Abstract
Background:
Chronic red blood cell transfusions reduce acute care utilization for sickle cell disease (SCD) pain. However, little is known about whether chronic transfusions treat or prevent the development of non-crisis pain. We investigated patient-report of pain in adults with SCD receiving chronic exchange transfusions (CET) compared to adults not on CET with similar disease characteristics.
Study Method and Design:
Eleven participants receiving chronic exchange transfusion (CET) for at least one year were compared to 33 participants not receiving CET. Participants completed validated patient-reported outcomes regarding pain impact and quality of life at regularly scheduled visits or before CET. One year of health care utilization and opioid prescriptions were examined.
Results:
After 1:1 propensity matching was performed for age, genotype, WBC and neutrophil counts, patients on CET had lower Pain Impact scores (−5.1, p=0.03) and higher Neuropathic (7.4, p<0.001) and Nociceptive Pain Quality (3.7, p<0.001) scores, all indicating worse pain. However, CET was associated with a reduction in annual all cause admissions (−3.1, p<0.001), length of stay (−2.1 days, p<0.001) and ED visits (−2.7, p<0.001). CET was not associated with differences in opioids dispensed.
Conclusions:
After adjusting for disease characteristics, CET was associated with worse pain impact and neuropathic and nociceptive pain quality, lower health care utilization and with similar levels of opioids dispensed. This data suggest that CET may reduce hospitalizations for acute pain but may not adequately treat nociceptive or neuropathic pain in SCD.
Keywords: sickle cell disease, exchange transfusion, chronic pain, pain crisis, opioid use
Introduction:
Sickle cell disease (SCD) results in red blood cells (RBCs) taking on a sickled shaped due to hemoglobin polymerization in the deoxygenated state. These “sickled” cells adhere to the endothelium along with leukocytes and platelets leading to hemolysis, vascular congestion, hypoxia, ischemia-reperfusion injury, and chronic inflammation.1,2 SCD is complicated by acute pain events (pain crises), chronic pain, other causes of non-crisis related pain, and cerebrovascular events among other multi-organ complications. There is some evidence that chronic RBC transfusions (simple or exchange) reduce health care encounters for acute pain crises in both children and adults, but little is known about their effect on pain not brought to medical attention.3,4
Pain not due to pain crisis is common in adults with SCD and is difficult to treat. It has been estimated that 30% of patients with SCD develop chronic pain, defined as pain present on most or all days for at least 6 months.5,6 Chronic pain often develops during adolescence and worsens with increasing age.5,7,8 This pain also continues to be punctuated by exacerbations of acute pain.5,9 SCD pain is varied within and between individuals; multiple pain phenotypes exist including nociceptive, neuropathic, and inflammatory pain and all may be worsened by the development of central or peripheral sensitization.10–14
Red blood cell transfusion reduces the circulating fraction of RBCs with sickle hemoglobin, but little is known about its effectiveness for non-crisis pain. Simple or exchange transfusions may be prescribed acutely to treat disease complications or chronically to prevent new or recurrent complications. As a result, many patients with SCD receive chronic transfusion therapy for years.15,16 However, the benefits of transfusion therapy must be weighed against the risks of red blood cell antibody formation, iron overload, and vascular access issues, among other considerations.17–19 Secondary analyses of both the Silent Cerebral Infarct Transfusion Trial (SIT) and the Stroke with Transfusions Changing to Hydroxyurea (SWiTCH) trial showed that chronic transfusions reduce health care encounters for acute pain events in children with SCD. Smaller, non-randomized studies show chronic transfusions also reduce acute pain events in adults.4,7,20,21 However, only one study has examined the effect of chronic transfusions on chronic pain in adults with SCD. Karafin et al found that 10 of 14 adults on regular simple or exchange transfusions reported pain on most days in a period of approximately two months.22 Thus it is unclear whether transfusions can improve non crisis related pain, alter the variable types of pain experienced, or reduce the development of chronic pain as patients age.
In a cross-sectional analysis at a single center, we sought to compare pain-related patient-reported outcomes measures and quality of life measures between adults undergoing CET to adults not on CET with similar characteristics of disease morbidity. We hypothesized that adults on CET would have fewer acute pain episodes but have similar patient-reports of pain quality and pain impact to those not on CET.
Methods:
Participants
This was a cross-sectional study. Participants enrolled were adults (≥ 18 years) with a diagnosis of SCD (HbSS, HbSC, HbSβ+ or HbSβ0-thalassemia) who presented to our adult sickle cell center for scheduled clinic or CET visits. We enrolled both participants receiving CET (cases) and participants not receiving CET (controls). Participants were asked if they had experienced either an acute pain crisis or any exacerbation of non-crisis pain in the past 7 days and if either were reported they were excluded from the study. Participants were also excluded if they had any illness or visit to a health care facility in the EMR in the past 7 days, were pregnant, unable to offer informed consent, or were receiving regular simple transfusions.
Cases were patients with SCD who were receiving chronic transfusions via CET for any indication for at least one year with at least three documented %HbS values post-transfusion below 20% within the past year. Leukoreduced, sickle negative RBCs were phenotypically matched at C/c, E/e,K and negative for any other antigens against which a participant was alloimmunized were utilized for automated RBC exchange, which was completed on the Spectra Optia or Cobe Spectra. Controls were patients with SCD who had not received regular scheduled transfusions (exchange or simple) for at least one year prior to study enrollment.
This study was approved by the Yale New Haven Hospital Institutional Review Board and informed consent was obtained prior to study procedures. Participants were financially compensated for their participation.
Outcomes
Primary Outcome
Our primary outcome was pain that was assessed using two validated patient-reported outcome measures (PROs). These measures included: 1) Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) measures for Pain Episode Frequency and Severity and Pain Impact, and 2) Patient Reported Outcome Measures Information System (PROMIS) measures for Neuropathic and Nociceptive Pain Quality. Participants completed all instruments using paper and pencil at a single regularly scheduled visit. For participants on CET, these PROs were obtained immediately prior to a scheduled transfusion. Blood samples for complete blood counts were obtained concurrently.
ASCQ-Me Pain Measures
The ASCQ-Me Pain Episode Frequency and Severity measure asks about acute pain events for the past 12 months. All other measures have a recall period of 7 days. The Pain Impact measure asks questions regarding the impact of pain on daily living in the past week. These measures were developed and validated using adults living with sickle cell disease. The median score and standard deviation representing the median amount of pain experienced by an adult with sickle cell disease is 50±10. For both Pain Episode Frequency and Severity higher scores represent worse pain. For Pain Impact lower scores represent worse pain and a difference of 3–5 points is considered clinically significant.23 Measures were scored using T scores using the ASCQ-Me scoring manual created by the domain developers.24
PROMIS Pain Measures
PROMIS nociceptive and neuropathic pain measures refer to the quality of pain experienced in the past 7 days and are validated to determine the presence or absence of neuropathic and nociceptive pain.23,25–28 They were developed using populations with known neuropathic or nociceptive pain. Both measures are scored using a T-score metric in which a score of 50 represents the median score of the reference population and 10 is the standard deviation of that population. Scoring was done using the HealthMeasures Scoring Service powered by Assessment Center.
Secondary outcomes
Other Patient-Reported Outcome Measures
As pain also impacts other aspects of patient functioning, we assessed additional domains pertinent to our primary outcome of pain as secondary outcomes. These included ASCQ-Me measures for Stiffness Impact, Sleep Impact, Emotional Impact and Social Functioning Impact which were developed and validated using adults living with sickle cell disease and the PROMIS measure for Anxiety (Short Form Anxiety 8a) which was developed in the general population. The Social Functioning Impact and Emotional Impact measures have a recall period of 30 days and all other measures have a recall period of 7 days. Median scores for all measures is 50 and standard deviation is 10, for the ASCQ-Me measures lower scores represent worse symptoms and for the Anxiety measure higher scores represent worse symptoms. ASCQ-Me measures were scored using the ASCQ-Me scoring manual and PROMIS measures were scored using the HealthMeasures Scoring Service powered by Assessment Center. 29,30
Health Care Utilization and Opioid Data
Clinical information for each subject was obtained from the electronic medical record including ICD10 codes for avascular necrosis and lower extremity ulcers as these are known comorbidities of SCD that cause pain. Total numbers of emergency department visits and hospital admissions to our center, and average length of stay in days, from 1/1/2016 to 12/31/2016 for each individual participant were recorded. Total milligrams of opioids dispensed from outpatient pharmacies from 1/1/2016 to 12/31/2016 was obtained from the Connecticut Prescription Monitoring and Reporting System (PMPAware) database and converted to daily oral morphine equivalents (OME).
Propensity Matched Analysis
Participants on CET may have a history of worse SCD complications such as stroke than participants not on CET, which could confound effects of CET on pain outcomes. To adjust for this, measures of pain and health care utilization in cases were compared to controls using 1:1 propensity matching for laboratory values and other clinical characteristics shown to correlate with poor clinical outcomes in previous studies. Factors selected were age, SCD genotype (HbSS/HbSβ0 vs HbSC/HbSβ+),WBC, and absolute neutrophil count.31–34 A p-value less than 0.05 was considered statistically significant. Missing PRO data was handled according to the instructions in the ASCQ-Me scoring manual or by the HealthMeasures Assessment Center.
Statistical Considerations
Participant demographics, health care utilization data, and complete blood counts, were summarized using means and standard deviations or medians and interquartile ranges as appropriate for cases and controls. Comparison of these data between cases and controls was done using Students T test for parametric data and Wilcoxon rank-sum test for non-parametric data. Direct comparison of ASCQ-Me and PROMIS domain scores between cases and controls was done using Student’s T test. Linear regression was used to examine the associations between time on CET or age starting CET, and pain impact scores or opioids dispensed.
Results
Eleven participants who had received CET at the center for at least one year and 33 participants who were not on CET were enrolled between 6/2017 to 12/2017. Of the 11, 10 were on CET for secondary prophylaxis against neurological events and one was on CET for primary prophylaxis against neurologic events. All patients had been on CET for at least one year and some participants had been on chronic simple transfusions before transitioning to CET; the median number of years on regular scheduled transfusions was 12.0 (IQR 3–15.5) and median age at initiation of transfusions was 14 years (IQR 5.5–29.5) (Table 1). Participants on CET had a mean pretransfusion %S of 42.3±8.3 and a mean posttransfusion %S of 16.1±2.8. There were no significant differences in gender, age, genotype, frequency of avascular necrosis or lower extremity ulcers between the those on CET and those not on CET (Table 1). Of those not on CET 49% were taking hydroxyurea.
Table 1.
Comparison of demographic, transfusion, medication, and hospitalization data for patients on chronic exchange transfusion therapy (CET) compared to those not on CET.
| Subjects on CET, N=11 | Subjects not on CET N=33 | P Value | |
|---|---|---|---|
| Age (Mean+/−SD) | 29.2±10.3 | 35.5±14.7 | 0.2 |
| Gender (% Female) | 45% | 61% | 0.4 |
| Genotype | SS/Sβ0 (82%) SC/Sβ+(18%) | SS/Sβ0 (58%) SC/Sβ+(42%) | 0.1 |
| Years on Chronic Transfusion Therapy Median (IQR) | 12.0 (3.0–15.5) | - | - |
| Opioids Dispensed (Daily OME) Median (IQR) | 3.9 (0.4–28.6) | 6.2 (0.8/102.9) | 0.5 |
| Ulcers (% with) | 9% | 9% | 1.0 |
| Avascular Necrosis (% with) | 9% | 15% | 0.3 |
| Hydroxyurea (%) | 0% | 49% | .004 |
| Hospital Admissions Median (IQR) | 1.0(0.0–1.50) | 2.0(0.0/5.0) | 0.2 |
| ED Visits Median (IQR) | 0.0 (0.0–0.50) | 1.0 (0.0/2.0) | 0.4 |
| Length of Stay (Days) (Mean ± SD) | 3.2±1.5 | 4.0±1.6 | 0.6 |
| Hemoglobin (g/dL) (Mean±SD) | 9.4±1.7 | 10.1±1.9 | 0.3 |
| MCV (fL) (Mean±SD) | 83.3±10.9 | 84.9±12.6 | 0.7 |
| Platelet (x10 9 /L) (Mean±SD) | 367.1±91.6 | 333.9±166.1 | 0.5 |
| WBC (x10 9 /L) (Mean±SD) | 11.6±3.8 | 9.6±3.4 | 0.2 |
| Neutrophil (x10 9 /L) (Mean±SD) | 7.8±2.7 | 5.4±2.1 | 0.006 |
| Lymphocyte (x109/L) (Mean±SD) | 2.4±1.0 | 2.9±1.4 | 0.3 |
| Monocyte (x10 9 /L) (Mean±SD) | 1.2±0.4 | 0.9±0.5 | 0.1 |
IQR=interquartile range.
Comparisons with Propensity Matching
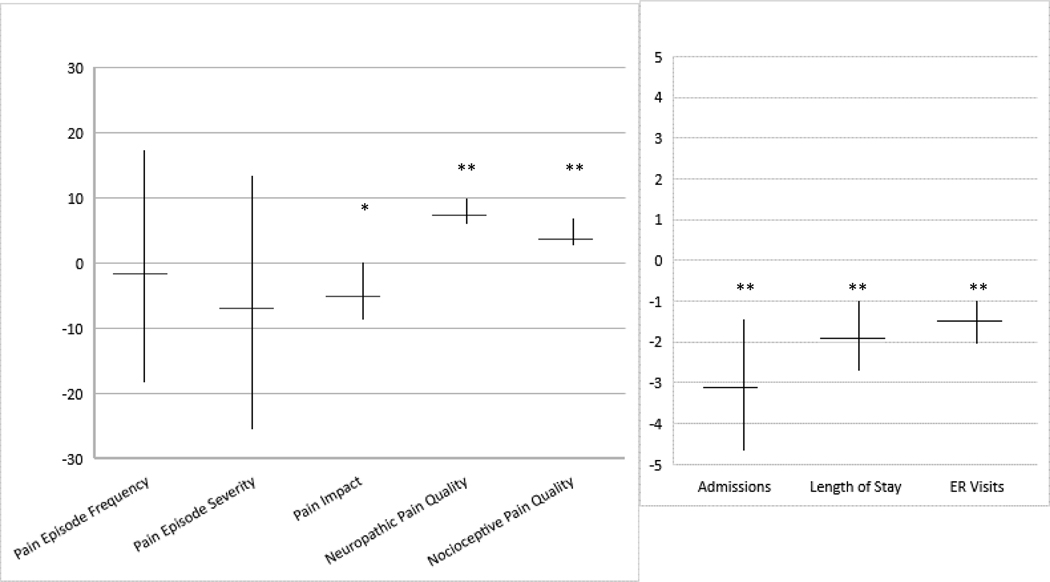
After propensity matching, participants on CET continued to have similar amounts of daily opioids dispensed as those not on CET (Figure 1). However, participants on CET had lower ASCQ-Me Pain Impact scores (−5.1, p=0.03) (Figure 1) indicating worse impact from their pain in the past 7 days and higher scores of PROMIS Neuropathic Pain Quality (7.4, p<0.001) and Nociceptive Pain Quality (3.7, p<0.001) indicating higher likelihood of the presence of nociceptive and neuropathic pain. CET was associated with a reduction in annual all cause admissions (−3.1, p<0.001) and length of stay (−2.1 days, p<0.001) and all-cause ER visits (−2.7, p<0.001). CET was not associated with differences in Pain Episode Frequency or Pain Episode Severity measure scores (Figure 3).
Figure 1. Effect of chronic exchange transfusions (CET) on Pain and Health Care Utilization Outcomes Using Propensity Matching.
Estimated effects of CET on pain outcomes after 1:1 propensity matching for age, genotype, WBC, and total neutrophil count. The coefficient marked with the horizontal line shows the estimated effect of CET on patient-reported outcome pain domains in the figure on the left (an increase in scores represent worse symptoms and a decrease in scores represent better symptoms in all domains except in Pain Impact where lower scores represent worse symptoms and higher scores represent better symptoms) and on hospital utilization in the figure on the right (a decrease represents an estimated decrease in utilization). The vertical line shows 95% confidence intervals of the estimated effect. *p=<0.05, **p=<0.001
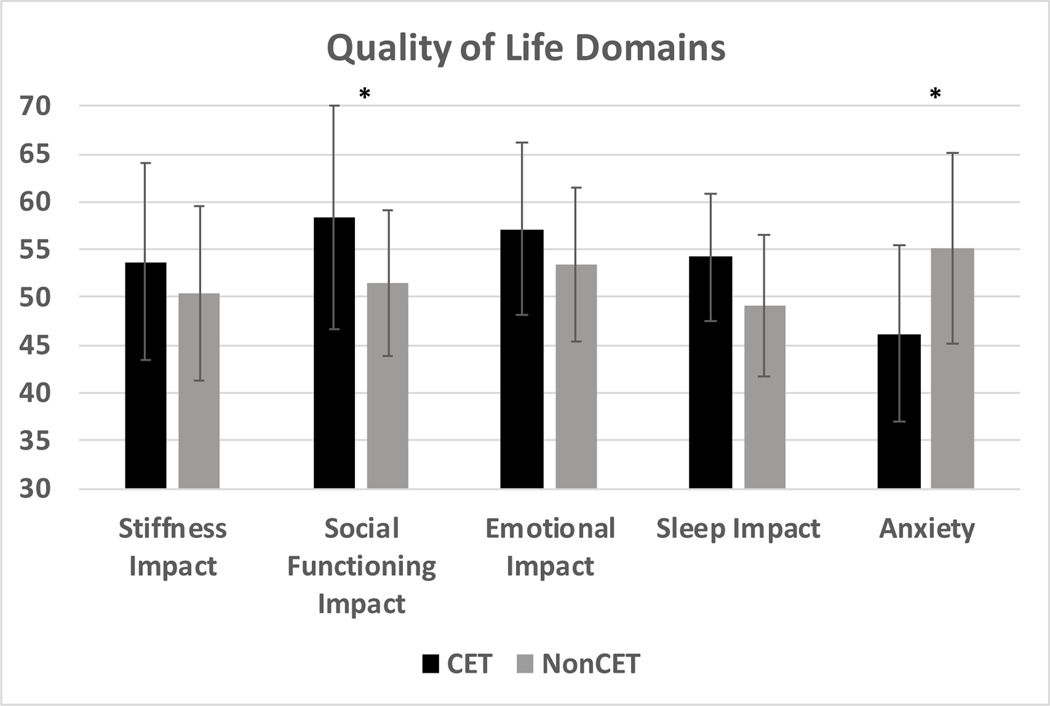
Figure 3. Quality of life domains for those on chronic exchange transfusions (CET) compared to those not on CET.
Quality of life domains as measured using the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) and the Patient Reported Outcomes Measurement Information System (PROMIS), in patients with SCD on CET (black bars) compared to those not on CET (grey bars). The y-axis shows ASCQ-Me (Stiffness Impact, Social Functioning Impact, Emotional Impact, Sleep Impact) and PROMIS (Anxiety) domain scores. A score of 50 is the median for the validation cohort in all domains. Lower scores represent worse symptoms and higher scores represent better symptoms in all domains except for Anxiety where higher scores represent worse symptoms and lower scores represent better symptoms. Ten is one standard deviation from the mean of the validation cohort for all domains. *p=<0.05
Direct Comparisons without Propensity Matching
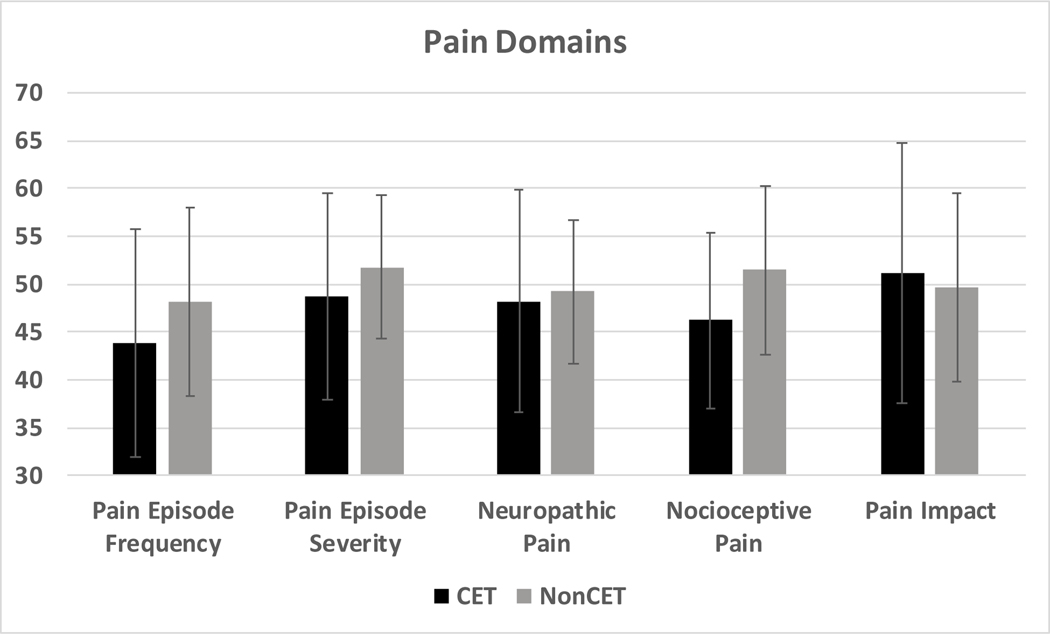
When compared directly without propensity matching participants on CET had similar ASCQ-Me Pain Impact Scores (51.1 vs 49.7, p=0.6), Pain Episode Severity (48.8 vs 51.8, p=0.3) and Pain Episode Frequency (43.9 vs 48.2, p=0.3), compared to those not on CET (Figure 2). In addition, both groups had similar PROMIS Neuropathic (48.1 vs 49.6, p=0.7) and Nociceptive (46.2 vs 51.4, p=0.2) Pain Quality scores to those not on CET (Figure 2). There was no association between age of starting transfusion therapy or years on chronic transfusions with Pain Impact, Pain Episode Frequency or Severity, Neuropathic Pain, Nociceptive Pain, or opioids dispensed.
Figure 2. Patient-reported Pain measures for those on chronic exchange transfusions (CET) compared to those not on CET.
Pain measures as assessed using the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) and the Patient Reported Outcomes Measurement Information System (PROMIS), in patients with SCD on CET (black bars) compared to those not on CET (grey bars). The y-axis shows ASCQ-Me (Pain Impact, Pain Episode Frequency, Pain Episode Severity) and PROMIS (Neuropathic Pain, Nociceptive Pain) domain scores. A score of 50 is the median for the validation cohort and higher scores represent worse symptoms and lower scores represent better symptoms in all domains except in Pain Impact where lower scores represent worse symptoms and higher scores represent better symptoms. Ten is one standard deviation of the validation cohort for all domains. There were no significant differences in scores between the two groups for all measures assessed (p=>0.05).
Participants on CET reported lower anxiety (46.2 vs 55.2, p=0.02) and social functioning impact (58.3 vs 51.5 p=0.04), compared to those not on CET (Figure 2). Stiffness Impact, Emotional Impact, and Sleep Impact were not different between the participants on CET compared to those not on CET (Figure 3).
There was no difference in numbers of median all-cause annual admissions (1 vs 2, p=0.2), median all-cause annual ER use (0 vs 1, p=0.4), and median length of admission (4.0 vs 3.2 days, p=0.6) between groups (Table 1). The two groups also had similar median milligrams of opioids dispensed (3.9 vs 6.2 mg daily OME, p=0.46).
Discussion:
After propensity matching, we found CET was associated with Pain Impact Scores suggesting worse pain outcomes and scores of Neuropathic and Nociceptive Pain Quality also suggesting worse pain. We also found that patients receiving CET did not report better scores on Pain Episode Frequency or Pain Episode Severity and did not have fewer opioids dispensed. Finally, CET was however associated with reductions in all-cause health care utilization.
In agreement with previous studies, we found that after propensity matching adults on CET had fewer and shorter hospital admissions than those not on CET. The SWITCH and SIT trials found that children receiving chronic transfusions had fewer acute care visits.7,20 Two recent retrospective studies, one in adults and one in children, also showed that initiation of chronic transfusions in patients who had frequent health care utilization for pain reduced acute pain admissions.3,21 While patients on CET in our study did not have fewer episodes of health care utilization when compared directly to patients not on CET, this may be due to the control cohort having characteristics suggestive of more end organ complications than the cases, as 10/11 cases had a history of stroke which itself is a marker of morbidity and mortality risk in SCD.35
Participants on CET had worse Pain Impact scores and worse scores of Neuropathic and Nociceptive pain, all of which reflect decreased functioning due to pain and more evidence of neuropathic and nociceptive pain. This suggests that while CET is associated with reduced health care utilization for acute pain, patients continue to report pain on pain-related PROs. One study of adults on chronic transfusions who completed daily pain diaries found that 63% of adults had chronic pain based on these daily diaries.36 There is otherwise a paucity of data that examine associations between chronic transfusions and the impact of non-crisis pain in adults with SCD. One possible explanation for these findings is that pain may have developed before chronic transfusions were started. However, the participants on CET in our study started on CET at a mean age of 14 years, before chronic pain or neuropathic pain typically develop in people with SCD.8,14 We also found no association between age of starting CET or years on transfusion therapy with the pain outcomes assessed. This suggests that CET may not be effective for the treatment of, or the development of, nociceptive or neuropathic pain in SCD.
Chronic inflammation can contribute to the development of neuropathic and nociceptive pain in SCD. Thus, a possible explanation for our findings is that chronic transfusion therapy does not completely reduce hemolysis and reductions in markers of inflammation such as platelets and neutrophil count seen after exchange transfusion rebound within days.32,37 Participants on CET had higher neutrophil counts compared to those not on CET, and neutrophils are thought to play a role in pain development in SCD. Nociceptive signaling in murine models of SCD is activated by elastase released by neutrophils, and signs of pain in these mice are reduced after treatment with an elastase inhibitor.32 Stroke itself is also associated with centrally-mediated pain and neuropathic pain, so it is possible that participants on transfusion therapy for secondary stroke prophylaxis may be more vulnerable to the development of chronic pain independent of CET.38 Future prospective studies should examine the effects of anti-inflammatory agents in combination with transfusion therapy on pain in adults with SCD. One such study combining hydroxyurea and transfusion (HAT, https://clinicaltrials.gov/ct2/show/NCT03644953) is ongoing.
We found that participants on CET reported lower anxiety and social functioning impact, compared to those not on CET. This may be partially explained by the reduction in annual admissions and ED visits. There may also be selection bias as good baseline social functioning is likely required to be able to regularly attend monthly appointments for transfusions. These findings deserve further study in larger cohorts followed longitudinally.
This study has limitations. This is a small single center study and we could not account for possible hospitalizations of patients outside of our institution. Though we performed propensity matching to account for confounding effects of disease morbidity, there may be other differences that could impact morbidity that we did not identify. We examined pain impact in the preceding week and the presence of neuropathic and nociceptive pain in participants who self-reported no acute pain for at least a week; however, we did not collect data to meet the strict definition of chronic pain (i.e., pain present on most days for at least 6 months). We examined all cause health care utilization, however, in other studies of adults with SCD, 74% of ED visits and 90% of admissions for adults with SCD were for uncontrolled pain, thus we believe our data provide a reasonable estimate of differences in pain related admissions between our two groups.39,40 Lifetime data about percent hemoglobin S and iron status were not available and can be associated with pain development.39,40 We also did not examined mental health diagnoses such as depression or opioid dependency which can be associated with frequent hospitalizations in patients with SCD.
In conclusion, CET was associated with worse Pain Impact, Neuropathic Pain and Nociceptive Pain, compared with participants not receiving CET with similar levels of select parameters associated with disease morbidity. Large multicenter studies are warranted to investigate the impact of CET on the development and treatment of chronic pain in children and adults with SCD, and to understand how CET may be combined with other therapies to minimize pain.
Acknowledgments
Funding:
NHLBI 5T32HL007974-17, UL1 TR001863, K23HL151884
During the time this study was started Susanna Curtis was a PhD Student in the Investigative Medicine Program at Yale which is supported by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Credit Author Statement
Susanna Curtis: Conceptualization, methodology, investigation, data curation, writing original draft. Raisa Balbuena-Merle: Methodology, investigation, data curation, writing – review and editing. John Roberts: Methodology, supervision, project administration, writing – review and editing. Jeanne Hendrickson: Methodology, supervision, writing – review and editing. Joanna Starrels: Writing – review and editing. Lesley Devine: Resources, data curation. Michelle DeVeaux: Data curation, methodology. Dan Zelterman: Data curation, methodology. Amanda Brandow: Conceptualization, methodology, supervision, writing – review and editing.
The authors declare no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wun T. The Role of Inflammation and Leukocytes in the Pathogenesis of Sickle Cell Disease; Haemoglobinopathy. Hematology. 2001;5(5):403–412. [PubMed] [Google Scholar]
- 2.Fadlon E, Vordermeier S, Pearson TC, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91(1):266–274. [PubMed] [Google Scholar]
- 3.Hilliard LM, Kulkarni V, Sen B, et al. Red blood cell transfusion therapy for sickle cell patients with frequent painful events. Pediatr Blood Cancer. 2018:e27423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsitsikas DA, Orebayo F, Agapidou A, Amos RJ. Distinct patterns of response to transfusion therapy for different chronic complications of sickle cell disease: A useful insight. Transfus Apher Sci. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Smith WR, Scherer M. Sickle-cell pain: advances in epidemiology and etiology. Hematology Am Soc Hematol Educ Program. 2010;2010:409–415. [DOI] [PubMed] [Google Scholar]
- 6.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT Diagnostic Criteria for Chronic Sickle Cell Disease Pain. J Pain. 2017;18(5):490–498. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez O, Yovetich NA, Scott JP, et al. Pain and other non-neurological adverse events in children with sickle cell anemia and previous stroke who received hydroxyurea and phlebotomy or chronic transfusions and chelation: results from the SWiTCH clinical trial. Am J Hematol. 2013;88(11):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandow AM, Zappia KJ, Stucky CL. Sickle cell disease: a natural model of acute and chronic pain. Pain. 2017;158 Suppl 1:S79–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith WR, McClish DK, Dahman BA, et al. Daily home opioid use in adults with sickle cell disease: The PiSCES project. J Opioid Manag. 2015;11(3):243–253. [DOI] [PubMed] [Google Scholar]
- 10.Molokie RE, Wang ZJ, Wilkie DJ. Presence of neuropathic pain as an underlying mechanism for pain associated with cold weather in patients with sickle cell disease. Med Hypotheses. 2011;77(4):491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ZJ, Wilkie DJ, Molokie R. Neurobiological mechanisms of pain in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2010;2010:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkie DJ, Molokie R, Boyd-Seal D, et al. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc. 2010;102(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tawfic QA, Faris AS, Eipe N. Sickle cell pain management: are we missing the role of pronociception and neuropathic pain? Paediatr Anaesth. 2013;23(11):1104–1105. [DOI] [PubMed] [Google Scholar]
- 14.Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. 2014;61(3):512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware RE, Helms RW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood. 2012;119(17):3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estcourt LJ, Fortin PM, Hopewell S, Trivella M, Wang WC. Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease. Cochrane Database Syst Rev. 2017;1:Cd003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarode R, Ballas SK, Garcia A, et al. Red blood cell exchange: 2015 American Society for Apheresis consensus conference on the management of patients with sickle cell disease. J Clin Apher. 2017;32(5):342–367. [DOI] [PubMed] [Google Scholar]
- 18.Biller E, Zhao Y, Berg M, et al. Red blood cell exchange in patients with sickle cell disease-indications and management: a review and consensus report by the therapeutic apheresis subsection of the AABB. Transfusion. 2018;58(8):1965–1972. [DOI] [PubMed] [Google Scholar]
- 19.Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4(2):327–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled Trial of Transfusions for Silent Cerebral Infarcts in Sickle Cell Anemia. New England Journal of Medicine. 2014;371(8):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsitsikas DA, Ekong A, Berg L, et al. A 5-year cost analysis of automated red cell exchange transfusion for the management of recurrent painful crises in adult patients with sickle cell disease. Transfus Apher Sci. 2017;56(3):466–469. [DOI] [PubMed] [Google Scholar]
- 22.Karafin MS, Chen G, Wandersee NJ, et al. Chronic pain in adults with sickle cell disease is associated with alterations in functional connectivity of the brain. PLoS One. 2019;14(5):e0216994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller S, Yang M, Treadwell MJ, Hassell KL. Sensitivity of alternative measures of functioning and wellbeing for adults with sickle cell disease: comparison of PROMIS(R) to ASCQ-Me. Health Qual Life Outcomes. 2017;15(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller S YM, Evensen C, Cowans T. ASCQ-Me® User’s Manual; 2017. [Google Scholar]
- 25.Treadwell MJ, Hassell K, Levine R, Keller S. Adult sickle cell quality-of-life measurement information system (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30(10):902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keller SD, Yang M, Treadwell MJ, Werner EM, Hassell KL. Patient reports of health outcome for adults living with sickle cell disease: development and testing of the ASCQ-Me item banks. Health Qual Life Outcomes. 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med. Care. 2007;45(5 Suppl 1):S3–11. 10.1097/01.mlr.0000258615.42478.55. PMID: 17443116; PMCID: PMC2829758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askew RL, Cook KF, Keefe FJ, et al. A PROMIS Measure of Neuropathic Pain Quality. Value Health 2016;19(5):623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmariah H, Garrett ME, De Castro LM, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89(5):530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran H, Gupta M, Gupta K. Targeting novel mechanisms of pain in sickle cell disease. Blood 2017;130(22):2377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Hematology Am Soc Hematol Educ Program. 2013;2013:362–369. [DOI] [PubMed] [Google Scholar]
- 33.Gladwin MT, Barst RJ, Gibbs JS, et al. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One. 2014;9(7):e99489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karafin MS, Mullins DE, Johnson ST, et al. Chronic pain persists in adults with sickle cell disease despite regular red cell transfusions. Transfus Apher Sci. 2019;58(4):434–438. [DOI] [PubMed] [Google Scholar]
- 35.Liem RI, O’Gorman MR, Brown DL. Effect of red cell exchange transfusion on plasma levels of inflammatory mediators in sickle cell patients with acute chest syndrome. Am J Hematol. 2004;76(1):19–25. [DOI] [PubMed] [Google Scholar]
- 36.Hansson P. Post-stroke pain case study: clinical characteristics, therapeutic options and long-term follow-up. Eur J Neurol. 2004;11 Suppl 1:22–30. [DOI] [PubMed] [Google Scholar]
- 37.Lanzkron S, Carroll CP, Haywood C Jr., The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. American journal of hematology. 2010;85(10):797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cronin RM, Hankins JS, Byrd J, et al. Risk factors for hospitalizations and readmissions among individuals with sickle cell disease: results of a U.S. survey study. Hematology. 2019;24(1):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamosauskaite J, Atkins JL, Pilling LC, et al. Hereditary Hemochromatosis Associations with Frailty, Sarcopenia and Chronic Pain: Evidence from 200,975 Older UK Biobank Participants. J Gerontol A Biol Sci Med Sci. 2019;74(3):337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng FX, Hou JM, Sun TS. In vivo evaluation of microglia activation by intracranial iron overload in central pain after spinal cord injury. J Orthop Surg Res. 2017;12(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]