletion resulted in the rapid rejection of the transplant in young mice. In contrast, aged mice treated with anti-CD20 had a 7-day delay in allograft rejection. This difference was attributed to ABCs and the adoptive transfer of ABCs into young mice reduced the skin allograft survival rate [115]. While the specific role of ABCs in human allograft reaction has yet to be directly addressed, it is tempting to speculate that they could have adverse effects on allograft survival.
Age-related changes in systemic cytokine/chemokine environment
Many studies reported that with aging, there is a subtle but significant increase in blood levels of inflammatory markers, including IL-6, TNF-α, C-reactive protein, IL-8, IL-18, IL1ra, macrophage inflammatory protein (MIP)-1b, and soluble TNF receptor (sTNFR) I and II [116–119]. The increase of those factors in blood can be interpreted as the existence of chronic, systemic, low-grade inflammation, which has been associated with many age-related diseases, including frailty and sarcopenia [119–124]. The effect of the age-related increase in inflammation is a problem from the standpoint of solid organ transplantation. Because of this increased proinflammatory status, older organs can exhibit more pronounced immunogenicity, may respond suboptimally to stress, and may repair less well than younger organs following transplantation [110, 125–127]. The inflammatory response in transplantation plays an important role in the allograft loss or dysfunction [128, 129]. It is reported that TNF-α, IL-6, IL-8, and IL-10 are expressed and released in circulation in the case of primary graft dysfunction (PGD) [130], and TNF-α and CCL2 have both been strongly associated with PGD development in lung transplant recipients [131]. Furthermore, pro-inflammatory cytokines IL-1, IL-6, and TNF-α are all upregulated in chronic lung allograft dysfunction (CLAD) [132, 133]. Many of those inflammatory factors are regulated by the TLR4-MyD88 pathway, and the upregulation of the MyD88 gene or TLR4 mRNA was reported to be associated with cell-mediated rejection [134] or kidney graft rejection [135].
Proinflammatory cytokines and chemokines, however, are not the only ones dysregulated and oversecreted with aging, and there is, unfortunately, an oversimplified tendency by many authors to describe the dysregulation of soluble mediators only in proinflammatory terms (often using the popular but misleading term “inflammaging”). Indeed, age-related dysregulation in soluble immune and inflammatory mediators affects equally strongly the mediators involved in the wound healing response, that are usually considered anti-inflammatory, most notably TGFβ and its family members, as well as other profibrotic mediators such as type 2 cytokines IL-4, 13 and others 1. TGFβ is overproduced by older organisms in many infections, including those with intracellular parasites [136, 137], alphaviruses 61 and flaviviruses (J.L. Uhrlaub, personal communication), although the basis of this overproduction has not been established. It will be of interest to evaluate to what extent this response can lead to scarring and fibrosis of transplanted organs.
The presence of cells carrying signs of cellular senescence has recently been documented in an increasing number of tissues, and their removal has been shown to improve the function of some tissues under certain disease conditions as well as in chronological aging [138]. With regard to transplantation, it has been shown that cells accumulated in the organs from older donors increase immunogenicity and elevate the risk of the rejection [139]. Many, but not all, of those senescent cells are characterized by a senescent-associated secretory phenotype (SASP), which is characterized by high expression of pro-inflammatory cytokines and chemokines such as IL-6, IL-8, TNF-α, and CCL2 [140]. The SASP can contribute to tissue dysfunction, development of chronic diseases, accelerated aging-like state, and impairing tissue homeostasis [138]. The removal of senescence cells has been shown to be effective to ameliorate age-related tissue dysfunctions in old mice [141–145]. Iske et al. found that senescent cell accumulation is a key source of cell-free mitochondrial DNA, which drives alloimmune responses to organs from older donors [146]. Therefore, the use of senolytics may represent a promising avenue to improve the outcome of older organ transplantation and prevent the spread of senescence.
Solid organ transplantation in older adults
Solid organ transplant is the most effective therapy for end-stage organ failure, even in older individuals. The number of people over 65 years of age receiving transplants is rising and so is the number of older adults on a waitlist to receive the organ transplant. However, our limited understanding of age-related changes, and their direct and indirect effects on donor organs and recipients, hinders the development of consensus protocols for solid organ transplantation in older adults (Table 1). Increased age of the donor organ negatively influences the longevity and outcome of transplantation, as it affects the organ’s homeostasis, inflammatory status, antigenicity, metabolic and bioenergetic activities, reparative capacity, and ability to handle a wide variety of stress and malignancies [147]. The kidney (> 55 yrs.) [148], heart (> 50 yrs.) [149], and lungs (> 60 yrs.) [150] from old donors have lower longevity compared to those from young adult donors, and this might be due to diminished functionality such as decreased glomerular number and function, chronotropic incompetence, and diminished airway epithelial function, as well as to the above inflammatory, wound healing/reparative, and immunogenic differences. The CMV seropositive status of the donor also affects the quality of the donor organ, as persistent CMV infection may be associated with some features of immunosenescence [151]. Further, IRI-related innate immune activation in the donor organ can contribute to faster allograft rejection [152]. It has been shown that aging compromises innate immunity and also induces chronic low-grade dysregulation of soluble mediators (elevated serum IL-6 and TNF-α) [153], elevated TGFβ following infection [61, 137]. In such a situation, aberrant innate immune activation in the donor organ itself can alter intrinsic repair mechanisms and induce the recipient’s immune effectors, fueling the alloimmune responses. Older organs transplanted into older recipients show lower rejection than those in younger recipients [154], suggesting that age-matching in addition to immunological matching can help achieve realistic goals in transplant settings. Supporting this notion, transplantation of older (18 months) or younger (2.5–3 months) murine cardiac allograft into younger recipient mice showed that older hearts are rejected more rapidly than younger hearts, and aged DCs played a decisive role in mediating recipient’s alloimmune response, as depletion of DCs prior to transplantation resulted in comparable survival of old and young donor hearts [125]. Such findings highlight that age-related alterations in the innate immune compartments of the donor organ may determine transplant outcome. In that context, targeting innate mechanisms in older organs may represent a logical avenue to help alleviate some of the negative effects of donor organ age on the outcome of a transplant, as well as to broaden the pool of donor organs available for transplantation. Along these lines, transplantation studies in transgenic mice that lack the components of innate signaling such as TLR2, TLR4, and MyD88, have shown beneficial effects by delaying both acute and chronic allograft rejection [155, 156].
Table 1.
Age-related changes in immune responses that may change transplant outcome
| Cellular compartment | Transplant type | |
|---|---|---|
| Solid organ | Bone marrow | |
| T cells |
- Bystander suppression by accumulated Tregs -Reduced frequency of T cell-mediated rejection |
-Increased bias away from lymphocytes in older donors -Delayed reconstitution of the T cell compartment |
| B cells |
-Accumulation of age-associated B cells (ABCs) -Lower risk of acute organ rejection as compared to pediatric recipients -Lower risk of developing de novo donor-specific HLA antibodies |
-Reduced B cell progenitors in aged mice and humans |
| Innate immune cells |
-Bias towards differentiation of infiltrating macrophages and monocytes towards proinflammatory phenotypes -Decreased DAMP recognition by PRRs, leading to faulty wound healing and IRI repair |
-Defective direct allopresentation by donor DCs, possibly leading to decreased alloreactive T cell tolerization |
Adjustment of immunosuppressive treatment in older adults
Since the pharmacokinetics and pharmacodynamics change with age, the administration of immunosuppressive drugs needs to be adjusted for age [157, 158]. Jacobson et al. analyzed calcineurin inhibitor (CNI) troughs in different age groups for 6 months following kidney transplant and showed that despite lower drug doses, the aged group (65–84 years old) showed higher troughs than younger groups [159].
The topmost adverse transplant outcome in older adults is death with a functioning graft [160, 161], and the most frequent cause of death more than 5 years after transplantation is an infection due to immunosuppression [162–166]. Since it has become widely accepted that older transplant recipients may encounter less acute rejection episodes after transplantation as compared to younger recipients due to immunosenescence [167–169], the application of a moderate immune suppression treatment strategy for the elderly seems to be reasonable. A rationale for age-adjusted immunosuppression in organ transplant is also reviewed by Krenzien et al. in 2015 [7]. However, many attempts to use lower immunosuppressive drug doses or withdrawal of drugs with high toxicity in kidney transplant patients result in a higher rejection rate [170–175]. These studies did not specifically target older adults, but included patients older than 65, and no age-associated benefit was found in those trials. A literature review of lowering or withdrawing immunosuppressive drugs in older kidney transplant patients by Swinski et al. concluded that the current data do not support definitive conclusions, but that there may be a possible benefit from lowering doses or withdrawing of CNI in low-risk populations [176]. Withdrawal or minimization of immunosuppressive drugs at late time points after liver transplant have been more promising, but they also require selection of low-risk patients and close monitoring to be successful [177, 178]. Those studies showed that fine adjustments will be required to find a balance between sufficient immunosuppression for successful allograft survival and maintenance of sufficient immune function to fend off infections or malignancies in older populations. We suggest that high-resolution immune profiling may be of use in these situations to dissect likely correlations and to appropriately monitor the immune system’s reactivity in the face of titrated immunosuppression.
Conclusion
Overall, aging abounds in changes to both the innate and adaptive immunity, to systemic cytokine and chemokine milieu, and encompasses alterations in immune cells, stromal cells, and the ECM. With regard to immunity to new infection, the net outcome is often manifested as lower immune reactivity and impaired immune defense, although these changes, like most of aging, exhibit great individual variability.
The situation is not quite as simple when it comes to the transplant setting, and the simple expectation that lowered immune reactivity would translate to better transplant acceptance is often incorrect. Despite the reduced efficacy of primary immune responses to new antigens, alloreactivity remains an exceedingly strong force that is built into the very nature of T and B cell receptor recognition. Therefore, while, for example, precursor frequencies of T cells specific for viral antigens drop by 60–80% in a mouse with aging [65, 179], and in absolute numbers down to 30–200 cells, alloreactive cell numbers remain in tens if not hundreds of thousands despite some numerical reduction. Moreover, increased inflammation, and reduced tolerogenicity of certain microenvironments (e.g., lymph node stroma), further play into allograft rejection.
Finally, older organs themselves tend to invite higher rejection rates, due to innate and inflammatory activation, with senescent cells being a potential culprit. Modulation of these processes by senolytics and by innate cell manipulations/depletions may expand the age of donor organs available to those in dire need of transplantation.
Abbreviations
- AID
Activation-induced cytidine deaminase\
- APC
Antigen presenting cell
- BAFF
B cell activating factor
- BECs
Blood endothelial cells
- CCL
Chemokine (C–C motif) ligand
- CCR
C-C motif chemokine receptor
- CD
Cluster of differentiation
- CMV
Cytomegalovirus
- CNI
Calcineurin inhibitor
- CSR
Class switch recombination
- CXCL
Chemokine (C-X-C motif) ligand
- DAMP
Damage-associated molecular pattern
- DCs
Dendritic cells
- Dll
Delta-like ligand
- E47
E2A-encoded transcription factor
- ECM
Extracellular matrix
- FRCs
Fibroblastic reticular cells
- GC
Germinal center
- GVHD
Graft versus host disease
- HEVs
High endothelial venule cells
- HVEM
Herpes virus entry mediator
- IL
Interleukin
- IRI
Ischemia-reperfusion injury
- LECs
Lymphatic endothelial cells
- LNs
Lymph nodes
- Ly6C
Lymphocyte-antigen 6
- MHC
Major histocompatibility complex
- NK
Natural killer
- PAMP
Pathogen-associated molecular pattern
- PGD
Primary graft dysfunction
- pLNs
Peripheral lymph nodes
- PTA
Peripheral tissue antigens
- SLOs
Secondary lymphoid organs
- Tem
T effector memory cells
- Temra
T effector memory cells expressing CD45RA
- Tfh
T follicular helper cells
- Tfr
T follicular regulatory cells
- TGF-β
Transforming growth factor β
- Th
T helper type
- TLR
Toll-like receptor
- TN
Naïve T cells
- TNF
Tumor necrosis factor
- Tregs
Regulatory T cells
- Tvm
Virtual memory T cells
Author contribution
All authors wrote the manuscript. KEM and JN-Ž edited the manuscript.
Funding
Supported by USPHS grants AG020719 and AG052359 and the Bowman Professorship in Medical Sciences to J.N-Ž.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 2.Albright JF, Albright JW. Aging, immunity, and infection. Humana Press. 2003, Totowa, NJ, USA.
- 3.DeSantis CE, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69:452–467. doi: 10.3322/caac.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helissey C, Vicier C, Champiat S. The development of immunotherapy in older adults: new treatments, new toxicities? J Geriatr Oncol. 2016;7:325–333. doi: 10.1016/j.jgo.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–37. doi: 10.1016/j.ctrv.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Seluanov A, Gladyshev VN, Vijg J, Gorbunova V. Mechanisms of cancer resistance in long-lived mammals. Nat Rev Cancer. 2018;18:433–441. doi: 10.1038/s41568-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krenzien F, et al. A rationale for age-adapted immunosuppression in organ transplantation. Transplant. 2015;99:2258–2268. doi: 10.1097/TP.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Bosch TP, Kannegieter NM, Hesselink DA, Baan CC, Rowshani AT. Targeting the monocyte-macrophage lineage in solid organ transplantation. Front Immunol. 2017;8:153. doi: 10.3389/fimmu.2017.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 11.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Metcalf TU, et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell. 2015;14:421–432. doi: 10.1111/acel.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SB, Kim JK, Cho KS. Complications of renal transplantation: ultrasonographic evaluation. J Ultrasound Med. 2007;26:615–633. doi: 10.7863/jum.2007.26.5.615. [DOI] [PubMed] [Google Scholar]
- 14.Granata, S. et al. Oxidative stress and ischemia/reperfusion injury in kidney transplantation: focus on ferroptosis, mitophagy and new antioxidants. Antioxidants (Basel) 2022;11. 10.3390/antiox11040769. [DOI] [PMC free article] [PubMed]
- 15.Liguori I, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, et al. A clinically relevant murine model unmasks a “two-hit” mechanism for reactivation and dissemination of cytomegalovirus after kidney transplant. Am J Transplant. 2019;19:2421–2433. doi: 10.1111/ajt.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde P, et al. DC-SIGN(+) Macrophages control the induction of transplantation tolerance. Immunity. 2015;42:1143–1158. doi: 10.1016/j.immuni.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong L, et al. Macrophage function in the elderly and impact on injury repair and cancer. Immun Ageing. 2021;18:4. doi: 10.1186/s12979-021-00215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2008;28:14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev. 2011;10:336–345. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varas A, et al. Age-dependent changes in thymic macrophages and dendritic cells. Microsc Res Tech. 2003;62:501–507. doi: 10.1002/jemt.10411. [DOI] [PubMed] [Google Scholar]
- 22.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 23.Ordemann R, et al. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109:1249–1256. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynoso ED, Lee JW, Turley SJ. Peripheral tolerance induction by lymph node stroma. Adv Exp Med Biol. 2009;633:113–127. doi: 10.1007/978-0-387-79311-5_10. [DOI] [PubMed] [Google Scholar]
- 25.Onder L, Ludewig B. A fresh view on lymph node organogenesis. Trends Immunol. 2018;39:775–787. doi: 10.1016/j.it.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Brown FD, Turley SJ. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J Immunol. 2015;194:1389–1394. doi: 10.4049/jimmunol.1402520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 28.Alexandre YO, Mueller SN. Stromal cell networks coordinate immune response generation and maintenance. Immunol Rev. 2018;283:77–85. doi: 10.1111/imr.12641. [DOI] [PubMed] [Google Scholar]
- 29.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 30.Grasso C, Pierie C, Mebius RE, van Baarsen LGM. Lymph node stromal cells: subsets and functions in health and disease. Trends Immunol. 2021;42:920–936. doi: 10.1016/j.it.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurty AT, Turley SJ. Lymph node stromal cells: cartographers of the immune system. Nat Immunol. 2020;21:369–380. doi: 10.1038/s41590-020-0635-3. [DOI] [PubMed] [Google Scholar]
- 32.Thompson HL, et al. Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates. Aging Cell. 2019;18:e12865. doi: 10.1111/acel.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becklund BR, et al. The aged lymphoid tissue environment fails to support naive T cell homeostasis. Sci Rep. 2016;6:30842. doi: 10.1038/srep30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richner JM, et al. Age-dependent cell trafficking defects in draining lymph nodes impair adaptive immunity and control of West Nile virus infection. PLoS Pathog. 2015;11:e1005027. doi: 10.1371/journal.ppat.1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwok TMS, Silva-Junior IA, Brown EM, Haug JC, Barrios MR, Morris KA and Lancaster JN. Age-associated changes to lymph node fibroblastic reticular cells. Frontiers in Aging 2022;838943. 10.3389/fragi.2022.838943. [DOI] [PMC free article] [PubMed]
- 36.Sonar SA, Uhrlaub JL, Coplen CP, Sempowski GD, Dudakov JA, van den Brink MRM, LaFleur BJ, Jergovic M, Nikolich-Zugich J. Early age-related atrophy of cutaneous lymph nodes precipitates an early functional decline in skin immunity in mice with aging. PNAS USA 2022:119(17);e202108119. 10.1073/pnas.2121028119 [DOI] [PMC free article] [PubMed]
- 37.Cohen JN, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol. 2015;15:350–361. doi: 10.1038/nri3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip L, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10:1026–1033. doi: 10.1038/ni.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fletcher AL, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 42.Dubrot J, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med. 2014;211:1153–1166. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kedl RM, et al. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat Commun. 2017;8:2034. doi: 10.1038/s41467-017-02247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena V, et al. Role of lymph node stroma and microenvironment in T cell tolerance. Immunol Rev. 2019;292:9–23. doi: 10.1111/imr.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Wu J, Abdi R, Jewell CM, Bromberg JS. Lymph node fibroblastic reticular cells steer immune responses. Trends Immunol. 2021;42:723–734. doi: 10.1016/j.it.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baptista AP et al. Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. Elife 2014;3. 10.7554/eLife.04433. [DOI] [PMC free article] [PubMed]
- 47.Nakayama Y, Bromberg JS. Lymphotoxin-beta receptor blockade induces inflammation and fibrosis in tolerized cardiac allografts. Am J Transplant. 2012;12:2322–2334. doi: 10.1111/j.1600-6143.2012.04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kokkonen TS, Augustin MT, Makinen JM, Kokkonen J, Karttunen TJ. High endothelial venules of the lymph nodes express Fas ligand. J Histochem Cytochem. 2004;52:693–699. doi: 10.1177/002215540405200513. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, et al. Tolerance induction towards cardiac allografts under costimulation blockade is impaired in CCR7-deficient animals but can be restored by adoptive transfer of syngeneic plasmacytoid dendritic cells. Eur J Immunol. 2011;41:611–623. doi: 10.1002/eji.201040877. [DOI] [PubMed] [Google Scholar]
- 50.Simon T, et al. Differential regulation of T-cell immunity and tolerance by stromal laminin expressed in the lymph node. Transplant. 2019;103:2075–2089. doi: 10.1097/TP.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, et al. The lymph node stromal laminin alpha5 shapes alloimmunity. J Clin Invest. 2020;130:2602–2619. doi: 10.1172/JCI135099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li, L. et al. Lymph node fibroblastic reticular cells preserve a tolerogenic niche in allograft transplantation through laminin alpha4. J Clin Invest 2022;132. 10.1172/JCI156994 [DOI] [PMC free article] [PubMed]
- 53.Nakayama Y, Brinkman CC, Bromberg JS. Murine fibroblastic reticular cells from lymph node interact with CD4+ T cells through CD40-CD40L. Transplant. 2015;99:1561–1567. doi: 10.1097/TP.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burrell BE, et al. Lymph node stromal fiber ER-TR7 modulates CD4+ T cell lymph node trafficking and transplant tolerance. Transplant. 2015;99:1119–1125. doi: 10.1097/TP.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masters AR, Jellison ER, Puddington L, Khanna KM, Haynes L. Attrition of T cell zone fibroblastic reticular cell number and function in aged spleens. Immunohorizons. 2018;2:155–163. doi: 10.4049/immunohorizons.1700062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warren KJ, Iwami D, Harris DG, Bromberg JS, Burrell BE. Laminins affect T cell trafficking and allograft fate. J Clin Invest. 2014;124:2204–2218. doi: 10.1172/JCI73683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suenaga F, et al. Loss of lymph node fibroblastic reticular cells and high endothelial cells is associated with humoral immunodeficiency in mouse graft-versus-host disease. J Immunol. 2015;194:398–406. doi: 10.4049/jimmunol.1401022. [DOI] [PubMed] [Google Scholar]
- 58.Chung J, et al. Fibroblastic niches prime T cell alloimmunity through Delta-like Notch ligands. J Clin Invest. 2017;127:1574–1588. doi: 10.1172/JCI89535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, et al. Lymph node fibroblastic reticular cells deposit fibrosis-associated collagen following organ transplantation. J Clin Invest. 2020;130:4182–4194. doi: 10.1172/JCI136618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aw D, et al. Disorganization of the splenic microanatomy in ageing mice. Immunol. 2016;148:92–101. doi: 10.1111/imm.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uhrlaub JL, et al. Dysregulated TGF-beta Production underlies the age-related vulnerability to Chikungunya virus. PLoS Pathog. 2016;12:e1005891. doi: 10.1371/journal.ppat.1005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masters AR, et al. Assessment of lymph node stromal cells as an underlying factor in age-related immune impairment. J Gerontol A Biol Sci Med Sci. 2019;74:1734–1743. doi: 10.1093/gerona/glz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 65.Rudd BD, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Zugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Britanova OV, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 68.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11c+CD4-CD8alpha- dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 71.Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grolleau-Julius A, Abernathy L, Harning E, Yung RL. Mechanisms of murine dendritic cell antitumor dysfunction in aging. Cancer Immunol Immunother. 2009;58:1935–1939. doi: 10.1007/s00262-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li G, Smithey MJ, Rudd BD, Nikolich-Zugich J. Age-associated alterations in CD8alpha+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium. Aging Cell. 2012;11:968–977. doi: 10.1111/j.1474-9726.2012.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jergovic M, Thompson HL, Renkema KR, Smithey MJ, Nikolich-Zugich J. Defective transcriptional programming of effector CD8 T cells in aged mice is cell-extrinsic and can be corrected by administration of IL-12 and IL-18. Front Immunol. 2019;10:2206. doi: 10.3389/fimmu.2019.02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uhrlaub JL, et al. Quantitative restoration of immune defense in old animals determined by naive antigen-specific CD8 T-cell numbers. Aging Cell. 2022;21:e13582. doi: 10.1111/acel.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGovern KE, Cabral CM, Morrison HW, Koshy AA. Aging with Toxoplasma gondii results in pathogen clearance, resolution of inflammation, and minimal consequences to learning and memory. Sci Rep. 2020;10:7979. doi: 10.1038/s41598-020-64823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deshpande NR, Parrish HL, Kuhns MS. Self-recognition drives the preferential accumulation of promiscuous CD4(+) T-cells in aged mice. Elife. 2015;4:e05949. doi: 10.7554/eLife.05949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beilhack A, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu YY, et al. T Cell Recruitment to the intestinal stem cell compartment drives immune-mediated intestinal damage after allogeneic transplantation. Immunity. 2019;51:90–103.e103. doi: 10.1016/j.immuni.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atif M, Conti F, Gorochov G, Oo YH, Miyara M. Regulatory T cells in solid organ transplantation. Clin Transl Immunol. 2020;9:e01099. doi: 10.1002/cti2.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 83.Paul G, Khare V, Gasche C. Inflamed gut mucosa: downstream of interleukin-10. Eur J Clin Invest. 2012;42:95–109. doi: 10.1111/j.1365-2362.2011.02552.x. [DOI] [PubMed] [Google Scholar]
- 84.Akkaya B, Shevach EM. Regulatory T cells: master thieves of the immune system. Cell Immunol. 2020;355:104160. doi: 10.1016/j.cellimm.2020.104160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 86.MacDonald G, Shi L, Vande Velde C, Lieberman J, Greenberg AH. Mitochondria-dependent and -independent regulation of Granzyme B-induced apoptosis. J Exp Med. 1999;189:131–144. doi: 10.1084/jem.189.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang Q, et al. Selective decrease of donor-reactive Tregs after liver transplantation limits Treg therapy for promoting allograft tolerance in humans. Sci Transl Med. 2022;14:eabo2628. doi: 10.1126/scitranslmed.abo2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Juneja T, Kazmi M, Mellace M, Saidi RF. Utilization of Treg cells in solid organ transplantation. Front Immunol. 2022;13:746889. doi: 10.3389/fimmu.2022.746889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 90.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 91.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. doi: 10.4049/jimmunol.155.3.1151. [DOI] [PubMed] [Google Scholar]
- 92.Muller-Sieburg CE, Sieburg HB, Bernitz JM, Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012;119:3900–3907. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alter-Wolf S, Blomberg BB, Riley RL. Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression. J Immunol. 2009;182:138–147. doi: 10.4049/jimmunol.182.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 95.Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. doi: 10.1182/blood.V91.1.75. [DOI] [PubMed] [Google Scholar]
- 96.Labrie JE, 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Lescale C, et al. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 99.Anspach J, Poulsen G, Kaattari I, Pollock R, Zwollo P. Reduction in DNA binding activity of the transcription factor Pax-5a in B lymphocytes of aged mice. J Immunol. 2001;166:2617–2626. doi: 10.4049/jimmunol.166.4.2617. [DOI] [PubMed] [Google Scholar]
- 100.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 101.Frasca D, et al. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- 102.Frasca D, et al. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- 103.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124:5191–5204. doi: 10.1172/JCI76861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lefebvre JS, et al. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell. 2012;11:732–740. doi: 10.1111/j.1474-9726.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sage PT, Tan CL, Freeman GJ, Haigis M, Sharpe AH. Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 2015;12:163–171. doi: 10.1016/j.celrep.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci Rep. 2016;6:25051. doi: 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cancro MP. Age-associated B cells. Annu Rev Immunol. 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]
- 110.Colvin MM, Smith CA, Tullius SG, Goldstein DR. Aging and the immune response to organ transplantation. J Clin Invest. 2017;127:2523–2529. doi: 10.1172/JCI90601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiebe C, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 112.Sellares J, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 113.von Moos S, Schalk G, Mueller TF, Laube G. Age-associated decrease in de novo donor-specific antibodies in renal transplant recipients reflects changing humoral immunity. Immun Ageing. 2019;16:9. doi: 10.1186/s12979-019-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barnett AN, Hadjianastassiou VG, Mamode N. Rituximab in renal transplantation. Transpl Int. 2013;26:563–575. doi: 10.1111/tri.12072. [DOI] [PubMed] [Google Scholar]
- 115.Mori DN, Shen H, Galan A, Goldstein DR. Aged B cells alter immune regulation of allografts in mice. Eur J Immunol. 2016;46:2650–2658. doi: 10.1002/eji.201646353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 117.Gerli R, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121:37–46. doi: 10.1016/s0047-6374(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 118.Mariani E, et al. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech Ageing Dev. 2001;122:1383–1395. doi: 10.1016/s0047-6374(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 119.Morrisette-Thomas V, et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139:49–57. doi: 10.1016/j.mad.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002;13:389–391. [PubMed] [Google Scholar]
- 121.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamerman D. Toward an understanding of frailty. Ann Intern Med. 1999;130:945–950. doi: 10.7326/0003-4819-130-11-199906010-00022. [DOI] [PubMed] [Google Scholar]
- 123.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yousefzadeh, M. J. et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell 2018;17. 10.1111/acel.12706. [DOI] [PMC free article] [PubMed]
- 125.Oberhuber R, et al. CD11c+ dendritic cells accelerate the rejection of older cardiac transplants via interleukin-17A. Circulation. 2015;132:122–131. doi: 10.1161/CIRCULATIONAHA.114.014917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tullius SG, Milford E. Kidney allocation and the aging immune response. N Engl J Med. 2011;364:1369–1370. doi: 10.1056/NEJMc1103007. [DOI] [PubMed] [Google Scholar]
- 127.Tullius SG, et al. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. 2010;252:662–674. doi: 10.1097/SLA.0b013e3181f65c7d. [DOI] [PubMed] [Google Scholar]
- 128.Ravindranath MH, El Hilali F, Filippone EJ. The impact of inflammation on the immune responses to transplantation: tolerance or rejection? Front Immunol. 2021;12:667834. doi: 10.3389/fimmu.2021.667834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sepe V, Libetta C, Gregorini M and Rampino T. The innate immune system in human kidney inflammaging. J Nephrol.10.1007/s40620-021-01153-4. [DOI] [PMC free article] [PubMed]
- 130.Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757–760. doi: 10.1002/bjs.1800790813. [DOI] [PubMed] [Google Scholar]
- 131.Bharat A, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–197. doi: 10.1016/j.athoracsur.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suwara MI, et al. Mechanistic differences between phenotypes of chronic lung allograft dysfunction after lung transplantation. Transpl Int. 2014;27:857–867. doi: 10.1111/tri.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Verleden SE, et al. Differential cytokine, chemokine and growth factor expression in phenotypes of chronic lung allograft dysfunction. Transplant. 2015;99:86–93. doi: 10.1097/TP.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 134.Sharbafi MH, et al. TLR-2, TLR-4 and MyD88 genes expression in renal transplant acute and chronic rejections. Int J Immunogenet. 2019;46:427–436. doi: 10.1111/iji.12446. [DOI] [PubMed] [Google Scholar]
- 135.Hosseinzadeh M, et al. Expression patterns of Toll like receptor (TLR)-2, TLR-4 and myeloid differentiation primary response gene 88 (MYD88) in renal transplant patients developing allograft dysfunction; a cohort study. Transpl Immunol. 2018;48:26–31. doi: 10.1016/j.trim.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 136.Cabral CM, McGovern KE, MacDonald WR, Franco J, Koshy AA. Dissecting amyloid beta deposition using distinct strains of the neurotropic parasite toxoplasma gondii as a novel tool. ASN Neuro. 2017;9:1759091417724915. doi: 10.1177/1759091417724915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhadra R, et al. Intrinsic TGF-beta signaling promotes age-dependent CD8+ T cell polyfunctionality attrition. J Clin Invest. 2014;124:2441–2455. doi: 10.1172/JCI70522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iske J, Matsunaga T, Zhou H, Tullius SG. Donor and recipient age-mismatches: the potential of transferring senescence. Front Immunol. 2021;12:671479. doi: 10.3389/fimmu.2021.671479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baar MP, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e116. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Farr JN, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moncsek A, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(-/-) ) mice. Hepatol. 2018;67:247–259. doi: 10.1002/hep.29464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu M, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Iske J, et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. 2020;11:4289. doi: 10.1038/s41467-020-18039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dreyer GJ, Hemke AC, Reinders ME, de Fijter JW. Transplanting the elderly: balancing aging with histocompatibility. Transplant Rev (Orlando) 2015;29:205–211. doi: 10.1016/j.trre.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 148.Tan RY, Allen JC, Kee T, Jafar TH. Predictors of low estimated glomerular filtration rate after living kidney donation in a Southeast Asian population from Singapore. Nephrol (Carlton) 2017;22:761–768. doi: 10.1111/nep.12845. [DOI] [PubMed] [Google Scholar]
- 149.Chau EM, et al. Increased incidence of chronotropic incompetence in older donor hearts. J Heart Lung Transplant. 1995;14:743–748. [PubMed] [Google Scholar]
- 150.De Perrot M, et al. Impact of donors aged 60 years or more on outcome after lung transplantation: results of an 11-year single-center experience. J Thorac Cardiovasc Surg. 2007;133:525–531. doi: 10.1016/j.jtcvs.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 151.Soderberg-Naucler C, Fornara O, Rahbar A. Cytomegalovirus driven immunosenescence-an immune phenotype with or without clinical impact? Mech Ageing Dev. 2016;158:3–13. doi: 10.1016/j.mad.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 152.Yi T, et al. Reperfusion injury intensifies the adaptive human T cell alloresponse in a human-mouse chimeric artery model. Arterioscler Thromb Vasc Biol. 2012;32:353–360. doi: 10.1161/ATVBAHA.111.239285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Solana R, et al. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 154.Frei U, et al. Prospective age-matching in elderly kidney transplant recipients–a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8:50–57. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 155.Wu H, et al. Absence of MyD88 signaling induces donor-specific kidney allograft tolerance. J Am Soc Nephrol. 2012;23:1701–1716. doi: 10.1681/ASN.2012010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ro H, et al. Roles of Toll-like receptors in allogeneic islet transplantation. Transplant. 2012;94:1005–1012. doi: 10.1097/TP.0b013e3182708dd3. [DOI] [PubMed] [Google Scholar]
- 157.Kuypers DR. Immunotherapy in elderly transplant recipients: a guide to clinically significant drug interactions. Drugs Aging. 2009;26:715–737. doi: 10.2165/11316480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 158.Staatz CE, Tett SE. Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs Aging. 2005;22:541–557. doi: 10.2165/00002512-200522070-00001. [DOI] [PubMed] [Google Scholar]
- 159.Jacobson PA, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12:3326–3336. doi: 10.1111/j.1600-6143.2012.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang E, et al. Intermediate-term outcomes associated with kidney transplantation in recipients 80 years and older: an analysis of the OPTN/UNOS database. Transplant. 2010;90:974–979. doi: 10.1097/TP.0b013e3181f5c3bf. [DOI] [PubMed] [Google Scholar]
- 161.Lemoine M, et al. Risk factors for early graft failure and death after kidney transplantation in recipients older than 70 years. Kidney Int Rep. 2019;4:656–666. doi: 10.1016/j.ekir.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fulop T, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 164.Martins PN, et al. Age and immune response in organ transplantation. Transplant. 2005;79:127–132. doi: 10.1097/01.tp.0000146258.79425.04. [DOI] [PubMed] [Google Scholar]
- 165.McKay D, Jameson J. Kidney transplantation and the ageing immune system. Nat Rev Nephrol. 2012;8:700–708. doi: 10.1038/nrneph.2012.242. [DOI] [PubMed] [Google Scholar]
- 166.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59:1539–1543. doi: 10.1046/j.1523-1755.2001.0590041539.x. [DOI] [PubMed] [Google Scholar]
- 167.Meier-Kriesche HU, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplant. 2000;69:885–889. doi: 10.1097/00007890-200003150-00037. [DOI] [PubMed] [Google Scholar]
- 168.Meier-Kriesche HU, Srinivas TR, Kaplan B. Interaction between acute rejection and recipient age on long-term renal allograft survival. Transplant Proc. 2001;33:3425–3426. doi: 10.1016/s0041-1345(01)02477-0. [DOI] [PubMed] [Google Scholar]
- 169.Rana A, et al. Profiling risk for acute rejection in kidney transplantation: recipient age is a robust risk factor. J Nephrol. 2017;30:859–868. doi: 10.1007/s40620-016-0354-x. [DOI] [PubMed] [Google Scholar]
- 170.Abramowicz D, et al. Cyclosporine withdrawal from a mycophenolate mofetil-containing immunosuppressive regimen: results of a five-year, prospective, randomized study. J Am Soc Nephrol. 2005;16:2234–2240. doi: 10.1681/ASN.2004100844. [DOI] [PubMed] [Google Scholar]
- 171.Asberg A, et al. Long-term outcomes after cyclosporine or mycophenolate withdrawal in kidney transplantation - results from an aborted trial. Clin Transplant. 2013;27:E151–156. doi: 10.1111/ctr.12076. [DOI] [PubMed] [Google Scholar]
- 172.Dugast E, et al. Failure of calcineurin inhibitor (tacrolimus) weaning randomized trial in long-term stable kidney transplant recipients. Am J Transplant. 2016;16:3255–3261. doi: 10.1111/ajt.13946. [DOI] [PubMed] [Google Scholar]
- 173.Hricik DE, et al. Adverse outcomes of tacrolimus withdrawal in immune-quiescent kidney transplant recipients. J Am Soc Nephrol. 2015;26:3114–3122. doi: 10.1681/ASN.2014121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Opelz G, Dohler B. Effect on kidney graft survival of reducing or discontinuing maintenance immunosuppression after the first year posttransplant. Transplant. 2008;86:371–376. doi: 10.1097/TP.0b013e31817fdddb. [DOI] [PubMed] [Google Scholar]
- 175.Roodnat JI, et al. 15-year follow-up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplant. 2014;98:47–53. doi: 10.1097/01.TP.0000442774.46133.71. [DOI] [PubMed] [Google Scholar]
- 176.Sawinski D, et al. Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: a systematic review and meta-analysis. Am J Transplant. 2016;16:2117–2138. doi: 10.1111/ajt.13710. [DOI] [PubMed] [Google Scholar]
- 177.Benitez C, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatol. 2013;58:1824–1835. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 178.Shaked A, et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am J Transplant. 2019;19:1397–1409. doi: 10.1111/ajt.15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smithey MJ, Li G, Venturi V, Davenport MP, Nikolich-Zugich J. Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J Immunol. 2012;189:5356–5366. doi: 10.4049/jimmunol.1201867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brinkman CC, et al. Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration. Nat Commun. 2016;7:12021. doi: 10.1038/ncomms12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piao W, et al. Regulation of T cell afferent lymphatic migration by targeting LTbetaR-mediated non-classical NFkappaB signaling. Nat Commun. 2018;9:3020. doi: 10.1038/s41467-018-05412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Turner VM, Mabbott NA. Structural and functional changes to lymph nodes in ageing mice. Immunol. 2017;151:239–247. doi: 10.1111/imm.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]