Abstract
Adequate coverage of complex, composite scalp defects in previously radiated, infected, or otherwise compromised tissue represents a challenge in reconstructive surgery. To provide wound closure with bony protection to the brain, improve cranial contour, and prevent or seal cerebrospinal fluid (CSF) leaks, composite free tissue transfer is a reliable and safe option. We report our experience with the latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap in the reconstruction of bony and soft tissue defects of the cranium and overlying scalp. The surgical technique, design, and outcomes of the latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap reconstruction in five patients with cranial defects between 2003 and 2007 were retrospectively evaluated. Patient characteristics, defect size, underlying cause, reconstructive details, and complications were analyzed. All patients (age 43 to 81) had composite defects ranging from 36 to 750 cm2 (mean size 230 cm2) for the bony component and from 16 to 400 cm2 (mean size 170 cm2) for the soft tissue defect. All patients had a history of prior or current infection of the affected area, and two patients had a CSF leak. Defects were due to malignancy and infection (n = 2), infiltrative cutaneous mucormycosis with osteomyelitis (n = 1), and hemorrhagic stroke requiring craniectomy (n = 2), complicated by infection and failed cranioplasty in one patient and continuous CSF leak in the other. The latissimus dorsi composite free flap consisting of skin, muscle, and vascularized rib can successfully cover large complex cranial defects, provide skeletal support, improve contour, and significantly enhance functional outcome with limited donor site morbidity.
Keywords: Scalp reconstruction, chimeric free flap reconstruction, latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap
Complex, composite defects of the scalp present a formidable challenge to the reconstructive surgeon. Trauma, infection, or combined-modality treatment of tumors with surgical resection and radiation can lead to tissue compromise, resulting in combined scalp and cranial defects with or without cerebrospinal fluid (CSF) leaks, requiring healthy vascularized tissue from outside the zone of injury (irradiation/infection/trauma). Available reconstructive options are influenced by defect size, the quality of the surrounding tissue, and exposure of vital structures such as dura or brain parenchyma. Radiation, scar, dural violation, and CSF leak usually preclude local flap options1 and, in fact, frequently require acceleration up the “reconstructive elevator”2 to free tissue transfer. Another requirement that is often disregarded in the initial stage of reconstruction is the need for skeletal support.1 This rather controversial issue is particularly important to consider when complex defects lie posterior or lateral to the vertex of the skull. In these situations, external pressure from a recumbent patient with a soft tissue-only reconstruction can theoretically transmit to increased intracranial pressure, resulting in negative neurological sequelae, such as seizuriform activity. Neurological impairment has also been described from external compression from the flap weight itself, especially for bony defects over the frontal lobe,3 as well as from compression of the cortex just from atmospheric pressure,4 the neurological sequelae of which is known as the syndrome of the trephined.5 Symptoms may be related to changes in CSF circulation6 or reduced venous return. One other indication for cranioplasty is the psychological distress and anxiety that manifests in some patients and their families who are worried about the lack of protection of their brain if they would sustain future trauma to the area where the cranium was removed.7
We present our experience utilizing the latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap for soft tissue and structural support in complicated scalp and calvarial defects.
METHODS AND PATIENTS
The surgical technique, design, and outcome of the latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap reconstruction in five patients with cranial defects between 2003 and 2007 at the University of Chicago Medical Center were retrospectively evaluated. Patient characteristics, defect size, underlying cause, reconstructive details, and complications were analyzed. This study was approved by the Institutional Review Board of The University of Chicago.
Surgical Technique
The treatment of all the patients involved a multidisciplinary approach, including debridement of the cranial defect by the neurosurgery team and simultaneous design and harvest of the composite latissimus free flap by the plastic surgery team. After evaluation of the soft tissue and bony defect and preparation of recipient vessels, the flap design was finalized.
The incision was carried down to the anterior border of the latissimus dorsi muscle, and the thoracodorsal vessels were identified. A thoracodorsal perforating vessel supplying a separate skin paddle was included in two patients. A template of the defect was designed to determine the amount of muscle and bone needed. Then the muscle was transected near its origin at the thoracolumbar fascia and the iliac crest elevating it until the intercostal perforators were identified. Depending on the amount of bone needed and its desired location within the flap the 8th, 9th, or 10th rib or a combination of those were visualized, after which the intercostal muscles were incised. The intercostal nerve was isolated and left intact at the donor site, the amount of rib needed for coverage of the bony defect was marked, and the periosteum was cleaned off the proximal and distal edges of the rib graft. The rib was lifted up with a Doyen elevator, cut proximally and distally, and the intercostal vessels were clipped proximally and distally leaving the customized vascularized rib construct attached by the intercostal perforator from the thoracodorsal system. Then the remaining muscle was elevated and detached at its insertion. After preparation of the recipient vessels, the thoracodorsal vessels were isolated, clamped, transected, and ligated, and the flap was inset into the cranial defect. The osteoplasty was done by removing the inner cortex of the rib for ~1 cm at the peripheral margin, fitting the rib into the bony defect as an onlay graft in a tongue-and-groove fashion, and securing it to the calvarium with self-drilling miniplates and screws. One patient had two ribs spanning the defect. In yet another patient, the rib construct was carefully bivalved along its long axis, keeping both halves attached to the periosteum invested by the perforating vessel. This maneuver effectively doubled the surface area for bony coverage without jeopardizing the vascularity of the bone, as evidenced by bleeding from the exposed cancellous surface. The microvascular anastomosis was performed to the superficial temporal artery and vein in three cases, to the superior thyroid artery and vein in one case, and to the external carotid artery and the internal jugular vein in another case. Then the muscle was draped over the vascularized rib graft and secured to the scalp edges, covered with split-thickness skin graft, and the cutaneous paddle was inset for postoperative flap monitoring.
RESULTS
All patients (age 43 to 81) had composite defects ranging from 36 to 750 cm2 (mean size 230 cm2) for the bony component and from 16 to 400 cm2 (mean size 170 cm2) for the soft tissue defect. All patients had a history of prior or current infection of the affected area, two patients had prior radiation, and two patients had persistent CSF leak (Table 1).
Table 1.
Patient Demographics, Etiology/Indication for Surgery, Defect Size, and Location
| Patient | Age/Gender | Etiology/Indication for Surgery | Defect Location and Size Bone/Soft Tissue |
|---|---|---|---|
| 1 | 81/M | Squamous cell carcinoma, osteomyelitis | Bifrontal and parietal region, 750 cm2/400 cm2 |
| 2 | 43/M | Calvarial mucor, osteomyelitis | Left parietal, bifrontal region, 110 cm2/60 cm2 |
| 3 | 43/F | Hemorrhagic stroke, craniectomy, infection | Right frontotemporal region, 150 cm2/280 cm2 |
| 4 | 51/M | Hemorrhagic stroke, craniectomy, CSF leak | Occipital region, 36 cm2/16 cm2 |
| 5 | 53/M | Brain tumor, CSF leak | Occipitoparietal region, 100 cm2/100 cm2 |
CSF, cerebrospinalfluid.
Surgical details, complications, and follow-up are summarized in Table 2.
Table 2.
Surgical Details Including Recipient Vessels, Previous Radiation, Infection, CSF Leak, Complications, Outcome, and Follow-up Period
| Patient | Surgical Details | Recipient Vessels | CSF Leak |
Radiation | Prior or Current Infection |
Complications | Outcome/Follow-up |
|---|---|---|---|---|---|---|---|
| 1 | Craniectomy, dural tumor resection, latissimus dorsi free flap with vascularized rib graft, STSG | Superficial temporal | No | Yes | Yes | Wound debridement,2 wk postoperation, VAC, STSG 4 wk later | Died of recurrence 1 y postoperative |
| 2 | Craniectomy, latissimus dorsi free flap with vascularized rib graft, STSG | Superficial temporal | No | No | Yes | Arterial thrombosis 24 h postoperation, salvaged with vein graft, 2 wk later partial flap necrosis treated with debridement and STSG | 35 mo |
| 3 | Craniectomy, latissimus dorsi free flap with 8th + 10th vascularized rib graft | Internal jugular vein, external carotid artery | No | No | Yes | Revision debridement for wound breakdown 4 wk postoperation | 29 mo |
| 4 | Craniectomy, latissimus dorsi free flap with vascularized rib graft | Superior thyroid | Yes | No | Yes | None | 15 mo |
| 5 | Craniectomy, latissimus dorsi free flap with vascularized rib graft, STSG | Superficial temporal | Yes | Yes | Yes | None | 12 mo |
CSF, cerebrospinal fluid; STSG, split-thickness skin graft; VAC, vacuum-assisted closure.
All patients had bony union. No complete flap losses and no donor site complications were noted. Complications included vascular compromise of one flap salvaged by vein graft, limited distal flap necrosis in three patients that was treated with local debridement, and topical antimicrobial agents. Three patients required a second procedure for wound debridement and additional split-thickness skin graft. Follow-up ranged from 12 months to 3 years.
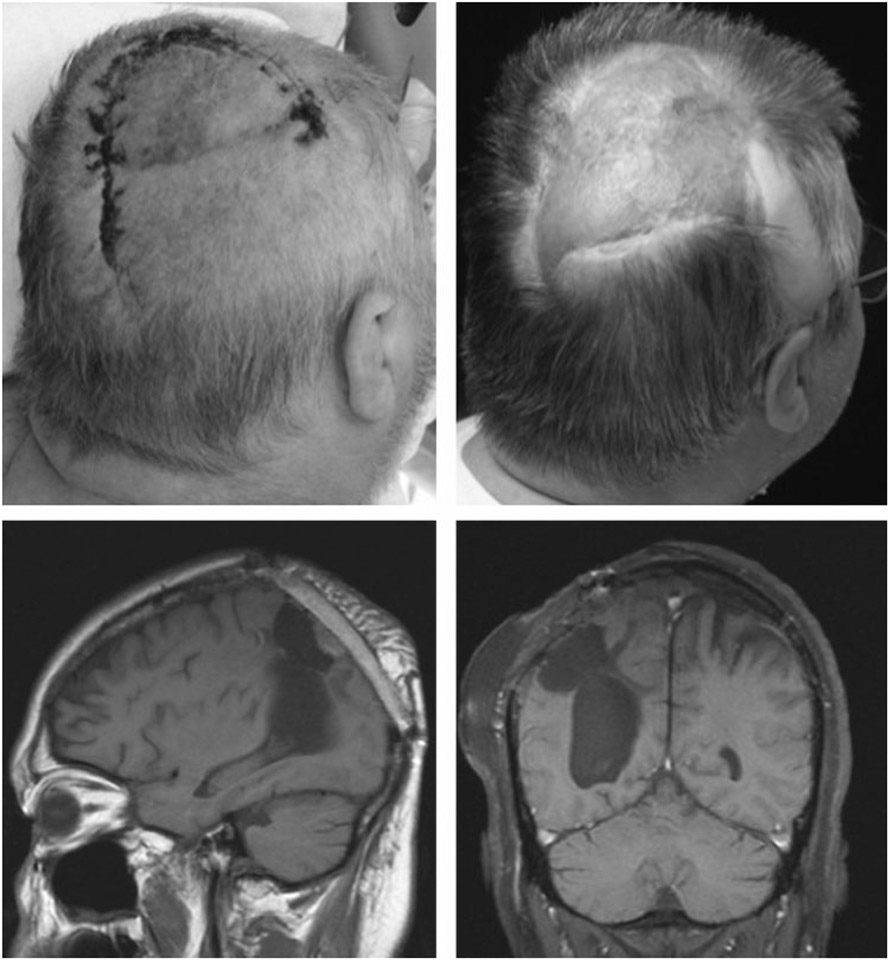
Case 3
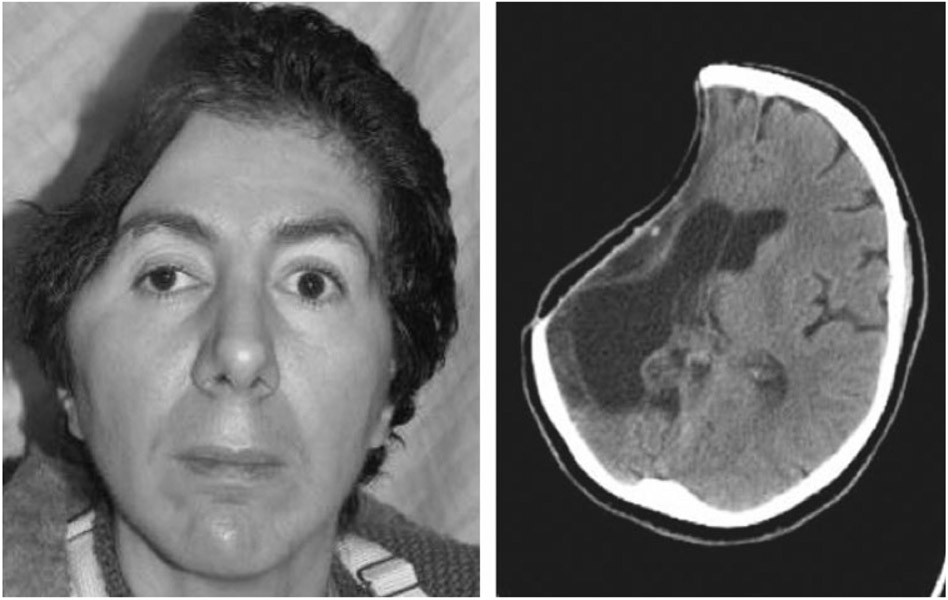
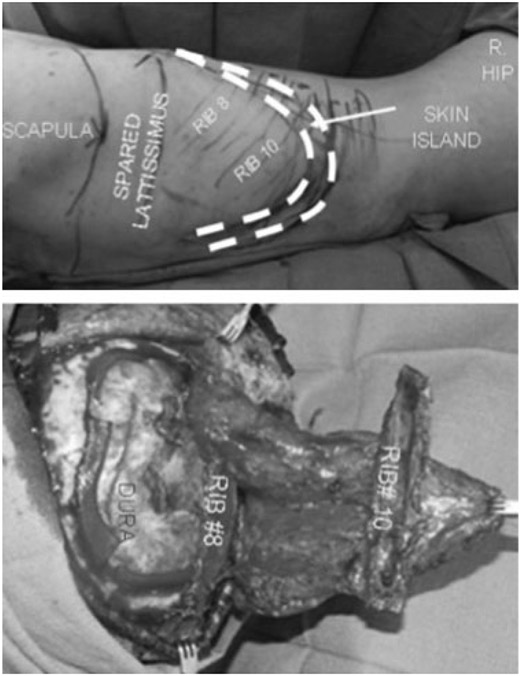
A 43-year-old woman with a history of protein-S deficiency sustained a hemorrhagic stroke requiring craniectomy with “banking” of her calvarial bone flap. Her banked bone was replaced 2 months later but had to be removed after another 2 months due to infection. Seven months later, she underwent cranioplasty with a synthetic cranial implant, which needed to be removed 4 months later due to infection (Fig. 1). Seven months later, she underwent craniectomy with debridement and reconstruction of a right frontotemporal 150-cm2 bony and 60-cm2 soft tissue defect with a latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap with 8th and 10th vascularized rib grafts (Fig. 2). Recipient vessels were the external carotid artery and internal jugular vein. The flap had a minor wound breakdown leading to revisional debridement 4 weeks postoperatively. She had no adverse neurological sequelae. The patient is doing well at over 2.5 years of follow-up (Fig. 3).
Figure 1.
Patient 3: preoperative picture and computed tomography of failed cranioplasty due to infection.
Figure 2.
Patient 3: design and insetting of latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap.
Figure 3.
Patient 3: immediately postoperative, at 3-month follow-up, and at 2 years postoperatively.
Case 5
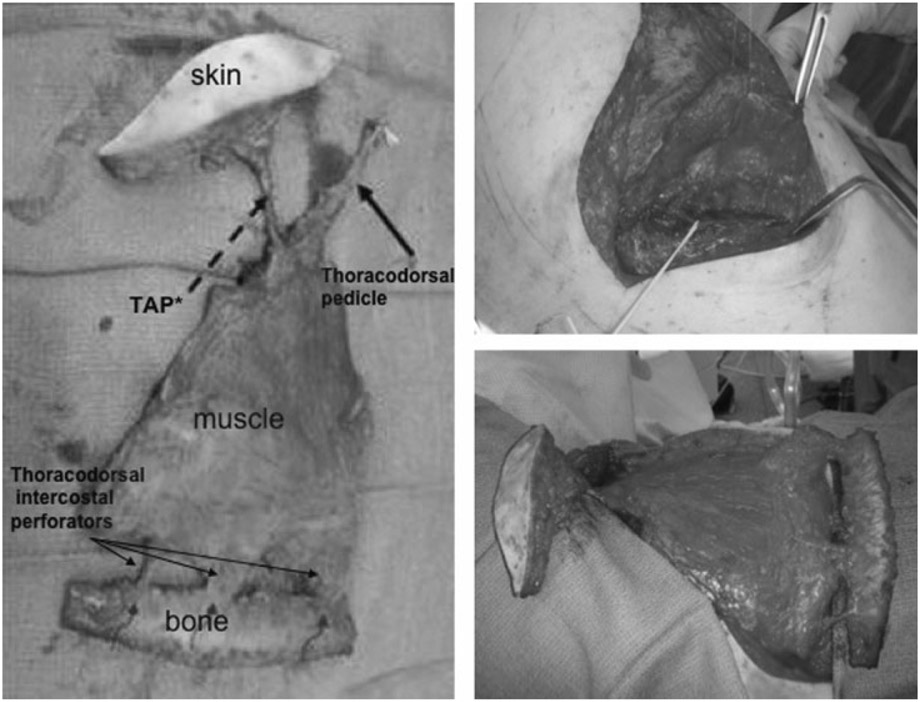
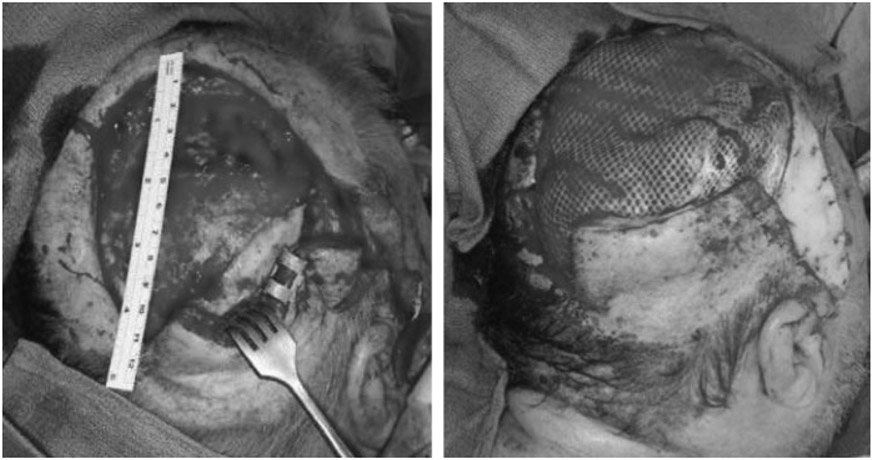
A 53-year-old man with recurrent astrocytoma underwent resection followed by radiation therapy. Wound closure was initially obtained using local tissue transfer. He developed infection of the bone flap and subsequent CSF leak, necessitating craniectomy and debridement followed by immediate reconstruction of an occipitoparietal bony and same-size soft tissue defect of 100 cm2 with a latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap (Figs. 4 and 5). There were no postoperative complications. The CSF leak is resolved, the wounds are healed, and the patient is doing well at 1-year follow-up (Fig. 6).
Figure 4.
Patient 5: intraoperative view of latissimus dorsi/rib intercostal perforator myo-osseocutaneous chimeric free flap, dissection of thoracodorsal pedicle, thoracodorsal artery perforator (TAP) with skin monitor, latissimus dorsi muscle, and vascularized rib graft attached by the intercostal perforators.
Figure 5.
Patient 5: intraoperative cranial defect and latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap with meshed split-thickness skin graft in place.
Figure 6.
Patient 5: preoperative picture (left upper corner) and postoperative picture after 11 months (right upper corner); postoperative computed tomography visualizing vascularized rib graft (pictures below).
DISCUSSION
There is a broad variety of reconstructive options for scalp and cranial defects ranging from primary closure, skin grafts, tissue expansion, local and regional flap options for small or medium-size defects, to free tissue transfer for larger, complex composite defects.8 In evaluating and treating those complex defects, several authors provided guidance in establishing reconstructive algorithms following the general principles of the reconstructive ladder8-10 and describing classification and staging systems of the defect.11,12 In our series of patients, we emphasize the following reconstructive priorities: to replace scarred, irradiated, infected tissue with well-vascularized tissue; treat or prevent CSF leaks; and restore cranial protection and form.
Given the complexity of our defects with missing bone, soft tissue, and skin, with or without CSF leak, in prior irradiated, traumatized, scarred or infected areas, we feel that composite tissue transfer including vascularized bone is preferable to soft tissue alone or soft tissue with prosthetic cranial replacement. The presence of a rigid structure can act to protect the brain in a pressure-prone area of the skull as well as restore contour. In our experience with the latissimus dorsi/rib intercostal perforator myo-osseocutaneous chimeric free flap, we consistently achieved wound closure with soft tissue coverage including sealing or preventing CSF leaks and bony protection of the brain in all patients. The anatomy of the intercostal perforating vessels from the thoracodorsal system was originally described by Schlenker et al in a dog model13 and since then has been investigated further by several authors in cadaveric studies14 and on patients intraoperatively during flap dissection.15 In Yamamoto et al’s cadaveric study, medial and lateral communicating perforators between the latissimus branch of the thoracodorsal and intercostal vascular system at the inferior margin of the 10th, 11th, and 12th ribs were explored. They found the vessel diameter to be 1.0 mm and the intercostal perforator pedicle length from costal margin to muscle entrance to be 25 mm. They chose the lower ribs in their study to provide a longer pedicle and greater arc of rotation and presumably minimize functional impairment to the thoracic wall.14 We have found these perforating vessels from the 8th to the 12th ribs.
We based our decision on which ribs to harvest on the size and location of the cranial defect and the desired location of bony support within the latissimus dorsi myocutaneous flap. The advantage of basing the rib on these intercostal perforating vessels, rather than just periosteal supply where the latissimus (or serratus) muscles attach to the ribs, is the increased ability to move the bone independently to the muscle thereby facilitating precise insetting.16 During dissection of the muscles flap, the intercostal perforators were visualized and evaluated for adequate size and good Doppler signal before harvest.
All of our patients had full-thickness scalp and cranial defects, compromise of the surrounding tissue, and a history of infection of the affected area. Furthermore, all patients had previously failed cranioplasties or had unsuccessful local tissue reconstructions. In addition, two patients had undergone previous irradiation and two patients had persistent CSF leaks. In those circumstances, it is important to provide wound closure and reliably seal CSF leaks with healthy, vascularized tissue.17 The complexity of the defect involving bone, soft tissue, and skin is important in the reconstructive decision making. The missing bony support needs to be addressed and in irradiated, infected, or otherwise traumatized tissue, it is preferable to provide vascularized bone for support to decrease the chances of infection and failure of the reconstruction.1,15,18 In two of our cases, the patients had prior reconstruction with synthetic material or nonvascularized bone graft. Due to the compromise of the local tissue, those reconstructions ultimately became infected and failed. The advantages of vascularized bone flaps include a higher survival rate, faster osteosynthesis, less resorption, and greater resistance to infection.1,15,18 On the other hand, it requires an experienced, highly skilled team and additional operating room time. The addition of skin has the advantage of easier monitoring of the flap and in selected cases allows for closure without tension (case No. 3) or addition of a thoracodorsal perforating component to position over the pedicle (case No. 5). The disadvantage of including skin is the abnormal contour, especially in obese patients (case No. 2).
To find the most appropriate reconstructive option for every patient, all of these components need to be taken into consideration. The initial evaluation of the defect includes size and potential violation/exposure of vital structures such as dura or brain parenchyma as evidenced by a persisting CSF leak. If the defect is small enough, local reconstructive options such as tissue rearrangement, skin grafts, tissue expansion, and local or regional flaps can be considered. If the defect is larger, classified by Beasley et al12 as greater than 200 cm2, or if there is compromise of surrounding tissue due to trauma, osteoradionecrosis, infection, pre- or postoperative radiation, previous local flap failure, violation/exposure of dura, brain parenchyma, or a persistent CSF leak, free tissue transfer may be justified to adequately reconstruct the defect.12,17 The evaluation of the defect also includes whether bone is missing and whether structural support will be needed to protect the brain or prevent negative neurological sequelae from external pressure or the weight of the flap tissue.3 If indeed structural support is indicated, different options are available including metal implants, alloplastic materials (e.g., polymethylmethacrylate, polyethylene), autologous bone graft, and allogeneic bone and bone substitutes placed either at the time of soft tissue reconstruction or in a delayed fashion. The advantages of autologous bone are long-term viability, potential for future growth, and resistance to infection. With vascularized bone, the chances of bone healing, specifically in compromised or previously infected tissue, increase. There are two different approaches to vascularized bone: one is a vascularized bone flap based on a perforator of the same vascular pedicle as the soft tissue free flap, and the other is the creation of neovascularized autogenous calvarial bone flaps, as described by Tsukagoshi et al,19 applying the principle of flap prefabrication by preservation of autogenous calvarial bone obtained during external cranial decompression and integrated into a rectus abdominus muscle flap with later transfer of the neovascularized bone within the flap and microvascular anastomosis of the flap at the cranial recipient site. In their results, complete vascularization of the calvarial bone flap was not achieved as indicated by their postoperative imaging.19 This approach not only requires a two-step operation but also requires a two-team approach of neurosurgery and plastic surgery at the time of emergent decompression to preserve and neovascularize the bone flap. Furthermore, this technique is not available to patients who are transferred in a nonurgent setting where prior craniectomy and cranioplasty have been performed and where reconstruction of the failed cranioplasty is indicated.
CONCLUSION
The latissimus dorsi composite free flap consisting of skin, muscle, and vascularized rib based of the thoracodorsal system can successfully cover large complex cranial defects, provide skeletal support, restore contour, and significantly improve functional outcome with limited donor site morbidity. Coverage with the latissimus dorsi/rib intercostal perforator myo-osseocutaneous free flap is especially important for patients with bony defects posterior or lateral to the vertex of the skull in the setting of compromised tissue. This well-vascularized composite flap provides structural support and thereby potentially mitigates negative neurological sequelae from external pressure.
ACKNOWLEDGMENTS
We would like to thank our neurosurgical colleagues, Drs. Daniel Curry, David M. Frim, Maciej S. Lesniak, and Bahktiar Yamini, as well as all of the residents involved from the Section of Plastic and Reconstructive Surgery and the Section of Neurosurgery.
Footnotes
Presented in part at: Congress of the World Society for Reconstructive Microsurgery, Buenos Aires, Argentina, 2005. Presented at: American Society of Plastic Surgery, Chicago, Illinois, November 2008, Poster Session, and American Society of Reconstructive Microsurgery, Maui, Hawaii, January 2009, Poster Session.
REFERENCES
- 1.Robson MC, Zachary LS, Schmidt DR, Faibisoff B, Hekmatpanah J. Reconstruction of large cranial defects in the presence of heavy radiation damage and infection utilizing tissue transferred by microvascular anastomoses. Plast Reconstr Surg 1989;83:438–442 [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb LJ, Krieger LM. From the reconstructive ladder to the reconstructive elevator. Plast Reconstr Surg 1994;93:1503–1504 [DOI] [PubMed] [Google Scholar]
- 3.Nahabedian MY, Chevray P, Olivi A, Manson P. Clinically manifested frontal lobe compression after anterior craniectomy and deep inferior epigastric perforator flap reconstruction. Plast Reconstr Surg 2003;112:1040–1045 [DOI] [PubMed] [Google Scholar]
- 4.Richaud J, Boetto S, Guell A, Lazorthes Y. [Effects of cranioplasty on neurological function and cerebral blood flow]. Neurochirurgie 1985;31:183–188 [PubMed] [Google Scholar]
- 5.Grant FC, Norcross NC. Repair of cranial defect by cranioplasty. Ann Surg 1939;110:488–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res 1997;19:311–316 [DOI] [PubMed] [Google Scholar]
- 7.Gladstone HB, McDermott MW, Cooke DD. Implants for cranioplasty. Otolaryngol Clin North Am 1995;28:381–400 [PubMed] [Google Scholar]
- 8.Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg 2008;122:195e–208e [DOI] [PubMed] [Google Scholar]
- 9.Hierner R, van Loon J, Goffin J, van Calenbergh F. Free latissimus dorsi flap transfer for subtotal scalp and cranium defect reconstruction: report of 7 cases. Microsurgery 2007;27:425–428 [DOI] [PubMed] [Google Scholar]
- 10.Kruse-Lösler B, Presser D, Meyer U, Schul C, Luger T, Joos U. Reconstruction of large defects on the scalp and forehead as an interdisciplinary challenge: experience in the management of 39 cases. Eur J Surg Oncol 2006;32:1006–1014 [DOI] [PubMed] [Google Scholar]
- 11.Lutz BS, Wei FC, Chen HC, Lin CH, Wei CY. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg 1998;51:186–190 [DOI] [PubMed] [Google Scholar]
- 12.Beasley NJ, Gilbert RW, Gullane PJ, Brown DH, Irish JC, Neligan PC. Scalp and forehead reconstruction using free revascularized tissue transfer. Arch Facial Plast Surg 2004;6:16–20 [DOI] [PubMed] [Google Scholar]
- 13.Schlenker JD, Indresano AT, Raine T, Meredith SC, Robson MC. A new flap in the dog containing a vascularized rib graft—the latissimus dorsi myoosteocutaneous flap. J Surg Res 1980;29:172–183 [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Sugihara T, Kawashima K, Qi F. An anatomic study of the latissimus dorsi-rib flap: an extension of the subscapular combined flap. Plast Reconstr Surg 1996;98:811–816 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt DR, Robson MC. One-stage composite reconstruction using the latissimus myoosteocutaneous free flap. Am J Surg 1982;144:470–472 [DOI] [PubMed] [Google Scholar]
- 16.Maruyama Y, Urita Y, Ohnishi K. Rib-latissimus dorsi osteomyocutaneous flap in reconstruction of a mandibular defect. Br J Plast Surg 1985;38:234–237 [DOI] [PubMed] [Google Scholar]
- 17.Wang HT, Erdmann D, Olbrich KC, Friedman AH, Levin LS, Zenn MR. Free flap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: lessons learned closing large, difficult wounds of the dura and skull. Plast Reconstr Surg 2007;119:865–872 [DOI] [PubMed] [Google Scholar]
- 18.Ohsumi N, Shimamoto R, Tsukagoshi T. Free composite latissimus dorsi muscle-rib flap not containing the intercostal artery and vein for reconstruction of bone and soft-tissue defects. Plast Reconstr Surg 1994;94:372–378 [DOI] [PubMed] [Google Scholar]
- 19.Tsukagoshi T, Satoh K, Hosaka Y. Cranioplasty with neovascularized autogenous calvarial bone. Plast Reconstr Surg 1998;102:2114–2118 [DOI] [PubMed] [Google Scholar]