Various methods have been used to treat onychomycosis, including application of topical and systemic antifungal agents and laser therapy. In current standard systemic treatment, the complete cure rate of toe nails is 38% with terbinafine and 14% with itraconazole. 1 In addition, recurrences (i.e., relapse [same infection after incomplete cure] or reinfection [same infection after complete cure]) occur at a rate of 20–25%. 1 Near‐infrared diode lasers, 1064 nm neodymium‐doped yttrium aluminum garnet (Nd:YAG) lasers, dual diode lasers, 1320 nm Nd:YAG lasers, and fractional CO2 lasers have all been used for the treatment of onychomycosis. 2 A recent review reported that 1064 nm Nd:YAG laser treatment resulted in lower mycologic cure rates (11%) than oral and topical therapies approved by the Food and Drug Administration (29–61%). 2 Thus, an effective and safe local target therapy for onychomycosis is needed. Plasma lasers have antibacterial and antifungal effects; these lasers are generated in the air by a strong electric field pulse generated by the ionization of air molecules into ozone, hydroxyl radicals, and nitric oxide, which exert antifungal properties. 3 In previous studies, nonthermal atmospheric‐pressure plasma (NTAP) inhibited the growth of Trichophyton rubrum in vitro, 4 achieving an overall clinical cure rate for infected toenails of 53.8%, and a mycological cure rate of 15.4% in an in vivo pilot study. 5
This study aimed to evaluate the feasibility of NTAP as a treatment option for onychomycosis. A total of five participants (four females and one male, mean age 46.4) with toenail onychomycosis provided informed consent to participate. Reflectance confocal microscopy (RCM, VivaScope® 1500; Lucid Inc., Rochester, NY, USA) was performed on the infected toenails before and immediately after NTAP (PLADUO; SHENB, Seoul, Korea), which generated plasma from argon and nitrogen gas sources, delivering pulses to the skin. The NTAP was applied to the affected nail plate with pulse energy 1.5 J, frequency 3 Hz of nitrogen gas and followed by 0.75 J, 3 Hz of argon gas, three passes, respectively.
The number of fungal spores and hyphae was counted in each 500 × 500 µm2 RCM image. To evaluate fungal spore dispersion, the RCM images were divided in a grid pattern. Wilcoxon signed‐rank test was then applied to compare the total number and dispersion effect of spores before and after NTAP treatment. Statistical significance was set at p < 0.05.
Before NTAP treatment, a spherical spore surrounded by a bright visible wall was observed in the RCM image; after NTAP treatment, cell wall disruption and fungal hyphae breakage were observed (Figure 1). The size of fungal spores is reported to range from 2 to 50 µm in diameter. 6 We measured the size of the fungal spores to be 2−8 µm using Image J. The spores were clustered; however, immediately after treatment, the spores became more disperse (p = 0.5), and the total number of spores decreased from 216 to 138 (p = 0.31) (Figure 2), although this difference was not statistically significant. On the RCM image, crack‐like changes in the nail plate were seen after treatment, which was thought to indicate a synergistic effect when combined with other treatments such as topical antifungal agent (Figure 3).
FIGURE 1.
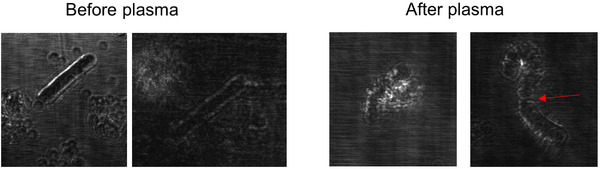
A hyphae with an intact cell wall was observed on reflectance confocal microscopy (RCM) image. After plasma treatment, disruption of the cell wall and broken hyphae (red arrow) were observed
FIGURE 2.
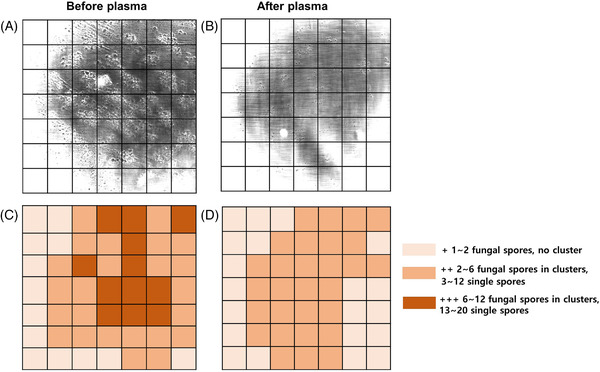
An RCM image (500 × 500 µm2) with grid pattern showing that clusters of fungal spores (A) were dispersed (B) following plasma treatment. The mean number of fungal spores of all participants were counted, and the grid pattern showed a decreased density after plasma treatment (C and D)
FIGURE 3.
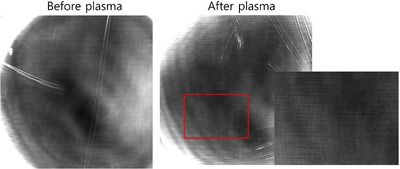
On the RCM image, reflectance was increased and fine lines were clearly visible, reflecting the nail plate changes immediately after treatment
Biofilms are structured sessile microbial communities comprising microorganisms adhering to a surface and each other via an extracellular polymeric matrix. 7 Similar to bacteria, fungi are also able to form biofilms; T. rubrum and T. mentagrophyte biofilms have been previously reported. 8 The adhesion of fungal spores to surfaces occurs readily and is a prerequisite for biofilm formation. 9 Recent studies have reported the positive effect of plasma on fungal biofilm inactivation. 10 , 11 Our prior in vitro study showed destruction of colonies of T. rubrum with waxy surface after NTAP irradiation and scanning electron microscopy, confirming that the exopolymeric matrix of the biofilm 8 disappeared (Figure S1). Despite the small sample size, this study revealed that NTAP treatment disrupted the cell wall and decreased the number and dispersed clusters of spores. These results suggest that NTAP treatment could exert preventive effects against fungal biofilms.
There were no serious side effects during NTAP irradiation, except for a minor heating sensation. To reduce this unpleasant sensation, it is necessary to keep moving the hand‐piece, instead of fixing it at one point. Although the effect may be weak as a single treatment, we suggest that irradiating onychomycosis with NTAP prior to topical antifungal or laser treatment could enhance the therapeutic effect.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Informed consents were obtained from all participants.
Supporting information
Supporting Information
ACKNOWLEDGMENT
This research was supported by a Paul Janssen grant from the Korean Dermatological Association, Republic of Korea, in 2022.
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853‐67. [DOI] [PubMed] [Google Scholar]
- 2. Gupta AK, Versteeg SG. A critical review of improvement rates for laser therapy used to treat toenail onychomycosis. J Eur Acad Dermatol Venereol. 2017;31:1111‐8. [DOI] [PubMed] [Google Scholar]
- 3. Ouf SA, El‐Adly AA, Mohamed AH. Inhibitory effect of silver nanoparticles mediated by atmospheric pressure air cold plasma jet against dermatophyte fungi. J Med Microbiol. 2015;64:1151‐61. [DOI] [PubMed] [Google Scholar]
- 4. Heinlin J, Maisch T, Zimmermann JL, et al. Contact‐free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Fut Microbiol. 2013;8:1097‐106. [DOI] [PubMed] [Google Scholar]
- 5. Lipner SR, Friedman G, Scher RK. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin Exp Dermatol. 2017;42:295‐8. [DOI] [PubMed] [Google Scholar]
- 6. Di Filippo PD, Pomata D, Riccardi C, Buiarelli F,Perrino C. Fungal contribution to size‐segregated aerosol measured through biomarkers. Atmos Environ. 2013;64:132–40. [Google Scholar]
- 7. Heydorn A, Ersbøll BK, Hentzer M, Parsek MR, Givskov M, Molin S. Experimental reproducibility in flow‐chamber biofilms. Microbiology (Reading). 2000;146:2409–15. [DOI] [PubMed] [Google Scholar]
- 8. Costa‐Orlandi CB, Sardi JC, Santos CT, Fusco‐Almeida AM, Mendes‐Giannini MJ. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling. 2014;30:719‐27. [DOI] [PubMed] [Google Scholar]
- 9. Liauw CM, Slate AJ, Butler JA et al. The effect of surface hydrophobicity on the attachment of fungal conidia to substrates of polyvinyl acetate and polyvinyl alcohol. J Polym Environ. 2020;28:1450–64. [Google Scholar]
- 10. Los A, Ziuzina D, Boehm D, Cullen PJ, Bourke P. Inactivation efficacies and mechanisms of gas plasma and plasma‐activated water against Aspergillus flavus spores and biofilms: a comparative study. Appl Environ Microbiol. 2020;86:e02619‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He M, Duan J, Xu J, et al. Candida albicans biofilm inactivated by cold plasma treatment in vitro and in vivo. Plasma Process Polym. 2020;17:1900068. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


