Abstract
Background
The transient receptor potential vanilloid 1 (TRPV1) provides a heat and pain sensation (nociception). Capsaicin, a TRPV1 agonist, has been shown to induce a refractory period in the nerve terminal expressing TRPV1 and create long‐term nerve terminal defunctionalization.
Objective
To evaluate the efficacy of capsaicin for pain reduction during microfocused ultrasound with visualization (MFU‐V) treatment.
Methods and materials
A randomized, split‐side study including 24 subjects was conducted. A combined 0.025% capsaicin gel and topical anesthetic were randomly applied on one side of the neck, and a topical anesthetic monotherapy was applied on the contralateral side for 30 min before MFU‐V treatment. Pain score (visual analog scale, 0–10) was evaluated at T1 (before MFU‐V), T2a (after the 4.5‐mm transducer treatment), T2b (after the 3.0‐mm transducer treatment), and T3 (after the entire treatment). Side effects were recorded.
Results
Mean pain scores at T2a for combined and single regimens were 5.19 (±2.26) and 6.91 (±1.72), respectively (p < 0.001). The capsaicin‐treated side had a lower pain score at T2b and T3 (p < 0.001). Redness was longer on the capsaicin‐treated side (112.67 vs. 10.68 min, p < 0.001). No other adverse events including contact dermatitis were reported.
Conclusion
A single application of a combined 0.025% capsaicin gel with topical anesthesia produces a significantly lesser pain score during the MFU‐V treatment. Defunctionalization of TRPV1 may explain the alleviation of painful sensations caused by heat from MFU‐V.
Keywords: anesthesia, chili, lidocaine, nociceptive pain, prilocaine, TRPV1 receptor
1. INTRODUCTION
Microfocused ultrasound with visualization (MFU‐V) is a well‐known modality for the treatment of facial skin laxity. Over the past years, the use of MFU‐V in the dermatologic field continues to grow owing to its effectiveness and safety demonstrated thoroughly in clinical studies and general practices. Unlike other noninvasive tissue tightening techniques, MFU‐V targets the dermis and the superficial musculoaponeurotic system. 1 The mechanism of MFU‐V is based on acoustic energy transmission, which causes molecular friction by generating vibration in the targeted tissue. A portion of this mechanical energy is converted to thermal energy, resulting in temperatures surpassing 60–70°C at predefined depths, which leads to collagen denaturation and neocollagenesis, while the surrounding tissue remains undisturbed. 2 , 3
In both literature and clinical practices, the safety profile of MFU‐V has been well established, 4 , 5 while most of the negative effects are temporary. Nonetheless, the thermal effect created by MFU‐V may cause pain or discomfort throughout the procedure, which is considered the drawback of this noninvasive modality. The severity of patients’ discomfort varies, and in certain cases, extreme discomfort has necessitated intravenous sedation. 5 Currently, a wide range of pain management methods for MFU‐V are being reported in practices with clinical study evidence including acetaminophen, nonsteroidal anti‐inflammatory drugs, antianxiety drugs, narcotics, nitrous oxide, the centrally acting analgesic tapentadol, injectable lidocaine, cooling devices, and combinations of the above. 2 , 6 , 7 Although these methods may lessen pain, there are a certain number of patients do not achieve an adequate level of comfort. Therefore, additional studies into pharmaceutical pain management in conjunction with nonpharmacological approaches employed during MFU‐V treatment are warranted.
Capsaicin (trans‐8‐methyl‐N‐vanillyl‐6‐nonenamide—C18H27NO3) is a naturally occurring substance derived from the plants of the genus Capsicum, family Solanaceae, which is known as chili pepper. Recently, it has been widely used in pain‐relief research and other clinical therapies. The mechanism of action of this substance has been proposed to be its role as a selectively nociceptive neurons stimulation or transient receptor potential vanilloid 1 (TRPV1) receptor agonist, which has evidence in pain perception and plays a critical role in sensory afferents. Capsaicin, in various forms, is now increasingly utilized for the treatment of a variety of painful disorders due to its TRPV1 agonist properties, such as complex regional pain syndromes and neuropathic pain, 8 , 9 , 10 , 11 , 12 postherpetic neuralgia, 13 and cluster headache prevention. 14
However, there is no evidence of its use in discomfort management during MFU‐V treatment. We expected that employing the substance that directly affects the processes behind procedure‐associated discomfort and attempting to apply procedure‐specific analgesics would benefit in optimizing the patient's comfort. Accordingly, the purpose of this study is to compare the pain perception during MFU‐V procedures treated with a combination of capsaicin gel and topical anesthesia versus topical anesthesia monotherapy.
2. MATERIALS AND METHODS
2.1. Study design
This was a prospective, single‐blinded, randomized controlled trial study conducted at a university‐based hospital (Ramathibodi hospital, Mahidol university, Bangkok, Thailand). We prospectively performed our study in compliance with the Declaration of Helsinki of 1975, as revised in 1983. The study protocol was approved by the Institutional Review Board of Human Rights Related to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University (Protocol number MURA2021/1009) and Thai Clinical Trials Registry (Identification number TCTR20220225006). All participants signed a written informed consent prior to study initiation.
2.2. Study subjects
A total of 24 healthy participants aged between 18 and 60 years with neck laxity and willingness to participate in the research were recruited. The exclusion criteria included pregnancy or active breastfeeding, active skin diseases as well as a history of surgery at the experimental sites within 6 months, metal implantation at the experimental sites, abnormal facial/neck sensation, or known allergy to local anesthetics or capsaicin‐containing gel. At the time of recruitment, each participant was given a brief explanation of the study.
2.3. Study material
The topical anesthesia used in this study was a compounded mixture of 2.5% lidocaine and 2.5% prilocaine (Racser, Galentic Pharma, Mumbai, India) while the capsaicin gel contained capsaicin 0.025% (Capsika‐25, Bangkok Lab & Cosmetic CO., LTD., Bangkok, Thailand). There were 5 grams of capsaicin gel and 2.5% lidocaine/prilocaine on the combination‐treated side, whereas the monotherapy side had only 10 grams of 2.5% lidocaine/prilocaine.
2.4. Study protocol
This split‐neck design compared two anesthetic options in the same patient. Each side of the neck was randomized to receive two different anesthetic alternatives: capsaicin plus topical anesthetic cream versus topical anesthetic cream monotherapy, as well as the sequence in which they were applied. This randomization was generated by a computer‐based generator and was hidden from the researcher enrolling subjects. Before MFU therapy, one of the anesthetic options with a total amount of 10 grams was randomly applied to one side of the neck with an occlusive dressing for 30 min. Thereafter, the applied cream was removed, and the MFU‐V (Ultherapy (Merz Aesthetics, Raleigh, NC) procedure was performed at the same side consisting of 45 treatment lines with a 4.5 mm transducer (4.5 MHz, 0.9 J), followed by 45 treatment lines with 3.0 mm transducer (7 MHz, 0.3). After the first side was done, the 30‐min application of another anesthetic option on the contralateral side was initiated, and MFU‐V was performed with a similar pattern. (Figure 1)
FIGURE 1.
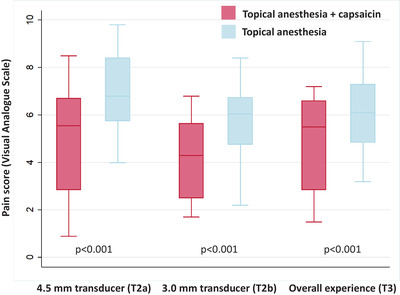
The mean pain score (VAS, 0–10) at immediately after 4.5‐mm transducer (T2a), immediately after 3.0‐mm transducer (T2b), and overall pain experience (T3). p‐Value compared between each anesthetic technique
2.5. Pain assessment
The visual analog scale (VAS), a common and accepted approach for evaluating pain levels, was used to assess pain perception in each participant. 15 The 10 cm‐VAS score (0, no pain; 10, worst possible pain) was measured at four‐time points: after removing the anesthetic option (T1), immediately after the 4.5 mm transducer (T2a), immediately after 3.0 mm transducer (T2b), and overall pain experience (T3). At each time point, the VAS score was compared between the side treated with topical anesthesia monotherapy and the combination of capsaicin and topical anesthesia.
2.6. Statistical analysis
All data analysis was performed by STATA/SE version 14.2 (STATA Corp, College Station, TX). Categorical data (e.g., sex, comorbidity, Fitzpatrick skin type) were shown as a percentage. Continuous data (e.g., pain score, redness duration) was presented in either mean with standard deviation or median with interquartile range. The difference in mean pain score at each time point (T1, T2a, T2b, and T3) was compared between 2 anesthetic options and analyzed using the mixed‐effect model. A p‐value less than 0.05 determined statistical significance.
3. RESULTS
A total of 24 participants were recruited and completed this study. Their average age was 44.86 ± 5.80 years. Twenty‐two participants were female (91.67%), and two participants were male (8.33%). Fifteen subjects had Fitzpatrick skin type III (62.50%), and nine had Fitzpatrick skin type IV (37.50%). Eight participants (33.33%) were naïve to focused ultrasound procedures for skin tightening or rejuvenation.
At baseline (T1), there were no significant differences in the mean VAS score between each experimental site. At T2a, the mean VAS score rated by participants was 5.19 ± 2.26 for combined capsaicin plus topical anesthesia and 6.91 ± 1.72 for topical anesthesia monotherapy (p < 0.001). The mean VAS score at T2b for the combined regimen was 4.18 ± 1.63 and 5.69 ± 1.71 for topical anesthesia monotherapy (p < 0.001). The VAS score for overall pain experienced (T3) was significantly lower in the combined capsaicin plus topical anesthesia group than in topical anesthesia monotherapy (4.94 ± 1.91 vs. 6.13 ± 1.64, p < 0.001) (Table 1 and Figure 1). The average treatment duration of the MFU‐V treatment on the combined side and monotherapy side was 19.46 (±2.26) and 19.29 (±2.18) min, respectively.
TABLE 1.
Pain perception during microfocused ultrasound procedure and duration of redness between each anesthetic technique
| Combined capsaicin gel with topical anesthesia | Topical anesthesia monotherapy | p‐Value | |
|---|---|---|---|
| Pain score (10‐cm visual analogue scale); mean (SD) | |||
| T1, after removing cream | 0.00 | 0.00 | 1.000 |
| T2a, after MFU 4.5 | 5.19 (2.26) | 6.91 (1.72) | <0.001* |
| T2b, after MFU 3.0 | 4.18 (1.63) | 5.69 (1.71) | <0.001* |
| T3, over all pain experience | 4.94 (1.91) | 6.13 (1.64) | <0.001* |
| Duration of skin redness, minutes; mean (SD) | |||
| From the first appearance | 112.67 (33.48) | 10.38 (9.27) | <0.001* |
In terms of adverse reactions, redness was noticed immediately after being wiped off on the combination side in all patients. The redness persisted until the end of the MFU‐V procedure. In the topical anesthetic monotherapy side, the redness was noticed in four patients after the removal of the anesthetic and disappeared during MFU‐V treatment. Regarding the duration of redness after the removal of the anesthetic cream, the combined regimen demonstrated 112.67 ± 33.48 min, whereas the monotherapy regimen showed 10.38 ± 9.27 min ( p < 0.001) (Table 1). Burning and stinging sensations were noted in all patients on the combined capsaicin plus anesthetic cream‐treated side. However, no patients required early removal of the cream. The burning and stinging sensation subsided spontaneously within 10 min after removing the cream. None of the patients reported contact dermatitis. No other complications (e.g., postinflammatiory hyperpigmentation) regarding the anesthetic application or procedure were noted (Figures 2 and 3).
FIGURE 2.
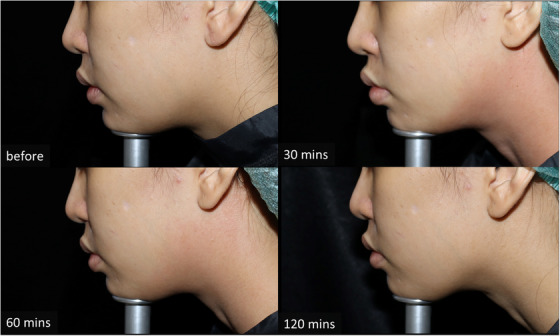
Subject's photographs at combined capsaicin and topical anesthesia treated side at different time points (before application, 30 min after removal, 60 min after removal, and 120 min after removal)
FIGURE 3.
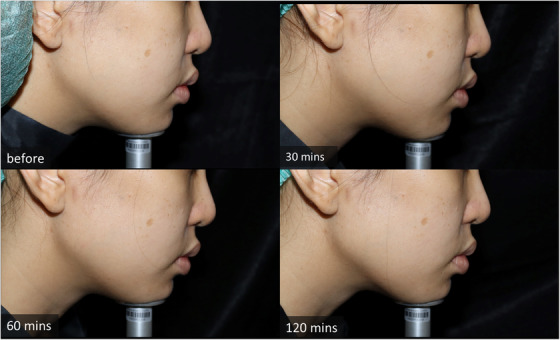
Subject's photographs at topical anesthesia monotherapy side at different time points (before application, 30 min after removal, 60 min after removal, and 120 min after removal)
4. DISCUSSION
MFU‐V is an established modality for treating skin laxity through its controlled heating of the dermis and deep layers, resulting in neocollagenesis and elastic tissue remodeling. As previously stated, MFU‐V waves may generate heat that reaches temperatures of 60–70°C in certain areas of dermal and subcutaneous tissue to establish thermal coagulation points with the depth of 1.5‐4.5 mm. This temperature breaks a collagen fibrils’ threshold temperature of 58–65°C leading to immediate collagen denaturing, contraction and followed by collagen genesis. 3 Although the mechanism of pain production during MFU‐V treatment has yet to be determined, a possible theory might point to TRPV1 channel stimulation by heat stimuli.
TRPV1, belonging to the transient release potential (TRP) ion channel family, is a nonselective cationic ligand‐gated channel that acts as a signal transducer by changing the membrane potential or intracellular calcium concentration. 16 This channel is located in small to medium‐sized neurons of the sensory and sympathetic ganglia, where it gives rise to C‐fibers and to a lesser degree to Aδ‐fibers. 17 It responds to several distinct agents, such as extracellular acidification, and high temperatures (>42°C), making it crucial for thermal and chemical nociception. 18 , 19 , 20 , 21 TRPV1 is also expressed in nonneuronal tissues such as the gastrointestinal epithelium, the cardiovascular system, the skin epidermis, and immune system cells. 22 Upon stimulation, TRPV1 transiently opens and allows the influx of sodium and calcium ions, resulting in the depolarization of membranes of sensory nerve cells. Then comes a sharp or prickly pain mediated by fast conducting Aδ‐fibers,‐V followed by a dull or burning pain mediated by slow‐conducting C‐fibers. 23 As a result, while providing MFU‐V energy, discomforts such as stinging, burning, and pain may occur. 24 In addition to high temperature, TRPV1 can be activated by a variety of exogenous and endogenous ligands such as protons, vanilloid compounds biding, such as capsaicin and capsaicin‐related compounds, and leukotriene B. 25
During the MFU‐V procedure, patients may have some degree of discomfort in the treated area, especially over bony prominences. 26 Without anesthesia, mean pain score has been reported as 7.35 during treatment with the 4.5 mm (100 lines, 1.20 J) and 3.0 (50 lines, 0.45 J) transducers. 27 A number of topical anesthetic regimens and systemic agents have been used to minimize discomfort. 28 , 29 , 30 , 31 , 32 , 33 , 34 According to a study by Polacco et al., a mean pain score of 5.1 ± 1.7 was reported when patients were pretreated with lorazepam (2 mg), ibuprofen (800 mg), and a topical compounded anesthetic (20% benzocaine, 4% lidocaine, 4% tetracaine) 15 min prior to the procedure. 35 Another study reported average pain scores in the submandibular region of 6.53 (range, 2–10) when receiving some form of premedication including oral medication (i.e., 5–10 mg of diazepam and 5/325 mg of hydrocodone/acetaminophen [1–2 tablets]) 30 min before treatment or intramuscular medication (60 mg of ketorolac tromethamine) 60 min before treatment. 36 Nevertheless, some medications with anti‐inflammatory property might potentially interfere with the wound healing process, resulting in poor outcome. 37
The majority of clinicians have been searching for pretreatment modalities that can minimize pain and other complications without affecting the result. The definitive therapy or standard regimen has yet to be defined. In this study, we evaluated the efficacy of capsaicin gel for discomfort management during MFU‐V therapy. According to the result of our study, the addition of 5% capsaicin in a gel vehicle showed to be a safe and effective option to decrease pain in patients undergoing MFU‐V treatment. We found that the mean pain score was significantly lower in the combined capsaicin plus anesthetic cream treated side when using both the 4.5 and 3 mm‐transducers and overall pain perception. The explanation underlying these findings might be the role of capsaicin in TRPV1 activation, which plays as an important role in the creation of pain from MFU‐V.
Capsaicin is an agonist of TRPV1 and reduces its heat activation threshold. 38 Capsaicin binds and stimulates these receptors, causing a burning sensation at the application site through the release of inflammatory mediators such as substance P. In contrast to other physiological stimuli, capsaicin has been reported to cause several effects, including an increase in intracellular calcium, activation of calcium‐dependent proteases, depolymerization of microtubules, and disruption of mitochondrial function. 39 The analgesic mechanism is theorized to be due to the “desensitization” or “de‐functionalization” of the TRPV1 receptors. After being stimulated with capsaicin, TRPV1 goes into a long refractory period, as a result, previously stimulated neurons on the skin that respond to painful stimuli decrease. 40 Capsaicin appears to cause analgesia through a series of processes that culminate in defunctionalization of nociceptive fibers. Regarding the duration of action, a single 60‐min patch application using 8% capsaicin had efficacy for the treatment of neuropathic pain syndromes last up to 12 weeks. 39 According to a study by Schnitzer et al., a four‐time daily application of 0.025% capsaicin cream demonstrated 50% pain reduction for osteoarthritis at day 14. 41 Therefore, a single application of a low concentration of capsaicin gel (i.e., 0.025%) 30 min before the procedure used in this study might exert a less analgesic effect compared to previous studies. However, we still see some beneficial effects regarding the use of the single application, low‐concentration formulation.
Amide local anesthetic agents, including lidocaine and prilocaine, exert their analgesic effect by primarily binding to voltage‐gated Na + channels, hence inhibiting the influx of Na + . As a result, the peak current of Na + channel is greatly decreased, leading to the reduction of sensory neuron's excitability, and ultimately, decrease in pain sensation. 42 , 43 As aforementioned, the mechanism of capsaicin‐mediated analgesic effect is different from that produced by amide local anesthetic agents. Therefore, the combination of two classes of drug can potentially abolish pain sensation by modulating the two distinct mechanisms.
Despite the efficacy of analgesia prior to MFU‐V, concerns have been raised regarding safety due to the adverse reaction caused by capsaicin (e.g., irritation). 44 As mentioned previously, capsaicin needs more time to cause desensitization to the TRPV1 receptor. To avoid side effects, the neck area and short contact time were chosen (instead of the face and long contact time). However, we still observe some benefits with this protocol. In addition, it is more applicable to a daily practice because topical anesthesia is typically applied 30–60 min before the procedure. In our study, redness and burning sensation at the application area were the most common reaction which was reported in all patients. However, they were temporary, and all participants were able to tolerate low concentration formulation. Additionally, we did not observe postinflammatory hyperpigmentation in our patient. Similar to the prospective study by Maihofner and Heskamp where an 8% capsaicin patch was used, erythema and pain at the application site were most commonly seen. 44
The limitation in our study was the small number of participants, which might affect the power of the results. In addition, we performed the treatment in the neck region, with a limited number of treatment lines. Further studies with a large number of participants with different anatomical locations, different concentrations or preparation, and different application schedules are recommended to establish the efficacy and safety of this agent.
In conclusion, the combination of topical capsaicin with topical anesthesia produces a significantly lesser pain score during MFU‐V treatment. The capsaicin‐mediated “defunctionalization” of TRPV1 may explain the alleviation of painful sensation caused by heat from MFU‐V.
Vachiramon V, Tanratana P, Anuntrangsee T, et al. The role of topical capsaicin gel in pain management during microfocused ultrasound treatment for neck laxity. Skin Res Technol. 2023;29:1–7. 10.1111/srt.13240
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 2013;32:18‐25. [PubMed] [Google Scholar]
- 2. Gutowski KA. Microfocused ultrasound for skin tightening. Clin Plast Surg. 2016;43:577‐82. [DOI] [PubMed] [Google Scholar]
- 3. Bozec L, Odlyha M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys J. 2011;101:228‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hitchcock TM, Dobke MK. Review of the safety profile for microfocused ultrasound with visualization. J Cosmet Dermatol. 2014;13:329‐35. [DOI] [PubMed] [Google Scholar]
- 5. Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269‐75. [DOI] [PubMed] [Google Scholar]
- 6. Sasaki GH, Tevez A. Microfocused ultrasound for nonablative skin and subdermal tightening to the periorbitum and body sites: preliminary report on eighty‐two patients. J Cosmet Derm Sci Appl. 2012;2:108‐16. [Google Scholar]
- 7. Fabi SG, Few JW, Moinuddin S. Practical guidance for optimizing patient comfort during microfocused ultrasound with visualization and improving patient satisfaction. Aesthet Surg J. 2020;40:208‐16. [DOI] [PubMed] [Google Scholar]
- 8. Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123‐39. [DOI] [PubMed] [Google Scholar]
- 9. Robbins WR, Staats PS, Levine J, et al. Treatment of intractable pain with topical large‐dose capsaicin: preliminary report. Anesth Analg. 1998;86:579‐83. [DOI] [PubMed] [Google Scholar]
- 10. Ellison N, Loprinzi CL, Kugler J, et al. Phase III placebo‐controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J Clin Oncol. 1997;15:2974‐80. [DOI] [PubMed] [Google Scholar]
- 11. Zis P, Apsokardos A, Isaia C, et al. Posttraumatic and postsurgical neuropathic pain responsive to treatment with capsaicin 8% topical patch. Pain Physician. 2014;17:E213‐218. [PubMed] [Google Scholar]
- 12. Derry S, Rice AS, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1:CD007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watson CP, Tyler KL, Bickers DR, et al. A randomized vehicle‐controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther. 1993;15:510‐26. [PubMed] [Google Scholar]
- 14. Fusco BM, Marabini S, Maggi CA, et al. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain. 1994;59:321‐5. [DOI] [PubMed] [Google Scholar]
- 15. Sriwatanakul K, Kelvie W, Lasagna L, et al. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234‐9. [DOI] [PubMed] [Google Scholar]
- 16. Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285‐7. [DOI] [PubMed] [Google Scholar]
- 17. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355‐84. [DOI] [PubMed] [Google Scholar]
- 18. Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature. 1997;389:816‐24. [DOI] [PubMed] [Google Scholar]
- 19. Du Q, Liao Q, Chen C, et al. The role of transient receptor potential vanilloid 1 in common diseases of the digestive tract and the cardiovascular and respiratory system. Front Physiol. 2019;10:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caterina MJ, Rosen TA, Tominaga M, et al. A capsaicin‐receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436‐41. [DOI] [PubMed] [Google Scholar]
- 21. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619‐47. [DOI] [PubMed] [Google Scholar]
- 22. Shuba YM. Beyond neuronal heat sensing: diversity of TRPV1 heat‐capsaicin receptor‐channel functions. Front Cell Neurosci. 2021;14:612480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev. 2010;34:177‐84. [DOI] [PubMed] [Google Scholar]
- 24. Sasaki GH, Tevez A. Clinical efficacy and safety of focused‐image ultrasonography: a 2‐year experience. Aesthet Surg J. 2012;32:601‐12. [DOI] [PubMed] [Google Scholar]
- 25. Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125:181‐95. [DOI] [PubMed] [Google Scholar]
- 26. Chaves Bellote TP, Miot HA. Microfocused ultrasound with visualization for face slimming: preliminary results in four women. Clin Cosmet Investig Dermatol. 2021;14:1613‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan NP, Shek SY, Yu CS, et al. Safety study of transcutaneous focused ultrasound for non‐invasive skin tightening in Asians. Lasers Surg Med. 2011;43:366‐75. [DOI] [PubMed] [Google Scholar]
- 28. Fabi SG, Massaki A, Eimpunth S, et al. Evaluation of microfocused ultrasound with visualization for lifting, tightening, and wrinkle reduction of the decolletage. J Am Acad Dermatol. 2013;69:965‐71. [DOI] [PubMed] [Google Scholar]
- 29. Goldberg DJ, Hornfeldt CS. Safety and efficacy of microfocused ultrasound to lift, tighten, and smooth the buttocks. Dermatol Surg. 2014;40:1113‐7. [DOI] [PubMed] [Google Scholar]
- 30. Rokhsar C, Schnebelen W, West A, et al. Safety and efficacy of microfocused ultrasound in tightening of lax elbow skin. Dermatol Surg. 2015;41:821‐6. [DOI] [PubMed] [Google Scholar]
- 31. Baumann L, Zelickson B. Evaluation of micro‐focused ultrasound for lifting and tightening neck laxity. J Drugs Dermatol. 2016;15:607‐14. [PubMed] [Google Scholar]
- 32. Sasaki GH, Abelev N, Papadopoulos L. A split face study to determine the significance of adding increased energy and treatment levels at the marionette folds. Aesthet Surg J. 2017;37:947‐60. [DOI] [PubMed] [Google Scholar]
- 33. Schlessinger J, Lupin M, McDaniel D, et al. Safety and effectiveness of microfocused ultrasound for treating erythematotelangiectatic rosacea. J Drugs Dermatol. 2019;18:522. [PubMed] [Google Scholar]
- 34. Lowe S. Single treatment, single depth superficial microfocused ultrasound with visualization for rhytid improvement. Plast Reconstr Surg Glob Open. 2021;9:e3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polacco MA, Butz DR, Bass R, et al. Nerve blocks prior to microfocused ultrasound treatment are safe and reduce patient discomfort. Aesthet Surg J. 2020;40:887‐91. [DOI] [PubMed] [Google Scholar]
- 36. Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099‐110. [DOI] [PubMed] [Google Scholar]
- 37. Murota SI, Abe M, Otsuka K, et al. Stimulative effect of prostaglandins on production of hexosamine‐containing substances by cultured fibroblasts (2) early effect of various prostaglandins at various doses. Prostaglandins. 1977;13:711‐7. [DOI] [PubMed] [Google Scholar]
- 38. Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008;24:142‐54. [DOI] [PubMed] [Google Scholar]
- 39. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high‐concentration capsaicin 8% patch. Br J Anaesth. 2011;107:490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159‐212. [PubMed] [Google Scholar]
- 41. Schnitzer TJ, Posner M, Lawrence ID. High strength capsaicin cream for osteoarthritis pain: rapid onset of action and improved efficacy with twice daily dosing. J Clin Rheumatol. 1995;1:268‐73. [PubMed] [Google Scholar]
- 42. Lenkey N, Karoly R, Epresi N, et al. Binding of sodium channel inhibitors to hyperpolarized and depolarized conformations of the channel. Neuropharmacology. 2011;60:191‐200. [DOI] [PubMed] [Google Scholar]
- 43. Scholz A. Mechanisms of (local) anaesthetics on voltage‐gated sodium and other ion channels. Br J Anaesth. 2002; 89:52‐61. [DOI] [PubMed] [Google Scholar]
- 44. Maihofner C, Heskamp ML. Prospective, non‐interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: first results of the QUEPP study. Curr Med Res Opin. 2013;29:673‐83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


