Abstract
Human endothelial as well as epithelial cells were shown to respond to lipopolysaccharides (LPSs). However, the expression and release of CD14 by these so-called CD14-negative cells have not been studied in detail. We investigated three human intestinal epithelial cell lines (ECLs), SW-480, HT-29, and Caco-2, for their expression of CD14 and CD11c/CD18 as well as their responsiveness to endotoxins. Fluorescence-activated cell sorter analysis revealed no expression of CD11c/CD18, but there was low expression of membrane-bound CD14 on HT-29, Caco-2, and SW-480 ECLs. Both Western blotting and reverse transcription-PCR confirmed the CD14 positivity of all three intestinal ECLs. No substantial modulation of CD14 expression was achieved after 6, 8, 18, 24, and 48 h of cultivation with 10-fold serial dilutions of LPS ranging from 0.01 ng/ml to 100 μg/ml. Interestingly, soluble CD14 was found in the tissue culture supernatants of all three ECLs. Finally, only HT-29 and SW-480, and not Caco-2, cells responded to LPS exposure (range, 0.01 ng/ml to 100 μg/ml) by interleukin 8 release. Thus, we show that HT-29, SW-480, and Caco-2 human intestinal ECLs express membrane-bound CD14. As Caco-2 cells did not respond to LPS, these cell lines might be an interesting model for studying the receptor complex for LPS. The fact that human intestinal epithelial cells are capable not only of expression but also of release of soluble CD14 may have important implications in vivo, e.g., in shaping the interaction between the mucosal immune system and bacteria in the gut and/or in the pathogenesis of endotoxin shock.
Endotoxin, the bacterial lipopolysaccharide (LPS), is a characteristic outer membrane entity of gram-negative bacteria and a potent inducer of inflammatory responses. Exposure to even low amounts of LPS leads to a dramatic release of inflammatory mediators that are thought to be responsible for the deleterious effects in septic shock, such as refractory hypotension, disseminated intravascular coagulation, and multiple organ failure, causing the high mortality rate in gram-negative sepsis (18).
Several cell surface structures such as CD11c/CD18, the scavenger receptor, and the d-galactose receptor have been found to bind LPS, as have a number of serum components, namely, albumin, transferrin, bactericidal/permeability-increasing protein (BPI), and high-density lipoproteins. Many of these are involved in LPS detoxification (reviewed in reference 45). On the other hand, CD14, a 53-kDa glycosylphosphatidylinositol (GPI)-anchored protein together with LPS-binding protein (LBP) have been shown to play a substantial role in LPS-mediated cell activation (31, 54). CD14 exists as a membrane GPI-anchored glycoprotein and a soluble plasma protein. Both forms of CD14 were shown to be involved in LPS signaling and cell activation, characterized by induction of tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6, and IL-8 (7, 45). While membrane CD14 (mCD14) is involved in LPS activation of CD14-positive cells via complexes of LPS and LBP (21, 49, 54), soluble CD14 (sCD14) was shown to mediate LPS activation on CD14-negative cells such as endothelial and epithelial cells (4, 16, 23, 40, 45, 49). mCD14 is a GPI-anchored protein; therefore, a transmembrane, signal-transducing molecule for LPS has been assumed for many years (45, 49, 50, 52). Human Toll-like receptor 2 and more recently Toll-like receptor 4 have been shown to mediate transmembrane LPS signaling by utilizing the NF-κB signaling pathway in an LBP-dependent and CD14-enhancing manner (1, 11, 26, 32, 55). The importance of CD14 as a coreceptor for LPS was clearly demonstrated by inhibition studies using blocking anti-CD14 antibodies (Abs) (7, 27, 38, 51).
CD14 is primarily expressed on monocytes/macrophages but also on polymorphonuclear as well as nonmyeloid cells such as B cells and gingival fibroblasts (45, 47, 53, 56). Endothelial and epithelial cell lines (ECLs) were used for studying the LPS effect on CD14-negative LPS-sensitive cells (16, 23, 40). However, the expression of mCD14 and thus also the release of sCD14 by these cells have been only poorly investigated, usually by negative immunohistochemical staining (45).
Protease-mediated shedding participates in generating sCD14, which reaches concentrations in plasma of 2 to 6 μg/ml and is increased together with levels of acute-phase proteins during sepsis (3, 47). The cellular source of sCD14 has not yet been clearly identified, as patients with paroxysmal nocturnal hemoglobinuria, who have a defect in GPI anchoring, do not express mCD14 on their monocytes yet possess normal levels of serum sCD14 (9). Prolonged exposure to LPS was reported to down-regulate membrane expression of CD14 on monocytes (27). In vivo, intestinal epithelial cells are continuously exposed to LPS in the gut and play an important role in mucosal innate immunity.
These facts led us to investigate in more detail the presence of both mCD14 and sCD14 as well as LPS activation of three human intestinal ECLs, SW-480, HT-29, and Caco-2. In this study we report that CD14 is expressed on, and more importantly also released as sCD14 by, human intestinal epithelial cells.
MATERIALS AND METHODS
Reagents and MAbs.
LPSs used in this study were the phenol extract LPS from Escherichia coli serotype O55:B5, purified by ion-exchange chromatography, and phenol-extracted LPS from Salmonella enterica serovar Minnesota (Sigma, St. Louis, Mo.). LPS suspensions were prepared as 2 mg of sonicates per ml in pyrogen-free phosphate-buffered saline (PBS; Gibco BRL, Grand Island, N.Y.). Human albumin (endotoxin, ≤0.1 ng/mg) and phosphatidylinositol-specific phospholipase C (PI-PLC) were purchased from Sigma. Mouse anti-human CD14 monoclonal Abs (MAbs) MEM-15 and MEM-18 (both immunoglobulin G1 [IgG1]) and IN-05 mouse anti-insulin (IgG1) MAb were kindly provided by V. Hořejší (Institute of Molecular Genetics, Czech Academy of Sciences Prague, Czech Republic). Compared to the MEM-15 anti-human CD14 MAb, the MEM-18 MAb displays a much greater inhibitory capacity on LPS-induced activation of CD14-positive cells (7). Other mouse anti-human CD14 MAbs, MoP9, MoP15, and MoS39, were obtained from the CD14 panel of the 4th Human Cluster of Differentiation Workshop (provided via V. Hořejší). Fluorescein isothiocyanate (FITC)-conjugated anti-CD11c and R-phycoerythrin (PE)-conjugated anti-CD18 MAbs were purchased from Immunotech (Marseille, France). Biotinylated (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) and FITC-conjugated goat anti-mouse IgG F(ab′)2 Ab and peroxidase-conjugated streptavidin (Immunotech) were used in secondary and tertiary labeling steps, respectively.
Cells and cell cultures.
HT-29, SW-480, and Caco-2 human colonic adenocarcinoma cell lines were obtained from the European Type Culture Collection (Salisbury, United Kingdom). HT-29 and Caco-2 human ECLs were cultivated in Dulbecco's modified Eagle's medium (DMEM) (Sigma) containing 4.5 g of glucose per liter and supplemented with heat-inactivated 10% fetal bovine serum and 2 mM l-glutamine, whereas SW-480 cells were grown in RPMI 1640 medium (10% fetal bovine serum, 2 mM l-glutamine) containing 2 g of glucose per liter. All complete media were tested by the Limulus amebocyte lysate assay (Sigma) to be free of endotoxin. No antibiotics were added to these media, and cell lines were repeatedly screened for mycoplasma (PCR-based test; Statens Serum Institut, Copenhagen, Denmark) with negative results. Cell cultures were incubated at 37°C in humidified air maintained at 5% CO2. The cells were grown in plastic tissue culture flasks (Nunc, Roskilde, Denmark) and subcultured at confluence by employing trypsin-EDTA (Sigma). For further assays, cells were plated into 6- or 24-well plates (Costar, Acton, Mass.) with 3 or 1 ml of medium, respectively. On reaching confluence, the cells were rinsed with the corresponding medium and incubated with 10-fold serial dilutions of LPSs (E. coli serotype O55:B5, Salmonella serovar Minnesota) ranging from 0.01 ng/ml to 100 μg/ml for 6, 8, 18, 24, and 48 h. Human albumin (Sigma) was used as a negative control.
In experiments evaluating a relation between the differentiation stage of epithelial cells and their level of mCD14 expression, HT-29 cells were cultivated in medium without glucose (Glc−), as described by Zweibaum et al. (57). In brief, HT-29 cells were cultivated in a special glucose-free DMEM (prepared at the Institute of Molecular Genetics, Czech Academy of Sciences), supplemented with 10% fetal bovine serum and 2 mM l-glutamine. After 20 days the surviving, selected HT-29 cells were subcultured using trypsin-EDTA (Sigma) and cultivated for the next three passages with a doubling time of approximately 5 days. At confluence, these differentiated, slowly growing HT-29 Glc− cells were then examined for their expression of CD14.
Finally, human peripheral blood mononuclear cells (PBMNC) were isolated from heparinized (10 U/ml) venous blood samples from healthy volunteers by Histopaque 1077 (Sigma) density gradient centrifugation (30 min at 400 × g at ambient temperature). Cells were then washed with RPMI 1640 medium and used as a positive control in subsequent assays.
FACS analysis.
Cell suspensions were prepared from confluent cells growing as monolayers in six-well plates (Costar) and detached from the surface using PBS with 0.02% EDTA (Sigma). Cells (5 × 105 cells per sample) were then used for direct or indirect immunofluorescence staining. Thus, epithelial cells were incubated for 30 min with the mouse anti-human CD14 MAb MEM-15 or MEM-18 at a 1:500 dilution and then incubated for 30 min with secondary FITC-conjugated, goat anti-mouse IgG F(ab′)2 at a 1:200 dilution (Immunotech). For direct immunofluorescence staining, cells were incubated with FITC-conjugated mouse anti-human CD11c or PE-conjugated mouse anti-human CD18 MAbs (Immunotech). FITC- and PE-conjugated isotype-matched (IgG1) antibodies (Immunotech) were used as controls. Furthermore, in order to exclude any nonspecific staining, both MEM-15 and MEM-18 anti-CD14 MAbs were tested on a CD14-negative human erythroblastic cell line, K562. Through the whole staining procedure cells were kept on ice in PBS containing 0.02% EDTA, 2% gelatine, and 0.01% NaN3 (Sigma) and were washed twice between each step. Fluorescence was measured on a FACScan (Becton Dickinson & Co., San Jose, Calif.), and data respecting 10,000 live cells from each sample were collected according to the propidium iodide (FL-3) discrimination method. Changes in mean fluorescence intensity (MFI) were analyzed by WinMDI software version 2.8.
SDS-PAGE and Western blotting.
Confluent epithelial cells were washed three times in corresponding culture media without fetal bovine serum, and 107 cells were incubated with 2 U of PI-PLC per ml in PBS at 37°C for 1 h. After centrifugation for 20 min at 1,000 × g, this GPI protein-enriched cell-free supernatant (as described by Pugin et al. [40]) was diluted in sample buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, and 10 mM EDTA, with a cocktail of the protease inhibitors aprotinin, phenylmethylsulfonyl fluoride, and leupeptin) (Sigma and Boehringer Mannheim, Mannheim, Germany) and heated at 90°C for 10 min, and 20 μl of each sample in 2% sodium dodecyl sulfate (SDS) was subjected under nonreducing conditions to SDS–10% polyacrylamide gel electroforeseis (PAGE) in the discontinuous buffer system of Laemmli (28). For detection of sCD14, cell-free supernatants which were obtained after 48 h of cell culture were concentrated three times on Centricon-10 concentrators (Amicon, Inc., Beverly, Mass.) and similarly assessed by SDS–10% PAGE. Separated proteins were transferred to nitrocellulose (Schleicher & Schuell, Feldbach, Germany) using a transblot cell (Bio-Rad, Hercules, Calif.). The nitrocellulose strips were blocked in PBS containing 2% low-fat milk (PBS-M) and 0.05% Tween 20 (Serva, Heidelberg, Germany) for 1 h and then incubated with a panel of primary anti-CD14 MAbs (MEM-15, MEM-18, MoP9, MoP15, and MoS39 at a dilution of 1:500 in PBS-M) as well as IN-05 mouse anti-insulin (IgG1) isotype control MAb (at a dilution of 1:200 in PBS-M), followed by biotinylated goat anti-mouse IgG F(ab′)2 Ab (1:1,000 dilution in PBS-M; Amersham) and peroxidase-conjugated streptavidin (1:200 dilution in PBS-M; Immunotech). The strips were washed three times in PBS with 0.05 Tween 20 after each step. Marked proteins were visualized by the enhanced-chemiluminescence detection system (Amersham).
RT-PCR and sequencing of PCR products.
Total RNA was isolated with Trizol (Gibco BRL), and single-stranded complementary cDNA was synthetized using 1 μg of total RNA and 200 U of Superscript II reverse transcriptase (Gibco BRL) in a solution containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, a 1.5 μM concentration of oligo(dT)12–18 primers, and a 0.5 mM concentration of each deoxynucleoside triphosphate for 1 h at 37°C followed by 5 min at 75°C. Subsequently, 2 μl of cDNA was specifically amplified by PCR with 2.5 U of Taq DNA polymerase (Top-Bio, Prague, Czech Republic) in a solution containing 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, and 0.5 μM each primer. The PCR conditions were as follows: 94°C for 1 min; 35 cycles of 94°C for 45 s, 57°C for 45 s, and 72°C for 2 min; and 72°C for 10 min as a final extension. Two sets of human-CD14-specific primers were used. The first one consisted of sense 5′ GCT GGA CGA TGA AGA TTT CC 3′ and antisense 5′ ATT GTC AGA CAG GTC TAG GC 3′ primers with expected product sizes of 535 bp. Contamination with genomic DNA was checked by omitting the Superscript II during reverse transcription (RT). Amplification without cDNA was carried out to assess later contaminations. The fact that the human CD14 gene contains a single intron after the initiation codon (15) has allowed us to design a set of spanning primers which give products of different sizes from genomic DNA and mRNA, 356 and 284 bp, respectively. The sequences of the primers were 5′ GCT GTG TAG GAA AGA AGC TA 3′ (sense) and 5′ TTT AGA AAC GGC TCT AGG TTG 3′ (antisense) (Genset, Paris, France). The PCR products and 100-bp PCR marker (Sigma) were run on 1.5% agarose gels stained with ethidium bromide and visualized with a UV transilluminator (UVP, Upland, Calif.). All amplifications, including RTs, were repeated at least three times.
Results were confirmed by manual sequencing of purified and cloned RT-PCR products obtained from the HT-29 ECL. Briefly, the band of interest (284 bp) was cut out from the agarose gel, boiled for 10 min, ethanol precipitated, and reamplified with the same set of primers and a trace of [α-32P]dCTP (NEN, Boston, Mass.). Two microliters of the PCR mixture was then subjected to single-strand conformation polymorphism analysis on native polyacrylamide gel (5% acrylamide, 5% glycerol; Sigma) at 5 W for 12 h. After autoradiography, the major purified band was cut out from the unfixed polyacrylamide gel, rereamplified by PCR, and cloned into the pCR2.1 vector using a TA cloning kit with INVαF′ competent cells from Invitrogen (San Diego, Calif.). Isolated plasmid DNA was sequenced by using the M13 (−20) forward primer and a T7 sequencing kit from Pharmacia Biotech (Uppsala, Sweden).
IL-8 ELISA.
The interleukin 8 (IL-8) concentration was determined in the undiluted cell culture supernatants collected after 8 and 24 h of cultivation with an enzyme-linked immunosorbent assay (ELISA) DuoSet kit from Genzyme (Cambridge, Mass.). The sensitivity of this assay was 20 pg/ml. An additional human IL-8 standard (1 μg/ml) was kindly provided by M. Ceska (Sandoz Forschungsinstitut, Vienna, Austria) and used in parallel. All experiments were performed in 96-well flat-bottom Immuno-Maxisorp microtiter plates (Nunc). O-Phenylenediamine (Sigma) was used as a substrate for peroxidase, and absorption was measured with a Uniskan II microplate reader (Labsystems, Helsinki, Finland) at 405 nm.
NO production.
Nitric oxide (NO) production was assessed as nitrite formation in 50 μl of cell culture supernatants. Samples were incubated for 10 min at 37°C with Griess reagent (1% sulfanilamide, 0.1% naphtylethylendiamine, 2.5% H3PO4) and measured at 540 nm with a Uniskan II microplate reader (Labsystems).
RESULTS
Detection of CD14 on the human intestinal SW-480, HT-29, and Caco-2 ECLs by FACS, Western blot, and RT-PCR analyses.
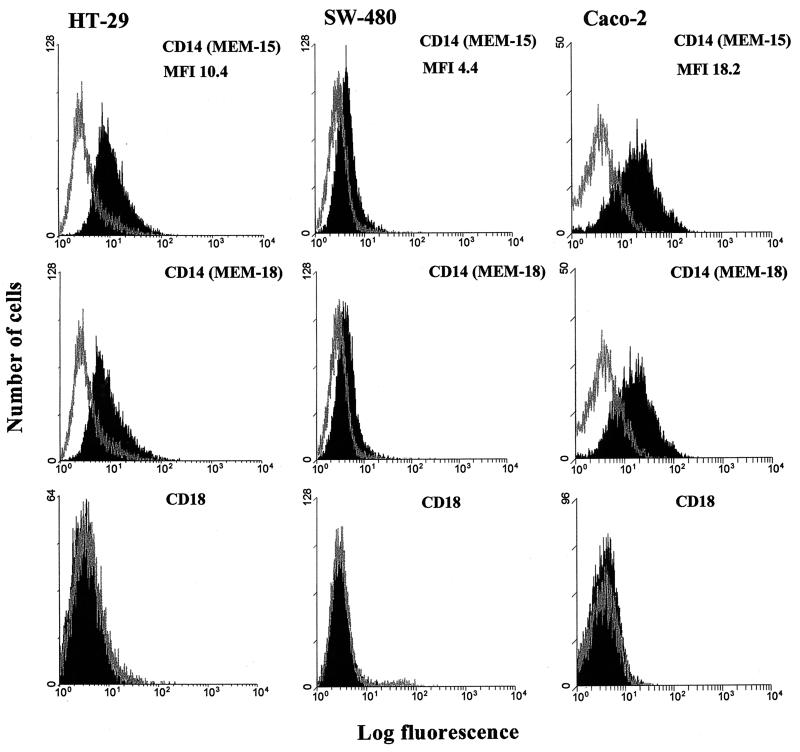
The cell surface expression of the LPS receptors CD14 and CD11c/CD18 on SW-480, HT-29, and Caco-2 human ECLs was first assessed by flow cytometry. FACS analysis revealed positivity for mCD14 on HT-29 and Caco-2 cell lines with MFIs of 10.4 and 18.2, respectively (Fig. 1). Only very low positivity for mCD14 was detected on the SW-480 ECL. The three cell lines were found negative for both CD11c and CD18, thus excluding expression of another LPS-signaling receptor, CD11c/CD18 (e.g., see the data for CD18 staining shown in Fig. 1). All FACS experiments were repeated at least two times but on average were repeated four to five times, and two anti-CD14 MAbs, MEM-15 and MEM-18, were used in parallel.
FIG. 1.
Flow cytometry analysis of CD14 and CD18 surface expression on HT-29, SW-480, and Caco-2 intestinal ECLs. Expression of CD14 was assessed by indirect immunofluorescence using anti-CD14, MEM-15, and MEM-18 MAbs (IgG1) at a 1:500 dilution followed by FITC-conjugated, goat anti-mouse IgG F(ab′)2 (black profile). CD18 expression was assessed by direct immunoflourescence using PE-conjugated mouse anti-human CD18 (IgG1) MAb (black profile). FITC- and PE-conjugated isotype-matched (IgG1) Abs and staining without primary MAb were used as negative controls (gray profile). The data are expressed as cell number versus log fluorescence and are representative of two to five independent experiments. The highest mCD14 positivity is documented by MFI values.
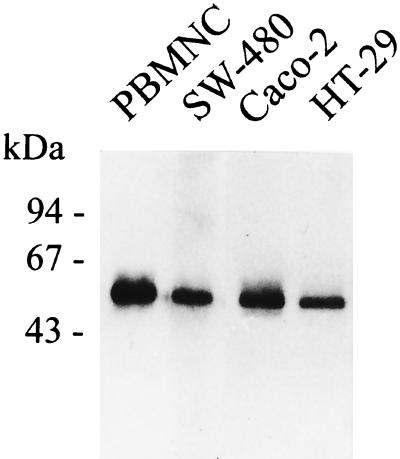
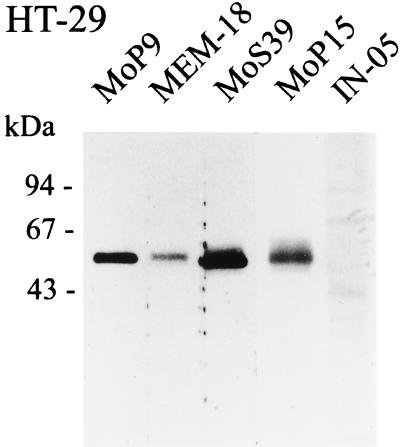
Western blot analysis of supernatants from PI-PLC-treated cells was performed on SW-480, HT-29, and Caco-2 cells. As shown in Fig. 2, the MEM-15 anti-CD14 MAb recognizes mCD14 on all three epithelial cell lines (Fig. 2). Human PBMNC were used as a positive control, and anti-insulin isotype-matched MAb IN-05 was used as another internal control of these experiments. The results were further confirmed by employing several anti-CD14 MAbs such as MoP9, MEM-18, MoS39, and MoP15 (data from the HT-29 ECL are shown in Fig. 3).
FIG. 2.
SDS-PAGE and Western blot detection of mCD14 on HT-29, SW-480, and Caco-2 intestinal ECLs. Cells (107) were incubated with 2 U of PI-PLC per ml at 37°C for 1 h, and 20 μl of the GPI protein-enriched fraction was run under nonreducing conditions on SDS–10% PAGE, transferred to a nitrocellulose membrane, and stained with MEM-15 (IgG1) mouse anti-human CD14 MAb at a dilution of 1:500, followed by biotinylated goat anti-mouse IgG F(ab′)2 Ab and peroxidase-conjugated streptavidin. Bound proteins were visualized by the enhanced-chemiluminescence detection system, and PBMNC were used as a positive control. The figure shows the results of one experiment representative of four.
FIG. 3.
SDS-PAGE and Western blot detection of mCD14 on the HT-29 intestinal ECL with multiple anti-CD14 MAbs. Using the same experimental design used to obtain the results shown in Fig. 2, mCD14 was detected by MEM-15, MEM-18, MoP9, MoP15, and MoS39 anti-CD14 MAbs (IgG1) at a dilution of 1:500. IN-05 mouse anti-insulin (IgG1) MAb at a dilution of 1:200 was used as an isotype-matched negative control. The figure presents data representative of two separate experiments.
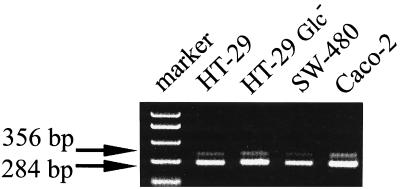
Finally, these results were further confirmed at the mRNA level by RT-PCR. We have used a set of primers spanning the intron sequence, which follows the initiation codon of the CD14 gene on chromosome 5 (15). These primers give products of different sizes from genomic DNA and mRNA, 356 and 284 bp, respectively (Fig. 4). The mRNA for CD14 was clearly and repeatedly detected in the Caco-2, HT-29, and SW-480 ECLs (Fig. 4). Final verification of CD14 mRNA was achieved by sequencing the reamplified, single-strand-conformation polymorphism, gel-purified, and cloned RT-PCR product from HT-29 cells as described in Materials and Methods.
FIG. 4.
Expression of CD14 mRNA in intestinal ECLs. Total RNA from the HT-29 SW-480, and Caco-2 ECLs, as well as HT-29 Glc− cells differentiated by cultivation in glucose-free medium as described in Materials and Methods, was reverse transcribed with Superscript II and amplified with CD14-specific primers. The expected sizes of PCR products from DNA and mRNA were 356 and 284 bp, respectively. The data are representative of results from three independent cell cultures and RT-PCR experiments.
Effect of differentiation stage of epithelial cells on CD14 expression.
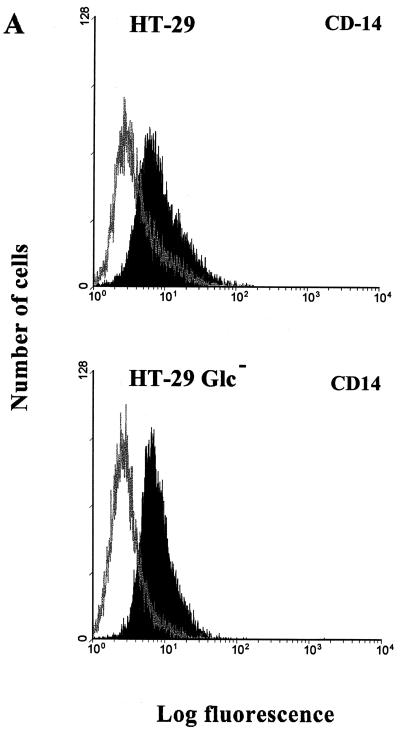
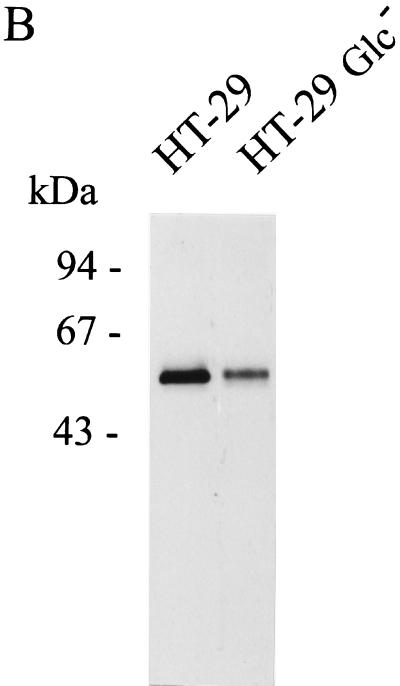
The three ECLs SW-480, HT-29, and Caco-2, when cultivated under standard conditions, represent good models as concerns the stage of differentiation of epithelial cells. The most undifferentiated cell line, SW-480, showed very low but detectable surface expression of CD14 (Fig. 1 and 2), whereas the most differentiated cell line, Caco-2, displayed the highest surface expression of CD14 (MFI, 18.2) as documented by FACS analysis (Fig. 1 and 5A). In order to further evaluate a relation between the differentiation stage of epithelial cells and their expression of mCD14, HT-29 cells were cultivated in Glc− DMEM. The absence of glucose leads to the structural and enzymatic differentiation of selected HT-29 cells as described by Zweibaum et al. (57). Both RT-PCR and Western blot analyses revealed clear CD14 expression on both undifferentiated and differentiated HT-29 cells (Fig. 4 and 5B). FACS analysis revealed no substantial differences in the levels of mCD14 expression between HT-29 and differentiated HT-29 Glc− cells (Fig. 5A).
FIG. 5.
Effect of differentiation stage of the HT-29 intestinal ECL on CD14 expression. The HT-29 intestinal ECL was differentiated by cultivation in Glc− DMEM supplemented with 10% fetal bovine serum as described in Materials and Methods and reference 57. (A) Flow cytometry analysis of the undifferentiated HT-29 and differentiated HT-29 Glc− ECLs. Cells (5 × 105 cells per sample) were stained with mouse anti-human CD14 MAb MEM-15 (IgG1) followed by secondary FITC-conjugated, goat anti-mouse IgG F(ab′)2 (black profile). FITC-conjugated isotype-matched (IgG1) antibody and staining without primary MAb were used as negative controls (gray profile). The data are representative of three independent experiments. (B) SDS-PAGE and Western blot detection of mCD14 in undifferentiated HT-29 and differentiated HT-29 Glc− ECLs using MEM-15 (IgG1) mouse anti-human CD14 MAb and the same experimental design as that described for Fig. 2. The data are representative of two separate experiments.
Modulation of CD14 expression on SW-480, HT-29, and Caco-2 epithelial cells.
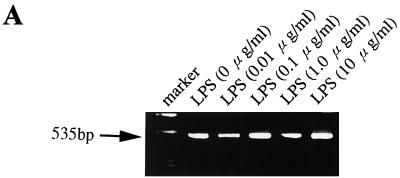
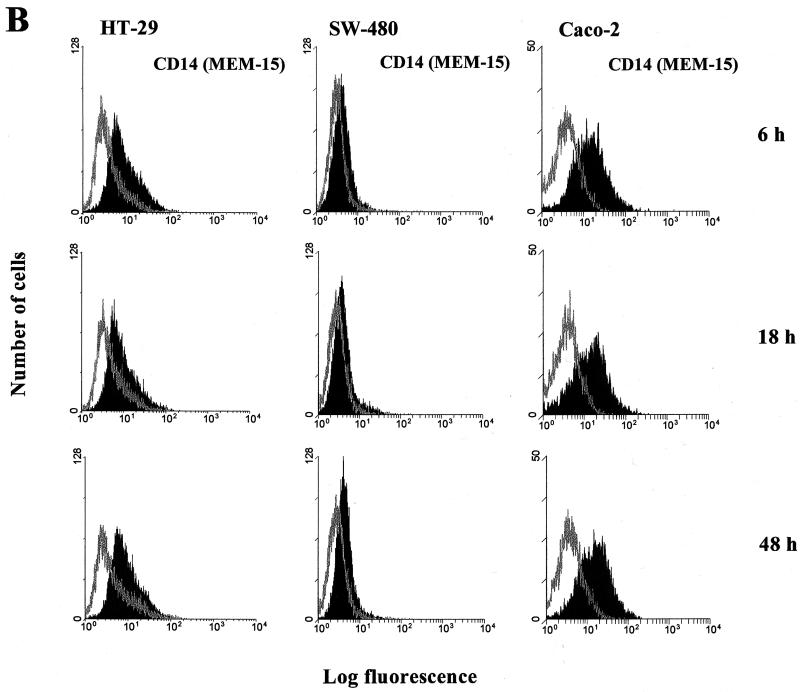
Figure 6a shows a constant presence of CD14 mRNA in the HT-29 cell line, irrespective of exposure to different doses of LPS. FACS analysis was employed for the final quantification of modulation of CD14 expression on the three ECLs. No remarkable differences measured as a shift in MFI (FL-1) were observed after cultivating the three epithelial cell lines with LPS from E. coli serotype O55:LB5 or Salmonella serovar Minnesota for 6, 18, 24, or 48 h. Taking into account the massive exposure of human enterocytes to LPS in the gut, quite high doses of LPSs were also included in our experiments, with concentrations from 0.01 ng/ml to 100 μg/ml. All experiments were repeated in at least two independent cell cultures, and the same concentrations of human albumin were used as negative controls. However, no substantial modulation of CD14 expression was recorded in any of the above-mentioned cultivating arrangements (some data are presented in Fig. 6B).
FIG. 6.
Modulation of mCD14 expression on HT-29, SW-480, and Caco-2 intestinal ECLs. (A) Effect of LPS on CD14 mRNA expression in the HT-29 intestinal ECL. CD14 mRNA was determined by RT-PCR using CD14-specific primers with an expected product size of 535 bp. RT-PCR was performed on total RNA from the HT-29 intestinal ECL cultivated in DMEM with 10% fetal bovine serum and with 0.01, 0.1, 1, and 10 μg of LPS per ml (serovar Minnesota) for 6 h. RT without Superscript II and PCR without template cDNA were carried out as negative controls. (B) Flow cytometry analysis of LPS's effect on modulation of mCD14 expression in HT-29, SW-480, and Caco2 intestinal ECLs. The cell lines were cultivated in complete medium supplemented with 10% fetal bovine serum and in the presence of 0.1 μg of LPS per ml (E. coli serotype O55:LB5) for 6, 18, and 48 h. The cells (5 × 105 cells per sample) were then used for indirect immunofluorescence staining as described for Fig. 1. The data are representative of two to four independent experiments.
Human intestinal ECLs release sCD14 into supernatants.
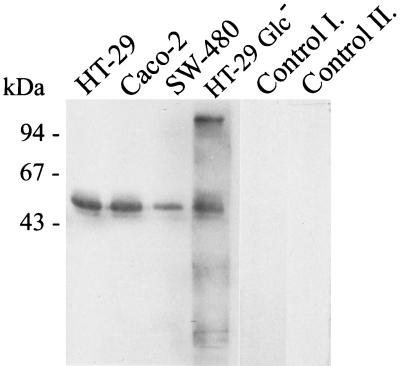
As sCD14 plays important roles in activation of both CD14-negative and CD14-positive cells and its cellular source remains unknown, we investigated the presence of sCD14 in the cell-free tissue culture supernatants of the three ECLs. Interestingly, SDS-PAGE and Western blot detection revealed a clear positivity for sCD14 in cell-free 48-h culture supernatants from SW-480, HT-29, and Caco-2 as well as HT-29 Glc− ECLs. Supernatants from the differentiated HT-29 Glc− ECL cultivated in glucose-free medium have repeatedly revealed a higher background than those from the SW-480, Caco-2, and HT-29 ECLs (Fig. 7). The spontaneously released form of CD14 displayed a typical multiple-band pattern of 53 and 48 kDa (Fig. 7) as described by Bažil et al. (2, 3). SW-480 cells with the lowest expression of surface mCD14 were also characterized by a weak release of sCD14 into the supernatant, especially as regards the smaller band, corresponding to the protease-shed CD14 (2, 3) of about 48 kDa (Fig. 7).
FIG. 7.
Detection of sCD14 in cell-free cell culture supernatants of HT-29, SW-480, Caco-2, and differentiated HT-29 Glc− intestinal ECLs. Cell-free supernatants were collected after 48 h of cell culture, concentrated three times using Centricon-10 tubes (cutoff, 10 kDa), and assessed by SDS–10% PAGE under nonreducing conditions followed by Western blotting and immunodetection as described for Fig. 2. Three times-concentrated complete DMEM with 10% fetal bovine serum and three times-concentrated 48 h supernatant of the HT-29 cell line (with the primary anti-CD14 MAb MEM-15 omitted) were used as control I and control II, respectively. The figure shows the results of one experiment representative of three.
Activation of human intestinal epithelial cells by LPS.
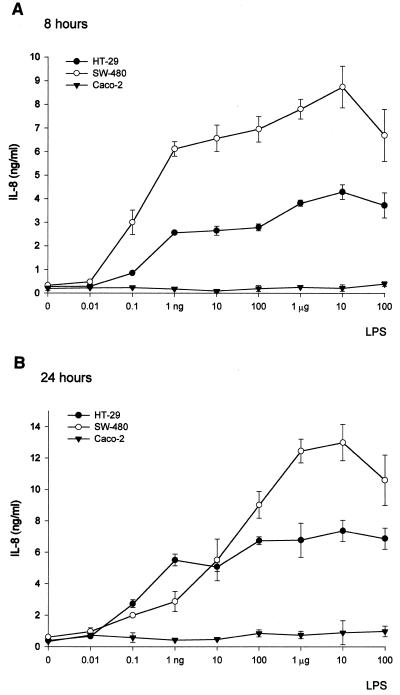
Both SW-480 and HT-29 cell lines released IL-8 in response to 10-fold concentrations of LPS (E. coli O55:LB5) ranging from 0.01 ng/ml to 100 μg/ml. A slight increase in the amount of IL-8 in supernatants was observed after 24 h of cultivation compared to that after 8 h (Fig. 8). Although the amount of IL-8 displays a dose dependency in both cell lines, the highest dose of 100 μg of LPS per ml already had an inhibitory effect. On the other hand, no LPS-induced production of IL-8 was detected in any of these cultivation arrangements with Caco-2 cells (Fig. 8). In order to further investigate the activation of epithelial cells by LPS, production of NO measured as nitrite production was assessed. However, no detectable NO production was found in SW-480, HT-29, or Caco-2 epithelial cells exposed to LPS (data not shown). Thus, although all three cell lines express surface mCD14, only the SW-480 and HT-29 cell lines responded by IL-8 release to exposures to different doses of LPS.
FIG. 8.
IL-8 secretion by HT-29 (filled circles), SW-480 (open circles), and Caco-2 (filled triangles) intestinal ECLs stimulated with LPS (E. coli serotype O55:LB5) for 8 (A) and 24 (B) h. LPS concentrations ranged from 0.01 ng/ml to 100 μg/ml. Cell-free supernatants were collected after 8 (A) or 24 (B) h of cell culture, and levels of IL-8 were determined by ELISA. Data are presented as means ± standard errors of values from three parallel cell cultures and are representative of three independent experiments.
DISCUSSION
In this study we demonstrate for the first time that CD14 is expressed as well as released as sCD14 from human intestinal epithelial cells. CD14 was detected on SW-480, HT-29, and Caco-2 human intestinal ECLs on both the protein and mRNA levels (Fig. 1 to 4). Its membrane expression was confirmed by repeated FACS and Western blot analyses using multiple anti-CD14 MAbs (Fig. 1 and 3). Interestingly, a soluble form of CD14 was detected in cell-free cell culture supernatants from all three ECLs (Fig. 7). Furthermore, only SW-480 and HT-29 and not Caco-2 ECLs were sensitive to LPS (Fig. 8).
CD14 expression has been reported on nonmyeloid cells, such as B cells, or gingival fibroblasts (45, 47). On the other hand, the astrocytoma cell line U373, smooth muscle cells, endothelial cells, and epithelial cells were used in studies dealing with LPS-mediated activation of CD14-negative cells via the sCD14 pathway (4, 16, 33, 40, 41, 45). However, unlike for smooth muscle cells (33), the expression of CD14 on human epithelial cells was not studied in detail. Our results showing that human intestinal adenocarcinoma ECLs express mCD14 (Fig. 1 to 4) are in agreement with and extend data gained at the mRNA level from other species. Thus, Diamond et al. (8) reported that bovine tracheal epithelial cells express CD14 mRNA. An in vivo study of mice showed extramyeloid, LPS-induced expression of CD14 mRNA in epithelial cells from several organs such as lung, kidney, and liver (12). In addition, the up-regulation of CD14 mRNA was shown to be mediated also by TNF-α and IL-1β (13, 14). No studies concerning the expression of CD14 mRNA by nonneoplastic human intestinal epithelial cells have been published.
Another cell surface molecule known to bind LPS, the β2 integrin of CD11c/CD18, was first considered in LPS clearance and detoxification (52). Later, CD11c/CD18 was reported to mediate an activation signal after exposure to LPS (25). The cell activation was not dependent on the presence of sera, and thus its role in infected tissues was proposed (25). We wish to point out, however, that no expression of CD11c or CD18 was detected on HT-29, SW-480, and Caco-2 human intestinal ECLs (Fig. 1).
Several studies reported, although with certain controversy, LPS-induced changes in the level of CD14 expression on myeloid cells. These effects occurred within a relatively short period of time (1 to 6 h) and were documented on both the protein and mRNA levels (3, 10, 34, 35, 56). In our hands, no substantial modulation of CD14 expression was observed in the three ECLs, in spite of our using a wide range of LPS doses as well as timings (some data are presented in Fig. 6). We think that this may be due to the differences in levels of LPS-induced CD14 expression in myeloid cells compared to that in epithelial cells, in which TNF-α participates in mediating CD14 gene expression (13). Although the three epithelial cell lines express mCD14 at levels correlating with their degree of differentiation (SW-480 < HT-29 < Caco-2) (Fig. 1), we were not able to induce a substantial increase in the level of mCD14 by differentiating the HT-29 cell line in glucose-free medium (57) (Fig. 5A).
The most differentiated, mCD14-positive ECL, Caco-2, which represents the closest model of in vivo enterocytes, does not secrete IL-8 after stimulation with LPS (Fig. 8) (10). It is of interest, however, that sodium butyrate, a metabolic product of intestinal bacterial fermentation of carbohydrates, enables IL-8 secretion in response to LPS as well as significantly enhancing IL-1β-induced IL-8 secretion by Caco-2 cells (17). Thus, additional stimuli, such as from physiological intestinal microflora, are necessary to promote LPS's effect on Caco-2 cells. Our recent experiments have shown that the blocking anti-CD14 MAb MEM-18 significantly reduced LPS-induced IL-8 release in both HT-29 and SW-480 ECLs, pointing to the involvement of mCD14 in their activation (unpublished data).
The fact that only SW-480 and HT-29, but not Caco-2, mCD14-positive ECLs responded to LPS by IL-8 release (Fig. 8) makes them a promising tool in studies of the role of the multiple receptor complex for LPS. The Toll-like 4 receptor was clearly shown as a trans-membrane signaling molecule for CD-14-enhanced LPS activation (1, 11, 32). Nevertheless, signal transduction via the GPI anchor, a component of glycolipid-reach microdomains, which are associated with cytoplasmic protein tyrosine kinases and G proteins (5, 24), cannot be ruled out, especially for CD14 receptor cross-linking (39).
Although LPS-induced production of NO was reported in myeloid and endothelial cells (44, 45), we observed no such effect with the intestinal ECLs. These data confirm the previous results of Salzman et al. (43), who documented that an additional stimulus, e.g., gamma interferon, is required for promoting LPS-induced NO production by epithelial cells.
Of interest is our finding that all three intestinal ECLs released soluble forms of CD14 (Fig. 7). In accordance with data published by Bažil and Strominger (3), sCD14 displayed a two-band pattern of 48 and 50 kDa on SDS-PAGE (Fig. 7). While the smaller form of 48 kDa has been considered a product of protease-mediated shedding, the 50-kDa band has been suggested to represent a directly released form of sCD14 (2, 3). Previous studies have shown opposite, dose-dependent effects of sCD14 on the activation of mCD14-positive cells (22) as well as its involvement in activating CD14-negative cells (16, 23, 40). Thus, sCD14, together with other LPS-binding factors such as LBP, BPI, and high-density lipoproteins, is thought to play a central role in regulating responses to encountered gram-negative bacteria (22).
It is generally known that human enterocytes in vivo do not express mCD14 at levels detectable by immunohistochemistry (40, 45, 46). However, taking into account the number of epithelial cells in the mucosal compartment and the fact that polarized, basolateral secretion of various cytokines, e.g., chemokines as well as acute-phase plasma proteins, was described for human ECLs (29, 37), even relatively low levels of release of sCD14 might maintain its level in serum. Several mechanisms can contribute to the decrease or loss of mCD14 expression. While monocytes and endothelial cells are activated by LPS during infection, intestinal epithelial cells are continuously exposed to LPS from gut microflora under physiological conditions. Prolonged exposure to LPS was shown to down-regulate mCD14 expression on monocytes and to establish LPS tolerance (27). Furthermore, the anti-inflammatory cytokines IL-4, a prevalent cytokine of the mucosal microenvironment, and IL-13 were reported to down-regulate CD14 expression on monocytes (6, 30). Interestingly, human lamina propria macrophages almost do not express mCD14 (19, 46) unless inflammation is present, such as in inflammatory bowel disease (20, 42) or celiac disease (48). In mice, TNF-α and IL-1β were shown to induce CD14 mRNA in nonintestinal epithelial cells (13, 14). In addition, Meijssen et al. reported expression of CD14 mRNA in intestinal epithelial cells of IL-2−/− mice, which develop inflammatory bowel disease resembling human ulcerative colitis (36). These data suggest that the healthy mucosal microenvironment down-regulates mCD14 expression but that inflammatory processes lead to its up-regulation (13, 14, 19, 20, 36, 42, 46).
Our observations that human intestinal ECLs express and release CD14 raise the possibility that enterocytes may be a source of sCD14 in humans. In conclusion, we suggest that the ability of intestinal epithelial cells to express and release CD14 may be of importance in maintaining the intricate balance between “self” and the external environment in the gut.
ACKNOWLEDGMENTS
We gratefully thank V. Hořejší for kindly providing us with anti-human CD14 (MEM-15, MEM-18) and anti-insulin IN-05 MAbs. We also thank M. Ceska for an additional IL-8 standard, as well as Petr Klement and Knud Josefsen for their help with SDS-PAGE and molecular biology techniques, respectively. P. Kašparová and D. Horáková are thanked for excellent technical assistance, and James Harries is thanked for his help in the preparation of the manuscript.
This work was supported by grants A7020808, A7020716, and C6020801 from the grant agency of the Czech Academy of Sciences; grants VS96149 and KONTAKT178 from the grant agency of the Ministry of Education; grant 306/98/0433/00/1373 from the grant agency of the Czech Republic; and a grant from the EU project QLGI-1999-00050.
REFERENCES
- 1.Akashi S, Ogata H, Kirikae F, Kirikae T, Kawasaki K, Nishijima M, Shimazu R, Nagai Y, Fukudome K, Kimoto M, Miyake K. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4–MD-2. Biochem Biophys Res Commun. 2000;268:172–177. doi: 10.1006/bbrc.2000.2089. [DOI] [PubMed] [Google Scholar]
- 2.Bažil V, Baudyš M, Hilgert I, Stefanová I, Low M G, Zbrozek J, Hořejší V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 3.Bažil V, Strominger J L. Shedding as a mechanism of down-regulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- 4.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H C, Podolsky D K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 5.Cebecauer, M., J. Černý, and V. Hořejší. Incorporation of leucocyte GPI-anchored proteins and protein tyrosinc kinases into lipid-rich membrane domains of COS-7 cells. Biochem. Biophys. Res. Commun. 243:706–710. [DOI] [PubMed]
- 6.Consentino G, Soprana E, Thienes C P, Siccardi A G, Viale G, Vercelli D. IL-13 down-regulates CD14 expression and TNF-alpha secretion in normal human monocytes. J Immunol. 1995;15:3145–3151. [PubMed] [Google Scholar]
- 7.Dentener M A, Bažil V, von Asmuth E J U, Ceska M, Buurman W A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-α, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 8.Diamond G, Russell J P, Bevins C L. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchow J, Marchant A, Crusiaux A, Husson C, Alonso-Vega C, De Groote D, Neve P, Goldman M. Impaired phagocyte response to LPS in paroxysmal nocturnal hemoglobinuria. Infect Immun. 1993;61:4280–4285. doi: 10.1128/iai.61.10.4280-4285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann I, Jung H C, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 11.Faure E, Equils O, Sieling P A, Thomas L, Zhang F X, Kirschning C J, Polentarutti N, Muzio M, Arditi M. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 12.Fearns C, Kravchenko V V, Ulevitch R J, Loskutoff D J. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995;181:857–866. doi: 10.1084/jem.181.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearns C, Loskutoff D J. Role of tumor necrosis factor alpha in induction of murine CD14 gene expression by lipopolysaccharide. Infect Immun. 1997;65:4822–4831. doi: 10.1128/iai.65.11.4822-4831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fearns C, Ulevitch R J. Effect of recombinant interleukin-1beta on murine CD14 gene expression in vivo. Shock. 1998;9:157–163. doi: 10.1097/00024382-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ferrero E, Goyert S M. Nucleotide sequence of the gene encoding the monocyte differentiation antigen, CD14. Nucleic Acids Res. 1988;16:4173. doi: 10.1093/nar/16.9.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey E A, Miller D S, Jahr T G, Sundan A, Bažil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fusunyan R D, Qinn J, Ohno Y, Richard P, MacDermott R P, Sanderson I R. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1beta and lipopolysaccharide. Pediatr Res. 1998;43:84–90. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Glauser M P, Zanetti G, Baumgartner J D, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 19.Grimm M C, Pavli P, Van de Pol E, Doe W F. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa—implications for pathogenesis. Clin Exp Immunol. 1995;100:291–297. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm M C, Pullmann W E, Bennett G M, Sullivan P J, Pavli P, Doe W F. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995;10:387–395. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 21.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 23.Haziot A, Rong G W, Silver J, Goyert S M. Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide J. Immunol. 1993;151:1500–1507. [PubMed] [Google Scholar]
- 24.Hořejší V, Drbal K, Cebecauer M, Ĉerný J, Brdička T, Angelisová P, Stockinger H. GPI-microdomains: a role in signalling via immunoreceptors. Immunol Today. 1999;20:356–361. doi: 10.1016/s0167-5699(99)01489-9. [DOI] [PubMed] [Google Scholar]
- 25.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschning C J, Wesche H, Ayers T M, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labeta M O, Durieux J J, Spagnoli G, Fernandez N, Wijdenes J, Herrmann R. CD14 and tolerance to lipopolysaccharide: biochemical and functional analysis. Immunology. 1993;80:415–423. [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lammers K M, Jansen J, Bijlsma P B, Ceska M, Tytgat G N, Laboisse C L, van Deventer S J. Polarised interleukin 8 secretion by HT 29/19A cells. Gut. 1994;35:338–342. doi: 10.1136/gut.35.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauener R P L, Goyert S M, Geha R S, Vercelll D. Interleukin 4 down-regulates the expression of CD14 in normal human monocytes. Eur J Immunol. 1990;20:2375–2381. doi: 10.1002/eji.1830201103. [DOI] [PubMed] [Google Scholar]
- 31.Lee J D, Kato K, Tobias P S, Kirkland T N, Ulevitch R J. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lien E, Means T K, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton M J, Oikawa M, Qureshi N, Monks B, Finberg R W, Ingalls R R, Golenbock D T. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Investig. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loppnow H, Stelter F, Schonbeck U, Schluter C, Ernst M, Schutt C, Flad H D. Endotoxin activates human vascular smooth muscle cells despite lack of expression of CD14 mRNA or endogenous membrane CD14. Infect Immun. 1995;63:1020–1026. doi: 10.1128/iai.63.3.1020-1026.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchant A, Duchow J, Delville J P, Goldman M. Lipopolysaccharide induces up-regulation of CD14 molecule on monocytes in human whole blood. Eur J Immunol. 1992;22:1663–1665. doi: 10.1002/eji.1830220650. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijssen M A, Brandwein S L, Reinecker H C, Bhan A K, Podolsky D K. Alteration of gene expression by intestinal epithelial cells precedes colitis in interleukin-2-deficient mice. Am J Physiol. 1998;274:G472–G479. doi: 10.1152/ajpgi.1998.274.3.G472. [DOI] [PubMed] [Google Scholar]
- 37.Molmenti E P, Ziambaras T, Perlmutter D H. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993;268:14116–14124. [PubMed] [Google Scholar]
- 38.Pugin J, Heumann D, Tomasz A, Kravchenko V, Akamatsu Y, Nishijama M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 39.Pugin J, Kravchenko V V, Lee J D, Kline L, Ulevitch R J, Tobias P S. Cell activation mediated by glycosylphosphatidylinositol-anchored transmembrane forms of CD14. Infect Immun. 1998;66:1174–1180. doi: 10.1128/iai.66.3.1174-1180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pugin J, Schurer-Maly C C, Letureq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read M A, Cordle S R, Veach R A, Carlisle C D, Hawiger J. Cell-free pool of CD14 mediates activation of transcription factor NF-kappa B by lipopolysaccharide in human endothelial cells. Proc Natl Acad Sci USA. 1993;90:9887–9891. doi: 10.1073/pnas.90.21.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogler G, Hausmann M, Spotti T, Vogi D, Aschenbrenner E, Andus T, Falk W, Scholmerich J, Gross V. T-cell co-stimulatory molecules are upregulated on intestinal macrophages from inflammatory bowel disease mucosa. Eur J Gastroenterol Hepatol. 1999;11:1105–1111. doi: 10.1097/00042737-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Salzman A L, Eaves-Pyles T, Linn S C, Denenberg A G, Szabó C. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology. 1998;114:93–102. doi: 10.1016/s0016-5085(98)70637-7. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder R A, delaTorre A, Kuo P C. CD14-dependent mechanism for endotoxin-mediated nitric oxide synthesis in murine macrophages. Am J Physiol. 1997;273:C1030–C1039. doi: 10.1152/ajpcell.1997.273.3.C1030. [DOI] [PubMed] [Google Scholar]
- 45.Schumann R R, Rietschel E T. The role of CD14 and LBP in the activation of different cell types by endotoxin. Med Microbiol Immunol. 1994;183:279–297. doi: 10.1007/BF00196679. [DOI] [PubMed] [Google Scholar]
- 46.Smith P D, Janoff E N, Mosteller-Barnum M, Merger M, Orenstein J M, Kearney J F, Graham M F. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Methods. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 47.Sugawara S, Sugiyama A, Nemoto E, Rikiishi H, Takada H. Heterogeneous expression and release of CD14 by human gingival fibroblasts: characterization and CD14-mediated interleukin-8 secretion in response to lipopolysaccharide. Infect Immun. 1998;66:3043–3049. doi: 10.1128/iai.66.7.3043-3049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ter Steege J, Buurman W, Arends J W, Forget P. Presence of inducible nitric oxide synthase, nitrotyrosine, CD68, and CD14 in the small intestine in celiac disease. Lab Investig. 1997;77:29–36. [PubMed] [Google Scholar]
- 49.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 50.Vita N, Lefort S, Sozzani P, Reeb R, Richards S, Borysiewicz L K, Ferrara P, Labeta M O. Detection and biochemical characteristics of the receptor for complexes of soluble CD14 and bacterial lipopolysaccharide. J Immunol. 1997;158:3457–3462. [PubMed] [Google Scholar]
- 51.Von Asmuth E J U, Dentener M A, Bažil V, Bouma M G, Leeuwenberg J F M. Anti-CD14 antibodies reduce responses of cultured human endothelial cells to endotoxin. Immunology. 1993;80:78–83. [PMC free article] [PubMed] [Google Scholar]
- 52.Wright S D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991;3:83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]
- 53.Wright S D, Ramos R A, Hermanowski-Vosatka A, Rockwell P, Detmers P A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J. CD14, a receptor for complexes of LPS and LPS/binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 55.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang H, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;17:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler-Heitbrock H W, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 57.Zweibaum A, Pinto M, Chevalier G, Dussaulx E, Triadou N, Lacroix B, Haffen K, Brun J-L, Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985;122:21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]