Abstract
Bacteroides forsythus, which has been reported to be associated with periodontitis but has not been recognized as a key pathogen, was found to induce cytolytic activity against HL-60 and other human leukemic cells. This cytolytic activity was demonstrated according to three different criteria: (i) loss of both mitochondrial membrane potential and membrane integrity in cells treated with bacterial extracts and then with Rh123 and propidium iodide, respectively, as demonstrated by flow cytometry; (ii) damage to cytoplasmic membrane, as revealed by scanning electron microscopy (SEM); and (iii) DNA ladder formation and activation of caspase-3. These results indicate that B. forsythus produced an apoptosis-inducing factor(s) found to be composed of protein as judged by heat and trypsin sensitivity. In addition to extracts from B. forsythus, the culture supernatant of this bacterium has the ability to induce a cytolytic effect against peripheral white blood cells, especially lymphocytes. For comparison with B. forsythus, the same analyses were applied to two strains with different serotypes of Actinobacillus actinomycetemcomitans, serotypes a (ATCC 43717) and c (ATCC 43719), in addition to previously reported apoptosis-inducing serotype b (ATCC 43718), which was used as a positive control. The strains of A. actinomycetemcomitans serotypes a and b induced apoptosis in HL-60 cells as judged by the above three criteria but to a slightly lesser extent than did B. forsythus, while the serotype c strain produced apoptosis to a negligible extent. Detailed SEM images showed that the A. actinomycetemcomitans serotype a strain induced large-pore formation and the serotype b strain produced small pores with typical blebbing, while B. forsythus induced severe membrane ruffling. Further DNA ladder formation and caspase-3 activation were observed in the serotype a and b strains but not in the serotype c strain. The present paper is the first report of a protein factor(s) from B. forsythus and the A. actinomycetemcomitans serotype a strain which induces apoptotic cell death.
Periodontitis, one of the most common human infectious diseases, is an acute or chronic infectious condition that can result in the inflammatory destruction of periodontal tissues such as periodontal ligaments and alveolar bone. Furthermore, recent studies have indicated that periodontitis might contribute to serious systemic diseases, such as cardiovascular diseases (18). Among more than 300 species of bacteria in the oral cavity, Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans are the major periodontopathic bacteria, and several virulence factors related to the pathogenesis of periodontitis have been reported (reviewed in references 3a, 8, and 26). In addition to these two bacteria, Bacteroides forsythus, a gram-negative, anaerobic, and fusiform bacterium (24), has recently been recognized as one of the agents associated with periodontitis. It has been reported that the presence of B. forsythus in subgingival flora was significantly associated with the severity of periodontitis, such as attachment loss and alveolar bone loss (5, 6). However, only a trypsin-like protease and a sialidase produced by this bacterium have been characterized to a limited extent as putative virulence factors (15, 24). In periodontitis, virulence factors could eliminate host immune cells through the induction of apoptosis or necrosis and facilitate bacterial colonization in periodontal tissues. Therefore, identification of certain virulence factors of B. forsythus and other periodontopathic bacteria would aid in the development of preventive strategies against periodontal diseases, especially severe periodontitis.
In this paper, we report the apoptosis-inducing activities of B. forsythus and two strains of A. actinomycetemcomitans (serotypes a and b).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. forsythus (ATCC 43037; American Type Culture Collection, Manassas, Va.) was grown in heart infusion broth (Difco Laboratories, Detroit, Mich.) containing hemin (5 mg/liter), menadione (1 mg/liter), l-cysteine (1%), and N-acetylneuraminic acid (15 mg/liter) under anaerobic conditions. A. actinomycetemcomitans ATCC 43717 (serotype a), ATCC 43718 (Y4, serotype b), and ATCC 43719 (serotype c) were cultured in Todd-Hewitt broth (Difco Laboratories) supplemented with 1% yeast extract at 37°C in an atmosphere of 5% CO2 in air. Escherichia coli containing plasmid pBluescript II SK+ (Stratagene, La Jolla, Calif.), used as a negative control, was grown in Luria-Bertani broth (Difco Laboratories) containing ampicillin (100 μg/ml).
Cells and culture conditions.
The human leukemia cell lines HL-60 (acute promyelocytic leukemia, precursor of monocytes, macrophages, and granulocytes) (3), Jurkat (acute T-cell leukemia) (19), and BL2 (Burkitt's lymphoma; B cell) (2) were maintained in RPMI 1640 medium (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum. Cultures were maintained at 37°C in a humidified 5% CO2 atmosphere and subcultured twice a week. Peripheral white blood cells (PWBC) from a healthy adult subject were maintained under the same conditions as the leukemic cell lines without passage.
Preparation of sonic extracts and culture supernatants.
The growing bacteria were collected by centrifugation, washed twice with cold phosphate-buffered saline (PBS), and sonicated for 5 min at 100 W with an Isonator model 200M sonicator (Kubota, Tokyo, Japan) with cooling with running water. The sonic extracts were centrifuged at 8,000 × g for 10 min, filtered (0.20-μm pore size; Sartorius, Göttingen, Germany), aliquoted, and stored at −80°C. In some experiments, sonic extracts were heated at 60°C for 1 h or preincubated with 0.1% trypsin at 37°C for 30 min. Culture supernatants used for analysis of cell death by flow cytometry were filtered before use.
Analysis of cell death by flow cytometry.
Target cells (HL-60) at a concentration of 2 × 105 per well (Jurkat, 8 × 104 per well; BL2, 3.2 × 105 per well) in 24-well plates were treated with bacterial extracts (50 μg of protein/ml) (except where indicated otherwise), adriamycin (10 μM), or PBS for 24 to 48 h at 37°C. Furthermore, PWBC (3 × 105 per well) were treated with culture supernatants (20, 500, and 1,000 μl/ml) for 48 h at 37°C. Uncultured medium was used as a negative control. The cells were harvested, Rh123 (final concentration, 10 μM; Wako, Tokyo, Japan) was added 15 min prior to the indicated times, and cells were washed twice with cold PBS, followed by the addition of 10 μM propidium iodide (PI; Wako) 10 min before analysis. The extent of cell death was evaluated by measuring the fluorescence intensity of Rh123 and PI using a FACScalibur flow cytometer (Becton-Dickinson Immunocytochemistry Systems, San Jose, Calif.) (20). All analyses were performed in duplicate experiments.
Scanning electron microscopy (SEM).
HL-60 cells (2.5 × 105) were incubated with or without bacterial sonic extracts for 48 h at 37°C, washed with PBS, and then pelleted by low-speed centrifugation. The pelleted cells were prefixed with 2.5% glutaraldehyde in PBS for 2 h, rinsed with PBS, and postfixed with 1% osmium tetroxide in PBS for 2 h. The samples were dehydrated in a series of ethanol rinses, followed by critical-point drying using an HCP-2 apparatus (Hitachi, Tokyo, Japan) employing CO2 as the transitional fluid. The specimens mounted on stubs were coated with platinum, examined with a scanning electron microscope (S-4500; Hitachi), and photographed.
Analysis of DNA fragmentation by agarose gel electrophoresis.
HL-60 cells (5 × 105) were treated with bacterial extracts (1 μg/ml) or PBS for 48 h at 37°C. Two micrograms of cellular DNA isolated using the Hirt method (7) was subjected to agarose gel electrophoresis and then stained with ethidium bromide.
Detection of caspase activation.
Ten micrograms of cellular lysate prepared from HL-60 cells (5 × 105) which had been treated with each bacterial extract at 1 μg/ml was subjected to Western blot analysis by using anti-procaspase-3 antibody (clone 19; Transduction Laboratory, Lexington, Ky.). The anti-tubulin antibody (clone YL 1/2; Biosys, S.A.) was used to monitor the amount loaded in each lane.
RESULTS
Apoptotic cell death of HL-60 cells with extracts from B. forsythus and three serotypes of A. actinomycetemcomitans.
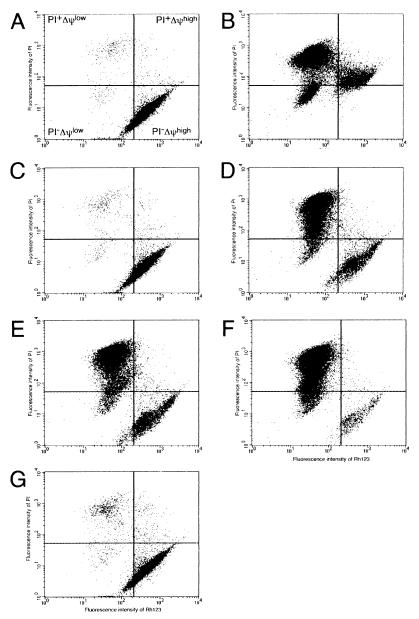
To examine apoptotic cell death induced by bacterial extracts, we used a leukemic cell line, HL-60, since this line is a precursor of and is differentiated in vitro into monocytes and macrophages (3), which could play essential roles in the host defense against bacterial infection and also have been used for apoptosis assay (12). Cells undergoing apoptosis display subtle molecular and biochemical alterations, including perturbations in the structure of their plasma membrane and the dissipation of mitochondrial transmembrane potentials (ΔΨ) (13). Decreased mitochondrial ΔΨ is one of the primary signals of chemical hypoxia-induced apoptotic cell death. Flow cytometry following Rh123 and PI staining was used to elucidate the cytotoxic effects of bacteria on human cells. The population of PI+ΔΨlow and PI−ΔΨlow cells accumulated to almost 90% after treatment with 10 μM adriamycin, a DNA topoisomerase II inhibitor used as a positive control for apoptosis induction (4) (Fig. 1B). The PI−ΔΨlow and PI+ΔΨlow subsets represent the preapoptotic and apoptotic cell populations, respectively. The extracts from B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b induced the transition of more than 80% of HL-60 cells from the PI−ΔΨhigh subset to the PI−ΔΨlow or PI+ΔΨlow subset (Fig. 1D, E, and F). Based on these results, most of the HL-60 cells exposed to these bacterial extracts underwent apoptotic cell death. In contrast, more than 90% of the cells exposed to A. actinomycetemcomitans serotype c extract remained in PI−ΔΨhigh (Fig. 1F), as was the case for cells treated with PBS or E. coli 421C extract (used as a negative control) (Fig. 1A and C). Similar results were obtained with two other leukemic cell lines (Jurkat and BL2) (data not shown).
FIG. 1.
Flow cytometric analysis of HL-60 cells incubated with or without 50 μg of protein of the extracts from various bacteria per ml. Panels: A, PBS; B, adriamycin (10 μM); C, E. coli; D, B. forsythus; E, A. actinomycetemcomitans serotype a; F, A. actinomycetemcomitans serotype b; G, A. actinomycetemcomitans serotype c. The extent of cell death was assessed by measuring fluorescence intensity using a FACScalibur flow cytometer after staining with PI and Rh123. All analyses were performed in duplicate experiments.
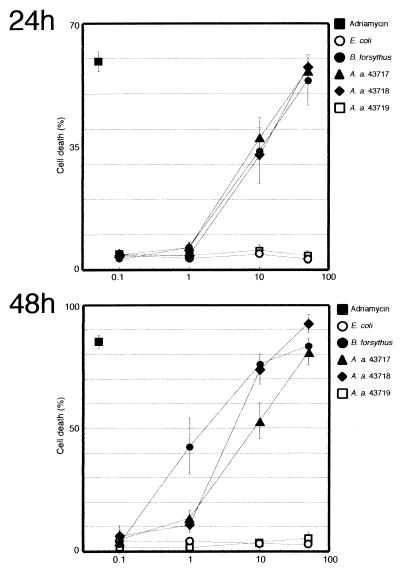
To determine doses sufficient to induce apoptotic cell death, HL-60 cells were incubated with various concentrations of bacterial extracts, ranging from 0.1 to 50 μg of protein/ml for 24 or 48 h, and assayed for apoptotic cell death. At 24 h, fractions of dead cells were proportional to protein doses at least up to 50 μg/ml, as evidenced by the dose-dependent increases in the numbers of cells in both the PI+ΔΨlow and PI−ΔΨlow subsets for B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b relative to cells exposed to PBS (Fig. 2A). All three strains showed similar kinetics regarding cell death activities, while incubation with the same concentrations of A. actinomycetemcomitans serotype c or E. coli did not result in cell death. At 48 h of incubation at 1 μg/ml, the cell death-inducing activity of the extract from B. forsythus was almost three times as high as that of A. actinomycetemcomitans serotype a or A. actinomycetemcomitans serotype b, suggesting that B. forsythus had the highest activity among the three strains that showed apoptotic activity (Fig. 2B).
FIG. 2.
Dose-dependent induction of apoptotic cell death by B. forsythus and A. actinomycetemcomitans strains. HL-60 cells were incubated in the presence of various concentrations of extracts, ranging from 0.1 to 50 μg of protein/ml for 24 or 48 h. All analyses were performed in duplicate experiments. The results shown are means and standard deviations.
Apoptotic cell death of normal PWBC with culture supernatants of B. forsythus.
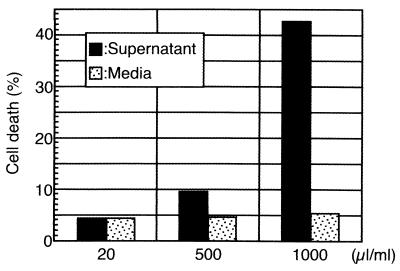
In order to examine the actual role of this cell lytic activity against the host defense mechanisms, normal PWBC were also used as target cells. The extent of the cell death of lymphocytes gated from PWBC was evaluated by using flow cytometry. As shown in Fig. 3, the total populations of PI+ΔΨlow, PI−ΔΨlow, and PI+ΔΨhigh cells among lymphocytes treated with 500 and 1,000 μl of culture supernatants were approximately two and eight times as high as that of the control, respectively. The results suggested that both bacterial extract and culture supernatants of B. forsythus had the ability to induce apoptotic cell death in normal lymphocytes in addition to leukemic cells.
FIG. 3.
Flow cytometric analysis of PWBC incubated with B. forsythus culture supernatant (20, 500, and 1,000 μl/ml) or corresponding volumes of uncultured medium. The extent of cell death caused by lymphocytes gated from PWBC was assessed by the method described in the legend to Fig. 1.
Ultrastructural cell surface changes in HL-60 cells treated with bacterial extracts.
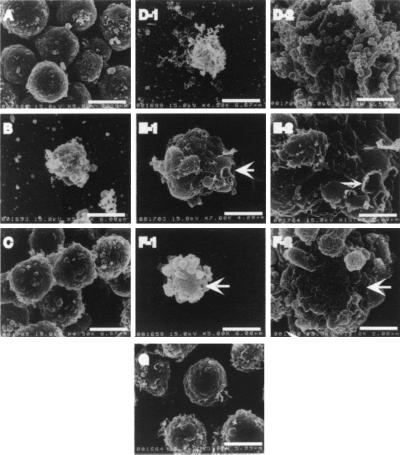
Mechanisms of cell death were further studied by analyzing ultrastructural changes of cell surface by the use of SEM. Morphological changes characteristic of apoptosis, such as apoptotic bodies, membrane blebbing, and overall shrinkage, were observed in cells treated with extracts from B. forsythus and A. actinomycetemcomitans serotype b, as observed in adriamycin-treated cells (Fig. 4D, F, and B, respectively). Severe membrane ruffling was observed in cells treated with B. forsythus but not in those treated with A. actinomycetemcomitans serotype a and A. actinomycetemcomitans serotype b. No swollen cells characteristic of necrosis were observed.
FIG. 4.
Ultrastructural changes of HL-60 cells incubated with or without 50 μg of protein of bacterial sonic extracts per ml. Panels: A, B, and C, control HL-60 cells (treated with PBS, 10 μM adriamycin, and E. coli extract, respectively); D, E, and F, cells incubated for 48 h in the presence of the extracts from B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b, respectively; D-2, E-2, and F-2, photographs of corresponding samples at a higher magnification; G, cells incubated in the presence of the extract from A. actinomycetemcomitans serotype c, exhibiting cell surfaces similar to those of untreated HL-60 cells. Arrows indicate pores on cell membranes. Bar lengths and magnifications: A, 5.96 μm, ×5,000; B, 6.00 μm, ×4,500; C, 6.67 μm, ×5,000; D-1, 6.67 μm, ×4,500; D-2, 1.50 μm, ×20,000; E-1, 4.29 μm, ×7,000; E-2, 2.00 μm, ×15,000; F-1, 6.00 μm, ×5,000; F-2, 2.00 μm, ×15,000; G, 5.99 μm, ×5,000. Overall shrinkage was observed in cells treated with B. forsythus and A. actinomycetemcomitans serotype a extracts, and the calculated diameters were 5.8 and 5.6 μm, respectively, while that of control cells was approximately 7.5 μm.
Interestingly, large-pore-forming activity on the cell surface without the typical membrane blebbing was found with A. actinomycetemcomitans serotype a extract, while A. actinomycetemcomitans serotype b extract induced small pores with the typical blebbing on the cell membrane (Fig. 4E and F).
The overall extent of destruction of the cells was strongest with the extract from B. forsythus, followed by those from A. actinomycetemcomitans serotype a and A. actinomycetemcomitans serotype b. No significant ultrastructural change was observed when cells were treated with extracts from A. actinomycetemcomitans serotype c and E. coli or PBS. (Fig. 4G, C, and A, respectively). These results may suggest that B. forsythus, A. actinomycetemcomitans serotype a and A. actinomycetemcomitans serotype b induced different effects in HL-60 cells. Differences in the intracellular staining patterns obtained with Annexin-V and PI were also revealed by fluorescence microscopy, which was consistent with the results of flow cytometry and SEM (data not shown).
DNA fragmentation and caspase-3 activation.
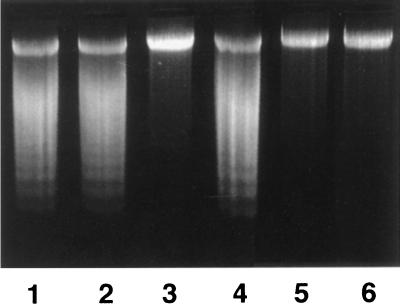
One hallmark of apoptotic cells is nuclear DNA fragmentation induced by specific endonucleases. Fragmented cellular DNA enriched by the Hirt extraction method (7) was subjected to agarose gel electrophoresis. Chromosomal DNAs were isolated and analyzed by agarose gel electrophoresis. As shown in Fig. 5, nucleosomal DNA ladders, characteristic of apoptotic cells, were observed in HL-60 cells treated with extracts of B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b but not in those treated with A. actinomycetemcomitans serotype c.
FIG. 5.
DNA ladder formation of HL-60 cells treated with bacterial extracts. HL-60 cells (5 × 105) were treated with bacterial extracts (1 μg/ml) or PBS for 48 h at 37°C. Two micrograms of cellular DNA isolated using the Hirt method (7) was subjected to agarose gel electrophoresis and then stained with ethidium bromide. Lanes: 1, A. actinomycetemcomitans serotype a; 2, A. actinomycetemcomitans serotype b; 3, A. actinomycetemcomitans serotype c; 4, B. forsythus; 5, E. coli; 6, PBS. Nucleosomal DNA ladders characteristic of apoptotic cells were observed in the cells treated with B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b lysates.
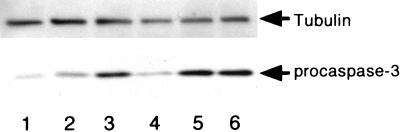
In addition to DNA fragmentation, caspase-3 is generally activated upon execution of apoptosis. Procaspase-3 (32 kDa) disappeared from HL-60 cells treated with the extracts from B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b but not from those treated with extract from A. actinomycetemcomitans serotype c, suggesting that caspase-3 was activated during apoptotic cell death induced by extracts from these bacteria (Fig. 6).
FIG. 6.
Activation of caspase-3 by treatment of HL-60 cells in the presence of periodontopathic bacterial extracts. Ten micrograms of cellular lysate prepared from HL-60 cells (5 × 105 cells) which had been treated with 1 μg of each bacterial extract per ml or with PBS was subjected to Western blot analysis by using anti-procaspase-3 antibody. Lanes: 1, A. actinomycetemcomitans serotype a; 2, A. actinomycetemcomitans serotype b; 3, A. actinomycetemcomitans serotype c; 4, B. forsythus; 5, E. coli; 6, PBS). Decreases in procaspase-3 levels represent the activation of caspase-3. The filter was also probed with anti-tubulin antibody to monitor the amount of loading in each lane.
These results confirmed biochemically that the sonic extracts of B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b induced apoptotic cell death in HL-60 cells, while that of A. actinomycetemcomitans serotype c did not.
Apoptosis-inducing factors are heat-labile proteins.
To study the nature of cell death-inducing factors, bacterial extracts were pretreated with heat or trypsin and then incubated in the presence of HL-60 cells. Heating of extracts at 60°C for 1 h or treatment with 0.1% trypsin at 37°C for 30 min diminished most of the death-inducing activities (data not shown), suggesting that these apoptotic cell death-inducing factors of B. forsythus, A. actinomycetemcomitans serotype a, and A. actinomycetemcomitans serotype b were most likely to be heat-labile factors composed of proteins.
DISCUSSION
In the present study, we demonstrated that periodontopathic bacteria, B. forsythus and two different-serotype strains of A. actinomycetemcomitans, produce factors composed of protein which induce cell death. This cell death is due to apoptosis, since each of the bacterial extracts induced (i) DNA ladder formation and caspase-3 activation and (ii) loss of both mitochondrial membrane potential and membrane integrity. Further, SEM analysis also indicated that cell death is induced by apoptosis, as cells treated with bacterial extracts showed shrinkage, which is one of the typical feature of apoptosis, but not by swelling. Among the three bacteria, B. forsythus extract, unlike the A. actinomycetemcomitans serotype a and A. actinomycetemcomitans serotype b strains, displayed the most severe membrane ruffling, extensive shrinkage, and cell membrane blebbing. In addition, the A. actinomycetemcomitans serotype a strain induced large pores on the cytoplasmic membrane but the A. actinomycetemcomitans serotype b strain induced small pores. Taken together, the cell death-inducing factors of these three bacteria could represent different apoptotic effects on leukemic cells.
Several kinds of apoptosis-inducing bacterial factors, such as hemolysin, leukotoxin, Shiga toxins, and verotoxins, etc., have been described (1, 9, 11, 16, 25). As for serotype b of A. actinomycetemcomitans, three cytotoxic factors, leukotoxin (12), cytolethal distending toxin (CDT) (20), and a toxin which induces both cell cycle arrest and apoptosis (17), have been reported. It has been reported by Korostoff et al. that leukotoxin induced apoptosis in HL-60 cells, which was consistent with the present results of SEM analyses (12). Sugai et al. reported that the mean size of CDT-treated HeLa cells became 10- to 18-fold larger than that of control cells (22); however, we observed shrinkage rather than expansion, suggesting that the present factor of the A. actinomycetemcomitans serotype b strain is different from CDT. Furthermore, it has been reported that both cell cycle arrest and apoptosis were induced by partially purified A. actinomycetemcomitans serotype b toxin in mouse hybridoma cell line HS-72 cells (17). It is likely that A. actinomycetemcomitans serotype b produces several types of toxins which induce different cytotoxic effects against mammalian cells; the relationship between these factors should be elucidated by purifying these cell death-inducing factors.
In contrast to the A. actinomycetemcomitans serotype b strain, cell death-inducing activity of B. forsythus or A. actinomycetemcomitans serotype a extract against leukemic cells has not been reported. Treatment with the extract from the A. actinomycetemcomitans serotype a strain induced pore formation on the cell membrane, nucleosomal DNA ladder formation, and caspase-3 activation. Many gram-negative bacteria have been reported to synthesize cytolytic toxins with similar activities (14). It may be assumed that killing through the formation of a stable pore on the target cell membrane results mostly in cell death with the characteristics of necrosis. However, recent evidence has indicated that other pore-forming toxins are able to induce morphologic and biochemical alterations that are consistent with apoptosis in susceptible target cells (10, 12, 21).
B. forsythus has recently been recognized as one of the agents associated with periodontitis (5, 6) in which we found apoptosis-inducing activity. During the course of infection, B. forsythus could invade periodontal tissue in combination with P. gingivalis (23) and might be attacked by PWBC of the host. In fact, we found the cell toxic activity in B. forsythus culture supernatant, as well as bacterial extract. One of the possible functions of this activity is the elimination of host immune or preimmune cells through the induction of cell death, which facilitates bacterial colonization of the oral cavity, especially in the subgingival area. Whether this activity is responsible for the initiation and progression of periodontitis has yet to be clarified biochemically and biologically. Since a single strain of standard B. forsythus was used in this study, multiple strains, including clinically isolated strains, should be analyzed to elucidate the pathogenesis of this bacterium.
ACKNOWLEDGMENTS
The Jurkat and BL2 cell lines were kindly provided by Y. Koyanagi (Department of Microbiology, Faculty of Medicine, Tokyo Medical and Dental University) and T. Ono (School of Medicine, Nihon University), respectively.
REFERENCES
- 1.Arab S, Murakami M, Dirks P, Boyd B, Hubbard S L, Lingwood C A, Rutka J T. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J Neurooncol. 1998;40:137–150. doi: 10.1023/a:1006010019064. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand S, Berger R, Philip T, Bernheim A, Bryon P A, Bertoglio J, Dore J F, Brunat-Mentigny M, Lenoir G M. Variant translocation in a non endemic case of Burkitt's lymphoma: t(8;22) in an Epstein-Barr virus negative tumor and in a derived cell line. Eur J Cancer. 1981;17:577–584. doi: 10.1016/0014-2964(81)90060-8. [DOI] [PubMed] [Google Scholar]
- 3.Collins S J, Gallo R C, Gallagher R E. Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature. 1977;270:347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- 3a.Fives-Taylor P M, Meyer D H, Mintz K P, Brisette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;29:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 4.Gewirtz D A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 5.Grossi S G, Zambon J J, Ho A W, Koch G, Dunford R G, Machtei E E, Norderyd O M, Genco R J. Assessment of risk for periodontal disease. I. Risk indicators for alveolar bone loss. J Periodontol. 1994;65:260–267. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 6.Grossi S G, Genco R J, Machtei E E, Ho A W, Koch G, Dunford R G, Zambon J J, Hausmann E. Assesment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol. 1995;66:23–29. doi: 10.1902/jop.1995.66.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 8.Holt S C, Kesavalu L, Walker S, Genco C A. Virulence factors of Porphyromonas gingivalis. Periodontol 2000. 1999;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson M L, Poxton I R, Govan J R. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect Immun. 1998;66:2033–2039. doi: 10.1128/iai.66.5.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiyokawa N, Taguchi T, Mori T, Uchida H, Sato N, Takeda T, Fujimoto J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J Infect Dis. 1998;178:178–184. doi: 10.1086/515592. [DOI] [PubMed] [Google Scholar]
- 12.Korostoff J, Wang J F, Kieba I, Miller M, Shenker B J, Lally E T. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect Immun. 1998;66:4474–4483. doi: 10.1128/iai.66.9.4474-4483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunology Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig A. Cytolytic toxins from gram-negative bacteria. Microbiologia. 1996;12:281–296. [PubMed] [Google Scholar]
- 15.Moncla B J, Braham P, Rabe L K, Hillier S L. Rapid presumptive identification of black-pigmented gram-negative anaerobic bacteria by using 4-methylumbelliferone derivatives. J Clin Microbiol. 1991;29:1955–1958. doi: 10.1128/jcm.29.9.1955-1958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris S J, Price G E, Barnett J M, Hiscox S A, Smith H, Sweet C. Role of neuraminidase in influenza virus-induced apoptosis. J Gen Virol. 1999;80:137–146. doi: 10.1099/0022-1317-80-1-137. [DOI] [PubMed] [Google Scholar]
- 17.Ohguchi M, Ishisaki A, Okabatashi N, Koide M, Koseki T, Yamato K, Noguchi T, Nishihara T. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in the G2/M phase and apoptosis. Infect Immun. 1998;66:5980–5987. doi: 10.1128/iai.66.12.5980-5987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scannapieco F A. Position paper of the American Academy of Periodontology: periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69:841–850. [PubMed] [Google Scholar]
- 19.Schuneider U, Schwenk H U, Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977;19:521–526. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Bcl-2 blocks loss of mitochondrial membrane potential while ICE inhibitors act at a different step during inhibition of death induced by respiratory chain inhibitors. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 21.Stevens P K, Czuprynski C J. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consistent with apoptosis in vitro. Infect Immun. 1996;64:2687–2694. doi: 10.1128/iai.64.7.2687-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugai M, Kawamoto T, Pérès S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takemoto T, Kurihara H, Dahlen G. Characterization of Bacteroides forsythus isolates. J Clin Microbiol. 1997;35:1378–1381. doi: 10.1128/jcm.35.6.1378-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner A C, Strzempko M N, Belsky C A, McKinley G A. API ZYM and API An-Ident reactions of fastidious oral gram-negative species. J Clin Microbiol. 1985;22:333–335. doi: 10.1128/jcm.22.3.333-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J F, Kieba I R, Korostoff J, Guo T L, Yamaguchi N, Rozmiarek H, Billings P C, Shenker B J, Lally E T. Molecular and biochemical mechanisms of Pasteurella haemolytica leukotoxin-induced cell death. Microb Pathog. 1998;25:317–331. doi: 10.1006/mpat.1998.0236. [DOI] [PubMed] [Google Scholar]
- 26.Weinrauch Y, Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]