Abstract
Abstract
Versatile nature of copper oxide nanoparticles (CuO NPs) has made them an imperative nanomaterial being employed in nanomedicine. Various physical, chemical, and biological methodologies are in use for the preparation of CuO NPs. The physicochemical and biological properties of CuO NPs are primarily affected by their method of fabrication; therefore, selectivity of a synthetic technique is immensely important that makes these NPs appropriate for a specific biomedical application. The deliberate use of CuO NPs in biomedicine questions their biocompatible nature. For this reason, the present review has been designed to focus on the approaches employed for the synthesis of CuO NPs; their biomedical applications highlighting antimicrobial, anticancer, and antioxidant studies; and most importantly, the in vitro and in vivo toxicity associated with these NPs. This comprehensive overview of CuO NPs is unique and novel as it emphasizes on biomedical applications of CuO NPs along with its toxicological assessments which would be useful in providing core knowledge to researchers working in these domains for planning and conducting futuristic studies.
Key Points
• The recent methods for fabrication of CuO nanoparticles have been discussed with emphasis on green synthesis methods for different biomedical approaches.
• Antibacterial, antioxidant, anticancer, antiparasitic, antidiabetic, and antiviral properties of CuO nanoparticles have been explained.
• In vitro and in vivo toxicological studies of CuO nanoparticles exploited along with their respective mechanisms.
Graphical Abstract

Keywords: CuO NPs, Toxicity, Green synthesis, Biocompatibility, ROS, Biomedicine
Introduction
Nanotechnology is an emerging field of twenty-first century and nanoparticles exhibit distinct structural, optical, electrical, magnetic, and mechanical characteristics due to their nanodimensional range (1–100 nm) as compared to their bulk counterparts (Bezza et al. 2020). There has been increasing attention given to transition metallic oxide nanoparticles for last two decades because of their wide range of applications in the fields of catalysis, biosensing, cosmetics, pharmacy, food and agriculture, electronics, dentistry, energy, and environment (Javed et al. 2022a; Katwal et al. 2015). Copper oxide (CuO) nanoparticles (NPs) have shown fascinating behavior in different areas of biomedicine and act as strong bactericidal, catalytic, anti-carcinogenic, and coating agents (Grigore et al. 2016). CuO NPs are highly abundant in nature and are low-cost materials. These NPs have interesting physicochemical features and are present in various oxidation states, viz., Cu0, CuI, CuII, and CuIII (Bhanushali et al. 2015). They have ~ 2 eV band gap energy and have attained captivating significance because of their chemical inertness and thermal stability (Naz et al. 2020a).
CuO NPs are inorganic and much more stable than organic NPs. These are p-type semiconductors that exist in monoclinic nanostructures. The controllability of synthetic methods is very crucial in obtaining NPs of desired size and morphology. High energy ball milling, laser ablation, and sputtering are the physical methods documented in literature for the fabrication of CuO NPs (Gawande et al. 2016). These NPs can easily be synthesized by various solution methods like co-precipitation, sol–gel, microemulsion, hydrothermal, sonochemical, and microwave irradiation. Capping agents or surfactants are added to the NPs using the chemical methods to get controlled growth and stability of CuO nanoproducts (Tran and Nguyen 2014). Biological methods for preparing CuO NPs utilize extracts of different plant parts (leaf, root, seed, stem, flower, fruit) as well as microbes (bacteria, fungi) and algae as reported in literature (Shamsuddin et al. 2019).
CuO NPs are widely used for nanomedical purposes because of their tremendous antimicrobial activity and use as potential disinfectants against nosocomial infections. These are applied in wound dressings due to strong bactericidal property against different Gram + ve and Gram –ve bacterial strains (Grigore et al. 2016). The fungicidal activity of CuO NPs against few fungal strains is also documented. CuO NPs are widely used as biosensors for sensing of glucose, dopamine, cholesterol, lactate, DNA, etc. Moreover, their potential role as antitumor agents in the treatment of lung, breast, prostate, kidney, and glioma cancer is indispensable. These are also being used as effective nanocarriers (Chevallet et al. 2017; Naz et al. 2018). Furthermore, it plays a vital role in the cellular respiration, regulating level of neurotransmitters, production of collagen protein, and metabolism of essential nutrients like iron which is important part of major enzymes and proteins (Naz et al. 2020a).
Despite the promising applications of CuO NPs in biomedicine, the major concern for their use for diagnostic and therapeutic purposes is the toxic effects elucidated by researchers working on different vertebrates (specifically mammalian cells) and invertebrates. CuO NPs have been reported to induce oxidative stress by the over-generation of reactive oxygen species (ROS) in living cells that leads to damage of DNA and cellular organelles. The size, surface charge, and dissolution of NPs are the major factors contributing to in vitro and in vivo toxicity caused by CuO NPs (Grigore et al. 2016; Katsumiti et al. 2018). Hence, biocompatibility and non-toxicity are the key selection parameters for a particular nanoparticle to be employed in clinical research.
This review provides a detailed information to the readers about CuO NPs, their fabrication techniques, biomedical applications (exclusively), and their toxicological analysis. CuO NPs are unique materials which have extensive applications in almost every field, but they have negative effects as well. Hence for biomedical applications, one must know which method is effective for the fabrication of CuO NPs to get a particular biological property with minimal in vitro and in vivo toxic effects. Although the promising influence of CuO NPs in the domain of biomedicine has been summarized previously. Similarly, the effects of toxicity have also been previously defined in the biological systems. Nevertheless, no single document exists in literature in which the different biological applications and toxicology of CuO nanoparticles could be comprehensively defined in interaction with one another. This review provides a wide knowledge about all these parameters which help readers to conduct their research accordingly.
Synthesis techniques
Fabrication of CuO NPs involves three general techniques, i.e., physical, chemical, and biogenic methods. These methods are further divided into other types as shown in Fig. 1.
Fig. 1.
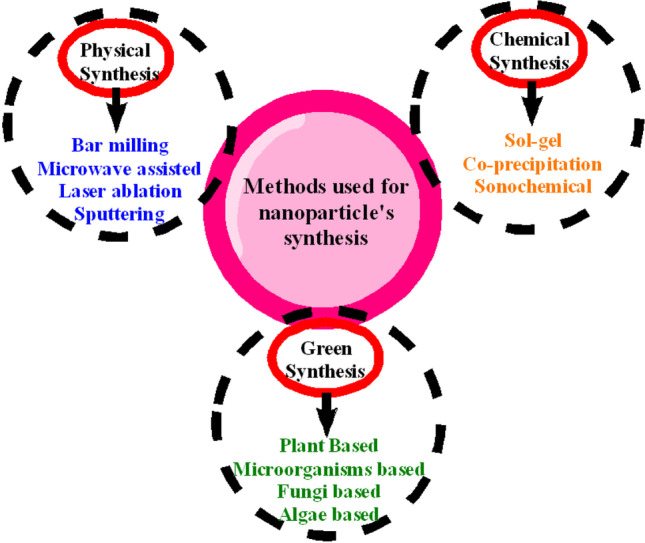
Highlight of the general methods involved in the synthesis of CuO NPs
Physical synthesis
All methods in this category utilize top-down synthetic approach. This is destructive methodology in which bulk materials are decomposed into smaller ones consequently transforming them in to NPs. In a recent study, CuO NPs were prepared by ultrasonic-assisted ball milling process which is the simplest mechanical method. The cupric acetate was used as precursor and milling speed was 256 r/min. At the end, 20 nm sized CuO NPs were produced (Yang and Chen 2017). In another study, 11 nm sized CuO NPs were prepared at 450 rpm milling speed and 0–60 h time (Khayati et al. 2013). Laser ablation is another process that has been utilized for CuO NPs fabrication by physical synthesis route. In this method, laser irradiation causes decomposition of precursor to form NPs. In a study, Nd-YAG laser source was used on Cu metal for 10 min to form CuO NPs of 8–10 nm. Pulse width, frequency, and wavelength applied was 7 ns, 1064 nm, and 5 Hz, respectively (Abdulateef et al. 2016). In another study, 9–26 nm of CuO NPs were fabricated using Nd-YAG laser source for 15 min duration on metallic Cu foil at 5 ns pulse width, 532 nm wavelength, and 10 Hz frequency (Gondal et al. 2013). Sputtering is another phenomenon that is reported for the physical synthesis of CuO NPs. It involves annealing of thin layer of NPs. Das et al. (2016) documented the production of 35 nm, 24 nm, and 22 nm sized CuO NPs using Cu as target material and 0.02 M,0.03 M, and 0.04 M solution of [Cu(NO3)2.3H2O] and C6H12N4 HTMA. The sputtering gas used was argon for a duration of 5 min at 3.3 m Torr pressure and room temperature. All physical methods produce pure nanoproducts but require expensive instruments and high energy.
Chemical synthesis
All chemical synthesis methods utilize bottom-up approach in which basic units assemble into larger structures ultimately forming NPs. These methods are cost-effective and result in the formation of uniform-sized and shaped products on large scale without requiring high-throughput equipments. Sol–gel is the most preferred method for chemical fabrication of CuO NPs because it is easy, scalable, and economical process. Recently, 100–140 nm crystalline sized CuO NPs of tunable surface area were prepared using copper carbonate species as precursors at a pH of 5.8 and > 250 °C calcination temperature (Dörner et al. 2019). In another study, CuO NPs of 32 nm size were prepared using copper nitrate precursor at a calcination temperature of 400 °C for 4 h (Muthuvel et al. 2020). Co-precipitation is very simple and efficient method for fabrication of CuO NPs. Phiwdang et al. (2013) prepared CuO NPs of different size and morphology using two different precursors, viz., copper nitrate and copper chloride by precipitation process. In another study, CuO NPs were prepared using different precursors, i.e., copper(II) sulfate, copper(II) chloride, and copper(II) acetate. In this way, different sized and shaped CuO NPs were fabricated (Gvozdenko et al. 2022).
Capping agents used as reducing and stabilizing agents are mostly added along with precursors at the start of reaction during chemical fabrication of different nanoparticles (Javed et al. 2020, 2022b). For instance, in an investigation, CuO NPs were fabricated using copper sulfate salt as precursor and aniline as capping agent (Rostami-Tapeh-Esmaeil et al. 2021). In another study, chitosan stabilized CuO NPs were fabricated via co-precipitation method using copper acetate precursor. 500 °C was the calcination temperature that produced < 35 nm sized NPs (Javed et al. 2021a). Similarly, PEG and PVP capped CuO NPs of 27 nm size were fabricated using co-precipitation by (Javed et al. 2017). Sonochemical method uses ultrasonic waves for the fabrication of NPs. Recently, CTAB stabilized CuO NPs were synthesized using copper sulfate precursor via this method by which NPs of 36 nm size were obtained (Silva et al. 2019).
Green synthesis
Biogenic fabrication or “green synthesis” of NPs occurs using plant and microbial extracts such as extracts of bacteria and fungi. The biogenic fabrication is ecofriendly, simple, low cost, and result in the formation of biocompatible and non-toxic NPs unlike physical and chemical methods that use hazardous and expensive chemicals (solvents, reducing, and stabilizing agents). The green synthesis using plant extracts does not require capping moieties because the secondary metabolites in extracts such as phenolics, alkaloids, flavonoids, and terpenoids and enzymes in microorganisms act as both reducing and capping agents, eventually producing stabilized NPs. However, microbes-based green synthesis is non-attractive because it requires aseptic cultivation and is very costly for fabrication of NPs on commercial scale. Moreover, downstream processing is very tedious. Besides, the algae-based green synthesis is approach is also not appealing because it is slow and time-taking (Javed et al. 2021b).
CuO NPs synthesis has been reported using different plant extracts as reducing agents and stabilizers as shown in Table 1.
Table 1.
List of different plants species in the green synthesis of CuO NPs
| Plant | Plant part | Size of CuO NPs (nm) | Shape of CuO NPs | Methods of Characterization | Reference |
|---|---|---|---|---|---|
| Acalypha indica | Leaf | 26–30 | Spherical | XRD, FTIR, SEM,TEM, EDX, UV–Vis spectroscopy | (Sivaraj et al. 2014a) |
| Tabernaemontana divaricata | Leaf | 48 ± 4 | Spherical | UV–Vis spectroscopy, SEM, EDX, TEM, FTIR | (Sivaraj et al. 2014b) |
| Albizia lebbeck | Leaf | < 100 | Spherical | UV–Vis spectroscopy, SEM, TEM, EDS, XRD | (Jayakumarai et al. 2015) |
| Theobroma cacao | Leaf | 40 | Spherical | TEM, EDS, FTIR, UV–Vis absorption spectroscopy | (Nasrollahzadeh and Sajadi 2015) |
| Thymus vulgaris | Leaf | 30 | Face-centered cubic | XRD, SEM, EDS, FTIR, TEM, TGA, DTA | (Nasrollahzadeh et al. 2016) |
| Punica granatum | Peel | 40 | NA | FTIR, SEM, UV–Vis absorption spectroscopy, XRD | (Ghidan et al. 2016) |
| Rubus glaucus | Fruit & Leaf | 43.3 | Spherical | UV–Vis spectroscopy, DLS, TEM, XRD | (Kumar et al. 2017) |
| Stachys lavandulifolia | Flower | 80 | Near-spherical | TEM, FTIR, UV–Vis spectroscopy, XRD | (Khatami et al. 2017) |
| Calotropis procera | Leaf | 40 | Cylindrical | XRD, FTIR, UV–Vis spectroscopy, SEM, EDX, TGA | (Reddy 2017) |
| Aloe vera | Leaf | 24–61 | Octahedral & Spherical | XRD, SEM, EDX, FTIR, UV–Vis spectroscopy | (Kerour et al. 2018) |
| Galeopsidis herba | NA | 10 ± 5 | Spherical | SEM, EDX, FTIR, UV–Vis spectroscopy, TEM | (Dobrucka 2018) |
| Citrofortunella microcarpa | Leaf | 54–68 | Spherical | XRD, FTIR, UV–Vis spectroscopy, SEM | (Rafique et al. 2020) |
| Ruellia tuberosa | Leaf | 83.23 | Nanorods | FTIR, SEM, EDS, TEM, DLS, zeta potential | (Vasantharaj et al. 2019) |
| Punica granatum | Leaf | 20.33 | Spherical | Zeta potential, TEM, SEM, EDX, XRD, UV–Vis spectroscopy | (Vidovix et al. 2019) |
| Psidium guajava | Leaf | 2–6 | Spherical | UV–Vis spectroscopy, FTIR, SEM, EDX, TEM, XRD, Zeta potential | (Singh et al. 2019) |
| Eupatorium odoratum, Acanthospermum hispidum | Leaf | NA | Spherical | UV–Vis spectroscopy, FTIR, XRD, SEM, TEM | (Gowri et al. 2019) |
| Annona muricata | Leaf | 30–40 | Spherical | XRD, FTIR, SEM, EDX, TEM, UV–Vis spectroscopy | (Kayalvizhi et al. 2020) |
| Carica papaya | Leaf | < 100 | Square or rectangle | UV–Vis spectroscopy, FTIR, XRD, SEM, TEM | (Turakhia et al. 2020) |
| Catha edulis | Leaf | ~ 25 | Spherical | XRD, SEM, EDS, TEM, FTIR | (Andualem et al. 2020) |
| Caesalpinia bonducella | Seed | NA | Rice-shaped | XRD, XPS, SEM, UV–Vis spectroscopy, FTIR, TGA | (Sukumar et al. 2020) |
| Eletteria cardamomum | Seed | 1–100 | Spherical | UV–Vis spectroscopy, SEM, XRD, DLS, FTIR | (Venkatramanan et al. 2020) |
| Cordia africana | Leaf | 9 | Spherical | XRD, FTIR, SEM, EDS | (Bekru et al. 2021) |
| Stachys lavandulifolia | Flower | 15–25 | Spherical | FTIR, SEM, EDS, TEM, XRD, TGA, UV–Vis spectroscopy | (Veisi et al. 2021) |
| Carica papaya | Peel | ~ 90 | Spherical | XRD, FTIR, SEM, EDS, TEM, UV–Vis spectroscopy | (Phang et al. 2021) |
| Aerva javanica | Leaf | 15–23 | Spherical | XRD, FTIR, SEM, UV–Vis spectroscopy | (Amin et al. 2021) |
| Camellia sinensis, Lavandula anguistifolia | Leaf | 50 | Rod-shaped, spherical | XRD, SEM, TEM | (Khaldari et al. 2021) |
| Muntingia calabura | Leaf | 23–79 | Rod-shaped | SEM, TEM, FTIR, XRD, XPS | (Salvanathan et al. 2021) |
| Eucalyptus globoulus | Leaf | 88 | Spherical | SEM, DLS, XRD, Zeta potential, FTIR, TEM | (Alhalili 2022) |
Synthesis of CuO NPs using bacterial extracts of Pseudomonas aeroginosa and marine Streptomyces sp. MHM38 has been reported (Bukhari et al. 2021; Khodair et al. 2019). Similarly, there are few reports about the biological synthesis of CuO NPs using different fungal extracts such as Trichoderma asperellum, Aspergillus terreus, Trichoderma harzianum, and Stereum hirsutum (Consolo et al. 2020; Cuevas et al. 2015; Mani et al. 2021; Saravanakumar et al. 2019). Additionally, Bhattacharya et al. (2019), Araya-Castro et al. (2021), and Taherzadeh Soureshjani et al. (2021) documented algae-mediated synthesis of CuO NPs using different algal species including Anabaena cylindrica, Macrocystis pyrifera, Cystoseira myrica, Sargassum latifolium, and Padina australis.
Biomedical applications
Generally, physical, chemical, and biogenic synthetic methods are extensively used for the preparation of CuO NPs. Among them, green synthesis route utilizing plant materials is of great significance due to its biocompatible nature and minimal toxicity. Moreover, it is very economical and easy to handle. Henceforth, our emphasis is on green chemistry using plant-based compounds for the fabrication of CuO NPs and their possible biomedical implications. Although the following sections describe biological activities of CuO NPs fabricated by any route, the main emphasis is on the various potent features of biologically prepared metallic oxide NPs, specifically CuO NPs in nanomedicine.
Antimicrobial activities of metal oxide NPs
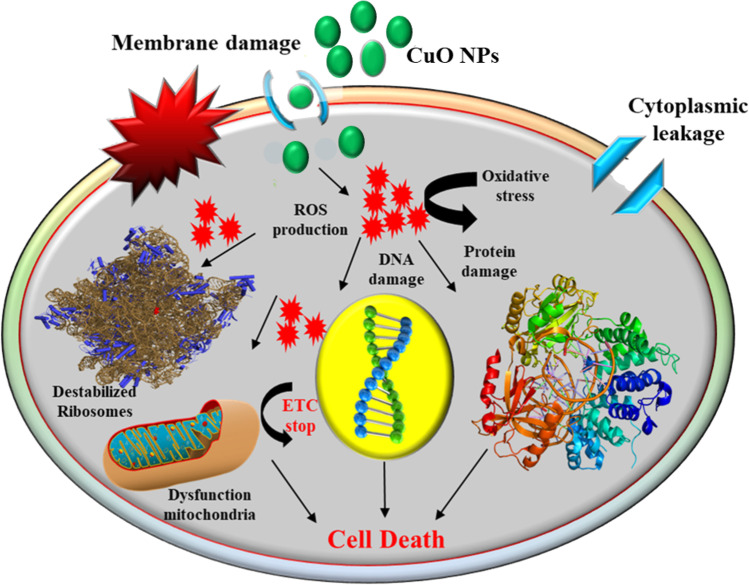
Microorganism-borne infectious diseases are a major culprit of human suffering throughout the world. Researchers are trying to develop new strategies to fight against microbial infections. One of the significant reasons behind continuous research is the development of resistance against conventional drugs, i.e., antibiotic resistance which subsequently limits the potentiality of traditional medicines to fight against microbial infections. Universal efforts were in progress to prevent and inhibit microbe-borne diseases; as a result, nanomaterials were explored as a novel antimicrobial candidate (Javed et al. 2017). Nowadays, nano-based therapies have been extensively applied to diagnose and treat diseases and formulate novel drugs. Among different kinds of green nanomaterials, metal oxide nanoparticles (MONPs) such as CuO NPs have been vigorously screened for their antimicrobial potential against different human pathogenic bacterial strains and showed substantial result (Halbus et al. 2017; Shkodenko et al. 2020). Also, CuO NPs are actively explored for their action as disinfectants, food processing agents, and in medical devices (Marambio-Jones and Hoek 2010). Figure 2 describes the antibacterial mechanism of CuO NPs. A brief insight on the antibacterial and antifungal applications of CuO NPs is given in the following subsections.
Fig. 2.
Antimicrobial Mechanism of CuO NPs
Antibacterial activity of CuO NPs
Biogenic CuO NPs as antibacterial agents have attained considerable attention due to their unique physico-chemical properties and biocompatibility. For instance, Nagore et al. (2021b) reported the green synthesis of CuO NPs using Polyalthia longifolia leaf extract. The CuO NPs exhibited significant antibacterial effects against various bacterial strains such as E.coli, Streptococcus pyogenes, Pseudomonas aeruginosa, and Staphylococcus aureus. In another study, CuO NPs were synthesized via Aloe vera leaf extract, and their antibacterial ability was investigated against various bacterial pathogens including Aeromonas hydrophila (MTCC 646), Flavobacterium branchiophilum (MTCC 671), and Pseudomonas fluorescens (ATCC 35,036) by agar well diffusion method. Significant antibacterial activity was shown by CuO NPs against all the tested organisms in dose-dependent manner, i.e., at higher dose (100 μg/mL), no bacterial growth was observed, with zones of inhibition being 21, 19, and 17 mm for A. hydrophila, P. fluorescens, and F. branchiophilum, respectively. The minimum inhibitory concentration (MIC) was determined via micro-dilution broth method. Zone of inhibition, i.e., 13, 15, and 11 mm was obtained against A. hydrophila, P. fluorescens, and F. branchiophilum, respectively, at MIC of 20 μg/mL (Kumar et al. 2015). CuO NPs synthesized via leaf extract of Malva sylvestris exhibited strong antibacterial activity as evaluated by disc diffusion method against Shigella and Listeria with 15 and 18 mm zone of inhibition, respectively (Awwad 2014). CuO NPs produced through the bark of Syzygium alternifolium stem were investigated for their antibacterial potential against a few bacterial strains including Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 25,922), Bacillus subtilis (ATCC 6633), Klebsiella pneumonia (ATCC 43,816), Proteus vulgaris (ATCC 13,315), Pseudomonas aeruginosa (ATCC 15,442), and Salmonella typhimurium (ATCC 14,028). Among all these tested strains, E. coli exhibited maximum antibacterial activity followed by S. aureus. Furthermore, MIC was calculated in various dilutions of 5, 10, 20, 40, and 80 lg/ml and demonstrated that 20 lg/ml exhibit minimum, while 80 lg/ml exhibit almost lethal effect against all microbial strains. The lethal dose (LD50) value of CuO NPs turned out to be 40 lg/ml in this case (Yugandhar et al. 2018). In a study conducted by Yalcinkaya and Kumarek (2019), CuO NPs have been proven efficacious bactericidal agents against the Gram-negative bacteria: E. coli.
Antifungal activity of CuO NPs
The antifungal assessment of CuO NPs synthesized from tea was carried out by agar dilution method against Fusarium solani at various concentrations, i.e., 5, 25, and 50 μg/ml (ppm). More than 90% mycelium growth inhibition was observed at 50 ppm of CuO NPs (Khatami et al. 2019). The antifungal effect of CuO NPs synthesized through Cissus quadrangularis extract demonstrated extreme antifungal activity against A. niger and A. flavus at 500 and 1000 ppm concentration as determined by the Clinical and Laboratory Standards Institute (CLSI) method. Maximum percent growth inhibition was observed against A. niger, i.e., 85 and 90% at 500 and 1000 ppm. Overall, the % mycelium inhibition was noteworthy against both strains compared to the positive standard. It depicts that CuO NPs exhibit potent antifungal activity against tested strains (Devipriya and Roopan 2017). The bark of Syzygium alternifolium was used for the synthesis of CuO NPs. Its antifungal potential 5, 10, 20, 40, and 80 lg/ml concentrations were tested against various fungal strains, including Alternaria solani (ATCC 32,904), Aspergillus flavus (ATCC 9643), Aspergillus niger (ATCC 16,404), Penicillium chrysogenum (ATCC 11,709), and Trichoderma harzianum (ATCC 20,476) through disc diffusion assay. Results revealed the highest zone of inhibition against T. harzianum with 40 lg/ml concentrations used as LD50 (Yugandhar et al. 2018).
As the citrus black rot disease is a major disease of citrus plants caused by Alternaria citri that results in 30–35% economic loss on annual basis, in a recent study by Sardar et al. (2022), antifungal applications of ZnO NPs, CuO NPs, and their mixture (CuO NPs/ZnO NPs) synthesized from lemon peels extract were explored against Alternaria citri. The maximum zone of inhibition, i.e., CuO NPs/ZnO NPs (53 ± 0.6 mm), was followed by CuO NPs (50 ± 0.5 mm) and ZnO NPs (51.5 ± 0.5 mm). Moreover, the minimum fungicidal concentration (MFC) and minimum inhibitory concentration (MIC) results revealed that 80 mg ml−1 NPs concentration showed potential antifungal activity while > 100 mg ml−1 completely inhibited the growth. In another study, antifungal potential of CuO NPs was found against a devastating pathogen named Colletotrichum gloeosporioides that causes anthracnose disease in a wide range of crops. 74.2% and 89% growth inhibition against the fungal hyphal pathogen was obtained at 500 mg/L and 1000 mg/L, respectively elucidating a dose-dependent response of CuO NPs (Oussou-Azo et al. 2020). Table 2 examplifies plant-based green synthetic route for CuO NPs exhibiting antimicrobial activities.
Table 2.
Antimicrobial applications of CuO NPs synthesized through green synthesis route
| Plant | NPs | Size (nm) and shape | Application | Reference |
|---|---|---|---|---|
| Moringa oleifera | CuO | 61 | Antibacterial activity | (Ingle et al. 2022) |
| Dodonaea angustifolia | CuO | NA | Antibacterial activity | (Andra et al. 2022) |
| Pseudomonas fluorescens | CuO |
40–100 Irregular |
Antifungal activity | (Sawake et al. 2022) |
| Trichoderma viride |
20–80 Spherical |
|||
| Quercus infectoria | CuO |
30 Spherical |
Antibacterial activity | (Khatamifar and Fatemi 2022) |
| Calotropis procera | CuO |
20–80 Spherical |
Antibacterial activity | (Shah et al. 2022) |
| Aspergillus terreus | CuO |
11–47 Spherical |
Antibacterial activity | (Shaheen et al. 2021) |
| Polyalthia longifolia | CuO |
40–90 NA |
Antibacterial and antifungal activity | (Nagore et al. 2021a) |
| Aspergillus terreus | CuO |
NA Spherical |
Antibacterial activity | (Mousa et al. 2021) |
| Citrus | CuO |
48–76 Spherical |
Antibacterial activity | (Tshireletso et al. 2021) |
| Sesbania aculeata | CuO | NA | Antibacterial & Antifungal activity | (Tamil Elakkiya et al. 2021) |
| Ginkgo biloba | CuO |
NA Short rod |
Antibacterial activity | (Huang et al. 2021) |
| Moringa oleifera | CuO |
45.30 NA |
Antibacterial activity | (Kalaiyan et al. 2021) |
| Almond gum | CuO |
16–25 Spherical |
Antibacterial activity | (Nithiyavathi et al. 2021) |
| Penicillium chrysogenum | CuO |
10.5–59.7 Spherical |
Antibacterial & Antifungal activity | (Mohamed et al. 2021) |
| Abies spectabilis | CuO |
50 Spherical |
Antibacterial activity | (Liu et al. 2020) |
| Cymbopogon citratus | CuO |
11.4–14.5 NA |
Antibacterial activity | (Cherian et al. 2020) |
| Monotheca buxifolia | CuO |
38, 47, and 62 NA |
Antibacterial & Antifungal activity | (Ahmad et al. 2010) |
| Ruellia tuberosa | CuO |
83.23 Rod |
Antibacterial activity | (Vasantharaj et al. 2019) |
| Asparagus racemosus | CuO |
50–100 Rod |
Antibacterial activity | (Panduranga Naga Vijay Kumar 2019) |
| Bauhinia tomentosa | CuO |
22–40 Spherical |
Antibacterial activity | (Sharmila et al. 2018) |
| Sida acuta | CuO |
50 NA |
Antibacterial activity | (Sathiyavimal et al. 2018) |
| Cordia sebestena | CuO |
20–35 Spherical |
Antibacterial activity | (Prakash et al. 2018) |
| Malus domestica | CuO & Ag-CuO |
18 and 20 Spherical |
Antibacterial activity | (Jadhav et al. 2018) |
|
Ocimum basilicum |
CuO |
70 Spherical |
Antibacterial activity | (Altikatoglu et al. 2017) |
| Seidlitzia rosmarinus | CuO |
30–222.5 NA |
Antibacterial activity | (Rezaie et al. 2017) |
| Cissus quadrangularis | CuO |
30 ± 2 Spherical |
Antifungal activity | (Devipriya and Roopan 2017) |
| Acanthospermum hispidum L | CuO |
5–25 Quasi-spherical |
Antibacterial, antifungal, antiviral, antimalarial, and antimycobacterial activity | (Pansambal et al. 2017) |
| Tecoma castanifolia | CuO |
Less than 100 Spherical |
Antibacterial activity | (Sharmila et al. 2016) |
| Malva parviflora | CuO | NA | Antibacterial activity | (Sulak 2021) |
Anticancer and cytotoxic activities of metal oxide NPs
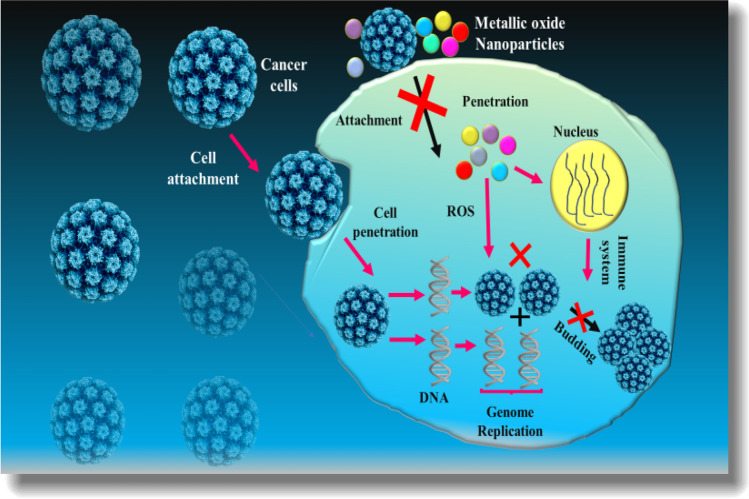
Cancer is characterized by rapid and uncontrolled cell division. It grows progressively and eventually breaches the cell matrix evading distant body areas from its origin via metastasis. As per GLOBOCAN 2020 reports, female breast, liver, and lung cancers remain the substantial causes of cancer death, covering 6.9%, 8.3%, and 18%, respectively (Abu‑Serie and Eltarahony 2021a). Cancer complexity and heterogeneity promotes the ferocious spreading of cancerous cells leading to substantial mortality (Naz et al. 2018). Presently, cancer is considered a lethal disease with higher incident rate worldwide. This demands imperative research to find out ways to target cancer cells specifically. Currently, NPs are being effectively used to combat cancer. NPs can efficiently attach to other metals, minerals, and drugs to be effective against cancer cells specifically (Bilek et al. 2019; Sisubalan et al. 2018; Suganthy et al. 2018). Recently, MONPs in treating cancer have become an attractive research area. Some nanoproducts have FDA approval for their use as anticancer agents while others are tested for effective anticarcinogenic potential and cytotoxic estimation. Figure 3 describes the anticancer mechanism of MONPs.
Fig. 3.
Anticancer Mechanism of MONPs
Anticancer and cytotoxic activities of CuO NPs
The anticancer properties of bimetallic ZnO-CuO NPs produced using S. nigra were examined against A549 and A375 cancer cells (Cao et al. 2021). Results depicted dose-dependent anticancer capability against A375 cells while showed low toxicity against A549 cells. The lower toxicity might be attributed to the different serum levels of trace elements in every cancer cell. The biosynthesized CuO NPs through aqueous black bean extract revealed a significant reduction in the cervical carcinoma cells (HeLa) through SRB cytotoxic with few changes in the structure of mitochondria (Nagajyothi et al. 2017). The cytotoxic potential of CuO NPs was measured up to 54.5% as compared to the normal cells (Chung et al. 2017). In another study, CuO NPs produced through leaf extract of Pterolobium hexapetalum revealed improved cytotoxicity against MDA-MB-231 human breast cancer cell line (Nagaraj et al. 2019a). In a study conducted by Giannousi et al. (2016), PEGylatyed CuO NPs showed anticancer activity against human cervical carcinoma HeLa cells. IC50 values of 11.91–25.78 μg/mL elucidated significant reduction in viability of tumor cells. DNA electrophoresis, nitroblue tetrazolium (NBT), and enzymatic assays revealed cell membrane damage by ROS production and anti-inflammatory activity. Recently, Al-Jawhari et al. (2022) reported CuO NPs synthesis from spinach leaf extract and evaluated their biomedical applications. For comparative studies, two types of CuO NPs were produced. One was synthesized through conventional route (CuO NPs)chem, and the other was a green approach using curcumin (CuO NPs)cur. The CuO NPs’ antioxidant properties were observed as (CuO NPs)sp > (CuO NPs)cur > (CuO NPs)chem at a concentration of 92, 86, and 84%, correspondingly. Also, the (CuO NPs)sp revealed promising antiproliferative activity (IC50 21 ± 6 g/ml) than (CuO NPs)cur and (CuO NPs)chem. These reports affirmed the significant anticancer potential of biosynthesized CuO NPs and declare them potent cancer therapeutic agents. Table 3 shows few examples CuO NPs fabricated via plant-based green synthesis revealing significant anticancer potential.
Table 3.
Anticancer application of green synthesized CuO NPs
| Plant | NPs | Size and shape | Anticancer effect | Reference |
|---|---|---|---|---|
| Artemisia deserti | CuO |
9.72 ± 7.80 nm Spherical |
A2780-CP cells | (Shahriary et al. 2022) |
| Lime juice | Co-CuO | 18–21 nm | MDA-MB-231 | (Sathyananda et al. 2022) |
| Starch | CuO |
20–30 nm Quasi-spherical |
Gastric cancer (AGS, KATO III), pancreatic cancer (AsPC-1, MIA PaCa-2), colon cancer (HCT 116 and HCT-8) | (Chen et al. 2022) |
| Pterocladia capillacea | CuO |
62 nm Spherical |
Hepatocellular carcinoma, breast cancer, and ovarian cancer cell | (Aboeita et al. 2022) |
| Sambucus nigra | ZnO-CuO |
20–130 nm Spherical |
Melanoma cancer cells | (Cao et al. 2021) |
| Ocimum americanum | CuO |
~ 68 nm Spherical |
A549 cells | (Manikandan et al. 2021; Rajamma and Nair 2020) |
| Atalantia monophylla | CuO |
~ 23 nm Spherical to rod-shaped |
HeLa cells | (Verma et al. 2021) |
| Cylindrospermum stagnale | CuO | 12.21 nm Spherical | HepG2 cell lines | (Sonbol et al. 2021) |
| Aspergillus terreus | CuO | NA | HT-29 | (Mani et al. 2021) |
| Nilgirianthus ciliatus | CuO |
20 nm Spherical |
Breast cancer cell line (MCF‐7) and lung cancer cell line (A549) | (Rajamma and Nair 2020) |
| Streptomyces pluricolorescence | CuO | 143.07 ± 1.13 nm | Breast, liver, and lung cancer cells | (Abu‑Serie and Eltarahony 2021b) |
| Scoparia dulcis L | CuO |
11.95–16.64 nm Spherical |
Adenocarcinomic human alveolar basal epithelial cells | (Navada et al. 2020) |
| Andrographis paniculata | CuO | 30 nm | A549 cells | (Kannan et al. 2020) |
| Alchornea cordifolia | Cu2O/CuO | 75.22 nm and 16.25 nm | HeLa cells | (Elemike et al. 2020) |
| Solanum tuberosum | CuO | 54 nm Monodispersed and spherical | MCF-7 breast cancer cells | (Alishah et al. 2017) |
| Dovyalis caffra | CuO | Monoclinic | MCF-7 breast cancer cell lines | (Adeyemi et al. 2022) |
| Pterolobium hexapetalum leaf extract | CuO |
10–50 nm Spherical |
MDA-MB-231 cell lines | (Nagaraj et al. 2019b) |
| Stigmaphyllon ovatum | Au-CuO |
6.40 nm Spherical |
Hela cells | (Elemike et al. 2019) |
| Azadirachta indica | CuO |
36 ± 8 nm Crystalline and spherical |
MCF-7 and Hela | (Dey et al. 2019) |
| Pomegranate peel and date stones | CuO |
6 nm PP-CuO-NPs 20 nm DS-CuO-NPs |
Human breast cancer (MCF7) cell | (Mahmoud et al. 2020) |
| Rhus punjabensis | CuO |
36.6 and 31.27 nm Spherical and circular |
HL-60 and PC-3 prostate cancer | (Naz et al. 2020b) |
| Beta vulgaris L | CuO |
33.47 nm Spherical and irregular |
A549 | (Chandrasekaran et al. 2020) |
Antioxidant activities of metal oxide NPs
Recently, the total antioxidant potential, total reducing power, and free radical scavenging activity of MONPs have become an attractive research area. During the phyto-mediated synthesis of these NPs, biological molecules bound on the surface of NPs might be linked with some antioxidant activities of NPs. Literature has reported various data where the antioxidant potential of MONPs has been assessed to estimate the overall antioxidant efficacy of these NPs. For instance, iron oxide NPs greenly produced from Camellia sinensis (Paulpandian et al. 2022), Achillea nobilis (Sepasgozar et al. 2022), ginger (Zingiber officinale), and cumin seeds (Cuminum cyminum L.) (Noor et al. 2022); ZnO NPs from Thymbra spicata L. (Gur et al. 2022), Caesalpinia crista (Donga and Chanda 2022), Cladosporium tenuissimum (Mani et al. 2022), Cystoseira crinite (Elrefaey et al. 2022); and CeO2 NPs from turmeric (Kalaycıoğlu et al. 2022), Spirulina platensis microalgae (Khaligh and Asoodeh 2022), etc., exhibited significant antioxidant potential. Herein, we briefly discuss the antioxidant activities of CuO NPs.
Antioxidant potential of CuO NPs
In a recent study conducted by Asghar et al. (2022), synthesis of CuO NPs from Rosa delicia (Rd), Rosa kardina (Rk), and Rosa foetida (Rf) petals was reported. Different sizes were exhibited by (CuO NPs)RF, (CuO NPs)RD, and (CuO NPs)RK which were 17.7, 14.9, and 26.3 nm, respectively. The maximum antioxidant potential observed by (CuO NPs)RK was 98.700.3 μg AAE/mg and reducing power capacity of 73.50.18 μg AAE/mg. Also, the (CuO NPs)RK depicted the highest DPPH scavenging activity of 60.9%. Vinothkanna et al. (2022) prepared CuO NPs from Rubia cordifolia bark and examined their antioxidant potential. Fabrication of CuO NPs from Andean blackberry fruit (ABF) and leaf (ABL) was done, and further synthesized NPs were evaluated for their antioxidant effect. Results demonstrated that ABF-mediated CuO NPs exhibited the highest antioxidant potential (89.02%) compared to ABL-mediated CuO NPs that revealed 75.92% in DPPH assay at a concentration of 1 mM (Kumar et al. 2017). A study conducted by Ruiz et al. (2015) elucidated induction of superoxide dismutase (SOD) and catalase (CAT) activities in different organs of mussels, Mytilus galloprovincialis upon CuO NPs’ exposure. In another study, antioxidant enzymatic activities of CuO NPs were revealed in mature female rats, i.e., Rattus norvegicus var. albinos. SOD, glutathione peroxidase (GPx), CAT, glutathione S-transferase (GST), and glutathione reductase (GR) were measured in the liver of rats. Transmission electron microscopy (TEM) analysis demonstrated significant variations in the antioxidant enzymes of liver after administration of CuO NPs (Canli et al. 2019).
Other biomedical applications of CuO NPs
Despite the aforementioned applications of CuO NPs, it can be widely used in many other applications, such as antiviral, antidiabetic, and antiparasitic. Yet, only limited reports are available regarding these applications of CuO NPs. Henceforth, we briefly discuss these applications.
Antiviral activity
Metal oxide NPs such as ZnO and CuO are extensively known to have antiviral capabilities. As SARS-CoV-2 being responsible for millions of deaths globally, researchers have found extraordinary antiviral applications of the mentioned NPs supported by several studies (Tortella et al. 2021). For instance, Merkl et al. (2021) reported deposition of these well-known antimicrobial materials on a solid flat surfaces and porous filter media. They checked their antiviral activity against SARS-CoV-2 viability and compared with viral plaque assays. The produced ZnO and CuO NPs displayed antiviral coatings on surfaces and on filter media to reduce the transmission. A research reported by Cui et al. (2021) produced CuO NPs incorporated electrospun nanofibers amalgamated with PVP to remove viruses. To demonstrate its antiviral application, H1N1 virus was utilized as a target material. Upon exposure to CuO NPs for 4 h, 70% of the viruses were inactivated. This indicates that CuO NPs exhibit significant antiviral efficiency. Moreover, antiviral properties of CuO NPs prepared from Syzygium alternifolium showed noticeable efficiency against Newcastle Disease Virus (NDV) (Yugandhar et al. 2018).
Antidiabetic activity
Metal oxide NPs have shown great potential against diabetes illness (Malaikozhundan et al. 2020). Faisal et al. (2022) reported antidiabetic activity of biosynthesized CuO NPs using Bacopa monnieri leaves extract. CuO NPs of size 34.4 nm vividly reduced the glucose levels in STZ-induced diabetic mice. Results showed 33.66% and 32.19% reduction in blood glucose levels in CuO NPs and CuO-NP/insulin induced mice. Murraya koenigii and Zingiber officinale derived Ag/CuO nanocomposites were evaluated for in vitro antidiabetic activity using α-amylase, α-glucosidase, glucose-6-phosphatase enzymes, and glucose uptake assays. For comparison, CuO NPs, AgO NPs, and Ag/CuO composites were also prepared by chemical method. The obtained results showed maximum antidiabetic potential of phytosynthesized Ag/CuO composite compared to other materials because of maximum phytoconstituents in these extracts (Arumai Selvan et al. 2022).
Antiparasitic activity
Different metal oxide NPs have been investigated for determination of their potential against various parasites and researchers have obtained good findings (do Carmo Neto et al. 2022; Franco et al. 2016). In a recent study by Faisal et al. (2021), CuO, NiO, and Cu/Ni hybrid NPs were greenly obtained by using Curcuma longa roots extract. At 400 μg/mL (for promastigote and amastigotes), maximum anti-leishmanial activity was shown by Cu/Ni hybrid NPs (60.5 ± 0.53 and 68.4 ± 0.59) than NiO NPs (53.2 ± 0.48 and 61.2 ± 0.44), and CuO-NPs (56.2 ± 0.45), respectively. Acanthospermum hispidum derived CuO NPs were tested for their in vitro antimalarial and antimycobacterial potential against Plasmodium falciparum strain and Mycobacterium tuberculosis (H37RV). CuO NPs demonstrated significant antimalarial activity against Plasmodium falciparum with MIC of 1.08 µg/ ml as compared to standard Chloroquine and Quinine (0.020 and 0.268 µg/ ml), respectively, while Mycobacterium tuberculosis H37RV was inhibited entirely at the MIC of 100 μg/ml (Pansambal et al. 2017).
Toxicological assessment of nanomaterials
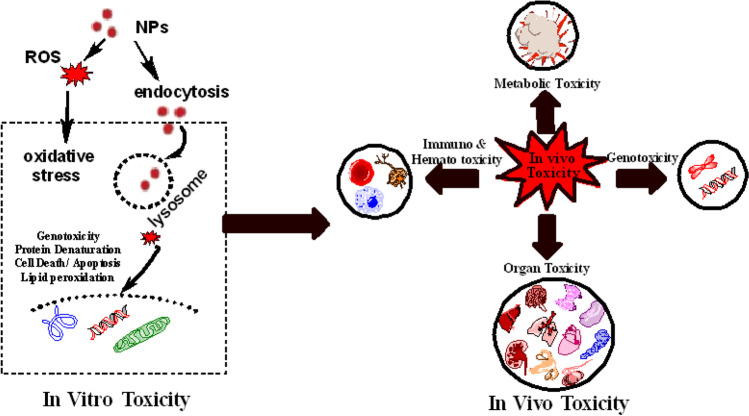
In the last few decades, the development of nanotechnology and its role in subsequent applications have increased the exposure of NPs to the environment and human beings. The occurrence of NPs in the environment is significant regarding their impact on human health (Galdiero et al. 2014; Jamil et al. 2018; Krug 2022; Solano et al. 2021). MONPs’ toxicity has been studied but exclusively focused on inducing cytotoxicity in living systems. In causing toxicity, the dissolution of metal ions from NPs and the environment in which the NPs are administered plays a vital role (Wang et al. 2012). The three primary screening schemes followed in the toxicity of nanomaterials are physiological characterization and in vitro and in vivo assays (Oberdörster et al. 2009). Among all these strategies, the in vivo assay is considered ethically safe, cost-effective, and has reduced risk assessment efficacy and reliability. Verily, the use of in vitro testing and the short-term procedure of NPs’ toxicity can play a tremendous role in demonstrating the mechanistic studies of nanotoxicology (Scherer et al. 2002) (Fig. 4). However, the available toxicological data is generally contradictory; hence, more in vitro and in vivo studies are required to assess the factors and mechanisms involved in the nanomaterial-mediated toxicity. This is a crucial challenge to standardize/regulate the investigation methods and establish a database comprising the risks linked to NPs that will be freely available for researchers, manufacturers, and consumers. There are very few reports available on the in vitro and in vivo toxicological assessment of biogenic MONPs compared to the other fabrication routes. Hence, in this section, we discuss the in vitro and in vivo toxicity of CuO NPs in general irrespective of the synthesis route.
Fig. 4.
Schematic illustration of in vitro and in vivo systems implemented for toxicity assessment of MONPs
In vitro toxicity of CuO NPs
Recently, the toxicity of CuO NPs has attained valuable attention and becoming a major trend. This portion highlights the in vitro toxicity of CuO NPs, induced oxidative stress, and apoptosis mechanism in different cell lines. Dose-dependent toxicity of CuO NPs was studied in vitro in human airway epithelial cells (Hep-2). Results showed altered morphology of HEP-cells when exposed to 1–40 µg/ml CuO NPs for 24 h. In addition, cytotoxicity was induced by oxidative stress that subsequently increased the lipid peroxidase (LPO) levels and ROS generation as well as lowered glutathione (GSH) and matrix metalloproteinase (MMP). The altered apoptotic genes in Hep-2 cells and activated caspase enzymes induced apoptosis (Farshori et al. 2022b). Toxicity of CuO NPs in Hep-2 cells was also investigated by Abudayyak et al. (2020). In another study, in vitro toxicity of novel L-valine polyviny alcohol/CuO NPs (PVA/CuO NPs) and PVA/carboxymethyl cellulose/CuO nanocomposite (PVA/CMC/CuO NCs) was elucidated against human embryonic kidney cell lines. The CuO NPs used for NCs films were produced via green synthesis from Euphorbia heterophylla. Results indicated > 80% cell viability by NC films (Amaregouda and Kamanna 2022). Moreover, in vitro toxicity of CuO NPs was studied in digestion model (Büttner et al. 2022) and CuO/ZnO NPs in lung and human melanoma cells (Cao et al. 2021). CuO NPs prepared through leaf extract of Rhus punjabensis exhibited concentration- and time-dependent toxicity against brine shrimp Leishmania tropica in in vitro study (Naz et al. 2021a).
Induction of in vitro cytotoxicity and oxidative stress of CuO NPs have been examined in A549 and HepG2 cells. Higher lipid peroxidation and ROS production was observed in A549 cells while lower antioxidant glutathione (GSH) levels in HepG2 (human hepatocellular carcinoma) cells. Researchers observed that malondialdehyde (MDA), a lipid peroxidation marker and antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT), significantly increased followed by reduction in glutathione (GSH) level. These outcomes suggested that oxidative strain might be the key mechanism behind the toxicity of CuO NPs (Akhtar et al. 2016; Farshori et al. 2022a). Other studies also reported the cytotoxicity of CuO NPs in the primary liver cells of catfish and HepG2 due to ROS generation (Piret et al. 2012; Wang et al. 2011). The cytotoxicity of CuO NPs was also observed in human cell lines like human lung epithelial A549, human cardiac microvascular endothelial, kidney, and neuronal cells (Akhtar et al. 2016; Fahmy and Cormier 2009; Maynard and Kuempel 2005; Perreault et al. 2012; Sun et al. 2011; Xu et al. 2013). Another work stated that there was no difference in the case of SOD activity while 25% and 29% inhibition in CAT and glutathione reductase (GR) activities was observed (Fahmy and Cormier 2009). Researchers found a 100% and 150% increase in 8-isoprostanes and glutathione peroxidase (GPx) activity upon exposure of Hep-2 cells to 80 lg/cm2 of CuO NPs. An increase (150%) in oxidized to total glutathione ratio showed that oxidized GSH directed the failure of epithelial cells to inhibit ROS produced by CuO NPs. This led to the generation of oxidative stress that was responsible for oxidative damage and cell death. Moreover, CuO NPs even caused genotoxicity in A549 cells that exhibited time- and dose-dependent genotoxicity through inducing lesions and damages that ultimately caused cytotoxicity (Costa et al. 2018). Exposure of CuO and PbO NPs on human fibroblasts was done and in vitro cytotoxicity was measured through the cellular dehydrogenase activity and ATP content, while continuous impedance-based measurement of the normalized cell index was carried out to study cell proliferation, viability, adherence to the substrate, and spreading. All these parameters revealed a marked damage induced by both CuO and PbO NPs on human fibroblasts in concentration-dependent manner (Bushueva et al. 2019). Similar was observed earlier where both CuO and PbO NPs exhibited similar cytotoxic effect studied through some non-specific in vivo toxicity (Minigalieva et al. 2017). Another study reported the comparative in vitro cytotoxic effect of both chemical and green synthesized CuO NPs. Results demonstrated that chemical CuO NPs induced severe toxicity in dose-dependent manner through the production of ROS and induced apoptosis and necrosis. Treated lymphocytes were characterized with hemolysis and reduced viable lymphocytes through higher intracellular deposition, elevation in NO generation, NADPH oxidase activity, MDA, LDH level, the pro-inflammatory cytokine TNF-α level, and pro-apoptotic proteins, while reduction in anti-inflammatory cytokine IL-10 and anti-apoptotic protein level (Dey et al. 2019).
Exposure of CuO NPs (50 nm) to pulmonary epithelial cells induced concentration-dependent manner DNA damage, mediated through lipid peroxidation and oxidative stress, leading to apoptosis (Ahmad et al. 2010). HepG2 cells treated with 22 nm CuO NPs encouraged ROS production, cytotoxicity, p53 and apoptotic gene caspase-3 upregulation, and apoptosis in HepG2 cells via mitochondrial pathway (Prabhu et al. 2010), while human bronchial epithelial cell line (BEAS-2B) exhibited size-dependent toxicity when treated with CuO NPs in the size range of 20–200 nm. These NPs caused cytotoxicity by inducing oxidative stress, cell cycle arrest, and apoptosis (Karlsson et al. 2009). These studies support that NPs cause cytotoxicity by generating oxidative stress followed by genotoxicity. In another report, Monotheca buxifolia derived CuO NPs exhibited pronounced dose-dependent cytotoxic effects against brine shrimp at various concentrations (200, 100, 50, and 25 μg/ml). Maximum mortality of 80 ± 0.970 was observed at 200 μg/ml followed by 40 ± 0.7 at lower tested dose. The IC50 value was 40.3 μg/ml determined via table curve 2D v5.01 software (Ali et al. 2020).
In vivo toxicity of CuO NPs
Recent study investigated the antioxidant and immuno-toxic effect of CuO NPs on 6-week-old female mice (ICR line) for a period of 6 weeks. Preferably higher accumulation of copper was observed in the lungs and liver as compared to other organs. Besides this, noteworthy increment was observed in the production of certain cytokines like IL-12p70; Th1-cytokine IFN-g and Th2-cytokines IL-4, IL-5; and proliferation of splenocytes and T-lymphocytes. Immunogenic assays demonstrated remarkable phagocytic activity in granulocytes with fewer respiratory burst, while no significant differences were observed in monocytes. No obvious difference was observed in the hematological parameters and percentages of CD3 + , CD3 + CD4 + , CD3 + CD8 + , and CD3-CD19 + cell subsets in spleen, thymus, and lymph nodes. Moreover, mice treated with CuO NPs expressed marked reduction in the GSH level depicting alteration in antioxidant status. These results demonstrated that CuO NPs lead to unnecessary variations in the immune response (Tulinska et al. 2022). Another study reported induced pulmonary inflammation upon sub-acute inhalation of CuO NPs. Researchers found an elevation in the level of lactate dehydrogenase, total cell counts, macrophages, neutrophils, inflammatory cytokines, iron in BALFs, and changes in lung weight. Moreover, a dosimetry study of lungs and BALF indicated a gradual increase in the concentration of Cu upon administration and a decrease after exposure. Marked increment occurred in the level of Cu in blood and heart depicting the possible translocation of Cu into the bloodstream and cardiac tissues. Elimination of Cu from the lungs followed first-order kinetics with 6.5 days of half-life. Furthermore, a significant weight increase in the kidneys and a decrease in the spleen demonstrated toxic effects of Cu on these organs along with reduced concentration of selenium in them revealing disturbance in the homeostasis of trace elements (Areecheewakul et al. 2022). Rainbow trout intestinal cell (RTgutGC) serving as an in vitro intestinal barrier was employed to assess the toxicity and translocation of various NPs including PVP-coated and uncoated Ag NPs, CuO, ZnO, and TiO2 NPs. Results demonstrated greater resistance to stress generation via NPs on cells cultured with permeable membranes with fewer to no impact on cell viability or barrier integrity in comparison to conventional monolayers on impermeable supports, although high levels of Ag, Cu, and Zn were observed in the basolateral side depicting translocation of these NPs and ions liberated from them via the epithelial cell. Among these NPs, CuO NPs were translocated as intact particles through apical caveolae-mediated endocytosis followed by delayed export onto the basolateral side (Geppert et al. 2021). Repeated exposure of CuO NPs (5 and 15 mg/kg) on 1-day-old broiler chickens showed a dose-dependent elevation in MDA levels, copper contents, percent DNA fragmentation, and a notable fall in catalase activity, weight gain, food conversion ratio, and antibody titer of both New Castle and Avian Influenza viruses. Histopathological analysis revealed noteworthy variations in dose-dependent manner (Morsy et al. 2021). CuO NPs/PEI (polyethyleneimine) and CuO NPs/ASC (ascorbate) in vivo pulmonary toxicity in dose-dependent manner was studied in rats for 5 days. Upon 6 and 27 day post-exposure, in both types (CuO NPs/PEI and CuO NPs/ASC), histopathological findings revealed alterations in bronchoalveolar lavage fluid (BALF), lung, and transcriptome. Also, in CuO NPs/ASC case, evidences regarding dysregulation of drug metabolism-linked genes were found in rats (Gosens et al. 2021). Comparative in vivo acute toxicity of CuO-NPs and CuO microparticles revealed that CuO NPs were accumulated in the liver tissues and feces and caused histopathological alterations including binucleation and megalocytosis as compared to counterparts (Maciel-Magalhães et al. 2019). Cytotoxic effects of CuO and ZnO NPs were studied on land snail (Cornu aspersum) that demonstrated agglomeration of these NPs in hemocytes along with elevated ROS production, lipid peroxidation, DNA damage, protein carbonyl content, ubiquitin conjugates, and breakdown of caspases conjugate levels, although the toxic effects of ZnO NPs were more obvious as compared to the CuO NPs (Feidantsis et al. 2020). Another study compared the toxic effects of biologically (B-CuO) and chemically synthesized CuO (C-CuO) NPs (500 mg/kg/ body weight) through oral administration in mice. Both B-CuO and C-CuO NPs caused leukocytosis, increased serum ATL, AST, urea, creatinine while in hepatic tissues, increased P53 mRNA and caspase-3 protein expression was also observed. Moreover, CuO NPs also caused necrosis and degeneration in hepatic, renal, and splenic tissues. Among both NPs, B-CuO NPs were found to provoke more persuasive toxicity as compared to C-CuO NPs (El Bialy et al. 2020). Exposure of zebrafish embryos with plant-based synthesized CuO NPs induced accumulation on skin surface and chorion which induced abnormalities in yolk sacs and pericardial edema (Santhoshkumar and Venkatkumar 2020). In vivo acute toxicity of CuO NPs was studied through exposure of chemical (C-CuO) and green (G-CuO) synthesized CuO NPs (100, 200, 500, and 1000 μg/Kg) in Balb/C mice via I.P route. Results demonstrated significant toxicity in C-CuO treated mice in dose-dependent manner characterized with significant body weight loss, while organ weight increased in case of the kidney and liver and decreased in the spleen, whereas no weight change was observed in heart. Serum chemistry demonstrated higher LDH, SGOT, and creatinine levels. Higher accumulation of C-CuO NPs was observed in the liver, kidney, lungs, heart, and intestine as compared to G-CuO NPs, while fecal elimination of C-CuO NPs was less as compared to G-CuO NPs. Moreover, histopathology revealed significant changes in the liver and kidney’s structure treated with high dose C-CuO NPs (Dey et al. 2019). Sub-acute oral administration of CuO NPs (250 and 500 mg/kg) for 2 weeks on rats effect their cognitive functioning by inducing minor alterations in memory and learning. Moreover, CuO NPs also effect locomotor activity of treated rats with higher anxiety index, liver, and stomach weights with few changes in biochemical parameters (Ouni et al. 2020). Swiss male albino mice orally administered with green synthesized Cu NPs demonstrated significant increase in weight of liver and kidney while reduction in weight of spleen which affects digestive system at higher dose at 800 mg/kg (Sulaiman et al. 2018). Intoxication of Danio rerio with CuO, ZnO, and NiO NPs showed that CuO NPs were more lethal among them. These NPs effected nucleic acid metabolism through variations in its binding. The NPs linked toxicity led to enhanced production of ROS and impairments in DNA replication and repair (Hou et al. 2018).
As per reports of Elsayed et al. (2021), in vivo toxicity of CuO NPs/chitosan/quercetin (CuO NPs/CH/Q) was assessed for anti-breast cancer activity in female rats. The research data exhibited remarkable reduction in breast tumor cells of 1,3-methylbutylamine (DMBA)-stimulated rats exposed to CuO NPS/CH/Q. The treatment induced apoptosis via enhanced p53 gene causing cell cycle arrestment and increased caspase-3 and c-3 (cytochrome c) leading to mammary cancer cells’ death. Moreover, the CuO NPs/CH/Q decreased the carcinoma cells’ proliferation by suppressing PCNA genes. In interesting research study, oral exposure effects of CuO NPs of 50 nm size (10 mg/kg) were explored in vivo in male and female Sprague–Dawley (SD) rat pups for four consecutive daily doses between 7 and 10 post-natal day (PND). Findings revealed that the stimulated gastric digestion in rates led towards CuO NPs dissolution at the PND14 and PND2. Moreover, hyperspectral imaging of intestinal cross sections showed the intestinal uptake of CuO NPs. Also, the immune cells increased in the intestinal tissues upon NPs’ administration (Mortensen et al. 2021). Neurotoxicological impacts of CuO NPs were examined in thirty Wister albino rats for 28 days. Pomegranate juice (PJ) is a rich source of polyphenols with enhanced antioxidant potentials. In this study, mechanistic role of PJ was also studied to reduce the CuO NPs’ toxicity. The substantial increase was observed in MDA levels and reduction in antioxidant capacity linked with significant changes in all brain parts (cerebellum, hippocampus, and cerebrum) was revealed. Rats exposed to CuO NPs without PJ showed progressive decline of memory and noticeable cognitive and psychiatric disturbances. Also, the continuous exposure of NPs resulted over expression of caspase-3, iNOS, GFAP, and down-regulation of HO-1/Nrf2 genes in whole brain tissues. In contrast, PJ co-treated rats expressed improvements in the entire neurotoxicological parameters. Overall, PJ reduced the oxidative stress damage through the up-regulation of HO-1/Nrf2 genes (Hassanen et al. 2021). Green synthesized CuO NPs exhibited dose- and time-dependent toxicity in both male and female SD rats. Notable variations were observed in liver functional tests (LFTs), antioxidant enzymes, total proteins, and nitrites in both genders, whereas no significant differences were observed in renal functional tests (RFTs), lipid profile, and histopathology in females; however, males exposed to high dose demonstrated significant toxicity. This finding revealed that CuO NPs at lower dose were somewhat biocompatible (Naz et al. 2021a). Another study demonstrated that among both chemical and green synthesized CuO NPs, chemically synthesized CuO NPs were found to be hepatotoxic at higher dose against both parents and off-springs supported by LFTs, histopathology, antioxidant enzyme assay, and genotoxicity studies (Naz et al. 2021b).
A comparative toxicity study was performed on the biogenic and chemically derived CuO NPs against Wistar rats to assess their nephrotoxic effect against LLC PK1 cells line (renal proximal epithelial cells) and isolated renal mitochondria. The biogenic CuO NPs employed in this study were synthesized through aqueous root extract of Desmodium gangeticum while chemically derived CuO NPs fabricated via standard chemical approach. Results revealed that chemical CuO NPs caused significant nephrotoxic effects against LLC PK1 cell lines characterized by the higher renal oxidative stress leading to renal tissue injury by compromising the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, uric acid, and creatinine. However, both NPs did not induce any toxicity in mitochondrial function (Ansari and Kurian 2018). Intoxication of rats with different CuO NPs’ concentrations (50, 100, and 200 mg/kg) reported significant biochemical and histopathological alterations in rats administered with 200 mg/kg compared to the control group. A five-fold increase in AST, bilirubin test (TB), creatinine (CRE), and blood urea nitrogen (BUN) was noticed, while about two-fold increase was observed in ALT, triglyceride (TG), and total bile. Furthermore, alkaline phosphatase (ALP) and total cholesterol (TCHO) level reduction occurred, while TCHO and TG were slightly increased in mid- and low-dose treated groups. Researchers observed a higher level of glucose, citrate, amino acid, acetate, lactate, succinate, and trimethylamine N-oxide (TAMO), while creatinine levels dropped in urine spectra of rats treated with Cu NPs. Furthermore, it was found that 200 mg/kg of nano Cu caused toxic effects, including prevalent necrosis in the proximal tubule and cell debris present in the tubule’s lumen where orange crystalline material was deposited in the renal tissues. At the same time, scattered dot necrosis in liver cells was also observed at the same dose. Moreover, other doses (50 and 100 mg/kg) caused proximal tubule swelling in renal tissues and did not exhibit any toxic sign in the liver (Lei et al. 2008). CuO NPs induced hemolysis of red blood cells (RBCs), resulting in decrease in level of RBCs, hemoglobin (HB), iron, hematocrit (HCT), and mean corpuscular volume (MCV); however, increase in reticulocytes (RET) was observed. Researchers also found that reduction in lymphocytes (LYM) percentage was due to the differential white blood cells’ (WBC) count that in turn affected defense system of the organisms. Meanwhile, increased percentages of monocytes (MON) and neutrophils (NEU) indicated inflammation in the exposed organs. Furthermore, dose-dependent increase in AST, ALP, ALT, TBIL, CRE, BUN, and LDH was noticed, while the level of total protein (TP) and triglyceride (TG) decreased significantly. Dysregulation in the electrolytes’ balance and an increase in protein, WBCs, ketone bodies, specific gravity, nitrite, and occult blood in urine were found. Moreover, the comparative effect of nano and micro Cu on the spleen, thymus, hepatic, and nephrotic tissues of rats suggested that nano Cu is more toxic and causes major alterations in these organs. In contrast, micro Cu did not cause any change. Major alterations found in these organs were the appearance of atrophic white pulp, yellow coloration, decreased cellularity, and follicular number observed in the spleen interrupted demarcation of cortex and medulla. Reduced cells and vacuolation in the cytoplasm was exhibited by thymus and hepatic tissues that represented sinusoid dilation, mononuclear cell infiltration, deteriorated or binucleated liver cells, tubule dilation, and cell fragments with purple or pink pigmented tubular casts, while nephrotic cells displayed disintegrated tubule cells along with inflammatory cell infiltration (Lee et al. 2016).
Comparative intoxication of male and female rats with CuO NPs was done previously (Wang et al. 2016). Rats treated with 1250 mg/kg CuO NPs showed a reduction in TG, Na, and Cl and elevation in ALT, AST, BUN, LDH, and TCHO in males, while increased CPK and LDH were found in female rats. Additionally, a significant increase in ALT, AST, TCHO, CPK, LDH, and total proteins, whereas a decrease in TG, Cl, and K was reported in female rats exposed to 2500 mg/kg CuO NPs. Histological alterations were noticed in the liver, spleen, and kidney in male (1250 mg/kg) and female (2500 mg/kg) rats. The liver of male and female rats with the doses mentioned above also displayed slight inflammatory cell infiltration, sinusoid dilation, and vacuolation. Similar was observed when male and female rats were treated with Cu ions (625 mg/kg). Inflammatory cell infiltration, cellular fragments deposition in the tubule, hyaline cast, tubular dilation, and glomerulus atrophy were found in the kidneys treated with Cu NPs. In contrast, mild cast and dilation were observed in the kidney’s tubule when exposed to Cu ions. Multinucleated spleen and decline in cell’s white pulp was observed in both sexes administered with Cu NPs, while no such alterations were found in Cu ion-treated rats (Wang et al. 2016). In another experiment, female rats were exposed to CuO NPs, and these NPs conjugated with quercetin at 3 mg/kg and 50 mg/kg, respectively. It was found that CuO NPs exhibit a significant increase in liver enzymes (ALT, AST, and ALP) in a dose-dependent manner as compared to the control. Moreover, CuO NPs induced severe damage to the liver like lobular liver structure, liver cells with ballooning and bi-nucleated cell infiltration, microsteatotic, dilated sinusoids, and congested central vein as compared to the control CuO NPs conjugated with quercetin (Arafa et al. 2017). By summation of all these reports, we have found that biogenic CuO NPs exhibit lesser toxic effects compared to chemically prepared CuO NPs but need further research to elucidate the comparative toxicity of both types of CuO NPs in different animal models.
Conclusions and future prospects
CuO NPs exhibit unique physicochemical properties which make them potent nanomaterials to be used in almost every field of science. This review provides essential knowledge regarding preparation, nanomedical application, and toxicity of CuO NPs. Synthesis of CuO NPs can be achieved through various processes each of them having their own advantages and limitations. Green chemistry is commonly used method for the synthesis of biocompatible nanomaterials and their applications in biomedical sciences due to their antimicrobial, anticancerous, and antioxidant nature. Plant-based moieties act as an effective and powerful reducing and capping agents during the synthesis of CuO NPs with noteworthy biocompatibility. Moreover, assessment of in vitro and in vivo toxicity of CuO NPs reveals that plant-based green synthesized CuO NPs exhibit lower toxicity in comparison to chemically synthesized CuO NPs. Toxicological studies have depicted that CuO NPs induce oxidative stress mediated toxicity through the production of reactive oxygen species (ROS). This consequently leads to the cascade of chemical reactions like effecting enzymes balance, lipid peroxidation, denaturation of proteins and nucleic acids, deterioration of organs, and their functions ultimately compromising hematological parameters, metabolites, etc. This review study concludes that CuO NPs synthesized through plant-based materials act as an effective nanomaterials with plentiful biomedical applications, and it aids in addition of useful knowledge to the existing one.
Although significant advancement has been done regarding different metal NPs, the metal oxide NPs research is still in its early stages. Therefore, tailoring of CuO NPs is required in context of its size, morphology, and surface composition by devising novel protocols for their synthesis. Different reaction parameters contribute in determining the appropriate structure–function relationship. Similarly, the dose and concentration of NPs are crucial in determining particular biological function and its associated toxicity. Moreover, the challenges of toxicity of CuO NPs should be properly tackled to enhance their broad-spectrum biomedical approaches. This could be done by the regulation of CuO nanomaterial interface that would augment their interaction with biomolecules of model animals (prokaryotes and eukaryotes) and humans. The hybrid CuO nanomaterials should also be studied for different areas of nanomedicine. The green synthesis methods reduce toxicity of CuO NPs by using non-hazardous biological extracts, while chemical synthesis can control the toxic effects of CuO NPs by using surface modifying capping agents. The surface modification would not only make the aggregated NPs stable but also enhance the functionalization of these NPs by minimizing their size and maximizing targeted and specific binding with the surface receptors. Hence, groundbreaking research should be done on the routes opted to reduce toxicity of CuO NPs and increase their efficacy in biological systems.
Author contribution
SN conceived the idea. RJ implemented and supervised the idea. SN, AG, and RJ contributed in writing. MZ did editing and proofreading. All authors have accepted the final version of manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulateef SA, MatJafri MZ, Omar AF, Ahmed NM, Azzez SA, Ibrahim IM, Al-Jumaili BEB. Preparation of CuO nanoparticles by laser ablation in liquid. 2016;1733(1):020035. doi: 10.1063/1.4948853. [DOI] [Google Scholar]
- Aboeita NM, Fahmy SA, El-Sayed MM, Azzazy HMES, Shoeib TJP. Enhanced anticancer activity of nedaplatin loaded onto copper nanoparticles synthesized using red algae. Pharmaceutics. 2022;14(2):418. doi: 10.3390/pharmaceutics14020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudayyak M, Guzel E, Özhan GJAPB. Cupric oxide nanoparticles induce cellular toxicity in liver and intestine cell lines. Adv Pharm Bull. 2020;10(2):213. doi: 10.34172/apb.2020.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Serie MM, Eltarahony M. Novel nanoformulation of disulfiram with bacterially synthesized copper oxide nanoparticles for augmenting anticancer activity: an in vitro study. Cancer Nanotechnol. 2021;12(1):25. doi: 10.1186/s12645-021-00097-5. [DOI] [Google Scholar]
- Adeyemi JO, Onwudiwe DC, Oyedeji AO. Biogenic synthesis of CuO, ZnO, and CuO-ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules (Basel, Switzerland) 2022;27(10):3206. doi: 10.3390/molecules27103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Rasheed N, Banu N, Palit G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress (amsterdam, Netherlands) 2010;13(4):355–364. doi: 10.3109/10253891003667862. [DOI] [PubMed] [Google Scholar]
- Akhtar MJ, Kumar S, Alhadlaq HA, Alrokayan SA, Abu-Salah KM, Ahamed M. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health. 2016;32(5):809–821. doi: 10.1177/0748233713511512. [DOI] [PubMed] [Google Scholar]
- Alhalili Z. Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus globoulus leaf extract: adsorption and design of experiments. Arab J Chem. 2022;15(5):103739. doi: 10.1016/j.arabjc.2022.103739. [DOI] [Google Scholar]
- Ali JS, Mannan A, Nasrullah M, Ishtiaq H, Naz S, Zia M. Antimicrobial, antioxidative, and cytotoxic properties of Monotheca buxifolia assisted synthesized metal and metal oxide nanoparticles. Inorg Nano-Met Chem. 2020;50(9):770–782. doi: 10.1080/24701556.2020.1724150. [DOI] [Google Scholar]
- Alishah H, Pourseyedi S, Ebrahimipour SY, Mahani SE, Rafiei NJRL (2017) Green synthesis of starch-mediated CuO nanoparticles: preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. 28(1):65–71
- Al-Jawhari H, Bin-Thiyab H, Elbialy N. In vitro antioxidant and anticancer activities of cupric oxide nanoparticles synthesized using spinach leaves extract. Nano-Struct Nano-Objects. 2022;29:100815. doi: 10.1016/j.nanoso.2021.100815. [DOI] [Google Scholar]
- Altikatoglu M, Attar A, Erci F, Cristache C, Isildak I. Green synthesis of copper oxide nanoparticles using Ocimum basilicum extract and their antibacterial activity. Fresenius Environ Bull. 2017;25(12):7832–7837. [Google Scholar]
- Amaregouda Y, Kamanna KJICE (2022) Physico-chemical, in-vitro cytotoxicity and antimicrobial evaluation of L-valine functionalised CuO NPs on polyvinyl alcohol and blended carboxymethyl cellulose films.1–10
- Amin F, Fozia KB, Alotaibi A, Qasim M, Ahmad I, Ullah R, Bourhia M, Gul A, Zahoor S, Ahmad R. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. Evid-Based Complement Altern Med : Ecam. 2021;2021:5589703. doi: 10.1155/2021/5589703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra S, Balu Sk, Ponnada S, Mohan S, Hossain MS, Sivakumar B, Palanivel B, Alsalme A, Muthalagu M (2022) Antimicrobial and toxicity studies of Dodonaea angustifolia extracts-mediated green synthesized copper oxide particles. 7(8):e202104017. 10.1002/slct.202104017
- Andualem WW, Sabir FK, Mohammed ET, Belay HH, Gonfa BA. Synthesis of copper oxide nanoparticles using plant leaf extract of Catha edulis and its antibacterial activity. J Nanotechnol. 2020;2020:2932434. doi: 10.1155/2020/2932434. [DOI] [Google Scholar]
- Ansari M, Kurian GA. Evaluating the effect of green synthesised copper oxide nanoparticles on oxidative stress and mitochondrial function using murine model. IET Nanobiotechnol. 2018;12(5):669–672. doi: 10.1049/iet-nbt.2017.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa A, Ghanem H, Soliman M, EL-Meligy E, Modulation effects of quercetin against copper oxide nanoparticles-induced liver toxicity in rats. Egypt Pharma J. 2017;16(2):78–86. doi: 10.4103/epj.epj_15_17. [DOI] [Google Scholar]
- Araya-Castro K, Chao T-C, Durán-Vinet B, Cisternas C, Ciudad G, Rubilar O. Green Synthesis of Copper Oxide Nanoparticles Using Protein Fractions from an Aqueous Extract of Brown Algae Macrocystis Pyrifera. 2021;9(1):78. [Google Scholar]
- Areecheewakul S, Adamcakova-Dodd A, Haque E, Jing X, Meyerholz DK, O'Shaughnessy PT, Thorne PS, Salem AK. Time course of pulmonary inflammation and trace element biodistribution during and after sub-acute inhalation exposure to copper oxide nanoparticles in a murine model. Part Fibre Toxicol. 2022;19(1):40. doi: 10.1186/s12989-022-00480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumai Selvan DS, Kumar RS, Murugesan S, Shobana S, Rahiman AK. Antidiabetic activity of phytosynthesized Ag/CuO nanocomposites using Murraya koenigii and Zingiber officinale extracts. J Drug Deliv Sci Technol. 2022;67:102838. doi: 10.1016/j.jddst.2021.102838. [DOI] [Google Scholar]
- Asghar M, Sajjad A, Hanif S, Ali JS, Ali Z, Zia M. Comparative analysis of synthesis, characterization, antimicrobial, antioxidant, and enzyme inhibition potential of roses petal based synthesized copper oxide nanoparticles. Mater Chem Phys. 2022;278:125724. doi: 10.1016/j.matchemphys.2022.125724. [DOI] [Google Scholar]
- Awwad AM (2014) Antibacterial activity of synthesized copper oxide nanoparticles using Malva sylvestris leaf extract
- Bekru AG, Zelekew OA, Andoshe DM, Sabir FK, Eswaramoorthy R. Microwave-assisted synthesis of CuO nanoparticles using Cordia africana Lam. leaf extract for 4-nitrophenol reduction. J Nanotechnol. 2021;2021:5581621. doi: 10.1155/2021/5581621. [DOI] [Google Scholar]
- Bezza FA, Tichapondwa SM, Chirwa EMN. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci Rep. 2020;10(1):16680. doi: 10.1038/s41598-020-73497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanushali S, Ghosh P, Ganesh A, Cheng W. 1D copper nanostructures: progress, challenges and opportunities. Small. 2015;11(11):1232–1252. doi: 10.1002/smll.201402295. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Swarnakar S, Ghosh S, Majumdar S, Banerjee S. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J Environ Chem Eng. 2019;7(1):102867. doi: 10.1016/j.jece.2018.102867. [DOI] [Google Scholar]
- Bilek O, Fohlerova Z, Hubalek J. Enhanced antibacterial and anticancer properties of Se-NPs decorated TiO2 nanotube film. PloS one. 2019;14(3):e0214066. doi: 10.1371/journal.pone.0214066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari SI, Hamed MM, Al-Agamy MH, Gazwi HSS, Radwan HH, Youssif AM. biosynthesis of copper oxide nanoparticles using Streptomyces MHM38 and its biological applications. J Nanomater. 2021;2021:6693302. doi: 10.1155/2021/6693302. [DOI] [Google Scholar]
- Bushueva T, Minigalieva I, Panov V, Kuznetsova A, Naumova A, Shur V, Shishkina E, Gurvich V, Privalova L, Katsnelson B. More data on in vitro assessment of comparative and combined toxicity of metaloxide nanoparticles. Food Chem Toxicol. 2019;133:110753. doi: 10.1016/j.fct.2019.110753. [DOI] [PubMed] [Google Scholar]
- Büttner J, Schneider T, Westermann M, Glei MJT. Artificial digestion of polydisperse copper oxide nanoparticles: investigation of effects on the human in vitro intestinal co-culture model Caco-2/HT29-MTX. Toxics. 2022;10(3):130. doi: 10.3390/toxics10030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli EG, Ila HB, Canli M. Response of the antioxidant enzymes of rats following oral administration of metal-oxide nanoparticles (Al(2)O(3), CuO, TiO(2)) Environ Sci Pollut Res Inter. 2019;26(1):938–945. doi: 10.1007/s11356-018-3592-8. [DOI] [PubMed] [Google Scholar]
- Cao Y, Dhahad HA, El-Shorbagy M, Alijani HQ, Zakeri M, Heydari A, Bahonar E, Slouf M, Khatami M, Naderifar MJSr, Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sic Rep. 2021;11(1):1–8. doi: 10.1038/s41598-021-02937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran R, Yadav SA, Sivaperumal SJJoCS. Phytosynthesis and characterization of copper oxide nanoparticles using the aqueous extract of Beta vulgaris L and evaluation of their antibacterial and anticancer activities. J Clust Sci. 2020;31(1):221–230. doi: 10.1007/s10876-019-01640-6. [DOI] [Google Scholar]
- Chen J, Karmakar B, Salem MA, Alzahrani AY, Bani-Fwaz MZ, Abdel-Daim MM, El-kott AFJAJoC. CuO NPs@ Starch as a novel chemotherapeutic drug for the treatment of several types of gastrointestinal system cancers including gastric, pancreatic, and colon cancers. Arab J Chem. 2022;15(4):103681. doi: 10.1016/j.arabjc.2021.103681. [DOI] [Google Scholar]
- Cherian T, Ali K, Saquib Q, Faisal M, Wahab R, Musarrat JJB. Cymbopogon Citratus Functionalized Green Synthesis of CuO-Nanoparticles: Novel Prospects as Antibacterial and Antibiofilm Agents. Biomolecules. 2020;10(2):169. doi: 10.3390/biom10020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallet M, Veronesi G, Fuchs A, Mintz E, Michaud-Soret I. Deniaud A (2017) Impact of labile metal nanoparticles on cellular homeostasis Current developments in imaging, synthesis and applications. Biochim Biophys Acta Gen Subj. 1861;6:1566–1577. doi: 10.1016/j.bbagen.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Chung IM, Abdul Rahuman A, Marimuthu S, Kirthi AV, Anbarasan K, Padmini P, Rajakumar G. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med. 2017;14(1):18–24. doi: 10.3892/etm.2017.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolo VF, Torres-Nicolini A, Alvarez VA. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci Rep. 2020;10(1):20499. doi: 10.1038/s41598-020-77294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PM, Gosens I, Williams A, Farcal L, Pantano D, Brown DM, Stone V, Cassee FR, Halappanavar S, Fadeel B. Transcriptional profiling reveals gene expression changes associated with inflammation and cell proliferation following short-term inhalation exposure to copper oxide nanoparticles. J Appl Toxicol. 2018;38(3):385–397. doi: 10.1002/jat.3548. [DOI] [PubMed] [Google Scholar]
- Cuevas R, Durán N, Diez MC, Tortella GR, Rubilar O. extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from Chilean forests. J Nanomater. 2015;2015:789089. doi: 10.1155/2015/789089. [DOI] [Google Scholar]
- Cui WY, Yoo HJ, Li YG, Baek C, Min J. Electrospun nanofibers embedded with copper oxide nanoparticles to improve antiviral function. J Nanosci Nanotechnol. 2021;21(8):4174–4178. doi: 10.1166/jnn.2021.19379. [DOI] [PubMed] [Google Scholar]
- Das A, Kushwaha A, Raj Bansal N, Suresh V, Dinda S, Chattopadhyay S, Kumar Dalapati G. Copper oxide nano-particles film on glass by using sputter and chemical bath deposition technique. J Adv Mater Lett. 2016;7(8):600–603. doi: 10.5185/amlett.2016.6433. [DOI] [Google Scholar]
- Devipriya D, Roopan SM. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mater Sci Eng c, Mater Biol Appl. 2017;80:38–44. doi: 10.1016/j.msec.2017.05.130. [DOI] [PubMed] [Google Scholar]
- Dey A, Manna S, Chattopadhyay S, Mondal D, Chattopadhyay D, Raj A, Das S, Bag BG, Roy SJJoSCS. Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J Saudi Chem Soc. 2019;23(2):222–238. doi: 10.1016/j.jscs.2018.06.011. [DOI] [Google Scholar]
- do Carmo Neto JR, Guerra RO, Machado JR, Silva ACA, da Silva MV. Antiprotozoal and anthelmintic activity of zinc oxide Nanoparticles. Curr Med Chem. 2022;29(12):2127–2141. doi: 10.2174/0929867328666210709105850. [DOI] [PubMed] [Google Scholar]
- Dobrucka R. Antioxidant and catalytic activity of biosynthesized CuO nanoparticles using extract of Galeopsidis herba. J Inorg Organomet Polym Mater. 2018;28(3):812–819. doi: 10.1007/s10904-017-0750-2. [DOI] [Google Scholar]
- Donga S, Chanda S. Caesalpinia crista seeds mediated green synthesis of zinc oxide nanoparticles for antibacterial, antioxidant, and anticancer activities. BioNanoScience. 2022;12(2):451–462. doi: 10.1007/s12668-022-00952-8. [DOI] [Google Scholar]
- Dörner L, Cancellieri C, Rheingans B, Walter M, Kägi R, Schmutz P, Kovalenko MV, Jeurgens LPH. Cost-effective sol-gel synthesis of porous CuO nanoparticle aggregates with tunable specific surface area. Sci Rep. 2019;9(1):11758. doi: 10.1038/s41598-019-48020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bialy BE, Hamouda RA, Abd Eldaim MA, El Ballal SS, Heikal HS, Khalifa HK, Hozzein WN. Comparative toxicological effects of biologically and chemically synthesized copper oxide nanoparticles on mice. Int J Nanomed. 2020;15:3827–3842. doi: 10.2147/ijn.S241922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemike EE, Onwudiwe DC, Nundkumar N. Singh MJICC. CuO and Au-CuO Nanoparticles Mediated by Stigmaphyllon Ovatum Leaf Extract and Their Anticancer Potential. 2019;104:93–97. [Google Scholar]
- Elemike EE, Onwudiwe DC, Singh MJJoI, Polymers O. Eco-friendly synthesis of copper oxide, zinc oxide and copper oxide–zinc oxide nanocomposites, and their anticancer applications. J Inorg Organomet Polym Mater. 2020;30(2):400–409. doi: 10.1007/s10904-019-01198-w. [DOI] [Google Scholar]
- Elrefaey AAK, El-Gamal AD, Hamed SM, El-belely EF. Algae-mediated biosynthesis of zinc oxide nanoparticles from Cystoseira crinite (Fucales; Sargassaceae) and it's antimicrobial and antioxidant activities. J Egypt J Chem. 2022;65(4):231–240. doi: 10.21608/ejchem.2021.87722.4231. [DOI] [Google Scholar]
- Elsayed AM, Sherif NM, Hassan NS, Althobaiti F, Hanafy NA, Sahyon HAJIJoBM. Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int J Biol Macromol. 2021;185:134–152. doi: 10.1016/j.ijbiomac.2021.06.085. [DOI] [PubMed] [Google Scholar]
- Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol Vitro. 2009;23(7):1365–1371. doi: 10.1016/j.tiv.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal S, Al-Radadi NS, Jan H, Abdullah Shah SA, Shah S, Rizwan M, Afsheen Z, Hussain Z, Uddin MN, Idrees M, Bibi N. Curcuma longa mediated synthesis of copper oxide, nickel oxide and Cu-Ni bimetallic hybrid nanoparticles: characterization and evaluation for antimicrobial, anti-parasitic and cytotoxic potentials. Coatings. 2021;11(7):849. doi: 10.3390/coatings11070849. [DOI] [Google Scholar]
- Faisal S, Jan H, Abdullah AI, Rizwan M, Hussain Z, Sultana K, Ali Z, Uddin MN. In vivo analgesic, anti-inflammatory, and anti-diabetic screening of Bacopa monnieri-synthesized copper oxide nanoparticles. ACS Omega. 2022;7(5):4071–4082. doi: 10.1021/acsomega.1c05410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshori NN, Siddiqui MA, Al-Oqail MM, Al-Sheddi ES, Al-Massarani SM, Ahamed M, Ahmad J, Al-Khedhairy AA. Copper oxide nanoparticles exhibit cell death through oxidative stress responses in human airway epithelial cells: a mechanistic study. Biol Trace Elem Res. 2022 doi: 10.1007/s12011-022-03107-8. [DOI] [PubMed] [Google Scholar]
- Farshori NN, Siddiqui MA, Al-Oqail MM, Al-Sheddi ES, Al-Massarani SM, Ahamed M, Ahmad J, Al-Khedhairy AAJBTER (2022b) Copper oxide nanoparticles exhibit cell death through oxidative stress responses in human airway epithelial cells: a mechanistic study 1–10 [DOI] [PubMed]
- Feidantsis K, Kalogiannis S, Marinoni A, Vasilogianni A-M, Gkanatsiou C, Kastrinaki G, Dendrinou-Samara C, Kaloyianni M. Toxicity assessment and comparison of the land snail’s Cornu aspersum responses against CuO nanoparticles and ZnO nanoparticles. Comp Biochem Physiol Part C Toxicol Pharmacol. 2020;236:108817. doi: 10.1016/j.cbpc.2020.108817. [DOI] [PubMed] [Google Scholar]
- Franco AM, Grafova I, Soares FV, Gentile G, Wyrepkowski CD, Bolson MA, Sargentini É, Jr, Carfagna C, Leskelä M, Grafov A. Nanoscaled hydrated antimony (V) oxide as a new approach to first-line antileishmanial drugs. Int J Nanomed. 2016;11:6771–6780. doi: 10.2147/ijn.S121096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero S, Falanga A, Cantisani M, Ingle A, Galdiero M, Rai M (2014) Silver nanoparticles as novel antibacterial and antiviral agents handbook of nanobiomedical research pp 565–594
- Gawande MB, Goswami A, Felpin F-X, Asefa T, Huang X, Silva R, Zou X, Zboril R, Varma RS. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev. 2016;116(6):3722–3811. doi: 10.1021/acs.chemrev.5b00482. [DOI] [PubMed] [Google Scholar]
- Geppert M, Sigg L, Schirmer K. Toxicity and translocation of Ag, CuO, ZnO and TiO2 nanoparticles upon exposure to fish intestinal epithelial cells. Environ Sci Nano. 2021;8(8):2249–2260. doi: 10.1039/D1EN00050K. [DOI] [Google Scholar]
- Ghidan AY, Al-Antary TM, Awwad AM. Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: effect on green peach Aphid. Environ Nanotechnol Monit Manag. 2016;6:95–98. doi: 10.1016/j.enmm.2016.08.002. [DOI] [Google Scholar]
- Giannousi K, Hatzivassiliou E, Mourdikoudis S, Vourlias G, Pantazaki A, Dendrinou-Samara C. Synthesis and biological evaluation of PEGylated CuO nanoparticles. J Inorg Biochem. 2016;164:82–90. doi: 10.1016/j.jinorgbio.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Gondal MA, Qahtan TF, Dastageer MA, Maganda YW, Anjum DH. Synthesis of Cu/Cu2O nanoparticles by laser ablation in deionized water and their annealing transformation into CuO nanoparticles. J Nanosci Nanotechnol. 2013;13(8):5759–5766. doi: 10.1166/jnn.2013.7465. [DOI] [PubMed] [Google Scholar]
- Gosens I, Costa PM, Olsson M, Stone V, Costa AL, Brunelli A, Badetti E, Bonetto A, Bokkers BG, de Jong WHJN. Pulmonary Toxicity and Gene Expression Changes after Short-Term Inhalation Exposure to Surface-Modified Copper Oxide Nanoparticles. 2021;22:100313. doi: 10.1016/j.impact.2021.100313. [DOI] [PubMed] [Google Scholar]
- Gowri M, Latha N, Rajan M. Copper oxide nanoparticles synthesized using Eupatorium odoratum, Acanthospermum hispidum leaf extracts, and its antibacterial effects against pathogens: a comparative study. BioNanoScience. 2019;9(3):545–552. doi: 10.1007/s12668-019-00655-7. [DOI] [Google Scholar]
- Grigore ME, Biscu ER, Holban AM, Gestal MC, Grumezescu AM. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals (Basel, Switzerland) 2016;9(4):75. doi: 10.3390/ph9040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur T, Meydan I, Seckin H, Bekmezci M, Sen F. Green synthesis, characterization and bioactivity of biogenic zinc oxide nanoparticles. Environ Res. 2022;204:111897. doi: 10.1016/j.envres.2021.111897. [DOI] [PubMed] [Google Scholar]
- Gvozdenko AA, Siddiqui SA, Blinov AV, Golik AB, Nagdalian AA, Maglakelidze DG, Statsenko EN, Pirogov MA, Blinova AA, Sizonenko MN, Simonov AN, Zhukov RB, Kolesnikov RO, Ibrahim SA. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci Rep. 2022;12(1):12843. doi: 10.1038/s41598-022-16878-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbus AF, Horozov TS, Paunov VN. Colloid particle formulations for antimicrobial applications. Adv Colloid Interface Sci. 2017;249:134–148. doi: 10.1016/j.cis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- Hassanen EI, Ibrahim MA, Hassan AM, Mehanna S, Aljuaydi SH, Issa MYJNR. Neuropathological and cognitive effects induced by CuO-NPs in rats and trials for prevention using pomegranate juice. Neurochem Res. 2021;46(5):1264–1279. doi: 10.1007/s11064-021-03264-7. [DOI] [PubMed] [Google Scholar]
- Hou J, Liu H, Wang L, Duan L, Li S, Wang X. Molecular toxicity of metal oxide nanoparticles in Danio rerio. Environ Sci Technol. 2018;52(14):7996–8004. doi: 10.1021/acs.est.8b01464. [DOI] [PubMed] [Google Scholar]
- Huang W, Fang H, Zhang S, Yu HJM, Letters N. Optimised Green Synthesis of Copper Oxide Nanoparticles and Their Antifungal Activity. 2021;16(7):374–380. [Google Scholar]
- Ingle PU, Biswas JK, Mondal M, Rai MK, Kumar PS, Gade AKJC. Assessment of in Vitro Antimicrobial Efficacy of Biologically Synthesized Metal Nanoparticles against Pathogenic Bacteria. 2022;291:132676. doi: 10.1016/j.chemosphere.2021.132676. [DOI] [PubMed] [Google Scholar]
- Jadhav MS, Kulkarni S, Raikar P, Barretto DA, Vootla SK, Raikar US. Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J Chem. 2018;42(1):204–213. doi: 10.1039/C7NJ02977B. [DOI] [Google Scholar]
- Jamil B, Javed R, Qazi AS, Syed MA. Nanomaterials: toxicity, risk management and public perception. In: Rai M, Biswas JK, editors. Nanomaterials: Ecotoxicity, Safety, and Public Perception. Cham: Springer International Publishing; 2018. pp. 283–304. [Google Scholar]
- Javed R, Ahmed M, Haq IU, Nisa S, Zia M. PVP and PEG doped CuO nanoparticles are more biologically active: antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater Sci Eng c, Mater Biol Appl. 2017;79:108–115. doi: 10.1016/j.msec.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Javed R, Zia M, Naz S, Aisida SO, Ain Nu, Ao Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnol. 2020;18(1):172. doi: 10.1186/s12951-020-00704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed R, Ghonaim R, Shathili A, Khalifa SAM, El-Seedi HR. Chapter 3 - Phytonanotechnology: a greener approach for biomedical applications. In: Patra C, Ahmad I, Ayaz M, Khalil AT, Mukherjee S, Ovais M, editors. Biogenic Nanoparticles for Cancer Theranostics. Elsevier; 2021. pp. 43–86. [Google Scholar]
- Javed R, Rais F, Kaleem M, Jamil B, Ahmad MA, Yu T, Qureshi SW, Ao Q. Chitosan capping of CuO nanoparticles: facile chemical preparation, biological analysis, and applications in dentistry. Int J Biol Macromol. 2021;167:1452–1467. doi: 10.1016/j.ijbiomac.2020.11.099. [DOI] [PubMed] [Google Scholar]
- Javed R, Ain NU, Gul A, Arslan Ahmad M, Guo W, Ao Q, Tian S. Diverse biotechnological applications of multifunctional titanium dioxide nanoparticles: an up-to-date review. IET Nanobiotechnol. 2022 doi: 10.1049/nbt2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed R, Sajjad A, Naz S, Sajjad H, Ao Q. Significance of capping agents of colloidal nanoparticles from the perspective of drug and gene delivery, bioimaging, and biosensing: an insight. Int J Mol Sci. 2022;23(18):10521. doi: 10.3390/ijms231810521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumarai G, Gokulpriya C, Sudhapriya R, Sharmila G, Muthukumaran C. Phytofabrication and characterization of monodisperse copper oxide nanoparticles using Albizia lebbeck leaf extract. Appl Nanosci. 2015;5(8):1017–1021. doi: 10.1007/s13204-015-0402-1. [DOI] [Google Scholar]
- Kannan K, Radhika D, Vijayalakshmi S, Sadasivuni KK, A. Ojiaku A, Verma UJIJoEAC (2020) Facile fabrication of CuO nanoparticles via microwave-assisted method: photocatalytic antimicrobial and anticancer enhancing performance 1–14
- Kalaiyan G, Suresh S, Prabu K, Thambidurai S, Kandasamy M, Pugazhenthiran N, Kumar SK, Muneeswaran TJJoECE. Bactericidal activity of Moringa oleifera leaf extract assisted green synthesis of hierarchical copper oxide microspheres against pathogenic bacterial strains. J Environ Chem Eng. 2021;9(1):104847. doi: 10.1016/j.jece.2020.104847. [DOI] [Google Scholar]
- Kalaycıoğlu Z, Geçim B, Erim FB. Green synthesis of cerium oxide nanoparticles from turmeric and kinds of honey: characterisations, antioxidant and photocatalytic dye degradation activities. Adv Nat Sci. 2022;13:015016. doi: 10.1088/2043-6262/ac5dc5. [DOI] [Google Scholar]
- Karlsson HL, Gustafsson J, Cronholm P, Möller L. Size-dependent toxicity of metal oxide particles–a comparison between nano- and micrometer size. Toxicol Lett. 2009;188(2):112–118. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Katsumiti A, Thorley AJ, Arostegui I, Reip P, Valsami-Jones E, Tetley TD, Cajaraville MP. Cytotoxicity and cellular mechanisms of toxicity of CuO NPs in mussel cells in vitro and comparative sensitivity with human cells. Toxicol Vitro. 2018;48:146–158. doi: 10.1016/j.tiv.2018.01.013. [DOI] [PubMed] [Google Scholar]
- Katwal R, Kaur H, Sharma G, Naushad M, Pathania D. Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J Ind Eng Chem. 2015;31:173–184. doi: 10.1016/j.jiec.2015.06.021. [DOI] [Google Scholar]
- Kayalvizhi S, Sengottaiyan A, Selvankumar T, Senthilkumar B, Sudhakar C, Selvam K. Eco-friendly cost-effective approach for synthesis of copper oxide nanoparticles for enhanced photocatalytic performance. Optik. 2020;202:163507. doi: 10.1016/j.ijleo.2019.163507. [DOI] [Google Scholar]
- Kerour A, Boudjadar S, Bourzami R, Allouche B. Eco-friendly synthesis of cuprous oxide (Cu2O) nanoparticles and improvement of their solar photocatalytic activities. J Solid State Chem. 2018;263:79–83. doi: 10.1016/j.jssc.2018.04.010. [DOI] [Google Scholar]
- Khaldari I, Naghavi MR, Motamedi E. Synthesis of green and pure copper oxide nanoparticles using two plant resources via solid-state route and their phytotoxicity assessment. RSC Adv. 2021;11(6):3346–3353. doi: 10.1039/D0RA09924D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaligh SF, Asoodeh A. Green synthesis and biological characterization of cerium oxide nanoemulsion against human HT-29 colon cancer cell line. Mater Technol. 2022;37:1–21. doi: 10.1080/10667857.2022.2031492. [DOI] [Google Scholar]
- Khatami M, Heli H, Mohammadzadeh Jahani P, Azizi H, Lima Nobre MA. Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnol. 2017;11(6):709–713. doi: 10.1049/iet-nbt.2016.0189. [DOI] [Google Scholar]
- Khatami M, Varma RS, Heydari M, Peydayesh M, Sedighi A, Agha Askari H, Rohani M, Baniasadi M, Arkia S, Seyedi F, Khatami S. Copper oxide nanoparticles greener synthesis using tea and its antifungal efficiency on Fusarium solani. Geomicrobiol J. 2019;36(9):777–781. doi: 10.1080/01490451.2019.1621963. [DOI] [Google Scholar]
- Khatamifar M, Fatemi SJ. Green synthesis of pure copper oxide nanoparticles using Quercus infectoria galls extract, thermal behavior and their antimicrobial effects. Part Sci Technol. 2022;40(1):18–26. doi: 10.1080/02726351.2021.1901810. [DOI] [Google Scholar]
- Khayati GR, Nourafkan E, Karimi G, Moradgholi J. Synthesis of cuprous oxide nanoparticles by mechanochemical oxidation of copper in high planetary energy ball mill. Adv Powder Technol. 2013;24(1):301–305. doi: 10.1016/j.apt.2012.07.006. [DOI] [Google Scholar]
- Khodair ZT, Alzubaidy MWM, Almohaidi AMS, Sultan AA, AL-Shimmary SMH, Albusultan SS. Synthesis of copper oxide nanoparticles (CuO-NPs) and its evaluation of antibacterial activity against. P. aeruginosa biofilm gene’s. 2019;2190(1):020006. doi: 10.1063/1.5138492. [DOI] [Google Scholar]
- Krug HF. Collection of controlled nanosafety data-the CoCoN-database, a tool to assess nanomaterial hazard. Nanomaterials (basel) 2022;12(3):441. doi: 10.3390/nano12030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PNV. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon. 2019;5(11):e02765. doi: 10.1016/j.heliyon.2019.e02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PPNV, Shameem U, Kollu P, Kalyani RL, Pammi SVN. Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. BioNanoScience. 2015;5(3):135–139. doi: 10.1007/s12668-015-0171-z. [DOI] [Google Scholar]
- Kumar B, Smita K, Cumbal L, Debut A, Angulo Y. Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf. J Saudi Chem Soc. 2017;21:S475–S480. doi: 10.1016/j.jscs.2015.01.009. [DOI] [Google Scholar]
- Lee IC, Ko JW, Park SH, Shin NR, Shin IS, Moon C, Kim JH, Kim HC, Kim JC. Comparative toxicity and biodistribution assessments in rats following subchronic oral exposure to copper nanoparticles and microparticles. Part Fibre Toxicol. 2016;13(1):56. doi: 10.1186/s12989-016-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei R, Wu C, Yang B, Ma H, Shi C, Wang Q, Wang Q, Yuan Y, Liao M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Toxicol Applied Pharmacol. 2008;232(2):292–301. doi: 10.1016/j.taap.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Liu H, Zheng S, Xiong H, Alwahibi MS, Niu XJAJoC. Biosynthesis of copper oxide nanoparticles using Abies spectabilis plant extract and analyzing its antinociceptive and anti-inflammatory potency in various mice models. Arab J Chem. 2020;13(9):6995–7006. doi: 10.1016/j.arabjc.2020.07.006. [DOI] [Google Scholar]
- Maciel-Magalhães M, Medeiros RJ, Bravin JS, Patricio BFC, Rocha HVA, Paes-de-Almeida EC, Santos LMG, Jacob SC, Savignon TCM, Amendoeira FC. Evaluation of acute toxicity and copper accumulation in organs of Wistar rats, 14 days after oral exposure to copper oxide (II) nano- and microparticles. J Nanoparticle Res. 2019;22(1):2. doi: 10.1007/s11051-019-4721-0. [DOI] [Google Scholar]
- Mahmoud NM, Mohamed HI, Ahmed SB, Akhtar SJCP. Efficient Biosynthesis of CuO Nanoparticles with Potential Cytotoxic Activity. 2020;74(9):2825–2835. [Google Scholar]
- Malaikozhundan B, Vinodhini J, Kalanjiam MAR, Vinotha V, Palanisamy S, Vijayakumar S, Vaseeharan B, Mariyappan A. High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of Withania somnifera leaf extract-assisted zinc oxide nanoparticle. Bioprocess Biosyst Eng. 2020;43(9):1533–1547. doi: 10.1007/s00449-020-02346-0. [DOI] [PubMed] [Google Scholar]
- Mani VM, Kalaivani S, Sabarathinam S, Vasuki M, Soundari AJPG, Das MA, Elfasakhany A, Pugazhendhi AJEr. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ Chem. 2021;201:111502. doi: 10.1016/j.envres.2021.111502. [DOI] [PubMed] [Google Scholar]
- Mani VM, Nivetha S, Sabarathinam S, Barath S, Das MPA, Basha S, Elfasakhany A, Pugazhendhi A. Multifunctionalities of mycosynthesized zinc oxide nanoparticles (ZnONPs) from Cladosporium tenuissimum FCBGr: antimicrobial additives for paints coating, functionalized fabrics and biomedical properties. Progr Org Coat. 2022;163:106650. doi: 10.1016/j.porgcoat.2021.106650. [DOI] [Google Scholar]
- Manikandan DB, Arumugam M, Veeran S, Sridhar A, Sekar RK, Perumalsamy B, Ramasamy TJES. Biofabrication of ecofriendly copper oxide nanoparticles using Ocimum americanum aqueous leaf extract: analysis of in vitro antibacterial, anticancer, and photocatalytic activities. Environ Sci Pollut Res. 2021;28(26):33927–33941. doi: 10.1007/s11356-020-12108-w. [DOI] [PubMed] [Google Scholar]
- Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 2010;12(5):1531–1551. doi: 10.1007/s11051-010-9900-y. [DOI] [Google Scholar]
- Maynard AD, Kuempel ED. Airborne nanostructured particles and occupational health. J Nanopart Res. 2005;7(6):587–614. doi: 10.1007/s11051-005-6770-9. [DOI] [Google Scholar]
- Merkl P, Long S, McInerney GM, Sotiriou GA. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials. 2021;11(5):1312. doi: 10.3390/nano11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigalieva IA, Katsnelson BA, Panov VG, Privalova LI, Varaksin AN, Gurvich VB, Sutunkova MP, Shur VY, Shishkina EV, Valamina IE, Zubarev IV, Makeyev OH, Meshtcheryakova EY, Klinova SV. In vivo toxicity of copper oxide, lead oxide and zinc oxide nanoparticles acting in different combinations and its attenuation with a complex of innocuous bio-protectors. Toxicology. 2017;380:72–93. doi: 10.1016/j.tox.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Abu-Elghait M, Ahmed NE, Salem SSJBter. Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Bio Trace Elem Res. 2021;199(7):2788–2799. doi: 10.1007/s12011-020-02369-4. [DOI] [PubMed] [Google Scholar]
- Morsy EA, Hussien AM, Ibrahim MA, Farroh KY, Hassanen EI. Cytotoxicity and genotoxicity of copper oxide nanoparticles in chickens. Biol Trace Elem Res. 2021;199(12):4731–4745. doi: 10.1007/s12011-021-02595-4. [DOI] [PubMed] [Google Scholar]
- Mortensen NP, Moreno Caffaro M, Aravamudhan S, Beeravalli L, Prattipati S, Snyder RW, Watson SL, Patel PR, Weber FX, Montgomery SAJN. Simulated gastric digestion and in vivo intestinal uptake of orally administered CuO nanoparticles and TiO2 E171 in male and female rat pups. Nanomaterials. 2021;11(6):1487. doi: 10.3390/nano11061487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, El-Sayed E-SR, Mohamed SS, Abo El-Seoud MA, Elmehlawy AA, Abdou DAJAM. Novel mycosynthesis of Co3O4, CuO, Fe3O4 NiO, and ZnO Nanoparticles by the Endophytic Aspergillus Terreus and Evaluation of Their Antioxidant and Antimicrobial Activities. Appl Microbiol Biotechnol. 2021;105(2):741–753. doi: 10.1007/s00253-020-11046-4. [DOI] [PubMed] [Google Scholar]
- Muthuvel A, Jothibas M, Manoharan C. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: photocatalytic degradation and in vitro antioxidant activity. Nanotechnol Environ Eng. 2020;5(2):14. doi: 10.1007/s41204-020-00078-w. [DOI] [Google Scholar]
- Nagajyothi PC, Muthuraman P, Sreekanth TVM, Kim DH, Shim J. Green synthesis: in-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab J Chem. 2017;10(2):215–225. doi: 10.1016/j.arabjc.2016.01.011. [DOI] [Google Scholar]
- Nagaraj E, Karuppannan K, Shanmugam P, Venugopal S. Exploration of bio-synthesized copper oxide nanoparticles using Pterolobium hexapetalum leaf extract by photocatalytic activity and biological evaluations. J Clust Sci. 2019;30(4):1157–1168. doi: 10.1007/s10876-019-01579-8. [DOI] [Google Scholar]
- Nagore P, Ghotekar S, Mane K, Ghoti A, Bilal M, Roy A. structural properties and antimicrobial activities of Polyalthia longifolia leaf extract-mediated CuO nanoparticles. BioNanoScience. 2021;11(2):579–589. doi: 10.1007/s12668-021-00851-4. [DOI] [Google Scholar]
- Nasrollahzadeh M, Sajadi SM. Green synthesis of copper nanoparticles using Ginkgo biloba L. leaf extract and their catalytic activity for the Huisgen [3+2] cycloaddition of azides and alkynes at room temperature. J Colloid Interface Sci. 2015;457:141–147. doi: 10.1016/j.jcis.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Sajadi SM, Rostami-Vartooni A, Hussin SM. Green synthesis of CuO nanoparticles using aqueous extract of Thymus vulgaris L. leaves and their catalytic performance for N-arylation of indoles and amines. J Colloid Interface Sci. 2016;466:113–119. doi: 10.1016/j.jcis.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Navada KM, Nagaraja G, D’Souza JN, Kouser S, Ranjitha R, Manasa DJAN. Phyto assisted synthesis and characterization of Scoparia dulcis L leaf extract mediated porous nano CuO photocatalysts and its anticancer behavior. Appl Nanosci. 2020;10(11):4221–4240. doi: 10.1007/s13204-020-01536-2. [DOI] [Google Scholar]
- Naz S, Shahzad H, Ali A, Zia M. Nanomaterials as nanocarriers: a critical assessment why these are multi-chore vanquisher in breast cancer treatment. Artif Cells Nanomed Biotechnol. 2018;46(5):899–916. doi: 10.1080/21691401.2017.1375937. [DOI] [PubMed] [Google Scholar]
- Naz S, Gul A, Zia M. Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol. 2020;14(1):1–13. doi: 10.1049/iet-nbt.2019.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz S, Tabassum S, Freitas Fernandes N, Mujahid M, Zia M, Carcache de Blanco EJJNPR. Anticancer and antibacterial potential of Rhus punjabensis and CuO nanoparticles. Natural Product Res. 2020;34(5):720–725. doi: 10.1080/14786419.2018.1495633. [DOI] [PubMed] [Google Scholar]
- Naz S, Hanif S, Ali H, Ali JS, Zia M. Synthesis, characterization, in vitro and in vivo toxicity of CuO nanoparticles fabricated through Rhus punjabensis leaf extract. BioNanoScience. 2021;11(4):946–956. doi: 10.1007/s12668-021-00906-6. [DOI] [Google Scholar]
- Naz S, Nasir B, Ali H, Zia M. Comparative toxicity of green and chemically synthesized CuO NPs during pregnancy and lactation in rats and offspring: Part I -hepatotoxicity. Chemosphere. 2021;266:128945. doi: 10.1016/j.chemosphere.2020.128945. [DOI] [PubMed] [Google Scholar]
- Nithiyavathi R, Sundaram SJ, Anand GT, Kumar DR, Raj AD, Al Farraj DA, Aljowaie RM, AbdelGawwad MR, Samson Y, Kaviyarasu KJJoi. Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J Infect Public Health. 2021;14(12):1893–1902. doi: 10.1016/j.jiph.2021.10.022. [DOI] [PubMed] [Google Scholar]
- Noor R, Yasmin H, Ilyas N, Nosheen A, Hassan MN, Mumtaz S, Khan N, Ahmad A, Ahmad P. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere. 2022;292:133201. doi: 10.1016/j.chemosphere.2021.133201. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol. 2009;9(8):4996–5007. doi: 10.1166/jnn.2009.gr02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouni S, Askri D, Jeljeli M, Abdelmalek H, Sakly M, Amara S. Toxicity and effects of copper oxide nanoparticles on cognitive performances in rats. Arch Environ Occup Health. 2020;75(7):384–394. doi: 10.1080/19338244.2019.1689376. [DOI] [PubMed] [Google Scholar]
- Oussou-Azo AF, Nakama T, Nakamura M, Futagami T, Vestergaard MCM. Antifungal potential of nanostructured crystalline copper and its oxide forms. Nanomaterials (Basel) 2020;10(5):1003. doi: 10.3390/nano10051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansambal S, Deshmukh K, Savale A, Ghotekar S, Pardeshi O, Jain G, Aher Y, Pore D. Phytosynthesis and biological activities of fluorescent CuO nanoparticles using Acanthospermum hispidum L Extract. J Nanostruct. 2017;7(3):165–174. doi: 10.18502/jns.v7i3.1. [DOI] [Google Scholar]
- Paulpandian P, Beevi IS, Somanath B, Kamatchi RK, Paulraj B, Faggio C. Impact of Camellia sinensis iron oxide nanoparticle on growth, hemato-biochemical and antioxidant capacity of blue gourami (Trichogaster trichopterus) fingerlings. Biol Trace Elem Res. 2022 doi: 10.1007/s12011-022-03145-2. [DOI] [PubMed] [Google Scholar]
- Perreault F, Pedroso Melegari S, Henning da Costa C, de Oliveira Franco Rossetto AL, Popovic R, Gerson Matias W. Genotoxic effects of copper oxide nanoparticles in Neuro 2A cell cultures. Sci Total Environ. 2012;441:117–24. doi: 10.1016/j.scitotenv.2012.09.065. [DOI] [PubMed] [Google Scholar]
- Phang Y-K, Aminuzzaman M, Akhtaruzzaman M, Muhammad G, Ogawa S, Watanabe A, Tey L-H. Green Synthesis and Characterization of CuO Nanoparticles Derived from Papaya Peel Extract for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME) 2021;13(2):796. [Google Scholar]
- Phiwdang K, Suphankij S, Mekprasart W, Pecharapa W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia. 2013;34:740–745. doi: 10.1016/j.egypro.2013.06.808. [DOI] [Google Scholar]
- Piret JP, Jacques D, Audinot JN, Mejia J, Boilan E, Noël F, Fransolet M, Demazy C, Lucas S, Saout C, Toussaint O. Copper(II) oxide nanoparticles penetrate into HepG2 cells, exert cytotoxicity via oxidative stress and induce pro-inflammatory response. Nanoscale. 2012;4(22):7168–7184. doi: 10.1039/c2nr31785k. [DOI] [PubMed] [Google Scholar]
- Prabhu BM, Ali SF, Murdock RC, Hussain SM, Srivatsan M. Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology. 2010;4(2):150–160. doi: 10.3109/17435390903337693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Elavarasan N, Venkatesan A, Subashini K, Sowndharya M, Sujatha V. Green synthesis of copper oxide nanoparticles and its effective applications in Biginelli reaction, BTB photodegradation and antibacterial activity. Adv Powder Technol. 2018;29(12):3315–3326. doi: 10.1016/j.apt.2018.09.009. [DOI] [Google Scholar]
- Rafique M, Shafiq F, Ali Gillani SS, Shakil M, Tahir MB, Sadaf I. Eco-friendly green and biosynthesis of copper oxide nanoparticles using Citrofortunella microcarpa leaves extract for efficient photocatalytic degradation of Rhodamin B dye form textile wastewater. Optik. 2020;208:164053. doi: 10.1016/j.ijleo.2019.164053. [DOI] [Google Scholar]
- Rajamma R, Nair SGJIn, Antibacterial and anticancer activity of biosynthesised CuO nanoparticles. IET Nanobiotechnology. 2020;14(9):833. doi: 10.1049/iet-nbt.2020.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KR. Green synthesis, morphological and optical studies of CuO nanoparticles. J Mol Struct. 2017;1150:553–557. doi: 10.1016/j.molstruc.2017.09.005. [DOI] [Google Scholar]
- Rezaie AB, Montazer M, Rad MM. Photo and biocatalytic activities along with UV protection properties on polyester fabric through green in-situ synthesis of cauliflower-like CuO nanoparticles. J Photochem Photobiol b: Biol. 2017;176:100–111. doi: 10.1016/j.jphotobiol.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Rostami-Tapeh-Esmaeil E, Golshan M, Salami-Kalajahi M, Roghani-Mamaqani H. Synthesis of copper and copper oxide nanoparticles with different morphologies using aniline as reducing agent. Solid State Commun. 2021;334–335:114364. doi: 10.1016/j.ssc.2021.114364. [DOI] [Google Scholar]
- Ruiz P, Katsumiti A, Nieto JA, Bori J, Jimeno-Romero A, Reip P, Arostegui I, Orbea A, Cajaraville MP. Short-term effects on antioxidant enzymes and long-term genotoxic and carcinogenic potential of CuO nanoparticles compared to bulk CuO and ionic copper in mussels Mytilus galloprovincialis. Mar Environ Res. 2015;111:107–120. doi: 10.1016/j.marenvres.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar J, Venkatkumar S. Green synthesis of copper oxide nanoparticles from Magnolia champaca floral extract and its antioxidant & toxicity assay using Danio Rerio. Int J Recent Technol Eng. 2020;8(5):5444–9. doi: 10.35940/ijrte.E6869.018520. [DOI] [Google Scholar]
- Saravanakumar K, Shanmugam S, Varukattu NB, MubarakAli D, Kathiresan K, Wang M-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J Photochem Photobiol b: Biol. 2019;190:103–109. doi: 10.1016/j.jphotobiol.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Sardar M, Ahmed W, Al Ayoubi S, Nisa S, Bibi Y, Sabir M, Khan MM, Ahmed W, Qayyum A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J Biol Sci. 2022;29(1):88–95. doi: 10.1016/j.sjbs.2021.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyavimal S, Vasantharaj S, Bharathi D, Saravanan M, Manikandan E, Kumar SS, Pugazhendhi A. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of gram negative and gram positive bacteria. J Photochem Photobiol b, Biol. 2018;188:126–134. doi: 10.1016/j.jphotobiol.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Sathyananda H, Prashanth P, Prashanth G, Dileep M, Boselin Prabhu S, Nagabhushana B, Shivakumara C, Nagendra HJAN. Evaluation of antimycobacterial, antioxidant, and anticancer activities of CuO nanoparticles through cobalt doping. Applied Nanoscience. 2022;12(1):79–86. doi: 10.1007/s13204-021-02156-0. [DOI] [Google Scholar]
- Sawake MM, Moharil MP, Ingle YV, Jadhav PV, Ingle AP, Khelurkar VC, Paithankar DH, Bathe GA, Gade AK. Management of Phytophthora parasitica causing gummosis in citrus using biogenic copper oxide nanoparticles. J Appl Microbiol. 2022;132(4):3142–3154. doi: 10.1111/jam.15472. [DOI] [PubMed] [Google Scholar]
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, Gänsbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9(2):102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- Selvanathan V, Aminuzzaman M, Tey LH, Razali SA, Althubeiti K, Alkhammash HI, Guha SK, Ogawa S, Watanabe A, Shahiduzzaman M, Akhtaruzzaman M. Muntingia calabura leaves mediated green synthesis of CuO nanorods: exploiting phytochemicals for unique morphology. Materials (Basel, Switzerland) 2021;14(21):6379. doi: 10.3390/ma14216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepasgozar S, Mohseni S, Feyzizadeh B, Morsali A. Eco-friendly synthesis of magnetic iron oxide nanoparticles using Achillea nobilis extract and evaluation of their antioxidant and antibacterial properties. J Food Biosci Technol. 2022;12(2):61–71. [Google Scholar]
- Shah IH, Ashraf M, Sabir IA, Manzoor MA, Malik MS, Gulzar S, Ashraf F, Iqbal J, Niu Q, Zhang YJJOMS. Green synthesis and characterization of copper oxide nanoparticles using Calotropis procera leaf extract and their different biological potentials. J Mol Struct. 2022;1259:132696. doi: 10.1016/j.molstruc.2022.132696. [DOI] [Google Scholar]
- Shaheen TI, Fouda A, Salem SS. Integration of cotton fabrics with biosynthesized CuO nanoparticles for bactericidal activity in the terms of their cytotoxicity assessment. Ind Eng Chem Res. 2021;60(4):1553–1563. doi: 10.1021/acs.iecr.0c04880. [DOI] [Google Scholar]
- Shamsuddin M, Nordin Raja, NJMJoF, Sciences A, Biosynthesis of copper(II) oxide nanoparticles using Murraya koenigii aqueous leaf extract and its catalytic activity in 4-nitrophenol reduction. Mal J Fund Appl Sci. 2019;15(2):218. doi: 10.11113/mjfas.v15n2.1390. [DOI] [Google Scholar]
- Shahriary S, Tafvizi F, Khodarahmi P, Shaabanzadeh MJBC, Biorefinery (2022) Phyto-mediated synthesis of CuO nanoparticles using aqueous leaf extract of Artemisia deserti and their anticancer effects on A2780-CP cisplatin-resistant ovarian cancer cells. Biomass Conv Bioref 1–17
- Sharmila G, Thirumarimurugan M, Sivakumar VM. Optical, catalytic and antibacterial properties of phytofabricated CuO nanoparticles using Tecoma castanifolia leaf extract. Optik. 2016;127(19):7822–7828. doi: 10.1016/j.ijleo.2016.05.142. [DOI] [Google Scholar]
- Sharmila G, Sakthi Pradeep R, Sandiya K, Santhiya S, Muthukumaran C, Jeyanthi J, Manoj Kumar N, Thirumarimurugan M. Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: Characterization and its antibacterial application. J Mol Struct. 2018;1165:288–292. doi: 10.1016/j.molstruc.2018.04.011. [DOI] [Google Scholar]
- Shkodenko L, Kassirov I, Koshel E. Metal oxide nanoparticles against bacterial biofilms: perspectives and limitations. Microorganisms. 2020;8(10):1545. doi: 10.3390/microorganisms8101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N, Ramírez S, Díaz I, Garcia A, Hassan N. Easy, quick, and reproducible sonochemical synthesis of CuO nanoparticles. Materials (Basel, Switzerland) 2019;12(5):804. doi: 10.3390/ma12050804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Kumar V, Kim K-H, Rawat M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ Res. 2019;177:108569. doi: 10.1016/j.envres.2019.108569. [DOI] [PubMed] [Google Scholar]
- Sisubalan N, Ramkumar VS, Pugazhendhi A, Karthikeyan C, Indira K, Gopinath K, Hameed ASH, Basha MHG. ROS-mediated cytotoxic activity of ZnO and CeO(2) nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ Sci Pollut Res Int. 2018;25(11):10482–10492. doi: 10.1007/s11356-017-0003-5. [DOI] [PubMed] [Google Scholar]
- Sivaraj R, Rahman PK, Rajiv P, Narendhran S, Venckatesh R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;129:255–258. doi: 10.1016/j.saa.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Sivaraj R, Rahman PKSM, Rajiv P, Salam HA, Venckatesh R. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricata leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim Acta A Mol Biomol Spectrosc. 2014;133:178–181. doi: 10.1016/j.saa.2014.05.048. [DOI] [PubMed] [Google Scholar]
- Solano R, Patiño-Ruiz D, Tejeda-Benitez L, Herrera A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: a review on the applications, nanotoxicological effects, and risk control strategies. Environ Sci Pollut Res. 2021;28(14):16962–16981. doi: 10.1007/s11356-021-12996-6. [DOI] [PubMed] [Google Scholar]
- Sonbol H, AlYahya S, Ameen F, Alsamhary K, Alwakeel S, Al-Otaibi S, Korany SJAN (2021) Bioinspired synthesize of CuO nanoparticles using Cylindrospermum stagnale for antibacterial anticancer and larvicidal applications 1–11
- Suganthy N, Sri Ramkumar V, Pugazhendhi A, Benelli G, Archunan G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ Sci Pollut Res Int. 2018;25(11):10418–10433. doi: 10.1007/s11356-017-9789-4. [DOI] [PubMed] [Google Scholar]
- Sukumar S, Rudrasenan A, Padmanabhan Nambiar D. Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega. 2020;5(2):1040–1051. doi: 10.1021/acsomega.9b02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman GM, Tawfeeq AT, Jaaffer MD. Biogenic synthesis of copper oxide nanoparticles using Olea europaea leaf extract and evaluation of their toxicity activities: an in vivo and in vitro study. Biotechnol Prog. 2018;34(1):218–230. doi: 10.1002/btpr.2568. [DOI] [PubMed] [Google Scholar]
- Sulak M. Preparation of G-CuO NPs and G-ZnO NPs with mallow leaves, investigation of their antibacterial behavior and synthesis of bis(indolyl)methane compounds under solvent-free microwave assisted dry milling conditions using G-CuO NPs as a catalyst. Turk J Chem. 2021;45(5):1517–1532. doi: 10.3906/kim-2105-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wang S, Zhao D, Hun FH, Weng L, Liu H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells: cytotoxicity, permeability, and inflammation of metal oxide nanoparticles. Cell Biol Toxicol. 2011;27(5):333–342. doi: 10.1007/s10565-011-9191-9. [DOI] [PubMed] [Google Scholar]
- Taherzadeh Soureshjani P, Shadi A, Mohammadsaleh F. Algae-mediated route to biogenic cuprous oxide nanoparticles and spindle-like CaCO3: a comparative study, facile synthesis, and biological properties. RSC Adv. 2021;11(18):10599–10609. doi: 10.1039/D1RA00187F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortella G, Rubilar O, Fincheira P, Pieretti JC, Duran P, Lourenço IM, Seabra AB. Bactericidal and virucidal activities of biogenic metal-based nanoparticles: advances and perspectives. Antibiotics (Basel, Switzerland) 2021;10(7):783. doi: 10.3390/antibiotics10070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Nguyen VT. Copper oxide nanomaterials prepared by solution methods, some properties, and potential applications: a brief review. Int Sch Res Notices. 2014;2014:856592. doi: 10.1155/2014/856592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshireletso P, Ateba CN, Fayemi OEJM. Spectroscopic and antibacterial properties of CuONPs from orange, lemon and tangerine peel extracts potential for combating bacterial resistance. Mol. 2021;26(3):586. doi: 10.3390/molecules26030586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulinska J, Mikusova ML, Liskova A, Busova M, Masanova V, Uhnakova I, Rollerova E, Alacova R, Krivosikova Z, Wsolova L, Dusinska M, Horvathova M, Szabova M, Lukan N, Stuchlikova M, Kuba D, Vecera Z, Coufalik P, Krumal K, Alexa L, Vrlikova L, Buchtova M, Dumkova J, Piler P, Thon V, Mikuska P. Copper oxide nanoparticles stimulate the immune response and decrease antioxidant defense in mice after six-week inhalation. Front Immunol. 2022;13:874253. doi: 10.3389/fimmu.2022.874253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamil Elakkiya V, Meenakshi R, Senthil Kumar P, Karthik V, Ravi Shankar K, Sureshkumar P, Hanan AJIJoES, Technology (2021) Green synthesis of copper nanoparticles using Sesbania aculeata to enhance the plant growth and antimicrobial activities 1–10
- Turakhia B, Divakara MB, Santosh MS, Shah S. Green synthesis of copper oxide nanoparticles: a promising approach in the development of antibacterial textiles. J Coat Technol Res. 2020;17(2):531–540. doi: 10.1007/s11998-019-00303-5. [DOI] [Google Scholar]
- Vasantharaj S, Sathiyavimal S, Saravanan M, Senthilkumar P, Gnanasekaran K, Shanmugavel M, Manikandan E, Pugazhendhi A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B Biol. 2019;191:143–149. doi: 10.1016/j.jphotobiol.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Veisi H, Karmakar B, Tamoradi T, Hemmati S, Hekmati M, Hamelian M. Biosynthesis of CuO nanoparticles using aqueous extract of herbal tea (Stachys lavandulifolia) flowers and evaluation of its catalytic activity. Sci Rep. 2021;11(1):1983. doi: 10.1038/s41598-021-81320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatramanan A, Ilangovan A, Thangarajan P, Saravanan A, Mani B. Green synthesis of copper oxide nanoparticles (CuO NPs) from aqueous extract of seeds of Eletteria cardamomum and its antimicrobial activity against pathogens. Current Biotechnol. 2020;9(4):304–311. doi: 10.2174/2211550109999201113095459. [DOI] [Google Scholar]
- Verma R, Khan AB, Khan MIK, Amar AK, Sah S, Jaiswal KK, Singh RKJCE. Microwave-assisted biosynthesis of CuO nanoparticles using Atalantia monophylla (L) leaf extract and its biomedical applications. Technology. 2021;44(8):1496–1503. [Google Scholar]
- Vidovix TB, Quesada HB, Januário EFD, Bergamasco R, Vieira AMS. Green synthesis of copper oxide nanoparticles using Punica granatum leaf extract applied to the removal of methylene blue. Mater Lett. 2019;257:126685. doi: 10.1016/j.matlet.2019.126685. [DOI] [Google Scholar]
- Vinothkanna A, Mathivanan K, Ananth S, Ma Y, Sekar S. Biosynthesis of copper oxide nanoparticles using Rubia cordifolia bark extract: characterization, antibacterial, antioxidant, larvicidal and photocatalytic activities. Environ Sci Poll Res Int. 2022 doi: 10.1007/s11356-022-18996-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo CX, Liu J, Chen T, Yang H, Li CM. CeO2 nanoparticles/graphene nanocomposite-based high performance supercapacitor. Dalton Trans. 2011;40(24):6388–6391. doi: 10.1039/C1DT10397K. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li N, Zhao J, White JC, Qu P, Xing B. CuO nanoparticle interaction with human epithelial cells: cellular uptake, location, export, and genotoxicity. Chem Res Toxicol. 2012;25(7):1512–1521. doi: 10.1021/tx3002093. [DOI] [PubMed] [Google Scholar]
- Wang D, Lin Z, Wang T, Yao Z, Qin M, Zheng S, Lu W. Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J Hazard Mater. 2016;308:328–334. doi: 10.1016/j.jhazmat.2016.01.066. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Z, Xu P, Xiao L, Yang Z. Nanosized copper oxide induces apoptosis through oxidative stress in podocytes. Arch Toxicol. 2013;87(6):1067–1073. doi: 10.1007/s00204-012-0925-0. [DOI] [PubMed] [Google Scholar]
- Yalcinkaya F, Komarek M. Polyvinyl Butyral (PVB) Nanofiber/nanoparticle-covered yarns for antibacterial textile surfaces. Int J Mol Sci. 2019;20(17):4317. doi: 10.3390/ijms20174317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chen D. Synthesis of CuO nanoparticles for catalytic application via ultrasound-assisted ball milling. Process Appl Ceram. 2017;11(1):39–44. doi: 10.2298/PAC1701039Y. [DOI] [Google Scholar]
- Yugandhar P, Vasavi T, Jayavardhana Rao Y, Uma Maheswari Devi P, Narasimha G, Savithramma N. Cost effective, green synthesis of copper oxide nanoparticles using fruit extract of Syzygium alternifolium (Wt.) Walp, characterization and evaluation of antiviral activity. J Clust Sci. 2018;29(4):743–755. doi: 10.1007/s10876-018-1395-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.