Background:
Acute pericarditis/myocarditis is a rare complication of the mRNA-based vaccines and although mostly self-limiting, long-term sequelae remain unclear.
Methods:
We enrolled all patients admitted to the emergency department between September 2021 and February 2022 meeting the CDC work case definition, with symptoms onset after mRNA-based COVID-19 vaccine. Alternative virologic causes were excluded. Clinical data, laboratory values, cardiologic evaluation, electrocardiogram (ECG), and echocardiogram (ECHO) were collected on admission, at discharge, and during follow-up in all patients. Cardiac Magnetic Resonance (CMR) was performed only in those with signs consistent with myocarditis.
Results:
We observed 13 patients (11M and 2F), median age 15 years, affected by acute pericarditis/myocarditis after COVID-19 mRNA vaccination (11 after Comirnaty® and 2 after Spikevax®). Symptoms’onset occurred at a median of 5 days (range, 1 to 41 days) after receiving mRNA vaccine (13 Prizer 2 Moderna): 4 patients (31%) after the 1st dose, 6 (46%) after the 2nd, and 3 (23%) after 3rd dose. Increased levels of high-sensitive troponin T (hsTnT) (median 519,5 ng/mL) and N-terminal-pro hormone BNP (NT-proBNP) (median 268 pg/mL) and pathognomonic ECG and ECHO abnormalities were detected. On admission, 7 of 13 (54%) presented with myopericarditis, 3 (23%) with myocarditis, and 3 (23%) with pericarditis; CMR was performed in 5 patients upon pediatric cardiologist prescription and findings were consistent with myocarditis. At 12 weeks of follow-up, all but one patient (92%), still presenting mild pericardial effusion at ECHO, were asymptomatic with normal hsTnT and NT-proBNP levels and ECG. On CMR 6 of 9 patients showed persistent, although decreased, myocardial injury. Higher hsTnT levels on admission significantly correlated with persistent CMR lesions.
Conclusion:
Evidence of persistent CMR lesions highlights the need for a close and standardized follow-up for those patients who present high hsTnT levels on admission.
Keywords: children, myocarditis, mRNA vaccines, COVID-19
INTRODUCTION
Safe and effective COVID-19 vaccines are a powerful tool for ensuring public health and controlling the SARS-CoV-2 infection. The global vaccine campaigns have indeed drastically changed the course of the pandemic.1
In May 2021, the European Medicines Agency (EMA) recommended an extension of indication for the BNT162b2 mRNA vaccine (Comirnaty®) to include use in adolescents between 12 and 15 years of age. Subsequently, in July 2021, EMA extended the indication for the mRNA-1273 vaccine (Spikevax®) for use in children 12 to 17 years old.2
Both Comirnaty® and Spikevax® vaccines are mRNA vaccines encoding the stabilized prefusion spike glycoprotein of SARS-CoV-2.
A study in adolescents demonstrated as the BNT162b2 mRNA vaccine was highly effective against COVID-19 hospitalization and critical illness, including among patients with underlying risk factors for severe disease, regardless of SARS-CoV-2 variants.3
Despite the undeniable public health benefits of COVID-19 vaccines, they also could result in potential adverse events. Pericarditis and myocarditis have been reported as rare complications after mRNA-based COVID-19 vaccination in adolescents and young adults.4 A recent study on 411 myocarditis after COVID-19 mRNA vaccines, showed an increased risk in men 18–25 years of age, especially after the second dose.5
Although cardiologic symptoms are self-limiting or non-steroidal anti-inflammatory drugs (NSAID) controlled, long-term cardiologic outcomes have not yet been fully investigated
In the present work, we aim to depict the cardiologic findings after COVID-19 vaccination in a pediatric population and to evaluate the short follow-up sequalae.
METHODS
Population
We performed a prospective evaluation of all patients admitted at the pediatric emergency department of the Children’s Hospital “Bambino Gesù” between September 2021 and February 2022 with chest pain, shortness of breath, or palpitations after COVID-19 vaccines who meeting the CDC work case definition for myocardial injuries. Accordingly, clinical cases have been categorized as probable myocarditis, confirmed myocarditis, or acute pericarditis.6 Only confirmed cases were enrolled in this study. The Institutional Ethical Committee approved the present study named “CACTUS” (2083_OPBG_2020 amendment). All patients’ parents or legal guardians enrolled in the study signed the informed consent.
Data Collection and Definitions
Clinical data including demographic characteristics, type of vaccination, previous SARS-CoV2 history, past cardiologic history, laboratory values, cardiologic evaluation, imaging, and a full range of clinical symptoms and signs were collected.
Pericarditis was defined as chest pain in association with electrocardiographic (ECG) abnormalities and/or positive ultrasound showing pericardial effusion. Myocarditis was defined as chest pain in association with elevated troponin levels above the upper limit of normal.6 The evidence of characteristics of both manifestations was defined as myopericarditis.7 ECG criteria to define pericarditis including widespread concave ST elevation and PR depression, reciprocal ST depression and PR elevation in lead aVR (±V1), sinus tachycardia and low voltages. ECG abnormalities to identify myocarditis were nonspecific ST-T–wave changes, T-wave inversion, low-voltage QRS complexes in the limb leads, and atrioventricular conduction delays.8
Echocardiography findings related to myocarditis included changes in global left ventricular (LV) or right ventricular systolic function, variable degrees of LV enlargement, thickened myocardium from wall edema, pericardial effusion, intracardiac thrombus, functional valvar regurgitation.9
Echocardiographic examination was performed with standard echocardiographic machines (Epic cVx model Philips Medical System, Andover, MA), and images were captured with a 2/3-dimensional probe X-5. Images were digitized, and measurements were taken offline according to the guidelines of the American Society of Echocardiography. We detected conventional parameters from the parasternal long-axis window over three consecutive M-mode cycles. The presence of pericardial effusion was analyzed as a categorical variable, while quantification of PE (mild, moderate, and severe) was performed in a semi-quantitative fashion by the mean diastolic dimension measured in diastole in parasternal views.
The 99th percentile cut-off point for high-sensitive troponin T (hsTnT) detectable through ECLIA method is defined as 0.014 ng/mL (14 pg/ml).10 hsTnT was measured on admission, at discharge, and during outpatient follow-up. Cardiac evaluation for all patients included an electrocardiogram (ECG) and echocardiogram (ECHO) that were performed on admission, at discharge, and during outpatient follow-up.
For patients who met the criteria for myocarditis or myopericarditis6 cardiac magnetic resonance (CMR) 1.5 Tesla was performed on admission upon pediatric cardiologist prescription and planned during outpatient follow-up on week 12.
The contrast agent used was gadoterate meglumine 0.3 mL/kg (0.15 mmol/kg). We used the pre and postcontrast Modified Look-Locker Inversion Recovery 5(3)3 (MOLLI) sequence to calculate Native T1 myocardial values and extracellular volume (ECV). The CMR study included cine images of LV/RV long-axis views and a cine short-axis stack covering the entire heart to calculate ventricular volumes and global systolic function. The same planes have been applied to T2 weighted images and postcontrast late gadolinium enhancement images.
Ventricular systolic dysfunction was defined as a left ventricular (LV) ejection fraction equal to or lower than 50% on echocardiogram and/or CMR results.
Markers of heart inflammation by CMR were defined according to the Lake Louise criteria11 and included high signal intensity on T2-weighted imaging, increased T2 and T times denoting extracellular volume (ECV) fraction, and myocardial thickening attributable to edema as a sign of hyperemia. Measures of necrosis included contrast retention 10 to 15 minutes after injection of gadolinium (late gadolinium enhancement, LGE), indicating either acute myocardial injury or scar formation.12
Statistical Analysis
Quantitative variables shown in the clinical table were described as median, range, and percentages. To compare the hsTnT, NT-proBNP, and CRP values between CMR positive and CMR negative, we performed statistical analyses using the non-parametric Wilcoxon-Mann-Whitney test with a significance threshold set at P < 0.05. Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Thirteen patients were admitted to the Bambino Gesù Children Hospital in Rome for the management of acute pericarditis and/or myocarditis after mRNA-based COVID-19 vaccines between September 2021 and February 2022. The median age was 15 years (range, 12 to 17 years) and 85% of them were male (Table 1). All patients but one had no history of cardiac disease. Indeed, one patient was previously followed for asymptomatic ventricular monomorphic extrasystoles occasionally observed during a stress test and he was out of therapy. The majority of patients received the BNT162b2 vaccine (85%) and most of the symptoms occurred after the second dose of this vaccine (Table 1).
TABLE 1.
Characteristic of Patients With Pericarditis and/or Myocarditis After mRNA-Based COVID-19 Vaccine
| Clinical characteristics, laboratory and cardiac findings | On admission | At discharge | Outpatient follow-up |
|---|---|---|---|
| Age, y, Median (range) | 15 (12–17) | 15 (12–17) | 16 (13–17) |
| Sex, M/F, No. (%) | 11/2 (85/15) | 11/2 (85/15) | 10/2 (84/16) |
| Onset of symptoms after vaccine, days in median (range) | 5 (1–41) | 5 (1–41) | 5 (1–41) |
| BNT162b2 mRNA vaccine (Comirnaty®) (%) | 11 (85) | 11 (85) | 10 (84) |
| mRNA-1273 vaccine (Spikevax®) (%) | 2 (15) | 2 (15) | 2 (16) |
| BNT162b2 mRNA vaccine (Comirnaty®) 1st dose, No. (%) | 3 (23) | 3 (23) | 3 (23) |
| BNT162b2 mRNA vaccine (Comirnaty®) 2nd dose, No. (%) | 5 (38) | 5 (38) | 5 (38) |
| BNT162b2 mRNA vaccine (Comirnaty®) 3rd dose, No. (%) | 3 (23) | 3 (23) | 3 (23) |
| mRNA-1273 vaccine (Spikevax®) 1st dose, No. (%) | 1 (8) | 1 (8) | 1 (8) |
| mRNA-1273 vaccine (Spikevax®) 2nd dose, No. (%) | 1 (8) | 1 (8) | 1 (8) |
| mRNA-1273 vaccine (Spikevax®) 3rd dose, No. (%) | 0 | 0 | 0 |
| Duration of symptoms, d. Median (range) | 3 (1–22) | 3 (1–22) | 3 (1–22) |
| Chest pain, No. (%) | 13 (100) | 0 (0) | 0 (0) |
| Fever, No. (%) | 5 (38) | 0 (0) | 0 (0) |
| Headache, No (%) | 2 (15) | 0 (0) | 0 (0) |
| Syncope, No (%) | 1 (8) | 0 (0) | 0 (0) |
| Palpitation, No (%) | 1 (8) | 0 (0) | 1 (8) |
| NSAID treatment, No. (%) | 11 (85) | 11 (85) | 0 (0) |
| Hospital length of stay, d. Median (range) | - | 9 (2–27) | - |
| Days of follow-up | - | - | 101 (88 to128) |
| CRP, mg/mL (normal <0,5). Median (range) | 1.16 (0,07–6,69) | 0,16 (0,04–1,31) | 0,1 (0,03–0,69) |
| Patients with elevated hsTnT and proBNP levels, No. (%) | 10 (77) | 0 (0) | 0 (0) |
| hsTnT, ng/mL (NV NV <14). Median (range) | 519,5 (168–1428) | 11 (6,3–26,9) | 4,5 (3–9,9) |
| NT-proBNP, pg/mL (NV <217). Median (range) | 268 (28,12–1591) | 85,33 (20,9–190) | 13,15 (5–23,2) |
| CRP of patients with elevated hsTNT and proBNP, mg/mL (normal <0,5). Median (range) | 1,76 (0,07– 6,69) | 0,26 (0,04–1,31) | 0,1 (0,03–0,61) |
| Time–normalization of cardiac enzymes, d. Median (range) | 6 (3–20) | – | – |
| Patients performed ECG, No. (%) | 13 (100) | 13 (100) | 12 (92%) |
| ST-segment elevation, No. (%) | 6 (46) | 0 (0) | 0 (0) |
| ST-segment depression, No. (%) | 2 (15) | 0 (0) | 0 (0) |
| Nonspecific ST-segment changes, No. (%) | 1 (8) | 1(8) | 0 (0) |
| T-wave inversion, No. (%) | 1 (8) | 0 (0) | 0 (0) |
| Patients performed Echocardiogram, No. (%) | 13 (100) | 13 (100) | 12 (92%) |
| Pericardial effusion, No (%) | 9 (69) | 2 (15) | 1 (8) |
| Pericardial hyperechogenicity, No. (%) | 3 (23) | 1(8) | 0 (0) |
| Decreased LV EF, No. (%) | 1 (8) | 0 (0) | 0 (0) |
| Patients performed Cardiac MRI, No. (%) (% calculated on patients with sign of myocarditis/myopericarditis at presentation = 10) | 5 (50) | – | 9 (90) |
| LV EF %. Median (range) | 70,6 (69,2–73,1) | – | 58,3 (55,9–65) |
| Decreased LV EF, No. (%) | 0 (0) | – | 0 (0) |
| Regional hyperintensity on T2-weighted imaging, No. (%) | 5 (100) | – | 1 (11) |
| Late gadolinium enhancement, No. (%) | 5 (100) | – | 6 (66) |
In the table the % of the clinic characteristcs, the laboratory values, the ECG, and the echocardiogram are calculated on the total of patients (13 at the time of admission and discharge and 12 during the outpatient follow-up). The % of the CMR are calculated on the total of patients who underwent CMR (5 at the time of admission and 9 during outpatient follow-up).
CMR, cardiac magnetic resonance; CRP, C-reactive protein; ECG, electrocardiogram; hsTnT, high-sensitive troponin T; NT-proBNP, N-terminal-pro hormone BNP; LVEF, left ventricular ejection fraction.
All patients but one, who experienced SARS-CoV-2 infection with molecular positive swab 8 months before the vaccination, had no history of infection and resulted negative for SARS-CoV2 nucleocapsid protein antibodies on admission. Alternative virologic causes were ruled out through serology and PCR for HHV6, HHV7, HHV8, adenovirus, parvovirus-B19, enterovirus, EBV, and CMV.
Symptoms’ onset occurred at median of 5 days (range, 1 to 41 days) after receiving any dose of vaccine and lasted 1 to 22 days. Four patients (31%) presented symptoms after any first dose vaccination and the symptoms onset was at median of 16 days. 6 patients (46%) showed symptoms after any second dose vaccination with the onset of symptoms at median of 3 days. 3 patients (23%) reported symptoms the day after the Comirnaty®’s vaccine III dose. All patients presented with chest pain. Other symptoms included fever (38%), headache (15%), syncope (8%), and palpitation (8%). Most patients (85%) were treated with ibuprofen 10 mg/kg and none of them received immunomodulatory treatments. No patients required intensive care. The hospital length of stay was at median 9 days (range, 2–27 days) (Table 1).
On admission, C-Reactive Protein (CRP) was mild elevated (median 1.16 mg/mL), hsTnT levels were elevated in 10 patients (77%) (median 51 9,5 pg/mL) as well as the levels of N-terminal-pro hormone BNP (NT-proBNP) (median 268 pg/mL). ECG records presented ST-segment elevation in 6 of 13 patients (46%), 2 patients (15%) had ST-segment depression and 1 patient (8%) showed T-wave inversion, while nonspecific ST-segment changes were observed in the remaining group of patients.
The most frequent finding at echocardiogram was pericardial effusion present in nine patients (69%). Three patients (23%) had pericardial hyperechogenicity and one patient (8%) showed global LV systolic ventricular dysfunction (EF 45%).
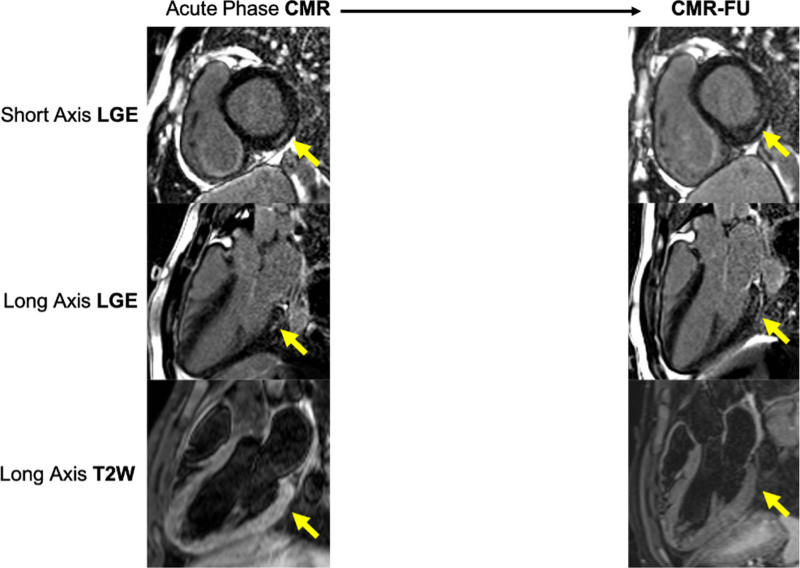
In those patients where CMR was available (n=5), hyperintensity on T2-weighted imaging and LGE were always evident in the inferior and inferolateral regions. In one patient was found an extensive involvement of the left ventricle (mid-ventricular septum, infero- and inferolateral mid-basal wall, and apex). The LV systolic function was normal in all patients (100%) with median ejection fraction of 70,6 % (Table 1, Figure 1).
FIGURE 1.
Comparison between the acute phase magnetic resonance and follow-up in one patient. CMR, cardiac magnetic resonance; CMR-FU, cardiac magnetic resonance follow-up; LGE, late gadolinium enhancement; T2W, T2-weighted images.
At discharge PCR resulted negative in all patients; in addition, cardiac enzymes decreased to normal levels after a median of 6 days (range, 3–20 days). All EGCs, but one, were normal, whereas 2 of 13 patients (15%) still presented mild signs of cardiac involvement detectable by ultrasound, such as mild pericardial effusion and pericardial hyperechogenicity (Table 1).
Follow-up evaluation after hospitalization was available for 12 of 13 patients and occurred at median of 101 days (range, 88 to 128 days). Many patients (92%) were asymptomatic with normal hsTnT and NT-proBNP levels. One patient was still referred persistent palpitation and chest pain. Further examinations in this case, including endocavitary electrophysiologic study (SEE). revealed an atrioventricular nodal reentrant tachycardia, requiring treatment with Flecainide, probably present before the myocarditis episode. One of the two patients discharged with positive echocardiography, still presented mild pericardial effusion on ECHO, without any ECG changes (Table 1).
CMR follow-up was performed in all patients with consistent signs of myocarditis on admission. Nine of 10 patients with presenting signs of myocardial involvement received a cardio-MRI during the follow-up. One patient did not perform the examination since his legal guardian withdrew the consent refusing further investigation.
Of the nine patients, seven showed CMR imaging abnormalities. LGE was present in seven patients in inferior, inferolateral, and anterolateral regions and two patients (17%) had regional hyperintensity on T2-weighted imaging. LV ejection fraction was normal, with a median of 58,3% (Table 1, Figure 1).
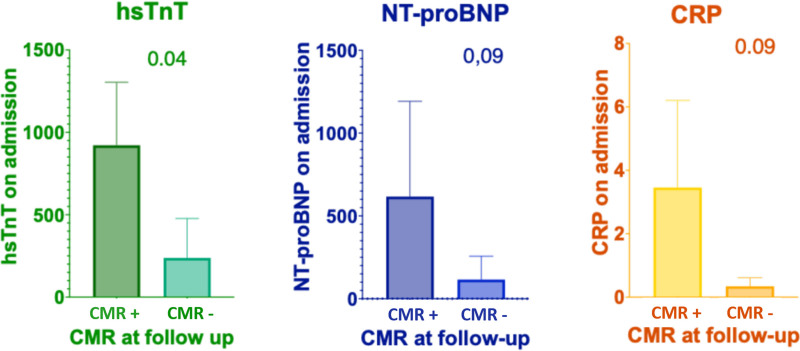
Higher levels of hsTnT on admission significantly correlated with persistent myocardial lesions, both LGE and regional hyperintensity on T2-weighted imaging, detectable trough CMR, performed at follow-up (P value < 0.05) with a log2 fold change of 1,9 (Figure 2). Conversely, no significant correlation was observed between NT-proBNP values, as well as CRP levels, and the presence of myocardial lesions (Figure 2).
FIGURE 2.
Correlation with hsTnT, NT-proBNP, and CRP values at admission and CMR results at 3-month follow-up. CMR+, positive cardiac magnetic resonance, CMR−, negative cardiac magnetic resonance; CRP, C-reactive protein; hsTnT, high-sensitive troponin T; NT-proBNP, N-terminal-pro hormone BNP.
DISCUSSION
Myocarditis and/or pericarditis related to mRNA-based COVID1-9 vaccination are rare adverse events. These reactions occur more often in male adolescents and young adults (<30 years of age) and mostly after a second dose.13
Since April 2021, increasing cases of myocarditis and pericarditis14,15 have been reported, but poorly in childhood.
Following these notifications, ACIP and international regulatory agencies rapidly arranged a regular meeting to review reported cases of myocarditis and pericarditis and discussed the benefits and risks of these kinds of vaccinations, particularly in children. It has been established that the benefits of using vaccines outweigh the risks in all populations, including adolescents and young adults and it has been recommended to monitor the adverse events and possible outcomes of myocardial injuries.16
However, definitive international guidelines to manage myocarditis due to mRNA-based COVID-19 vaccine in the acute phase and during the follow-up are unknown.
A very recent study describes five adolescents with myocarditis COVID-19-vaccine-related and subsequent three months follow-up. The CMR revealed persistent myocardium injury features although clinical recovery.15 These data are consistent with our results reporting CMR alterations of myocardial tissue detectable after 12 weeks of follow-up, despite normalized hsTnT levels and ECG or echocardiographic abnormalities.
In addition to the above study, our data showed a correlation between the initial troponin values and CMR findings. Higher troponin levels have been previously associated with poor outcomes17 in postinfective/vaccine myocarditis in pre-COVID-19-era. In adult patients, elevations of hsTnT and CRP were demonstrated as useful tools in the diagnostic approach to detect suspected myocardial inflammation.18
Several theories on the mechanisms underlying the inflammation induced by mRNA-based COVID-19 vaccines have been hypothesized. Among these, the activation of aberrant innate and acquired immune response to mRNA and molecular mimicry between the spike protein of SARS-CoV-2 and cardiac self-antigens have been proposed.19
The finding of persistent cardiac lesions in a group of patients, albeit limited by the sample size, could suggest a role for a proper immunomodulatory treatment such as corticosteroids and/or intravenous immunoglobulin (IVIG) at symptoms’ onset in those patients with higher troponin levels. However, the most recent guidelines regarding the management of myocarditis in children do not include the use of any immunotherapy as first-line treatment and these approaches remain center and practitioner-specific12. A similar pattern of myocarditis has just been seen in Multisystemic Inflammatory Syndrome in Children (MIS-C) related to SARS-CoV2 infection.20,21 The combination therapy with IVIG and corticosteroids has been proven successful in patients affected by MIS-C and data published on six months follow-up showed no myocardial edema or fibrosis at CMR performed during recovery.22
A recent study by Dionne et al. analyzed data from outpatient follow-ups in adolescents with myocarditis after the BNT162b2 mRNA vaccine.23 These findings are similar to our cases for clinical presentation and the benign course while the CMR data are difficult to compare because of the different time points of imaging execution. The patients underwent CMR after about 6–13 days from hospital admission and about 50% of them experienced treatment with immunomodulatory drugs. To date, there are still no data available on follow-up for these patients.
Considering the relevance of myocarditis among mRNA vaccines’ related adverse events some countries started to draw up specific indications for the pediatric population.24,25 There are evidence that a longer interval of 8 weeks between the first and second dose of mRNA-based COVID-19 vaccine may be appropriate to further reduce the risk of myocarditis or pericarditis.15,26,27 Additionally, the risk of myocarditis has been reported to be lower for Comirnaty® in comparison to Spikevax® and thus administration of the mRNA-1273 vaccine could be recommended for the primary vaccine series or booster in males under 30 years of age.28 A recent study highlighted as individualized pediatric COVID‐19 vaccination strategies could led to better results in preventing adverse events.29
Finally, our data, along with the other published evidence, outline the need to carry on further investigations to depict the mechanistic insight of such conditions to define clear therapeutic indications.
Furthermore, the identification of a standardized follow-up is necessary to early detect myocardial injury and related complications. In this view higher levels of troponin on admission, suggest a more careful follow-up and potentially different treatment approaches. Additional larger prospective studies are needed to identify the threshold level guiding such personalized strategies.
Footnotes
This work was supported by Bambino Gesù Children’s Hospital ricerca corrente 2020 to NC, ricerca corrente 2019 to PP and by 5x1000 from Bambino Gesù to DA.
There are no conflicts of interest.
Contributor Information
Emma Concetta Manno, Email: emmaconcetta.manno@opbg.net.
Donato Amodio, Email: donato.amodio@opbg.net.
Nicola Cotugno, Email: nicola.cotugno@opbg.net.
Chiara Rossetti, Email: chiara.rossetti@opbg.net.
Carmela Giancotta, Email: carmela.giancotta@opbg.net.
Veronica Santilli, Email: veronica.santilli@opbg.net.
Paola Zangari, Email: paola.zangari@opbg.net.
Gioacchino Andrea Rotulo, Email: gandrea.rotulo@opbg.net.
Alberto Villani, Email: alberto.villani@opbg.net.
Emanuele Giglioni, Email: emanuele.giglioni@opbg.net.
Attilio Turchetta, Email: a.turchetta@opbg.net.
Giulia Cafiero, Email: giulia.cafiero@opbg.net.
Alessio Franceschini, Email: alessio.franceschini@opbg.net.
Marcello Chinali, Email: marcello.chinali@opbg.net.
Ottavia Porzio, Email: ottavia.porzio@opbg.net.
Aurelio Secinaro, Email: aurelio.secinaro@opbg.net.
REFERENCES
- 1.Dye C. The benefits of large scale covid-19 vaccination. BMJ. 2022;377:o867. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency (EMA). Available at: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu. Accessed September 2, 2022.
- 3.Olson SM, Newhams MM, Halasa NB, et al. ; Overcoming Covid-19 Investigators. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med. 2022;386:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong H-L, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. The Lancet. 2022;399:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Clinical considerations: myocarditis and pericarditis after receipt of mRNA COVID-19 vaccines among adolescents and young adults. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. Accessed September 2, 2022.
- 7.Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhamnani S, Fuisz A, Lindsay J. The spectrum of electrocardiographic manifestations of acute myocarditis: an expanded understanding. J Electrocardiol. 2014;47:941–947. [DOI] [PubMed] [Google Scholar]
- 9.Skouri HN, Dec GW, Friedrich MG, et al. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006;48:2085–2093. [DOI] [PubMed] [Google Scholar]
- 10.Xu R-Y, Zhu X-F, Yang Y, et al. High-sensitive cardiac troponin T. J Geriatr Cardiol. 2013;10:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira VM, Schulz-Menger J, Holmvang G, et al. cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 12.Law YM, Lal AK, Chen S, et al. ; American Heart Association Pediatric Heart Failure and Transplantation Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Stroke Council. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144:e123–e135. [DOI] [PubMed] [Google Scholar]
- 13.Wong H-L, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148:e2021052478. [DOI] [PubMed] [Google Scholar]
- 15.Puchalski M, Kamińska H, Bartoszek M, et al. COVID-19-vaccination-induced myocarditis in teenagers: case series with further follow-up. Int J Environ Res Public Health. 2022;19:3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butts RJ, Boyle GJ, Deshpande SR, et al. Characteristics of clinically diagnosed pediatric myocarditis in a contemporary multi-center cohort. Pediatr Cardiol. 2017;38:1175–1182. [DOI] [PubMed] [Google Scholar]
- 18.Schwuchow-Thonke S, Göbel S, Emrich T, et al. Increased C reactive protein, cardiac troponin I and GLS are associated with myocardial inflammation in patients with non-ischemic heart failure. Sci Rep. 2021;11:3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. 2021;73:e13–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capone CA, Misra N, Ganigara M, et al. Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics. 2021;148:e2021050973. [DOI] [PubMed] [Google Scholar]
- 23.Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiology. 2021;6:14461446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Australian Technical Advisory Group on Immunisation (ATAGI), Cardiac Society of Australia and New Zealand (CSANZ), Royal Australian College of General Practitioners (RACGP), et al. Guidance on myocarditis and pericarditis after mRNA COVID-19 vaccines. Available at: https://www.health.gov.au/sites/default/files/documents/2022/05/covid-19-vaccination-guidance-on-myocarditis-and-pericarditis-after-mrna-covid-19-vaccines.pdf. Accessed September 2, 2022.
- 25.National Advisory Committee On Immunization (NACI) . Available at: https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/rapid-response-recommendation-use-covid-19-vaccines-individuals-aged-12-years-older-myocarditis-pericarditis-reported-following-mrna-vaccines/summary.pdf.
- 26.Oliver S. Summary and work group interpretation. extended intervals for mRNA COVID-19 vaccines. Available at: https://stacks.cdc.gov/view/cdc/114165. Accessed September 2, 2022.
- 27.Public Health Agency of Canada. Canadian experience and evidence with COVID-19 vaccine primary series extended intervals. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-04/09-covid-tunis-508.pdf. Accessed September 2, 2022.
- 28.Abraham N, Spruin S, Rossi T, et al. Myocarditis and/or pericarditis risk after mRNA COVID-19 vaccination: a Canadian head to head comparison of BNT162b2 and mRNA-1273 vaccines. SSRN. 2022:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug A, Stevenson J, Høeg TB. BNT162b2 vaccine-associated myo/pericarditis in adolescents: a stratified risk-benefit analysis. Eur J Clin Invest. 2022;52:e13759. [DOI] [PMC free article] [PubMed] [Google Scholar]