PURPOSE
In patients with resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma, surgery plus perioperative platinum-based chemotherapy is the standard of care. Perioperative chemotherapy remains debatable for gastric/GEJ adenocarcinoma with deficient mismatch repair (dMMR)/microsatellite instability–high (MSI-H).
PATIENTS AND METHODS
NEONIPIGA (ClinicalTrials.gov identifier: NCT04006262) phase II study evaluated neoadjuvant nivolumab 240 mg once every two weeks ×6 and ipilimumab 1 mg/kg once every six weeks ×2, followed by surgery and adjuvant nivolumab 480 mg once every four weeks (nine injections) in patients with locally advanced resectable dMMR/MSI-H, clinical (c) tumor (T)2-T4 node (N)x metastasis (M)0 gastric/GEJ adenocarcinoma. The primary end point was a pathological complete response (pCR) rate.
RESULTS
Between October 2019 and June 2021, 32 patients with dMMR/MSI-H gastric/GEJ adenocarcinoma were enrolled. The median age was 65.5 years (range, 40-80). Clinical stages were cT2-T3N0 (n = 9), cT2-T3N1 (n = 22), and cT3N1M1 (n = 1, wrongly included). With a median follow-up of 14.9 months (95% CI, 10.6 to 17.6), 32 patients received neoadjuvant immunotherapy (27 patients completed all cycles). Neoadjuvant therapy-related grade 3/4 adverse events occurred in six patients (19%). Twenty-nine patients underwent surgery; three did not have surgery and had complete endoscopic response with tumor-free biopsies and a normal computed tomography scan (two refused surgery and one had metastasis at inclusion). The rate of surgical morbidity (Clavien-Dindo classification) was 55% (one postoperative death occurred). All 29 patients had an R0 resection, and 17 (58.6%; 90% CI, 41.8 to 74.1) had pCR (pathological T0N0). Becker tumor regression grades 1a, 1b, 2, and 3 were observed in 17 patients, three (including two pathological T0N1), two, and seven patients, respectively. Of the 29 patients with surgery, 23 received adjuvant nivolumab. At database lock, no patient had relapse and one died without relapse.
CONCLUSION
Nivolumab and ipilimumab-based neoadjuvant therapy is feasible and associated with no unexpected toxicity and a high pCR rate in patients with dMMR/MSI-H resectable gastric/GEJ adenocarcinoma.
INTRODUCTION
Gastric cancer is the third most common cause of cancer-related deaths and the fifth most common diagnosed cancer globally.1 The standard of care for patients with operable gastric adenocarcinoma is perioperative chemotherapy and surgical resection.2-4 Locally advanced mismatch repair deficient (dMMR)/microsatellite instability–high (MSI-H) gastric or gastroesophageal junction (GEJ) adenocarcinomas have better prognosis than mismatch repair proficient/microsatellite stable tumors.5-9 The dMMR/MSI-H phenotype is found in about 10% of gastric and GEJ adenocarcinomas and can reach 48% in patients older than 85 years.7-10 In individual patient data-based meta-analysis study from four trials, including the MAGIC study, evaluating perioperative platinum and fluoropyrimydine-based chemotherapy in locally advanced gastric cancer, dMMR/MSI-H status was a negative predictive factor of the chemotherapy efficacy in terms of disease-free survival (DFS) and overall survival (OS).6,8 Contrarily, a recent meta-analysis on adjuvant chemotherapy for locally advanced dMMR/MSI-H gastric/GEJ adenocarcinoma suggested a benefit of adjuvant treatment in terms of OS but not DFS.9 These data led to the discussion on chemotherapy value in the neoadjuvant and/or adjuvant setting in routine clinical practice for patients diagnosed with locally advanced dMMR/MSI-H gastric/GEJ adenocarcinoma.11,12
CONTEXT
Key Objective
NEONIPIGA evaluates the pathological complete response (pCR) rate after surgery and the safety of neoadjuvant nivolumab plus low-dose ipilimumab followed by adjuvant nivolumab in patients with deficient mismatch repair (dMMR)/microsatellite instability–high (MSI-H) locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma.
Knowledge Generated
Durable and high tumor response rates with anti–programmed death-1 with/without anti–cytotoxic T-lymphocyte associated antigen-4 or anti–programmed death-1 are reported. We detected pCR in 59% of the 29 patients who underwent surgery, without unexpected immune-related adverse events and/or postoperative morbidity/mortality. After a median follow-up of 14.9 months, one patient had an event (death 3 days after surgery).
Relevance
Perioperative nivolumab and low-dose ipilimumab immunotherapy is feasible and associated with a high rate of pCR in dMMR/MSI-H gastric/GEJ adenocarcinoma. This result may pave the way for other studies for the standard of care–changing treatment. NEONIPIGA raises the question whether surgery can be avoided by using a watch-and-wait approach in some patients with locally advanced dMMR/MSI-H gastric/GEJ adenocarcinoma with clinical complete response to immune checkpoint inhibitors.
The dMMR/MSI-H phenotype has now become a major predictive biomarker for the efficacy of immune checkpoint inhibitors (ICIs) in advanced disease, including gastric/GEJ adenocarcinoma, independently of tumor type.13 `In the NICHE phase II trial, patients with dMMR/MSI-H nonmetastatic colorectal cancer who received the ipilimumab-nivolumab combination before surgery achieved a pathological complete response (pCR) of 60%.14 The results from post hoc or subgroup analyses of several studies have shown that dMMR/MSI-H status predicts the efficacy of anti–programmed death-1 ICIs combined with chemotherapy in patients with advanced gastric/GEJ adenocarcinoma.15,16 In locally advanced gastric/GEJ cancer, pCR after neoadjuvant chemotherapy is associated with improved DFS and OS and could be used as a surrogate marker of survival in a phase I/II trial.17-22
We conducted this phase II study to evaluate the pCR rate after treatment with a perioperative nivolumab and ipilimumab combination in patients with resectable locally advanced dMMR/MSI-H gastric/GEJ adenocarcinoma.
PATIENTS AND METHODS
Patients and Study Design
NEONIPIGA is a French single-arm, multicenter academic phase II study promoted by GERCOR evaluating preoperative nivolumab and ipilimumab and postoperative nivolumab in patients with resectable dMMR/MSI-H gastric/GEJ adenocarcinoma. Patients were required to be at least 18 years of age or older (trial was amended in April 2020 to recommend 75 years and younger as an upper limit of age for inclusion) with histologically confirmed locally advanced gastric/GEJ adenocarcinoma. Patients must have had clinical (c) stage c tumor (T)2-T4 node (N)0/N + metastasis (M)0 according to the seventh edition of the International Union Against Cancer after thoraco-abdomino-pelvic computed tomography (CT) scan and esophagogastric endoscopic ultrasound (EUS), if feasible; dMMR and/or MSI-H status; an Eastern Cooperative Oncology Group Performance Status of 0 or 1; adequate organ function; and be eligible for surgery as determined by a local multidisciplinary team meeting in each participating center. Main exclusion criteria were previous cancer therapy for gastric/GEJ adenocarcinoma, conditions requiring corticosteroids or other immunosuppressive medications within 2 weeks before starting treatment, or history of malignancy within the past 5 years except for cured localized cancers. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All eligible patients provided written informed consent. Approval of the Protocol (online only) was obtained from an independent ethics committee.
Treatment
Patients received neoadjuvant therapy for 12 weeks, including nivolumab 240 mg administered intravenously (IV) over 30 minutes once every 2 weeks (six doses) and ipilimumab 1 mg/kg administered IV over 30 minutes once every 6 weeks (two doses; cycle 1 and cycle 4). Neoadjuvant therapy was followed by surgery performed 5 weeks ± 1 week after the last cycle of treatment. Between 4 and 8 weeks after surgery, patients received nine cycles of adjuvant therapy with nivolumab 480 mg administered IV over 30 minutes once every 4 weeks. Adjuvant treatment was given upon the investigator decision depending on the efficacy (ie, tumor regression grade [TRG] on surgical tumor specimen), the tolerance of treatment, and the general postoperative condition.
Assessments and Outcomes
The dMMR/MSI-H status was initially evaluated by local laboratories. Tumor samples (archival or freshly obtained biopsy specimens from primary or metastatic lesions) were collected at the Pathological Department of Saint-Antoine Hospital (central collection), retrospectively reviewed for histological tumor subtype, dMMR status and programmed death-ligand 1 determination by combined positive score (CPS; method in Appendix 1 [online only]), and subjected to MSI-H status analysis by polymerase chain reaction (Promega panel) in case of Becker grade > 1b and to additional translational studies. The clinical TNM stage was defined by a thoraco-abdomino-pelvic CT scan and EUS when feasible. In the case of an obstructing tumor hampering the ability to perform EUS, but not invading the adjacent organs on CT scan, tumor was classified as T3N+. Such procedure was based on the observation from the previous studies in which obstructing tumors represented locally advanced disease in most cases.23,24 The primary objective of this study was the pCR rate defined as complete disappearance of tumor cells at pathological examination in primary tumor surgical specimen and lymph nodes. The intent-to-treat (ITT) population included all patients regardless of their eligibility and treatment received; the per-protocol population was defined as all patients who received at least one dose of treatment and presented no major deviations from the protocol, and the modified ITT (mITT) population included all patients who underwent surgery and had surgery results available for the primary end point analysis. The pCR was graded using the Mandard TRG and Becker TRG grading.17-19 Secondary end points included event-free survival (EFS) defined as the time from first dose of treatment to first documented progression or death from any cause and OS defined as time from the first dose of treatment to death from any cause. After surgery, an evaluation (clinical and biological with and CT) was performed every 3 months for 2 years and then every 6 months until 5 years. Safety assessments, including adverse events (AEs), were performed at baseline and at each study visit continuously throughout treatment and minimum 100 days after surgery or treatment discontinuation using the Common Terminology Criteria for Adverse Events v5.0.25 Treatment-related AEs (TRAEs), defined as AEs with potential immunologic etiology, were grouped by category. Immune-modulating medications, including corticosteroids and immunosuppressive agents, were used to manage TRAEs per-protocol–specified algorithms.26 Postoperative morbidity within a 90-day time postoperative period was assessed according to the Clavien-Dindo surgical classification.27
Statistical Methods
The sample size of patients was calculated based on unilateral α = 5%, β = 20%, and the expected effect size using the A'Hern design28 with the following assumptions: a pCR of 5% was considered unacceptable and of 20% as acceptable (details oxf sample size calculation and continuous and categorical variables definition are provided in Appendix 1).
RESULTS
Baseline Patient Disposition and Characteristics
Between October 23, 2019, and June 4, 2021, 39 patients with resectable locally advanced gastric and GEJ adenocarcinoma were screened at 11 participating centers (Fig 1A). Of them, seven did not meet the inclusion criteria and were not included, leaving a total of 32 eligible patients with dMMR status who were enrolled and given the multimodality treatment schedule. The accrual and treatment summary is depicted in Figure 1A. At data cutoff (February 28, 2022), the median duration of follow-up (time from first dose to the cutoff date) was 14.9 months (95% CI, 10.6 to 17.6; range, 3.4-25.8).
FIG 1.
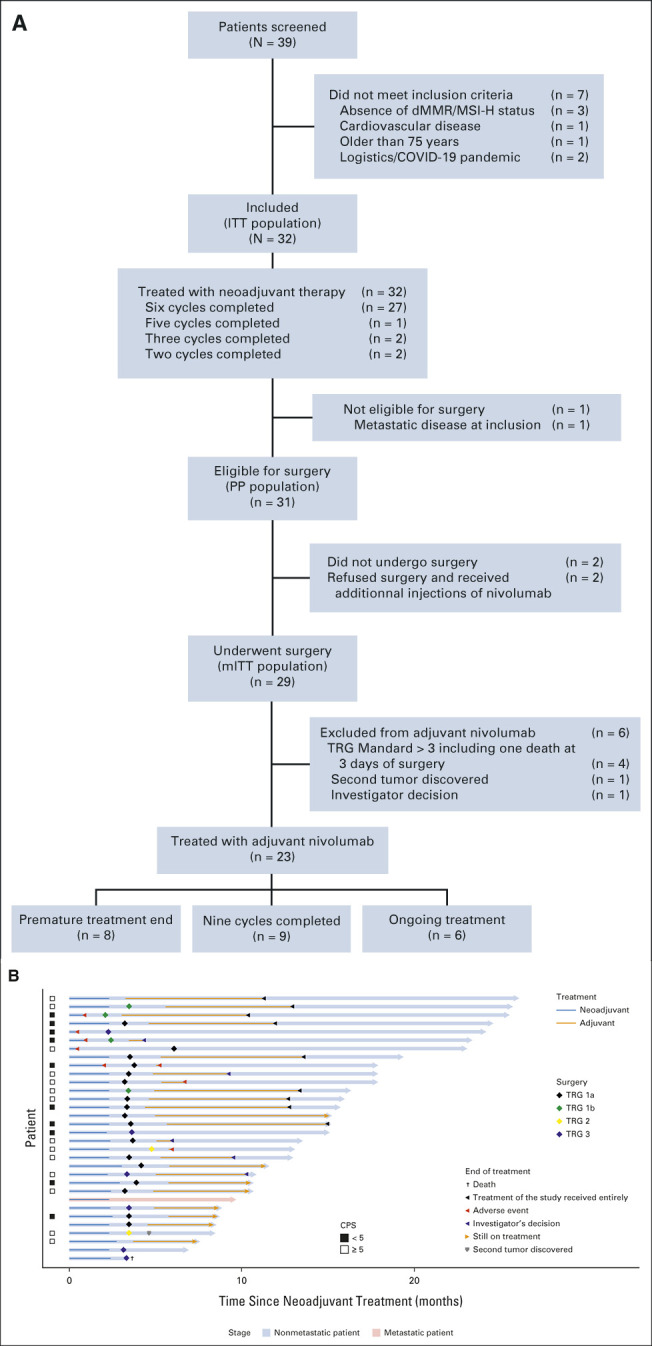
(A) Flow chart. (B) Swimmer plot showing patients' current situation and treatment status in the ITT population (N = 32). dMMR/MSI-H, DNA mismatch repair deficient/microsatellite instability–high; ITT, intent-to-treat; mITT, modified intent-to-treat; PP, per protocol; TRG, tumor regression grade.
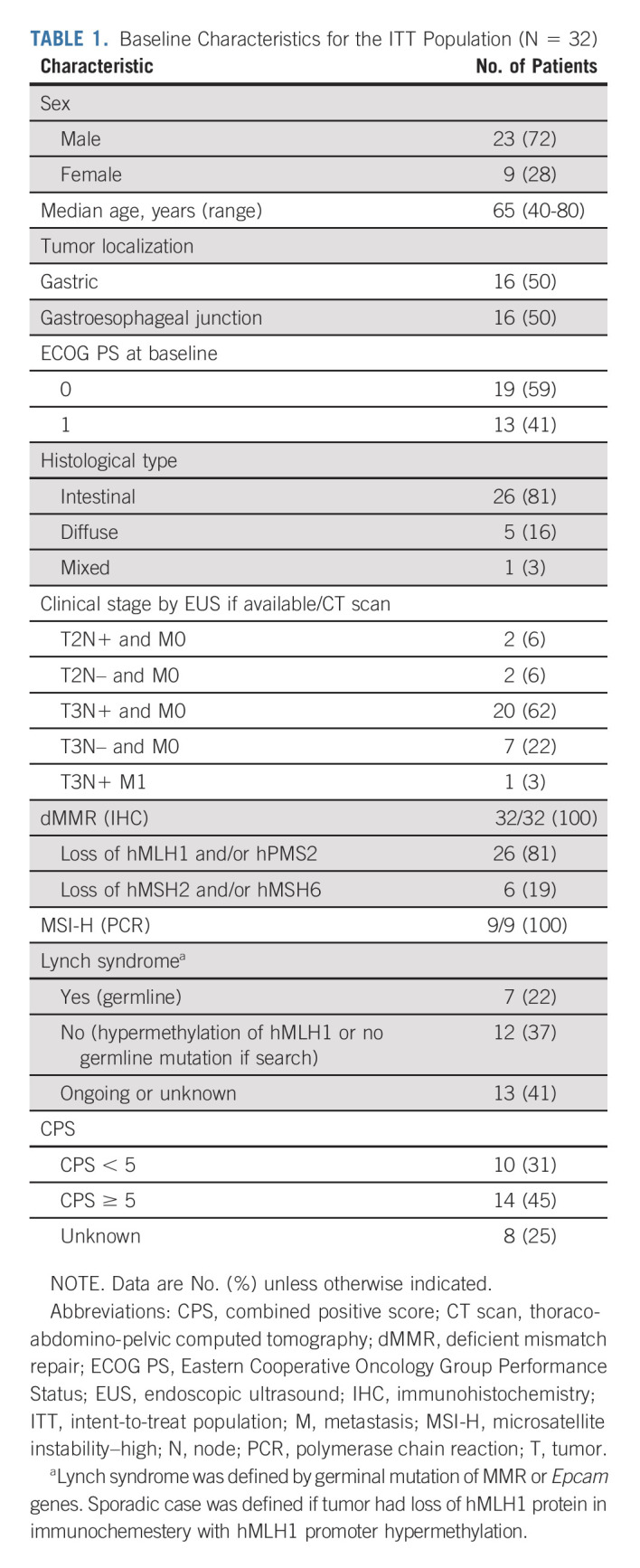
Demographic and baseline characteristics are listed in Table 1. All the 32 patients included were dMMR (local and centrally confirmed). Although polymerase chain reaction testing was not available for all cases, we performed this analysis retrospectively for nine patients with Becker TRG ≥ 2 to avoid misdiagnosis of dMMR status. cTNM were cT2-T3N0 (n = 9), cT2-T3N1 (n = 22), and cT3N1M1 (n = 1, wrongly included); six patients did not undergo EUS because tumor was not passable with echo-endoscope including one with metastatic disease who was classified T3N1M1.
TABLE 1.
Baseline Characteristics for the ITT Population (N = 32)
Neoadjuvant Ipilimumab Plus Nivolumab
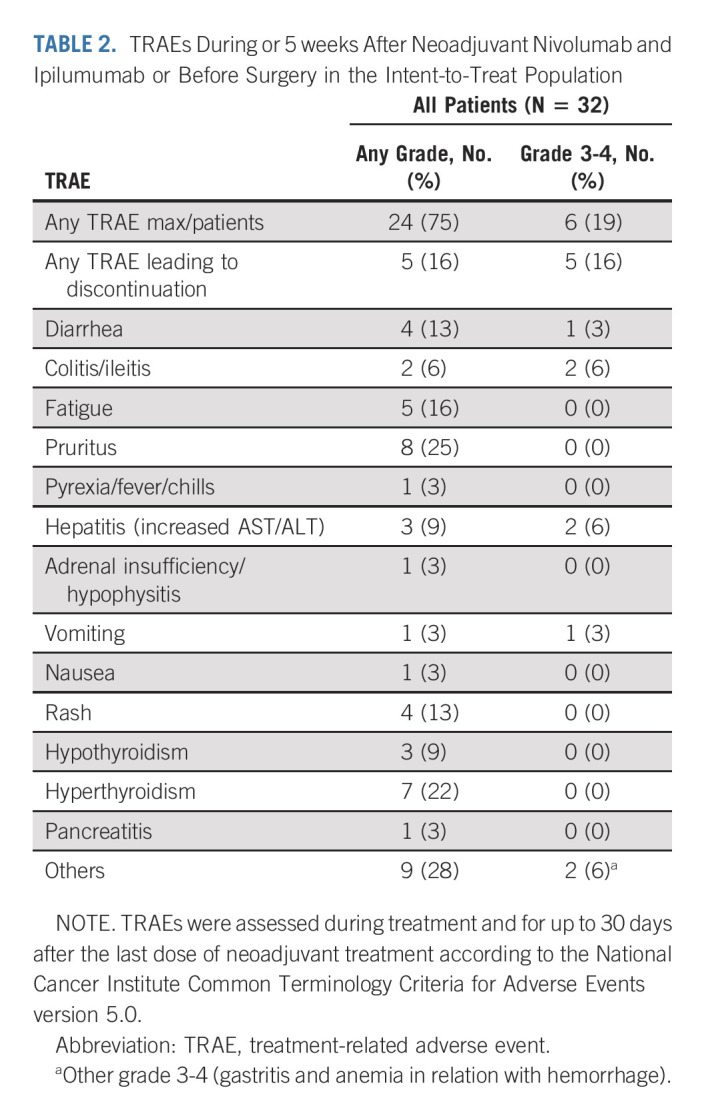
Of the included 32 patients, 27 (84%) completed the planned six cycles of neoadjuvant therapy, one patient (3%) completed five cycles, two patients (6%) completed three cycles, and two patients (6%) completed two cycles (Fig 1A). Grade 3-4 TRAEs were recorded among six patients (19%; Table 2), five of which led to discontinuation of neoadjuvant treatment before surgery (colitis/ileitis, n = 2; gastritis, n = 1; hepatitis, n = 1; and stenosis of the pylorus in relation with a pseudoprogression with pCR at biopsy during endoscopy with vomiting, n = 1). The most common grade 3-4 TRAEs during neoadjuvant treatment were colitis/ileitis (n = 2) and hepatitis (n = 2). The RECIST 1.1 evaluation was performed prior surgery and after neoadjuvant treatment: complete response (n = 5), partial response (n = 12), stable disease (n = 11), and not evaluable (n = 4).
TABLE 2.
TRAEs During or 5 weeks After Neoadjuvant Nivolumab and Ipilumumab or Before Surgery in the Intent-to-Treat Population
Surgery
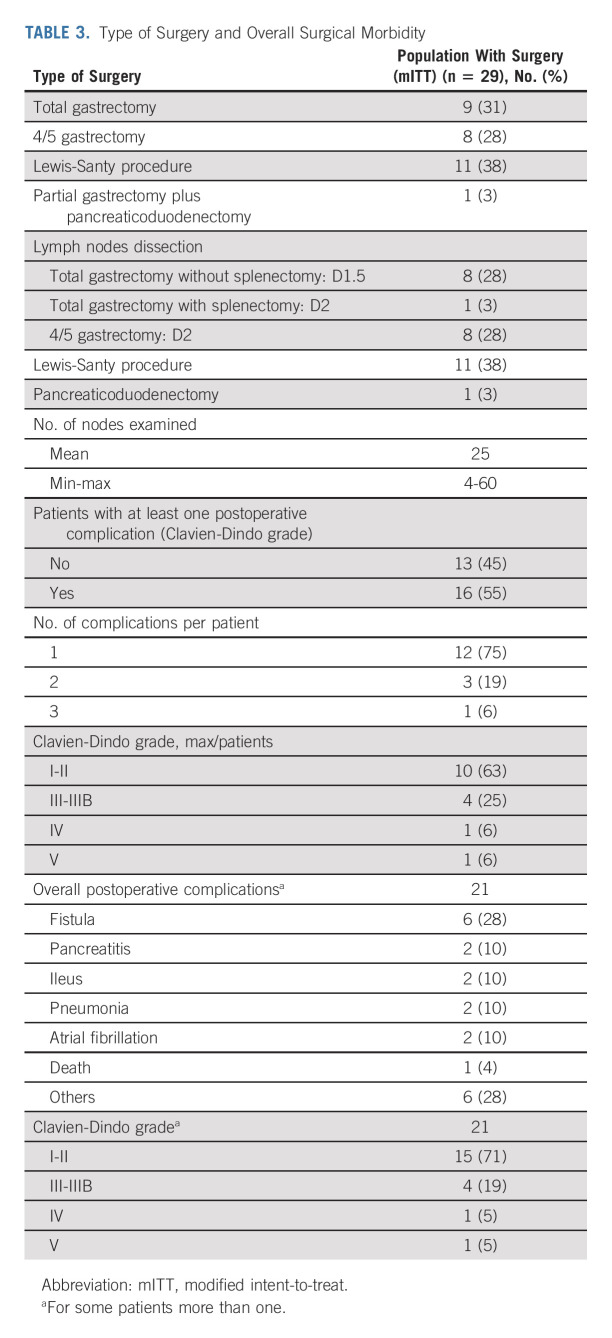
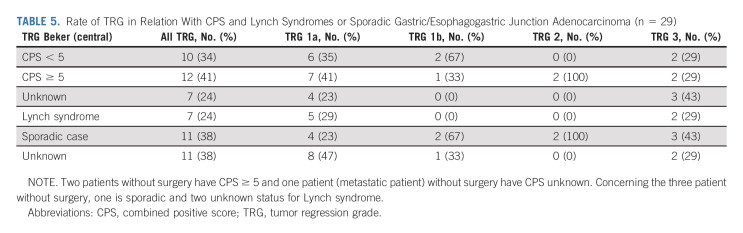
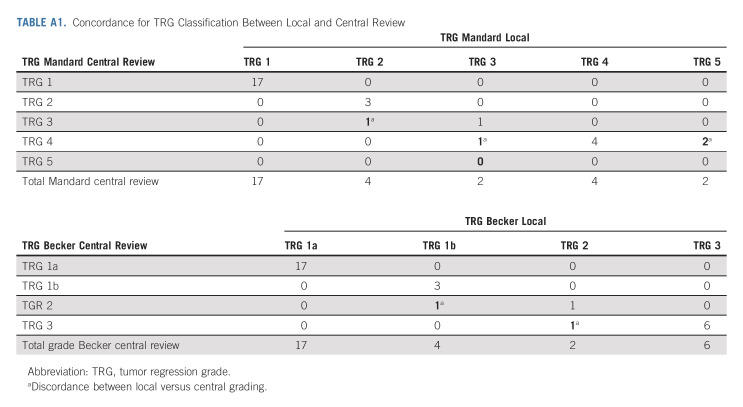
After neoadjuvant treatment, a total of 29 patients (91%) underwent surgery and were evaluable in mITT. Reasons for canceled surgery were metastatic disease in one patient and patients' own decision in two cases. The median delay between the last cycle of neoadjuvant treatment and surgery was 5 weeks (range, 4-24). The type of surgery and the overall surgical morbidity are shown in Table 3. One patient died within 3 days of surgery because of cardiovascular AE, not related to the neoadjuvant ICIs. Complete R0 resection was performed in all 29 patients (Table 4). Seventeen patients (58.6%; 90% CI, 41.8 to 74.1) achieved pCR in primary tumor and lymph nodes. Two additional cases were T0, but with some tumoral cells in one node, and for this reason, these were not considered as pCR. TRG results (central evaluation) by the Becker and Mandard score are summarized in Table 4 with few discrepancies between local and central evaluation (Appendix Table A1, online only). The two patients who refused surgery and the patient who had metastasis at inclusion (tumoral supraclavicular and axillary nodes confirmed by lymph node biopsy after inclusion) showed complete response on CT scan and complete endoscopic response after six cycles of neoadjuvant treatment, tumor-free biopsies, and normal EUS if performed (n = 2 of 3). The rate of Becker TRG (central) by CPS (< 5 or ≥ 5) and by Lynch syndrome or sporadic gastric/OGJ adenocarcinoma is given in Table 5. The rate of Becker TRG (central) by CPS (0-75) and by patient is given in Appendix Table A2 (online only).
TABLE 3.
Type of Surgery and Overall Surgical Morbidity
TABLE 4.
Pathologic Characteristics of Patients Who Underwent Surgery (the mITT population)
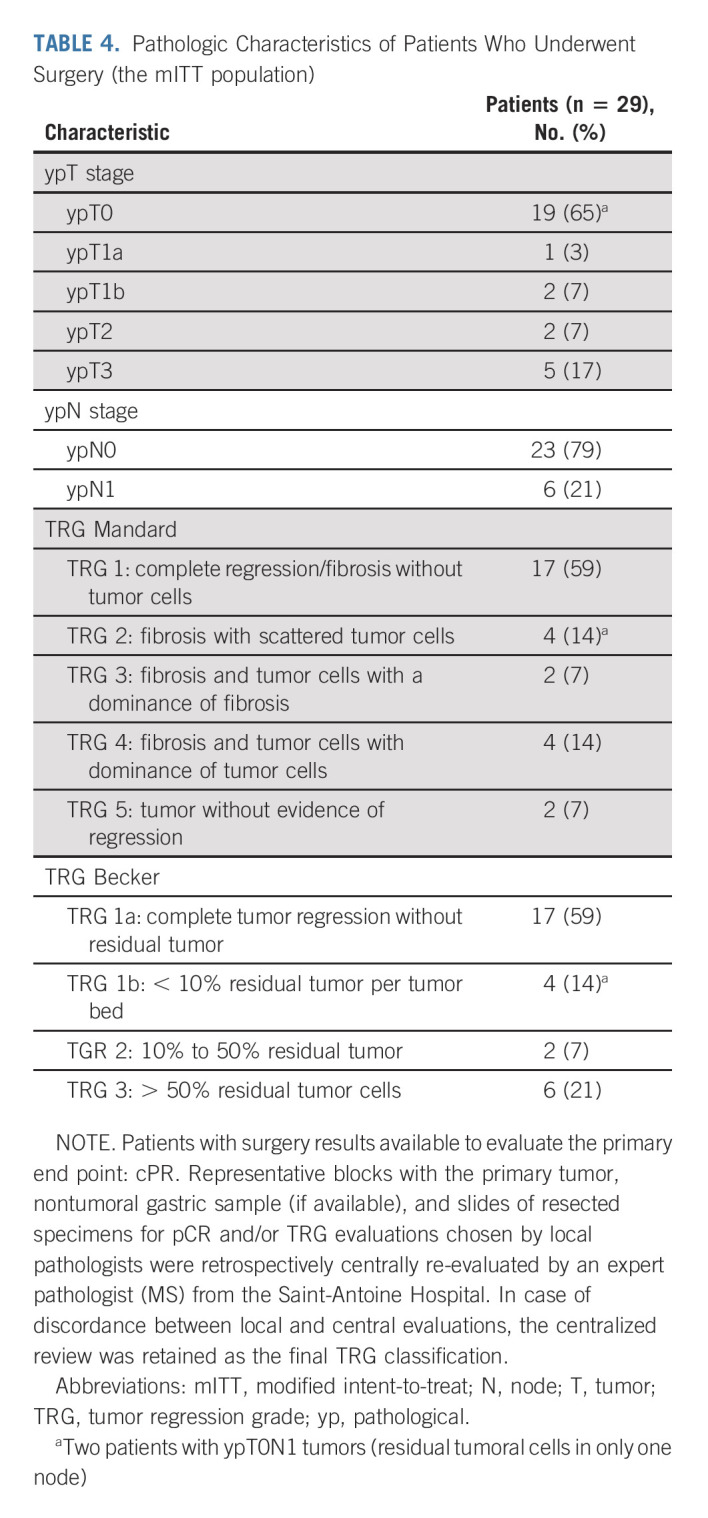
TABLE 5.
Rate of TRG in Relation With CPS and Lynch Syndromes or Sporadic Gastric/Esophagogastric Junction Adenocarcinoma (n = 29)
Postoperative Nivolumab
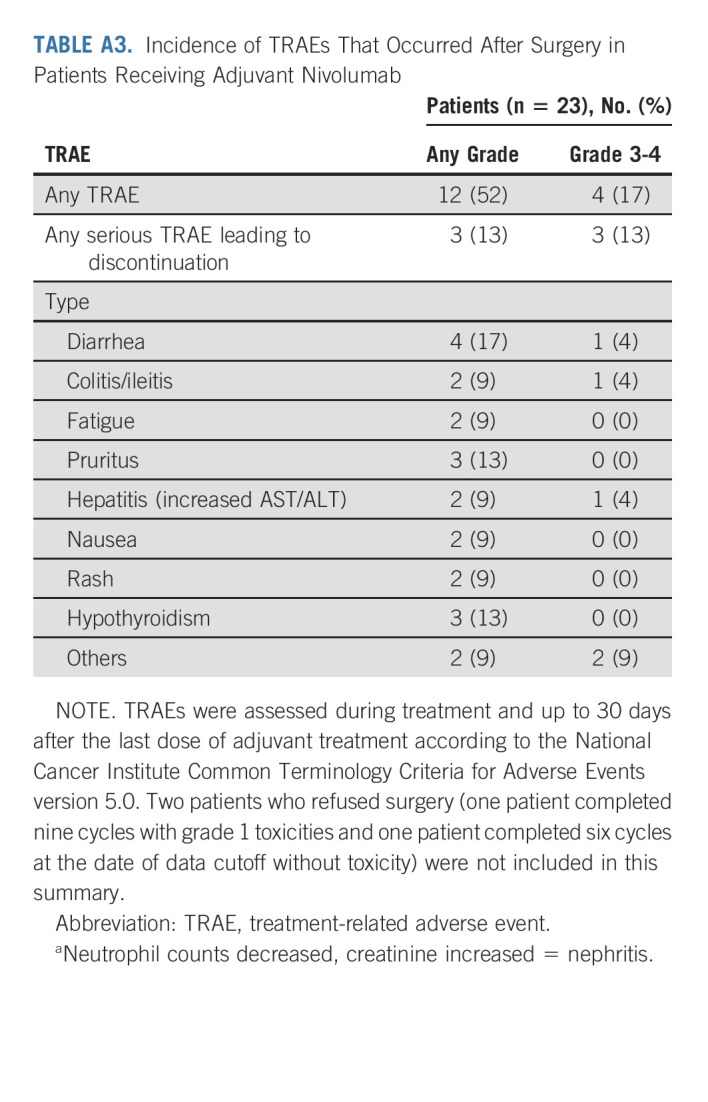
Among the 29 patients who underwent surgery, 23 (79%) received adjuvant nivolumab. The two patients who refused surgery and were in clinical complete response (endoscopic biopsy without tumoral cells) after neoadjuvant treatment received adjuvant nivolumab as termed in the study protocol while those who had metastatic disease did not (investigator decision). The investigator decisions for nonprescribing adjuvant nivolumab (n = 6) in patients who underwent surgery were Mandard TRG > 3 (n = 3), postoperative death with a Mandard score of 3 (n = 1), second non-dMMR/MSI-H gastric tumor (n = 1), and investigator decision (n = 1). Of the 23 patients who initiated adjuvant nivolumab, five and three patients stopped treatment because of TRAEs and investigator decision, respectively. Nine patients received nine cycles of nivolumab and six were still on treatment at the data cutoff. After surgery and/or during adjuvant nivolumab, patients were assessed for TRAEs (Appendix Table A3, online only).
EFS and OS
Thirty-one eligible patients were included in the per-protocol survival analysis (cutoff date: February 28, 2022). The patient with metastatic disease wrongly included was excluded of this survival analysis. Of the 31 eligible patients, 30 (97%) were alive and free of recurrence/progression; one patient died suddenly 3 days after surgery (severe cardiovascular comorbidity–related death). The Kaplan-Meier curves for OS and EFS are presented in Appendix Figures A1A and A1B (online only), respectively. The patient with metastatic disease at inclusion (retrospective review of baseline CT scan) was free of events at the data cutoff date after a follow-up of 9.4 months. Figure 1B shows CPS, treatment duration, and Becker TRG centralized grading for each patient.
DISCUSSION
The GERCOR NEONIPIGA phase II study reached its primary objective for pCR (59% for the primary end point of 20%) with four additional patients showing < 10% of residual tumor cells in surgical specimen with a major Becker TRG of 79% (TRG 1 plus TRG 2) for the 29 patients with pCR evaluable at surgery. Three patients did not have surgery and were considered in complete response because of (1) disappearance of the tumor lesion/ulceration at endoscopy, (2) normal CT scan, and (3) cancer cells absence in biopsy specimens.29
The use of perioperative/adjuvant chemotherapy in patients with locally advanced dMMR/MSI-H gastric/GEJ adenocarcinoma remains controversial mainly because of two issues. First, dMMR/MSI-H tumors provide better prognosis than microsatellite stable/mismatch repair proficient tumors, but with a low pCR rate between 3% and 11% if treated with platinum and fluoropyrimidine-based neoadjuvant chemotherapy in this particular molecular subtype.2,30,31 Second, survival benefit from this strategy has not been clearly shown.6,8 For these reasons, testing for dMMR/MSI-H phenotype nonmetastatic gastric/GEJ adenocarcinoma is in our opinion required.
Unlike for perioperative combination of fluoropyrimidines and platinum salts for which studies suggested the lack of the efficacy in the subgroup of dMMR/MSI-H metastatic gastric/GEJ adenocarcinoma, the efficacy of the perioperative docetaxel, oxaliplatin, fluorouracil, and leucovorin (FLOT) regimen was demonstrated with few data known.4,32 In two studies, rates of pCR in patients with gastric/GEJ adenocarcinoma treated with neoadjuvant FLOT were 16% in cases with unknown MMR status and 38% (5 of 13) in those with dMMR/MSI-H tumors, respectively.32,33 These high rates suggest that neoadjuvant FLOT is a treatment option for selected subgroup of patients with dMMR/MSI-H.
To our knowledge, NEONIPIGA is the first study to test this scheme of treatment for patients with resectable locally advanced gastric/GEJ adenocarcinoma. Limitation of this study is a selected and limited number of patients undergoing surgical resection in a phase II without random assignment. In this phase II trial, with a limited number of patients, CPS and the fact that the gastric/OGJ adenocarcinoma falls within Lynch syndrome vs. sporadic do not seem to be predictive factors for pathologic response after neoadjuvant therapy.
Moreover, following recommendation of the steering committee, a study amendment was performed to exclude patients age older than 75 years. This decision was conditioned by the fact that the 80-year-old patient who was included in the study refused surgery and, therefore, was not evaluable for the pathological evaluation, the primary end point of this study. In elderly patients, morbidity after gastrectomy is high, and its benefit is controversial; the incidence of dMMR/MSI-H increases with age.7,10 The watch-and-wait strategy for elderly patients in case of complete response at endoscopy with biopsies without tumor cells will be an option.
In our study, half of patients had gastric tumor while the other 50% had GEJ adenocarcinoma. This high frequency of dMMR/MSI-H in GEJ adenocarcinoma is unusual, compared with other studies where the majority of dMMR/MSI-H gastric/GEJ adenocarcinoma were found to arise mostly from the body of the stomach.8,34 This greater number of GEJ tumors in our series (11 patients had a Lewis-Santy procedure) may explain the high rate of surgical complication observed, in particular fistula; 16 patients experienced one or more surgical complications during a 90-day postsurgical period (Table 3). Unfortunately, the role of nivolumab and ipilimumab in these postsurgical complications could not be determined.
Discordant cases between local and centralized histological response scores were rare (Table 2, Appendix 1) and did not involve cases with TRG 1a/1b by Becker classification or TRG 1/2 by Mandard classification. Two cases were in complete histological response of the tumor with some residual tumor cells in one lymph node. We preferred not to count them as pCR cases, given that the Mandard and Becker classifications only consider the tumor.
The morbidity rates after oncological gastrectomy (partial/total) or esogastrectomy (the Lewis-Santy procedure) reported in published studies, particularly in elderly patients, are high and mostly attributed to anastomotic leaks.35 The reported 90-day mortality and morbidity rates from gastrectomy in a French national cohort were 7% and 45%, respectively, with reintervention required in 9% of patients.36 Complications after gastrectomy/esogastrectomy include frequent weight loss, possible dumping syndrome, nausea and vomiting, and a vitamin B12 deficiency. Elderly patients had more comorbidities than their younger counterparts, with a limited rate of survivors at 1 year and 2 years.37,38 An individualized approach to treatment decisions is required to improve outcomes in the elderly, especially for those with dMMR/MSI-H tumors.
For all these reasons, avoiding surgery in patients with pCR will be a new challenge. Based on the encouraging NEONIPIGA findings, one treatment avenue that may be explored as an alternative to surgery is a watch-and-wait approach for patients with dMMR/MSI-H gastric/GEJ adenocarcinoma. The three patients treated in the study without surgery, including one ineligible with metastatic disease at the data cutoff, were event-free and in complete response at endoscopy, which illustrates the feasibility for this strategy. Considering the morbidity rate associated with esophagogastrectomy/gastrectomy and their negative impact on patients' quality of life, one might wonder whether the surgery could be avoided in patients with localized dMMR/MSI-H gastric/GEJ adenocarcinoma with complete response at endoscopy and biopsies (according to a protocol that remains to be defined) free of tumoral cells after neoadjuvant ICIs, with a watch-and-wait approach. There remain many unanswered questions with the use of neoadjuvant treatment in this setting. What is the best regimen? An anti–programmed death-1 agent alone or combined with an anti–cytotoxic T-lymphocyte associated antigen-4 despite an increased toxicity or with chemotherapy? What is the optimal treatment duration and delay time before surgery? How to develop this approach? How to be sure that the primary tumor is in pCR without surgery and without nodes histology?
Is it possible to conduct randomized trials evaluating watch-and-wait approaches? Translational studies to identify and validate biomarkers predictive of response to immunotherapy should be a priority and the development of tools with ability to diagnose pCR without the surgical specimen of the primary tumor.39
Other clinical trials currently question the efficacy of ICIs in locally advanced dMMR/MSI-H gastric/GEJ adenocarcinoma (ClinicalTrials.gov identifiers: NCT04795661, NCT04817826 and NCT04152889) and will enhance knowledge and understanding about the management in early-stage disease.
In conclusion, neoadjuvant nivolumab plus low-dose ipilimumab and adjuvant nivolumab is feasible in patients with resectable locally advanced dMMR/MSI-H resectable gastric/GEJ adenocarcinoma, with a high rate of pCR and complications rate as expected. Although not practice changing at the present time, these findings pave the way for other studies to change the standard of care in this group of patients.
ACKNOWLEDGMENT
The authors thank the patients and their families for making the study possible. The authors acknowledge the GERCOR clinical study teams and investigators and study teams in all centers and also Pierre Bourgoin for technical assistance for CPS determination. We thank Bristol Myers Squibb for supplying nivolumab and ipilimumab and for the partial financial support and the ARCAD (Aide et Recherche en Cancérologie Digestive) Foundation for partial financial support. Medical writing and editorial assistance were provided by Magdalena Benetkiewicz (DSc). This assistance was funded by GERCOR.
APPENDIX 1. COMPLEMENTARY METHOD ASSESSMENTS
Tumor Regression Grade System
Mandard tumor regression grade (TRG) 1: complete regression/fibrosis with no evidence of tumor cells, TRG 2: fibrosis with scattered tumor cells, TRG 3: fibrosis and tumor cells with predominance of fibrosis, TRG 4: fibrosis and tumor cells with predominance of tumor cells, and TRG 5: tumor without evidence of regression). Becker TRG grading (TRG 1a: complete tumor regression without residual tumor: TRG 1b: < 10% residual tumor per tumor bed; TRG 2: 10%-50% carcinoma present; and TRG 3: > 50% carcinoma present).17-19
Mismatch Repair and Microsatellite Instability Determination and Combined Positive Score Determination
The deficient mismatch repair/microsatellite instability-high status was initially evaluated by local laboratories and defined as the loss of expression of at least one mismatch repair protein detected by immunohistochemistry (antibodies directed against hMLH1, hMSH2, hMSH6, and hPMS2) and/or as a positivity of at least three DNA markers (Promega panel) by polymerase chain reaction testing. The histological tumor subtype was determined centrally on pretreated tumor biopsy by both the Lauren and WHO 2019 classifications of gastric cancers. Immunohistochemistry was performed on biopsy of the primary tumor before therapy for programmed death-ligand 1 (PD-L1) determination by combined positive score (CPS). Slides were incubated with the clone QR1 (Diagomics, 1/100 dilution) on unstained tissue sections (4 μm) using the Leica Bond platform automated stainer according to the manufacturers' recommendations. Tumor cell PD-L1 expression was defined as the percentage of viable tumor cells with partial or complete membrane staining in at least 100 viable tumor cells. CPS was generated by scoring PD-L1 stained slides using the CPS algorithm, defined as the number of PD-L1–positive tumor cells (partial or complete membrane staining), lymphocytes, and macrophages (membrane staining or intracellular staining or both) divided by the total number of viable tumor cells multiplied by 100.
Sample Size and Decision Rule
The sample size of patients was calculated based on unilateral α = 5%, β = 20% and the expected effect size using the A'Hern design28 with the following assumptions: a pathological complete response (pCR) of 5% was considered unacceptable (H0) and of 20% as acceptable (H1).
Twenty-seven patients were required to be enrolled for the primary objective (pCR) analysis to test the previous hypothesis. Considering a rate of 15% for uninformative patients, a total of 32 patients needed to be included. With the previous assumptions, the design consisted of the following decision rule in the first 27 evaluable patients:
• If at most three (11.1%) responses are observed, the treatment will be declared insufficiently active.
• If at least four (14.8%) responses are observed, the treatment will be declared active and promising for further evaluation.28
Continuous and categorical variables were described with median (range) and frequency (percentage), respectively. The pCR estimation was provided with 90% binomial CIs.
Event-free survival and overall survival were estimated with the Kaplan-Meier method. Median follow-up with 95% CI was estimated with the reverse Kaplan-Meier method. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC) and R software version 3.6.1 (Vienna, Austria).
FIG A1.
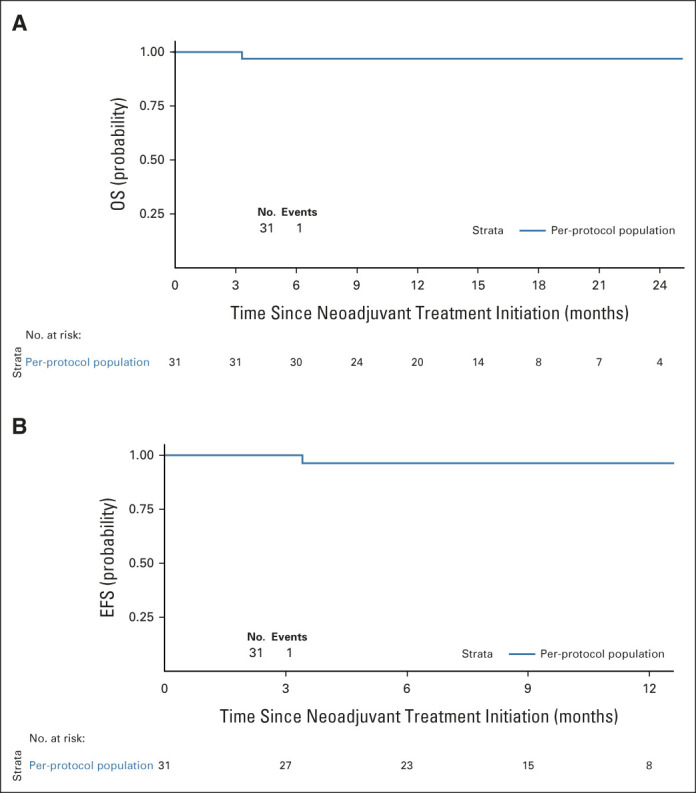
Kaplan-Meier plots for (A) OS and (B) EFS in the per-protocol population (n = 31). EFS, event-free survival; OS, overall survival.
TABLE A1.
Concordance for TRG Classification Between Local and Central Review
TABLE A2.
CPS by Patient (N = 32)
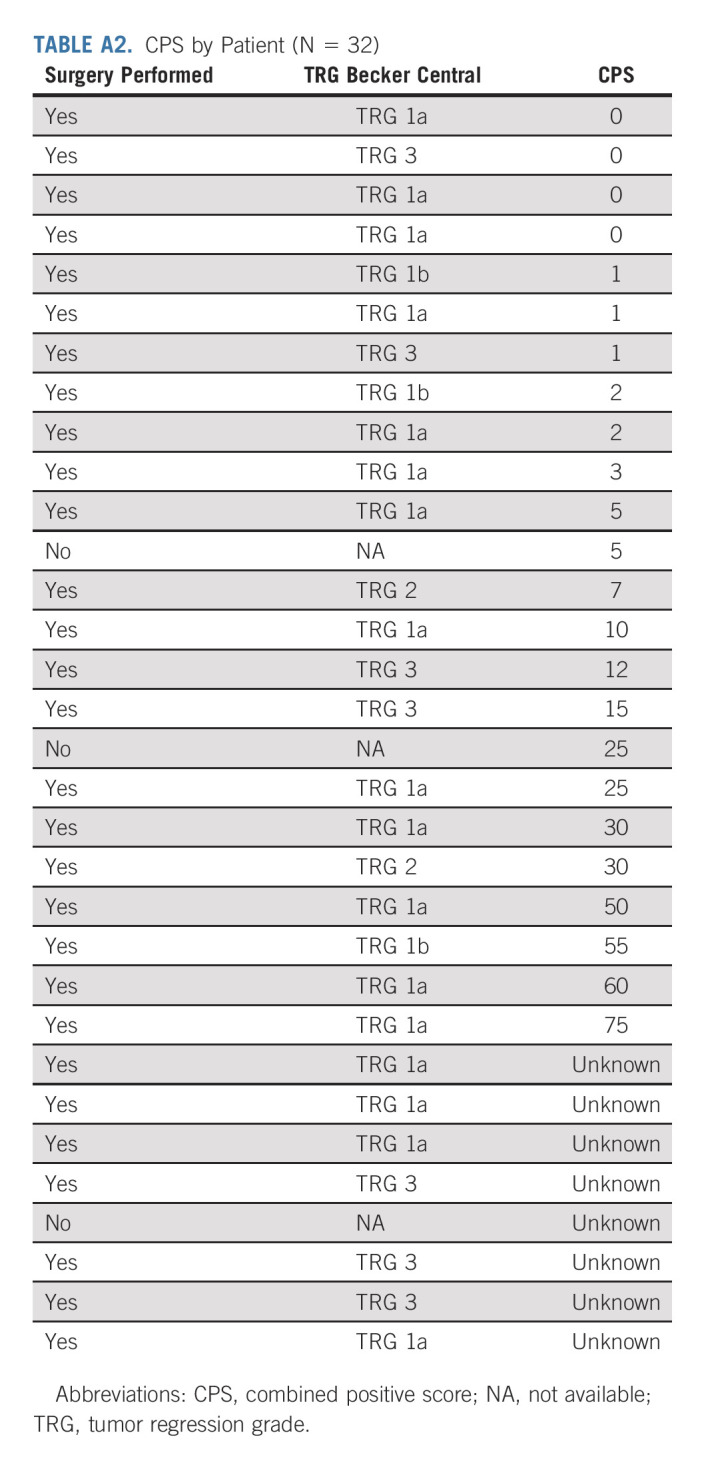
TABLE A3.
Incidence of TRAEs That Occurred After Surgery in Patients Receiving Adjuvant Nivolumab
Thierry André
Honoraria: Roche/Genentech, Bristol Myers Squibb, Servier, Amgen, Pierre Fabre, Ventana Medical Systems, GlaxoSmithKline, Merck
Consulting or Advisory Role: Amgen, Bristol Myers Squibb, MSD Oncology, Servier, AstraZeneca/MedImmune, Tesaro, Pierre Fabre, GamaMabs Pharma, Astellas Pharma, Kaleido Biosciences, Gritstone Bio, GlaxoSmithKline, Seattle Genetics, Transgene
Travel, Accommodations, Expenses: MSD Oncology
Uncompensated Relationships: ARCAD Foundation, Adjuvant Colon Cancer End Points (ACCENT) Collaborative Group, Gercor
David Tougeron
Honoraria: Amgen, Roche, Sanofi, Bristol Myers Squibb, Merck Serono, MSD, Servier/Pfizer, Ipsen
Consulting or Advisory Role: Sanofi, MSD, Pierre Fabre, AstraZeneca
Research Funding: AstraZeneca (Inst), Servier (Inst), Roche (Inst), MSD (Inst), BTG (Inst)
Travel, Accommodations, Expenses: Sanofi, Amgen, Bristol Myers Squibb
Guillaume Piessen
Consulting or Advisory Role: Bristol Myers Squibb, Nestle Health Science, MSD Oncology, Stryker, Astellas Pharma
Travel, Accommodations, Expenses: Medtronic
Uncompensated Relationships: Medtronic
Christelle de la Fouchardière
Honoraria: Merck Serono, Roche
Consulting or Advisory Role: Lilly, Bayer, Bristol Myers Squibb, Amgen, Servier, Roche, Pierre Fabre, Incyte, Eisai, MSD Oncology, Ipsen
Speakers' Bureau: Ipsen
Research Funding: Roche, Pierre Fabre (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Roche, Celgene, Amgen, Bristol Myers Squibb, Servier
Other Relationship: Incyte, MSD Oncology
Christophe Louvet
Consulting or Advisory Role: Merck, Roche, Amgen, MSD Oncology
Travel, Accommodations, Expenses: Roche, Merck
Antoine Adenis
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Merck Serono, Astellas Pharma, Arcus Biosciences
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb
Research Funding: Sanofi (Inst), Bayer (Inst), Roche (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Servier
Marine Jary
Consulting or Advisory Role: Incyte
Speakers' Bureau: Pierre Fabre, Servier
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Pierre Fabre, Bayer, Roche, Servier
Christophe Tournigand
Honoraria: Roche, Sanofi, Bayer, Bristol Myers Squibb, MSD
Consulting or Advisory Role: Bayer
Research Funding: Roche
Travel, Accommodations, Expenses: Roche, Bayer, MSD
Thomas Aparicio
Honoraria: Servier, AstraZeneca, Pierre Fabre, Amgen
Consulting or Advisory Role: MSD Oncology, Servier, Pierre Fabre, Sirtex Medical
Travel, Accommodations, Expenses: MSD Oncology
Jérôme Desrame
Expert Testimony: Roche
Travel, Accommodations, Expenses: Hospira
Astrid Lièvre
Honoraria: Amgen, Bayer, Bristol Myers Squibb (Mexico), Incyte, Ipsen, LEO Pharma, Mylan, Novartis, Pierre Fabre, Roche, Servier, Viatris, Advanced Accelerator Applications
Consulting or Advisory Role: Advanced Accelerator Applications, Astellas Pharma, Bristol Myers Squibb (Mexico), Incyte, Pierre Fabre, Servier
Research Funding: Bayer (Inst), Lilly (Inst), IntegraGen (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Ipsen, Mylan, MSD Oncology, Pierre Fabre, Roche, Servier
Romain Cohen
Honoraria: MSD Oncology, Pierre Fabre, Bristol Myers Squibb
Consulting or Advisory Role: Exeliom Biosciences, Enterome
Research Funding: Servier
Travel, Accommodations, Expenses: Amgen, Bristol Myers Squibb, Mylan
Dewi Vernerey
Consulting or Advisory Role: OSE Immunotherapeutics, Janssen-Cilag, HalioDx, Pfizer, CellProthera, GERCOR, Incyte, Fondazione Smith Kline, INVECTYS, AC BioScience
Travel, Accommodations, Expenses: MSD
Jérémie H. Lefevre
Honoraria: Intuitive Surgical, Braun Travacare
Consulting or Advisory Role: SafeHeal
Research Funding: SafeHeal (Inst), SafeHeal
Travel, Accommodations, Expenses: Biom'up, SafeHeal
Magali Svrcek
Honoraria: Sanofi/Aventis, MSD Oncology, Owkin, BMS, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Astellas Pharma
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2022 ASCO Gastrointestinal Cancers Symposium (abstract 244), San Francisco, CA, January 20-22, 2022.
SUPPORT
Bristol Myers Squibb, Princeton, NJ (nivolumab and ipilimumab supply), GERCOR, and the ARCAD (Aide et Recherche en Cancérologie Digestive) Foundation.
CLINICAL TRIAL INFORMATION
NCT04006262; EUDRACT 2018-004712-22
AUTHOR CONTRIBUTIONS
Conception and design: Thierry André, Christophe Louvet, Christophe Tournigand, Thomas Pudlarz, Romain Cohen, Dewi Vernerey, Magali Svrcek
Administrative support: Thierry André
Provision of study materials or patients: David Tougeron, Christelle de la Fouchardière, Christophe Louvet, Antoine Adenis, Christophe Tournigand, Thomas Aparicio, Jérôme Desrame, Astrid Lièvre, Marie-Line Garcia-Larnicol, Romain Cohen, Jérémie H. Lefevre, Magali Svrcek
Collection and assembly of data: David Tougeron, Guillaume Piessen, Christelle de la Fouchardière, Christophe Louvet, Marine Jary, Christophe Tournigand, Thomas Aparicio, Jérôme Desrame, Astrid Lièvre, Marie-Line Garcia-Larnicol, Salomé Memmi, Jérémie H. Lefevre, Magali Svrcek
Data analysis and interpretation: Thierry André, David Tougeron, Guillaume Piessen, Christophe Louvet, Antoine Adenis, Christophe Tournigand, Astrid Lièvre, Romain Cohen, Salomé Memmi, Dewi Vernerey, Julie Henriques, Jérémie H. Lefevre, Magali Svrcek
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability–High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Thierry André
Honoraria: Roche/Genentech, Bristol Myers Squibb, Servier, Amgen, Pierre Fabre, Ventana Medical Systems, GlaxoSmithKline, Merck
Consulting or Advisory Role: Amgen, Bristol Myers Squibb, MSD Oncology, Servier, AstraZeneca/MedImmune, Tesaro, Pierre Fabre, GamaMabs Pharma, Astellas Pharma, Kaleido Biosciences, Gritstone Bio, GlaxoSmithKline, Seattle Genetics, Transgene
Travel, Accommodations, Expenses: MSD Oncology
Uncompensated Relationships: ARCAD Foundation, Adjuvant Colon Cancer End Points (ACCENT) Collaborative Group, Gercor
David Tougeron
Honoraria: Amgen, Roche, Sanofi, Bristol Myers Squibb, Merck Serono, MSD, Servier/Pfizer, Ipsen
Consulting or Advisory Role: Sanofi, MSD, Pierre Fabre, AstraZeneca
Research Funding: AstraZeneca (Inst), Servier (Inst), Roche (Inst), MSD (Inst), BTG (Inst)
Travel, Accommodations, Expenses: Sanofi, Amgen, Bristol Myers Squibb
Guillaume Piessen
Consulting or Advisory Role: Bristol Myers Squibb, Nestle Health Science, MSD Oncology, Stryker, Astellas Pharma
Travel, Accommodations, Expenses: Medtronic
Uncompensated Relationships: Medtronic
Christelle de la Fouchardière
Honoraria: Merck Serono, Roche
Consulting or Advisory Role: Lilly, Bayer, Bristol Myers Squibb, Amgen, Servier, Roche, Pierre Fabre, Incyte, Eisai, MSD Oncology, Ipsen
Speakers' Bureau: Ipsen
Research Funding: Roche, Pierre Fabre (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Roche, Celgene, Amgen, Bristol Myers Squibb, Servier
Other Relationship: Incyte, MSD Oncology
Christophe Louvet
Consulting or Advisory Role: Merck, Roche, Amgen, MSD Oncology
Travel, Accommodations, Expenses: Roche, Merck
Antoine Adenis
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Merck Serono, Astellas Pharma, Arcus Biosciences
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb
Research Funding: Sanofi (Inst), Bayer (Inst), Roche (Inst), BeiGene (Inst), Bristol Myers Squibb (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Servier
Marine Jary
Consulting or Advisory Role: Incyte
Speakers' Bureau: Pierre Fabre, Servier
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Pierre Fabre, Bayer, Roche, Servier
Christophe Tournigand
Honoraria: Roche, Sanofi, Bayer, Bristol Myers Squibb, MSD
Consulting or Advisory Role: Bayer
Research Funding: Roche
Travel, Accommodations, Expenses: Roche, Bayer, MSD
Thomas Aparicio
Honoraria: Servier, AstraZeneca, Pierre Fabre, Amgen
Consulting or Advisory Role: MSD Oncology, Servier, Pierre Fabre, Sirtex Medical
Travel, Accommodations, Expenses: MSD Oncology
Jérôme Desrame
Expert Testimony: Roche
Travel, Accommodations, Expenses: Hospira
Astrid Lièvre
Honoraria: Amgen, Bayer, Bristol Myers Squibb (Mexico), Incyte, Ipsen, LEO Pharma, Mylan, Novartis, Pierre Fabre, Roche, Servier, Viatris, Advanced Accelerator Applications
Consulting or Advisory Role: Advanced Accelerator Applications, Astellas Pharma, Bristol Myers Squibb (Mexico), Incyte, Pierre Fabre, Servier
Research Funding: Bayer (Inst), Lilly (Inst), IntegraGen (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Ipsen, Mylan, MSD Oncology, Pierre Fabre, Roche, Servier
Romain Cohen
Honoraria: MSD Oncology, Pierre Fabre, Bristol Myers Squibb
Consulting or Advisory Role: Exeliom Biosciences, Enterome
Research Funding: Servier
Travel, Accommodations, Expenses: Amgen, Bristol Myers Squibb, Mylan
Dewi Vernerey
Consulting or Advisory Role: OSE Immunotherapeutics, Janssen-Cilag, HalioDx, Pfizer, CellProthera, GERCOR, Incyte, Fondazione Smith Kline, INVECTYS, AC BioScience
Travel, Accommodations, Expenses: MSD
Jérémie H. Lefevre
Honoraria: Intuitive Surgical, Braun Travacare
Consulting or Advisory Role: SafeHeal
Research Funding: SafeHeal (Inst), SafeHeal
Travel, Accommodations, Expenses: Biom'up, SafeHeal
Magali Svrcek
Honoraria: Sanofi/Aventis, MSD Oncology, Owkin, BMS, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, Astellas Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Smyth EC, Verheij M, Allum W, et al. : Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38-v49, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11-20, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Al-Batran S-E, Homann N, Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948, 19572019 [DOI] [PubMed] [Google Scholar]
- 5.Choi YY, Bae JM, An JY, et al. : Is microsatellite instability a prognostic marker in gastric cancer?: A systematic review with meta-analysis: MSI and gastric cancer. J Surg Oncol 110:129-135, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Smyth EC, Wotherspoon A, Peckitt C, et al. : Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol 3:1197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran-Minh M-L, Lehmann-Che J, Lambert J, et al. : Prevalence and prognosis of microsatellite instability in oesogastric adenocarcinoma, NORDICAP 16-01. Clin Res Hepatol Gastroenterol 45:101691, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Pietrantonio F, Miceli R, Raimondi A, et al. : Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol 37:3392-3400, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Nie RC, Chen GM, Yuan SQ, et al. : Adjuvant chemotherapy for gastric cancer patients with mismatch repair deficiency or microsatellite instability: Systematic review and meta-analysis. Ann Surg Oncol 29:2324-2331, 2022 [DOI] [PubMed] [Google Scholar]
- 10.Polom K, Marrelli D, Roviello G, et al. : Molecular key to understand the gastric cancer biology in elderly patients—The role of microsatellite instability: MSI and elderly gastric cancer patients. J Surg Oncol 115:344-350, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Lordick F: Chemotherapy for resectable microsatellite instability-high gastric cancer? Lancet Oncol 21:203, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Eads JR: Is it time yet for adjuvant immunotherapy for patients with DNA mismatch repair deficient gastric cancer? Ann Surg Oncol 29:2141-2143, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalabi M, Fanchi LF, Dijkstra KK, et al. : Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 26:566-576, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Chao J, Fuchs CS, Shitara K, et al. : Assessment of pembrolizumab therapy for the treatment of microsatellite instability–high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol 7:895, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandard A-M, Dalibard F, Mandard J-C, et al. : Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680-2686, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Becker K, Mueller JD, Schulmacher C, et al. : Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy: Response of gastric carcinoma to chemotherapy. Cancer 98:1521-1530, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Becker K, Langer R, Reim D, et al. : Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: A summary of 480 cases. Ann Surg 253:934-939, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Mansour JC, Tang L, Shah M, et al. : Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol 14:3412-3418, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Fujitani K, Mano M, Hirao M, et al. : Posttherapy nodal status, not graded histologic response, predicts survival after neoadjuvant chemotherapy for advanced gastric cancer. Ann Surg Oncol 19:1936-1943, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Blackham AU, Greenleaf E, Yamamoto M, et al. : Tumor regression grade in gastric cancer: Predictors and impact on outcome: TRG in gastric cancer. J Surg Oncol 114:434-439, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Mariette C, Balon JM, Maunoury V, et al. : Value of endoscopic ultrasonography as a predictor of long-term survival in oesophageal carcinoma. Br J Surg 90:1367-1372, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Noiret B, Branche J, Piessen G: Uselessness and danger of dilating obstructive esophageal for staging purpose: A confirmation. Ann Thorac Surg 114:357-358, 2022 [DOI] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services: National Cancer Institute : Common Terminology criteria for adverse events (CTCAE) (version 5.0). Index of /ftp1/CTCAE/CTCAE_5.0 (nih.gov)
- 26.Brahmer JR, Lacchetti C, Schneider BJ, et al. : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36:1714-1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien P-A: Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205-213, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A'Hern RP: Sample size tables for exact single-stage phase II designs. Stat Med 20:859-866, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Tahara M, Ohtsu A, Hironaka S, et al. : Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol 35:316-323, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Haag GM, Czink E, Ahadova A, et al. : Prognostic significance of microsatellite-instability in gastric and gastroesophageal junction cancer patients undergoing neoadjuvant chemotherapy. Int J Cancer 144:1697-1703, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Wang Y, Ying X, et al. : Prognostic and predictive value of mismatch repair deficiency in gastric and gastroesophageal junction adenocarcinoma patients receiving neoadjuvant or adjuvant chemotherapy. J Surg Oncol 124:1356-1364, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Al-Batran S-E, Hofheinz RD, Pauligk C, et al. : Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 17:1697-1708, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Al-Batran S-E, Lorenzen S, Homann N, et al. : Pathological regression in patients with microsatellite instability (MSI) receiving perioperative atezolizumab in combination with FLOT vs. FLOT alone for resectable esophagogastric adenocarcinoma: Results from the DANTE trial of the German Gastric Group at the AIO and SAKK. Ann Oncol 32, 2021. (suppl 5; abstr 1069) [Google Scholar]
- 34.Marrelli D, Polom K, Pascale V, et al. : Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric cancer. Ann Surg Oncol 23:943-950, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Baiocchi GL, Giacopuzzi S, Reim D, et al. : Incidence and grading of complications after gastrectomy for cancer using the GASTRODATA Registry: A European Retrospective Observational Study. Ann Surg 272:807-813, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Challine A, Voron T, Dousset B, et al. : Postoperative outcomes after laparoscopic or open gastrectomy. A national cohort study of 10,343 patients. Eur J Surg Oncol 47:1985-1995, 2021 [DOI] [PubMed] [Google Scholar]
- 37.Joharatnam-Hogan N, Shiu KK, Khan K: Challenges in the treatment of gastric cancer in the older patient. Cancer Treat Rev 85:101980, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Shen Y, Hao Q, Zhou J, et al. : The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: A systematic review and meta-analysis. BMC Geriatr 17:188, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon M, An M, Klempner SJ, et al. : Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov 11:2168-2185, 2021 [DOI] [PubMed] [Google Scholar]