Abstract
Background
A stepwise surgical approach with hemithyroidectomy and completion thyroidectomy was used to achieve definite characterization of follicular thyroid carcinoma (FTC). Choosing appropriate candidates for completion thyroidectomy has been controversial.
Objective
The aim of this study was to clarify the selection criteria for completion thyroidectomy using telomerase reverse transcriptase (TERT) promoter mutation.
Methods
A total of 87 FTC patients who had information about TERT promoter mutation from August 1995 to November 2020 were investigated. The cumulative risk of initial distant metastasis, disease recurrence, and cancer-specific death according to primary tumor size in each of the World Health Organization (WHO) 2017 classifications were calculated.
Results
Of the 87 patients, 8 (9.2%) had initial distant metastasis and 15 (17.2%) had persistent disease or developed structural recurrence. The threshold diameter for initial distant metastasis, disease recurrence, and cancer-specific death was 2 cm in minimally invasive FTC (MI-FTC) with mutant TERT (M-TERT) and in encapsulated angioinvasive FTC (EA-FTC) with M-TERT, while that in MI-FTC with wild-type TERT (WT-TERT) and EA-FTC with WT-TERT was 4 cm. The cumulative risk of initial distant metastasis, disease recurrence, and cancer-specific death according to primary tumor size in each WHO 2017 classification was significantly different only in patients with WT-TERT (p = 0.001, p = 0.019, and p = 0.005, respectively).
Conclusions
The data suggest 2 cm as a critical threshold diameter for performance of completion thyroidectomy in MI-FTC with M-TERT and EA-FTC with M-TERT. TERT promoter mutational status can help select candidates for completion thyroidectomy.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-022-13089-5.
Follicular thyroid carcinoma (FTC) is the second most common form of thyroid carcinoma, accounting for about 10% of all cases.1 FTC is defined as a malignant epithelial tumor arising from the follicular cells and lacking the diagnostic nuclear features of papillary thyroid carcinoma (PTC).2 Classic PTC demonstrates hallmark nuclear changes such as overlap, crowding, or palisading nuclei, and many cells show nuclear clefts and intranuclear cytoplasmic pseudo inclusions or vacuoles.3 However, those nuclear features are not found in FTC, making its diagnosis difficult by fine-needle aspiration. Furthermore, pathological examination of capsular and vascular invasion is important to distinguish FTC from benign follicular adenoma. Thereby, diagnosis of FTC based on cytological findings is limited.
For these reasons, a stepwise surgical approach with hemithyroidectomy and completion thyroidectomy has been used based on the definite histopathological diagnosis of follicular neoplasm.4 However, the subjects who should be chosen for completion thyroidectomy are controversial. In American Thyroid Association (ATA) guidelines, completion thyroidectomy is recommended when patients would have undergone initial total thyroidectomy if postoperative histopathological diagnosis had been available.5 Total thyroidectomy is recommended in patients with tumor size > 4 cm, gross extrathyroidal extension (ETE), presence of node metastasis, or presence of initial distant metastasis.6–11 In addition, ATA guidelines suggest that encapsulated angioinvasive FTC (EA-FTC) with invasion of four or more blood vessels should undergo completion thyroidectomy.
Traditionally, FTC has been classified into minimally invasive FTC (MI-FTC) and widely invasive follicular carcinoma (WI-FTC). However, an important change was made to the World Health Organization (WHO) 2017 classification based on the prognostic significance of vascular invasion in MI-FTC.12–14 Furthermore, the Armed Forces Institute of Pathology (AFIP) suggested four categories considering the prognostic significance of vascular invasion extent.15, 16 ATA guidelines also suggest consideration of extent of vascular invasion in the decision to perform completion thyroidectomy. Additional evidence is needed to reach consensus on this treatment.
Previous studies revealed that telomerase reverse transcriptase (TERT) promoter mutations are an important prognostic marker in thyroid cancer.17–21 In a recent study, the presence of TERT promoter mutations was significantly associated with poor survival in FTC patients.22 Of note, patients with mutant TERT (M-TERT) in EA-FTC or WI-FTC showed significantly worse prognoses than wild-type TERT (WT-TERT). Thus, TERT promoter mutations might be an important clue when deciding the completion thyroidectomy. The aim of this study was to clarify which FTC patients will need completion thyroidectomy based on TERT promoter mutational status.
Materials and Methods
Patients
A total of 87 patients who were pathologically diagnosed with FTC and had information about TERT promoter mutation status from August 1995 to November 2020 were included in this study; however, patients with Hurthle cell carcinoma and poorly differentiated thyroid carcinoma (PDTC) were excluded. The study protocol was approved by the Institutional Review Board of Samsung Medical Center (IRB no. 2021-04-085). Patient consent was waived by the committee owing to the retrospective design of the study.
Treatment and Follow-up Protocol
In Korea, there were no significant changes in the treatment or follow-up strategy during the study period.23–25 The initial surgical extent was decided based on guidelines, and completion thyroidectomy was performed when needed. Patients underwent regular follow-up at 6–12 months with physical examination, neck ultrasonography, thyroid function test, serum thyroglobulin (Tg) test, and anti-Tg antibody test. Patients with total thyroidectomy were treated with levothyroxine to suppress thyrotropin to < 0.10mIU/L, and radioactive iodine ablation was considered when patients were at high risk for recurrence. Computed tomography (CT), magnetic resonance imaging (MRI), 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET), diagnostic I-131, and 99mTc whole-body bone scintigraphy (99mTc WBS) were performed as needed. The frequency of follow-up visits and imaging tests was based on the clinical course.
Clinicopathological Data
Pathology reports and surgical records were reviewed to refine tumor categories based on the WHO 2017 classification and the 8th edition of the American Joint Committee on Cancer/Tumor-Node-Metastasis (AJCC/TNM) classification. For patients who were described as having multifocality or cervical lymph node metastasis in the initial pathology report, pathology slides were reviewed by a pathologist (YLO) to exclude the possibility of initial misdiagnoses, such as follicular variant PTC (FV-PTC). Clinical data were obtained from review of the electronic medical record (EMR) system.
Initial distant metastasis was defined as detection of distant metastasis within 6 months of initial surgery. Distant metastasis was detected by CT, MRI, 18FDG-PET, diagnostic I-131 scan, and/or 99mTc WBS. Disease-free survival (DFS) was defined as the time from initial surgery to the date of the first structural recurrence, defined as persistent or recurrent disease. This was determined cytologically or pathologically and/or by the presence of highly suspicious metastatic lesions on imaging. Cancer-specific survival (CSS) was defined as the time from initial surgery to the date of thyroid cancer-specific death. Survival status and cause of death were obtained from the EMR or the Korean Statistical Information Service (KOSIS).
Detection of Telomerase Reverse Transcriptase (TERT) Promoter Mutation
TERT promoter mutations were identified using semi-nested polymerase chain reaction and direct Sanger sequencing of the hot spots (chr5:1,295,228C > T and chr5:1,295,250C > T, commonly termed C228T and C250T, respectively), as described in previous studies.20, 26
Statistical Analysis
Continuous variables are presented as mean and standard deviation (SD) and categorical variables are presented as number and percentage. The Jonckheere–Terpstra test was used to analyze P for trend for continuous variables, and linear-by-linear association was used to analyze P for trend for categorical variables. The cumulative risks of initial distant metastasis, disease recurrence, and cancer-specific death were calculated using the Kaplan–Meier method and the results were compared using the log-rank test.27 Statistical analysis was performed using SPSS version 25.0 for Windows (IBM Corporation, Armonk, NY, USA).
Results
Baseline Characteristics
The baseline clinicopathological characteristics are described in Table 1. Of the total 87 patients, 67 (77.0%) were female and 20 (23.0%) were male. The mean (SD) primary tumor size was 3.7 (2.1) cm, and 15 (17.2%), 43 (49.4%), and 29 (33.3%) patients had tumors ≤ 2 cm, > 2.0 cm but ≤ 4.0 cm, and > 4 cm, respectively. Considering completion thyroidectomy as total thyroidectomy, 60 (69.0%) patients underwent initial total thyroidectomy or lobectomy followed by completion thyroidectomy. Initial distant metastases were present in 8 (9.2%) patients, and TERT promoter mutations were present in 16 (18.4) patients. When patients were classified by the WHO 2017 classification, 47 (54.0%), 25 (28.7%), and 15 (17.2) patients were classified as MI-FTC, EA-FTC, and WI-FTC, respectively.
Table 1.
Baseline characteristics of 87 patients
| Characteristics | |
|---|---|
| Age, years (mean ± SD) | 42.4 ± 15.4 |
| Sex | |
| Female | 67 (77.0) |
| Male | 20 (23.0) |
| Size | |
| Mean, cm (mean ± SD) | 3.7 ± 2.1 |
| ≤ 2 cm | 15 (17.2) |
| > 2 cm and ≤ 4 cm | 43 (49.4) |
| > 4 cm | 29 (33.3) |
| Surgical extent | |
| Total or subtotal thyroidectomy | 60 (69.0) |
| Lobectomy | 27 (31.0) |
| Gross ETE | |
| Absent | 84 (96.6) |
| Present | 3 (3.4) |
| Initial distant metastasis | |
| Absent | 79 (90.8) |
| Present | 8 (9.2) |
| TERT promoter mutations | |
| Wild-type | 71 (81.6) |
| Mutation | 16 (18.4) |
| WHO classification | |
| Minimally invasive FTC | 47 (54.0) |
| Encapsulated angioinvasive FTC | 25 (28.7) |
| Widely invasive FTC | 15 (17.2) |
| AJCC/TNM 8th stage | |
| I | 74 (85.1) |
| II | 10 (11.5) |
| III/IV | 3 (3.4) |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, ETE extrathyroidal extension, TERT telomerase reverse transcriptase, WHO World Health Organization, FTC follicular thyroid carcinoma, AJCC/TNM American Joint Committee/Tumor-Node-Metastasis
The study population was divided into three groups according to primary tumor size (Table 2). There were no significant clinicopathological factors, such as age, WHO 2017 classification, surgical extent, gross ETE, initial distant metastasis, and TERT promoter mutational status, between the groups. On the other hand, age, surgical extent, gross ETE, initial distant metastasis, TERT promoter mutations, and AJCC/TNM stage were significantly different between groups when patients were divided using the WHO 2017 classification (Table 3).
Table 2.
Clinicopathological characteristics according to primary tumor size
| ≤ 2 cm | > 2 cm and ≤ 4 cm | > 4 cm | p for trend | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 44.3 ± 13.4 | 40.2 ± 15.4 | 44.7 ± 16.4 | 0.794* |
| Sex | ||||
| Female | 14 (93.3) | 35 (81.4) | 18 (62.1) | 0.016 |
| Male | 1 (6.7) | 8 (18.6) | 11 (37.9) | |
| WHO classification | ||||
| MI-FTC | 9 (60.0) | 25 (58.1) | 13 (44.8) | 0.663 |
| EA-FTC | 1 (6.7) | 14 (32.6) | 10 (34.5) | |
| WI-FTC | 5 (33.3) | 4 (9.3) | 6 (20.7) | |
| Surgical extent | ||||
| Total or subtotal thyroidectomy | 9 (60.0) | 29 (67.4) | 22 (75.9) | 0.534 |
| Lobectomy | 6 (40.0) | 14 (32.6) | 7 (24.1) | |
| Gross ETE | ||||
| Absent | 15 (1000) | 42 (97.7) | 27 (93.1) | 0.201 |
| Present | 0 (0.0) | 1 (2.3) | 2 (6.9) | |
| Initial distant metastasis | ||||
| Absent | 13 (86.7) | 40 (93.0) | 26 (89.7) | 0.878 |
| Present | 2 (13.3) | 3 (7.0) | 3 (10.3) | |
| TERT promoter mutations | ||||
| Wild-type | 11 (73.3) | 38 (88.4) | 22 (75.9) | 0.866 |
| Mutation | 4 (26.7) | 5 (11.6) | 7 (24.1) | |
| AJCC/TNM 8th stage | ||||
| I | 13 (86.7) | 40 (93.0) | 21 (72.4) | 0.266 |
| II | 2 (13.3) | 1 (2.3) | 7 (24.1) | |
| III/IV | 0 (0.0) | 2 (4.7) | 1 (3.4) | |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, WHO World Health Organization, MI-FTC minimally invasive FTC, EA-FTC encapsulated angioinvasive FTC, WI-FTC widely invasive FTC, FTC follicular thyroid carcinoma, ETE extrathyroidal extension, TERT telomerase reverse transcriptase, AJCC/TNM American Joint Committee/Tumor-Node-Metastasis
*p for trend for continuous variables was analyzed using the Jonckheere–Terpstra test
Table 3.
Clinicopathological characteristics according to the WHO 2017 classification
| MI-FTC | EA-FTC | WI-FTC | p for trend | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 39.7 ± 13.1 | 42.5 ± 18.4 | 50.8 ± 14.8 | 0.063* |
| Sex | ||||
| Female | 38 (80.9) | 17 (68.0) | 12 (80.0) | 0.651 |
| Male | 9 (19.9) | 8 (32.0) | 3 (20.0) | |
| Size | ||||
| Mean, cm (mean ± SD) | 3.2 ± 1.5 | 4.0 ± 1.7 | 4.9 ± 3.4 | 0.063* |
| ≤ 2 cm | 9 (19.1) | 1 (4.0) | 5 (33.3) | 0.688 |
| > 2 cm and ≤ 4 cm | 25 (53.2) | 14 (56.0) | 4 (26.7) | |
| > 4 cm | 13 (27.7) | 10 (40.0) | 6 (40.0) | |
| Surgical extent | ||||
| Total or subtotal thyroidectomy | 23 (48.9) | 22 (88.0) | 15 (100.0) | < 0.001 |
| Lobectomy | 24 (51.1) | 3 (12.0) | 0 (0.0) | |
| Gross ETE | ||||
| Absent | 47 (100.0) | 24 (96.0) | 13 (86.7) | 0.017 |
| Present | 0 (0.0) | 1 (4.0) | 2 (13.3) | |
| Initial distant metastasis | ||||
| Absent | 47 (100.0) | 23 (92.0) | 9 (60.0) | < 0.001 |
| Present | 0 (0.0) | 2 (8.0) | 6 (40.0) | |
| TERT promoter mutations | ||||
| Wild-type | 42 (89.4) | 20 (80.0) | 9 (60.0) | 0.013 |
| Mutation | 5 (10.6) | 5 (20.0) | 6 (40.0) | |
| AJCC/TNM 8th stage | ||||
| I | 47 (100.0) | 19 (76.0) | 8 (53.3) | < 0.001 |
| II | 0 (0.0) | 4 (16.0) | 6 (40.0) | |
| III/IV | 0 (0.0) | 2 (8.0) | 1 (6.7) | |
Data are expressed as n (%) unless otherwise specified
SD standard deviation, WHO World Health Organization, MI-FTC minimally invasive FTC, EA-FTC encapsulated angioinvasive FTC, WI-FTC widely invasive FTC, FTC follicular thyroid carcinoma, ETE extrathyroidal extension, TERT telomerase reverse transcriptase, AJCC/TNM American Joint Committee/Tumor-Node-Metastasis
*p for trend for continuous variables was analyzed using the Jonckheere–Terpstra test
Cumulative Risks of Initial Distant Metastasis, Disease Recurrence, and Distant Metastasis
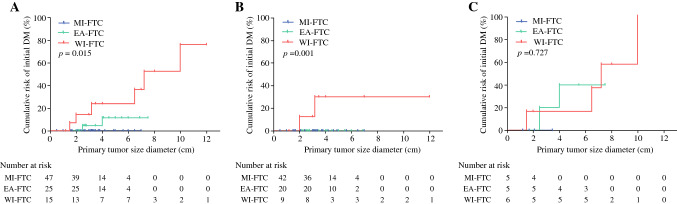
The cumulative risks of initial distant metastasis according to primary tumor size in each WHO 2017 group are shown in Fig. 1. The cumulative risk was significantly higher in the aggressive categories (Fig. 1a). The cumulative risks increased linearly with increasing tumor size in EA-FTC and WI-FTC, but initial distant metastasis was not found in MI-FTC regardless of primary tumor size. Interestingly, when patients were stratified according to TERT promoter mutational status (WT-TERT vs. M-TERT), initial metastasis was not found in EA-FTC with WT-TERT patients regardless of primary tumor size (Fig. 2b). Furthermore, cumulative risk of initial distant metastasis according to primary tumor size was not significantly different between EA-FTC with M-TERT and WI-FTC with M-TERT (Fig. 1c). The threshold diameter for initial distant metastasis in EA-FTC with M-TERT patients was 2 cm.
Fig. 1.
Cumulative risk of initial distant metastasis according to primary tumor size in each WHO 2017 classification. A Overall patients; B patients with wild-type TERT; and (C) patients with mutant TERT. EA-FTC encapsulated angioinvasive FTC, FTC follicular thyroid carcinoma, MI-FTC minimally invasive FTC, TERT telomerase reverse transcriptase, WI-FTC widely invasive FTC
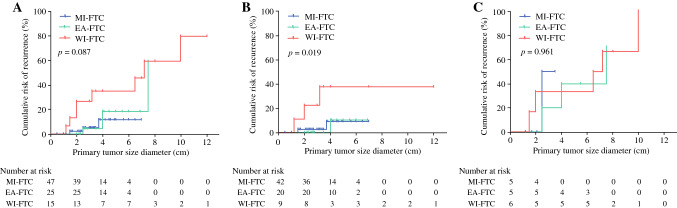
Fig. 2.
Cumulative risk of disease recurrence according to primary tumor size in each WHO 2017 classification. A Overall patients; B patients with wild-type TERT; and C patients with mutant TERT. EA-FTC encapsulated angioinvasive FTC, FTC follicular thyroid carcinoma, MI-FTC minimally invasive FTC, TERT telomerase reverse transcriptase, WI-FTC widely invasive FTC
Figure 2 shows the cumulative risk of disease recurrence according to primary tumor size. The slope of the curve for disease recurrence was steeper in WI-FTC than in EA-FTC and MI-FTC, but the difference was not statistically different (Fig. 2a). However, when patients were stratified according to TERT promoter mutational status, cumulative risk was significantly different according to aggressiveness of the WHO 2017 classification in patients with WT-TERT (Fig. 2b). Hence, cumulative risk of disease recurrence was not different according to the WHO 2017 classification in patients with M-TERT (Fig. 2c). The threshold diameter for disease recurrence in MI-FTC and EA-FTC with M-TERT was 2 cm, while that in MI-FTC with WT-TERT and EA-FTC with WT-TERT was 4 cm. We further discuss this in the Discussion section using two MI-FTC patients with WT-TERT who experienced disease recurrence despite primary tumor size < 2 cm.
Similar trends were shown in curves for cumulative risk of CSS. Cumulative risk of CSS was not significantly different between the WHO 2017 classifications (electronic supplementary Fig. 1a), but when patients were stratified using TERT promoter mutational status, the cumulative risk of CSS was significantly different between three WHO 2017 groups (electronic supplementary Fig. 1b). CSS was not different in patients with M-TERT (electronic supplementary Fig. 1c). The threshold diameter for CSS was 2 cm in EA-FTC with M-TERT, but 4 cm in MI-FTC with WT-TERT and EA-FTC with WT-TERT.
Prognostic Outcomes According to Tumor Diameter and TERT Promoter Mutations
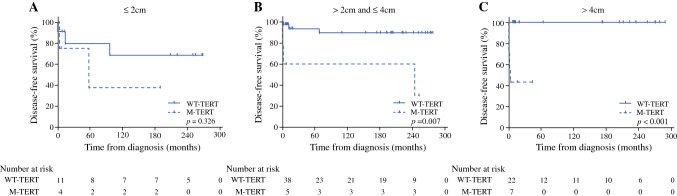
DFS according to TERT promoter mutational status in three primary tumor size categories is shown in Fig. 3. TERT promoter mutations were significantly associated with DFS when primary tumor size was larger than 2 cm. Similarly, CSS according to TERT promoter mutational status was significantly different when primary tumor size was larger than 2 cm (electronic supplementary Fig. 2). When primary tumor size was ≤ 2 cm, DFS and CSS were not different according to TERT promoter mutational status.
Fig. 3.
Disease-free survival according to the presence of TERT promoter mutations in three primary tumor size categories. A ≤ 2 cm; B > 2.0 cm but ≤ 4.0 cm; and C > 4 cm. M-TERT mutant TERT, TERT telomerase reverse transcriptase, WT-TERT wild-type TERT
Discussion
The purpose of this study was to assess whether TERT promoter mutation, an emerging molecular prognostic marker, can be useful to clarify proper candidates for completion thyroidectomy. During a mean follow-up period of 10.6 years, we found that the WHO 2017 classification was significantly associated with oncologic outcomes only in patients with WT-TERT. The thresholds of primary tumor diameter for initial distant metastasis, disease recurrence, and cancer-specific death were smaller according to tumor aggressiveness in the WHO 2017 classification. In patients with M-TERT, disease recurrence and cancer-specific death occurred at a much smaller tumor size and there was no significant difference between the WHO 2017 classifications. In addition, initial distant metastasis did not occur in EA-FTC with WT-TERT but did occur in EA-FTC with M-TERT. Based on these results, we suggest 2 cm as a critical threshold diameter for performance of completion thyroidectomy in MI-FTC with M-TERT and EA-FTC with M-TERT. In addition, 4 cm may be a critical threshold diameter in MI-FTC with WT-TERT and EA-FTC with WT-TERT. Patients with WI-FTC will need completion thyroidectomy regardless of primary tumor diameter and TERT promoter mutational status.
Selection of candidates for completion thyroidectomy has been controversial over time. Recent recommendations from the British Thyroid Association (BTA), European Society of Endocrine Surgeons (ESES), and German Association of Endocrine Surgeons (CAEK) are total thyroidectomy for patients diagnosed with EA-FTC and WI-FTC. However, ATA guidelines suggest completion thyroidectomy when EA-FTC patients had high risk (> 4 vessels with angioinvasion) of structural disease recurrence. The ATA does not recommend completion thyroidectomy in low-risk patients compared with other guidelines.5, 28–30
In the present study, initial distant metastasis, disease recurrence, and cancer-specific death occurred regardless of TERT promoter mutational status when the primary tumor diameter was < 2 cm in WI-FTC. Furthermore, disease recurrence and cancer-specific death occurred when the primary tumor diameter was > 4 cm even though the pathological diagnosis was EA-FTC or MI-FTC. These findings support current guidelines that completion thyroidectomy will be needed when the primary tumor diameter is > 4 cm or in patients with WI-FTC. Moreover, in real-world practice, entire histological examination of all specimens is practically impossible. Thus, the possibility of a small poorly differentiated component in a large tumor cannot be completely eliminated.31 Considering the cost of a labor-intensive evaluation to completely exclude any tiny poorly differentiated component in a large tumor, a size criterion of 4 cm is acceptable.
The 8th AJCC/TNM staging suggests that primary tumor size ≤ 2 cm be classified as T1 and that primary tumor size > 2 cm and ≤ 4 cm be classified as T2.32 Furthermore, Machens et al. reported that the critical threshold for distant metastasis is > 2 cm in differentiated thyroid carcinoma patients.33 This study showed that initial distant metastasis, disease recurrence, and death occurred at 2–4 cm in MI-FTC and EA-FTC when a TERT promoter mutation was present. Based on these results, completion thyroidectomy followed by radioiodine remnant ablation or adjuvant therapy might be needed when a TERT promoter mutation is found at 2–4 cm in size in MI-FTC and EA-FTC patients. However, disease recurrence and death occurred in only one patient in 2–4 cm-sized MI-FTC and EA-FTC when TERT promoter mutations were not present. Although a 3.7 cm MI-FTC with WT-TERT patient died of FTC according to KOSIS information, detailed information about the clinical course was not available because this patient transferred out during follow-up. Current guidelines recommend completion thyroidectomy in EA-FTC;28–30 however, EA-FTC with WT-TERT seems to be low risk. Poor prognosis was seen only in EA-FTC with M-TERT, while prognosis of EA-FTC with WT-TERT was similar to that of MI-FTC.22 Thus, lobectomy might be sufficient in 2–4 cm-sized MI-FTC and EA-FTC when TERT promoter mutations are not present.
Additionally, TERT promoter mutations tend to be found in larger tumors,34 while the clinical implication of TERT promoter mutations in small tumors (T1 tumor) is unclear. In this study, DFS and CSS according to the presence of TERT promoter mutation were significantly different when the primary tumor diameter was > 2 cm. When the primary tumor diameter is < 2 cm, lobectomy might be sufficient for MI-FTC and EA-FTC regardless of TERT promoter mutational status. Among the MI-FTC and EA-FTC patients with primary tumor diameter < 2 cm, only one experienced disease recurrence. In this patient, the primary tumor diameter was 1.5 cm and initial diagnosis was MI-FTC with WT-TERT. Pathological diagnosis at the recurred metastatic site was FTC with poorly differentiated components. Thus, it seems that FTC transformed into PDTC during follow-up. Thus, with no transformation into PDTC, lobectomy might be sufficient in patients with MI-FTC and EA-FTC with primary tumor ≤ 2 cm. However, as relatively small numbers of MI-FTC and EA-FTC patients were classified into primary tumor size ≤ 2 cm with M-TERT, external validation of this result is needed. Considering the poor prognostic outcome when a TERT promoter mutation is present, we recommend close monitoring in such patients after lobectomy.
A prognostic role of vascular invasion has been emphasized in patients with FTC.12, 13, 15, 16, 35 Furthermore, previous studies reported that patients with limited invasion of vessels have a better prognosis than those with extensive vascular invasion.15, 16 Thus, ATA 2015 guidelines suggest that more than four foci of vascular invasion be considered high risk and fewer than four foci of vascular invasion be considered low risk; however, this proposal has not been sufficiently validated.5 Furthermore, the role of an experienced endocrine pathologist is important to correctly evaluate the extent of vessel invasion, however it is difficult to retain an experienced specialist in every hospital. Thus, the clinical implication of vascular invasion extent is limited.
There is consensus that total thyroidectomy is necessary for high-risk patients, such as WI-FTC or primary tumor size ≥ 4 cm. However, consideration should be given to the need for completion thyroidectomy among low- to intermediate-risk patients. Based on previous reports, patients with the presence of ETE and/or presence of regional lymph node metastasis are considered candidates for completion thyroidectomy. However, the suggestions for candidates for completion thyroidectomy were mainly based on PTC patients.7, 9–11 Unlike PTC, FTC is a unifocal disease and is less likely to have direct tumor extension or regional lymph node metastasis.36 Based on this study, we suggest primary tumor size and TERT promoter mutational status are important criteria for completion thyroidectomy (Fig. 4). Furthermore, assays for TERT promoter mutations on preoperative fine-needle aspiration biopsies are currently available. Combining the findings of this study with preoperative TERT promoter mutation results can assist in the decision regarding surgical extent preoperatively. For instance, if TERT promoter mutational status is available preoperatively, total thyroidectomy should be offered to patients for whom completion thyroidectomy would have been recommended.
Fig. 4.
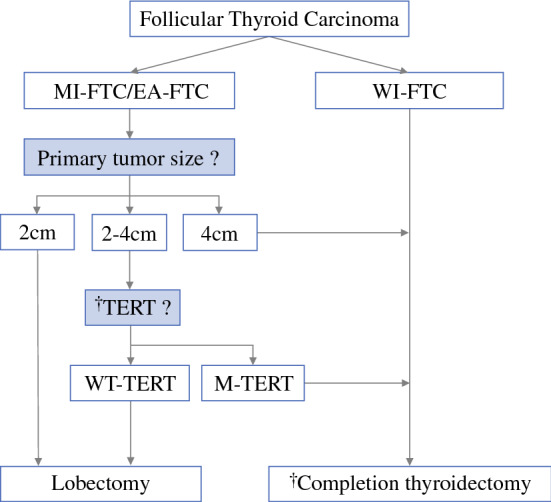
Proposal of a candidate for completion thyroidectomy. EA-FTC encapsulated angioinvasive FTC, FTC follicular thyroid carcinoma, M-TERT mutant TERT, MI-FTC minimally invasive FTC, TERT telomerase reverse transcriptase, WI-FTC widely invasive FTC, WT TERT wild-type TERT
This study has several limitations. First, the study had a retrospective nature and involved a relatively small number of FTC patients due to its low prevalence in Korea, which is an iodine-sufficient area. Of 87 patients, 16 had TERT promoter mutations. Thus, further studies are required using a larger cohort to obtain more solid evidence to corroborate the results of this study. Second, we could not investigate other promising genetic markers37 that TERT promoter mutational status because they could not be obtained in this cohort. Third, this study was conducted in a single tertiary referral hospital, thus there is a possibility of selection bias.
Conclusion
In summary, primary tumor size and TERT promoter mutational status can be important criteria for selecting candidates for completion thyroidectomy. In 2–4 cm MI-FTC and EA-FTC patients, completion thyroidectomy should be considered when a TERT promoter mutation is present. Hence, lobectomy might be sufficient in 2–4 cm MI-FTC and EA-FTC when no TERT promoter mutation is present.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgment
The abstract of this study was posted as a late-breaking abstract in the ENDO 2022 Annual Conference, held on 11–14 June 2022 in Atlanta, GA, USA.
Author Contributions
HP: Conceptualization (equal); writing – original draft preparation (lead); writing – review and editing (equal); formal analysis (lead). JH: Data curation (equal); methodology (supporting). C-SK: Data curation (supporting); resources (equal). JHS: Investigation (supporting); resources (equal). YLO: Investigation (supporting); resources (equal). YIS: Investigation (supporting); resources (equal). JSK: Investigation (supporting); resources (equal). SWK: Conceptualization (supporting); supervision (supporting). JHC: Conceptualization (supporting); supervision (supporting). TYK: Conceptualization (supporting); supervision (supporting). THK: Conceptualization (lead); supervision (lead); writing – review and editing (lead). J-HK: Conceptualization (lead); supervision (lead).
Funding
This research did not receive any specific grants from any funding agency in the public, commercial, or not-for-profit sector.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Disclosures
Hyunju Park, Jung Heo, Chang-Seok Ki, Jung Hee Shin, Young Lyun Oh, Young Ik Son, Jee Soo Kim, Sun Wook Kim, Jae Hoon Chung, Tae Yong Kim, Tae Hyuk Kim, and Jung-Han Kim declare that there are no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tae Hyuk Kim and Jung-Han Kim contributed equally to this work.
Contributor Information
Tae Hyuk Kim, Email: taehyukmd.kim@samsung.com.
Jung-Han Kim, Email: jinnee.kim@samsung.com.
References
- 1.Sobrinho-Simoes M, Eloy C, Magalhaes J, Lobo C, Amaro T. Follicular thyroid carcinoma. Mod Pathol. 2011;24(Suppl 2):S10–S18. doi: 10.1038/modpathol.2010.133. [DOI] [PubMed] [Google Scholar]
- 2.LiVolsi VA, Baloch ZW. Follicular neoplasms of the thyroid: view, biases, and experiences. Adv Anat Pathol. 2004;11(6):279–287. doi: 10.1097/01.pap.0000138143.34505.02. [DOI] [PubMed] [Google Scholar]
- 3.LiVolsi VA. Papillary thyroid carcinoma: an update. Mod Pathol. 2011;24(Suppl 2):S1–9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 4.Staubitz JI, Musholt PB, Musholt TJ. The surgical dilemma of primary surgery for follicular thyroid neoplasms. Best Pract Res Clin Endocrinol Metab. 2019;33(4):101292. doi: 10.1016/j.beem.2019.101292. [DOI] [PubMed] [Google Scholar]
- 5.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144(6):1070–7. doi: 10.1016/j.surg.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzu K, Sugino K, Masudo K, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014;38(1):68–79. doi: 10.1007/s00268-013-2224-1. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246(3):375–81. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant CS, Hay ID, Gough IR, Bergstralh EJ, Goellner JR, McConahey WM. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988;104(6):954–962. [PubMed] [Google Scholar]
- 11.Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124(6):958–64. doi: 10.1016/S0039-6060(98)70035-2. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol. 2011;37(2):181–185. doi: 10.1016/j.ejso.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Sung JY, Oh YL, et al. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head Neck. 2014;36(12):1695–1700. doi: 10.1002/hed.23511. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. WHO Classification of Tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
- 15.Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: a clinicopathologic study of 276 cases. Hum Pathol. 2015;46(12):1789–1798. doi: 10.1016/j.humpath.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito Y, Hirokawa M, Masuoka H, et al. Prognostic factors of minimally invasive follicular thyroid carcinoma: extensive vascular invasion significantly affects patient prognosis. Endocr J. 2013;60(5):637–642. doi: 10.1507/endocrj.EJ12-0419. [DOI] [PubMed] [Google Scholar]
- 17.Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98(9):E1562–E1566. doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20(4):603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99(5):E754–E765. doi: 10.1210/jc.2013-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TH, Kim YE, Ahn S, et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer. 2016;23(10):813–823. doi: 10.1530/ERC-16-0219. [DOI] [PubMed] [Google Scholar]
- 21.Park J, Lee S, Kim K, et al. TERT Promoter Mutations and the 8th Edition TNM Classification in Predicting the Survival of Thyroid Cancer Patients. Cancers (Basel). 2021;13(4):648. [DOI] [PMC free article] [PubMed]
- 22.Park H, Shin HC, Yang H, et al. Molecular classification of follicular thyroid carcinoma based on TERT promoter mutations. Mod Pathol. 2022;35(2):186–192. doi: 10.1038/s41379-021-00907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156(19):2165–2172. doi: 10.1001/archinte.1996.00440180017002. [DOI] [PubMed] [Google Scholar]
- 24.Yi KH, Park YJ, Koong SS, et al. Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2010;3(2):65–96. [Google Scholar]
- 25.Yi KH, Lee EK, Kang HC, et al. 2016 Revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016;9(2):59–126. doi: 10.11106/ijt.2016.9.2.59. [DOI] [Google Scholar]
- 26.Sohn SY, Park WY, Shin HT, et al. Highly concordant key genetic alterations in primary tumors and matched distant metastases in differentiated thyroid cancer. Thyroid. 2016;26(5):672–682. doi: 10.1089/thy.2015.0527. [DOI] [PubMed] [Google Scholar]
- 27.Hennekens CH, Buring JE. Epidemiology in Medicine. Boston/Toronto: Little Brown and Company; 1987.
- 28.Dralle H, Musholt TJ, Schabram J, et al. German Association of Endocrine Surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch Surg. 2013;398(3):347–375. doi: 10.1007/s00423-013-1057-6. [DOI] [PubMed] [Google Scholar]
- 29.Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81(Suppl 1):1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 30.Dionigi G, Kraimps JL, Schmid KW, et al. Minimally invasive follicular thyroid cancer (MIFTC): a consensus report of the European Society of Endocrine Surgeons (ESES) Langenbecks Arch Surg. 2014;399(2):165–184. doi: 10.1007/s00423-013-1140-z. [DOI] [PubMed] [Google Scholar]
- 31.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31(8):1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid. 2017;27(6):751–756. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–2273. doi: 10.1002/cncr.21055. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: a systematic review and meta-analysis. Endocrine. 2020;67(1):44–57. doi: 10.1007/s12020-019-02117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collini P, Sampietro G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-Hurthle cell follicular carcinoma of the thyroid gland: a clinicopathological study of 18 consecutive cases from a single institution with a 11-year median follow-up. Histopathology. 2004;44(1):35–39. doi: 10.1111/j.1365-2559.2004.01729.x. [DOI] [PubMed] [Google Scholar]
- 36.Chow SM, Law SC, Au SK, et al. Differentiated thyroid carcinoma: comparison between papillary and follicular carcinoma in a single institute. Head Neck. 2002;24(7):670–677. doi: 10.1002/hed.10080. [DOI] [PubMed] [Google Scholar]
- 37.Yip L, Gooding WE, Nikitski A, et al. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: a matched case-control study. Cancer. 2021;127(11):1779–1787. doi: 10.1002/cncr.33421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.