Abstract
Hexanucleotide repeat expansion (HRE) within C9orf72 is the most common genetic cause of frontotemporal dementia (FTD). Thalamic atrophy occurs in both sporadic and familial FTD but is thought to distinctly affect HRE carriers. Separately, emerging evidence suggests widespread derepression of transposable elements (TEs) in the brain in several neurodegenerative diseases, including C9orf72 HRE-mediated FTD (C9-FTD). Whether TE activation can be measured in peripheral blood and how the reduction in peripheral C9orf72 expression observed in HRE carriers relates to atrophy and clinical impairment remain unknown. We used FreeSurfer software to assess the effects of C9orf72 HRE and clinical diagnosis (n = 78 individuals, male and female) on atrophy of thalamic nuclei. We also generated a novel, human, whole-blood RNA-sequencing dataset to determine the relationships among peripheral C9orf72 expression, TE activation, thalamic atrophy, and clinical severity (n = 114 individuals, male and female). We confirmed global thalamic atrophy and reduced C9orf72 expression in HRE carriers. Moreover, we identified disproportionate atrophy of the right mediodorsal lateral nucleus in HRE carriers and showed that C9orf72 expression associated with clinical severity, independent of thalamic atrophy. Strikingly, we found global peripheral activation of TEs, including the human endogenous LINE-1 element L1HS. L1HS levels were associated with atrophy of multiple pulvinar nuclei, a thalamic region implicated in C9-FTD. Integration of peripheral transcriptomic and neuroimaging data from human HRE carriers revealed atrophy of specific thalamic nuclei, demonstrated that C9orf72 levels relate to clinical severity, and identified marked derepression of TEs, including L1HS, which predicted atrophy of FTD-relevant thalamic nuclei.
SIGNIFICANCE STATEMENT Pathogenic repeat expansion in C9orf72 is the most frequent genetic cause of FTD and amyotrophic lateral sclerosis (ALS; C9-FTD/ALS). The clinical, neuroimaging, and pathologic features of C9-FTD/ALS are well characterized, whereas the intersections of transcriptomic dysregulation and brain structure remain largely unexplored. Herein, we used a novel radiogenomic approach to examine the relationship between peripheral blood transcriptomics and thalamic atrophy, a neuroimaging feature disproportionately impacted in C9-FTD/ALS. We confirmed reduction of C9orf72 in blood and found broad dysregulation of transposable elements—genetic elements typically repressed in the human genome—in symptomatic C9orf72 expansion carriers, which associated with atrophy of thalamic nuclei relevant to FTD. C9orf72 expression was also associated with clinical severity, suggesting that peripheral C9orf72 levels capture disease-relevant information.
Keywords: C9orf72, dementia, neurodegeneration, radiogenomics, thalamus, transposable elements
Introduction
Hexanucleotide repeat expansion (HRE) intronic to C9orf72 is the most common genetic cause of frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). Because of the association of C9orf72 HRE with FTD and ALS more than a decade ago (Dejesus-Hernandez et al., 2011; Renton et al., 2011), several classes of pathogenic mechanisms have been characterized and invoked to explain HRE pathogenicity. These mechanisms are categorized broadly as involving gains of toxic function and partial loss of C9orf72 protein function (for review, see Balendra and Isaacs, 2018; Vatsavayai et al., 2019; Braems et al., 2020). Gain-of-function (GOF) mechanisms include the formation of repeat-containing RNA foci thought to result in sequestration of RNA-binding proteins, and the generation of dipeptide repeat proteins noncanonically translated from the expanded GGGGCC (G4C2) repeat present within the C9orf72 mRNA of HRE carriers. Haploinsufficiency, on the other hand, has received increasing interest in the past several years and has been proposed to act synergistically with GOF mechanisms to drive pathogenicity in expansion carriers. Precisely how these putative mechanisms lead to loss of nuclear TAR DNA-binding protein 43 (TDP-43) and TDP-43 aggregation that is characteristic of C9orf72-associated ALS/FTD (C9-ALS/FTD) neuropathology remains unknown.
Neuroanatomically, the earliest cortical regions thought to be affected in the behavioral variant of FTD (bvFTD), which has an impact on social behavior and emotional processing, are anterior cingulate and frontoinsular cortices (Seeley et al., 2008, 2012). However, prominent atrophy of the thalamus also occurs in both sporadic and familial forms of FTD (Bocchetta et al., 2018), including FTD because of C9orf72 HRE (hereafter referred to as C9-FTD) as well as pathogenic variation in MAPT, GRN, and other FTD-associated genes. Intriguingly, several lines of evidence suggest that the thalamus may be disproportionately affected in C9-FTD, and specific nuclei such as the medial pulvinar may be uniquely affected (Sha et al., 2012; Yokoyama and Rosen, 2012; Lee et al., 2014; Yokoyama et al., 2014; Vatsavayai et al., 2016; Bocchetta et al., 2020).
The discoveries that repetitive element (RE) transcripts are elevated in C9-ALS/FTD brains (Prudencio et al., 2017), that TDP-43 binds transposable element (TE) transcripts (Li et al., 2012), and that loss of nuclear TDP-43 is associated with decondensation of REs such as long interspersed nuclear elements (LINEs) and consequent LINE1 retrotransposition (Liu et al., 2019) have provided an additional pathobiological mechanism to consider. This phenomenon is unlikely to be restricted to C9-ALS/FTD; a subset of postmortem brain tissue from sporadic ALS also exhibits a profile of increased retrotransposon expression reminiscent of that observed in C9-ALS/FTD (Tam et al., 2019). Moreover, TE activation may occur in the context of other proteinopathies as well—tau neuropathology also appears to induce TE expression (Guo et al., 2018; Sun et al., 2018). Remarkably, a Drosophila model of pathogenic CHMP2B variation, which causes FTD characterized by atypical TDP-43- and tau-negative neuropathology (Skibinski et al., 2005; Mackenzie and Neumann, 2016), also involves augmented TE expression (Fort-Aznar et al., 2020), suggesting that heightened TE expression and retrotransposition may represent a general mechanism underlying multiple forms of neurodegeneration. However, to our knowledge, it is unknown whether this activation occurs in the periphery outside the brain.
In this article, we integrated peripheral blood RNA sequencing (RNA-seq) data and neuroimaging analyses from C9orf72 HRE carriers from across the clinical spectrum and healthy controls to (1) confirm global thalamic atrophy and reduced peripheral expression of C9orf72 in HRE carriers, (2) identify disproportionate atrophy of specific thalamic nuclei in HRE carriers, (3) show that peripheral C9orf72 expression associates with clinical impairment independent of thalamic atrophy, (4) discover global peripheral derepression of TEs in affected HRE carriers, (5) demonstrate strikingly increased expression of the human-specific LINE1 element L1HS in symptomatic HRE carriers, and (6) show that peripheral L1HS levels associate with thalamic nuclei volumes in FTD-relevant regions. Our results indicate that derepression of TE expression in C9-ALS/FTD patients is not restricted to the CNS. Peripheral upregulation of TEs such as L1HS may therefore enable novel, blood-based biomarkers for C9-ALS/FTD.
Materials and Methods
Study participants
All participants or their surrogates provided written informed consent before study participation, and all aspects of the studies described here were approved by the University of California, San Francisco (UCSF) or University of California, San Diego (UCSD) Institutional Review Boards.
Neuroimaging study
Seventy-eight individuals (n = 44 cognitively normal controls and n = 34 C9orf72 HRE carriers) participated in this study. Individuals were recruited from the San Francisco Bay Area as part of ongoing studies of normal aging and FTD at the UCSF Memory and Aging Center (MAC). C9orf72 HRE carriers had a range of clinical diagnoses seen across the spectrum of C9orf72 HRE-related disease, including cognitively normal (presymptomatic; n = 10), mild cognitive impairment (MCI; n = 7), bvFTD (n = 13), and bvFTD with motor neuron disease (n = 4). There were significant differences between normal controls and C9orf72 HRE carriers when compared by age and education (Table 1). All individuals (n = 78; 27 males, 51 females) in the neuroimaging study also participated in the RNA-seq study (see below).
Table 1.
Demographic characteristics of neuroimaging cohort
|
C9orf72 HRE carrier |
||||||
|---|---|---|---|---|---|---|
| Variable | Control (n = 44) | FTD (n = 13) | FTD-ALS (n = 4) | MCI (n = 7) | Presymp (n = 10) | p value |
| Age, years [mean, (SD)] | 62.5 (5.5) | 61.9 (8.2) | 56.3 (10.8) | 52.0 (10.7) | 46.0 (9.3) | <0.001 |
| Sex, n male (%) | 17 (38.6) | 4 (30.8) | 3 (75.0) | 2 (28.6) | 1 (10.0) | NS |
| Education, years [mean, (SD)] | 17.2 (1.9) | 16.2 (3.1) | 16.5 (1.0) | 13.7 (2.1) | 16.6 (1.4) | 0.003 |
| CDR-SB score [mean, (SD)] | 0.00 (0.00) | 6.7 (2.5) | 10.3 (3.4) | 1.1 (0.5) | 0.00 (0.00) | <0.001 |
| Scan type (%) | 0.007 | |||||
| 1.5T | 5 (11.4) | 3 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 3T Trio | 38 (86.4) | 5 (38.5) | 2 (50.0) | 4 (57.1) | 7 (70.0) | |
| 3T Prisma | 0 (0.0) | 3 (23.1) | 1 (25.0) | 3 (42.9) | 2 (20.0) | |
| 4T | 1 (2.3) | 2 (15.4) | 1 (25.0) | 0 (0.0) | 1 (10.0) | |
CDR-SB, Clinical Dementia Rating scale Sum of Boxes; FTD, frontotemporal dementia; FTD-ALS, frontotemporal dementia with motor neuron disease; MCI, mild cognitive impairment; Presymp, Presymptomatic; SD, Standard deviation; T, Tesla; NS, not significant.
RNA-seq study
For whole-blood donors, participants carrying a pathogenic HRE in C9orf72, defined as >30 repeats (n = 49; 19 males, 30 females; C9orf72+; Renton et al., 2011), were assessed and clinically diagnosed at the UCSF MAC. No participants in this study carried other known neurodegenerative disease-causing pathogenic variants. Participants with mild cognitive or behavioral symptoms were classified as having MCI, whereas C9orf72+ participants who did not display any symptoms were classified as presymptomatic. Cognitively normal, healthy older adult controls (n = 65; mean age, 61.3 ± 6.7 years; 27 males, 38 females) were recruited to the UCSF MAC as part of ongoing longitudinal studies of aging. Demographic information for participants in this study is included in Table 2. Differences in participant demographics were assessed by one-way ANOVA followed by Tukey's test for post hoc analysis, or chi-square test. A p < 0.05 was considered statistically significant. For the peripheral blood mononuclear cell (PBMC) RNA-seq study, patients diagnosed with ALS met the modified El Escorial criteria for ALS (Brooks et al., 2000; Extended Data Fig. 5-1, demographic information).
Table 2.
Demographic characteristics of RNA-seq cohort
|
C9orf72 HRE carrier |
||||||
|---|---|---|---|---|---|---|
| Variable | Control (n = 65) | FTD (n = 20) | FTD-ALS (n = 7) | MCI (n = 10) | Presymp (n = 12) | p value |
| Age, years [mean (SD)] | 61.3 (6.7) | 60.6 (8.2) | 57.7 (11.2) | 57.5 (13.1) | 45.7 (9.2) | <1 × 10–5 |
| Sex, n male (%) | 27 (41.5) | 6 (30.0) | 5 (71.4) | 5 (50.0) | 3 (25.0) | NS |
| CDR-SB Score [mean (SD)] | 0 (0) | 7.6 (2.9) | 8.4 (3.5) | 1.3 (0.9) | 0 (0) | <1 × 10–5 |
CDR-SB, Clinical Dementia Rating scale Sum of Boxes; FTD, frontotemporal dementia; FTD-ALS, frontotemporal dementia with motor neuron disease; MCI, mild cognitive impairment; Presymp, Presymptomatic; SD, Standard deviation; T, Tesla; NS, not significant.
Clinical assessment
Study participants underwent multistep screening before an in-person clinical evaluation at the UCSF MAC that included a neurologic examination, cognitive assessment, and medical history (Rankin et al., 2005; Miller et al., 2013). In addition, each participant's study partner was interviewed regarding the participant's functional abilities. A multidisciplinary team composed of a behavioral neurologist, neuropsychologist, and registered nurse then established clinical diagnoses for cases according to consensus criteria for MCI (Petersen et al., 1999, 2014), FTD and its subtypes (Gorno-Tempini et al., 2011; Rascovsky et al., 2011), ALS (Brooks et al., 2000), and FTD-ALS (Strong et al., 2017). Controls and presymptomatic C9orf72 HRE carriers had a Clinical Dementia Rating scale Sum of Boxes (CDR-SB; Morris, 1993) score of zero, a Mini Mental State Exam (Folstein et al., 1975) score >26, and a normal neurologic examination. All participants were screened for comorbid physical and psychiatric health conditions that could confound their diagnosis of FTD or ALS as well as a substance abuse history. In addition, before inclusion in the study, all patients underwent an MRI to evaluate for cerebrovascular disease such as a stroke that might confound their diagnosis.
Neuroimaging
Study participants underwent structural T1-weighted magnetic resonance imaging (MRI) at one of two imaging centers at UCSF; 65 participants were scanned at the UCSF Neuroscience Imaging Center (NIC; n = 38 controls, n = 27 C9orf72 HRE carriers) on a 3T scanner, whereas 13 participants (n = 7 controls, n = 6 C9orf72 HRE carriers) were imaged at the San Francisco Veterans Affairs Medical Center on either a 1.5T or 4T scanner. Of note, the UCSF NIC scanner was updated during the study from a Siemens Magnetom Trio to a Magnetom Prisma model. To reduce the potential for confounding by this upgrade, images acquired on the Trio and Prisma models were treated statistically as coming from distinct scanners. Additional cohort details and the distribution of scans by scanner type are provided in Table 1.
All images were processed using FreeSurfer version 7.1 software (Fischl et al., 2002; Desikan et al., 2006), manually checked for segmentation accuracy and corrected as needed. We used FreeSurfer because it enables manual correction of segmentation errors in severely atrophied brains (Desikan et al., 2006); facilitates analysis of cortical thickness, which provides a more sensitive measure of atrophy than gray matter volume (Winkler et al., 2010); and enables the use of unique software packages not available on other imaging pipelines (Iglesias et al., 2018). Cortical regions of interest were defined using the Desikan–Killiany atlas (Desikan et al., 2006). Thalamic nuclei volumes were estimated using an extension of the FreeSurfer package (Iglesias et al., 2018), and all segmentations were manually checked. Cortical and thalamic illustrations of neuroimaging findings were generated using Freeview, the image-viewing software distributed with FreeSurfer.
Experimental design and statistical analysis
General
Statistical analyses described below were completed using R version 4.1.2 software.
Whole thalamic volume analyses
Using multiple regression, we began our analyses by testing whether whole thalamic volumes were significantly different in C9orf72 HRE carriers compared with normal controls, controlling for the effects of age, sex, CDR-SB score, education, MRI scanner type, and total intracranial volume (TIV). Following this, we tested whether total thalamic volumes varied by clinical diagnosis (normal control, presymptomatic C9orf72 HRE carrier, MCI, FTD, or FTD-ALS) using the same covariates.
Thalamic nucleic volume analyses
Using FreeSurfer-estimated thalamic nuclei volumes, we next used hierarchical clustering to identify relationships between nucleic and clinical groupings. To ensure comparability across nuclei during hierarchical clustering, all nucleic volumes were normalized to TIV and z-scored before analysis. Hierarchical clustering analyses were performed, and the resulting heat map was generated using the ComplexHeatmap package in R (Gu et al., 2016).
Based on the findings of the hierarchical clustering analyses, we next tested whether individual thalamic nucleic volumes significantly differed in C9orf72 HRE carriers compared with normal controls, controlling for CDR-SB scores, age, sex, education, MRI scanner type, and TIV. As a sensitivity analysis to determine whether individual nuclei provided information independent of global thalamic atrophy, we next tested whether the nucleic volumes differed in HRE carriers versus controls, controlling for thalamic volumes, CDR-SB scores, age, sex, education, and MRI scanner type.
C9orf72 RNA expression analyses
We began the next stage of our analyses by confirming that C9orf72 RNA expression was lower in peripheral blood samples from C9orf72 HRE carriers relative to noncarriers, a finding suggested by prior literature. Multiple regression analysis was used to compare C9orf72 RNA expression levels, covarying for age, sex, education, CDR-SB score, and batch.
To determine whether C9orf72 expression provided clinically relevant, disease-related information, we tested whether it predicted CDR-SB scores, covarying for age, sex, education, and batch. Following this, we examined whether C9orf72 expression provided information about clinical severity independent of thalamic atrophy in a combined multiple regression model, covarying for age, sex, education, MRI scanner type, TIV, and batch, using likelihood ratio testing to compare the combined model to models in which CDR-SB was predicted by C9orf72 expression alone or thalamic volumes alone.
Cortical thickness analyses
We concluded our neuroimaging analyses by examining associations between cortical thickness and the following four biomarkers of C9orf72 HRE-related disease: clinical impairment as estimated by CDR-SB score, C9orf72 HRE status, C9orf72 expression, and volume of the top thalamic nucleus discovered in the above analyses. All cortical thicknesses were estimated using FreeSurfer as described above, and all analyses were performed using multiple regression models covarying for age, sex, education, MRI scanner type, and TIV. When analyzing C9orf72 expression, batch was also included as a covariate.
Peripheral blood mononuclear cell isolation
Blood samples from participants at UCSD diagnosed with ALS (both sporadic and because of C9orf72 HRE) and healthy controls were collected into sodium heparin tubes and stored at room temperature for no longer than 30 h from the time of draw. PBMCs were isolated via Ficoll density gradient centrifugation. Residual red blood cells were lysed in ammonium chloride–containing hemolytic buffer, then counted before freezing in 7% dimethyl sulfoxide in fetal bovine serum. PBMC samples were initially stored at −80°C, then transferred to a liquid nitrogen freezer within 72 h.
RNA extraction
For whole-blood analyses, blood was drawn from participants at UCSF within 90 d of clinical assessment and stored in PAXgene blood RNA tubes (Qiagen) in liquid nitrogen. Study participants were not required to fast before their blood draw. Briefly, total RNA was extracted from whole-blood samples using a MagMAX isolation kit (Thermo Fisher Scientific), and RNA quality was assessed with a Bioanalyzer (Agilent). For PBMC samples, RNA was isolated via an RNeasy kit (Qiagen). Samples with an RNA integrity score >7 underwent library preparation for sequencing.
RNA-seq
Library preparation and sequencing were performed at the UCLA Neuroscience Genomics core as previously described (Parikshak et al., 2016). The TruSeq Stranded Total RNA with Ribo-Zero Globin kit (Illumina) was used per manufacturer protocol to prepare RNA for sequencing. Samples were sequenced in two batches; batch 1 (35 C9orf72 HRE carriers, 10 sporadic ALS, 37 controls) was sequenced on a HiSeq 2500 generating 50 base pair paired-end reads, whereas batch 2 (24 C9orf72 HRE carriers, 36 controls) was sequenced on a HiSeq 4000 generating 75 base pair paired-end reads. Samples in both batches were sequenced over multiple lanes and at an average depth of 50–60 m paired reads per sample.
Sequencing data processing
Gene and TE abundance was determined in RNA-seq data as previously described (Jin and Hammell, 2018) using TEcount from the TEToolkit suite (Jin et al., 2015; http://hammelllab.labsites.cshl.edu/software/). Reads were aligned to the GRCh38 build of the human reference genome using STAR version 2.7.3a software (Dobin et al., 2013) and a prebuilt GTF (gene transfer format) file of gene and TE annotation provided with TEtranscripts using the following parameters: STAR –runThreadN 8 –genomeDir/Index –readFilesIn sample_ID.R1.fastq.gz sample_ID.R2. fastq.gz –readFilesCommand zcat –outFileNamePrefix sample_ID –outSAMtype BAM Unsorted –sjdbOverhang 100 –winAnchorMultimapNmax 200 –outFilterMultimapNmax 100 –sjdbGTFfile/Index/GRCh38_GENCODE_rmsk_TE.gtf.
Gene and TE abundance were estimated from the resulting BAM (Binary Alignment Map) files using TEcount, a comprehensive gene annotation GTF file from GENCODE release 34, and a prebuilt TE annotation index provided with TEtranscripts using the following parameters: TEcount -b sample_ID.Aligned.out.bam –format BAM –stranded reverse –mode multi –minL 1 -i 100 –TE GRCh38_GENCODE_rmsk_TE.gtf –GTF gencode.v34.annotation.gtf –project sample_ID.
Differential expression analysis
Differential gene and TE expression was assessed using the DESeq2 package (Love et al., 2014) in R. Participant sex and age and batch were included as covariates in the linear model when assessing differential expression of normalized counts in DESeq2. A change in gene or TE expression was considered statistically significant at a Benjamini–Hochberg false discovery rate (FDR)-adjusted p (pFDR) < 0.05.
Gene Set Enrichment Analysis
Gene lists were generated from DESeq2 analyses and ranked by log2 fold-change (logFC). All preranked lists were analyzed using the GSEAPreranked tool in the Gene Set Enrichment Analysis (GSEA) software version 4.1.0 (Mootha et al., 2003; Subramanian et al., 2005) together with curated, pregenerated gene sets in the Hallmark Molecular Signatures Database supplemented with gene sets representing the senescence-associated secretory and type I interferon (IFN-I) response pathways (De Cecco et al., 2019). Sample permutation (n = 10,000) was used to correct for multiple testing.
Data availability
RNA-seq data have been uploaded to the FAIR Data Sharing Portal within the Alzheimer's Disease Workbench, which is supported by the Alzheimer's Disease Data Initiative, and can be accessed at https://www.alzheimersdata.org/ad-workbench.
Results
To explore the relationship between C9orf72 HRE carrier status and thalamic atrophy, we began by assessing whole thalamic volumes according to gene carrier status and diagnosis. As expected, we confirmed results from prior studies (Sha et al., 2012; Bocchetta et al., 2018, 2020), finding that HRE carrier status was significantly associated with reduced total thalamic volume (Fig. 1A). When analyzed by diagnosis, presymptomatic C9orf72 HRE carriers as well as those diagnosed with MCI and FTD showed significant reductions in total thalamic volume, whereas the smaller FTD-ALS group did not reach significance (Fig. 1B).
Figure 1.
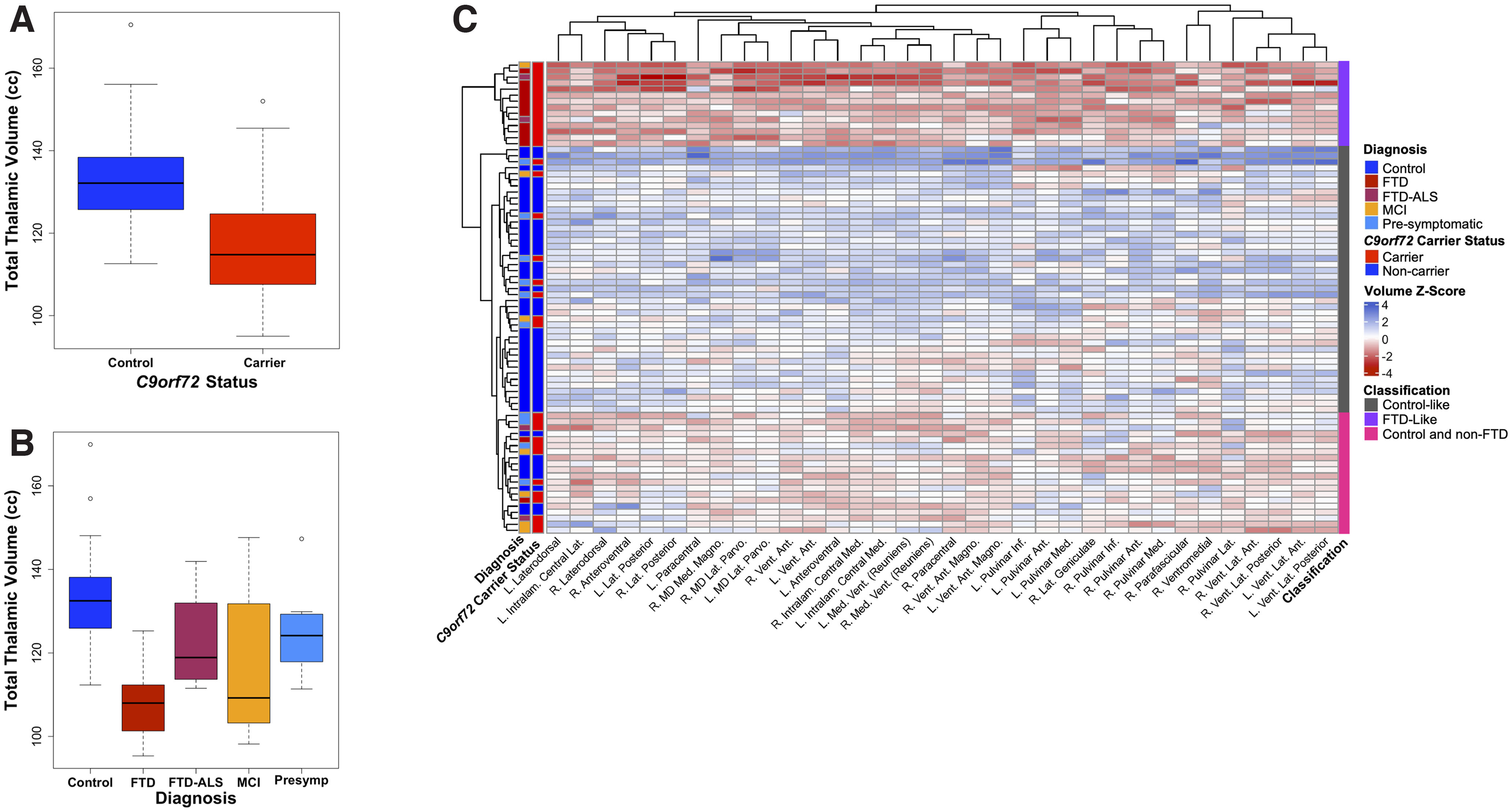
Comparisons of whole thalamic volume and thalamic nuclei volumes in C9orf72 HRE carriers versus controls. A, A box plot illustrates the results of a multiple regression analysis comparing total thalamic volume in C9orf72 carriers of all diagnoses to controls. Across all diagnoses and accounting for the presence as well as severity of a disease (as measured by the CDR-SB score), C9orf72 carriers demonstrated lower total thalamic volumes compared with controls (p = 1.57 × 10−6). Additional covariates included in this analysis were age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and TIV. B, A box plot illustrates the results of a multiple regression analysis comparing total thalamic volumes in C9orf72 HRE carriers versus controls by diagnosis. Of the four diagnostic groupings, three demonstrated a statistically significant difference versus controls, FTD (p = 0.005), MCI (p = 3.03 × 10−6), and presymptomatic (presymp; p = 0.003). The FTD-ALS group was not significantly different from controls (p = 0.16). Covariates included in this analysis were age, sex, education, MRI scanner type, CDR-SB score, and TIV. C, Hierarchical clustering analyses of all thalamic nuclei volumes, which significantly differed in C9orf72 HRE carriers compared with controls at pFDR < 0.05, are shown. The analysis revealed three primary groups—an FTD-like group (composed almost exclusively of FTD cases, plus two FTD-ALS cases and an MCI case), a control-like group (composed mostly of controls, plus presymptomatic C9orf72 carriers, and an MCI case), and a more heterogeneous group consisting of controls and largely non-FTD cases (presymptomatic C9orf72 carriers, MCI cases, and FTD-ALS cases). Ant, Anterior; Inf, Inferior; Intralam, Intralaminar; L, left; Lat, lateral; Magno, magnocellular; MD, mediodorsal; Med, medial; Parvo, parvocellular; Vent, ventral. The covariates used in our multiple regression analyses comparing individual thalamic nuclei by C9orf72 carrier status were the same as in B. Extended Data Figure 1-1 shows full results from the regression analyses.
Thalamic volume differences in C9orf72 HRE carriers compared to controls covarying for total intracranial volume. Comparisons of thalamic nuclei volumes in C9orf72 HRE carriers versus controls. Results from all 50 thalamic nuclei volumes estimated using FreeSurfer 7.1 software are shown above with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 1-1, DOCX file (19.5KB, docx) .
To clarify the relationship between individual thalamic nuclei volumes and diagnosis, we performed hierarchical clustering following thalamic nuclei segmentation performed using an extension of the FreeSurfer pipeline (Iglesias et al., 2018). Hierarchical clustering revealed three broad groupings—an FTD-predominant group composed largely of HRE carriers diagnosed with FTD, a control-like group composed largely of noncarrier controls and several presymptomatic carriers, and a third, more heterogeneous, group consisting largely of controls, presymptomatic carriers, and carriers with MCI (Fig. 1C). The FTD-predominant group showed a generally consistent atrophy pattern across 34 subthalamic regions that were significantly associated with C9orf72 carrier status (Fig. 1C, Extended Data Fig. 1-1).
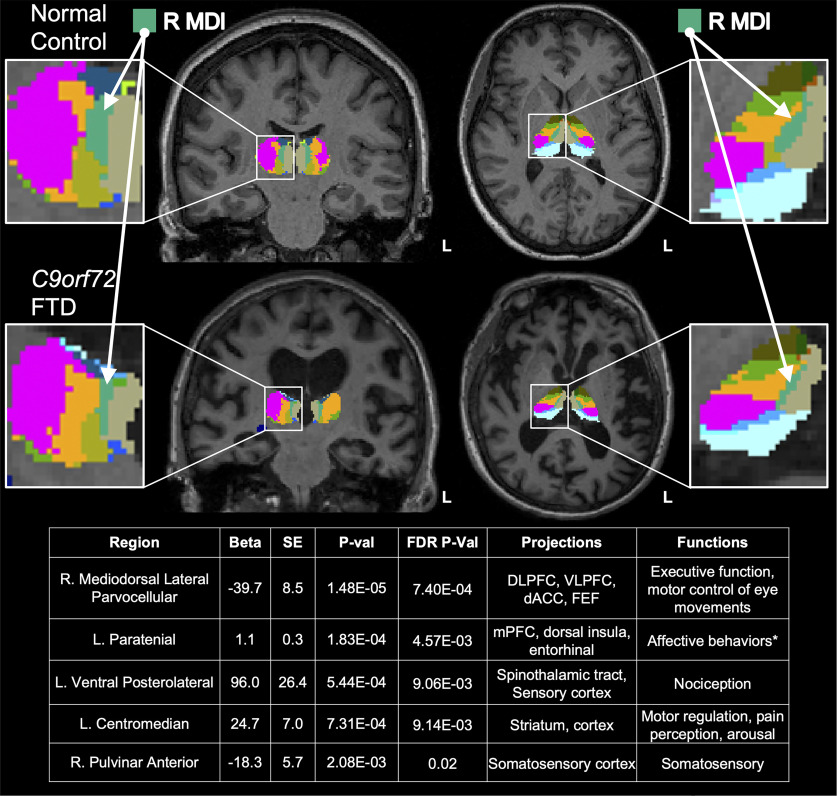
We next sought to determine whether any individual thalamic nuclei were disproportionately affected by C9orf72 carrier status. We reasoned that the most atrophied thalamic nuclei in HRE carriers would remain significantly associated with C9orf72 carrier status even after accounting for global thalamic atrophy. Intriguingly, we found that after including total thalamic volume as a covariate in our regression analyses, the right mediodorsal lateral parvocellular (R MDl) nucleus showed highly significant atrophy (Fig. 2), consistent with a disproportionate effect on this nucleus. The R MDl nucleus projects to multiple regions of prefrontal cortex (PFC), dorsal anterior cingulate cortex (dACC), and frontal eye fields (FEF), and is thought to be involved in executive function as well as motor control of eye movements (Fig. 2, table at bottom; Mitchell and Chakraborty, 2013; Ouhaz et al., 2018). Full results from the regression analyses described above are provided in Extended Data Figures 1-1 and 2-1.
Figure 2.
Mediodorsal lateral nucleus shows significant atrophy in C9orf72 HRE carriers, independent of global thalamic atrophy. Five thalamic nuclei were significantly different in C9orf72 HRE carriers versus controls at pFDR < 0.05. Covariates in the multiple regression included total thalamic volume, age, sex, education, and MRI scanner type. The most significant association was observed in the R MDl nucleus (p = 1.48 × 10−5). To illustrate the extent of volumetric differences observed in HRE carriers compared with controls, representative coronal and axial images (radiologic orientation) from a normal healthy control (Control) and an FTD case (C9orf72 FTD). The R MDl nucleus is shown in turquoise and indicated by white arrows (insets). Of note, several nuclei that were significantly different in carriers versus controls, including the R MDl and left paratenial nuclei, are notable for connectivity to cortical regions frequently implicated in FTD (Vertes and Hoover, 2008; Mitchell and Chakraborty, 2013; Vertes et al., 2015; Ouhaz et al., 2018). Further, these nuclei are implicated in behavioral changes prominently affected in C9-FTD, including executive function and affect (Mitchell and Chakraborty, 2013; Vertes et al., 2015; Ouhaz et al., 2018). Additional nuclei significantly associated with HRE carrier status include the left ventral posterolateral nucleus, which projects to the spinothalamic tract and sensory cortex and is involved in nociception (Al-Chaer et al., 1996; Darian-Smith et al., 1999; Krause et al., 2012); the left centromedian nucleus, which projects to motor cortex and is involved in motor regulation, pain perception, and arousal (Mai and Forutan, 2012; Ilyas et al., 2019); and the right pulvinar anterior nucleus, which projects to the somatosensory cortex and is involved in somatosensory function (Mai and Forutan, 2012). Extended Data Figure 2-1 shows full results from the regression analyses.
Thalamic volume differences in C9orf72 HRE carriers compared to controls covarying for total thalamic volume. Sensitivity analyses comparing of thalamic nuclei volumes in C9orf72 HRE carriers versus controls after covarying for total thalamic volume rather than total intracranial volume. Results from all 50 thalamic nuclei volumes estimated using FreeSurfer 7.1 software are shown above with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total thalamic volumes. L, Left. Download Figure 2-1, DOCX file (19.4KB, docx) .
Previous reports have demonstrated that peripheral blood expression of C9orf72 is reduced in HRE carriers (Rizzu et al., 2016; McCauley et al., 2020). Using a novel bulk RNA-seq dataset generated from whole blood (described below), we confirmed a significant reduction in C9orf72 RNA in HRE carriers in our neuroimaging cohort (Fig. 3A, Extended Data Fig. 3-1). Given that carriers showed both thalamic atrophy and reduced peripheral expression of C9orf72, we next asked whether thalamic volumes or peripheral C9orf72 RNA levels were associated with clinical impairment, as measured by CDR-SB scores. Strikingly, we found that both thalamic volumes and peripheral C9orf72 levels were associated with CDR-SB scores (Fig. 3B). To determine whether these two factors independently predict clinical impairment, we included both variables in a combined regression model and found that both remained significant, indicating independent contributions. Likelihood ratio testing evaluating goodness of fit revealed that the combined model was superior to the models examining thalamic volumes alone (p = 0.04) or C9orf72 expression alone (p = 2.14 × 10−4).
Figure 3.
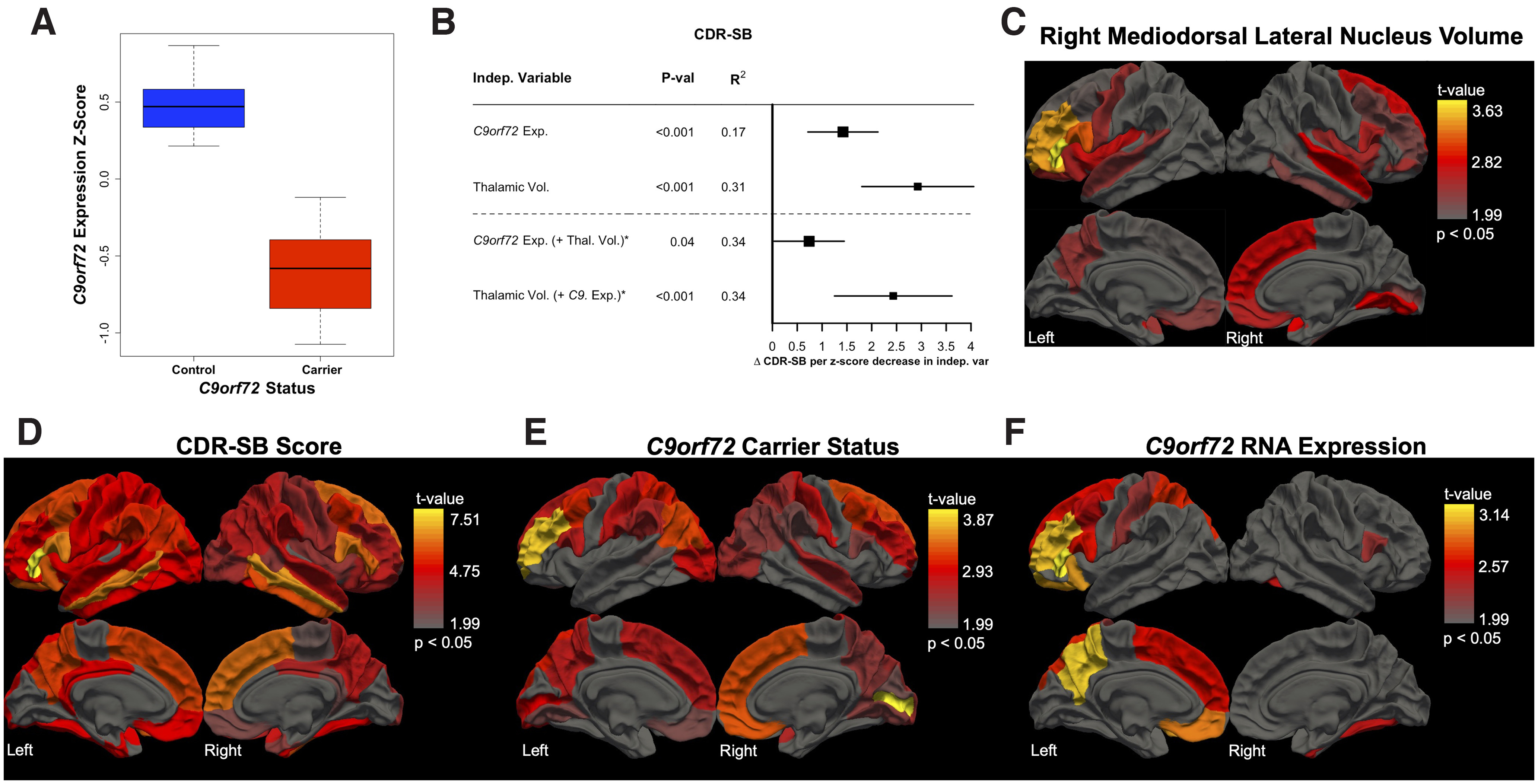
Peripheral C9orf72 expression captures unique, disease-relevant information related to clinical severity. A, C9orf72 expression is significantly decreased in HRE carriers versus noncarrier controls (p = 5.56 × 10−4) in a multiple regression model covarying for age, sex, education, CDR-SB score, and sample processing batch. B, C9orf72 expression associates with clinical impairment, as measured by CDR-SB score (p = 1.84 × 10−4), even after correcting for age, sex, education, and batch. Given our finding of significantly lower total thalamic volumes in HRE carriers compared with controls (Figure 1A; p = 1.57 × 10−6), we explored the relationship among C9orf72 expression, thalamic volumes, and CDR-SB scores. We found that thalamic volumes also predicted CDR-SB scores, again accounting for relevant covariates (p = 2.94 × 10−6; covariates: age, sex, education, MRI scanner type, and TIV). Both variables remained significant in a combined model, C9orf72 expression (p = 0.04) and thalamic volumes (p = 1.47 × 10−4), suggesting that C9orf72 expression and thalamic volumes provide distinct, disease-relevant information. C–F, Three-dimensional brain renderings depict results of multiple regression analyses (covarying for age, sex, education, clinical severity, MRI scanner type, and TIV) evaluating the relationship between cortical thickness and R MDl nucleus volumes (C), CDR-SB scores (D), C9orf72 HRE carrier status (E), and C9orf72 expression (F). R MDl nucleus volumes associate with cortical thickness in multiple FTD-relevant regions such as prefrontal cortex and orbitofrontal cortex (C). CDR-SB scores track global atrophy with relative sparing only of medial occipital cortex (D). C9orf72 carrier status associates with bifrontal thinning in HRE carriers with notable sparing of bilateral motor cortex (E). C9orf72 expression associates with left prefrontal cortex and left parietal cortex volumes with less prominent involvement of left orbitofrontal cortex and middle frontal gyrus (F). Extended Data Figures 3-1, 3-2, 3-3, 3-4, and 3-5 show full results from the regression analyses described above. Indep, Independent; Thal, Thalamic; Exp, Expression; Vol, Volume.
C9orf72 expression in C9orf72 HRE carriers versus controls. Multiple regression analyses demonstrate that C9orf72 expression in C9orf72 HRE carriers versus controls is significantly decreased after covarying for the effects of sex, age, education, clinical severity (as estimated by CDR-SB score), and RNA-seq batch. Download Figure 3-1, DOCX file (14KB, docx) .
Right mediodorsal lateral parvocellular nucleus volumes associate with cortical thicknesses. Associations between right mediodorsal lateral parvocellular nucleus volumes and cortical thicknesses are shown. Results for all 68 cortical regions of interest from the Desikan–Killiany atlas with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-2, DOCX file (20.5KB, docx) .
CDR-SB score associations with whole-brain cortical thicknesses. Associations between CDR-SB score and cortical thicknesses are shown. Results for all 68 cortical regions of interest from the Desikan–Killiany atlas with associated p values shown before and after FDR correction for multiple testing. All regression analysis covaried for age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-3, DOCX file (20.8KB, docx) .
Cortical thickness differences in C9orf72 HRE carriers compared to controls. Associations between C9orf72 HRE carrier status and cortical thicknesses are shown for all 68 cortical regions of interest from the Desikan–Killiany atlas with associated p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-4, DOCX file (20.4KB, docx) .
Cortical thickness associations with C9orf72 expression in HRE carriers and controls. Associations of C9orf72 expression and cortical thicknesses are shown for all 68 cortical regions of interest from the Desikan–Killiany atlas with associated p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-5, DOCX file (20.8KB, docx) .
We next asked whether R MDl nucleus volumes were associated with cortical thickness and found significant associations with multiple FTD-relevant regions, including PFC (Fig. 3C, Extended Data Fig. 3-2). As expected, we found that CDR-SB scores tracked global atrophy (Fig. 3D, Extended Data Fig. 3-3), whereas HRE carrier status was associated with bifrontal thinning with notable sparing of bilateral motor cortex (Fig. 3E, Extended Data Fig. 3-4). Finally, we found that peripheral C9orf72 expression predicted left PFC and left parietal cortex volumes with less pronounced involvement of middle frontal gyrus (Fig. 3F, Extended Data Fig. 3-5).
Together, our neuroimaging analyses confirm global thalamic atrophy as well as significantly reduced peripheral C9orf72 expression in HRE carriers. More significantly, we found that total thalamic volume and peripheral C9orf72 expression levels independently predicted clinical impairment, and through parcellation of individual thalamic nuclei, we found that HRE carriers showed significant atrophy of the R MDl nucleus independent of global thalamic atrophy. Strikingly, we found that R MDl nucleus volumes, HRE carrier status, and peripheral C9orf72 levels all associated with cortical thickness in FTD-relevant regions. The remarkable finding that peripheral C9orf72 expression is associated with clinical impairment even after accounting for thalamic atrophy suggests that global peripheral transcriptomic changes in HRE carriers may capture additional disease-relevant biology. We therefore analyzed our whole-blood RNA-seq dataset with a particular focus on peripheral dysregulation of TEs because of their recent emergence in multiple neurodegenerative diseases along the FTD-ALS spectrum.
C9orf72 HRE carriers (n = 49) and cognitively normal controls (n = 65) with available whole-blood-derived RNA were recruited for RNA-seq analyses (Table 2). HRE carriers had a range of clinical symptoms at the time of blood draw, with symptomatic participants having clinical diagnoses of MCI, FTD, ALS, and FTD-ALS. Also included were 12 presymptomatic HRE carriers, who did not meet consensus criteria for any of the aforementioned clinical diagnoses. As expected, a significant difference in CDR-SB scores by clinical diagnostic group was observed (p < 1 × 10−5). Presymptomatic HRE carriers were also significantly younger than both the control (p < 1 × 10−5) and symptomatic carriers (p < 1 × 10−4). There was no difference in sex across groups, and all participants self-reported race as White.
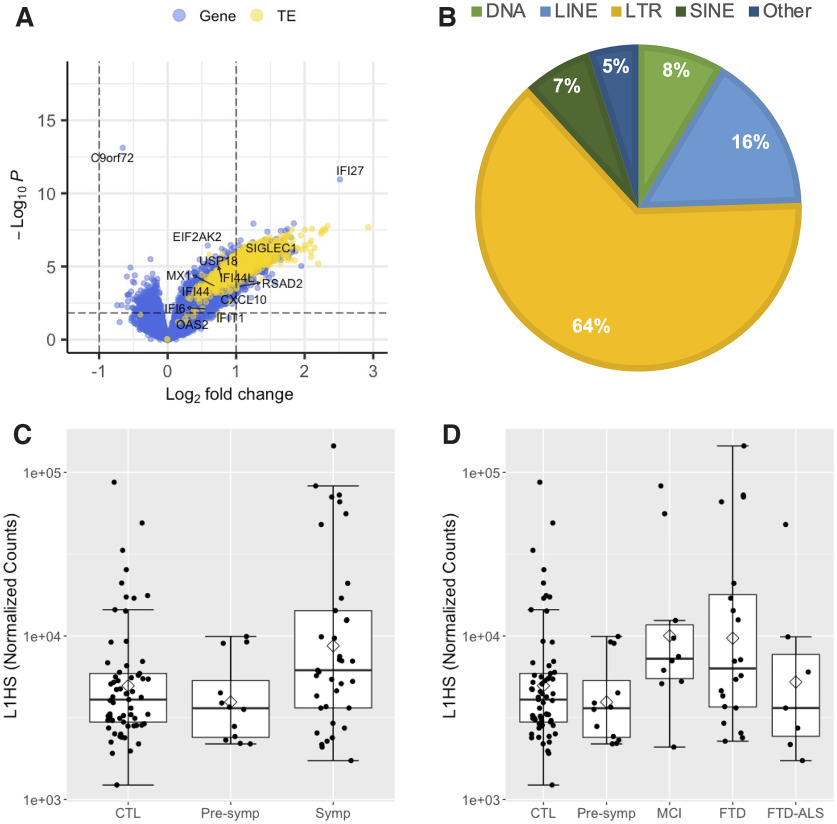
Total RNA derived from whole blood (n = 114) was deep sequenced to assess gene and TE expression across diagnostic groups. Because elevated TE expression—and in particular LINE1 element expression—has previously been reported in brain tissue from HRE carriers (Prudencio et al., 2017; Pereira et al., 2018; Tam et al., 2019; Zhang et al., 2019), we assessed total aggregate L1HS expression in cognitively normal older controls and presymptomatic and symptomatic HRE carriers. After including sex, age, and batch as covariates in the differential expression (DE) analysis, we observed significantly elevated L1HS levels in whole blood from symptomatic carriers relative to controls (logFC = 1.1, pFDR = 0.0001), whereas presymptomatic carriers had L1HS expression levels similar to controls (Fig. 4C). Beyond L1HS, nearly all detected TEs (1109 of 1112) had significantly elevated expression in symptomatic HRE carriers, with the long terminal repeat subclass followed by the LINE superfamily making up the majority of the observed upregulated TE expression (Fig. 4A,B). The latter observation is consistent with a general transcriptional derepression of TEs, including the human-specific LINE-1 element L1HS detected in blood from symptomatic C9orf72 HRE carriers.
Figure 4.
TE and type I interferon gene expression is elevated in symptomatic C9orf72 HRE carrier whole blood. A, Volcano plot of 17,220 genes (blue dots) and 1112 TEs (yellow dots) included in the DE analysis comparing symptomatic C9orf72 HRE carriers (n = 37) with healthy controls (n = 65). LogFC and raw p value determined by Wald test using DESeq2, including sex, age, and batch as covariates. Positive logFC indicates increased expression in C9orf72 HRE carriers. The horizontal dashed line represents pFDR = 0.05, with pFDR < 0.05 considered statistically significant (i.e., data points above the horizontal dashed line, including C9orf72). Select type I interferon genes are highlighted. B, The proportion of significantly elevated TEs in (A) by class and superfamily. C, L1HS expression is significantly increased in symptomatic C9orf72 HRE relative to control whole blood (logFC = 1.1, pFDR = 0.0001). The mean (diamond) and median (solid line) are shown for each group. D, L1HS expression by diagnostic group. C9orf72 HRE carriers diagnosed with FTD and MCI have significantly elevated L1HS relative to controls (FTD, logFC = 1.22, pFDR = 0.001; MCI, logFC = 1.30, pFDR = 0.02). SINE, Short interspersed element; CTL, healthy controls. Extended Data Figure 4-1 shows detailed gene set enrichment analysis results, Extended Data Figure 4-2 shows detailed type I interferon gene differential expression analysis results, and Extended Data Figure 4-3 shows full regression analysis results for L1HS expression.
Gene set enrichment analysis of differential expression results comparing whole blood from symptomatic C9orf72 HRE carriers versus healthy controls. Positive ESs indicate enrichment in C9orf72 HRE carriers. Download Figure 4-1, XLSX file (15.5KB, xlsx) .
Differential expression of type I interferon genes in whole blood from symptomatic C9orf72 HRE carriers versus healthy controls. Positive log2(fold-change) values indicate increased expression in C9orf72 HRE carriers. ND, Not detected. Download Figure 4-2, XLSX file (15.9KB, xlsx) .
Thalamic volume associations with L1HS expression. Associations of thalamic nuclei volumes with L1HS expression in a combined cohort of C9orf72 HRE carriers and controls. Results from all 50 thalamic nuclei volumes estimated using FreeSurfer 7.1 software are shown above with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 4-3, DOCX file (19KB, docx) .
To determine whether specific diagnostic groups of symptomatic carriers underlie the observed increase in L1HS expression, we divided the symptomatic HRE carrier group by clinical diagnosis (Fig. 4D). Carriers diagnosed with an FTD-spectrum disorder or MCI had significantly elevated L1HS expression levels relative to cognitively normal older controls (FTD, logFC = 1.22, pFDR = 0.001; MCI, logFC = 1.30, pFDR = 0.02). No other clinical diagnostic group had statistically significant changes in L1HS expression relative to controls.
Changes in gene expression were also assessed in whole blood from HRE carriers. Of 17,220 genes, expression of 731 and 3606 genes was significantly decreased and increased, respectively, in symptomatic C9orf72+ participants (pFDR < 0.05; Fig. 4A). Strikingly, C9orf72 was the top differentially expressed gene (DEG) with reduced expression in symptomatic HRE carriers relative to controls (logFC = −0.66, pFDR = 1.38 × 10−09; Fig. 4A). Presymptomatic carriers also had reduced C9orf72 expression, but this difference did not reach statistical significance, likely because of the small sample size of this group (logFC = −0.45, praw = 0.005, pFDR = 0.18). As IFN-I signaling genes become activated by transcriptional derepression of LINE-1 elements (De Cecco et al., 2019), and IFN-I signaling genes are upregulated in peripheral myeloid cells in C9orf72 HRE carriers (McCauley et al., 2020), we further focused on these genes. Although only a trend toward enrichment of the Interferon Alpha Response and type I Interferon Response gene sets in symptomatic HRE carrier blood was observed (Extended Data Fig. 4-1), most genes in both pathways had increased expression (76 of 114 detected genes), including 27 with significantly increased expression and the second most significant DEG, IFI27 (pFDR < 0.05; Fig. 4A, Extended Data Fig. 4-2). These findings support and extend previous observations of C9orf72 HRE-associated patterns of gene expression dysregulation in peripheral blood cells by suggesting a possible role for L1HS in the observed transcriptional changes.
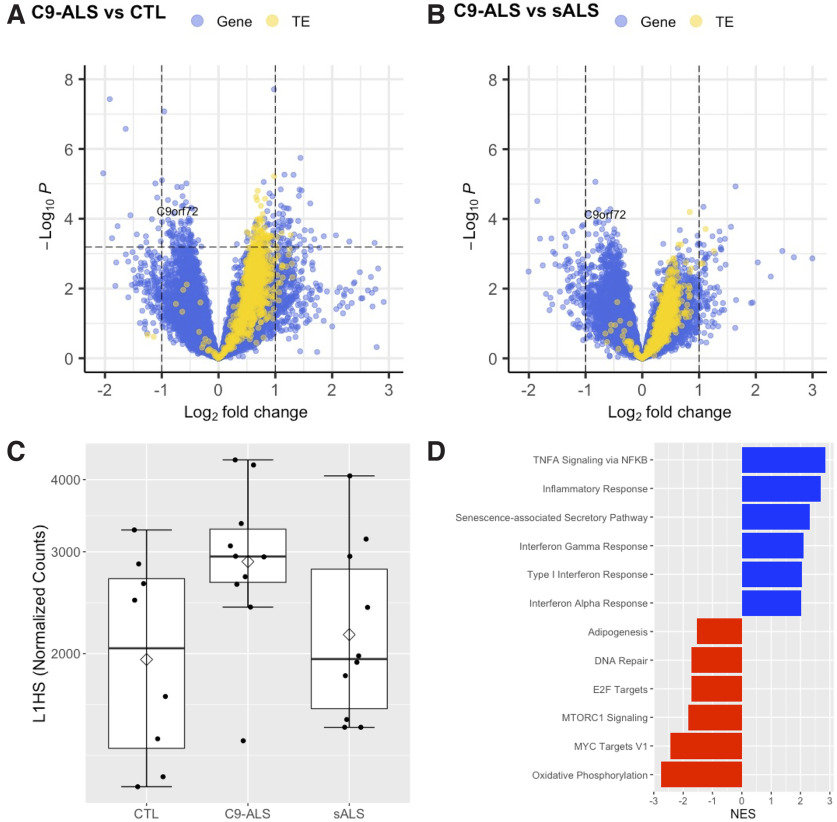
To determine whether the above findings could be replicated in an independent cohort, we performed RNA-seq analyses on PBMCs isolated from C9orf72 HRE carriers diagnosed with ALS (n = 10), individuals with sporadic ALS (n = 10), and healthy controls (n = 8). As expected, we again observed a significant reduction in C9orf72 expression in HRE carriers compared with noncarriers (Fig. 5A,B). More importantly, we observed a global upregulation of TEs in C9-ALS compared with control PBMCs, a subset of which reached significance (Fig. 5A). In comparing C9-ALS to sporadic ALS, we observed a similar trend of increased TE expression, although most individual TEs did not reach significance (Fig. 5B). In particular, L1HS expression trended toward increased expression in C9-ALS relative to control PBMCs (logFC = 0.71, pFDR = 0.075; Fig. 5C), suggesting derepression of L1HS similar to that observed in whole-blood-derived RNA from symptomatic C9orf72 HRE carriers (Fig. 4C,D). Finally, using GSEA, we identified significant enrichment of gene sets previously associated with L1HS activation (De Cecco et al., 2019), including the senescence-associated secretory pathway (SASP) and IFN-I response pathway (Fig. 5D).
Figure 5.
TE and type I interferon gene expression is elevated in PBMCs from C9orf72 HRE carriers diagnosed with ALS. A, B, Volcano plot of 18,185 genes (blue dots) and 1081 TEs (yellow dots) included in the DE analysis comparing C9orf72+ ALS (n = 10) with healthy control (n = 8; A), or sporadic ALS (n = 10; B) PBMCs. LogFC and raw p values were determined by Wald test using DESeq2, including sex and age as covariates. Positive logFC indicates increased expression in C9orf72 HRE carriers. The horizontal dashed line represents pFDR = 0.05, with pFDR < 0.05 considered statistically significant (i.e., data points above the horizontal dashed line, including C9orf72). C, L1HS expression trends toward a significant increase in C9orf72+ ALS relative to control PBMCs (logFC = 0.71, pFDR = 0.075). The mean (diamond) and median (solid line) are shown for each group. D, GSEA results of the select enriched (blue) and suppressed (red) gene sets in C9orf72+ ALS relative to control PBMCs. Expression of genes in inflammatory pathways associated with L1HS activity are elevated in C9orf72+ ALS, including the interferon alpha response, type I interferon response, and senescence-associated secretory pathway gene sets. Extended Data Figure 5-1 shows demographic characteristics of the cohort. Full gene set enrichment results are shown in Extended Data Figure 5-2. NES, Normalized enrichment score.
Demographic characteristics of PBMC RNA-seq cohort. Download Figure 5-1, DOCX file (13.4KB, docx) .
Gene set enrichment analysis of differential expression results comparing PBMCs from symptomatic ALS C9orf72 HRE carriers versus healthy controls. Positive ESs indicate enrichment in C9orf72 HRE carriers. Download Figure 5-2, XLSX file (15.6KB, xlsx) .
Given the marked upregulation of L1HS observed in the peripheral blood of symptomatic HRE carriers, we finally asked whether peripheral L1HS levels could predict clinical impairment and atrophy of thalamic nuclei in our neuroimaging cohort. Strikingly, we found that L1HS expression predicts CDR-SB scores (p = 0.02), whole thalamic volumes (p = 0.01), and volumes of several thalamic nuclei in the pulvinar (top three regions, praw < 7.9 × 10−3; Extended Data Fig. 4-3), a region of the thalamus previously shown to be affected in C9-FTD and implicated in motor function (Vatsavayai et al., 2016, 2019; Bocchetta et al., 2020). Of note, unlike C9orf72 expression, L1HS expression did not predict CDR-SB scores independent of thalamic volumes (p = 0.33).
Discussion
Our radiogenomic integration of C9orf72 HRE carrier neuroimaging and peripheral transcriptomic data enabled discoveries related to atrophy of specific thalamic nuclei and revealed an association between C9orf72 expression and clinical impairment occurring independent of thalamic atrophy. In addition, we discovered globally upregulated TE expression in peripheral blood of symptomatic HRE carriers in two independent cohorts. We also demonstrated strikingly increased expression of L1HS in affected HRE carriers and found that peripheral L1HS levels associated with thalamic nuclei volumes in FTD-relevant regions. Strikingly, our results indicate that derepression of TE expression in C9-ALS/FTD patients is not restricted to the CNS. Moreover, the peripheral upregulation of TEs such as L1HS we identified could be related to augmented expression of peripheral IFN-I signaling genes observed here and reported previously (De Cecco et al., 2019; McCauley et al., 2020).
Our neuroimaging analyses confirmed previous findings of global thalamic atrophy in C9orf72 HRE carriers (Sha et al., 2012; Bocchetta et al., 2018, 2020) and also identified atrophy of many individual thalamic nuclei, including those previously associated with HRE expansion (Lee et al., 2014, 2017; Yokoyama et al., 2014; Vatsavayai et al., 2016; Bocchetta et al., 2020). Of note, these findings are remarkably consistent despite significant differences in analytic technique; our analyses used parametric multiple regression models in keeping with prior work from our center (Sha et al., 2012), whereas other groups have used both parametric (Bocchetta et al., 2018, 2020) and nonparametric models (Schönecker et al., 2018) and hybrid frameworks (McKenna et al., 2022). Beyond this, by controlling for total thalamic volume we identified the R MDl nucleus as disproportionately atrophied in HRE carriers independent of global thalamic atrophy. The MDl nucleus projects to multiple regions of PFC, dACC, and FEF, and may influence executive function as well as motor control of eye movements (Mitchell and Chakraborty, 2013; Ouhaz et al., 2018). Given these connections, our group previously speculated that the MDl nucleus was a plausible candidate region for disproportionate dysfunction in C9-FTD (Yokoyama et al., 2014). In addition, presymptomatic C9orf72 HRE carriers were recently shown to display dysfunctional saccadic eye movements (Behler et al., 2021), a function highly influenced by the FEF and superior colliculus (Purves and Williams, 2001). Our finding of greater MDl nucleus atrophy in HRE carriers suggests an intriguing possible mechanism connecting this emerging clinical biomarker of presymptomatic disease to a neuroanatomic region projecting to the FEF. Finally, our finding that L1HS expression predicts both CDR-SB and global thalamic volumes but does not predict CDR-SB scores independent of thalamic volumes stands in contrast with our findings for C9orf72 expression. Although not conclusive, this distinction suggests that the effects of L1HS expression on clinical impairment and brain structure may be downstream of C9orf72 expression.
C9orf72 HRE carriers and a subset of sporadic FTD/ALS cases show evidence of LINE1 element derepression and retrotransposition, events thought to be related to loss of nuclear TDP-43 (Prudencio et al., 2017; Liu et al., 2019; Tam et al., 2019) and suggested by independent work in Drosophila TDP-43 models (Krug et al., 2017; Chang and Dubnau, 2019). In addition, heightened TE expression occurs in the context of tauopathies (Guo et al., 2018; Sun et al., 2018) and a distinct FTD-related proteinopathy because of pathogenic CHMP2B variation (Skibinski et al., 2005; Mackenzie and Neumann, 2016; Fort-Aznar et al., 2020). Collectively, these findings suggest that TE derepression may represent a general phenomenon occurring in multiple forms of neurodegeneration. However, to our knowledge, these findings are thus far reported exclusively within the CNS; we provide new evidence that TE upregulation can also be measured in peripheral blood and may capture disease-relevant information. Future studies will be critical to determine whether peripheral derepression of TEs also occurs in sporadic FTD patients.
Recent work in mice indicates a heightened inflammatory response in peripheral myeloid cells lacking C9orf72 (McCauley et al., 2020). Consistent with these findings, C9orf72 HRE in humans also appears to be associated with a heightened inflammatory response (Pinilla et al., 2021) driven by IFN-I signaling. Although the enhanced IFN-I signaling observed in C9-ALS myeloid cells is associated with a reduction in the peripheral expression of C9orf72, the primary mechanism driving inflammation is unknown. Impaired degradation of a promoter of IFN signaling has been proposed (McCauley et al., 2020), but gut microbiota (Burberry et al., 2020) and heightened expression of cytoplasmic double-stranded RNA in C9-ALS/FTD brains may also be important contributors (Rodriguez et al., 2021). Our RNA-seq findings confirm the expected increase in IFN-I signaling gene expression in symptomatic HRE carriers (McCauley et al., 2020) and, importantly, also provide an additional plausible explanation for this phenomenon—the upstream transcriptional derepression of TEs such as L1HS, which is known to trigger interferon signaling (Bourque et al., 2018).
Haploinsufficiency was suggested as a potential disease mechanism in C9-FTD/ALS in the initial reports characterizing C9orf72 HRE (Dejesus-Hernandez et al., 2011; Renton et al., 2011; Gijselinck et al., 2012), and evidence in support of this idea soon emerged in model organisms (Ciura et al., 2013). Until recently, this mode of pathogenicity has received comparatively less attention than the proposed GOF mechanisms. However, the identification of C9orf72 as a key regulator of peripheral macrophage and brain microglia function in mice represented a key advance and suggested plausible mechanisms for haploinsufficiency (O'Rourke et al., 2016). Further, in vitro studies of induced motor neurons derived from C9-ALS patients suggest that haploinsufficiency may synergize with GOF mechanisms, ultimately leading to cell death (Shi et al., 2018). More recent work using mouse models supports the notion of pathogenic synergy between gains and loss of function (Zhu et al., 2020). Our findings demonstrating that C9orf72 expression in peripheral blood captures information related to clinical severity, independent of an association with global thalamic atrophy, are potentially consistent with the notion that C9orf72 haploinsufficiency contributes to pathogenicity in the context of C9orf72 HRE (Shi et al., 2018). We presume that peripheral blood levels of C9orf72 relate to C9orf72 expression in the CNS, although we have not directly tested this. Any potential pathologic effects of haploinsufficiency are likely to be mediated by haploinsufficient cells residing in the CNS, although a contributory role for haploinsufficient peripheral and/or infiltrative immune cells remains possible.
Building on these findings and hypotheses, we used a novel radiogenomic approach to identify specific thalamic nuclei atrophied in C9orf72 HRE carriers, establish peripheral C9orf72 expression as a potential predictor of clinical impairment independent of thalamic atrophy, and characterize the cortical correlates of R MDl atrophy. Prior work has demonstrated that the mediodorsal thalamus is atrophied in most genetic forms of FTD and that atrophy of the pulvinar is unique to C9orf72 HRE carriers (Bocchetta et al., 2020), although these analyses were conducted covarying for TIV. Our analyses replicate and build on these findings by demonstrating that mediodorsal (i.e., R MDl nucleus) and pulvinar (i.e., R anterior pulvinar nucleus) atrophy remain significantly associated with C9orf72 HRE status even after correcting for thalamic atrophy, a unique and important finding given the pronounced global thalamic atrophy observed in HRE carriers (Bocchetta et al., 2020; Spinelli et al., 2021). To our knowledge, our finding that peripheral C9orf72 expression predicts clinical impairment independent of thalamic atrophy is novel and has potential not only as an adjunct to standard neuroimaging biomarkers of disease progression but also as a noninvasive biomarker that could be used to stratify patients in clinical trials or to measure treatment response. By characterizing the cortical regions most closely associated with R MDl atrophy, we identify atrophy in regions known to receive afferent connections from the MDl, possibly indicating degeneration of functionally connected regions.
Our study has several limitations. First, although we have replicated our primary RNA-seq findings—globally upregulated peripheral TE expression and upregulation of IFN-I signaling genes—in symptomatic HRE carriers in an independent cohort, the replication cohort was smaller, and therefore some findings replicated with only marginal significance (e.g., increased expression of L1HS in C9-ALS vs control PBMCs). In addition, some of our neuroimaging findings were marginally significant (e.g., the association of peripheral L1HS levels with pulvinar nuclei volumes). It will therefore be important in future work not only to validate our RNA-seq findings but also to replicate our novel neuroimaging findings in large, independent, and diverse cohorts. Large-scale studies using multimodal imaging (e.g., combined structural and functional MRI) and interconnected genetic networks will be required to better understand the biological processes contributing to the structural changes observed on MRI and may help to precisely delineate the neuroanatomic regions that atrophy symmetrically versus asymmetrically as well as the underlying connectivity patterns that may drive our observations.
Our study raises many critical questions. First, why do TEs, and L1HS in particular, become derepressed peripherally in symptomatic HRE carriers? This finding could be related to peripheral haploinsufficiency of C9orf72 protein, although CNS derepression of TEs in sporadic neurodegenerative disease suggests an alternative mechanism. Loss of nuclear TDP-43 has been proposed as a potential mechanism, although this is unlikely to occur in the peripheral blood cells contributing to the signal in our RNA-seq data. Future studies will also be required to determine whether derepression and increased expression of L1HS lead to increased rates of transposition. Second, does peripheral L1HS upregulation explain the upregulation of IFN-I signaling and SASP-related genes observed by us and others (De Cecco et al., 2019; McCauley et al., 2020)? Alternatively, is peripheral inflammation related more directly to cross talk with CNS inflammatory processes (Bettcher et al., 2021)? Third, do elevated peripheral L1HS levels in symptomatic HRE carriers correlate with elevated levels of L1HS in the CNS? Fourth, can the peripheral elevation of L1HS or other TEs serve as a useful biomarker in C9orf72 HRE carriers for prognosis or in clinical trials? Answers to these questions will be crucial for achieving a better understanding of C9orf72 HRE-mediated disease and other forms of neurodegeneration.
Footnotes
This work was supported by National Institutes of Health (NIH)–National Institute on Aging (NIA) Grants K01AG049152, R01AG062588, R01AG057234, P30AG062422, P01AG019724 and NIH–National Institute of Neurological Disorders and Stroke Grant U54NS123985 to J.S.Y.; Association for Frontotemporal Degeneration Susan Marcus Memorial Fund; Rainwater Charitable Foundation; Larry L. Hillblom Foundation; Bluefield Project to Cure Frontotemporal Dementia; Alzheimer's Association; Global Brain Health Institute; French Foundation; Mary Oakley Foundation; and Transposon Therapeutics. S.E.L. was supported by NIH–NIA Grant R01AG058233. V.E.S. was supported by NIH–NIA Grant R01AG052496. A.L.B. was supported by NIH–NIH Grants U19AG063911, R01AG038791, and R01AG073482; Tau Research Consortium; Association for Frontotemporal Degeneration; Bluefield Project to Cure Frontotemporal Dementia; Corticobasal Degeneration Solutions; Alzheimer's Drug Discovery Foundation; Alzheimer's Association; Biogen; Eisai; and Regeneron. We thank Giovanni Coppola for long-standing support and collaboration.
J.S.Y. serves on the scientific advisory board for the Epstein Family Alzheimer's Research Collaboration, and A.L.B. has served as a consultant for Aeovian, Applied Genetic Technologies Corp, Alector, Arkuda, Arvinas, AviadoBio, Boehringer Ingelheim, Denali, GSK (formerly GlaxoSmithKline), Life Edit, Humana, Oligomerix, Oscotec, Roche, Transposon, TrueBinding, and Wave. All the other authors declare no competing financial interests.
References
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD (1996) Visceral nociceptive input into the ventral posterolateral nucleus of the thalamus: a new function for the dorsal column pathway. J Neurophysiol 76:2661–2674. 10.1152/jn.1996.76.4.2661 [DOI] [PubMed] [Google Scholar]
- Balendra R, Isaacs AM (2018) C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat Rev Neurol 14:544–558. 10.1038/s41582-018-0047-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behler A, Knehr A, Finsel J, Kunz MS, Lang C, Müller K, Müller H-P, Pinkhardt EH, Ludolph AC, Lulé D, Kassubek J (2021) Eye movement alterations in presymptomatic C9orf72 expansion gene carriers. J Neurol 268:3390–3399. 10.1007/s00415-021-10510-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Tansey MG, Dorothée G, Heneka MT (2021) Peripheral and central immune system crosstalk in Alzheimer disease—a research prospectus. Nat Rev Neurol 17:689–701. 10.1038/s41582-021-00549-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta M, Gordon E, Cardoso MJ, Modat M, Ourselin S, Warren JD, Rohrer JD (2018) Thalamic atrophy in frontotemporal dementia—not just a C9orf72 problem. Neuroimage Clin 18:675–681. 10.1016/j.nicl.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta M, Iglesias JE, Neason M, Cash DM, Warren JD, Rohrer JD (2020) Thalamic nuclei in frontotemporal dementia: mediodorsal nucleus involvement is universal but pulvinar atrophy is unique to C9orf72. Hum Brain Mapp 41:1006–1016. 10.1002/hbm.24856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque G, Burns KH, Gehring M, Gorbunova V, Seluanov A, Hammell M, Imbeault M, Izsvák Z, Levin HL, Macfarlan TS, Mager DL, Feschotte C (2018) Ten things you should know about transposable elements. Genome Biol 19:199. 10.1186/s13059-018-1577-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braems E, Swinnen B, Van Den Bosch L (2020) C9orf72 loss-of-function: a trivial, stand-alone or additive mechanism in C9 ALS/FTD? Acta Neuropathol 140:625–643. 10.1007/s00401-020-02214-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- Burberry A, Wells MF, Limone F, Couto A, Smith KS, Keaney J, Gillet G, van Gastel N, Wang J-Y, Pietilainen O, Qian M, Eggan P, Cantrell C, Mok J, Kadiu I, Scadden DT, Eggan K (2020) C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582:89–94. 10.1038/s41586-020-2288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-H, Dubnau J (2019) The gypsy endogenous retrovirus drives non-cell-autonomous propagation in a Drosophila TDP-43 model of neurodegeneration. Curr Biol 29:3135–3152.e4. 10.1016/j.cub.2019.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A, Kabashi E (2013) Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol 74:180–187. 10.1002/ana.23946 [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Tan A, Edwards S (1999) Comparing thalamocortical and corticothalamic microstructure and spatial reciprocity in the macaque ventral posterolateral nucleus (VPLc) and medial pulvinar. J Comp Neurol 410:211–234. [DOI] [PubMed] [Google Scholar]
- De Cecco M, et al. (2019) L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566:73–78. 10.1038/s41586-018-0784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejesus-Hernandez M, et al. (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fort-Aznar L, Ugbode C, Sweeney ST (2020) Retrovirus reactivation in CHMP2BIntron5 models of frontotemporal dementia. Hum Mol Genet 29:2637–2646. 10.1093/hmg/ddaa142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijselinck I, et al. (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 11:54–65. 10.1016/S1474-4422(11)70261-7 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, et al. (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Eils R, Schlesner M (2016) Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32:2847–2849. 10.1093/bioinformatics/btw313 [DOI] [PubMed] [Google Scholar]
- Guo C, Jeong H-H, Hsieh Y-C, Klein H-U, Bennett DA, De Jager PL, Liu Z, Shulman JM (2018) Tau activates transposable elements in Alzheimer's disease. Cell Rep 23:2874–2880. 10.1016/j.celrep.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, van der Kouwe A, Fischl B, Caballero-Gaudes C, Paz-Alonso PM (2018) A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 183:314–326. 10.1016/j.neuroimage.2018.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas A, Pizarro D, Romeo AK, Riley KO, Pati S (2019) The centromedian nucleus: anatomy, physiology, and clinical implications. J Clin Neurosci 63:1–7. 10.1016/j.jocn.2019.01.050 [DOI] [PubMed] [Google Scholar]
- Jin Y, Hammell M (2018) Analysis of RNA-seq data using TEtranscripts. Methods Mol Biol 1751:153–167. 10.1007/978-1-4939-7710-9_11 [DOI] [PubMed] [Google Scholar]
- Jin Y, Tam OH, Paniagua E, Hammell M (2015) TEtranscripts: a package for including transposable elements in differential expression analysis of RNA-seq datasets. Bioinformatics 31:3593–3599. 10.1093/bioinformatics/btv422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause T, Brunecker P, Pittl S, Taskin B, Laubisch D, Winter B, Lentza ME, Malzahn U, Villringer K, Villringer A, Jungehulsing GJ (2012) Thalamic sensory strokes with and without pain: differences in lesion patterns in the ventral posterior thalamus. J Neurol Neurosurg Psychiatry 83:776–784. 10.1136/jnnp-2011-301936 [DOI] [PubMed] [Google Scholar]
- Krug L, Chatterjee N, Borges-Monroy R, Hearn S, Liao W-W, Morrill K, Prazak L, Rozhkov N, Theodorou D, Hammell M, Dubnau J (2017) Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLOS Genet 13:e1006635. 10.1371/journal.pgen.1006635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, et al. (2014) Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 137:3047–3060. 10.1093/brain/awu248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Sias AC, Mandelli ML, Brown JA, Brown AB, Khazenzon AM, Vidovszky AA, Zanto TP, Karydas AM, Pribadi M, Dokuru D, Coppola G, Geschwind DH, Rademakers R, Gorno-Tempini ML, Rosen HJ, Miller BL, Seeley WW (2017) Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin 14:286–297. 10.1016/j.nicl.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Jin Y, Prazak L, Hammell M, Dubnau J (2012) Transposable elements in TDP-43-mediated neurodegenerative disorders. PLoS One 7:e44099. 10.1371/journal.pone.0044099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu EY, Russ J, Cali CP, Phan JM, Amlie-Wolf A, Lee EB (2019) Loss of nuclear TDP-43 is associated with decondensation of LINE retrotransposons. Cell Rep 27:1409–1421.e6. 10.1016/j.celrep.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M (2016) Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem 138:54–70. 10.1111/jnc.13588 [DOI] [PubMed] [Google Scholar]
- Mai JK, Forutan F (2012) Thalamus. In: The human nervous system (Mai JK, Paxinos G, eds), pp 618–677. Amsterdam: Elsevier Academic Press. [Google Scholar]
- McCauley ME, O'Rourke JG, Yáñez A, Markman JL, Ho R, Wang X, Chen S, Lall D, Jin M, Muhammad AKMG, Bell S, Landeros J, Valencia V, Harms M, Arditi M, Jefferies C, Baloh RH (2020) C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature 585:96–101. 10.1038/s41586-020-2625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Shing SLH, Murad A, Lope J, Hardiman O, Hutchinson S, Bede P (2022) Focal thalamus pathology in frontotemporal dementia: phenotype-associated thalamic profiles. J Neurol Sci 436:120221. 10.1016/j.jns.2022.120221 [DOI] [PubMed] [Google Scholar]
- Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, Lobach IV, Bettcher BM, Wu TQ, Rabinovici GD, Graff-Radford NR, Miller BL, Gorno-Tempini ML (2013) Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 136:3461–3473. 10.1093/brain/awt242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Chakraborty S (2013) What does the mediodorsal thalamus do? Front Syst Neurosci 7:37. 10.3389/fnsys.2013.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, et al. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273. 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- O'Rourke JG, Bogdanik L, Yáñez A, Lall D, Wolf AJ, Muhammad AKMG, Ho R, Carmona S, Vit JP, Zarrow J, Kim KJ, Bell S, Harms MB, Miller TM, Dangler CA, Underhill DM, Goodridge HS, Lutz CM, Baloh RH (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. 10.1126/science.aaf1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhaz Z, Fleming H, Mitchell AS (2018) Cognitive functions and neurodevelopmental disorders involving the prefrontal cortex and mediodorsal thalamus. Front Neurosci 12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikshak NN, Swarup V, Belgard TG, Irimia M, Ramaswami G, Gandal MJ, Hartl C, Leppa V, Ubieta L, de la T, Huang J, Lowe JK, Blencowe BJ, Horvath S, Geschwind DH (2016) Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature 540:423–427. 10.1038/nature20612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira GC, Sanchez L, Schaughency PM, Rubio-Roldán A, Choi JA, Planet E, Batra R, Turelli P, Trono D, Ostrow LW, Ravits J, Kazazian HH, Wheelan SJ, Heras SR, Mayer J, García-Pérez JL, Goodier JL (2018) Properties of LINE-1 proteins and repeat element expression in the context of amyotrophic lateral sclerosis. Mob DNA 9:35. 10.1186/s13100-018-0138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L (2014) Mild cognitive impairment: a concept in evolution. J Intern Med 275:214–228. 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla G, Kumar A, Floaters MK, Pardo CA, Rothstein J, Ilieva H (2021) Increased synthesis of pro-inflammatory cytokines in C9ORF72 patients. Amyotroph Lateral Scler Frontotemporal Degener 22:517–527. 10.1080/21678421.2021.1912100 [DOI] [PubMed] [Google Scholar]
- Prudencio M, Gonzales PK, Cook CN, Gendron TF, Daughrity LM, Song Y, Ebbert MTW, van Blitterswijk M, Zhang Y-J, Jansen-West K, Baker MC, DeTure M, Rademakers R, Boylan KB, Dickson DW, Petrucelli L, Link CD (2017) Repetitive element transcripts are elevated in the brain of C9orf72 ALS/FTLD patients. Hum Mol Genet 26:3421–3431. 10.1093/hmg/ddx233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Williams SM (2001) Neuroscience. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Rankin KP, Kramer JH, Miller BL (2005) Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 18:28–36. 10.1097/01.wnn.0000152225.05377.ab [DOI] [PubMed] [Google Scholar]
- Rascovsky K, et al. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, et al. (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzu P, Blauwendraat C, Heetveld S, Lynes EM, Castillo-Lizardo M, Dhingra A, Pyz E, Hobert M, Synofzik M, Simón-Sánchez J, Francescatto M, Heutink P (2016) C9orf72 is differentially expressed in the central nervous system and myeloid cells and consistently reduced in C9orf72, MAPT and GRN mutation carriers. Acta Neuropathol Commun 4:37. 10.1186/s40478-016-0306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S, Sahin A, Schrank BR, Al-Lawati H, Costantino I, Benz E, Fard D, Albers AD, Cao L, Gomez AC, Evans K, Ratti E, Cudkowicz M, Frosch MP, Talkowski M, Sorger PK, Hyman BT, Albers MW (2021) Genome-encoded cytoplasmic double-stranded RNAs, found in C9ORF72 ALS-FTD brain, propagate neuronal loss. Sci Transl Med 13:eaaz4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönecker S, et al. (2018) Atrophy in the thalamus but not cerebellum is specific for C9orf72 FTD and ALS patients—an atlas-based volumetric MRI study. Front Aging Neurosci 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML (2008) Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 65:249–255. 10.1001/archneurol.2007.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Zhou J, Kim E-J (2012) Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist 18:373–385. 10.1177/1073858411410354 [DOI] [PubMed] [Google Scholar]
- Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, Khan B, Karydas A, Baker MC, Dejesus-Hernandez M, Pribadi M, Coppola G, Geschwind DH, Rademakers R, Lee SE, Seeley W, Miller BL, Boxer AL (2012) Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology 79:1002–1011. 10.1212/WNL.0b013e318268452e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, et al. (2018) Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med 24:313–325. 10.1038/nm.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sørensen SA, Gydesen S, Fisher EMC, Collinge J (2005) Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37:806–808. 10.1038/ng1609 [DOI] [PubMed] [Google Scholar]
- Spinelli EG, Ghirelli A, Basaia S, Cividini C, Riva N, Canu E, Castelnovo V, Domi T, Magnani G, Caso F, Caroppo P, Prioni S, Rossi G, Tremolizzo L, Appollonio I, Silani V, Carrera P, Filippi M, Agosta F (2021) Structural MRI signatures in genetic presentations of the frontotemporal dementia-motor neuron disease spectrum. Neurology 97:e1594–e1607. 10.1212/WNL.0000000000012702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, Mioshi E, Roberts-South A, Benatar M, HortobáGyi T, Rosenfeld J, Silani V, Ince PG, Turner MR (2017) Amyotrophic lateral sclerosis–frontotemporal spectrum disorder (ALS–FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18:153–174. 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Samimi H, Gamez M, Zare H, Frost B (2018) Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci 21:1038–1048. 10.1038/s41593-018-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam OH, Rozhkov NV, Shaw R, Kim D, Hubbard I, Fennessey S, Propp N, NYGC ALS, Consortium Fagegaltier D, Harris BT, Ostrow LW, Phatnani H, Ravits J, Dubnau J, Gale Hammell M (2019) Postmortem cortex samples identify distinct molecular subtypes of ALS: retrotransposon activation, oxidative stress, and activated glia. Cell Rep 29:1164–1177.e5. 10.1016/j.celrep.2019.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsavayai SC, Yoon SJ, Gardner RC, Gendron TF, Vargas JNS, Trujillo A, Pribadi M, Phillips JJ, Gaus SE, Hixson JD, Garcia PA, Rabinovici GD, Coppola G, Geschwind DH, Petrucelli L, Miller BL, Seeley WW (2016) Timing and significance of pathological features in C9orf72 expansion-associated frontotemporal dementia. Brain 139:3202–3216. 10.1093/brain/aww250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsavayai SC, Nana AL, Yokoyama JS, Seeley WW (2019) C9orf72-FTD/ALS pathogenesis: evidence from human neuropathological studies. Acta Neuropathol 137:1–26. 10.1007/s00401-018-1921-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508:212–237. 10.1002/cne.21679 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB (2015) Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev 54:89–107. 10.1016/j.neubiorev.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC (2010) Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53:1135–1146. 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama JS, Rosen HJ (2012) Neuroimaging features of C9ORF72 expansion. Alzheimers Res Ther 4:45. 10.1186/alzrt148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama JS, Sirkis DW, Miller BL (2014) C9ORF72 hexanucleotide repeats in behavioral and motor neuron disease: clinical heterogeneity and pathological diversity. Am J Neurodegener Dis 3:1–18. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-J, et al. (2019) Heterochromatin anomalies and double-stranded RNA accumulation underlie C9orf72 poly(PR) toxicity. Science 363:eaav2606. 10.1126/science.aav2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Jiang J, Gendron TF, McAlonis-Downes M, Jiang L, Taylor A, Diaz Garcia S, Ghosh Dastidar S, Rodriguez MJ, King P, Zhang Y, La Spada AR, Xu H, Petrucelli L, Ravits J, Da Cruz S, Lagier-Tourenne C, Cleveland DW (2020) Reduced C9ORF72 function exacerbates gain of toxicity from ALS/FTD-causing repeat expansion in C9orf72. Nat Neurosci 23:615–624. 10.1038/s41593-020-0619-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thalamic volume differences in C9orf72 HRE carriers compared to controls covarying for total intracranial volume. Comparisons of thalamic nuclei volumes in C9orf72 HRE carriers versus controls. Results from all 50 thalamic nuclei volumes estimated using FreeSurfer 7.1 software are shown above with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 1-1, DOCX file (19.5KB, docx) .
Thalamic volume differences in C9orf72 HRE carriers compared to controls covarying for total thalamic volume. Sensitivity analyses comparing of thalamic nuclei volumes in C9orf72 HRE carriers versus controls after covarying for total thalamic volume rather than total intracranial volume. Results from all 50 thalamic nuclei volumes estimated using FreeSurfer 7.1 software are shown above with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total thalamic volumes. L, Left. Download Figure 2-1, DOCX file (19.4KB, docx) .
C9orf72 expression in C9orf72 HRE carriers versus controls. Multiple regression analyses demonstrate that C9orf72 expression in C9orf72 HRE carriers versus controls is significantly decreased after covarying for the effects of sex, age, education, clinical severity (as estimated by CDR-SB score), and RNA-seq batch. Download Figure 3-1, DOCX file (14KB, docx) .
Right mediodorsal lateral parvocellular nucleus volumes associate with cortical thicknesses. Associations between right mediodorsal lateral parvocellular nucleus volumes and cortical thicknesses are shown. Results for all 68 cortical regions of interest from the Desikan–Killiany atlas with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-2, DOCX file (20.5KB, docx) .
CDR-SB score associations with whole-brain cortical thicknesses. Associations between CDR-SB score and cortical thicknesses are shown. Results for all 68 cortical regions of interest from the Desikan–Killiany atlas with associated p values shown before and after FDR correction for multiple testing. All regression analysis covaried for age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-3, DOCX file (20.8KB, docx) .
Cortical thickness differences in C9orf72 HRE carriers compared to controls. Associations between C9orf72 HRE carrier status and cortical thicknesses are shown for all 68 cortical regions of interest from the Desikan–Killiany atlas with associated p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-4, DOCX file (20.4KB, docx) .
Cortical thickness associations with C9orf72 expression in HRE carriers and controls. Associations of C9orf72 expression and cortical thicknesses are shown for all 68 cortical regions of interest from the Desikan–Killiany atlas with associated p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 3-5, DOCX file (20.8KB, docx) .
Gene set enrichment analysis of differential expression results comparing whole blood from symptomatic C9orf72 HRE carriers versus healthy controls. Positive ESs indicate enrichment in C9orf72 HRE carriers. Download Figure 4-1, XLSX file (15.5KB, xlsx) .
Differential expression of type I interferon genes in whole blood from symptomatic C9orf72 HRE carriers versus healthy controls. Positive log2(fold-change) values indicate increased expression in C9orf72 HRE carriers. ND, Not detected. Download Figure 4-2, XLSX file (15.9KB, xlsx) .
Thalamic volume associations with L1HS expression. Associations of thalamic nuclei volumes with L1HS expression in a combined cohort of C9orf72 HRE carriers and controls. Results from all 50 thalamic nuclei volumes estimated using FreeSurfer 7.1 software are shown above with p values shown before and after FDR correction for multiple testing. All regression analysis covaried for clinical severity (as estimated by CDR-SB score), age, sex, education, MRI scanner type (1.5T, 3T, or 4T), and total intracranial volume. L, Left. Download Figure 4-3, DOCX file (19KB, docx) .
Demographic characteristics of PBMC RNA-seq cohort. Download Figure 5-1, DOCX file (13.4KB, docx) .
Gene set enrichment analysis of differential expression results comparing PBMCs from symptomatic ALS C9orf72 HRE carriers versus healthy controls. Positive ESs indicate enrichment in C9orf72 HRE carriers. Download Figure 5-2, XLSX file (15.6KB, xlsx) .
Data Availability Statement
RNA-seq data have been uploaded to the FAIR Data Sharing Portal within the Alzheimer's Disease Workbench, which is supported by the Alzheimer's Disease Data Initiative, and can be accessed at https://www.alzheimersdata.org/ad-workbench.