PURPOSE
Bone marrow biopsies (BMB) are performed before/after therapy to confirm complete response (CR) in patients with lymphoma on clinical trials. We sought to establish whether BMB add value in assessing response or predict progression-free survival (PFS) or overall survival (OS) outcomes in follicular lymphoma (FL) subjects in a large, multicenter, multitrial cohort.
METHODS
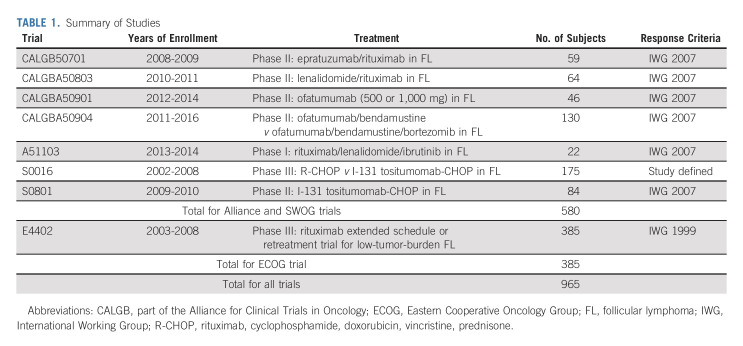
Data were pooled from seven trials of 580 subjects with previously untreated FL through Alliance for Clinical Trials in Oncology (Alliance) and SWOG Cancer Research Network (SWOG) completing enrollment from 2008 to 2016.
RESULTS
Only 5/580 (0.9%) had positive baseline BMB, CR on imaging, and subsequent positive BMB (P < .0001). Therefore, BMB were irrelevant to response in 99% of subjects. A sensitivity analysis of 385 FL subjects treated on an Eastern Cooperative Oncology Group study was included. In the Eastern Cooperative Oncology Group cohort, 5/385 (1.3%) had BMB that affected response assessment. Since some subjects do not undergo confirmatory BMB, we performed a landmark survival analysis from first radiologic CR with data from 580 subjects from Alliance and SWOG. Of subjects with CR on imaging (n = 187), PFS and OS were not significantly different among those with negative BMB to confirm CR (n = 47) versus those without repeat BMB (n = 140; PFS: adjusted hazard ratio, 1.10, 95% CI, 0.62 to 1.94, log-rank P = .686; OS: hazard ratio, 0.59, 95% CI, 0.23 to 1.53, log-rank P = .276).
CONCLUSION
We conclude that BMB add little value to response assessment in subjects with FL treated on clinical trials and we recommend eliminating BMB from clinical trial requirements. BMB should also be removed from diagnostic guidelines for FL except in scenarios in which it may change management including confirmation of limited stage and assessment of cytopenias. This would reduce cost, patient discomfort, resource utilization, and potentially remove a barrier to trial enrollment.
INTRODUCTION
Clinical trial requirements can be burdensome and deter patients from participation. Initiatives through ASCO focus on simplifying such protocols in a patient-centered approach to encourage enrollment.1-3 In clinical practice of follicular lymphoma (FL), utility of bone marrow biopsies (BMB) is controversial. The National Comprehensive Cancer Network (NCCN) guidelines for FL recommend BMB and aspiration in certain circumstances including to confirm stage I-II disease in patients being considered for radiotherapy or in those who require investigation of cytopenias. They note that BMB can be omitted for patients being observed without treatment.4 The European Society for Medical Oncology Clinical Practice Guidelines advise that BMB and aspiration should be done in all patients with newly diagnosed FL, particularly those with suspected early-stage disease.5 Response criteria for clinical trials in lymphoma require that BMB be performed at baseline and then repeated to confirm complete response (CR) in those with positive baseline BMB.6-8
CONTEXT
Key Objective
Are bone marrow biopsies (BMB) relevant for response assessment in follicular lymphoma (FL) clinical trials?
Knowledge Generated
In 99% of subjects with FL enrolled on National Clinical Trials Network clinical trials, response is unchanged on the basis of BMB results. Among subjects with complete response on imaging, there is no progression-free survival or overall survival difference in those with negative confirmatory BMB versus those who do not undergo the procedure.
Relevance (S. Lentzsch)
-
BMB should be removed from diagnostic guidelines for FL except in scenarios in which it may change management including confirmation of limited stage and assessment of cytopenias.*
*Relevance section written by JCO Associate Editor Suzanne Lentzsch, MD, PhD.
We hypothesized that only rarely do subjects have a positive baseline BMB, CR on imaging, and then a positive subsequent BMB—the only scenario in which BM assessment could change response determination. We then investigated 99 subjects with FL treated on clinical trials at a single institution and found that 1.0% had a BMB that could have affected response assessment.9 We performed a similar analysis of the randomized GALLIUM clinical trial, which enrolled untreated subjects with FL to obinutuzumab plus chemotherapy versus rituximab plus chemotherapy followed by obinutuzumab or rituximab maintenance.10 We found that only 5/1,202 subjects (0.4%) had BMB that affected response assessment when computed tomography (CT)–based International Working Group (IWG) 2007 response criteria were used.11 The GALLIUM trial required fluorodeoxyglucose-positron emission tomography (PET) imaging in the first 170 enrolled subjects, and PET was optional in subsequent subjects. PET was performed in 282 patients with positive/indeterminate baseline BMB. Two hundred thirteen of these patients underwent confirmatory BMB. Of 213 subjects with positive or indeterminate baseline BMB who underwent PET and repeat BMB after treatment, BMB were relevant for response assessment in a maximum of 10 (4.7%, five positive BMB and five indeterminate). We sought to confirm these results in subjects with untreated FL enrolled on National Cancer Institute National Clinical Trials Network (NCTN) trials. Our goals were to validate findings of preliminary studies to foster efforts to simplify future clinical trial requirements for subjects who may be discouraged from participation in clinical trials, and to change practice guidelines requiring BMB in the majority of patients with FL. In addition to encouraging patient participation in clinical trials and minimizing patient discomfort, this effort would also decrease cost and utilization of resources.
METHODS
We identified all clinical trials completing enrollment in the modern therapeutic era from 2008 to 2016 by the Alliance for Clinical Trials (Alliance and legacy Cancer and Leukemia Group B [CALGB]) and SWOG Cancer Research Network (SWOG) of subjects with untreated FL for which BMB results at baseline and during response assessment, and best response by imaging, were available. All studies were approved by the institutional review board at each participating site, and informed consent forms were signed by all subjects enrolled on the trials. For each study, and for all studies combined, we calculated the proportion of subjects with positive baseline BMB, CR on imaging after treatment, and positive repeat BMB using the total number of subjects enrolled as the denominator. These are the only subjects in whom BMB would affect response assessment. The majority of studies used IWG 2007 guidelines with five of eight protocols stating that BMB morphology and immunohistochemistry were required to be negative (with goal > 20 mm core) but that bone marrow aspirate was not required to be negative to confirm CR. Those five protocols indicated that a small population of clonal lymphocytes by flow cytometry in the bone marrow aspirate was considered a CR until data became available demonstrating a clear difference in patient outcome. Similarly, IWG 2007 guidelines indicate that histologically normal bone marrows with a small (< 2%) B-cell population detected by flow cytometry should be considered normal.7 The SWOG and Eastern Cooperative Oncology Group (ECOG) studies did require both biopsies and aspirates to be negative to confirm CR.
Statistical analysis was conducted by the Alliance Statistics and Data Center. We tested against the null hypothesis that this proportion was ≥ 10%, versus the alternative hypothesis that this proportion was < 10% (the threshold below which BMB would be considered inconsequential for response assessment), using a one-sided exact binomial test. With 500 subjects, we would have 99% power to reject that this proportion is > 10% when the true proportion is 5% using a binary test of a single proportion with one-sided alpha = .025. The power calculation was not a design feature of the study. This is a secondary analysis using existing data from completed NCTN clinical trials. Response criteria were CT-based. Imaging was not used to assess for BM involvement. BMB were unilateral in six clinical trials and bilateral in two.
Because confirmatory BMB were not completed in all indicated subjects, landmark survival analyses compared progression-free survival (PFS) and overall survival (OS) of subjects with CR on imaging and negative BMB versus subjects with CR on imaging without repeat BMB. This analysis was performed in subjects with a positive baseline BMB. Subjects with CR on imaging were categorized as having negative repeat BMB or no repeat BMB within 60 days of the first CR on imaging. PFS was defined from time of CR to progression or death. OS was defined as time of first CR to death. The time-to-event end points were calculated using a landmark analysis approach from first radiologic CR and estimated using Kaplan-Meier, and compared using log-rank tests, as well as univariate and multivariate Cox models adjusted for age, sex, stage, and Follicular Lymphoma International Prognostic Index (FLIPI) score, and stratified by treatment arm within study. An ECOG trial was analyzed separately as a sensitivity analysis, as it included only one time point for follow-up bone marrow assessment (13 weeks), in contrast to the Alliance and SWOG studies with repeated evaluations of response by imaging and BMB.
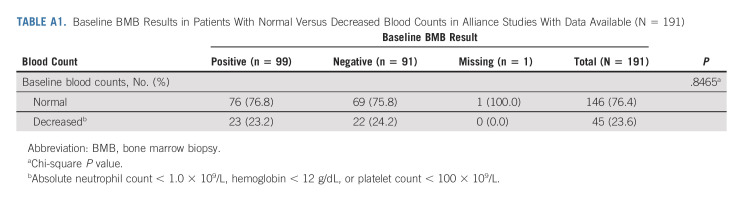
To investigate whether cytopenias were associated with positive findings of FL on initial BMB, we identified subjects enrolled on the five Alliance clinical trials whose baseline blood counts were available and met any of the following criteria: absolute neutrophil count < 1.0 × 109/L, hemoglobin < 12 g/dL, or platelet count < 100 × 109/L. We compared baseline BMB results of subjects with and without one or more cytopenias by these criteria.
RESULTS
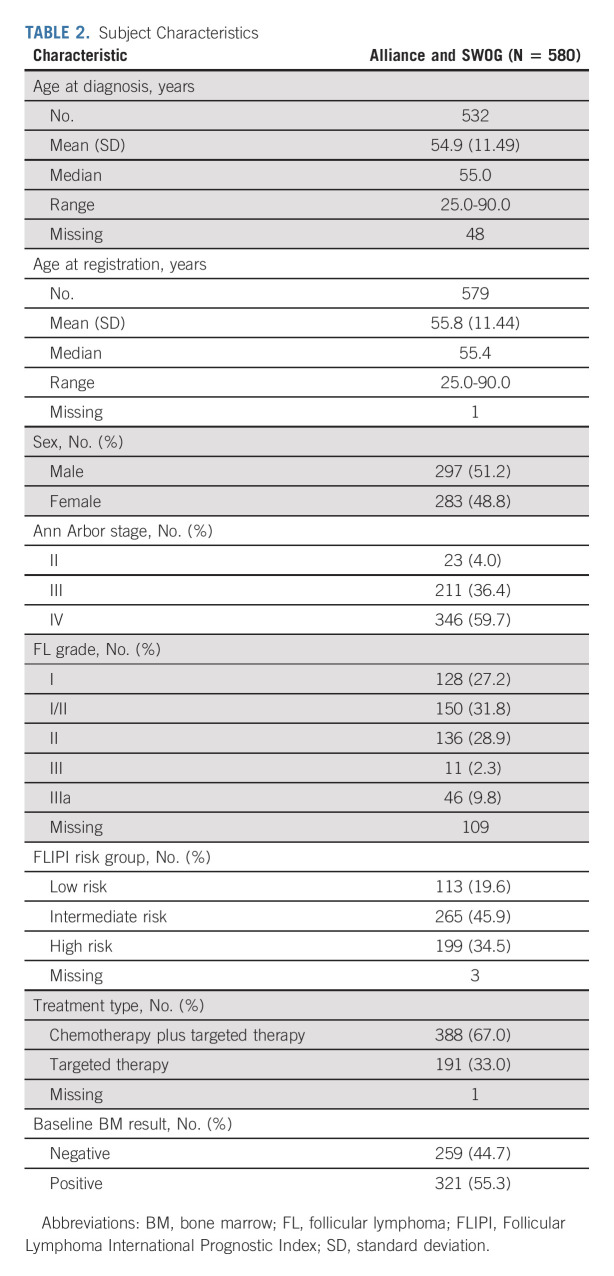
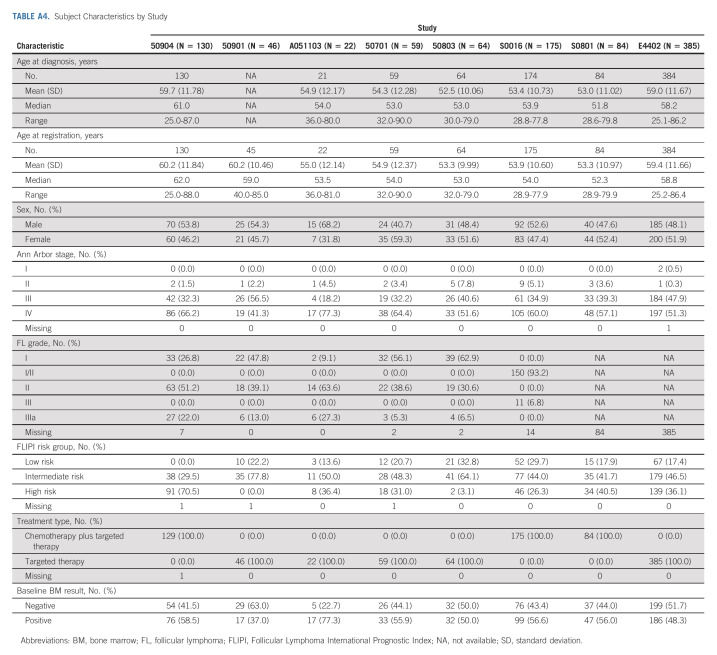
We identified seven studies meeting inclusion criteria that completed enrollment of a total of 580 subjects with FL, the majority of whom had advanced stage disease, from 2008 to 2016 through Alliance and SWOG (Table 1). One patient with no baseline BMB result was excluded. Five studies were phase II and one each were phase I and phase III. Six of the studies used IWG 2007 criteria for response assessment and one used study-defined criteria. Subject characteristics are listed in Table 2. Median age was 55 years (51% male, 96% stage III-IV, and 88% grade I-II). FLIPI scores were low in 20%, intermediate in 46%, and high risk in 35%. Chemotherapy-based regimens were administered to 67% of subjects. Baseline BMB were positive in 55%. Of subjects enrolled on Alliance clinical trials with available information (n = 191), there was no association between subjects with one or more cytopenia at baseline and positive initial BMB results (P = .8465; Appendix Table A1 [online only]).
TABLE 1.
Summary of Studies
TABLE 2.
Subject Characteristics
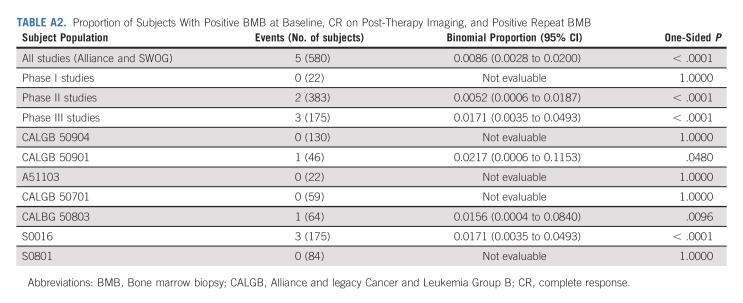
Only 5/580 (0.8%) FL subjects in Alliance and SWOG trials had positive baseline BMB, CR on imaging, and subsequent positive BMB (P < .0001; Appendix Table A2, online only). Of 344 subjects with a CR on imaging after treatment, 1.5% (5/344) had BMB that altered response assessment. See Appendix Figures A1A and A1B (online only) for CONSORT diagrams for Alliance and SWOG, and ECOG trials.
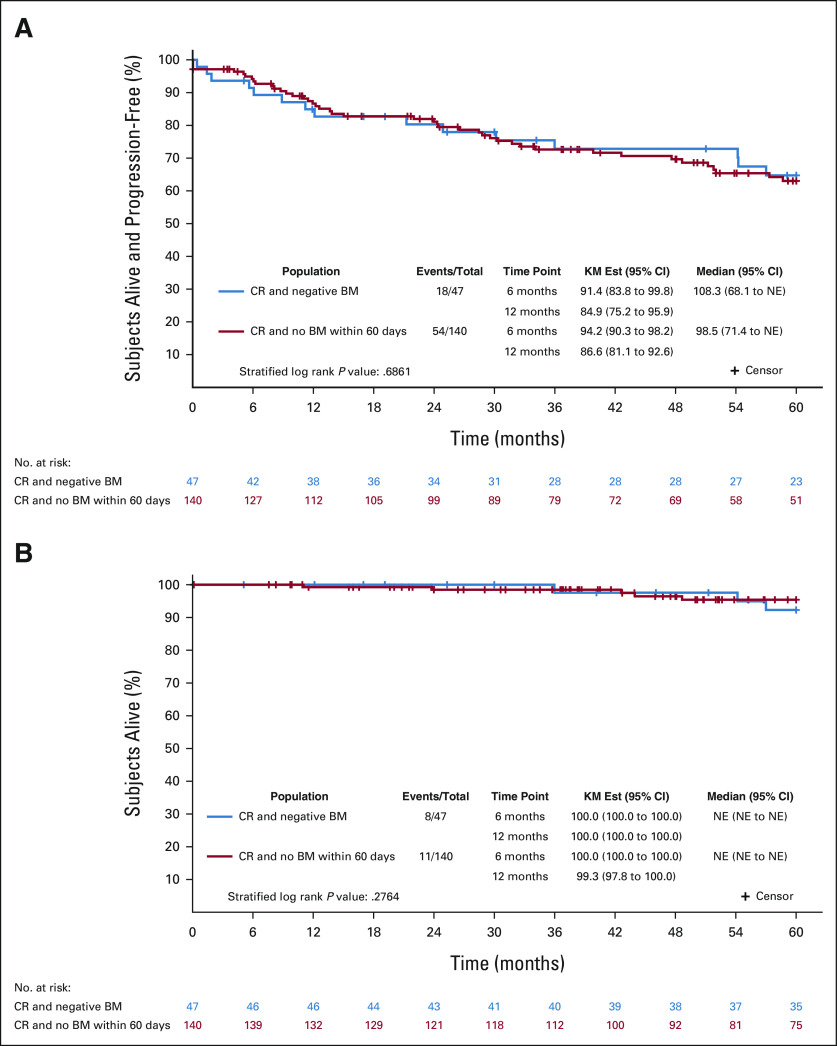
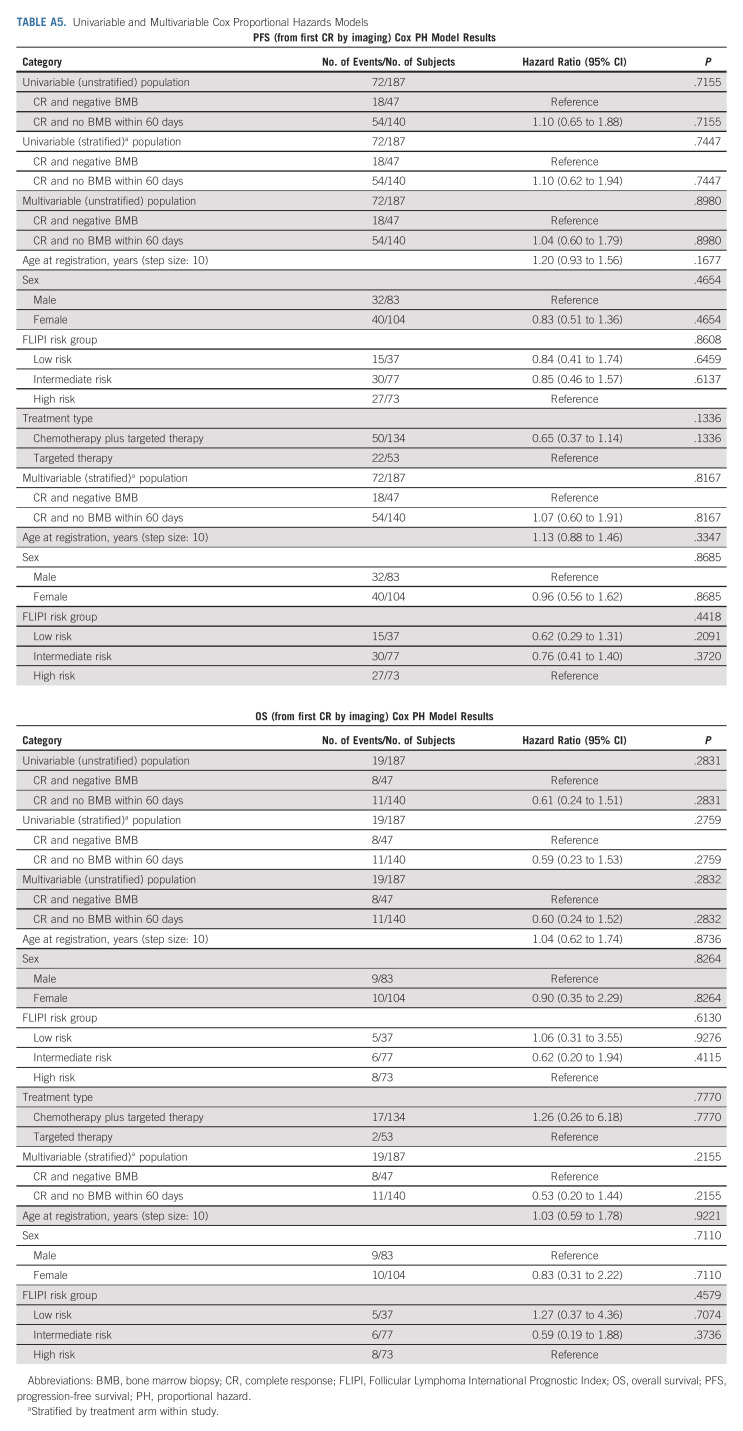
The landmark survival analysis from time of first radiologic CR was performed in subjects with previously untreated FL enrolled on Alliance and SWOG trials. Of subjects with CR on imaging (n = 187), PFS and OS were not different among subjects with negative BMB within 60 days of CR on imaging (n = 47) versus subjects without repeat BMB within 60 days of imaging (n = 140; PFS: HRadj = 1.10, 95% CI, 0.62 to 1.94, log-rank P = .686; OS: HR = 0.59, 95% CI, 0.23 to 1.53, log-rank P = .276; Fig 1 and Appendix Table A2).
FIG 1.
Landmark survival analysis. For subjects with positive BMB at baseline and subsequent CR on imaging, (A) progression-free survival and (B) overall survival were not different between those patients who had negative BMB within 60 days of the first CR on imaging (n = 47) and those who did not undergo repeat BMB within 60 days (n = 140). BM, bone marrow; BMB, bone marrow biopsy; CR, complete response; KM, Kaplan-Meier; NE, not evaluable.
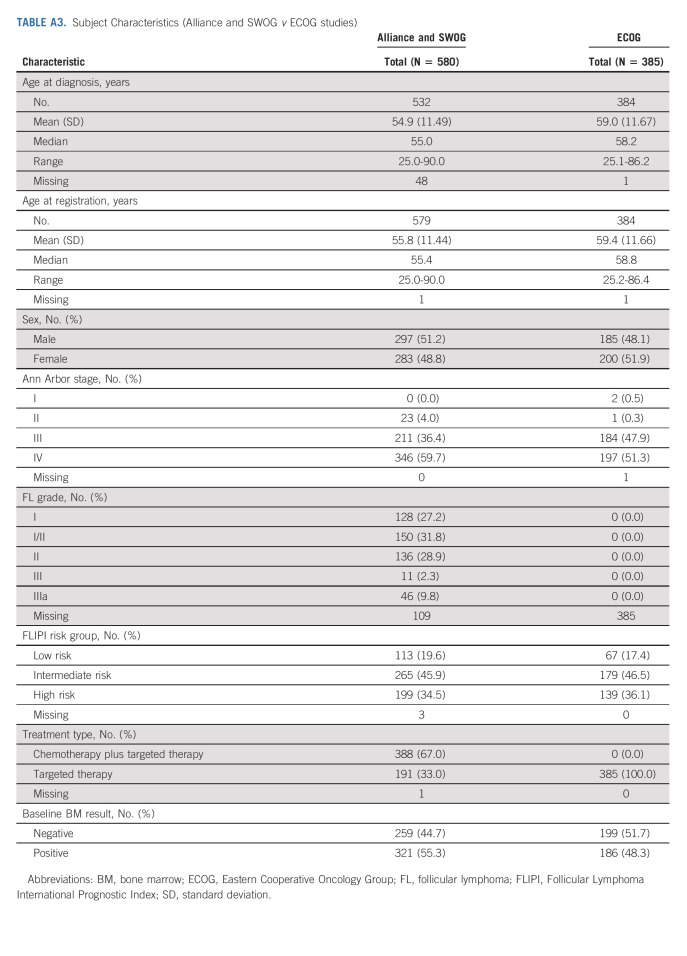
Two clinical trials conducted by ECOG that met inclusion criteria were identified (E4402 and E2408). E2408 was excluded because the data were not available at the time of our data collection. A sensitivity analysis was conducted on the 385 subjects with untreated FL on RESORT (E4402), a phase III trial of rituximab extended schedule versus retreatment at progression. Because imaging results were mandated only at an interim point (and therefore the results are not necessarily reflective of best response), we were unable to combine the analysis with that from the Alliance and SWOG trials. Characteristics of subjects on the ECOG trial were similar to those enrolled on Alliance and SWOG trials (Appendix Tables A3 and A4, online only). Of 385 subjects enrolled on the ECOG trial, only five (1.3%) had BMB that affected response assessment (Appendix Fig A1B).
DISCUSSION
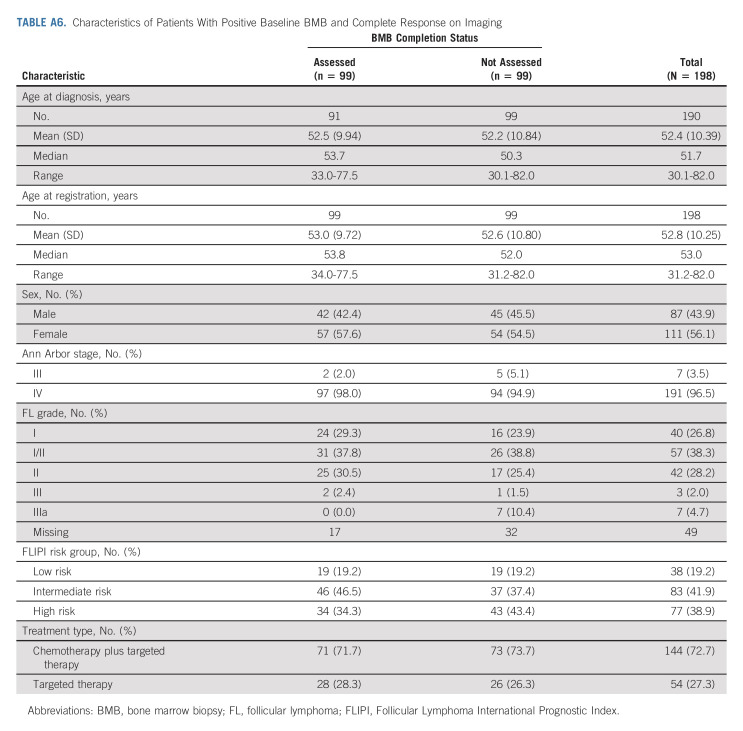
In conclusion, we establish that BMB do not affect response assessment in NCTN clinical trials enrolling subjects with untreated FL. We analyzed two different data sets with close to 1,000 total subjects, and the results of BMB changed response assessment in only 10 subjects (about 1%). On average, in the primary data set, one subject's response assessment was altered for every 116 subjects required to undergo BMB. A significant percentage of subjects with CR on imaging did not undergo confirmatory BMB (99/198, 50% in Alliance/SWOG trials; and 22/47, 47% in the ECOG trial). Although reasons for the omissions were not recorded, we presume that both patient and physician preference may have been the primary contributing factors (Appendix Tables A5 and A6, online only).
The landmark survival analysis was performed to compare outcomes in subjects who did not undergo BMB with those who did have confirmatory BMB, and we found no difference in PFS and OS. Therefore, BMB do not enable identification of distinct PFS/OS outcomes in FL patients with positive findings at baseline.
In another subtype of lymphoma, classical Hodgkin lymphoma, BMB were formerly required as part of diagnostic workup beginning with the guidelines established by the Committee on Hodgkin's Disease Staging Classification in 1971.12 With incorporation of CT imaging into practice guidelines, recommendations were changed in 1989 to include BMB as a requirement in only those HL patients with stage III-IV disease or stage II disease with high-risk features in whom bone marrow involvement would change management.13 When PET-CT became routinely used in staging of HL, this modality was established to accurately assess for bone marrow involvement in that disease.14 A retrospective study of 454 patients with HL concluded that BMB do not alter risk assessment or treatment decisions in these patients, and the procedures were subsequently eliminated from staging requirements.8,15-17 In diffuse large B-cell lymphoma (DLBCL), multiple studies have determined that PET-CT accurately reveals bone marrow involvement.18-20 Therefore, NCCN guidelines state that BMB are not necessary in DLBCL if PET-CT detects bone disease.4 Our findings from a retrospective review of the GOYA study indicate that BMB have minimal impact on response assessment in patients with DLBCL as well.11
Guidelines for FL still require routine staging BMB, although the procedures are inconsistently done in clinical practice.4,5 NCCN guidelines do specifically note that the procedures can be eliminated in those who are being followed without treatment.4 Our study indicates that BMB are unnecessary for response assessment and do not affect PFS or OS.
BMB in FL are still useful in select circumstances including for confirmation of stage I disease in which radiotherapy can be administered with curative intent. The stage I FL patient population was studied prospectively in the National LymphoCare Study, and those who underwent rigorous staging including imaging and BMB had longer PFS compared with those who did not.21 In addition, in FL patients with significant cytopenias, BMB can help determine the etiology. Interestingly, in our study, there was no association found between baseline cytopenias and positive findings on BMB.
Limitations of our study include its retrospective nature and response criteria variation in NCTN FL clinical trials. The CT-based IWG 2007 criteria were the response criteria in place at the time when the trials included in our study were performed. Current clinical trials primarily use the PET-based Lugano criteria. The two do not differ with respect to BMB recommendations; therefore, we expect our findings and conclusions to also apply when Lugano criteria are used to determine response. Our prior investigation of the GALLIUM data set found that a minimal number of subjects with FL who underwent PET imaging had alteration in response assessment on the basis of BMB. Because of differences in timing of BMB requirements, we were unable to include FL subjects from the ECOG trial in the primary analysis; however, findings with this cohort were similar to those in the primary group. We cannot rule out the influence of tumor bulk in the patient population studied in our analysis, but we think it is unlikely that this would affect our findings. The broad inclusion criteria are an advantage of our study, which make our conclusions applicable to the wide variety of patients with FL receiving frontline therapy. We note that bone marrow assessments may have been limited by technical issues including size of core, as well as lack of central review. In addition, a significant number of subjects did not undergo confirmatory BMB. This may indicate the hesitancy of both patients and physicians regarding invasive procedures, especially when the results would not change management. The landmark survival analysis confirms there is no difference in PFS or OS in subjects with CR on imaging regardless of BMB results.
Less invasive diagnostic tests including liquid biopsies are currently being investigated in lymphomas. Circulating tumor DNA levels of BCL2/IgH rearrangement and V(D)J immunoglobulin sequences correlate with PFS in FL.22-25 In mantle cell lymphoma, higher levels of circulating tumor DNA are associated with bone marrow involvement.26 These types of assays may eventually provide a surrogate for bone marrow involvement in lymphomas and become part of standard staging and monitoring of lymphomas including FL.
BMB requirements may discourage participation in clinical trials and add pain, expense, and time without providing necessary information in enrolled FL subjects. Furthermore, for 99% of patients with FL (including 96% with advanced stage disease), we show no benefit to marrow assessment on either prognosis or response assessment, validating previously published smaller experiences. Based upon these results, BMB should be eliminated from diagnostic guidelines in FL, and no longer incorporated as response assessments in clinical trials for patients with FL. This strategy is consistent with initiatives including the American Board of Internal Medicine Foundation's Choosing Wisely Campaign, as well as joint efforts by ASCO and Friends of Cancer Research to maximize value of medical interventions and modernize eligibility criteria and therefore make clinical care and trial enrollment more patient-focused.1-3,27
APPENDIX
FIG A1.
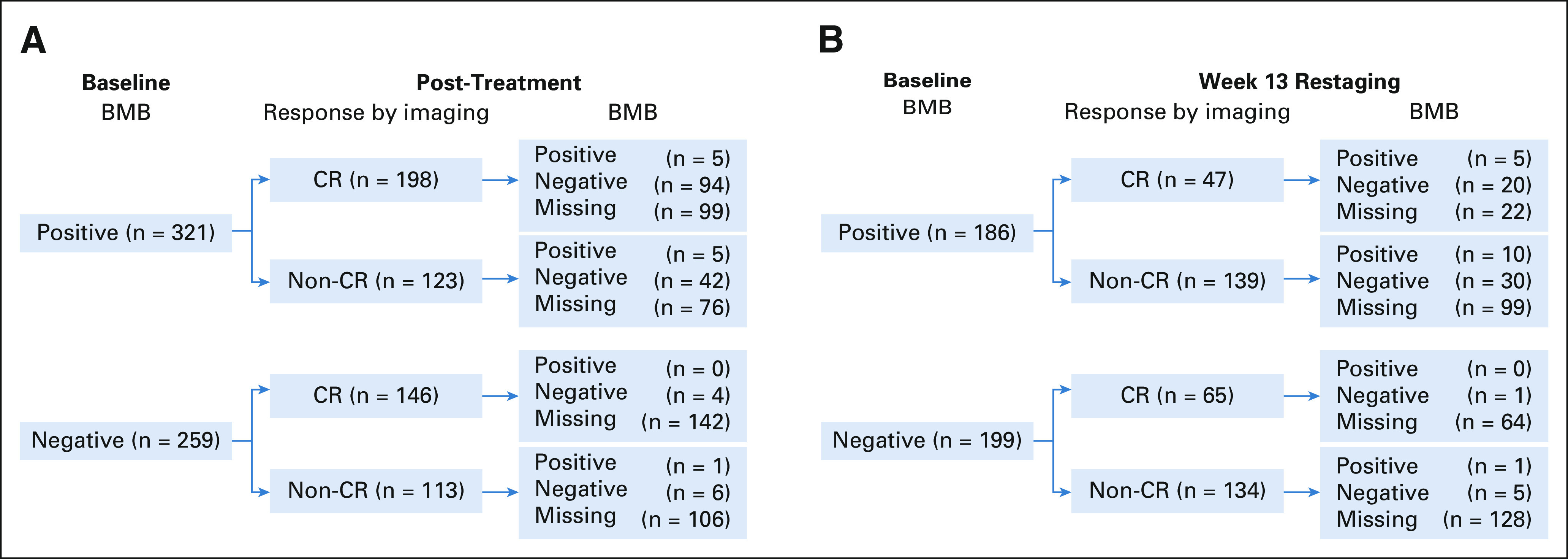
(A) Flow diagram for Alliance and SWOG trials. (B) Flow diagram for the ECOG trial. BMB, bone marrow biopsy; CR, complete response; ECOG, Eastern Cooperative Oncology Group.
TABLE A1.
Baseline BMB Results in Patients With Normal Versus Decreased Blood Counts in Alliance Studies With Data Available (N = 191)
TABLE A2.
Proportion of Subjects With Positive BMB at Baseline, CR on Post-Therapy Imaging, and Positive Repeat BMB
TABLE A3.
Subject Characteristics (Alliance and SWOG v ECOG studies)
TABLE A4.
Subject Characteristics by Study
TABLE A5.
Univariable and Multivariable Cox Proportional Hazards Models
TABLE A6.
Characteristics of Patients With Positive Baseline BMB and Complete Response on Imaging
Sarah C. Rutherford
Consulting or Advisory Role: Kite, a Gilead company, Dova Pharmaceuticals, ADC Therapeutics, Juno/Celgene, Karyopharm Therapeutics
Research Funding: Genentech (Inst), Karyopharm Therapeutics (Inst)
Jun Yin
Employment: Mayo Clinic
Mazyar Shadman
Consulting or Advisory Role: AbbVie, Genentech, AstraZeneca, Sound Biologics, Cellectar, Pharmacyclics, BeiGene, Bristol Myers Squibb/Celgene, MorphoSys, Innate Pharma, Kite, a Gilead company, Adaptive Biotechnologies, Epizyme, Fate therapeutics, Lilly, Regeneron, Adaptimmune, MustangBio, TG Therapeutics, MEI Pharma
Research Funding: Pharmacyclics (Inst), Acerta Pharma (Inst), Merck (Inst), TG Therapeutics (Inst), BeiGene (Inst), Celgene (Inst), Genentech (Inst), MustangBio (Inst), AbbVie (Inst), Sunesis Pharmaceuticals (Inst), Bristol Myers Squibb/Celgene
Michael L. LeBlanc
Consulting or Advisory Role: Agios
Vaishalee P. Kenkre
Research Funding: Novartis (Inst), MEI Pharma (Inst), Celgene/Bristol Myers Squibb (Inst), Seattle Genetics (Inst), Abbott Laboratories (Inst), Gilead Sciences (Inst)
Fangxin Hong
Employment: Pfizer
Consulting or Advisory Role: Merck Sharp & Dohme
Kristie A. Blum
Honoraria: American Society of Hemotology, Leidos Biomedical Research/NCI
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), BMSi (Inst)
Travel, Accommodations, Expenses: American Society of Hemotology
Peter Martin
Consulting or Advisory Role: Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, Takeda
Research Funding: Karyopharm Therapeutics (Inst)
Sin-Ho Jung
Consulting or Advisory Role: Samsung
Cara Rosenbaum
Stock and Other Ownership Interests: Merck/Organon
Consulting or Advisory Role: Takeda, Janssen Biotech, Akcea Therapeutics, GlaxoSmithKline, Oncopeptides
Research Funding: Amgen, GlaxoSmithKline, Janssen Oncology, Karyopharm Therapeutics
Chaitra Ujjani
Honoraria: AstraZeneca, Pharmacyclics, Kite/Gilead, AbbVie, Genentech, MorphoSys, TG Therapeutics, Janssen, Incyte, BeiGene, Lilly
Consulting or Advisory Role: AstraZeneca, Epizyme, Atara Biotherapeutics
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Lilly, AstraZeneca/MedImmune, Adaptive Biotechnologies, Kite, a Gilead company
Paul M. Barr
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Pharmacyclics, AbbVie, Seattle Genetics, Genentech, Novartis, Infinity Pharmaceuticals, Janssen, Merck, TG Therapeutics, MorphoSys, AstraZeneca, BeiGene, MEI Pharma, Bristol Myers Squibb/Celgene, Bayer
Research Funding: Pharmacyclics (Inst), AstraZeneca (Inst)
Bruce D. Cheson
Leadership: SymBio Pharmaceuticals
Consulting or Advisory Role: TG Therapeutics, AbbVie, Pharmacyclics/Janssen, Morphosys, Celgene, Karyopharm Therapeutics, Epizyme, Gilead Sciences, SymBio Pharmaceuticals, Parexel, Lilly, Imaging Endpoints
Speakers' Bureau: MorphoSys/Incyte, BeiGene, Lilly, TG Therapeutics
Research Funding: TG Therapeutics (Inst), Seattle Genetics (Inst), Bristol Myers Squibb (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: SymBio Pharmaceuticals
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics, Roche/Genentech, ADC Therapeutics, BTG, Acerta Pharma
Research Funding: Seattle Genetics (Inst), Kite, a Gilead company (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Immune Design (Inst), Forty Seven (Inst), Janssen (Inst), Pharmacyclics (Inst), Millennium (Inst), ADC Therapeutics (Inst), Autolus (Inst), Roche/Genentech (Inst), Pfizer (Inst), Affimed Therapeutics (Inst)
Brad Kahl
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Celgene, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, Kite/Gilead, MorphoSys, Janssen, Bristol Myers Squibb, Incyte, Genmab
Research Funding: Genentech (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst), Celgene (Inst)
Jonathan W. Friedberg
This author is the Editor-in-Chief of Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Research Funding: Enterome (Inst)
Patents, Royalties, Other Intellectual Property: Patient on bone marrow microenvironment signals
Sumithra J. Mandrekar
Honoraria: BeiGene
Consulting or Advisory Role: Flatiron Health, Harbinger Oncology, Inc
Other Relationship: Beigene
John P. Leonard
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, ADC Therapeutics, MEI Pharma, AstraZeneca, Merck, Morphosys, Karyopharm Therapeutics, Sutro Biopharma, Miltenyi Biotec, Regeneron, Akcea Therapeutics, Sandoz, Nordic Nanovector, Genmab, AbbVie, Incyte, Janssen Oncology, Eisai, MustangBio, Second Genome
Research Funding: Celgene (Inst), Alliance for Clinical Trials in Oncology (Inst), Takeda (Inst), Pfizer (Inst), National Cancer Institute (Inst), Janssen Oncology (Inst), Epizyme (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: BeiGene
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRIOR PRESENTATION
Presented in abstract form at the American Society of Clinical Oncology Annual Meeting, virtual, May 29-31, 2020.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233339, UG1CA233247, UG1CA233253, UG1CA233277, UG1CA233328, U10CA180888, and U10CA180819. https://acknowledgments.alliancefound.org. Also supported in part by Celgene (50803) and GSK (50901, 50904).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data sharing requests should be made to sar2014@med.cornell.edu for deidentified data. Such requests will be considered by the study team after publication following review and approval of proposals, and with appropriate data-sharing agreements in place.
AUTHOR CONTRIBUTIONS
Conception and design: Sarah C. Rutherford, Jun Yin, Levi Pederson, Sumithra J. Mandrekar, John P. Leonard
Provision of study materials or patients: Mazyar Shadman, Hongli Li, Michael L. LeBlanc, Vaishalee Kenkre, Fangxin Hong, Kristie A. Blum, Travis Dockter, Peter Martin, Sin-Ho Jung, Barbara Grant, Cara Rosenbaum, Chaitra Ujjani, Paul M. Barr, Joseph M. Unger, Bruce D. Cheson, Nancy L. Bartlett, Brad Kahl, Jonathan W. Friedberg, Sumithra J. Mandrekar, John P. Leonard
Collection and assembly of data: Sarah C. Rutherford, Jun Yin, Levi Pederson, Gabriela Perez Burbano, Mazyar Shadman, Hongli Li, Michael L. LeBlanc, Vaishalee P. Kenkre, Fangxin Hong, Kristie A. Blum, Travis Dockter, Barbara Grant, Chaitra Ujjani, Joseph M. Unger, Bruce D. Cheson, Nancy L. Bartlett, Brad Kahl
Data analysis and interpretation: Sarah C. Rutherford, Jun Yin, Levi Pederson, Gabriela Perez Burbano, Betsy LaPlant, Mazyar Shadman, Vaishalee P. Kenkre, Kristie A. Blum, Peter Martin, Sin-Ho Jung, Cara Rosenbaum, Paul M. Barr, Joseph M. Unger, Nancy L. Bartlett, Brad Kahl, Jonathan W. Friedberg, Sumithra J. Mandrekar, John P. Leonard
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Relevance of Bone Marrow Biopsies for Response Assessment in US National Cancer Institute National Clinical Trials Network Follicular Lymphoma Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sarah C. Rutherford
Consulting or Advisory Role: Kite, a Gilead company, Dova Pharmaceuticals, ADC Therapeutics, Juno/Celgene, Karyopharm Therapeutics
Research Funding: Genentech (Inst), Karyopharm Therapeutics (Inst)
Jun Yin
Employment: Mayo Clinic
Mazyar Shadman
Consulting or Advisory Role: AbbVie, Genentech, AstraZeneca, Sound Biologics, Cellectar, Pharmacyclics, BeiGene, Bristol Myers Squibb/Celgene, MorphoSys, Innate Pharma, Kite, a Gilead company, Adaptive Biotechnologies, Epizyme, Fate therapeutics, Lilly, Regeneron, Adaptimmune, MustangBio, TG Therapeutics, MEI Pharma
Research Funding: Pharmacyclics (Inst), Acerta Pharma (Inst), Merck (Inst), TG Therapeutics (Inst), BeiGene (Inst), Celgene (Inst), Genentech (Inst), MustangBio (Inst), AbbVie (Inst), Sunesis Pharmaceuticals (Inst), Bristol Myers Squibb/Celgene
Michael L. LeBlanc
Consulting or Advisory Role: Agios
Vaishalee P. Kenkre
Research Funding: Novartis (Inst), MEI Pharma (Inst), Celgene/Bristol Myers Squibb (Inst), Seattle Genetics (Inst), Abbott Laboratories (Inst), Gilead Sciences (Inst)
Fangxin Hong
Employment: Pfizer
Consulting or Advisory Role: Merck Sharp & Dohme
Kristie A. Blum
Honoraria: American Society of Hemotology, Leidos Biomedical Research/NCI
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), BMSi (Inst)
Travel, Accommodations, Expenses: American Society of Hemotology
Peter Martin
Consulting or Advisory Role: Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, Takeda
Research Funding: Karyopharm Therapeutics (Inst)
Sin-Ho Jung
Consulting or Advisory Role: Samsung
Cara Rosenbaum
Stock and Other Ownership Interests: Merck/Organon
Consulting or Advisory Role: Takeda, Janssen Biotech, Akcea Therapeutics, GlaxoSmithKline, Oncopeptides
Research Funding: Amgen, GlaxoSmithKline, Janssen Oncology, Karyopharm Therapeutics
Chaitra Ujjani
Honoraria: AstraZeneca, Pharmacyclics, Kite/Gilead, AbbVie, Genentech, MorphoSys, TG Therapeutics, Janssen, Incyte, BeiGene, Lilly
Consulting or Advisory Role: AstraZeneca, Epizyme, Atara Biotherapeutics
Research Funding: Pharmacyclics (Inst), AbbVie (Inst), Lilly, AstraZeneca/MedImmune, Adaptive Biotechnologies, Kite, a Gilead company
Paul M. Barr
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Pharmacyclics, AbbVie, Seattle Genetics, Genentech, Novartis, Infinity Pharmaceuticals, Janssen, Merck, TG Therapeutics, MorphoSys, AstraZeneca, BeiGene, MEI Pharma, Bristol Myers Squibb/Celgene, Bayer
Research Funding: Pharmacyclics (Inst), AstraZeneca (Inst)
Bruce D. Cheson
Leadership: SymBio Pharmaceuticals
Consulting or Advisory Role: TG Therapeutics, AbbVie, Pharmacyclics/Janssen, Morphosys, Celgene, Karyopharm Therapeutics, Epizyme, Gilead Sciences, SymBio Pharmaceuticals, Parexel, Lilly, Imaging Endpoints
Speakers' Bureau: MorphoSys/Incyte, BeiGene, Lilly, TG Therapeutics
Research Funding: TG Therapeutics (Inst), Seattle Genetics (Inst), Bristol Myers Squibb (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: SymBio Pharmaceuticals
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics, Roche/Genentech, ADC Therapeutics, BTG, Acerta Pharma
Research Funding: Seattle Genetics (Inst), Kite, a Gilead company (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Immune Design (Inst), Forty Seven (Inst), Janssen (Inst), Pharmacyclics (Inst), Millennium (Inst), ADC Therapeutics (Inst), Autolus (Inst), Roche/Genentech (Inst), Pfizer (Inst), Affimed Therapeutics (Inst)
Brad Kahl
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Celgene, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, Kite/Gilead, MorphoSys, Janssen, Bristol Myers Squibb, Incyte, Genmab
Research Funding: Genentech (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst), Celgene (Inst)
Jonathan W. Friedberg
This author is the Editor-in-Chief of Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Research Funding: Enterome (Inst)
Patents, Royalties, Other Intellectual Property: Patient on bone marrow microenvironment signals
Sumithra J. Mandrekar
Honoraria: BeiGene
Consulting or Advisory Role: Flatiron Health, Harbinger Oncology, Inc
Other Relationship: Beigene
John P. Leonard
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, ADC Therapeutics, MEI Pharma, AstraZeneca, Merck, Morphosys, Karyopharm Therapeutics, Sutro Biopharma, Miltenyi Biotec, Regeneron, Akcea Therapeutics, Sandoz, Nordic Nanovector, Genmab, AbbVie, Incyte, Janssen Oncology, Eisai, MustangBio, Second Genome
Research Funding: Celgene (Inst), Alliance for Clinical Trials in Oncology (Inst), Takeda (Inst), Pfizer (Inst), National Cancer Institute (Inst), Janssen Oncology (Inst), Epizyme (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: BeiGene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rubin EH, Scroggins MJ, Goldberg KB, et al. : Strategies to maximize patient participation in clinical trials. Am Soc Clin Oncol Ed Book 37:216-221, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Lichtman SM, Harvey RD, Damiette Smit MA, et al. : Modernizing clinical trial eligibility criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol 35:3753-3759, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Nipp RD, Hong K, Paskett ED: Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Ed Book 39:105-114, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Zelenetz AD, Gordon LI, Abramson JS, et al. : NCCN guidelines insights: B-cell lymphomas, version 3.2019. J Natl Compr Canc Netw 17:650-661, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Ghielmini M, Rule S, et al. : Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 32:298-308, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Cheson BD, Horning SJ, Coiffier B, et al. : Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Pfistner B, Juweid ME, et al. : Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutherford SC, Li V, Ghione P, et al. : Bone marrow biopsies do not impact response assessment for follicular lymphoma patients treated on clinical trials. Br J Haematol 179:242-245, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Marcus R, Davies A, Ando K, et al. : Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 377:1331-1344, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Rutherford SC, Herold M, Hiddemann W, et al. : Impact of bone marrow biopsy on response assessment in immunochemotherapy-treated lymphoma patients in GALLIUM and GOYA. Blood Adv 4:1589-1593, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone PP, Kaplan HS, Musshoff K, et al. : Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 31:1860-1861, 1971 [PubMed] [Google Scholar]
- 13.Lister TA, Crowther D, Sutcliffe SB, et al. : Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7:1630-1636, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Hutchings M, Loft A, Hansen M, et al. : Position emission tomography with or without computed tomography in the primary staging of Hodgkin's lymphoma. Haematologica 91:482-489, 2006 [PubMed] [Google Scholar]
- 15.El-Galaly TC, d'Amore F, Mylam KJ, et al. : Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 30:4508-4514, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. : Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048-3058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichenauer DA, Aleman BMP, André M, et al. : Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv19-iv29, 2018. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 18.Khan AB, Barrington SF, Mikhaeel NG, et al. : PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 122:61-67, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Berthet L, Cochet A, Kanoun S, et al. : In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med 54:1244-1250, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Adams HJA, Kwee TC, de Keizer B, et al. : FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: Systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 41:565-574, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Friedberg JW, Byrtek M, Link BK, et al. : Effectiveness of first-line management strategies for stage I follicular lymphoma: Analysis of the National LymphoCare study. J Clin Oncol 30:3368-3375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galimberti S, Luminari S, Ciabatti E, et al. : Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: The FIL FOLL05 trial. Clin Cancer Res 20:6398-6405, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Zohren F, Bruns I, Pechtel S, et al. : Prognostic value of circulating Bcl-2/IgH levels in patients with follicular lymphoma receiving first-line immunochemotherapy. Blood 126:1407-1414, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Sarkozy C, Huet S, Carlton VEH, et al. : The prognostic value of clonal heterogeneity and quantitative assessment of plasma circulating clonal IG-VDJ sequences at diagnosis in patients with follicular lymphoma. Oncotarget 8:8765-8774, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delfau-Larue MH, van der Gucht A, Dupuis J, et al. : Total metabolic tumor volume, circulating tumor cells, cell-free DNA: Distinct prognostic value in follicular lymphoma. Blood Adv 2:807-816, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal R, Chan YC, Tam CS, et al. : Dynamic molecular monitoring reveals that SWI-SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat Med 25:119-129, 2019 [DOI] [PubMed] [Google Scholar]
- 27.ABIM Foundation; ACP-ASIM Foundation; European Federation of Internal Medicine : Medical professionalism in the new millennium: A physician charter. Ann Intern Med 136:243-246, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing requests should be made to sar2014@med.cornell.edu for deidentified data. Such requests will be considered by the study team after publication following review and approval of proposals, and with appropriate data-sharing agreements in place.