Introduction
The COVID-19 pandemic has introduced numerous challenges to clinical practice, including potential staff and patient illness, travel restrictions, and limiting exposure for patients who may be immunocompromised. Although telehealth technologies and home health services were not new, pandemic-related policies addressing Health Insurance Portability and Accountability Act and payment parity rendered the regular use of these approaches operationally and financially feasible.1-3 More than 2 years into the pandemic, we have started to see the emergence of a virtual-first model where telehealth often serves as the default option for select clinical situations.4
These developments may be particularly relevant to clinical trials. Trial participation may pose time, cost, and logistical burdens on patients, who may be required to travel to a study center not only for treatment administration, but also for additional monitoring, biospecimen collection, or survey completion. Closing the gap between routine clinical care and virtual-first clinical care has made virtual-first trials more plausible. Capitalizing on increased operational capacity through mobile technologies and home health platforms, virtual-first trials—alternately referred to as remote or decentralized trials—have emerged to bring clinical research opportunities to individuals who might not otherwise have access to a traditional trial site. These trials incorporate decentralized supply chains and existing health care facilities, such as laboratories, imaging centers, urgent care centers, or emergency departments.5 Patient screening, enrollment, and trial logistics are managed by a central, remote team.6 Similar to virtual-first clinical care generally, virtual-first clinical trial participants can visit physical clinical sites for procedures not achievable by telehealth or home health.
As demonstrated in fields such as dermatology, psychiatry, and cardiology,7-9 a decentralized approach currently appears feasible for studies featuring oral therapies, end points limited to patient-reported outcomes or visual assessments, and drug toxicities expected to be mild. For example, 476 patients with heart failure participated in the double-blind, randomized CHIEF-HF trial of canagliflozin entirely without in-person interactions with clinicians. Participants were required to have access to an iPhone 6/Samsung S7 or later and be willing to wear a Fitbit. At 12 weeks, patients completed a Kansas City Cardiomyopathy Questionnaire.9
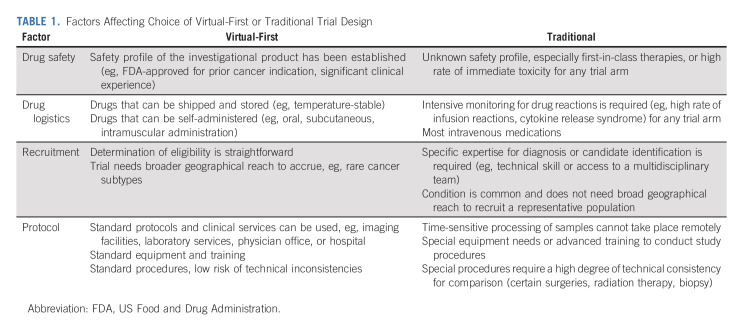
Despite such innovation in other fields, to date, implementation of decentralized clinical trials in oncology remains rare, an observation that may reflect recruitment mechanisms, protocol requirements, and treatment characteristics (Table 1). In cancer clinical trials, end points often involve advanced imaging, blood tests, and biopsies.10 Treatment may entail infusional therapies with narrow therapeutic windows. Patients may require high-acuity care because of the burden of cancer-related symptoms. Yet, early experiences, such as the phase II Alpha-T trial (ClinicalTrials.gov identifier: NCT04644315), demonstrate feasibility of fully decentralized oncology clinical trials.5 In this trial, treating oncologists are notified when a patient's tumor (other than lung cancer) is found to harbor an anaplastic lymphoma kinase alteration by next-generation sequencing. The clinician is then linked to a remote trial team. After approval from the treating oncologist, potentially eligible patients are offered enrollment and consented via a remote study team that completes the screening process and confirms eligibility. Enrolled patients are asked to take alectinib, an oral anaplastic lymphoma kinase inhibitor initially approved in 2015, twice daily at home. Clinical research coordinators and nurses visit patients at their homes to collect end point data through physical examination, phlebotomy, and questionnaires. A trial investigator completes clinical assessment using telemedicine tools. The study uses local radiology facilities, which enables patients to remain in their local area. The remote trial team is responsible for training and onboarding of local imaging centers. Standard operating protocols promote consistency and transfer of digital imaging. Similarly, the remote trial team is responsible for selection of and contracting with any home health or phlebotomy services.
TABLE 1.
Factors Affecting Choice of Virtual-First or Traditional Trial Design
Referral and Recruitment
Because patients' geographic location may pose a barrier to participation, decentralized trials have the potential to recruit, and retain, a larger and more inclusive patient population. However, referral mechanisms—which may vary according to patient population and disease indication—will require deliberate consideration. One approach uses a public-facing recruitment website for screening and enrollment. In this model, patients self-refer to trials via a website, a strategy that would rely heavily on direct-to-participant marketing. Another approach relies on clinical practices to identify potential trial participants. Clinic staff screen electronic health record or registry data for eligible patients, such that patients can be referred by their medical team or be contacted directly by the virtual team managing the study when there is a potential match.11
Supporting this approach, the Office of Human Research Protection of the US Department of Health and Human Services does not consider the act of referring a patient to a clinical trial at another center to be engaging in research.12 Specifically, the guidance states that a clinician who provides patients with literature about a research study at another institution, including a copy of the informed consent document, and obtains permission from the patient to provide the patient's name and telephone number to investigators is not engaging in research.12 Therefore, referring clinics are not required to have formal site approval if they are not involved in subsequent research-related procedures, which permits a wide referral base without the financial and logistical burden of activating multiple trial sites.
The role of the referring physician remains vital to success of clinical trial recruitment.13-15 A primary oncologist's consideration of trial potential may be even more crucial for trials of targeted therapies, which rely heavily on the physician to order tumor molecular profiling to identify specific genomic subsets. A study of factors preventing oncologists from referring patients to trials revealed that time and effort required to explain trials to patients and inadequate reimbursement from research sponsors were among the strongest barriers, while the fear of losing patients to another clinical practice was a weak barrier.15 These potential obstacles will need to be addressed for virtual-first trials to achieve their full potential.
From regulatory and operational perspectives, virtual-first decentralized trials present both familiar and novel considerations. Principal investigator roles and responsibilities do not differ from those seen in a traditional model. The investigator needs to ensure adequacy of resources, compliance with the protocol, safety, and communication with the relevant oversight groups (eg, institutional review board). By contrast, patient evaluation represents a major departure from conventional trials. In some circumstances, the investigator and study team may complete assessments, manage toxicities, and render treatment decisions via telemedicine encounters. However, given the potential seriousness and acuity of disease- and treatment-related complications, patients with cancer are likely to require in-person evaluation by local clinicians and health care facilities as well. In such scenarios, decentralized trials resemble existing clinical trials that enroll patients who live at considerable distance from the physical trial site. A relevant example is clinical trials performed at the National Institutes of Health, which historically have covered transportation and lodging costs for trial participants. This level of support permits patients from across the United States to participate. For many of these trials, patients return home in between study visits. If during those times, patients require routine or urgent in-person medical evaluation, they receive it from local clinicians who are not trial investigators. As such, a primary oncologist may remain involved in the standard clinical care of the patient after enrollment. Data resulting from local interventions or assessments are collected and reported by the study team. The treating nonstudy clinicians may contact the study team for bidirectional information exchange as needed, similar to existing communication practices among medical centers whether or not patients are on trials. However, these individuals would not be asked to perform any study-related tasks, whether concomitant medication recording, study-required performance status/vital signs/physical examination, efficacy assessment, toxicity grading and attribution, and the like. Decentralized trials take this approach even further, with the infrequent in-person evaluation potentially replaced by telehealth or study personnel home visits. We recognize that this difference is not incremental but potentially foundational, and represents one of the greatest challenges in implementation and acceptance of decentralized trials.
Treatment Logistics and Safety
Oral, subcutaneous, or intramuscular therapies with established safety profiles—which can be shipped directly to patients for self-administration—may be particularly suitable for virtual-first trials. To estimate the proportion of registrational trials featuring therapies meeting these characteristics, we reviewed cancer treatments approved in 2020 using the Drugs@FDA database.16 These approvals were based on 61 trials, of which 37 (61%) evaluated drugs already approved for other cancer indications with well-characterized toxicity. In 12 trials (20%), all study arms included oral, intramuscular, or subcutaneous agents. Such trials may represent a good starting point for the implementation of virtual-first clinical trials.
As a next step, trials involving infusional therapies with established safety profiles and which have been successfully administered at home previously (n = 5 trials; 8%) could be considered for virtual-first platforms. However, the role of at-home infusion in routine cancer care remains debated. Although some clinical practice organizations oppose this approach citing safety concerns,17 other groups argue that patient risk can be mitigated by careful drug selection.18
Recent pilot studies of at-home infusion of cytotoxic therapies provide early insight into potential feasibility of this approach.18-23 For example, Penn Medicine's Cancer Care at Home has administered and monitored 13 common cancer treatments at patients' homes.23 The University of Utah Huntsman at Home found that patients could receive acute-level care safely at home, with fewer hospitalizations and shorter lengths of stay if hospitalization did occur, thereby reducing potential harms of inpatient and emergency department care, such as hospital-acquired infections.22 These experiences suggest that certain home infusions may be feasible and safe, a prerequisite for many home-based cancer trials.18 Nevertheless, the generalizability of these reports for widespread adoption requires further investigation to determine the practicality of home infusion among diverse patient populations, the impact on caregiver burden, and protocols for emergency response in the home setting.
A commonly proposed model for decentralized trials involves a trained clinical trial nurse to administer drugs according to safety protocols and observe for acute reactions. The remote trial monitoring team arranges transportation should the patient need acute care. On-call investigators and board-certified oncologists coordinate remotely to perform adverse assessment grading, attribution of toxicity, dose adjustment, and response determination. Although technically feasible, home infusions still pose certain logistical challenges and inefficiencies. Where multiple patients could be managed simultaneously at a single infusion center, administration of intravenous therapies in a decentralized trial requires an infusion nurse to travel frequently to patients' homes and manage one patient at a time. For these reasons, drugs administered by oral, subcutaneous, or intramuscular routes, where a patient may self-administer, are more practical for decentralized trials at this time.19,24
Other Considerations
Cost considerations factor centrally in the implementation of decentralized clinical trials. Because oncology clinical trials frequently include standard therapies and assessments not paid for by trial sponsors, government and private insurance payers may need to provide coverage of at-home treatment and monitoring. This decision, in turn, may require endorsement of home-based cancer care by practice-guiding organizations such as the National Comprehensive Cancer Network. Only a few states have laws that ensure nonresearch activities related to clinical trials are covered by insurance.15 As there is a shift in resources to operate decentralized trials, sponsors will be required to fund the use of remote monitoring technologies, shipment of products to individual homes, tracking to ensure proper drug storage and access, and transportation costs for traveling coordinators.6 Nonetheless, decentralized trials may achieve overall cost savings and operational efficiency if the centralized team can reduce the tasks involved with trial management, as a whole, by eliminating redundancy, such as those seen with activating a trial across multiple traditional sites.6
Without having to pay for transportation and potentially lodging, patients and caregivers may benefit financially from virtual-first clinical trials. However, digital access and literacy become greater concerns, as decentralized trials rely heavily upon regular use of computers and/or mobile devices, as well as equitable broadband access.25 The impact on caregivers, who might have greater responsibilities to support decentralized trial participation, also needs to be elucidated. Given the need for imaging to evaluate tumor response, another consideration is availability of state-of-the-art radiology technology such as positron emission tomography, computerized tomography, and magnetic resonance imaging, which are not ubiquitous in rural settings.26-28
In conclusion, decentralized approaches will require a major shift in the consideration, implementation, and conduct of cancer clinical trials. From a regulatory standpoint, this strategy requires clear separation between study-related tasks (completed only by study investigators and personnel) and other aspects of clinical care. Beyond regulatory considerations, it will be critical that patients' local clinical teams not feel they are providing extra services for which they receive neither compensation nor credit. Indeed, because of their complexity and potential toxicities, many cancer clinical trials may not be suitable for home-based settings. Nevertheless, advances in drug delivery, digital technologies, and at-home care models have clearly increased the feasibility of decentralized trials in oncology. Potentially available to patients across the country and beyond, the virtual-first paradigm may substantially increase access to clinical trials, thereby enhancing participant diversity and result generalizability. Sustaining this model will require continuation of the COVID-19–related federal and payor policies that support telehealth-based medical care. Achieving the promise of this novel approach will require optimizing referral patterns, defining the roles of the longitudinal cancer care and clinical trial teams, and ensuring the safe and efficient delivery and monitoring of cancer treatments in patients' homes.
Sherry Fu
Employment: Aetio Biotherapy Inc
Leadership: Aetio Biotherapy Inc
Stock and Other Ownership Interests: Aetio Biotherapy Inc
Patents, Royalties, Other Intellectual Property: Patent pending: “Compositions and Methods for Treating Viral Infections” (publication number WO2021158907A1)
David E. Gerber
Stock and Other Ownership Interests: Gilead Sciences
Consulting or Advisory Role: Samsung Bioepis, Catalyst Pharmaceuticals, Mirati Therapeutics, Janssen Oncology, BeiGene, Sanofi, Regeneron
Research Funding: BerGenBio (Inst), Karyopharm Therapeutics (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Oxford University Press from two books, Royalties from Decision Support in Medicine from the Clinical Decision Support--Oncology online program, Patent pending: “Prediction and Treatment of Immunotherapeutic Toxicity” (provisional, application number 62/461,455) (Inst)
Uncompensated Relationships: Bristol Myers Squibb
Muhammad Shaalan Beg
Employment: Science 37
Consulting or Advisory Role: Ipsen, Array BioPharma, AstraZeneca/MedImmune, Cancer Commons, Legend Biotech, Foundation Medicine
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Merck Serono (Inst), Five Prime Therapeutics (Inst), MedImmune (Inst), Genentech (Inst), ImmuneSensor Therapeutics (Inst), Tolero Pharmaceuticals (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by NCI UM1CA186644 available to M.S.B. and NCI UG1CA233302-01 available to D.E.G.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Decentralized Clinical Trials in Oncology: Are We Ready for a Virtual-First Paradigm?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sherry Fu
Employment: Aetio Biotherapy Inc
Leadership: Aetio Biotherapy Inc
Stock and Other Ownership Interests: Aetio Biotherapy Inc
Patents, Royalties, Other Intellectual Property: Patent pending: “Compositions and Methods for Treating Viral Infections” (publication number WO2021158907A1)
David E. Gerber
Stock and Other Ownership Interests: Gilead Sciences
Consulting or Advisory Role: Samsung Bioepis, Catalyst Pharmaceuticals, Mirati Therapeutics, Janssen Oncology, BeiGene, Sanofi, Regeneron
Research Funding: BerGenBio (Inst), Karyopharm Therapeutics (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Oxford University Press from two books, Royalties from Decision Support in Medicine from the Clinical Decision Support--Oncology online program, Patent pending: “Prediction and Treatment of Immunotherapeutic Toxicity” (provisional, application number 62/461,455) (Inst)
Uncompensated Relationships: Bristol Myers Squibb
Muhammad Shaalan Beg
Employment: Science 37
Consulting or Advisory Role: Ipsen, Array BioPharma, AstraZeneca/MedImmune, Cancer Commons, Legend Biotech, Foundation Medicine
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca/MedImmune (Inst), Merck Serono (Inst), Five Prime Therapeutics (Inst), MedImmune (Inst), Genentech (Inst), ImmuneSensor Therapeutics (Inst), Tolero Pharmaceuticals (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bekelman JE, Emanuel EJ, Navathe AS: Outpatient treatment at home for Medicare beneficiaries during and after the COVID-19 pandemic. JAMA 324:21-22, 2020 [DOI] [PubMed] [Google Scholar]
- 2.1135 Waivers. US Centers for Medicare and Medicaid Services. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/1135-Waivers [Google Scholar]
- 3.Telehealth : Delivering Care Safely During COVID-19. US Department of Health and Human Services. https://www.hhs.gov/coronavirus/telehealth/index.html [Google Scholar]
- 4.Whitehead DC, Mehrotra A: The growing phenomenon of “Virtual-First” primary care. JAMA 326:2365-2366, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Kurzrock R, MacKenzie AR, Jurdi AA, et al. : Alpha-T: An innovative decentralized (home-based) phase 2 trial of alectinib in ALK-positive (ALK+) solid tumors in a histology-agnostic setting. J Clin Oncol 39, 2021. (suppl 15; abstr TPS3155) [Google Scholar]
- 6.Van Norman GA: Decentralized clinical trials: The future of medical product development? JACC Basic Transl Sci 6:384-387, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali Z, Zibert JR, Thomsen SF: Virtual clinical trials: Perspectives in dermatology. Dermatology 236:375-382, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Spertus JA, Birmingham MC, Butler J, et al. : Novel trial design: CHIEF-HF. Circ Heart Fail 14:e007767, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertus JA, Birmingham MC, Nassif M, et al. : The SGLT2 inhibitor canagliflozin in heart failure: The CHIEF-HF remote, patient-centered randomized trial. Nat Med 28:809-813, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parseghian CM, Tam AL, Yao J, et al. : Assessment of reported trial characteristics, rate of publication, and inclusion of mandatory biopsies of research biopsies in clinical trials in oncology. JAMA Oncol 5:402-405, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randell RL, Singler L, Cunningham A, et al. : Delivering clinical trials at home: Protocol, design and implementation of a direct-to-family paediatric lupus trial. Lupus Sci Med 8:e000494, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OHRP : Engagement of Institutions in Human Subjects Research. US Department of Health & Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/guidance/guidance-on-engagement-of-institutions/index.html [Google Scholar]
- 13.Baer AR, Michaels M, Good MJ, Schapira L: Engaging referring physicians in the clinical trial process. JCO Oncol Pract 8:e8-e10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Getz KA: US physician and nurse proclivity to refer their patients into clinical trials. Ther Innov Regul Sci 54:404-410, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Kaplan CP, Nápoles AM, Dohan D, et al. : Clinical trial discussion, referral, and recruitment: Physician, patient, and system factors. Cancer Causes Control 24:979-988, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Center for Drug Evaluation and Research : Drugs@FDA. US Food and Drug Administration. www.fda.gov/drugsatfda [Google Scholar]
- 17.COA : COA’s Position Statement on Home Infusion. Community Oncology Alliance. https://communityoncology.org/coas-position-statement-on-home-infusion/ [Google Scholar]
- 18.Kadakia KT, Halperin DM, Offodile AC II: Operationalizing virtual trials in oncology—From aspiration to action. JCO Clin Cancer Inform 5:953-957, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Voelker R: Breast cancer drug can Be given at home or in the clinic. JAMA 324:433-433, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Denys H, Martinez-Mena CL, Martens MT, et al. : Safety and tolerability of subcutaneous trastuzumab at home administration, results of the phase IIIb open-label BELIS study in HER2-positive early breast cancer. Breast Cancer Res Treat 181:97-105, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen FO, Christiansen AB, Rishøj A, et al. : Safety and feasibility of home-based chemotherapy. Dan Med J 65:A5482, 2018 [PubMed] [Google Scholar]
- 22.Mooney K, Titchener K, Haaland B, et al. : Evaluation of oncology hospital at home: Unplanned health care utilization and costs in the huntsman at home real-world trial. J Clin Oncol 39:2586-2593, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughlin AI, Begley M, Delaney T, et al. : Accelerating the delivery of cancer care at home during the Covid-19 pandemic. NEJM Catalyst Innov Care Deliv, 2020. 10.1056/CAT.20.0258 [Google Scholar]
- 24.Tan AR, Im SA, Mattar A, et al. : Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): A randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol 22:85-97, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Offodile AC 2nd, Seitz AJ, Peterson SK: Digital health navigation: An enabling infrastructure for optimizing and integrating virtual care into oncology practice. JCO Clin Cancer Inform 5:1151-1154, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaliq AA, Deyo D, Duszak R Jr: The impact of hospital characteristics on the availability of radiology services at critical access hospitals. J Am Coll Radiol 12:1351-1356, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Ginde AA, Foianini A, Renner DM, et al. : Availability and quality of computed tomography and magnetic resonance imaging equipment in U.S. emergency departments. Acad Emerg Med 15:780-783, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Burdorf BT: Comparing MRI and CT machine accessibility among urban and rural county hospitals. J Public Health Res 11:2527, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]