Abstract
Background
Prolonged face mask usage, a daily practice for the public due to the COVID‐19 pandemic, creates high levels of humidity underneath the mask, which may cause unexpected skin concerns.
Objective
To investigate the impact of repeated mask usage on the face by comparing skin properties inside and outside of the mask‐covered areas.
Methods
A double‐blinded, randomized, split‐face clinical study was conducted with 21 healthy female participants who wore face masks at least 6 h every day for 1 week, with one side of their face treated with a moisturizer three times daily. On day 8, after 5 h of wearing the mask, skin properties (sebum, hydration, and trans‐epidermal water loss [TEWL]) were evaluated at 15, 60, and 120 min post‐mask removal, followed by barrier disruption and recovery assessment.
Results
Mask usage weakened stratum corneum (SC) on facial skin compared to uncovered areas, including reduced SC hydration (p < 0.02 at 15 min) and increased TEWL in response to tape stripping challenge (p < 0.03 after stripping). In addition, sebum production also increased after mask removal (p < 0.01 at 15 min). Notably, a daily moisturizer mitigated these effects by increasing SC hydration (p < 0.001) and improving SC resilience against barrier disruption.
Conclusion
Daily prolonged usage of a facial mask, essential due to the COVID‐19 situation, generated a high‐humidity microenvironment and led to compromised SC, which was revealed by a barrier challenge technique. Moreover, proper facial moisturization may help to maintain skin homeostasis and prevent the barrier impairment caused by repeated mask usage.
Keywords: face mask, moisturizer, skin hydration, skin occlusion, stratum corneum barrier
1. INTRODUCTION
As the protective layer between the body and the external environment, human skin has adapted to develop an optimal barrier with active and continuous adjustment to cope with extreme environmental changes, including temperature, humidity, pressure, sun exposure, and other external insults. 1 , 2 , 3 Nevertheless, humidity changes may cause skin concerns. Conventional belief and some literature suggest that higher humidity may introduce excess sweating and acne aggravation, 4 , 5 and that a low‐humidity environment exacerbates skin xerosis such as during air flight or abrupt decrease of environmental humidity. 6 , 7 However, there is also evidence that low humidity could enhance skin barrier functions, and the effects of repeated humidity changes on human skin have not been investigated thoroughly. 8 , 9
The COVID‐19 pandemic has created one of the biggest crises for our contemporaneous human society. One prominent lifestyle change is a new norm of wearing face masks. Daily and prolonged usage of a facial mask has become an essential personal and public health practice. The effects of semiocclusive masks (from medical N95 to nonwoven 3‐layer disposable or cloth protective versions) on the skin have generated great interest even before the pandemic. 10 , 11 The obvious consequence of mask‐wearing is the localized relatively high humidity on facial skin under the mask. Early reports have demonstrated some irritation and discomfort from longtime mask‐wearing, not only for healthcare workers, but also for the general public. 12 , 13 The changes of facial skin properties due to mask coverage have been investigated; however, the results are inconsistent due to different study designs, materials (mask types, etc.), and evaluation technologies. 14 , 15 , 16
Human skin epidermis is a dynamic and constantly evolving system that maintains protective homeostasis through a sensitive balance of proliferation and differentiation of the keratinocytes. 17 , 18 Moreover, the upper epidermal layers have been tuned to sense and signal the environmental changes and adapt quick responses in order to fulfill the protective functions. 19 We hypothesize that the repeated environmental changes on the skin (mask effects) can impact barrier homeostasis, which the recovery process could reveal after response to the skin barrier disruption (such as tape stripping). This study was designed to investigate skin properties after prolonged and repeated use of face masks by comparing facial skin inside and outside the mask‐covered areas. We also assessed the efficacy of a skin moisturizer against potential skin impairment induced by mask coverage.
2. METHODS
The clinical study was reviewed and approved by an independent Institutional Review Board (IRB) (IntegReview, Austin, Texas) and was conducted following the principles of the Declaration of Helsinki, the ICH‐GCP guidelines, as well as the protective measures for the safety and well‐being of participants during the COVID‐19 situation. All participants signed written informed consent and photo release forms and participated in the study voluntarily. For each visit to the study center, the participants were asked about their health history, possible exposure to COVID‐19, and had their temperature checked for safety assurance. During the extended stay in the study center, proper social distancing and other safety measures were in accordance with the Centers for Disease Control and Prevention guidelines. All IRB approval and consenting documents are available for verification.
2.1. Study design
Healthy female volunteers with self‐perceived sensitive skin and self‐described regular mask use were recruited for the double‐blinded, randomized, split‐face study. Each participant applied a moisturizer on one randomly assigned side (left or right) of the face, designated as treated (TX), and the other side as untreated (NT). The protective mask (protective disposable face masks made of polypropylene with ear loops, Fisher Scientific, Waltham, Massachusetts) allowed the front face to be covered and left enough space on the cheek areas to be free of mask coverage. As a result, each side of the face could be further divided into two unique areas: masked‐covered regions close to the nose (IN) and no‐mask (uncovered) regions close to the ears (OUT). Hence, four facial sites could be assessed on each participant's face: treated inside or outside of the mask (TX‐IN, TX‐OUT), and untreated inside or outside of the mask (NT‐IN, NT‐OUT), as designated in Figure 1A.
FIGURE 1.

(A) Four designated testing sites on face were evaluated by instrumental measurements: TX‐OUT (treated outside mask); TX‐IN (treated inside mask); NT‐IN (untreated inside mask); NT‐OUT (untreated, outside mask). Treatment side is randomized. (B) Visual evaluation of mask impact on skin erythema, dryness, and pores, only on treated and untreated sides (no further split). Expert grading did not show significant difference for any of the parameters compared to baseline values at the start of the study. N = 21, error bars = standard errors (SE)
During the first visit (baseline‐Day 1), after acclimation (20–24°C) without wearing a face mask for at least 20 min, facial photography and visual evaluations were performed, followed by instrumental measurements of skin properties described later. Then, the participants were instructed to apply a facial moisturizer (see description later) on one randomly assigned side of their faces, and they were asked to use the moisturizer on the same side three times a day (morning, midday when changing mask, and evening) for the next 7 days while continuing wearing the provided protective masks for at least 6 h a day, with free replacement. On the second visit (Day 8), the participants applied the moisturizer one more time before wearing the face mask and stayed at the study center for 5 h. Afterward, they removed their face masks and remained seated in the isolated temperature‐controlled room. Their skin properties were measured at 15, 60, and 120 min after mask removal, including an image capture and visual grading after the 15‐min session. After the 120‐min evaluations, sequential tape stripping using D‐SQUAME tape (Clinical & Derm, Dallas, Texas) was conducted on 4 designated sites for 10 layers per site. This was followed by one more barrier measurement right after tape stripping. On Day 9, about 20 h after tape stripping, the participants came back to the study center and had one more barrier measurement at all sites for recovery assessment.
2.2. Facial imaging and visual grading
At baseline (Day 1) and Day 8 (15 min after mask removal), digital images were taken for each participant's face using VISIA‐CR photo station (Canfield Imaging Systems, Fairfield, New Jersey) with a Canon Mark II digital SLR camera (Canon Incorporated, Tokyo, Japan). The skin erythema, dryness, and pore appearance were also evaluated by an expert grader for possible changes caused by mask usage, all with 0 (minimal) to 9 (maximal) grading scales.
2.3. Instrument—sebumeter
Sebumeter SM 815 (Courage + Khazaka electronic GmbH, Köln, Germany) was used to measure the sebum quantity on the skin using a photometric method at baseline (Day 1) and Day 8 post‐mask removal time points at all four designated sites.
2.4. Instrument—GPSkin barrier light
The portable GPSkin Barrier Light (GPower Inc, Seoul, South Korea) device has been validated as a reliable tool for stratum corneum hydration (SCH) and trans‐epidermal water loss (TEWL) measurements. 20 , 21 , 22 All procedures were controlled using the GPSkin mobile app on an Apple iPhone 6S through Bluetooth connection. The measurements were taken at baseline (Day 1) and on Day 8 (post‐mask removal) at positions close to the sebum probing spots and the same location for tape stripping, including post tape stripping follow‐ups.
2.5. Face care and moisturizer
A testing facial moisturizing lotion was selected to investigate its effects on managing potential skin changes due to mask usage. The key ingredients included moisturizing agents such as glycerin and butylene glycol, hydroxyethyl urea, sodium polyaspartate, and high water bonding sodium hyaluronate in an emollient base (pH 5.7). The participants were asked to apply about 0.3 g of the moisturizer on one side of their faces (randomized among participants) three times a day during the 7‐day mask‐wearing period. In addition, a facial cleansing product (Clinique Liquid Facial Soap–Mild, New York) was provided to the participants for daily general cleansing.
2.6. Data analysis
All visual grading results were treated as nonparametric data for statistical analysis. For instrumentation (Sebumeter, GPSkin) measurements, triplicate readings from each site were averaged prior to analysis. The mean change from baseline (CFB, defined as post‐baseline value minus baseline value) was calculated at applicable time points for probing mask effects (inside vs. outside) and moisturizer effects (treated vs. untreated). The null hypothesis, that the mean CFB was equal between sides and treatments at post‐baseline time points, was tested using paired t test (for the instrumentation results) or Wilcoxon signed rank test (for visual grading parameters). In addition, a second statistical baseline was defined as Day 8 at 120 min (before tape stripping) to compare barrier integrity after tape stripping and at Day 9 (recovery).
3. RESULTS
Twenty‐four healthy female volunteers (ages 18–60) were recruited in the study managed by SGS Stephens, Inc. in Phoenix, Arizona between August and September 2020, of which 21 participants (40.7 years old ± 16.3 years) completed the study. The study panel was composed of Caucasian, Hispanic, and Asian ethnic groups, with Fitzpatrick skin type ranging from I to IV.
3.1. Visual and image analysis
For visual and image analysis, the two half‐face sides (treated and untreated) were evaluated separately without further separation by mask coverage. Visual evaluations of skin erythema, dryness, and pores showed no significant differences when comparing between moisturizer‐treated (TX) or untreated (NT) sides of the face after mask removal, indicating that the protective face mask commonly used by many people exhibited no significant measurable visual impairment even after prolonged daily usage (Figure 1B). Image analysis for redness (a* value under cross‐polarized lighting) also confirmed that no significant difference was observed on face when comparing between Day 1 baseline and Day 8 (post‐mask usage), or between moisturizer‐treated or untreated sides (data not shown).
3.2. Sebum production
Facial sebum production was significantly increased only inside the mask on the untreated side (NT‐IN) at 15 min post‐mask removal on Day 8, indicating a transient sebum increase in mask‐covered skin. There was no significant difference for sebum values at any time point for the treated side (TX‐IN or TX‐OUT), or for untreated side outside the mask (NT‐OUT) compared to their baseline values (Figure 2A). Furthermore, CFB comparison showed a significant increase in sebum production inside compared to outside the mask on the untreated side at 15 min. There was no significant difference between the treated and untreated side (either inside or outside the mask) at any other time point afterward (Figure 2B).
FIGURE 2.

Sebum production on face after 1 week of mask‐wearing. (A) Sebum changes after mask removal for all testing sites up to 120 min on Day 8. (B) Sebum change from baseline (CFB) cross comparisons among four testing sites for mask or treatment effects at different time points. Significant differences to baseline (in part A) and between sites (in part B) are designated by “*” (p < 0.05) and “**” (p < 0.01). N = 21, error bars = standard errors (SE) on both charts
3.3. Stratum corneum hydration (SCH)
On Day 8 after mask removal, there were significant decreases in SCH from 15 through 120 min at the mask‐covered site on the untreated side (NT‐IN), suggesting skin dehydration caused by mask usage (Figure 3A), whereas on the treated side at the mask‐covered site (TX‐IN), there were significant increases in SCH from 15 through 120 min (also in Figure 3A), indicating protection against mask‐induced dehydration with the moisturizer. When comparing treatment effects using CFB values, on the untreated side of the face, a significantly larger SCH reduction was observed inside the mask (NT‐IN) than outside the mask‐covered area (NT‐OUT) at 15 min post‐mask removal. In addition, the treated side of the face displayed a significant increase in SCH both inside and outside the mask cover compared with the untreated side at all time points (Figure 3B). Furthermore, the moisturizer significantly increased SCH inside the mask cover (TX‐IN), with about twofold greater hydration than non‐treated skin (NT‐IN) at all time points (Figure 3B), suggesting that the mask cover led to a temporary SCH reduction after mask removal, and the moisturizer treatment was able to protect the skin against the dehydrating effect after mask removal.
FIGURE 3.
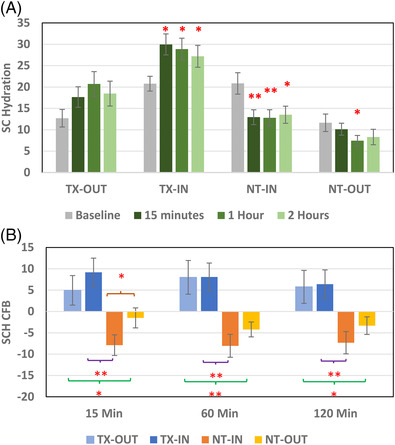
Stratum corneum hydration (SCH) measured by GPSkin after 1 week of mask‐wearing. (A) SCH changes after mask removal on all testing sites up to 120 min on Day 8, with significant differences to baseline marked by “*” (p < 0.05) and “**” (p < 0.01). (B) SCH change from baseline (CFB) cross comparisons among the four testing sites for mask or treatment effects at different time points. Significant differences for mask effects are designated on the top; treatment effects are designated at the bottom marked by “*” (p < 0.05), "**” (p < 0.01), and “***” (p < 0.001). N = 21, error bars = standard errors (SE) on both charts
3.4. Skin barrier integrity and tape stripping challenge by TEWL measurements
Similar to results shown in a previous study, 12 no significant TEWL changes could be detected at any test site (comparing to its own original baseline) after mask‐wearing for 7 days (data not shown). However, after a 10‐layer tape stripping challenge at 120 min post‐mask removal on Day 8, significantly higher TEWL was observed on all testing sites, with the two mask‐covered facial sites (IN) appearing to increase more than the two uncovered sites (OUT), respectively, suggesting skin might be more susceptible to challenge after mask usage (Figure 4A). To quantify these differences with CFB comparisons, we used “before tape stripping at 120 min” as the second baseline. It showed that on the untreated side of the face, in addition to significant TEWL increase right after tape stripping, even 20 h later, TEWL remained significantly higher on the mask‐covered site versus the uncovered site, indicating a more severe barrier damage and a slower barrier recovery without treatment (Figure 4B).
FIGURE 4.

Skin barrier assessment using trans‐epidermal water loss (TEWL) measured by GPSkin. (A) Changes of TEWL due to tape stripping challenge on Day 8 at 120 min after mask removal, and post 20 h recovery (Day 9), with significant differences to the second baseline (120 min) marked by “*” (p < 0.05), “**” (p < 0.01), and “***” (p < 0.001). (B) Using 120 min post‐mask removal as the second baseline, to compensate some mask‐induced imbalance, the change from baseline (CFB) of TEWL was compared among the four testing sites for mask effect or treatment effect after tape stripping challenge. Significant differences for mask effects are designated on the top, and treatment effects are designated at the bottom by “*” (p < 0.05) and “**” (p < 0.01). (C) TEWL CFB on Day 8 after mask removal depicting the small but significant changes due to mask coverage and moisturizer use as marked by “*” (p < 0.05). N = 21, error bars = standard errors (SE) on all charts
In terms of treatment with moisturizer, even before tape stripping, a significant reduction of TEWL was observed for the treated side versus the untreated side under the mask at all time points (15–120 min) after mask removal (CFB comparisons in Figure 4C). Furthermore, the tape stripping challenge resulted in a significantly less TEWL increase (about half) on the treated side versus untreated side under the mask, and no significant mask‐induced barrier difference on treated side of the face (Figure 4B), further demonstrating that applying a moisturizer boosted a stronger skin barrier resilience against disruption under the mask.
4. DISCUSSION
This clinical study aimed to explore the effects of prolonged and repeated mask use on facial skin properties by comparing the skin inside and outside mask‐covered areas, as well as testing the potential skin benefits of applying a moisturizer in a period of face mask usage. Although the protective face mask commonly used by the general public presented no visible changes on the facial skin features (erythema, dryness, and pore appearance) as evaluated by expert visual grading 15 min after mask removal, instrumental measurements revealed some significant changes caused by prolonged mask usage. Our study design allowed the direct comparisons of adjacent measurements on the cheek between covered and uncovered areas to show mask effects, which were more comparable than using forehead as control skin. 15 Overall, mask usage temporarily dehydrated the facial skin, weakened skin barrier, and increased transient sebum production. The tested moisturizer appeared to maintain hydration and strengthen skin barrier against a challenge.
To reveal the impact of mask‐wearing on skin, several different design considerations were implemented, compared with early studies run during the COVID‐19 pandemic. 13 , 14 , 15 In addition to four cheek sites for close comparisons, the evaluations were not conducted immediately after mask removal, to leave time for the initial evaporation of any moisture that might have been trapped under the skin surface due to semiocclusive protective mask cover. Previous studies on hygiene product impacts on skin have determined that excess water would accumulate on the skin surface under high‐humidity occlusion and may overhydrate the skin, resulting in mechanical or chemical irritations. 23 , 24 Once the occlusion is removed, the extra moisture would evaporate to restore normal hydration. The initial water release could be separated into two distinct types of water loss, skin surface water loss (SSWL) and TEWL. 25 , 26 SSWL (like moisture) does not represent actual skin hydration, and this initial SSWL and vapor permeability might be mistaken as increased skin hydration. To overcome the hydration artifact (as some studies might have reported), we set all measurements 15 min after the mask removal.
Contrary to the results in previous studies, in our study, the SCH significantly decreased under the mask coverage 15 min after mask removal on the untreated side of the face. Therefore, although mask coverage might temporarily cause increased humidity that the wearer commonly feels, the data suggest that the skin surface becomes temporarily dehydrated after mask removal. This finding raises the importance of skin care during mask usage as the loss of skin hydration needs to be addressed to prevent repeated skin perturbation and damage. In fact, applying a moisturizer in our study mitigated these drying effects by significantly increasing and maintaining SCH under the mask‐covered area, demonstrating that a properly designed moisturizer is beneficial to skin hydration when wearing a face mask for a long time.
The impact of the face mask was more striking when we compared the skin barrier integrity using TEWL as measurement. More importantly, a skin challenge method, sequential tape stripping, was deployed to disrupt skin homeostasis and to reveal barrier differences due to mask coverage. Although TEWL increased at all sites after tape stripping as predicted, mask coverage resulted in nearly threefold increase of TEWL compared to the uncovered area on the untreated side, signifying a weakened barrier. The barrier recovery remained delayed after 20 h on Day 9 and did not recover to original levels. Similarly, applying a moisturizer prevented the barrier disruption and strengthened SC integrity with lower TEWL increase. The greater TEWL changes resulting from mask covering validated the necessity of barrier disruption (such as tape stripping) to reveal a weakened SC structure and offered additional evidence of benefits for using a moisturizer during prolonged mask usage.
Occlusive or semiocclusive face mask‐wearing introduces local high humidity to facial skin. Our results confirmed that, like early eczema studies reported, 27 relatively high humidity even by regular protective mask cover could cause reduced SCH and weakened skin barrier. Other in vitro and modeling studies also supported the basis of our finding. First, several important skin barrier pathways, such as filaggrin degradation and caspase‐14 activity, could be inhibited under high‐humidity conditions. 28 , 29 , 30 Second, a low‐humidity environment might stimulate the lamellar body secretory system for barrier repair. 31 These reports suggest skin barrier deterioration for both lipid synthesis and humectant productions under high humidity compared to normal (or low humidity) conditions. We speculate that high local semiocclusive humidity due to repeat prolonged protective mask usage may lead to less hydrated skin and a weakened barrier. The possible mechanism of action could be explained by the comparison illustrated in Figure 5.
FIGURE 5.

Dynamic epidermal hemostasis as modulated by relative humidity. Left: Stratum corneum (SC) maturation under normal humidity conditions sustains a larger water gradient; right: at high humidity, SC favors less of a lipid bilayer (thinner) and lower levels of humectants (natural moisturizing factors [NMFs], etc.) because of a shallower water gradient. The shift back to normal (lower) humidity (left) may lead to dry skin and impaired barrier under the larger water gradient due to the lack of barrier development. Source: The epidermis model is reprinted from. 32 Copyright (2018), with permission from Elsevier
In this model, 32 under normal humidity, the epidermis maintains a healthy SC layer that can maintain a steeper water gradient (such as from 40% outside at the skin surface to 80% inside at upper granulosum). Both lipid synthesis and corneocyte natural moisturizing factor (NMF) production, mainly free amino acids from filaggrin degradation and other small molecules, operate at a normal rate. Once changed to high humidity, however, the SC adopts to a shallow water gradient (e.g., 70% outside to 80% inside). The epidermis senses this environmental shift and responds by reducing lipid synthesis and NMF production. These high‐humidity conditions are similar to those for facial skin under repeat and prolonged mask coverage. The problem arises once the skin returns to normal or low humidity, such as after mask removal. The increase of water gradient requires more protective layers and moisturizing components, but the reduced lipid/NMF production, an outcome of the prolonged exposure to high humidity, cannot compensate for the drastic shift, leading to loss of hydration (dry skin) and weakened barrier (with thinner SC exposed). In fact, an early report about skin response to humidity changes using an experimental model not only presented the evidence of the loss of filaggrin under high‐humidity conditions, but also a clear visualization of the thinning of SC, 33 which could be predicted and explained by our model. This is also consistent with the early reports of enhanced barrier quality under low‐humidity conditions. 8 , 34
In summary, the results of this clinical study demonstrated that daily and prolonged usage of face masks, which is an essential personal and public health practice during the COVID‐19 pandemic, 35 can create a high‐humidity microenvironment, which may negatively impact skin properties, including increased sebum production, reduced skin hydration, and weakened SC barrier integrity. However, facial moisturizer with high water binding ingredients and less occlusive emollients can alleviate these issues and help maintaining epidermal homeostasis to protect the facial skin under the mask. In addition to educating the general public about the proper moisturization of facial skin during the pandemic mask usage, the underlying physiological modulations of epidermal structures and functions corresponding to the microenvironmental changes warrant further investigations.
FUNDING INFORMATION
Estée Lauder Companies.
CONFLICT OF INTEREST
The authors have a conflict of interest to declare: YW received honorarium fees from the Estée Lauder Companies. Other authors are full‐time employees of the Estée Lauder Companies that commercializes skincare products.
ACKNOWLEDGMENTS
The authors thank Timothy Gray and Kathleen James for preparing the testing moisturizer; Esther Qin from SGS Stephens, Inc for data analysis; staff at SGS Stephens, Inc for the clinical study execution.
Feng L, Zhang Q, Ruth N, Wu Y, Saliou C, Yu M. Compromised skin barrier induced by prolonged face mask usage during the COVID‐19 pandemic and its remedy with proper moisturization. Skin Res Technol. 2023;29:1–8. 10.1111/srt.13214
DATA AVAILABILITY STATEMENT
Research data are not shared
REFERENCES
- 1. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease‐affected skin. J Clin Aesthet Dermatol. 2011;4:22‐42. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3175800/ [PMC free article] [PubMed] [Google Scholar]
- 2. Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta. 2006;1758:2080‐2095. 10.1016/j.bbamem.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 3. Goad N, Gawkrodger DJ. Ambient humidity and the skin: the impact of air humidity in healthy and diseased states. J Eur Acad Dermatol Venereol. 2016;30:1285‐1294. 10.1111/jdv.13707 [DOI] [PubMed] [Google Scholar]
- 4. Walling HW. Primary hyperhidrosis increases the risk of cutaneous infection: a case‐control study of 387 patients. J Am Acad Dermatol. 2009;61:242‐246. 10.1016/j.jaad.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 5. Narang I, Sardana K, Bajpai R, Garg VK. Seasonal aggravation of acne in summers and the effect of temperature and humidity in a study in a tropical setting. J Cosmet Dermatol. 2019;18:1098‐1104. 10.1111/jocd.12777 [DOI] [PubMed] [Google Scholar]
- 6. Guéhenneux S, Gardinier S, Morizot F, Fur IL, Tschachler E. Skin surface hydration decreases rapidly during long distance flights. Skin Res Technol. 2012;18:238‐240. 10.1111/j.1600-0846.2011.00560.x [DOI] [PubMed] [Google Scholar]
- 7. Sato J, Denda M, Chang S, Elias PM, Feingold KR. Abrupt decreases in environmental humidity induce abnormalities in permeability barrier homeostasis. J Invest Dermatol. 2002;119:900‐904. 10.1046/j.1523-1747.2002.00589.x [DOI] [PubMed] [Google Scholar]
- 8. Denda M, Sato J, Masuda Y, et al. Exposure to a dry environment enhances epidermal permeability barrier function. J Invest Dermatol. 1998;111:858‐863. 10.1046/j.1523-1747.1998.00333.x [DOI] [PubMed] [Google Scholar]
- 9. Bouwstra JA, Wouter H, Groenink W, Kempenaar JA, Romeijn SG, Ponec M. Water distribution and natural moisturizer factor content in human skin equivalents are regulated by environmental relative humidity. J Invest Dermatol. 2008;128:378‐388. 10.1038/sj.jid.5700994 [DOI] [PubMed] [Google Scholar]
- 10. Al Badri FM. Surgical mask contact dermatitis and epidemiology of contact dermatitis in healthcare workers. Curr Allergy Clin Immunol. 2017;30:183‐188. https://journals.co.za/doi/abs/10.10520/EJC‐c3ca4ee66 [Google Scholar]
- 11. Roberge R, Benson S, Kim JH. Thermal burden of N95 filtering facepiece respirators. Ann Occup Hyg. 2012;56:808‐814. 10.1093/annhyg/mes001 [DOI] [PubMed] [Google Scholar]
- 12. Gottlieb M, Long B. Dermatologic manifestations and complications of COVID‐19. Am J Emerg Med. 2020;38:1715‐1721. 10.1016/j.ajem.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szepietowski JC, Matusiak Ł, Szepietowska M, Krajewski PK, Białynicki‐Birula R. Face mask‐induced itch: a self‐questionnaire study of 2,315 responders during the COVID‐19 pandemic. Acta Derm Venereol. 2020;100:adv00152. 10.2340/00015555-3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hua W, Zuo Y, Wan R, et al. Short‐term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115‐121. Epub 2020 Jun 8. 10.1111/cod.13601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park SR, Han J, Yeon YM, Kang NY, Kim E. Effect of face mask on skin characteristics changes during the COVID‐19 pandemic. Skin Res Technol. 2020;27:554‐559. 10.1111/srt.12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J, Yoo S, Kwon OS, Jeong ET, Lim JM, Park SG. Influence of quarantine mask use on skin characteristics: one of the changes in our life caused by the COVID‐19 pandemic. Skin Res Technol. 2020;27:599‐606. 10.1111/srt.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maranduca MA, Hurjui LL, Branisteanu DC, et al. Skin—a vast organ with immunological function (review). Exp Ther Med. 2020;20:18‐23. 10.3892/etm.2020.8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson CJ, Tønseth KA, Utheim TP. Cultured epidermal stem cells in regenerative medicine. Stem Cell Res Ther. 2017;8:155. 10.1186/s13287-017-0587-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoath SB, Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. J Invest Dermatol. 2003;121:1440‐1446. 10.1046/j.1523-1747.2003.12606.x [DOI] [PubMed] [Google Scholar]
- 20. Ye L, Wang Z, Li Z, Lv C, Man MQ. Validation of GPSkin Barrier® for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res Technol. 2019;25:25‐29. 10.1111/srt.12590 [DOI] [PubMed] [Google Scholar]
- 21. Grinich EE, Shah AV, Simpson EL. Validation of a novel smartphone application‐enabled, patient‐operated skin barrier device. Skin Res Technol. 2019;25:612‐617. 10.1111/srt.12692 [DOI] [PubMed] [Google Scholar]
- 22. Logger JGM, Driessen RJB, de Jong EMGJ, van Erp PEJ. Value of GPSkin for the measurement of skin barrier impairment and for monitoring of rosacea treatment in daily practice. Skin Res Technol. 2020;27:15‐23. 10.1111/srt.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schäfer P, Bewick‐Sonntag C, Capri MG, Berardesca E. Physiological changes in skin barrier function in relation to occlusion level, exposure time and climatic conditions. Skin Pharmacol Appl Skin Physiol. 2002;15:7‐19. 10.1159/000049384 [DOI] [PubMed] [Google Scholar]
- 24. Berardesca E, Distante F, Vignoli GP, Rabbiosi G. Effects of site and menstrual cycle on barrier function and stratum corneum water‐holding capacity. Skin Res Technol. 1996;2:88‐90. 10.1111/j.1600-0846.1996.tb00065.x [DOI] [PubMed] [Google Scholar]
- 25. Gerhardt L‐C, Strässle V, Lenz A, Spencer ND, Derler S. Influence of epidermal hydration on the friction of human skin against textiles. J R Soc Interface. 2008;5:1317‐1328. 10.1098/rsif.2008.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parnham A, Copson D, Loban T. Moisture‐associated skin damage: causes and an overview of assessment, classification and management. Br J Nurs. 2020;29:S30‐S37. 10.12968/bjon.2020.29.12.s30 [DOI] [PubMed] [Google Scholar]
- 27. Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004‐2012. J Invest Dermatol. 2014;134:51‐57. 10.1038/jid.2013.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cau L, Pendaries V, Lhuillier E, et al. Lowering relative humidity level increases epidermal protein deimination and drives human filaggrin breakdown. J Dermatol Sci. 2017;86:106‐113. 10.1016/j.jdermsci.2017.02.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denda M, Tsuchiya JT, Elias PM, Feingold KR. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111:873–878. 10.1046/j.1523-1747.1998.00364.x [DOI] [PubMed] [Google Scholar]
- 30. Hoste E, Kemperman P, Devos M, et al. Caspase‐14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233‐2241. 10.1038/jid.2011.153 [DOI] [PubMed] [Google Scholar]
- 31. Mieremet A, Boiten W, van Dijk R, et al. Unravelling effects of relative humidity on lipid barrier formation in human skin equivalents. Arch Dermatol Res. 2019;311:679‐689. 10.1007/s00403-019-01948-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nafisi S, Maibach HI. Skin penetration of nanoparticles. In: Shegokar R, Souto EB, eds. Emerging Nanotechnologies in Immunology. Elsevier; 2018:51. 10.1016/B978-0-323-40016-9.00003-8 [DOI] [Google Scholar]
- 33. Katagiri C, Sato J, Nomura J, Denda M. Changes in environmental humidity affect the water‐holding property of the stratum corneum and its free amino acid content, and the expression of filaggrin in the epidermis of hairless mice. J Dermatol Sci. 2003;31:29‐35. 10.1016/s0923-1811(02)00137-8 [DOI] [PubMed] [Google Scholar]
- 34. Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30:223‐249. 10.1111/jdv.13301 [DOI] [PubMed] [Google Scholar]
- 35. Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS‐CoV‐2. Science. 2020;368:1422‐1424. https://science.sciencemag.org/content/368/6498/1422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared


