Abstract
Background
It was well known that the human body would produce an uncomfortable sensation when the fabric exerted a certain amount of pressure irritation on the skin. The amygdala had long been thought to be the source of negative emotion perception. However, up to now, the brain signal changes in the amygdala evoked by skin exposure pressure had not been known.
Materials and methods
In this work, a series of gradually increasing contact pressure stimulus from boneless corsets was repeatedly applied to the body's waist and abdomen, and the technology of functional magnetic resonance imaging (fMRI) was adopted to detect the brain response synchronously.
Results
The results shown that both subjective comfort score and percent signal changes (PSCs) of amygdala decreased with the increase of skin contact pressure. When the skin pressure applied to the waist and abdomen of the human body exceeded about 1 kPa, blood oxygen level dependent signal in the amygdala was negatively activated. Besides, the degree of response of PSCs was intense than subjective evaluation, and the standard deviations of PSCs between individuals were much smaller than subjective evaluations.
Conclusion
It was suggested that skin contact pressure stimulus caused the attention of the amygdala brain area. The greater the stimulus, the higher the attention, but such attention was caused by negative activation of the amygdala induced by skin discomfort. In addition, skin comfort representation based on brain perception was superior to subjective representation due to its higher response sensitivity and antipsychological interference ability.
Keywords: blood oxygen level dependent (BOLD), functional magnetic resonance imaging (fMRI), percent signal changes (PSCs), skin pressure stimulus
1. INTRODUCTION
Aiming at the problem of uncomfortable perception caused by the stimulation of contact pressure on human skin evoked by fabric, subjective comfort evaluation system deemed that when the various subjective evaluation scores under wearing state, such as sense of roughness, restraint, oppression, smoothness, itching, softness, thickness, comfort, were lower than a certain value (generally was zero), it could be regarded as uncomfort. 1 , 2 But, there was no doubt that this method of representation could be disturbed by individual psychological factor. Therefore, it was important to identify the root brain region of skin contact discomfort and its relationship with skin irritation. Amygdala brain region had long been recognized as the root brain region of negative perception of fear, 3 tactile discomfort, 4 , 5 and even pain. 6 , 7 Previous research about the processing of the unpleasantness of resistive load‐induced dyspnea by using functional magnetic resonance imaging (fMRI) had proved the affective dimension (unpleasantness) of perceived breathing uncomfortableness in humans was processed in the right anterior insula and the amygdala. 8 Kanosue 9 found blood oxygen level dependent (BOLD) signal of fMRI on bilateral amygdala were enhanced when human body felt more thermal tactile uncomfortable during the cooling process. Tan 10 studied and analyzed the fMRI of human brain under the stimulation of fabric contact thermal pain, obtained that contralateral amygdala was activated at 41°C, and bilateral amygdala were activated at 51°C. In the previous study, we also found that amygdala brain regions would produce a certain amount of negative activation under large fabric pressure stimulation. 11 Wang 12 found that repetitive prickling stimulation from the single fiber applied to the volar forearm aroused activation in amygdala.
In addition, studies on emotions also indicated that the amygdala was the key brain area for the generation and regulation of cognitive function and emotional behavior, which always responsible for fear, 13 phantom limb pain, 14 anxiety, 15 mental stress, 16 and so on. According to the general theory, when people were in the state of “mindfulness,” which was a kind of concentrated and peaceful mind, bilateral amygdala activity would be reduced. 17 Moreover, the mindfulness trait was negatively correlated with bilateral amygdala activity, and the depressive symptoms were positively correlated with right amygdala activity. 18 Liu 19 and Duan 20 successively found that amygdala brain region had significant changes in the perception of heat pain and negative emotions. Activation intensity and range of amygdala brain regions increased with the increase of stimulus intensity, and functional connectivity also changed longitudinally. 21 After amygdala was removed, no discomfort was felt. 22
To sum up, although a minority of studies had suggested a corresponding role on the amygdala in appetitive and affectively positive emotion, 23 the critical functional role of the amygdala was still often characterized as negative, which had been frequently proved by numerous studies based on fMRI, electroencephalogram, 24 single photon emission computed tomography, 25 positron emission tomography 26 as well as vivo brain morphometry using structural MRI. 27 The most typical case was that a significantly stronger activation in amygdala would occurred when uncomfortable visceral sensations produced. 28 Furthermore, even uncomfortable residential environments could also induced significant activation in amygdala. 29 In one word, “the amygdala: sensory gateway to the emotions,” as Aggleton and Mishkin said. 30 All of above suggested that the amygdala played a critical role in the origin of fabric tactile uncomfort in all probability. Therefore, the present neuroimaging study had examined the neuronal mechanisms associated with skin oppressing uncomfortable perception by exploring the relationship between amygdala and skin pressure.
2. MATERIALS AND METHODS
2.1. Participants
The study included six healthy female volunteers with no mental illness and no metal implants. They were of similar age and body size, with an average age of 25. The average height was 1.54 cm, the average weight was 48.74 kg, the average body mass index was 20.6 kg/m2, the average abdominal fat thickness was 0.95 cm, the average lower chest circumference was 74 cm, the average waist circumference was 69 cm, and the average abdominal circumference was 74 cm. All participants had a full understanding of the experimental procedure after the fMRI scanning procedure training, and voluntarily signed the informed consent. The study was approved by the ethics committee of Zhejiang Sci‐Tech University.
2.2. Materials and contact pressure test
Five types of boneless corsets were used as the experimental samples. The length and width of the corsets were 50 and 25 cm, respectively. When the fixed elongation was 20%, the elastic recovery rates were 94.55%, 91.45%, 87.82%, 94.75%, and 93.19%, respectively, which proved all of them had a good elastic recovery. The experimental corsets could be stretched or relaxed to control continuous and uniform changes in clothing pressure, easy to operate, and sustainable and smooth.
Clothing pressures were measured by AMI3037 Air‐pack Type Contact Pressure Measurement System, ranging from 0 to 34kpa, output voltage 0–3.4v, accuracy ± 0.2–0.45kpa. The pressure value of the measuring point was controlled by adjusting the length of the adhesive belt, which was successively 0 kpa, 0.5 kpa, 1 kpa, and 1.5 kpa, and the marks were recorded on the sample clothes, and the measurement was repeated for three times.
2.3. Subjective questionnaire survey of fabric samples
The subjective questionnaire was conducted 30 minutes after the participants entered the laboratory to ensure that their bodies had adjusted to the ambient temperature and humidity. The participants were asked to put on the sample. Different pressures of 0 kpa, 0.5 kpa, 1 kpa, and 1.5 kpa were applied through tightening the magic tape. Each pressure test lasted 1 min, and the pressures were averaged after removing the maximum and minimum values. Simultaneously, a subjective evaluation questionnaire survey was conducted as shown in Figure 1.
FIGURE 1.

Z score subjective rating scale
2.4. FMRI scanning
FMRI scanning were conducted on the Ingenia 3.0T medical fMRI equipment. TE = 30 s, TR = 3 s, and layer thickness = 3 mm in functional and structural images, the total functional and structural images scanning time were 190 s (prescanning time = 10 s and scanning time = 180 s) and 300s, respectively. 3D ‐ Gradient echo (GRE) T1WI sequence structure image scanning was from left to right. As for fMRI experiments, a block design 31 was adopted. Under fabric pressure of 0 kPa, 0.5 kpa, 1 kpa, and 1.5kpa, respectively, each subject was asked lying flat with her eyes closed but keeping brain awake. To avoid the interference of noise, the subjects were also given earplugs. After resting for 30 s, the fabric pressure was applied and lasted for 30 s, repeating each process for three times.
2.5. Data analysis
Anatomy 32 was used to select the amygdala brain region to make a region of interest (ROI). The so‐called ROI brain region referred to a mask file, which was used to filter and remove all the activated areas that were not in this brain region. The remaining activation clumps within the mask brain region were analyzed. 33 In other words, all the analysis of this topic was only conducted in this ROI. SPM12 (Statistical Parametric Mapping) was used for image preprocessing, individual analysis and group analysis. 34 Finally, Marsbar was utilized to extract the percent signal changes (PSCs, Percent BOLD Signal Changes or Percent fMRI Signal Changes) of all subjects under different fabric contact pressures.
The so‐called PSCs were calculated through the time series, which could be simply equivalent to dividing the average value of a certain section (for block design) or the value of a certain point (for event‐related design) of the entire time course by the average value of the entire time course, and multiplying by 100. This experiment was a block design experiment. Therefore, the average value of blood oxygen level dependent (BOLD) signals in a certain period (pressure stage) was selected for calculation. The calculation formula 35 was:
PSC: Percent Signal Change
βtask: A signal estimate of a task time series in the ROI
max(HRF): The maximum regression of task events
βconst: An estimated constant value for the signal of the particular ROI within the entire range of the sequence
Obviously, the PSC itself was calculated relative to the baseline (which could be the mean of the entire time period) and thus contained the concept of the baseline itself. Therefore, there was no need for the control condition, which was more intuitive and understandable than the activation intensity value obtained by the comparison calculation. 36 The practical significance was that in the ROI, the BOLD signal values of all voxels in the task time period accounted for the percentage of BOLD signal values of all voxel in the whole time series. The greater the absolute value of the PSC, the higher the attention of the ROI to the stimulus task. 33 The positive value of PSC represented the BOLD signal during the period of the task stimulation was positive in the ROI. Under the stimulation condition, the ROI was excited, and the brain activity was intense, while the opposite was inhibited.
3. RESULTS AND DISCUSSION
3.1. Making the amygdala into ROI
In order to avoiding the interference effect of other brain regions, the amygdala was made into a brain ROI, named ROI‐amygdala, and all analysis was only performed in ROI‐amygdala, rather than in the whole brain. The ROI‐amygdala shown in Figure 2 was made, calculated and depicted by Anatomy, Marsbar, 37 and BrainNet Viewer, 38 respectively.
FIGURE 2.
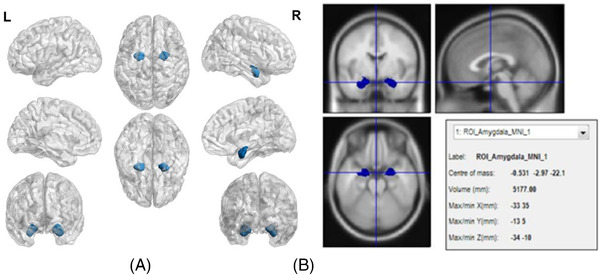
(A) Full view of ROI‐amygdala on a transparent brain background. (B) Anatomical map of brain region of ROI‐amygdala.
3.2. The results of PSCs in ROI‐amygdala under a series of increasing fabric pressures
By SPSS test, after eliminating outliers, the average PSC value and subjective evaluation value of comfort of each fabric under the stimulation of incremental contact pressure were obtained, and the results were shown in Table 1 and Figure 3.
TABLE 1.
Percent signal changes in region of interest (ROI)‐amygdala and subjective evaluation score Z under fabric contact pressure stimulation at 0 kpa, 0.5 kpa, 1 kpa, and 1.5 kpa
| Percent signal change/% | Subjective evaluation score Z | |||||||
|---|---|---|---|---|---|---|---|---|
| Subjects | 0 kpa | 0.5 kpa | 1 kpa | 1.5 kpa | 0 kpa | 0.5 kpa | 1 kpa | 1.5 kpa |
| 1 | 0.381 | 0.149 | 0.277 | −0.854 | −4.667 | −2.667 | 4.000 | 2.667 |
| 2 | 0.554 | 0.329 | −0.144 | −1.478 | −0.667 | −0.667 | −2.667 | −5.333 |
| 3 | 0.401 | 0.927 | −0.959 | −1.059 | −2.000 | −2.000 | −3.333 | −6.000 |
| 4 | 0.202 | ‐0.018 | −0.313 | 0.799 | 0.667 | −2.667 | −3.333 | −6.000 |
| 5 | 0.384 | 0.54 | −0.128 | 0.469 | 2.667 | 0.000 | −1.333 | −3.333 |
| 6 | 0.439 | −0.074 | −0.377 | 0.032 | 4.667 | 4.000 | 0.667 | −3.333 |
| Average | 0.394 | 0.250 | −0.241 | −0.627 | 0.111 | −0.667 | −1.000 | −3.556 |
| Standard Deviation | 0.104 | 0.208 | 0.107 | 0.273 | 3.041 | 2.309 | 2.632 | 2.998 |
FIGURE 3.
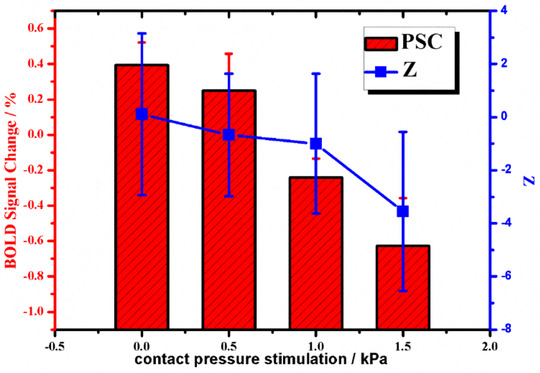
Mean (± SD) percent signal changes (PSCs) of region of interest (ROI)‐amygdala and subjective evaluation score Z of comfort under increasing contact pressure
Combined with Figure 3 and Table 1, we found that the amygdala exhibited some unique features when fabric contact pressure was applied to the skin.
Firstly, it could be seen that there was a consistent trend between subjective comfort and PSCs in ROI‐ amygdala, indicating that there was a certain correlation between the amygdala brain region and the perceived comfort of fabric touching skin, suggesting that the perceived pressure of fabric touching skin successfully attracted the attention of the amygdala brain region, which provided a good basis for using the amygdala as the characteristic sensing brain region for the comfort of fabric contact pressure.
Secondly, with the increasing of exposure pressure, the score of subjective comfort and PSCs in ROI‐amygdala decreased continuously, indicating that the increase of exposure pressure caused discomfort, especially when the pressure exceeded 1 kPa, the PSC in the amygdala brain region changed from about zero to negative. According to the definition formula of PSC, this might be caused by the negative activation of the amygdala brain region caused by excessive contact pressure. On one hand, the anatomical evidence suggested that somatic information was processed in a serious of parallel pathways that originated in the fields comprising Primary sensory cortex (SI) and eventually converge in Secondary sensory cortex (SII), which also accessed to the amygdala complex through the granular and dysgranular fields of the insula. 39 On the other hand, a large proportion of neurons in and around Central amygdala had the ability to receive somatosensory information of a nociceptive nature. Eighty‐one percent neurons in human amygdala cortex were exclusively inhibited by noxious stimuli, such as pinch, squeeze, thermal (>44°C), etc. 40 Nociceptive information carried along Aδ‐Fibers and C‐Fibers was sent from the spinal cord to thalamus and hypothalamus by the spinothalamic tract. After processed, polymodal information then reached the lateral amygdala via the granular and dysgranular insular cortex, or the anterior cingulate cortex. 41
Thirdly, by comparing the PSC of 0.5 kpa, 1 kPa, and 1.5kpa pressure stimulation, the Z value of the amygdala decreased more than that of the subjective evaluation. It could be seen that the attention of the amygdala to contact pressure increased with the increase of pressure stimulation, and the response degree was more intense than that of the subjective evaluation. This might be because the body's final subjective evaluation was being interfered with by psychological effects, so the subjective tolerance was higher, but in fact, the brain had been already reacting violently. This result demonstrated the antipsychological interference function and authenticity of brain perception compared with subjective evaluation of skin tactile comfort.
Finally, Standard Deviation (SD) values of subjective evaluation were all larger than SD of PSCs in ROI‐amygdala, indicating that interference of individual differences was more obvious in subjective evaluation, reflecting the superiority of using brain perception for comfort evaluation.
4. CONCLUSIONS
As could be seen from above, the amygdala brain region, which cognized and regulated pain and various negative emotions, had a significant correlation between PSCs and the fabric contact pressure, which implied that the attention of the amygdala brain region to skin perception with a negative effect increased significantly with the increase of fabric pressure. The finding suggested that the amygdala might be the characteristic assessment area of the nociceptive tactile pressure perception (such as: oppressive feeling and pressure‐pain). In the future, the fabric factors and human factors affecting this brain region will be further explored.
ACKNOWLEDGMENTS
The authors acknowledge all the subjects for their participation in the fMRI experiment. This research was funded by National Natural Science Foundation of China (grant number: 52003245), Fundamental Research Funds of Zhejiang Sci‐Tech University (grant number: 2019Q077), Zhejiang Provincial Natural Science Foundation of China (grant number: LQ18E030007), General Scientific Research Fund of Education Department of Zhejiang Province (grant number: 113129A4F21075), Education and Scientific Research Foundation for Middle‐aged and Young Scientist of Fujian Province, China (grant number: JAS180331), Open Funding of “Key Laboratory of Advanced Textile Materials and Manufacturing Technology (Zhejiang Sci‐Tech University), Ministry of Education” and “Zhejiang Provincial Key Laboratory of Fiber Materials and Manufacturing Technology, Zhejiang Sci‐Tech University” (grant number: 2019QN05), Natural Science Foundation of Shandong Province (grant number: ZR2020QF115), Research Foundation of Zhejiang Sci‐tech University (grant number: 11313132612042) Research Foundation of Zhejiang Sci‐tech University (grant number: 11313132612042) and National Natural Science Foundation of China (grant number:52106205).
Yuan J, Wang Q, Shang S, Lei Y, Lou L. Analysis of brain signal change response in amygdala evoked by skin pressure stimulus. Skin Res Technol. 2023;29:1–6. 10.1111/srt.13238
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Binns H. The discrimination of wool fabrics by the sense of touch. British Journal of Psychology General Section. 1926;16(3):237‐247. [Google Scholar]
- 2. Jacobsen M, Fritz A, Dhingra R, et al. A psychophysical evaluation of the tactile qualities of hand knitting yarns. Text Res J. 1992;62:557‐566. [Google Scholar]
- 3. Hagihara KM, Bukalo O, Zeller M, et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature. 2021;594(7863):403‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luyck K, Goode TD, Masson HL, et al. Distinct activity patterns of the human bed nucleus of the stria terminalis and amygdala during fear learning. Neuropsychol Rev. 2019;29(2):181‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song X, Bhinge S, Quiton RL, et al. An ICA based approach for steady‐state and transient analysis of task fMRI data: application to study of thermal pain response. J Neurosci Methods. 2019;326:108356. [DOI] [PubMed] [Google Scholar]
- 6. Allen HN, Bobnar HJ and Kolber BJ. Left and right hemispheric lateralization of the amygdala in pain. Prog Neurobiol. 2021;196:101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson TD, Valdivia S, Khan A, et al. Dual and opposing functions of the central amygdala in the modulation of pain. Cell Rep. 2019;29:332‐346.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am J Respir Crit Care Med. 2008;177(9):1026‐1032. [DOI] [PubMed] [Google Scholar]
- 9. Kanosue K, Sadato N, Okada T, et al. Brain activation during whole body cooling in humans studied with functional magnetic resonance imaging. Neurosci Lett. 2002; 329(2):157‐160. 10.1016/s0304-3940(02)00621-3 [DOI] [PubMed] [Google Scholar]
- 10. Tan J, Wang X, Luo C, et al. The brain functional magnetic resonance imaging characteristics induced by contact heat stimulations in normal adults. Chinese Journal of Neurology. 2014;47(5):331‐335. [Google Scholar]
- 11. Yuan J, Yu WD, Chen KM, et al. A potential new fabric evaluation approach by capturing brain perception under fabric contact pressure. Text Res J. 2019;89(1):3312‐3325. 10.1177/0040517518811939 [DOI] [Google Scholar]
- 12. Wang Q, Tao Y, Sun T, et al. Analysis of brain functional response to cutaneous prickling stimulation by single fiber. Skin Research and Technology. 2021;27(4):494‐500. [DOI] [PubMed] [Google Scholar]
- 13. Fonseca R, Madeira N, Simoes C. Resilience to fear: the role of individual factors in amygdala response to stressors. Mol Cell Neurosci. 2021;110:103582. [DOI] [PubMed] [Google Scholar]
- 14. Altered cortical reorganization and brain functional connectivity in phantom limb pain: a functional MRI study. Pain practice : the official journal of World Institute of Pain. 2020. [DOI] [PubMed]
- 15. Chen F, Yang J, Zhang H, et al. The role of amygdala neurosin/serpinb6 pathway in the regulation of gastric hypersensitivity and anxiety by electroacupuncture. Journal of Digestive Diseases. 149‐56, 2022. [DOI] [PubMed] [Google Scholar]
- 16. Tang Y, Zhou J. Exploring the application of mindfulness meditation in the local government executives’ mental stress management. 2018.
- 17. Creswell JD, Way BM, Eisenberger NI, et al. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69(6):560‐565. [DOI] [PubMed] [Google Scholar]
- 18. Way BM, Creswell JD, Eisenberger NI, et al. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self‐referential neural activity during rest. Emotion. 2010;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duan G, He Q, Pang Y, et al. Altered amygdala resting‐state functional connectivity following acupuncture stimulation at BaiHui (GV20) in first‐episode drug‐naïve major depressive disorder. Brain imaging and behavior. 2020;14(6):2269‐2280. [DOI] [PubMed] [Google Scholar]
- 20. Liu N, Yao L, Zhao X. Evaluating the amygdala network induced by neurofeedback training for emotion regulation using hierarchical clustering. Brain Res. 2020;1740:146853. 10.1016/j.brainres.2020.146853 [DOI] [PubMed] [Google Scholar]
- 21. Martynova O, Tetereva A, Balaev V, et al. Longitudinal changes of resting‐state functional connectivity of amygdala following fear learning and extinction. Int J Psychophysiol. 2020;149:15‐24. [DOI] [PubMed] [Google Scholar]
- 22. Smirni D, Smirni P, Carotenuto M, et al. Noli me tangere: social touch, tactile defensiveness, and communication in neurodevelopmental disorders. Brain Sciences. 2019;9(12):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamann S and Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13(1):15‐19. [DOI] [PubMed] [Google Scholar]
- 24. Manssuer L, Qiong D, Wei L, et al. Integrated amygdala, orbitofrontal and hippocampal contributions to reward and loss coding revealed with human intracranial EEG. J Neurosci. 2756‐71, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baeken C, Xu Y, Wu G‐R, et al. Hostility in medication‐resistant major depression and comorbid generalized anxiety disorder is related to increased hippocampal–amygdala 5‐HT2A receptor density. Eur Arch Psychiatry Clin Neurosci. 2021;271(7):1369‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frick A, Björkstrand J, Lubberink M, et al. Dopamine and fear memory formation in the human amygdala. Mol Psychiatry. 2021:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simonetti A, Saxena K, Koukopoulos AE, et al. Amygdala structure and function in paediatric bipolar disorder and high‐risk youth: a systematic review of magnetic resonance imaging findings. The World Journal of Biological Psychiatry. 2021:1‐24. [DOI] [PubMed] [Google Scholar]
- 28. Rubio A, Pellissier S, Van Oudenhove L, et al. Brain responses to uncertainty about upcoming rectal discomfort in quiescent Crohn's disease ‐ a fMRI study. Neurogastroenterol Motil. 2016;28:1419‐1432. 10.1111/nmo.12844 [DOI] [PubMed] [Google Scholar]
- 29. Kim GW, Jeong GW. Brain activation patterns associated with the human comfortability of residential environments: 3.0‐T functional MRI. Neuroreport. 2014;25(12):915‐920. 10.1097/wnr.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 30. Aggleton JP, Mishkin M. The amygdala: sensory gateway to the emotions. Biological Foundations of Emotion. Elsevier; 1986. [Google Scholar]
- 31. Fu X, Zhang Q, Zhang Y, et al. FMRI comparative study of hand motion block design and event related design. Practice radiology. 2006:5‐8. [Google Scholar]
- 32. Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325‐1335. [DOI] [PubMed] [Google Scholar]
- 33. Zhu Y. Image cognition based on functional magnetic resonance Imaging. Dalian Maritime University; 2013. [Google Scholar]
- 34. Ashburner J, Barnes G, Chen C, et al. SPM12 manual. Wellcome Trust Centre for Neuroimaging; 2014. [Google Scholar]
- 35. Glascher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7(1):73‐82. 10.1007/s12021-008-9042-x [DOI] [PubMed] [Google Scholar]
- 36. Yuan J, Xu C, Wang Q, et al. Brain signal changes of sensory cortex according to surface roughness of boneless corsets. Text Res J. 2020;90(1):76‐90. 10.1177/0040517519858758 [DOI] [Google Scholar]
- 37. Brett M, Anton J‐L, Valabregue R, et al. Region of interest analysis using an SPM toolbox. In: 8th international conference on functional mapping of the human brain 2002, 497. Sendai. [Google Scholar]
- 38. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Friedman DP, Murray EA, O'Neill JB, et al. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252(3):323‐347. [DOI] [PubMed] [Google Scholar]
- 40. Bernard J, Huang G, Besson J. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68(2):551‐569. [DOI] [PubMed] [Google Scholar]
- 41. Strobel C, Hunt S, Sullivan R, et al. Emotional regulation of pain: the role of noradrenaline in the amygdala. Science China Life Sciences. 2014;57(4):384‐390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


