Abstract
The early and massive vaccination campaign in Israel with the mRNA-LNP Comirnaty® (Pfizer-BioNTech) vaccine against the SARS-CoV-2 virus made available large amounts of data regarding the efficacy and safety of this vaccine. Adverse reactions to mRNA-based SARS-CoV-2 vaccines are rare events, but due to large mediatic coverage they became feared and acted as a potential source of delay for the vaccination of the Israeli population. The experience with the reactogenicity of the polyethylene glycol (PEG) moiety of PEGylated liposomes, PEGylated proteins and other PEGylated drugs raised the fear that similar adverse effects can be associated with the PEG lipid which is an essential component of currently used mRNA-LNP vaccines against COVID-19. In this study we quantified the levels of anti-PEG IgG, IgM and IgE present in the blood of 79 volunteers immediately before and 3 weeks after receiving a first dose of Comirnaty® vaccine. Our in vitro results show that different humanized anti-PEG antibodies bind the PEGylated nano-liposomes in a concentration-dependent manner, but they bind with a lower affinity to the Comirnaty vaccine, despite it having a high mole% of neutral PEG2000-lipid on its surface. We found an increase in IgG concentration in the blood 3 weeks after the first vaccine administration, but no increase in IgM or IgE. In addition, no severe signs of adverse reactions to the Comirnaty vaccine were observed in the population studied despite the significant pre-existing high titers of IgG before the first dose of vaccine in 2 donors.
Keywords: mRNA-based SARS-CoV-2 vaccine, COVID-19 vaccine, PEG, Anti-PEG antibodies, LNP
Graphical abstract
1. Introduction
During the COVID-19 pandemic, Pfizer-BioNTech and Moderna mRNA- lipid nanoparticles (LNP) vaccines saved the lives of millions [1,2]. These vaccines are based on modified mRNA molecules that code for the viral spike glycoprotein of SARS-CoV-2 that are encapsulated in LNPs. These LNPs are composed of four lipid components: L-α-di-stearoyl phosphatidylcholine (DSPC), cholesterol, an ionizable cationic lipid (ALC 315) with a pKa at pH ∼6.5, and a neutral PEG-lipid (ALC 0159). This PEG-lipid has two medium-length myristoyl (C14) acyl chains and, like the “helper lipids” DSPC and cholesterol, is critical for LNP stability during production and storage [3,4] thanks to the high mobility of the bulky, highly hydrated PEG head moiety (up to 200 water molecules per PEG2000 [5,6]). The unique structure of ALC 0159 enhances stability during production and storage while allowing internalization into cells after injection. The latter is explained by the structure of ALC 0159 which due to its two myristoyl acyl chains is having relatively high CMC [7] and relatively weak van-der-Waals interactions with the other lipids in the LNP, Therefore ALC 0159 is rapidly released from the LNP surface upon dilution in body fluids. This, in turn, enable effective LNP uptake by muscle and immune cells at the site of intramuscular administration [8]. Other physiochemical properties of the vaccine are detailed in supplementary Table S1.
Antibodies that bind to PEG are found not only in patients after treatment with PEGylated drugs [[9], [10], [11]], but also in the blood of many healthy individuals in the absence of any treatment involving PEG [[11], [12], [13], [14], [15], [16]]. The presence of pre-existing anti-PEG antibodies in the blood of patients, especially IgG and IgM, combined with low levels of PEGylated drug in the blood can accelerate their clearance (a phenomenon known as accelerated blood clearance, abbreviated ABC) and therefore decrease their efficacy [17]. This may be related to these vaccines as their concentration in blood must be very low. Moreover, anti-PEG antibodies can alter the physical properties and stability of PEGylated nanodrugs [18,19]. In addition, IgG and IgM anti-PEG antibodies (either pre-existing or elicited after exposure to the PEGylated drug) are expected to play an important role in the onset of infusion (hypersensitivity) reactions to the first administration of PEGylated drugs and nanodrugs with symptoms ranging from relatively benign to life-threatening [17,19].
Induction of antibodies against PEG after immunization with mRNA-LNP vaccines is of potential concern with the wide-spread use of these vaccines due to the presence of PEG-lipids in the LNP [17,20]. Although hypersensitive reactions to the SARS-CoV-2 mRNA-LNP vaccines are very rare [21], anti-PEG IgG and IgM antibodies have been associated with these reactions [22]. The question of whether these vaccines elicit anti-PEG IgG and IgM antibodies is also highly relevant due to their potential effects on increasing the reactogenicity to other PEGylated drugs. In this study, we investigated if anti-PEG antibodies can bind to Comirnaty mRNA-LNP since this is the first prerequisite for the induction of infusion reactions. Importantly, we quantified the increase in the level of anti-PEG IgG, IgM and IgE in the blood of 79 volunteers immediately before and 3 weeks after the first dose of Comirnaty.
2. Material and methods
2.1. Formulations used in the study
Lipodox (generic doxorubicin hydrochloride liposomes) was acquired from Sun Pharmaceutical Industries Ltd., India. The Comirnaty vaccine was received from Hadassa Ein Kerem Hospital from non-injected vials that remained for 2 h at RT and then were kept at 4–10 °C. All the assays were performed within 3 days of dilution of the vaccine. For characterization (See supplementary Table S1) Doxil batch #1300667 and Comirnaty batch #EW3344 were used. For in vitro experiments, Lipodox batch #JKSO322A and Comirnaty batch #FG9984 were used.
Liposomes with low PEG content and the non-PEGylated liposomes were prepared as described earlier [23]. Lipids were hydrated by ammonium sulfate. These nano-liposomes differ in their DSPE-PEG2000 mol% (0.3 vs. 0).
2.2. Characterization of LNP
Size was determined by dynamic light scattering (DLS) measurements that were performed immediately after thawing a Comirnaty vial and diluting NPs with saline (performed by a healthcare professional). Size (diameter, D), size distribution (polydispersity index, PDI) and zeta potential (Zp) of the NPs were determined with a Malvern Zetasizer Nano ZS instrument (Malvern, Worcestershire, UK). Zeta potential was determined at low ionic strength of 1.5 mM sodium nitrate.
2.3. Anti-PEG antibodies binding to formulations: Comirnaty vaccine, Lipodox (High 5.4 mol% DSPE-PEG), low (0.3 mol% DSPE-PEG) and nano-liposomes lacking DSPE-PEG
Binding of humanized anti-PEG antibodies to the different formulations was compared using sandwich ELISA. Maxisorp 96-well microplates were coated with 0.25 μg/well with either mouse AGP4 or rat AGP6 IgM anti-PEG antibodies and incubated at 4 °C overnight. The plates were then washed with PBS and blocked with 5% skim milk for 2 h at RT. After washing the plates, graded concentrations of the formulations were added to the plate, starting at a concentration of 8 μg mL−1 phospholipids for all formulations then diluted serially 6-fold in 2% skim milk, and incubated for 2 h at RT. The plates were then washed 3 times with PBS-Tween (0.05% Tween-20) if the detection antibody was 3.3-biotin or with PBS (with 15-2b-biotin) to remove unbound nanoparticles. The detection antibody (3.3-biotin or 15-2b-biotin) was then added at 5 μL mL−1 for 1 h at RT followed by washes as previously described. The plates were then incubated with streptavidin-HRP (1 μg mL−1) for 1 h at RT followed by washes. ABTS substrate was added for 30 min RT in the dark and the absorbance at 405 nm was read using a BioTek Synergy™ 4 Hybrid Microplate Reader. EC50 values were determined using Phoenix WinNonlin (Certara™, NJ, USA, Version 6.3). A figure illustrating this assay is available in Supplementary Materials (Fig. S2).
2.4. Serum samples
Human serum samples tested were obtained from the Blood Bank of Hadassa Ein Kerem Hospital (Jerusalem, Israel). The samples were collected after getting informed consent from volunteers as part of clinical trial 0672–13-HMO, but the samples were processed as part of clinical trial 0032–21-HMO (Helsinki Committee of Hadassa Ein Kerem, Jerusalem, Israel).
2.5. Anti-PEG antibody quantification
Chimeric humanized monoclonal anti-PEG antibodies c3.3-IgG and cAGP4-IgM were generated in Dr. Roffler's lab as previously described [12,24,25]. c3.3-IgG is a human IgG1 antibody whereas cAGP4-IgM is a human IgM antibody, both of which bind to the repeating ethylene oxide subunits of the PEG backbone. A humanized IgE anti-PEG antibody (hu6.3 IgE) was generated by combining the light chain and variable region of the heavy chain of human 6.3 with human IgE heavy chain constant regions using recombinant DNA technology [26]. Anti-PEG antibody concentrations in the patients' sera were quantified using a direct ELISA as described previously [27]. Briefly, sera samples were serially diluted in 2% (w/v) skim milk (25×, 50× and 100×), with the last 2 dilutions in dilution buffer containing 4% human serum that tested negative for the presence of anti-PEG IgG and IgM antibodies. The presence of negative plasma in the dilution buffer decreases the impact of matrix effects. Standard curves were obtained by assay of serial dilution of c3.3 (IgG), cAGP4 (IgM), or hu6.3 IgE, the highest concentration being 2.5, 2, or 2 μg mL−1, respectively, in dilution buffer with negative serum. The diluted serum samples and antibody standards were incubated on microplates coated with 0.5 μg/well NH2-PEG10K-NH2, followed by washing with 0.1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, Sigma Aldrich, Rehovot, Israel) in PBS, then PBS only twice. Horseradish peroxidase (HRP)-conjugated secondary antibodies (goat F(ab')2 anti-human IgG Fc or goat F(ab')2 anti-human IgM) were added to the IgG or IgM detection plates, respectively, for 1 h RT. IgE was detected by serial addition of biotin-conjugated mouse anti-human IgE and HRP-conjugated streptavidin. The plates were washed as previously described and incubated for 30 min RT in the dark with ABTS substrate. The antibodies were quantified by reading absorbance at 405 nm with a BioTek Synergy™ 4 Hybrid Microplate Reader (Winooski, VT, USA). Samples with absorbance values at least 3 times greater than the mean background (dilution buffer) were considered positive. The relative concentrations of anti-PEG IgG and IgM in positive samples were calculated by comparison with their relative standard curves. Positive samples were confirmed by an established PEG competition assay as described previously [12,27]. Briefly, PEG-liposomes containing 5 mol% PEG were generated as previously described [28] and were diluted to 200 μg mL−1 in a final concentration of 2% (w/v) skim milk powder in PBS (“dilution buffer”). On plates previously coated with NH2-PEG10 000-NH2 (same concentration used for antibody quantification), the liposomes or dilution buffer (no competition control) were added (50 μL/well) for 30 min at room temperature. Serum samples that tested positive using the ELISA assay were diluted 25-fold in 2× dilution buffer (8% human reference serum, 4% (w/v) skim milk powder in PBS). The c3.3-IgG and cAGP4-IgM control antibodies were diluted to 1 μg mL−1 in the 2× dilution buffer. The human plasma samples and control antibodies were added (50 μL) to the wells containing PEGylated liposomes (competition) or dilution buffer (no competition) at room temperature for 1 h. The plates were washed twice with 0.1% CHAPS in PBS followed by one wash with PBS. 50 μL of HRP-conjugated goat F(ab’)2 antihuman IgG Fc or HRP-conjugated goat F(ab’)2 anti-human IgM Fc5μ at 0.25 μg mL−1 in dilution buffer were added and incubated at room temperature for 1 h. After washing, the bound peroxidase activity was measured by adding 150 μL/well ABTS solution and measuring the absorbance (405 nm) of wells in a microplate reader. Samples were considered positive if there was a reduction of at least 35% in the absorbance reading of samples in the presence of excess PEGylated liposomes as compared to wells without addition of PEGylated liposomes.
2.6. Statistical evaluation
The paired differences of IgG or IgM antibody concentrations before and after vaccination were calculated for each subject a paired t-test was used to test for the difference of paired differences from zero. For sensitivity purpose, the nonparametric Wilcoxon signed rank test was also used to confirm the statistical outcomes. Readings below the limits of quantification (BLQ) were set at 0.2 μg mL−1 for IgG and 0.3 μg mL−1 for IgM and a first analysis was performed using these values. The analysis was repeated after treating the low values as missing. One outlier was identified in IgG data and another in IgM data. Statistical tests were performed with and without those outliers. Test results with P-values below 0.05 are considered statistically significant. Statistical analyses were performed by Oren Bar-Ilan, DataSights Ltd. (Israel) using JMP® Pro Statistical Discovery software, version 16.1.0 from SAS® Institute Inc., Cary NC.
3. Results
We first examined if humanized anti-PEG antibodies can bind to the Comirnaty vaccine with a sandwich ELISA using anti-PEG IgM antibodies to capture nanoparticles. The LNP include 1.5 mol% PEG lipids however most of it is on the LNP surface where its mol% is significantly higher [29]. Lipodox containing 5.4 mol% DSPE-PEG, low PEG liposomes containing 0.3 mol% DSPE-PEG, and liposomes lacking DSPE-PEG were used as controls. Anti-PEG antibodies targeting either the repeating ethylene-oxide subunits of the PEG backbone (Fig. 1A) or the terminal methoxy PEG functionality (Fig. 1B) bound to the vaccine LNP, but with less avidity than to Lipodox or even to liposomes with much lower (0.3 mol%) DSPE-PEG2000 (Table 1 ). It is worth noting that although the PEG-lipid content in the mRNA-LNP vaccine is only 1.5 mol% (which is significantly lower than in Doxil/Lipodox) the actual amount of the PEG-lipid in the LNP external lipid layer is higher than expected as most of the PEG-lipid molecules reside there [30]. Binding of anti-PEG antibodies requires the presence of PEG-lipid molecules on the nanoparticles surface as no binding was observed to liposomes without PEG lipid (Fig. 1). The explanation to the lower avidity of anti-PEG antibody binding to LNP compared to Doxil liposomes or to Doxil-like formulation having only 0.3 mol% DSPE-PEG is discussed later in the Conclusions.
Fig. 1.
Binding of anti-PEG antibodies to nano-liposomes differing in their mole% of DSPE-PEG2000: Lipodox 5.4 mol%, Low PEG liposomes 0.3 mol%, No PEG 0.0%, Comirnaty (Pfizer-BioNTech vaccine) 1.5 mol% PEG lipid ALC 0159. Serial dilutions of these nanoliposomes or LNPs were assayed on ELISA plates coated with (A) AGP4 for capture and 3.3-biotin for detection or (B) AGP6 for capture and 15-2b-biotin for detection. The characterization of nano-formulations used in this study are described in supplementary Table S1.
Table 1.
EC50 values for anti-PEG antibody binding to various nano-formulations. (calculated from Fig. 1).
| Nano-Liposomes used⁎ | Capture AGP4 Detection 3.3-biotin (μg mL−1 phospholipids) | Capture AGP6 Detection 15-2b-biotin (μg mL−1 phospholipids) |
|---|---|---|
| Lipodox (5.4 mol% DSPE-PEG) | 0.03 | 0.02 |
| Low PEG (0.3 mol% DSPE-PEG) | 0.06 | 0.14 |
| No PEG (0.0 mol% DSPE-PEG) | No binding# | No Binding# |
| Comirnaty (1.5 mol% neutral PEG Lipid) | 0.58 | 0.50 |
Supplementary Table S1 describes the physico-chemical characterization of the nano-formulations used in this study.
See Fig. 1.
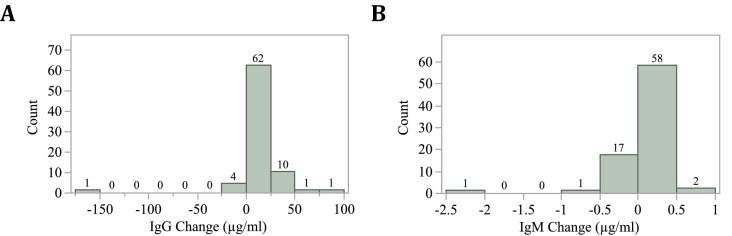
Plasma samples collected from 79 subjects just before vaccination with Comirnaty Covid-19 mRNA-LNP vaccine and three weeks later were assayed for anti-PEG IgG, IgM and IgE antibodies using an established ELISA method [12]. The study population comprised 47 women and 32 men with median ages of 35.2 and 34.3 years, respectively (Table 2 ). Before the first dose of Comirnaty, anti-PEG IgG was detected in 29 individuals (36.7%) and IgM were detected in 11 donors (13.9%). Three weeks after the first dose, the number of individuals with anti-PEG antibodies increased from 29 to 46 and from 11 to 31 for IgG and IgM, respectively. The mean concentration of anti-PEG IgG significantly increased (P < 0.0001 for both paired t-test and Wilcoxon signed rank test) from 7.8 μg mL−1 before immunization to 17.5 μg mL−1 at three weeks after immunization (Fig. 2A). By contrast, no change in the concentration of plasma anti-PEG IgM antibodies was observed before and after immunization (0.38 vs 0.35 μg mL−1) (Fig. 2B). No anti-PEG IgE antibodies were detected in the plasma of donors before or after vaccine administration. The majority of donors experienced a modest increase in anti-PEG IgG concentration after immunization, but two of the 79 individuals experienced large (> 50 μg mL−1 increases (Fig. 3 ). The data from the assays is available in Supplementary Materials (Table S3 and S4).
Table 2.
Characteristics of the population in the study.
| n= | 79 (32 men, 47 women) |
|---|---|
| age range (years) | 18.3–82.2 |
| age median (years) | 34.5 |
| IgG - before vaccine | 29 (36.7%) |
| IgG - after vaccine | 46 (58.2%) |
| average before vaccine IgG (ug/mL) | 7.8 |
| average after vaccine IgG (ug/mL) | 17.5 |
| IgM - before vaccine | 11 (13.9%) |
| IgM - after vaccine | 31 (39.2%) |
| average before vaccine IgM (ug/mL) | 0.38 |
| average after vaccine IgM (ug/mL) | 0.35 |
Fig. 2.
Concentrations of anti-PEG IgG (A) and IgM (B) antibodies before and 3 weeks after vaccination with Comirnaty vaccine.
Fig. 3.
Distribution of changes (μg mL−1) in concentration levels of anti-PEG IgG (A) and IgM (B) in individuals between the 2 doses of Comirnaty (Pfizer-BioNTech) Covid-19 vaccine. The observed outliers in IgG and IgM are of different individuals.
4. Discussion
The administration of Comirnaty (Pfizer-BioNTech) vaccine successfully decreased the number of hospitalizations of severely affected patients and potentially saved millions of lives [1,2]. But the fear of adverse reaction, despite being rare, is still present especially considering such large scale of vaccination (billion size population) in a short time. In patients who experienced such adverse reactions to COVID-19 mRNA vaccines, high levels of IgG and IgM anti-PEG antibodies have been observed [29]. Our study showed that, as previously reported [11,12,17] a non-negligible part of the population possesses pre-existing anti-PEG antibodies, i.e. antibodies directed against PEG found in the blood of people without a history of previous treatment with PEGylated drug. These findings can be explained by the exposure of humans to poly(ethylene)glycol (PEG) through common consumer products [17,[31], [32], [33], [34]].
This study confirms previous data [17,27] that IgG are more predominant than IgM (17.7% vs. 3.8% of the studied population respectively) and found in higher titers (14. 57 μg mL−1) than IgM (0.51 μg mL−1). IgE couldn't be detected in any sample. IgE is mostly bound to innate immune cells such as basophils and mast cells that express the high affinity Fcε receptor (FcɛRI), which may make it difficult to detect low concentrations of circulating IgE in blood [35].
We also showed that anti-PEG antibodies can bind PEGylated liposomes in a PEG concentration dependent manner. But the avidity for the Comirnaty vaccine (1.5 mol% lipid-PEG) was significantly smaller than for the nano-liposomes with low PEG (0.3 mol% of DSPE-PEG2000). The most likely reason is rapid shedding (desorption) of ALC 0159 from the vaccine as its acyl chains (myristoyl C14) is shorter by 4 methylene groups as compared to the two stearoyl (C18) acyl chains of the DSPE-PEG2000 and therefore its critical micellar concentration is much higher [7]. This leads to a dilution-induced reduction of ALC0159 from the Comirnaty vaccine surface which should reduce anti-PEG antibody binding to the LNPs. Since the avidity to Comirnaty is lower than the avidity to the Doxil-like nano-liposomes with only 0.3 mol% DSPE-PEG, it suggests that Comirnaty retained a concentration of PEG-lipid at the LNP surface below 0.3 mol%. It is claimed that approximately 2% ALC0159 (of the 1.5 mol%) is desorbed per minute when the vaccine is injected IV [8]. However, the desorption rate is dependent on the magnitude of dilution, the larger the dilution the larger the desorption rate. Dilution due to I.M. administration is unknown, and lack of knowledge prevent us and others to compare the PEG-lipids in the undiluted and freshly diluted vaccines and therefore we do not have confirmation of the hypothesis that major PEG-lipid desorption from the vaccine surface occurs. A second explanation to the very low avidity could be that the PEG-lipid of the Comirnaty vaccine is electrostatically charge-less while Doxil is negatively charged [36]. However, the Doxil-like nanoliposomes with 0.3 mol% DSPE-PEG are uncharged (see Zeta potential values in supplementary Table S1) and therefore this explanation does not hold. The third potential explanation that requires attention is that PEG on the Comirnaty LNP surface may be in a dense brush conformation such as in the case for DSPE-PEG micelles which induce no or weak complement activation [36]. Evaluation of this possibility requires a study quantifying the induction of anti-PEG antibodies by liposomes having on their surface PEG in a dense brush conformation (i.e. ∼9 mol% [6]).
The first injection of vaccine resulted in a significant increase in IgG from 7.8 μg mL−1 to 17.5 μg mL−1 after 3 weeks but not in IgM or IgE, suggesting no acute reaction to the vaccine occurred. Two donors had high titers of anti-IgG before the first dose (312.8 and 121.3 μg mL−1) and after the second dose one exhibited a decrease of 50% in IgG and the other had a titer that slightly (10%) increased. Interestingly, even with such high titers no signs of reactogenicity to the injection of PEGylated LNP was reported. But given the extremely low occurrence of high titers in this small population no conclusions can be drawn. Interestingly, a previous study [37] also found high titers of IgG in some participants and also had a cohort of participants who experienced vaccine-associated side effects. However, no correlation was found between side effects and high titers of anti-PEG IgG and, unfortunately, IgM and IgE were not measured in this study.
Recent studies also measured anti-PEG antibodies following vaccination with Comirnaty and Spikevax (Moderna COVID-19 vaccine). A study from Ju et al. [22] compared anti-PEG IgG and IgM levels in samples from a population receiving two doses of either Comirnaty or Spikevax, or not vaccinated. Their results showed a significant increase in both anti-PEG IgG and IgM, the increase in IgM being higher (2.64 fold) than the IgG (1.78 fold) after vaccination with Comirnaty. The endpoints for antibody quantification were later than in our study (2–7 weeks after the first boost, so 5–10 weeks after the first dose assuming a 3 week-interval between the doses).
Another study (from Guerrini et al. [38]) quantified the levels of anti-PEG IgG and IgM one week after the first dose, the first boost (second dose) and the second boost (third dose). Although they measured a significant increase in anti-PEG IgM after the first dose, anti-PEG IgM levels decreased and were close to baseline after the first boost. In contrast to other studies, they did not observe any significant variation in IgG levels before and after vaccine administration. In this study, the cohort (n = 69) received Comirnaty for the first and second injection, but for the second boost (6 months after the first boost), 34.32% of the population received Comirnaty while 65.68% were administered Spikevax. The authors noticed an increase in anti-PEG IgM production following Spikevax administration for the second boost as compared to Comirnaty. In the study from Ju et al., the authors also observed an increase in antibody production after Spikevax compared to Comirnaty. In an additional study [37], Carreño et al. compared anti-spike and anti-PEG IgG 19 days after the first and second dose of both vaccines. Despite a small population sample (n = 10 in each group), they demonstrated that vaccination with Spikevax elicited immunogenicity whereas Comirnaty did not trigger a change in antibody titers. The relatively low reactogenicity toward Comirnaty observed consistently in several studies could explain the results that we obtained, with a low (but significant) increase in anti-PEG IgG after the first vaccination. Despite the discrepancy, the ranges of anti-PEG antibodies are in line with the wide range previously reported [[11], [12], [13], [14], [15], [16]].
It is arduous to compare the results of the different studies because, despite trying to answer the same question (quantification of anti-PEG antibodies after COVID vaccine administration), the studies vary in many parameters: the designs are different (non-vaccinated control group only in the study from Ju et al.), the size of the cohorts vary (from 20 to 130), the time points for blood sample collection are also different (Guerrini et al. checked 1 week after each dose, in our study and Carreño et al. the samples were collected before the following doses and in the study led by Ju et al., the samples were collected 2–7 weeks after the boost). In addition, the techniques used in the different studies to detect and quantify the antibodies were different: Ju et al. and Carreño et al. used in-house ELISA without standard curves to quantify the antibodies, Guerrini et al. used a commercial assay but with nominal units, whereas we used a previously described technique [24,25] in which anti-PEG antibody concentrations are measured by comparison to standard curves prepared using humanized anti-PEG antibodies.
Our study did not have a group of unvaccinated participants as a negative control to confirm that the increase in IgG observed is due solely to vaccination. A study [34] measuring anti-PEG antibodies in healthy individuals over time showed that in some individuals, levels of anti-PEG antibodies fluctuate even without previous administration of PEGylated biopharmaceuticals. It is interesting to note that this could explain the wide array of anti-PEG titers measured in healthy populations. But in their study that included a group of unvaccinated participants, Ju et al. did not find any significant change in anti-PEG antibodies at a 6-month interval, with only a very minor decrease (mean fold change of 0.92 for IgG and 0.98 for IgM). In addition, we found significantly lower percentage of participants with anti-PEG antibodies before vaccination as compared to their study (36.7% vs. 71% for IgG and 13.9% vs. 68% for IgM) for cohorts of similar size (n = 79 in our study vs. n = 75 for vaccinated population in their study). So, it seems that it is more likely that the increase in IgG we measured is due to vaccination than to natural fluctuations.
In our study we did not observe an increase in IgM after the vaccine injection, as opposed to the results of Ju et al. and Guerrini et al. We measured the antibodies levels 3 weeks after the vaccine injection. Typically IgM are the first antibodies to rise after exposure to an antigen and are thought to be rapidly declining to very low levels, usually withing 3 weeks. But we know that a non-negligible percentage of IgM can last longer than 3 weeks, including secondary IgM synthetized after B cells have undergone somatic hypermutation following antigen exposure. In addition, studies [39,40] showed that in patients with COVID, anti-spike IgM were measured later than 3 weeks after exposure to the virus or appearance of the symptoms. It is now widely known that the anti-PEG antibodies found in the healthy population are sex-, age- but also geography-dependent. It is interesting to point out that in a previous study [27] we measured anti-PEG IgG and IgM in healthy population in the same city as our current study and we found significantly lower IgM positive individuals than a study in Taiwan [12] using the exact same ELISA assay (3.5% vs. 27.1% in Taiwan). It is therefore possible that the lack of increase in IgM following vaccination is not an artefact due to the small population but due to the specificity of the regional population.
Another important point is that our study uses plates coated with NH2-PEG10 000-NH2, which allows detection of anti-PEG antibodies directed against the repeating ethylene oxide subunits of the PEG backbone but not the methoxy end of PEG. Most PEGylated compounds use methoxy-PEG, so it is possible that we underestimate the totality of anti-PEG antibodies generated. This point needs to be addressed and it is critical to quantify IgG at different times after injection and upon subsequent boosts in order to see if the titers of anti-PEG antibodies rise after several boosts (or with each boost). A rise in anti-PEG antibodies would not only possibly increase the risk for adverse reactions upon injection of PEGylated drug in the bloodstream, but also could be problematic because of the potential risk of Accelerated Blood Clearance (ABC) associated with these antibodies. The ABC phenomenon is characterized by very fast elimination of a drug from the plasma upon its second or subsequent injections due to the synthesis and binding of anti-drug antibodies. This phenomenon has already been observed with PEGylated drugs, especially those given at low dose [[9], [10], [11]] and causes a decrease in the drug efficacy due to its very shortened residence time in the bloodstream. ABC has been observed with PEGylated drugs that weren't LNPs, but the binding of anti-PEG antibodies to PEGylated formulations has others consequences, namely activation of the complement cascade [41] and disruption of the LNP membrane, causing the formation of Membrane attack Complex (MAC) and leaking of the payload into the bloodstream [18,42]. The impact of high titers of either pre-existing or elicited anti-PEG antibodies have previously been linked to accelerated blood clearance, loss of therapeutic efficacy of pegylated pharmaceuticals administered intravenously and infusion reactions [18,42]. Therefore the impact of the increase in plasma anti-PEG antibodies caused by the Comirnaty vaccine and its boosters on cross-reaction with other PEGylated pharmaceuticals requires further investigation.
5. Conclusions
In conclusion, we showed that humanized anti-PEG antibodies bind to the Comirnaty COVID-19 vaccine in vitro, although with much lower avidity than to PEGylated liposomes. We also observed in a population sample that after the first administration of Comirnaty, there is a significant increase in IgG but not in IgM. In addition, no severe signs of adverse reactions to the Comirnaty vaccine were observed in the population studied, despite the significant pre-existing high titers of IgG before the administration of the vaccine in 2 study participants.
Funding
The Barenholz fund was established with a portion of Barenholz royalties, which the Hebrew University assigned to support research in the Barenholz lab, including this study. This work was also supported by Yissum and the Innovative Materials and Analysis Technology Exploration Program of Academia Sinica (AS-iMATE-107-97).
Potential conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Yaelle Bavli: Methodology, Data curation, Writing – original draft. Bing-Mae Chen: Methodology, Writing – review & editing. Guy Gross: Resources, Writing – review & editing. Alon Hershko: Conceptualization, Writing – review & editing. Keren Turjeman: Resources, Writing – review & editing. Steve Roffler: Methodology, Supervision, Writing – review & editing. Yechezkel Barenholz: Conceptualization, Supervision, Writing – review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2022.12.039.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Wang H., Paulson K.R., Pease S.A., Watson S., Comfort H., Zheng P., Aravkin A.Y., Bisignano C., Barber R.M., Alam T. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet (Lond. England) 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson O.J., Barnsley G., Toor J., Hogan A.B., Winskill P., Ghani A.C. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 2022 doi: 10.1016/s1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garbuzenko O., Zalipsky S., Qazen M., Barenholz Y. Electrostatics of PEGylated micelles and liposomes containing charged and neutral lipopolymers. Langmuir. 2005;21:2560–2568. doi: 10.1021/la0479105. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni J.A., Witzigmann D., Leung J., Tam Y.Y.C., Cullis P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11:21733–21739. doi: 10.1039/c9nr09347h. [DOI] [PubMed] [Google Scholar]
- 5.Tirosh O., Barenholz Y., Katzhendler J., Priev A. Hydration of polyethylene glycol-grafted liposomes. Biophys. J. 1998;74:1371–1379. doi: 10.1016/S0006-3495(98)77849-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbuzenko O., Barenholz Y., Priev A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem. Phys. Lipids. 2005;135:117–129. doi: 10.1016/j.chemphyslip.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Priev A., Zalipsky S., Cohen R., Barenholz Y. Determination of critical micelle concentration of lipopolymers and other amphiphiles: Comparison of sound velocity and fluorescent measurements. Langmuir. 2002;18:612–617. doi: 10.1021/la0110085. [DOI] [Google Scholar]
- 8.Mui B.L., Tam Y.K., Jayaraman M., Ansell S.M., Du X., Tam Y.Y.C., Lin P.J.C., Chen S., Narayanannair J.K., Rajeev K.G., Manoharan M., Akinc A., Maier M.A., Cullis P., Madden T.D., Hope M.J. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucl. Acids. 2013;2 doi: 10.1038/mtna.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong J.K., Hempel G., Koling S., Chan L.S., Fisher T., Meiselman H.J., Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 10.Sundy J.S., Ganson N.J., Kelly S.J., Scarlett E.L., Rehrig C.D., Huang W., Hershfield M.S. Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum. 2007;56:1021–1028. doi: 10.1002/art.22403. [DOI] [PubMed] [Google Scholar]
- 11.Hershfield M.S., Ganson N.J., Kelly S.J., Scarlett E.L., Jaggers D.A., Sundy J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014;16:R63. doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B.-M., Su Y.-C., Chang C.-J., Burnouf P.-A., Chuang K.-H., Chen C.-H., Cheng T.-L., Chen Y.-T., Wu J.-Y., Roffler S.R. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal. Chem. 2016;88:10661–10666. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 13.Leger R.M., Arndt P., Garratty G., Armstrong J.K., Meiselman H.J., Fisher T.C. Transfusion. Amer Assoc Blood Banks; 2001. Normal donor sera can contain antibodies to polyethylene glycol (PEG) pp. 29S-30S. [Google Scholar]
- 14.Armstrong J.K., Leger R., Wenby R.B., Meiselman H.J., Garratty G., Fisher T.C. Blood. Amer Soc Hematology; 2003. Occurrence of an antibody to poly (ethylene glycol) in normal donors. pp. 556A-556A. [Google Scholar]
- 15.Tillmann H., Ganson N.J., Patel K., Thompson A.J., Abdelmalek M., Moody T., McHutchison J.G., Hershfield M.S. 307 High prevalence of pre-existing antibodies angainst polyethylene glycol (PEG) in hepatitic C (HCV) patients which is not associated with impaired response to PEG-interferon. J. Hepatol. 2010;52:S129. doi: 10.1016/S0168-8278(10)60309-1. [DOI] [Google Scholar]
- 16.Yang Q., Lai S.K. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015;7:655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B.M., Cheng T.L., Roffler S.R. Polyethylene glycol immunogenicity: theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano. 2021;15:14022–14048. doi: 10.1021/acsnano.1c05922. [DOI] [PubMed] [Google Scholar]
- 18.Chen E., Chen B.M., Su Y.C., Chang Y.C., Cheng T.L., Barenholz Y., Roffler S.R. Premature drug release from polyethylene glycol (PEG)-coated liposomal doxorubicin via formation of the membrane attack complex. ACS Nano. 2020;14:7808–7822. doi: 10.1021/acsnano.9b07218. [DOI] [PubMed] [Google Scholar]
- 19.Szebeni J., Simberg D., Gonzalez-Fernandez A., Barenholz Y., Dobrovolskaia M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018;22:18–273. doi: 10.1038/s41565-018-0273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szebeni J., Storm G., Ljubimova J.Y., Castells M., Phillips E.J., Turjeman K., Barenholz Y., Crommelin D.J.A., Dobrovolskaia M.A. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat. Nanotechnol. 2022;17:337–346. doi: 10.1038/s41565-022-01071-x. [DOI] [PubMed] [Google Scholar]
- 21.Anis E., Preis S.A., Cedar N., Tal Y., Hershkowitz I., Hershko A.Y. Reporting of allergic reactions during Pfizer-BioNTech BNTT162B2 vaccination in Israel. J Allergy Clin Immunol Pract. 2022 doi: 10.1016/j.jaip.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju Y., Lee W.S., Pilkington E.H., Kelly H.G., Li S., Selva K.J., Wragg K.M., Subbarao K., Nguyen T.H.O., Rowntree L.C., Allen L.F., Bond K., Williamson D.A., Truong N.P., Plebanski M., Kedzierska K., Mahanty S., Chung A.W., Caruso F., Wheatley A.K., Juno J.A., Kent S.J. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano. 2022;16:11769–11780. doi: 10.1021/acsnano.2c04543. [DOI] [PubMed] [Google Scholar]
- 23.Schilt Y., Berman T., Wei X., Nativ-Roth E., Barenholz Y., Raviv U. Effect of the ammonium salt anion on the structure of doxorubicin complex and PEGylated liposomal doxorubicin nanodrugs. Biochim. Biophys. Acta Gen. Subj. 1865;2021:129849. doi: 10.1016/j.bbagen.2021.129849. [DOI] [PubMed] [Google Scholar]
- 24.Cheng T.-L.L., Cheng C.-M.M., Chen B.-M.M., Tsao D.-A.A., Chuang K.-H.H., Hsiao S.-W.W., Lin Y.-H.H., Roffler S.R. Monoclonal antibody-based quantitation of poly(ethylene glycol)-derivatized proteins, liposomes, and nanoparticles. Bioconjug. Chem. 2005;16:1225–1231. doi: 10.1021/bc050133f. [DOI] [PubMed] [Google Scholar]
- 25.Su Y.C., Chen B.M., Chuang K.H., Cheng T.L., Roffler S.R. Sensitive quantification of PEGylated compounds by second-generation anti-poly(ethylene glycol) monoclonal antibodies. Bioconjug. Chem. 2010;21:1264–1270. doi: 10.1021/bc100067t. [DOI] [PubMed] [Google Scholar]
- 26.Su Y.C., Burnouf P.A., Chuang K.H., Chen B.M., Cheng T.L., Roffler S.R. Conditional internalization of PEGylated nanomedicines by PEG engagers for triple negative breast cancer therapy. Nat. Commun. 2017;8 doi: 10.1038/ncomms15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bavli Y., Chen B.-M., Roffler S.R., Dobrovolskaia M.A., Elnekave E., Ash S., Barenholz Y., Turjeman K. Vol. 25. 2020. PEGylated Liposomal Methyl Prednisolone Succinate does not Induce Infusion Reactions in Patients: A Correlation Between in Vitro Immunological and in Vivo Clinical Studies, Mol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turjeman K., Bavli Y., Kizelsztein P., Schilt Y., Allon N., Katzir T.B., Sasson E., Raviv U., Ovadia H., Barenholz Y. Nano-drugs based on nano sterically stabilized liposomes for the treatment of inflammatory neurodegenerative diseases. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim X.R., Leung B.P., Ng C.Y.L., Tan J.W.L., Chan G.Y.L., Loh C.M., Tan G.L.X., Goh V.H.H., Wong L.T., Chua C.R., Tan S.C., Lee S.S.M., Howe H.S., Thong B.Y.H., Leong K.P. Pseudo-anaphylactic reactions to pfizer bnt162b2 vaccine: report of 3 cases of anaphylaxis post pfizer bnt162b2 vaccination. Vaccines. 2021;9 doi: 10.3390/vaccines9090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arteta M.Y., Kjellman T., Bartesaghi S., Wallin S., Wu X., Kvist A.J., Dabkowska A., Székely N., Radulescu A., Bergenholtz J., Lindfors L. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E3351–E3360. doi: 10.1073/pnas.1720542115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freire Haddad H., Burke J.A., Scott E.A., Ameer G.A. clinical relevance of pre-existing and treatment-induced anti-poly(ethylene glycol) antibodies. Regen. Eng. Transl. Med. 2022;8:32–42. doi: 10.1007/s40883-021-00198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garay R.P., El-Gewely R., Armstrong J.K., Garratty G., Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012;9:1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q., Jacobs T.M., McCallen J.D., Moore D.T., Huckaby J.T., Edelstein J.N., Lai S.K. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal. Chem. 2016;88:11804–11812. doi: 10.1021/acs.analchem.6b03437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubich C., Allacher P., de la Rosa M., Bauer A., Prenninger T., Horling F.M., Siekmann J., Oldenburg J., Scheiflinger F., Reipert B.M. The mystery of antibodies against polyethylene glycol (PEG) - what do we know? Pharm. Res. 2016;33:2239–2249. doi: 10.1007/s11095-016-1961-x. [DOI] [PubMed] [Google Scholar]
- 35.Prussin C., Metcalfe D.D. 5. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2006;117 doi: 10.1016/j.jaci.2005.11.016. S450–S456. [DOI] [PubMed] [Google Scholar]
- 36.Szebeni J., Bedocs P., Rozsnyay Z., Weiszhar Z., Urbanics R., Rosivall L., Cohen R., Garbuzenko O., Bathori G., Toth M., Bunger R., Barenholz Y. Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine. 2012;8:176–184. doi: 10.1016/j.nano.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Carreño J.M., Singh G., Tcheou J., Srivastava K., Gleason C., Muramatsu H., Desai P., Aberg J.A., Miller R.L., P. study group, Pardi N., Simon V., Krammer F. mRNA-1273 but not BNT162b2 induces antibodies against polyethylene glycol (PEG) contained in mRNA-based vaccine formulations. Vaccine. 2022;40:6114–6124. doi: 10.1016/j.vaccine.2022.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guerrini G., Gioria S., Sauer A.V., Lucchesi S., Montagnani F., Pastore G., Ciabattini A., Medaglini D., Calzolai L. Monitoring anti-PEG antibodies level upon repeated lipid nanoparticle-based COVID-19 vaccine administration. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23168838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu L.H., Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., Zhang X.X., Xiang J.L., Du H.X., Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li J.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 40.Ruggiero A., Piubelli C., Calciano L., Accordini S., Valenti M.T., Carbonare L.D., Siracusano G., Temperton N., Tiberti N., Longoni S.S., Pizzato M., Accordini S., Fantoni T., Lopalco L., Beretta A., Bisoffi Z., Zipeto D. SARS-CoV-2 vaccination elicits unconventional IgM specific responses in naïve and previously COVID-19-infected individuals. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neun B., Barenholz Y., Szebeni J., Dobrovolskaia M. Understanding the role of anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules. 2018;23:1700. doi: 10.3390/molecules23071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estapé Senti M., de Jongh C.A., Dijkxhoorn K., Verhoef J.J.F., Szebeni J., Storm G., Hack C.E., Schiffelers R.M., Fens M.H., Boross P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release Off. J. Control. Release Soc. 2022;341:475–486. doi: 10.1016/j.jconrel.2021.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.