Abstract
This study explored the application of transperineal ultrasound (TPUS) combined with shear wave elastography (SWE) in evaluating the pelvic structure function of women after total hysterectomy. Seventy healthy women and 76 women who underwent total hysterectomy were selected for ultrasound examination. They were divided into normal (nulliparous) group, (parous) group without hysterectomy, and (parous) group with hysterectomy. TPUS combined with SWE was used to evaluate the pelvic floor structure and function in the 3 groups of women. Posterior urethrovesical angle in resting and maximal Valsalva state, anteroposterior diameter of hiatus in the 3 states, the bladder neck descent, the urethral rotation angle, the Young modulus of left and right puborectalisis muscle in resting state, and the incidence of pelvic floor dysfunction diseases were all higher in the group with hysterectomy than in the group without hysterectomy (P < .05). Bladder neck-symphyseal distance and anorectal junction-symphyseal distance in the maximum Valsalva state, and the difference in Young modulus between the left and right PR before and after anus contraction were all lower in the group with hysterectomy than the group without hysterectomy (P < .05). The incidence of pelvic floor dysfunction in postmenopausal patients in the group with hysterectomy was higher than that in premenopausal patients (P < .05). Total hysterectomy had negative effects on female pelvic floor structure and function. TPUS combined with SWE can be used to evaluate pelvic floor function in multiple dimensions.
Keywords: pelvic floor dysfunction (PFD), puborectalisis muscle, shear wave elastography (SWE), total hysterectomy, transperineal ultrasound (TPUS)
1. Introduction
While total hysterectomy can effectively treat a variety of benign and malignant diseases of the uterus, complications inevitably occur. Studies have pointed out that total hysterectomy is one of the independent influencing factors of pelvic floor dysfunction (PFD).[1] PFD mainly includes stress urinary incontinence (SUI), pelvic organ prolapse (POP), fecal incontinence, sexual dysfunction, etc. It is not only a physical disease, but also causes many social and psychological problems. It has many negative effects and disturbs physical and mental health. In recent years, total hysterectomy has been widely used, and statistics show that 15 to 20% of women in China have their uterus removed for various reasons.[2] With people’s requirements for the quality of life, PFD caused by hysterectomy has gradually become a key issue in the clinical practice.
There are many studies on the changes of pelvic floor function after hysterectomy, but the in-depth imaging study is still insufficient. Pelvic floor is divided into 3 interacting compartments (anterior, middle, and posterior), and thus imaging is needed to examine the anatomy and functionality of this region. Recently, various imaging techniques have been developed to evaluate the structure and function of the pelvic floor. Traditional imaging techniques for pelvic floor include X-ray and magnetic resonance. Recently, transperineal ultrasound (TPUS) is developed for noninvasive, objective, and dynamic assessment of the pelvic floor function. The probe placed on the perineum close to the symphysis pubis (SP). Images can be acquired at rest, during contraction, and throughout the Valsalva maneuvers. TPUS allows a dynamic evaluation of pelvic floor. Shear wave elasticity imaging (SWE) is a new ultrasonic technology, which can provide information about the elastic characteristics of tissues.[3] We hope that the change of pelvic floor muscle elasticity can reflect the supporting function of pelvic floor muscle. This study intends to evaluate the pelvic floor structure and function of women after total hysterectomy by TPUS combined with SWE, to provide a multi-dimensional diagnosis for PFD after total hysterectomy and the anatomical basis for clinical selection of treatments.
2. Materials and methods
2.1. Research subjects
Seventy healthy women who underwent ultrasound examination in Affiliated Nantong Hospital 3 of Nantong University from September 2019 to September 2021 were selected, including 30 normal nulliparous cases and 40 parous cases without hysterectomy. In the same period, 76 women after total hysterectomy were examined by ultrasonography in the hospital, with a total of 146 cases. They were divided into 3 groups: normal nulliparous group (group I), parous group without hysterectomy (group II) and parous group with hysterectomy (group III). Group III was further divided into IIIA and IIIB subgroups according to whether menopause had occurred before operation. The number of women with childless hysterectomy was too small to be included in the study.
The age, body mass index (BMI), history of pregnancy and childbirth, causes of operation, history of menopause, dystocia, chronic cough or constipation, and other clinical data were recorded.
Exclusion criteria: those with BMI >28 or >80 years old; patients with cough, constipation and other diseases leading to increased abdominal pressure and serious cardiopulmonary insufficiency; patients with a history of radiotherapy and chemotherapy; patients with severe pelvic adhesion and pelvic floor rehabilitation experience; patients unable to complete the Valsalva maneuver correctly and with poor image quality; patients suffering from diabetes, and long-term smokers; patients in group II and III <10 years after delivery, <1 year after hysterectomy; ≥3 pregnancies; history of twin or multiple births, or macrosomia.
This study was reviewed and approved by the ethics committee of the hospital (EL20210007), and all subjects signed the informed consent form.
2.2. Instruments and methods
2.2.1. Instruments.
PHILIPS (iu-elite) ultrasonic diagnostic instrument (Philips, Eindhoven, Netherlands) with ElastPQ elastic imaging function was used, the probe model was C5-1 convex array probe, and the frequency was 1 to 5 MHz.
2.2.2. Two-dimensional pelvic floor ultrasonography.
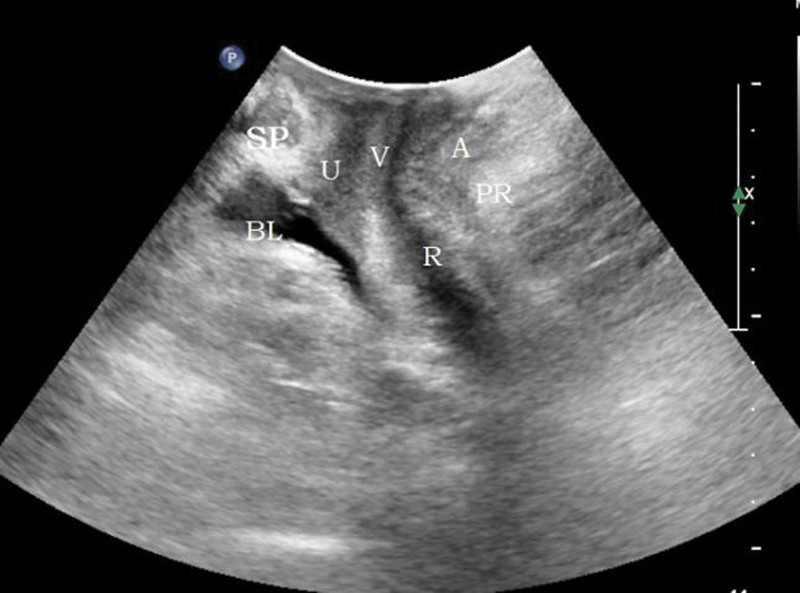
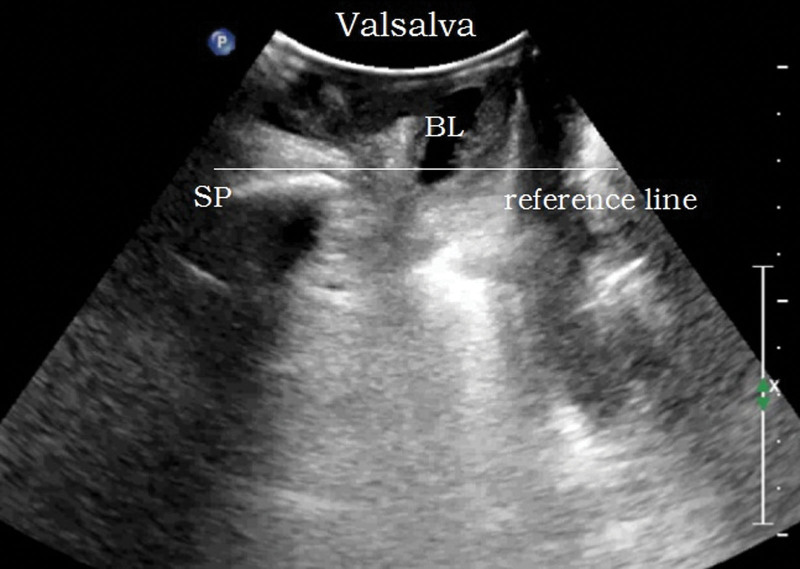
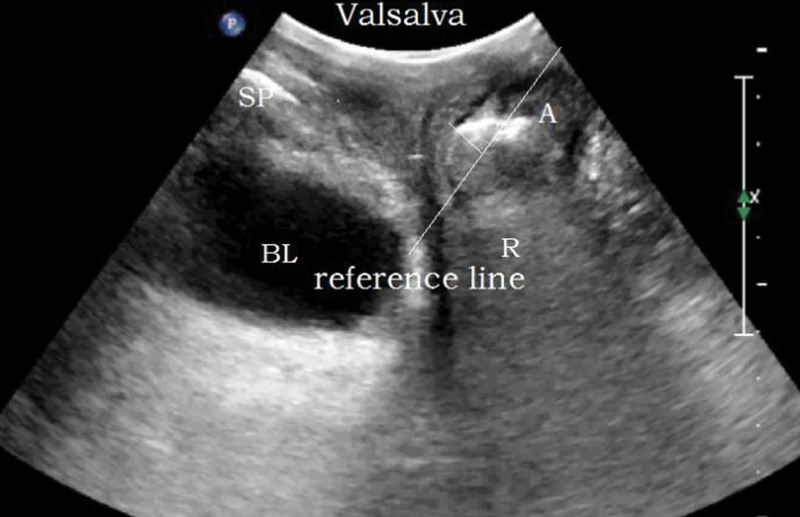
Methods: the bladder was emptied before examination (the residual urine volume should not exceed 50 mL). The lithotomy position was adopted. The probe was coated with coupling agent and covered with disposable condom to completely empty the air under the probe. The probe was vertically placed in the center of perineum to obtain a median sagittal plane. The pubic symphysis, urethra, bladder, vagina, anorectal junction, and the central part of the dorsal levator plate were displayed from ventral to dorsal side, as shown in Figure 1. The movement changes of pelvic floor organs and tissues at the resting (R) state, maximum Valsalva (V), and anus contraction (C) were recorded, and the lowest point was measured for 3 times.
Reference line: a horizontal line made through the posterior lower edge of SP.
Phase and action: rest, maximum Valsalva and anus contraction were indicated by the letters R, V and C, respectively. The standard Valsalva maneuver is to hold your breath after deep inhalation, increase abdominal pressure and move the pelvic organs to the caudal side for 6 seconds. The standard C action is to contract the anus and move the pelvic organs towards the head side for 3 seconds.
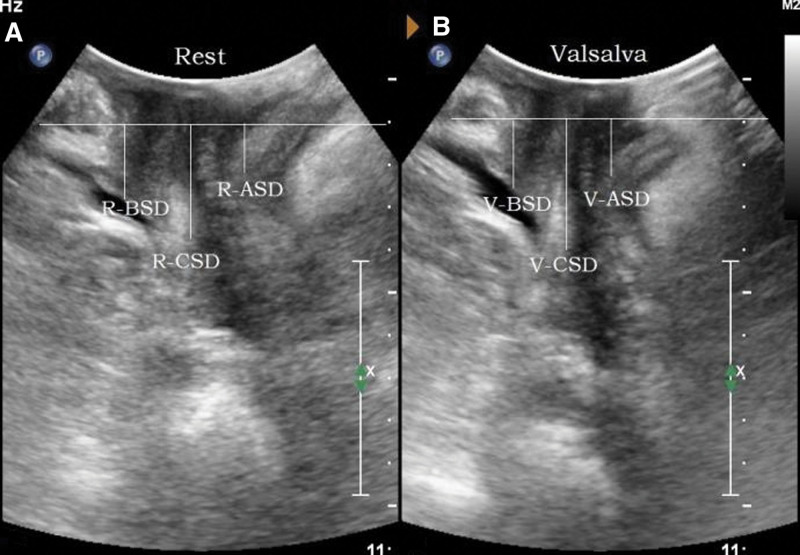
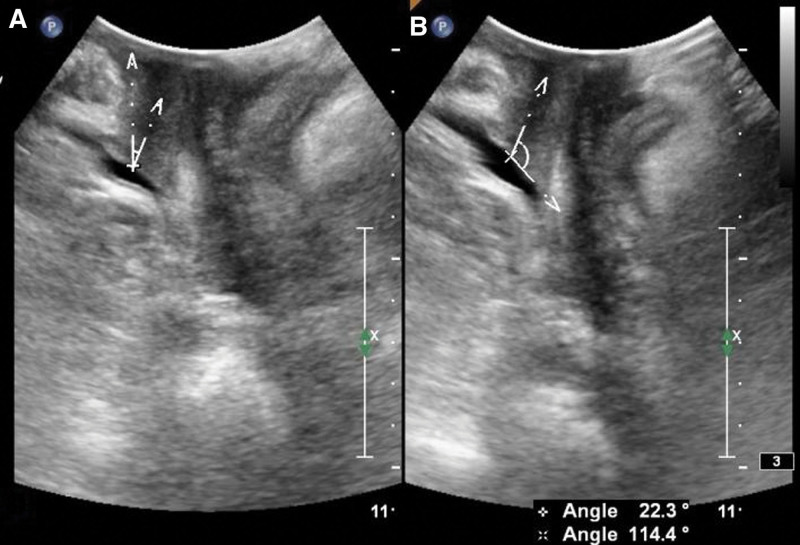
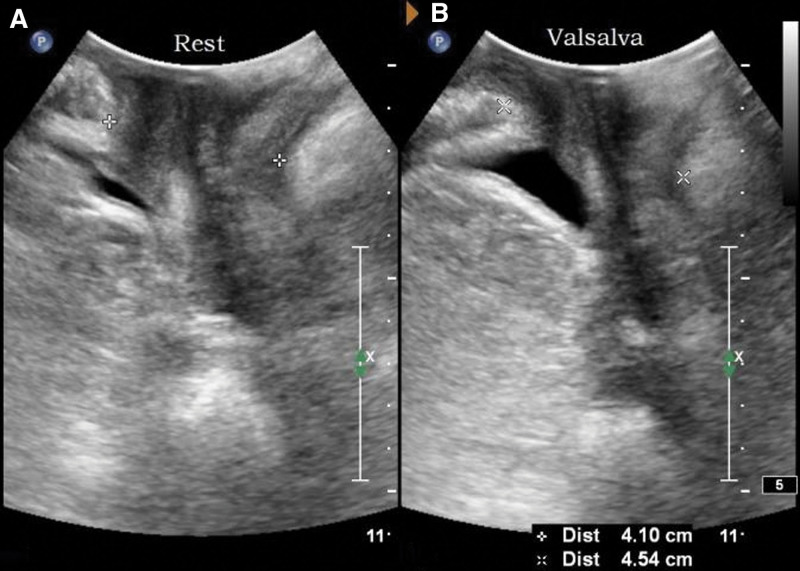
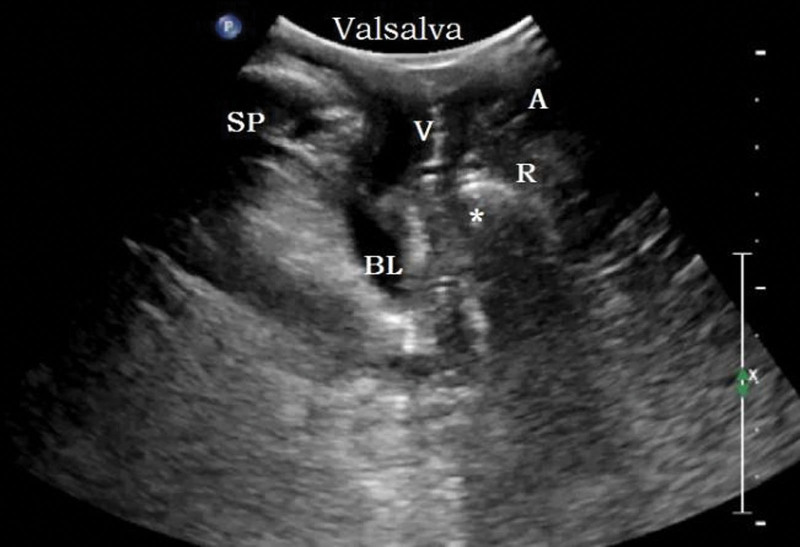
Parameters: the distance between the bladder neck, the external cervixorifice, and the anorectal junction from the reference line (positive values above the line and negative values below the line) are the bladder neck-symphyseal distance (BSD), cervix-symphyseal distance, and anorectal junction-symphyseal distance (ASD), respectively, as shown in Figure 2. The angles between the proximal end of the urethra and the posterior wall of the bladder and the central axis of the human body are the posterior urethrovesical angle (PUA) and the urethral obliquity angle, respectively. Positive values were to the ventral side and negative values were to the dorsal side, as shown in Figure 3. The anteroposterior diameter of the levator hiatus is the distance between the medial edge of the SP and the medial edge of the levator plate, as shown in Figure 4. Bladder neck descent (BND) is the difference between resting and V-BSD; urethral rotation angle (UA) is the difference between resting and V-UOA.
Figure 1.
Two-dimensional median sagittal plane of pelvic floor through perineum. BL = bladder, SP = symphysis pubis, U = urethra, V = vagina, R = rectum, A = anal canal, PR = puborectalis muscle.
Figure 2.
Schematic diagram of pelvic floor function parameters at rest and maximum Valsalva state. (A) is the resting state, (B) is the maximum Valsalva state. ASD = anorectal junction-symphyseal distance, BSD = the distance from the bladder neck to the reference line, CSD = the distance from the external cervix to the reference line.
Figure 3.
Schematic diagram of the urethral obliquity angle (UOA) and the posterior urethrovesical angle (PUA). (A) is a schematic diagram of the urethral obliquity angle (UOA), and (B) is a schematic diagram of the posterior urethrovesical angle (PUA).
Figure 4.
Schematic diagram of the anteroposterior diameter of the levator hiatus. (A) is the resting state and (B) is the maximum Valsalva state.
2.2.3. SWE of the puborectalisis muscle.
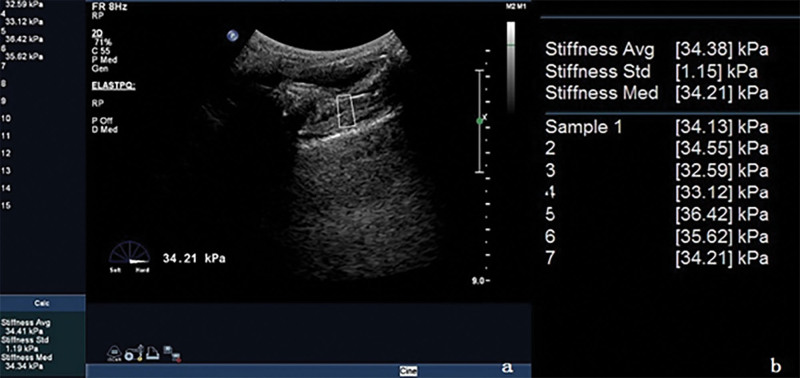
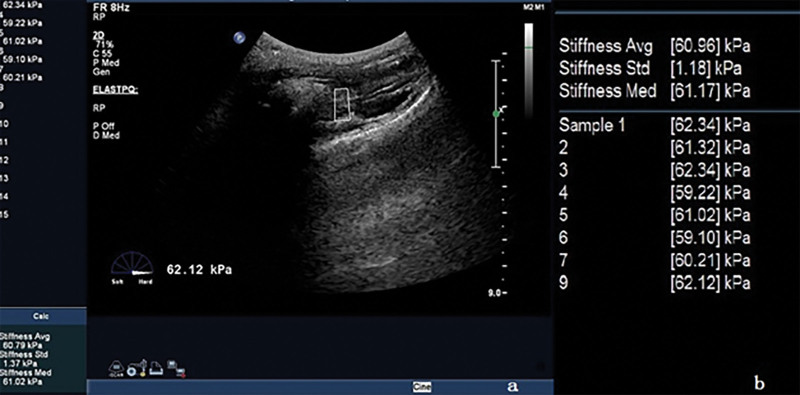
We moved the probe laterally, and the sound beam was obliquely to both sides of the puborectalisis muscle (PR), clearly showing a slightly higher echo of the PR on both sides, we switched to the elastic imaging mode, selected the front of PR in the region of interest,[4] and started the measurement key. The system automatically calculated the average hardness value (Young modulus) of the tissue in the region of interest, measured it for >5 consecutive times, and took the median when the standard deviation is <1.5 kPa. As shown in Figures 5 and 6, the left and right PR Young modulus were measured at rest and anal constriction, respectively. The size and placement of region of interest were the same during measurement.
Figure 5.
Measurement PR elasticity (resting state). (A) is the image of elasticity measurement, and (B) is the reported parameters. PR = puborectalisis muscle.
Figure 6.
Measurement PR elasticity (anus retraction state). (A) is the image of elasticity measurement, and (B) is the reported parameters. PR = puborectalisis muscle.
2.2.4. Diagnostic criteria of PFD.
SUI: according to the diagnostic criteria of the International Association of Urinary Control, when the abdominal pressure increases under coughing, laughing, exercise, etc, urine involuntarily leaking from the urethra is SUI.[5]
Prolapse of pelvic floor organs: according to Professor Dietz’s recommendation, the bladder neck 10 mm below the reference line, the external cervical orifice 15 mm from the reference line, and the anus 15 mm below the reference line are the cutoff values for the diagnosis of pelvic organ tissue prolapse.[6]
Rectocele: in the state of V, the rectal ampulla is pouch-shaped or finger-like protruding into the vagina, and the vertical distance between the highest point of the rectal ampulla and the straight line extending the front contour of the anal sphincter is the height of the bulge. A bulge >10 mm is considered a significant rectocele.[7]
Sexual dysfunction: it is diagnosed according to the latest guidelines for the clinical management of FSD issued by the American College of Obstetricians and Gynecologists in 2019.[8]
Others: intestinal hernia (the small intestine enters between the rectum and vagina), increased bladder neck mobility (BND >25 mm), excessive perineal body movement (rectal ampulla moves down to 15 mm below the reference line, and the internal anal sphincter echoes continuously), etc.
2.3. Statistics
The collected clinical information and physical and chemical examination results were written into Excel tables, and SPSS21.0 software (Armonk, NY: IBM Corp) was used for statistical analysis, and α = 0.05 was used as the test level for comparison between groups. The measurement data was tested for the normality by the Shapiro–Wilk test. Most of the measurement data were in accordance with the normal distribution (P > .05), so the measurement data were described by the mean ± standard deviation (x̅ ± s), and the categorized data were expressed in sums and percentages (%).
Comparison of measurement data between the 2 groups was analyzed by 2 sample independent t tests.
For comparing categorized data between groups, chi-square test or Fisher exact test was used.
Repeated measurements: the variance model for repeated measurement was adopted, and pairwise comparison between groups was performed by least significant difference t method.
Graphs were plotted with Graphpad Prism 7.0 software (GraphPad, Inc., San Diego, CA).
3. Results
3.1. General information
The etiology of total hysterectomy included 51 cases of multiple uterine fibroids, 11 cases of adenomyosis, 7 cases of cervical dysplasia, 3 cases of endometrial polyps, 5 cases of refractory dysfunctional uterine bleeding, and 4 other cases. Five cases were excluded according to the standard. There were 73 normal healthy women, and 3 cases were excluded according to the standard. In the end, there were 146 subjects that met the standard. There were 30 cases in the normal nulliparous group (group I), 40 cases in the parous group without hysterectomy (group II), and 76 cases in the parous group with hysterectomy (group III). For group III, there were 30 cases in group IIIa and 46 cases in group IIIb. The general information of age, BMI, gravidity, and parity are shown in Table 1.
Table 1.
Summary of the general information of the 3 groups of research subjects.
| Index | Normal nulliparous group (n = 30) | Normal parous group (n = 40) | Uterus removed (n = 76) |
|---|---|---|---|
| Age | 24.433 ± 2.687 | 53.500 ± 5.822 | 52.566 ± 5.913 |
| BMI (kg/m2) | 21.565 ± 1.867 | 23.142 ± 1.295 | 22.971 ± 1.788 |
| Parity | 0 | 1.350 ± 0.483 | 1.263 ± 0.443 |
| Gravidity | 0 | 2.125 ± 0.723 | 2.067 ± 0.759 |
BMI = body mass index.
The general data between the group II and group III, including age, BMI, gravidity, and parity, were not statistically significantly different (P > .05), that is, the baseline data of the 2 groups were consistent and comparable. The results are shown in Table 2.
Table 2.
Comparison of the general information between the 2 groups.
| Index | Normal parous group (n = 40) | Uterus removed group (n = 76) | t | P |
|---|---|---|---|---|
| Age | 53.500 ± 5.822 | 52.566 ± 5.913 | 0.813 | .418 |
| BMI (kg/m2) | 23.142 ± 1.295 | 22.971 ± 1.788 | 0.590 | .557 |
| Parity | 1.350 ± 0.483 | 1.263 ± 0.443 | 0.972 | .333 |
| Pregnancy | 2.125 ± 0.723 | 2.067 ± 0.759 | 0.399 | .691 |
BMI = body mass index.
3.2. Functional performance of the pelvic floor structure and PR SWE of normal nulliparous women
In the R state, the pelvic floor organs were on the head side of the reference line. Valsalva maneuvers, the pelvic organs moved towards the foot and dorsal sides, and the BAD, cervix-symphyseal distance and ASD were shortened (P < .01). The proximal urethra rotated posterior and inferior, and the PUA was increased (P < .01). There is no significant change of the urethral obliquity angle (P = .063). The anteroposterior diameter of the levator hiatus was increased (P < .01), reaching the maximum value of Valsalva maneuver. The pelvic floor organs were still on the headside of the reference line. In the R state, the long axis of the PR was slightly hyperechoic, and the hyperechoic muscle membrane and hypoechoic muscle fibers were intertwined inside, and the boundary with the surrounding tissues was clear. It clearly showed the anterior part of the PR (that is, the connection between the PR and the descending pubic branch), as well as the middle and posterior part of the PR (i.e., where PR surrounds the anorectal junction). When the anus was contracted, the pelvic floor organs moved to the head and ventral side, the anteroposterior diameter of the levator hiatus was reduced, and the PR became shorter and thicker and moved toward the inner ventral side. The Young modulus of the anterior part of the left and right PR was increased compared with the R state (P < .01). The results are shown in Table 3.
Table 3.
Comparison of various states of pelvic floor structure in normal nulliparous women.
| Index | Resting state | Valsalva state | Anus contraction state | P |
|---|---|---|---|---|
| BSD (mm) | 29.533 ± 3.665 | 20.967 ± 4.115 | – | <.001 |
| CSD (mm) | 36.767 ± 3.137 | 34.667 ± 3.055 | – | <.001 |
| ASD (mm) | 20.967 ± 3.243 | 18.667 ± 3.089 | – | <.001 |
| UOA (°) | 10.300 ± 8.370 | 6.000 ± 9.158 | – | .063 |
| PUA (°) | 114.700 ± 9.735 | 124.700 ± 8.347 | – | <.001 |
| Anteroposterior diameter of the hiatus (mm) | 45.810 ± 1.971 | 48.303 ± 1.517 | 41.733 ± 1.975 | <.001 |
| Young’s modulus of right PR (kPa) | 30.770 ± 3.535 | – | 62.600 ± 3.987 | <.001 |
| Young’s modulus of left PR (kPa) | 30.997 ± 3.558 | – | 63.037 ± 3.470 | <.001 |
Bold indicates data is statistically significant.
ASD = distance from the anorectal junction to the lower edge of the pubic symphysis, BND = bladder neck descent, BSD = distance from the bladder neck to the lower edge of the pubic symphysis, CSD = distance from the cervix to the lower edge of the pubic symphysis, PR = puborectalisis muscle, PUA = posterior urethrovesical angle, UA = rotation angle of the urethra, UOA = obliquity angle of the urethra.
3.3. Changes of pelvic floor parameters after total hysterectomy
Comparison of various indexes between the group with hysterectomy and the group without hysterectomy showed that the R-PUA, R-anteroposterior hiatus diameter, BND, UA, V-PUA, V-anteroposterior hiatus diameter, S-anteroposterior hiatus diameter, R-R-PR elasticity, R-L-PR elasticity and the incidence of PFD were higher in the group with hysterectomy than in the normal parous group, and the difference was statistically significant (P < .05). In contrast, V-BSD, V-ASD, R-elasticity difference, L-elasticity difference was lower in the group with hysterectomy than in the normal parous group, and the difference was statistically significant (P < .05). The differences in other indicators were not statistically significant (P > .05). The results are shown in Table 4.
Table 4.
Comparison of pelvic floor parameters between the 2 groups.
| Index | Women without hysterectomy (n = 40) | Women with hysterectomy (n = 76) | t/χ2 | P |
|---|---|---|---|---|
| R-BSD (mm) | 27.435 ± 4.049 | 26.459 ± 3.535 | 1.343* | .182 |
| R-UOA (°) | 12.788 ± 13.996 | 14.533 ± 18.100 | −0.575* | .566 |
| R-PUA (°) | 113.040 ± 6.947 | 126.836 ± 9.354 | −8.206* | <.001 |
| R-ASD (mm) | 18.555 ± 2.044 | 17.697 ± 3.614 | 1.631* | .106 |
| R-hiatus anteroposterior diameter (mm) | 47.588 ± 2.551 | 50.201 ± 3.353 | −4.313* | <.001 |
| V-BSD (mm) | 18.258 ± 5.111 | 12.974 ± 8.417 | 3.630* | <.001 |
| BND (mm) | 9.178 ± 3.618 | 13.486 ± 6.902 | −3.685* | <.001 |
| V-UOA (°) | 9.963 ± 21.882 | 14.787 ± 25.344 | −1.020* | .310 |
| UA (°) | 23.065 ± 4.701 | 27.254 ± 8.006 | −3.546* | .001 |
| V-PUA (°) | 135.230 ± 9.623 | 142.374 ± 11.467 | −3.364* | .001 |
| V-ASD (mm) | 13.548 ± 1.881 | 11.126 ± 4.911 | 3.000* | .003 |
| V-hiatus anteroposterior diameter (mm) | 54.835 ± 2.394 | 56.541 ± 3.596 | −3.047* | .003 |
| C-hiatus anteroposterior diameter (mm) | 41.360 ± 2.594 | 44.930 ± 3.013 | −6.353* | <.001 |
| R-R-PR elasticity (kPa) | 27.588 ± 4.017 | 33.674 ± 3.425 | −8.563* | <.001 |
| R-L-PR elasticity (kPa) | 27.560 ± 3.909 | 33.822 ± 3.249 | −9.189* | <.001 |
| C-R-PR elasticity (kPa) | 57.868 ± 1.438 | 57.615 ± 3.832 | 0.509* | .612 |
| C-L-PR elasticity (kPa) | 58.238 ± 1.395 | 57.780 ± 3.928 | 0.911* | .364 |
| R-elasticity difference (kPa) | 30.280 ± 4.274 | 23.942 ± 3.589 | 8.456* | <.001 |
| L-elasticity difference (kPa) | 30.678 ± 4.364 | 23.958 ± 3.504 | 9.004* | <.001 |
| PFD incidence (No/Yes) | 36/4 | 39/37 | 17.161† | .001 |
Bold indicates data is statistically significant.
ASD = distance from the anorectal junction to the lower edge of the pubic symphysis, BND = bladder neck descent, BSD = distance from the bladder neck to the lower edge of the pubic symphysis, CSD = distance from the cervix to the lower edge of the pubic symphysis, PFD = pelvic floor dysfunction, PR = puborectalisis muscle, PUA = posterior urethrovesical angle, R = resting state, S = anus retraction state, UA = rotation angle of the urethra, UOA = obliquity angle of the urethra, V = maximum Valsalva state.
: t test. †: chi-square test.
3.4. The influence of menopause on the incidence of PFD after total hysterectomy
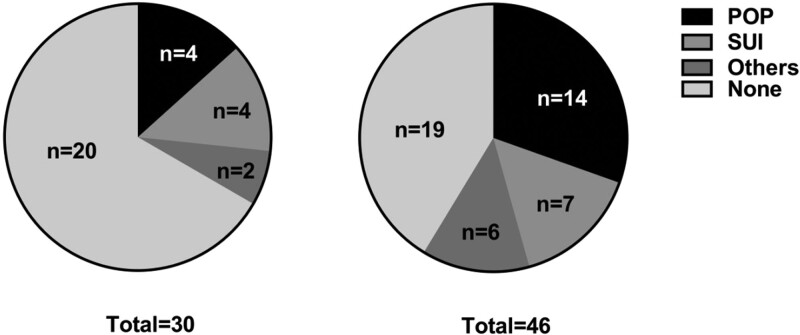
Comparison of the incidence of PFD between the group IIIa and group IIIb showed that the incidence of PFD in group IIIa was lower than that in group IIIb, and the difference was statistically significant (P < .05). The results are shown in Table 5. Group IIIa had 4 cases of POP, 4 cases of SUI, and 2 other cases; group IIIb had 14 cases of POP, 7 cases of SUI, and 6 other cases, as shown in Figure 7. POP includes bladder prolapse (see Fig. 8), rectocele (see Fig. 9), and intestinal hernia (see Fig. 10).
Table 5.
Comparison of the incidence of PFD between the 2 groups of women who have undergone hysterectomy before and after menopause.
| Age group | n | PFD | No PFD |
|---|---|---|---|
| IIIa (before menopause) | 30 | 10 (33.3) | 20 (66.7) |
| IIIb (after menopause) | 46 | 27 (58.7) | 19 (41.3) |
| χ 2 | 4.227 | ||
| P | .039 | ||
PFD = pelvic floor dysfunction.
Figure 7.
Pie chart of PFD in the 2 age groups. PFD = pelvic floor dysfunction.
Figure 8.
The 58-year-old menopausal patient underwent total hysterectomy for uterine fibroids 2 years ago. The picture shows a prolapsed bladder. In the state of maximum Valsalva, the bladder moved downward to below the horizontal line of the posterior lower edge of the pubic symphysis.
Figure 9.
The 51-year-old menopausal patient underwent total hysterectomy due to birth control ring incarceration and cervical lesions 1 year ago. The picture shows a rectal bulge with a height of 11 mm.
Figure 10.
The 62-year-old patient underwent total hysterectomy 8 years ago due to uterine fibroids and has been menopausal. The picture shows an intestinal hernia; * indicates the small intestine that hernias into the rectum and vagina.
3.5. The diagnostic value of TPUS combined with SWE for PFD
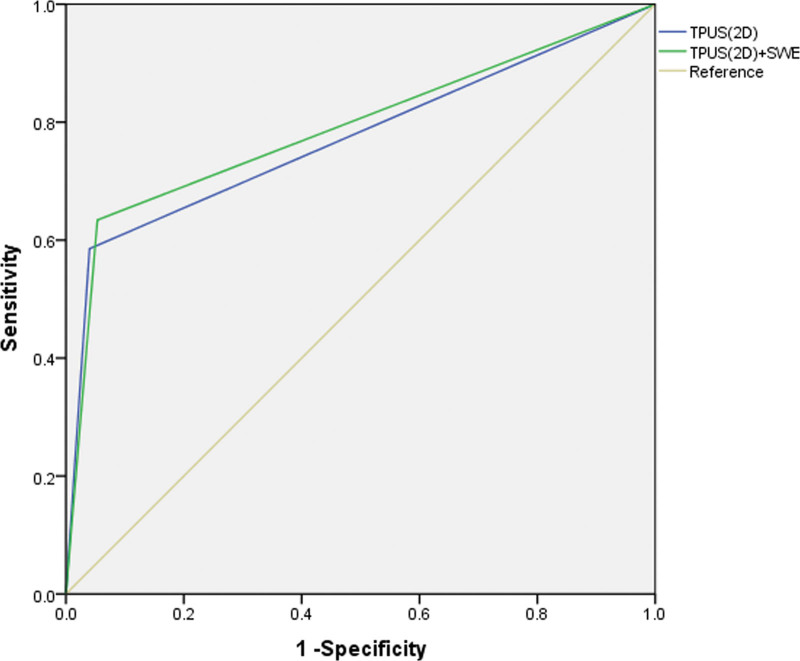
In order to further evaluate the diagnostic value of TPUS combined with SWE for the diagnosis of PFD, the 2 indicators, that is, the TPUS (2D) and the TPUS (2D) combined with SWE were selected, and the receiver operating characteristic curves were analyzed (Fig. 11). The results are shown in Table 6, indicating that the TPUS (2D) and the TPUS (2D) combined with SWE are both good in diagnosing PFD, but the TPUS (2D) combined with SWE is better than the TPUS (2D).
Figure 11.
ROC curve. ROC = receiver operating characteristic.
Table 6.
ROC results.
| Methods | AUC | SE | P value | 95% CI | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|---|
| TPUS (2D) | 0.773 | 0.051 | .000 | 0.673–0.873 | 0.585 | 0.960 | 0.545 |
| TPUS (2D) + SWE | 0.790 | 0.049 | .000 | 0.694–0.887 | 0.634 | 0.947 | 0.581 |
AUC = area under the curve, CI = confidence interval, ROC = receiver operating characteristic, SE = standard error, SWE = shear wave elastography, TPUS = transperineal ultrasound.
4. Discussion
4.1. The pelvic floor anatomy and damage to the pelvic floor by total hysterectomy
The pelvic floor is the lowest point of the trunk when the human body walks upright and is covering all organs and tissues in the abdominal cavity. The structure of the female pelvic floor is more complex, and the bony structure provides a fixed point for the pelvic floor support system. The pubic symphysis is an important reference point for TPUS to quantify the pelvic floor function.[6] The pelvic floor muscles, fascia, ligaments, and nerves close the pelvic outlet and carry the pelvic organs, leaving only the levator hiatus, which penetrates the urethra, vagina, and rectum. The levator ani muscle group is the most critical supporting structure of the pelvic floor. PR is the most powerful part of the levator ani muscle group. The left and right muscle fibers are connected to form a U-shaped sling to firmly hang the urethra, vagina, and rectum on the pubic bone, which can contract and close. The levator hiatus is of great significance. Previous reports showed that although the pelvic floor muscles and fascial ligaments jointly maintain the stability of the pelvic floor, the pelvic floor muscles provide the main support force under normal conditions, and the ligaments composed of connective tissue only provide temporary support when the muscles are damaged.[9] Therefore, evaluating the performance of pelvic floor muscles has great clinical value.
The uterus is an important organ of the pelvic floor and plays a vital role. The removal of the uterus is bound to cause changes in the structure and function of the pelvic floor. According to the “three-level” theory,[10] surgery destroys the first level, that is, apical support, which can cause vaginal vault prolapse and break the stability of the “hammocks.”[11] When abdominal pressure increases, the urethra cannot be closed normally, leading to SUI. Destroying the second and third levels will further cause the front and back walls of the vagina to bulge. According to the holistic theory,[12] the removal of the uterus creates emptiness in the middle pelvic cavity and destroys the perfection of the pelvic floor. Surgery also destroys the sympathetic or parasympathetic nerves distributed on the pelvic floor fascia and ligaments.[13] The functions of the bladder, bowel and vagina innervated by these nerves will be impaired to varying degrees. The drop in estrogen levels caused by surgery can also affect the pelvic floor support.
4.2. Evaluation of pelvic floor function by TPUS
Among the many methods of pelvic floor function assessment, imaging has obvious advantages and can directly observe the anatomical structure of the pelvic floor. X-ray examinations are gradually eliminated by clinics due to the complicated operations, large amounts of radiation, and not being able to directly display the shape and injury site of muscle fascia. The magnetic resonance imaging can display the subtle anatomical structures of pelvic organs, muscles, fascia, and pelvic floor from multiple angles and levels, but it is expensive and has many contraindications and limitations in the clinical practice.[14] Ultrasound can show observations dynamically in real time, has no radiation, and is low-cost. Transabdominal ultrasonography has limitations in the display of pelvic floor tissue, and during transvaginal ultrasound exploration, the probe will change the position of the bladder neck and compress the urethra, which brings troubles to the diagnosis.[15] TPUS has become the most-used examination method for postpartum pelvic floor function assessment due to its validity, real-time dynamic presentations, economic value, convenience in use, non-invasiveness,[16] and it is also suitable for observation and evaluation of functional structures of pelvic floor after total hysterectomy.
TPUS enables anatomical-like imaging of the pelvic floor and observes the pelvic floor structure of normal nulliparous women. It was found that the pelvic floor organs moved toward the dorsal side of the foot during the Valsalva maneuvers, the proximal urethra rotated backward and downward, and the anteroposterior diameter of the levator hiatus was increased; when contracting the anus, the pelvic floor organs moved to the head and ventral side, and the anterior and posterior diameter of the levator hiatus was reduced. In addition, TPUS can not only be used to dynamically observe the anatomical structure and position changes of the pelvic floor organs and tissues in real time, but also to quantitatively evaluate the pelvic floor function.
At present, it is recognized that the pathogenesis of SUI after hysterectomy is the destruction of the levator plate and pelvic floor support structure, which makes the pelvic floor supportive tissue weak, and the pressure cannot be passed along the pubic bladder fascia and vaginal anterior wall support structure to the bladder neck and proximal urethra, but directly to the bladder, so that the bladder pressure is greater than the urethral closure pressure, which triggers SUI. Increased bladder neck mobility and urethral mobility are independent predictors of SUI, and the greater the mobility, the higher the risk.[17] The mobility of the bladder neck can be quantitatively evaluated by BND, and the mobility of the urethra can be quantitatively evaluated by UA and PUA, which are also the main indicators for the diagnosis of SUI. This study found that the BND, UA, R-PUA, and V-PUA of the group with hysterectomy were greater than those of the group without hysterectomy, and the difference was statistically significant. This indicates that women have higher bladder neck and urethra mobility after hysterectomy. It can be inferred that the hysterectomy group also has a higher risk of SUI. The increased mobility of the urethra and bladder neck is due to the changes in the spatial position of the pelvic floor organs after the removal of the uterus. The main ligament and the sacral ligament are cut during the operation, the postoperative pelvic floor tissue and nerves are damaged, and the pelvic floor support is weak, which is consistent with the results of Wang et al.[18]
Levator hiatus plays a key role in the support and maintenance of pelvic floor organs. Previous studies have shown that the size of the hiatus is positively correlated with the severity of POP.[14] In this study, the anteroposterior diameter of the hiatus was used to reflect the hiatus size. The anteroposterior diameters of the hiatus in the 3 states of the hysterectomy group were larger than those in the group without hysterectomy. After total hysterectomy, the pelvic support function of women was weakened, which led to an increased risk of PFD. The tension of the levator ani muscle was decreased in the R state; the elasticity of the levator ani muscle was insufficient in the V state, and the hiatus expanded with the descending pelvic organs; the levator ani muscle cannot effectively contract due to the contraction function defect when the anus is retracted. The mechanism of these observations is that the pelvic floor muscles and their innervating nerves are damaged by the operation, the muscle elasticity and compliance are weakened, the hiatus size is increased, and the anteroposterior diameter is increased accordingly. These results are consistent with the research of Jin et al.[19]
The ASD can reflect the structure and function of the posterior pelvic cavity. In this study, the ASD in the Valsalva state of the group with hysterectomy was smaller than that in the group without hysterectomy, which indicates that the pelvic organs moved down more significantly after the Valsalva maneuvers after total hysterectomy, and the risk of prolapse was also increased, which is consistent with the study of Yao et al.[20] This study found 3 patients with intestinal hernia after total hysterectomy. The analysis showed that the original position of the uterus was replaced by the small intestine and sigmoid colon, and the autonomic nerves that innervated the intestine were damaged during the operation, resulting in disturbances in the coordinated contraction of the smooth muscles of the intestine and forced defecation. When the small intestine herniates into the weak rectum and vaginal septum to compress the distal rectum, patients will have difficulty in defecation.[21] This study also found 6 cases of rectocele after total hysterectomy. Rectocele is caused by a weak vaginal rectal septum, that is, the front wall of the rectum protrudes to the posterior wall of the vagina. At this time, the internal rectal pressure cannot be directly transmitted to the anus, resulting in secondary constipation. Long-term constipation causes an increase in abdominal pressure and then causes POP.[22]
4.3. Evaluation of pelvic floor function by SWE
At present, SWE is mostly used for tumor classification and assessment of tissue changes in breast, prostate, and liver. In recent years, the use of SWE in muscle research has also become a hot spot.[23,24] SWE can effectively evaluate and quantify the biological properties of muscle stiffness and elasticity, and muscle stiffness and elasticity in turn determine its contraction ability. SWE can quantitatively evaluate the changes in PR muscle biological performance by monitoring its hardness and contraction ability,[25] and its elasticity and contractility can reflect the support function of pelvic floor muscles. The research on the evaluation value of SWE on the postpartum PR elasticity is still in exploration,[26,27] and there are few reports on the evaluation of PR elasticity after total hysterectomy.
Compared with the Oxford muscle strength classification and pelvic floor muscle strength test,[28] which are subjective and have poor reliability, and magnetic resonance imaging examination for qualitative diagnosis, SWE can quantitatively evaluate muscle elasticity, and the results are objective and reliable with high repeatability. In the R state, the left and right PR long axes are in a band with slightly higher echoes, with strong echogenic muscle membranes and hypoechoic muscle fibers intertwined with each other. After retracting the anus, the PR becomes shorter and thicker, and moves toward the inside of the abdomen. Selecting the anterior part of PR to measure Young modulus shows good repeatability.[3] This study found statistically significant difference in PR Young modulus between the R and the receding anus states of normal nulliparous women, which is consistent with previous reports.[29] The greater the Young modulus, the greater the hardness of the structure and the lower the elasticity. In this study, the Young modulus of PR under the R state of the group with hysterectomy was higher than that of the group without hysterectomy, indicating that PR muscle stiffness was increased and elasticity was decreased after hysterectomy. The deterioration of PR elasticity in women after hysterectomy may be due to changes in PR components. In denervated muscles, elastic muscle fibers atrophy and degenerate, fibroblasts without diastolic function increase and deform, and the significant reduction of intramuscular vascular beds accelerates the degeneration of muscle cells. The arrangement of sarcomere is disordered, and the stripes are irregular during muscle cell regeneration. These factors lead to an increase in PR hardness.[30,31] The contractile force of PR can be quantified by the difference in Young modulus before and after contraction. In the study, the difference in PR Young modulus of the left and right PR before and after contraction in the group with hysterectomy was lower than that in the group without hysterectomy. It can be inferred that PR contractility was decreased after total hysterectomy. Zhan et al studied 400 women after total hysterectomy and found that the pelvic floor muscle strength was decreased after hysterectomy, which is consistent with this study.[32]
4.4. Diagnosis of PFD
The comparison between the group with hysterectomy and the group without hysterectomy found that the incidence of PFD in women with hysterectomy was significantly higher than that of women without hysterectomy, which is consistent with previous reports.[1]
In comparison within the hysterectomy group, it was found that the PFD rate of postmenopausal women was higher than that of premenopausal women. It can be speculated that in addition to direct damage to pelvic floor function, factors that reduce estrogen levels also matter. Estrogen can promote the proliferation of fibroblasts and the synthesis and maturation of collagen in the supporting tissues of the pelvic floor. The firmness and elasticity provided by collagen maintain the stability and plasticity of the pelvic floor structure.[33] Hysterectomy causes damage to the blood supply of the ovaries and decreases ovarian estrogen secretion.[34] The operation also damages the estrogen receptors widely distributed on the pelvic floor tissues such as the main sacral ligament, levator ani muscle, vaginal wall tissue, etc.[35] Decreased levels of estrogen and its receptors after hysterectomy lead to weakening of the strength of the pelvic floor support structure, which increases the risk of PFD. This is consistent with the research of Yang et al.[36]
In addition, this article further analyzed the diagnostic value of the TPUS (2D) combined with SWE in the diagnosis of PFD. The receiver operating characteristic curves showed that TPUS (2D) and the TPUS (2D) combined with SWE were both effective in diagnosing PFD. However, the TPUS (2D) combined with SWE was better than the TPUS (2D) alone, indicating that TPUS combined with SWE can improve the diagnosis of PFD in women’s pelvic floor function, and suggesting it is worthy of clinical promotion. In this study, the TPUS was applied to evaluate the pelvic floor function after total hysterectomy and achieved an ideal diagnosis effect.
4.5. Limitations
This study lacked examinations on the middle pelvic cavity and did not pay attention to the vaginal vault prolapse. Also, it had a short follow-up period and small sample size and did not discuss the long-term effects and outcomes of PFD after total hysterectomy.
5. Conclusion
In summary, total hysterectomy has a negative impact on pelvic floor support. TPUS can qualitatively and quantitatively evaluate female pelvic floor function, SWE can quantify the biological performance of pelvic floor muscles, and the combination of the 2 can be a multi-dimensional assessment of pelvic floor function, which improves the evaluation pelvic floor function and provides a comprehensive and reliable basis for early clinical prevention, intervention, and delay of PFD. The author believes that women after total hysterectomy should promptly evaluate pelvic floor function just like postpartum women and actively strengthen pelvic floor function to improve the quality of life.
Author contributions
Conceptualization: Runyan Ji, Bosheng He, Jing Wu.
Data curation: Runyan Ji.
Formal analysis: Runyan Ji, Bosheng He, Jing Wu.
Funding acquisition: Bosheng He, Jing Wu,Runyan Ji.
Investigation: Runyan Ji.
Project administration: Bosheng He, Jing Wu.
Resources: Runyan Ji.
Software: Runyan Ji.
Validation: Runyan Ji, Bosheng He, Jing Wu.
Writing – original draft: Runyan Ji.
Writing – review & editing: Bosheng He, Jing Wu.
Abbreviations:
- ASD =
- anorectal junction-symphyseal distance
- BMI =
- body mass index
- BND =
- bladder neck descent
- BSD =
- bladder neck-symphyseal distance
- C =
- anus contraction
- PFD =
- pelvic floor dysfunction
- POP =
- pelvic organ prolapse
- PR =
- puborectalisis muscle
- PUA =
- posterior urethrovesical angle
- R =
- resting
- SP =
- symphysis pubis
- SUI =
- stress urinary incontinence
- SWE =
- shear wave elastography
- TPUS =
- transperineal ultrasound
- UA =
- urethral rotation angle
- UOA =
- urethral obliquity angle
- V =
- maximum Valsalva
The research was supported by “333” Talent Funding Project of Jiangsu Province (BRA2020198), General Project B of Nantong Municipal Health Commission (MB2021055), and Nantong Municipal Science and Technology Plan (MSZ18214).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Ji R, He B, Wu J. Application of transperineal ultrasound combined with shear wave elastography in pelvic floor function assessment after hysterectomy. Medicine 2023;102:2(e32611).
Contributor Information
Runyan Ji, Email: jry003@163.com.
Jing Wu, Email: 464870834@qq.com.
References
- [1].Kocaay AF, Oztuna D, Su FA, et al. Effects of hysterectomy on pelvic floor disorders: a longitudinal study. Dis Colon Rectum. 2017;60:303–10. [DOI] [PubMed] [Google Scholar]
- [2].Liu J, Zhuang H, Kong LL. Application of mind mapping in the prevention of pelvic floor dysfunction after total hysterectomy. Chinese J Family Planning Gynecotokol. 2019;11:93–6. [Google Scholar]
- [3].Wang Y, Qu X, She Y. Reproducibility study of real-time shear wave elastography assessment in female puborectal muscle. J China Med Univ. 2017;46:360–2. [Google Scholar]
- [4].Guidelines for the diagnosis and treatment of female stress urinary incontinence. Chin J Obstet Gynecol. 2017;52:289–93. [DOI] [PubMed] [Google Scholar]
- [5].Dietz HP, Kamisan Atan I, Salita A. Association between ICS POP-Q coordinates and translabial ultrasound findings: implications for definition of “normal pelvic organ support.”. Ultrasound Obstet Gynecol. 2016;47:363–8. [DOI] [PubMed] [Google Scholar]
- [6].Dietz HP, Zhang X, Shek KL, et al. How large does a rectocele have to be to cause symptoms? a 3D/4D ultrasound study. Int Urogynecol J. 2015;26:1355–9. [DOI] [PubMed] [Google Scholar]
- [7].Female sexual dysfunction: ACOG practice bulletin clinical management guidelines for obstetrician-gynecologists, number 213. Obstet Gynecol. 2019;134:e1–e18. [DOI] [PubMed] [Google Scholar]
- [8].Zhu L. New concepts of female pelvic floor anatomy. J Pract Obstet Gynecol. 2005;21:129–30. [Google Scholar]
- [9].DeLancey JO. The anatomy of the pelvic floor. Curr Opin Obstet Gynecol. 1994;6:313–6. [PubMed] [Google Scholar]
- [10].DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–20. discussion 203. [DOI] [PubMed] [Google Scholar]
- [11].Petros PEP, Ulmsten UI. An integral theory of female urinary incontinence. experimental and clinical considerations. Acta Obstet Gynecol Scand. 1990;69:7–31. [DOI] [PubMed] [Google Scholar]
- [12].Forsgren C, Altman D. Long-term effects of hysterectomy: a focus on the aging patient. Aging Health. 2013;9:179–87. [Google Scholar]
- [13].Dietz HP. Pelvic floor ultrasound: a review. Clin Obstet Gynecol. 2017;60:58–81. [DOI] [PubMed] [Google Scholar]
- [14].Umek WH, Obermair A, Stutterecker D, et al. Three-dimensional ultrasound of the female urethra: comparing transvaginal and transrectal scanning. Ultrasound Obstet Gynecol. 2001;17:425–30. [DOI] [PubMed] [Google Scholar]
- [15].Wang FB, Rong R, Xu JJ, et al. Impact of pelvic floor ultrasound in diagnosis of postpartum pelvic floor dysfunction: a protocol of systematic review. Medicine (Baltimore). 2020;99:e21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dietz HP, Clarke B, Herbison P. Bladder neck mobility and urethral closure pressure as predictors of genuine stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:289–93. [DOI] [PubMed] [Google Scholar]
- [17].Wang L, Liu H, Wei X, et al. Transperineal ultrasonography in evaluation of pelvic floor structure changes in post-hysterectomy patients. Chin J Med Imag Technol. 2018;34:416–8. [Google Scholar]
- [18].Jin J, Yuan C, Jin M, et al. The value of four-dimensional transperineal pelvic floor ultrasound stereo imaging in detecting abnormal pelvic floor function in women after total hysterectomy. Mod Pract Med. 2019;31:808–9 + 50. [Google Scholar]
- [19].Yao J. Evaluation of the effect of total hysterectomy on pelvic floor function by transperineal ultrasound. Guangxi: Guangxi Medical University. 2019. [Google Scholar]
- [20].Zhao J, Sun Y, Hu Y. Impaction of total hysterectomy on defecate function. Chin Med Record. 2014;15:66–7. [Google Scholar]
- [21].Ma Y, Zhu Y, Tan C, et al. Relationship between posterior vaginal prolapse and bowel symptoms among patients with pelvic organ prolapse. Chin J Clin Obstet Gynecol. 2020;21:378–80. [Google Scholar]
- [22].Sigrist RMS, Liau J, Kaffas AE, et al. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu J, Hug F, Fu SN. Stiffness of individual quadriceps muscle assessed using ultrasound shear wave elastography during passive stretching. J Sport Health Sci. 2018;7:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wenz H, Dieckmann A, Lehmann T, et al. Strain ultrasound elastography of muscles in healthy children and healthy adults. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2019;191:1091–8. [DOI] [PubMed] [Google Scholar]
- [25].Gachon B, Nordez A, Pierre F, et al. In vivo assessment of the levator ani muscles using shear wave elastography: a feasibility study in women. Int Urogynecol J. 2019;30:1179–86. [DOI] [PubMed] [Google Scholar]
- [26].Jiang Y, Pan Y, Pan H, et al. Evaluation of postpartum female pelvic floor function by transperineal three-dimensional ultrasound combined with real-time shear wave elastography. Biomed Engin Clin Med. 2020;24:45–9. [Google Scholar]
- [27].Liu J, Zhou A. Influence of shear wave elastography on elasticity of the puborectalis muscle according to delivery mode. Chin J Med Imag. 2018;026:126–9. [Google Scholar]
- [28].da Silva JB, de Godoi Fernandes JG, Caracciolo BR, et al. Reliability of the PERFECT scheme assessed by unidigital and bidigital vaginal palpation. Int Urogynecol J. 2021;32:3199–207. [DOI] [PubMed] [Google Scholar]
- [29].Wang J, Wang H, Chen H, et al. Real-time shear wave elatography in measurement of the Young’s modulus of the female puborectalis muscle. Chin J Med Imag Technol. 2015;31:586–9. [Google Scholar]
- [30].Tang J, Chen Z, Wei W, et al. Quantifying levator ani muscle elasticity under normal and prolapse conditions by shear wave elastography. J Ultrasound Med. 2020;39:1379–88. [DOI] [PubMed] [Google Scholar]
- [31].Carlson BM. The biology of long-term denervated skeletal muscle. Eur J Transl Myol. 2014;24:3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhan F, Zhao R, Wu Y. Impact of panhysterectomy on pelvic floor function. Chin J N Clin Med. 2018;011:982–6. [Google Scholar]
- [33].Rahkola-Soisalo P, Savolainen-Peltonen H, Gissler M, et al. Postmenopausal hormone therapy is accompanied by elevated risk for uterine prolapse. Menopause (New York, NY). 2019;26:140–4. [DOI] [PubMed] [Google Scholar]
- [34].Yao W, Tang J, Fang F, et al. Observation on the effects of different modes of metrectomy on secretion level of sex hormones, perimenopausal symptoms, and sexual function of patients. Matern Child Health Care China. 2019;34:307–11. [Google Scholar]
- [35].Han L, Wang L, Wang Q, et al. Association between pelvic organ prolapse and stress urinary incontinence with collagen. Exp Ther Med. 2014;7:1337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang D, Nie F, Bu L, et al. Study on the changes of pelvic floor structure in different time after hysterectomy by transperineal ultrasound. Chin J Med Ultrasound (Electronic Edition). 2018;15:707–12. [Google Scholar]