PURPOSE
Infections pose a significant risk during therapy for childhood cancer. However, little is known about the risk of infection in long-term survivors of childhood cancer.
METHODS
We performed a retrospective observational study of children and adolescents born in Washington State diagnosed with cancer before age 20 years and who survived at least 5 years after diagnosis. Survivors were categorized as having a hematologic or nonhematologic malignancy and were matched to individuals without cancer in the state birth records by birth year and sex with a comparator:survivor ratio of 10:1. The primary outcome was incidence of any infection associated with a hospitalization using diagnostic codes from state hospital discharge records. Incidence was reported as a rate (IR) per 1,000 person-years. Multivariate Poisson regression was used to calculate incidence rate ratios (IRR) for cancer survivors versus comparators.
RESULTS
On the basis of 382 infection events among 3,152 survivors and 771 events among 31,519 comparators, the IR of all hospitalized infections starting 5 years after cancer diagnosis was 12.6 (95% CI, 11.4 to 13.9) and 2.4 (95% CI, 2.3 to 2.6), respectively, with an IRR 5.1 (95% CI, 4.5 to 5.8). The survivor IR during the 5- to 10-year (18.1, 95% CI, 15.9 to 20.5) and > 10-year postcancer diagnosis (8.3, 95% CI, 7.0 to 9.7) periods remained greater than comparison group IRs for the same time periods (2.3, 95% CI, 2.1 to 2.6 and 2.5, 95% CI, 2.3 to 2.8, respectively). When potentially vaccine-preventable infections were evaluated, survivors had a greater risk of infection relative to comparators (IRR, 13.1; 95% CI, 7.2 to 23.9).
CONCLUSION
Infectious complications continue to affect survivors of childhood cancer many years after initial diagnosis. Future studies are needed to better understand immune reconstitution to determine specific factors that may mitigate this risk.
INTRODUCTION
In the past five decades, the 5-year survival rate for all pediatric cancer has steadily increased to exceed 80%.1 This improvement in survival is primarily attributed to advancements in therapy, risk stratification, and supportive care.2,3 Although these treatment protocols are lifesaving, they have undesirable consequences, including prolonged delays in immune reconstitution leaving patients vulnerable to infection well beyond completing chemotherapy.4-6 The Childhood Cancer Survivor Study reported that survivors of all cancer were 1.6-2.7 times more likely to be hospitalized for infection > 5 years following the completion of therapy compared with age- and sex-matched individuals in the general population.7 As a result, infection remains a significant cause of morbidity and mortality for the estimated 500,000 survivors of childhood cancer in the United States.5,7,8
CONTEXT
Key Objective
There is a paucity of data documenting the incidence of specific infections in long-term cancer survivors, specifically differentiating risk in patients with hematologic versus nonhematologic malignancies. This population-based retrospective observational follow-up analysis sought to determine whether survivors of pediatric cancer still have an elevated risk of infection > 5 years from initial diagnosis.
Knowledge Generated
Survivors of pediatric cancer remain at increased risk of infections associated with a hospitalization or death long after their initial diagnosis. This risk is elevated in survivors of both hematologic and nonhematologic malignancies for infections across most of the organ systems and pathogen groups assessed.
Relevance
It is vital for patients, caregivers, and their providers to appreciate this long-term infection risk assessment. This allows for better counseling by physicians and recommendations of care for survivors that will reduce the morbidity of infection throughout the survivorship period.
Although prior studies highlight the overall infection risk for children after completing therapy for any cancer, there is a paucity of data documenting the incidence of specific infections in long-term cancer survivors, including potentially vaccine-preventable illnesses severe enough to require hospitalization.9 In the absence of these data, clinicians, in particular, pediatricians in the community who care for these children following completion of therapy, are unable to have informed discussions regarding infection risk with parents and their patients in this post-treatment period. The aim of the current study is to describe the overall incidence rate (IR) of infections requiring hospitalization for survivors of childhood and adolescent cancers at least 5 years from initial cancer diagnosis. IRs will also be calculated for specific infection types and by subgroups of survivors of hematologic and nonhematologic malignancies. Evaluating infections separately in survivors of hematologic and nonhematologic cancers allows for a better understanding of malignancy-specific risks and addresses the specific knowledge gap of immune impairment after treatment in nonhematologic malignancies.9 As therapeutic options improve and the number of childhood cancer survivors increases, it is important to define infection risk in the post-therapy period to guide counseling and encourage investigations aimed at developing effective preventative strategies.
METHODS
Study Design and Patient Population
We conducted a population-based retrospective observational follow-up analysis using linked cancer-birth-hospital discharge records from Washington State–born children and adolescents with and without cancer. We calculated IRs of infections associated with a hospitalization or death overall and during different follow-up time periods that each started 5 years after cancer diagnosis. These rates were separately calculated for children with hematologic and nonhematologic malignancies and compared with rates for children and adolescents without cancer.
Identification of Cancer Survivors
Cancer survivors were identified from two population-based cancer registries in Washington State: the Cancer Surveillance System (part of the National Cancer Institute's Surveillance, Epidemiology and End Results Program) and the Washington State Cancer Registry (part of the Centers for Disease Control and Prevention's North American Association of Central Cancer Registries). The former registry began in 1974 and includes > 70% of the state's population; the latter began in 1995. Cancer registry records of individuals age < 20 years at the time of primary cancer diagnosis were linked to Washington State birth records to identify those born in state (N = 5,605). Only children diagnosed between 1982 and 2008 and who survived at least 5 years from diagnosis were included in this analysis (N = 3,152), with last date of follow-up through December 2013. An index date of 5 years from initial cancer diagnosis was assigned to each survivor.
Comparator Subjects
Each cancer survivor was randomly matched by birth year and sex to 10 children identified in the remaining Washington State birth records. Comparator subjects representing the general population were not known to have died before the survivor's index date on the basis of further linkage to the state death records.
Outcome
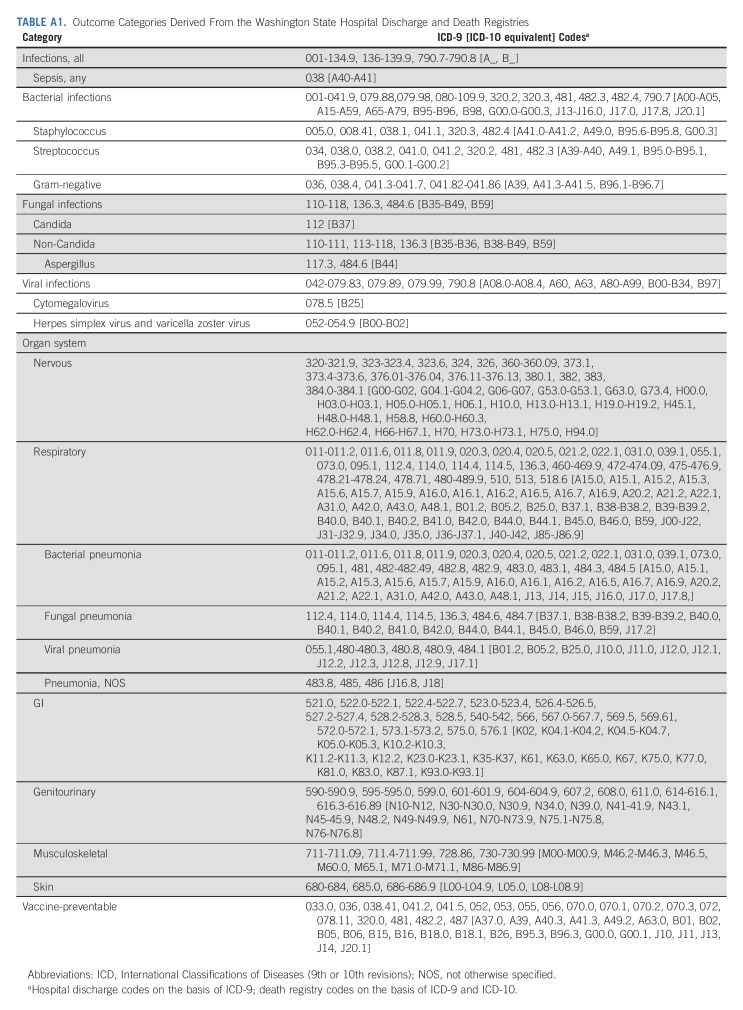
Record linkage occurred in 2014-2015. Records of all subjects were linked to state hospital discharge and death records beginning at the index date, as described previously.8 Subjects without a death or hospitalization record identified during this time were assumed to be alive and event-free through December 2013. The primary outcome was incidence of any infection associated with a hospitalization or death occurring 5 or more years after the cancer diagnosis through December 2013. Time to event was measured from index date, with median potential follow-up10 of 9.3 years (interquartile range [IQR], 4.4-14.5 years) for both cases and comparators. Infections were defined using International Classification of Disease, Version 9 and 10 (ICD-9/-10), codes according to Medicare-Medicaid billing standards (Appendix Table A1, online only). State hospital discharge data include records of all inpatient discharges (full and observation admissions) at non-Federal facilities and contain up to 25 discharge diagnoses. State death records contain one underlying primary and up to 10 contributing causes of death. Death because of infection without an associated hospitalization was extremely rare, and thus the primary outcome is referred to more simply as infection associated with a hospitalization. As done previously, infections were classified by organism type (bacterial, fungal, or virus), by organ system/infection type (nervous system, respiratory, GI, genitourinary, musculoskeletal, skin, or sepsis), and by whether it is potentially vaccine-preventable (influenza A and B virus, varicella zoster virus, H. influenzae, hepatitis B, or S. pneumoniae).11 Subjects may have had more than one hospitalization associated with infection.
Covariate Data
Race/ethnicity, birth year, and sex for cancer survivors and comparator subjects were available from the state birth records. Cancer-specific variables including International Classification of Diseases for Oncology (ICD-O) morphology and topography codes, date of diagnosis, and age at diagnosis were available from the cancer registries. Cancer types were classified per the International Classification of Childhood Cancer, 3rd edition.12 Cancer treatment duration and relapse status were not available from these registries.
Statistical Analysis
IRs of infection associated with a hospitalization per 1,000 person-years (PY) were calculated. Poisson regression was used to estimate the incidence rate ratios (IRR) and associated 95% CIs comparing infection rates between children with and without cancer.13 For each infection type, the count of infections (ranging from 0 to 15) was the dependent variable, with years under observation included as an offset term in the Poisson model. Birth year (continuous) and sex were included as covariates. Survivors were categorized as having a hematologic (n = 1,244) or nonhematologic (n = 1,908) malignancy, with calculation of respective IRs and IRRs. We examined the infection-related IRs and IRRs overall for 5+ years after diagnosis, and more specifically during 5 to < 10 years after diagnosis, and ≥ 10 years after diagnosis. IRs and IRRs for infections associated with a hospitalization were also calculated by infection type, organ system, and status as potentially vaccine-preventable. We used Stata version 17 (College Station, TX) for all analyses.14
RESULTS
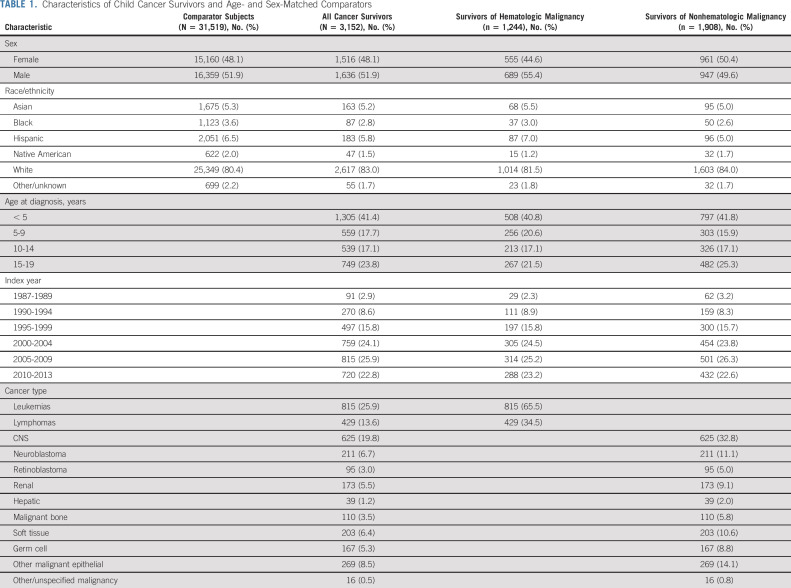
The assembled cohort included 3,152 cancer survivors: 1,244 (39.5%) survivors of hematologic malignancy and 1,908 (60.5%) survivors of nonhematologic malignancy (Table 1). Leukemia (25.9%), CNS tumors (19.8%), and lymphoma (13.6%) were the most common cancers. Sex and age distribution of cancer survivors and comparators were similar by design. Each group consisted of 48.1% females and 51.9% males. The median age at cancer diagnosis was 7 years (range, 0-19 years). Median follow-up after index date was 8.8 years (range, 0.05-27.0 years; IQR, 4.1-14.2 years) for cancer survivors and 9.3 years (range, 0.1-27.0 years; IQR, 4.4-14.5 years) for age- and sex-matched comparators.
TABLE 1.
Characteristics of Child Cancer Survivors and Age- and Sex-Matched Comparators
Overall and Specific Inpatient Infection Rates
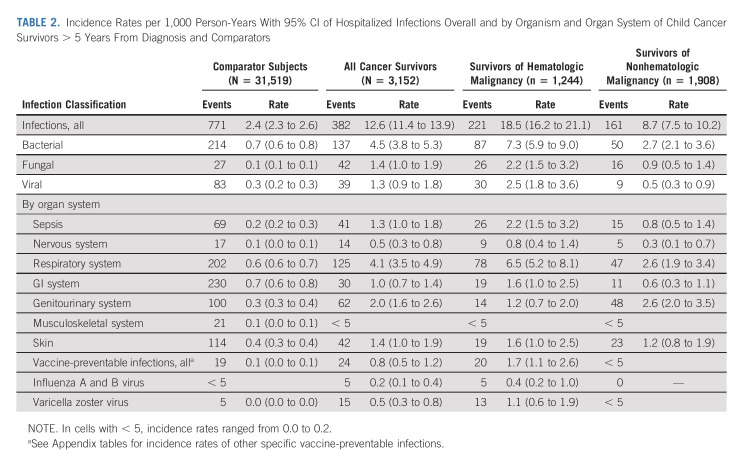
In the follow-up period, there were 382 infections diagnosed during a hospitalization among 3,152 cancer survivors and 771 infections diagnosed in the inpatient setting among 31,519 comparators. This resulted in an IR of 12.6 inpatient infections per 1,000 PY (95% CI, 11.4 to 13.9) in cancer survivors and 2.4 inpatient infections per 1,000 PY rate (95% CI, 2.3 to 2.6) in the comparator subjects. Among survivors of hematologic and nonhematologic malignancy, the IR for all infections associated with a hospitalization was 18.5 (95% CI, 16.2 to 21.1) and 8.7 (95% CI, 7.5 to 10.2), respectively. Among infection types, bacterial was the most common in both survivors of hematologic and nonhematologic malignancy (IR, 7.3; 95% CI, 5.9 to 9.0; and IR, 2.7; 95% CI, 2.1 to 3.6, respectively). By organ system, respiratory infections were most common in survivors of hematologic malignancy (IR, 4.1; 95% CI, 3.5 to 4.9), whereas survivors of nonhematologic malignancy had a very similar incidence of genitourinary and respiratory infections (IR, 2.6; 95% CI, 2.0 to 3.5; and IR, 2.6; 95% CI, 1.9 to 3.4, respectively). Sepsis was also among the most frequent inpatient infection in both survivors of hematologic and nonhematologic malignancy (IR, 2.2; 95% CI, 1.5 to 3.2; and IR, 0.8; 95% CI, 0.5 to 1.4, respectively; Table 2).
TABLE 2.
Incidence Rates per 1,000 Person-Years With 95% CI of Hospitalized Infections Overall and by Organism and Organ System of Child Cancer Survivors > 5 Years From Diagnosis and Comparators
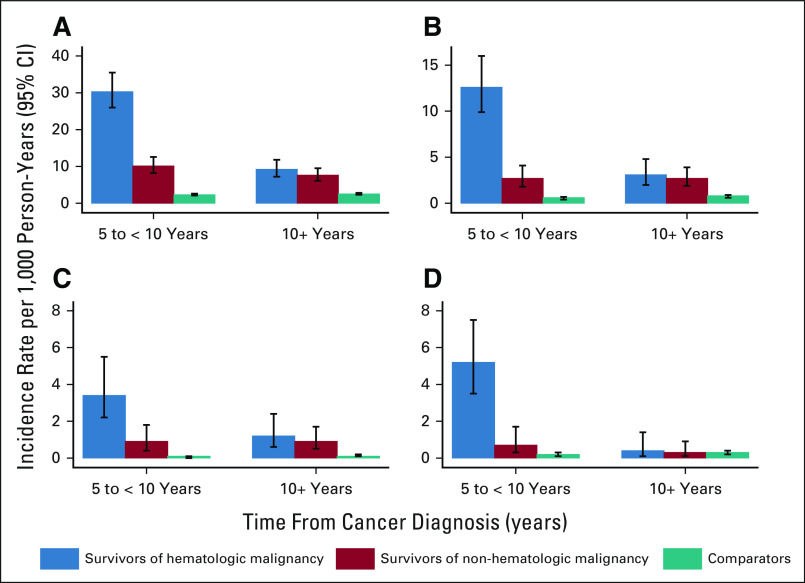
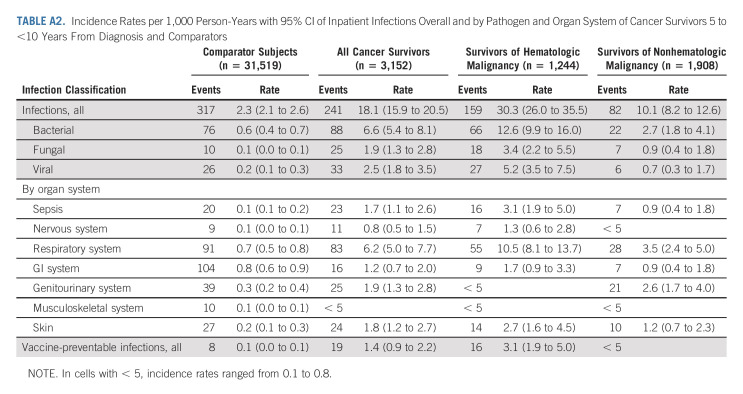
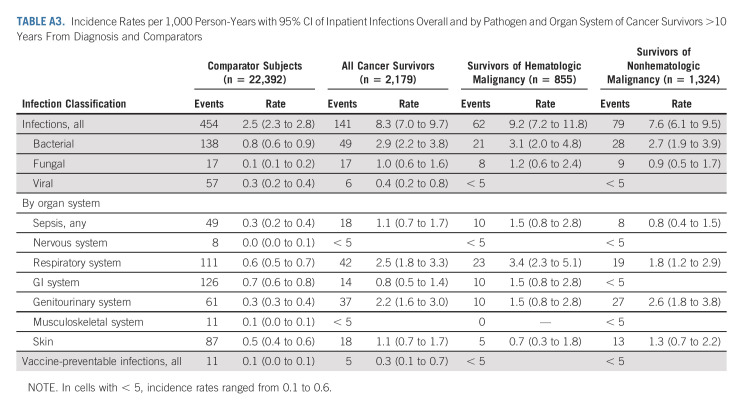
The incidence of infections associated with a hospitalization per 1,000 PY declined for cancer survivors, both of hematologic and nonhematologic malignancy, in the > 10-year postdiagnosis follow-up period (IR, 8.3; 95% CI, 7.0 to 9.7) as compared with 5- to 10-year follow-up period (IR, 18.1; 95% CI, 15.9 to 20.5; Figs 1 and 2). However, these IRs were higher for cancer survivors in both follow-up periods relative to rates of infection associated with a hospitalization for age- and sex-matched comparators during the same follow-up windows (IR, 2.5; 95% CI, 2.3 to 2.8; and IR, 2.3; 95% CI, 2.1 to 2.6, respectively; Appendix Tables A2 and A3 [online only]).
FIG 1.
Rates of infection by organism type: (A) all infections, (B) bacterial infections, (C) fungal infections, and (D) viral infections.
FIG 2.
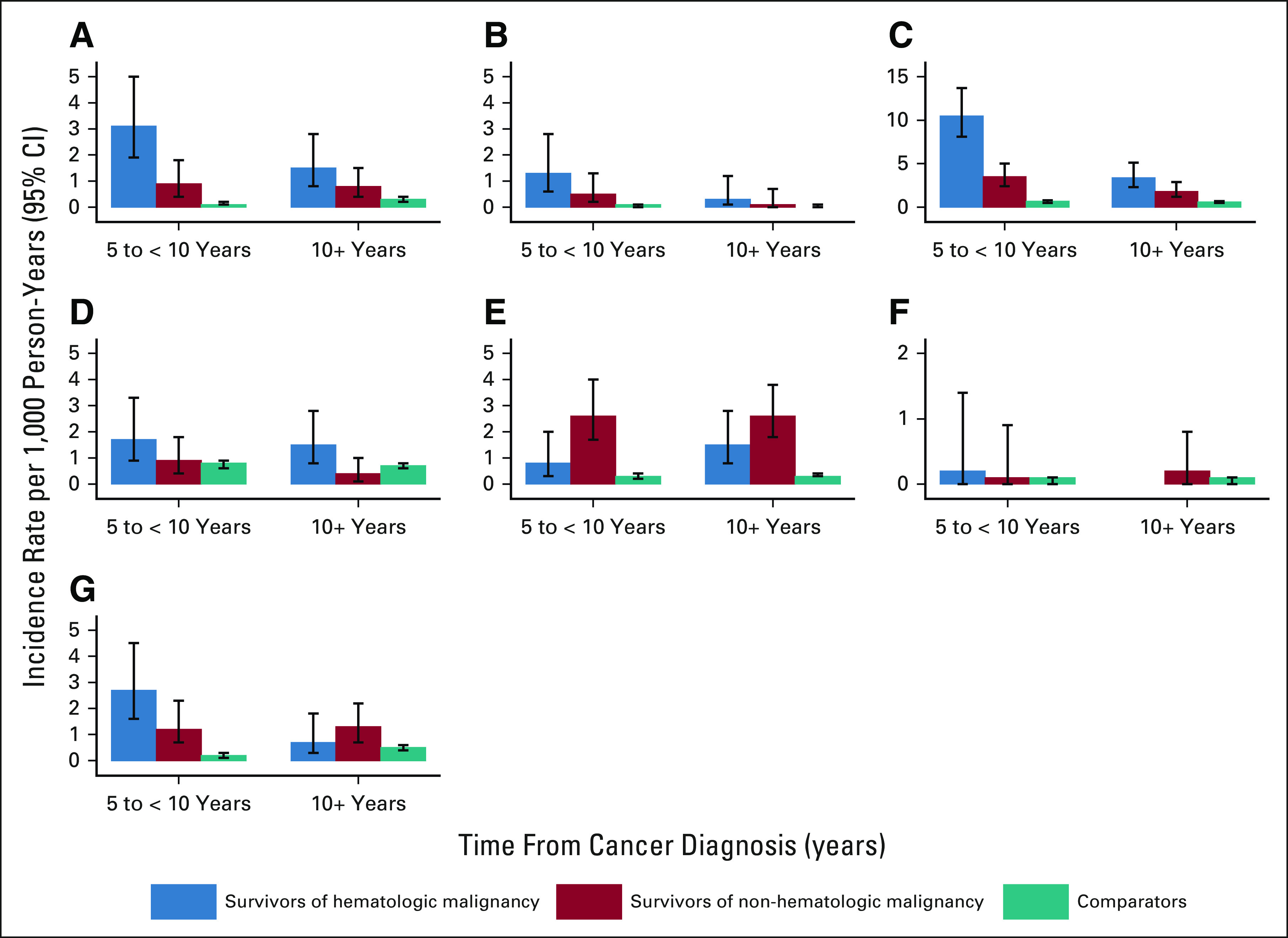
Rates of infection by organ system: (A) sepsis, (B) nervous system, (C) respiratory, (D) gastrointestinal, (E) genitourinary, (F) musculoskeletal, and (G) skin.
Relative Risk of Infections Associated With Hospitalization
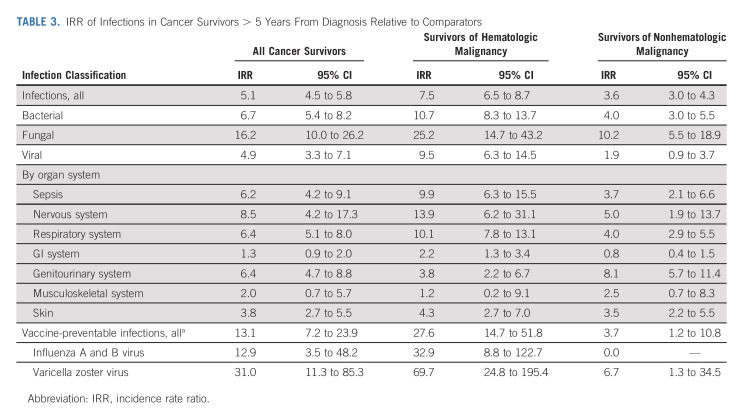
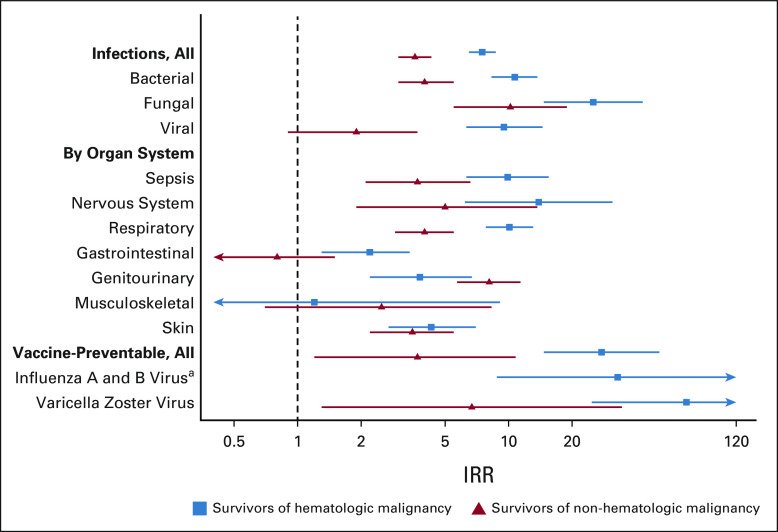
The IRR of infections associated with hospitalization in all cancer survivors was 5.1 (95% CI, 4.5 to 5.8). Although bacterial infections were more numerous in survivors of both hematologic and nonhematologic malignancy, the relative risk was highest for fungal infections in both groups (IRR, 25.2; 95% CI, 14.7 to 43.2; and IRR, 10.2; 95% CI, 5.5 to 18.9, respectively) followed by bacterial (IRR, 10.7; 95% CI, 8.3 to 13.7; and IRR, 4.0; 95% CI, 3.0 to 5.5, respectively). When evaluating organ systems, the IRR of survivors of hematologic malignancy relative to comparators was highest for infections of the nervous system (IRR, 13.9; 95% CI, 6.2 to 31.1) followed by respiratory infections (IRR, 10.1; 95% CI, 7.8 to 13.1), whereas relative risk for infections of the genitourinary followed by nervous system (IRR, 8.1; 95% CI, 5.7 to 11.4; and IRR, 5.0; 95% CI, 1.9 to 13.7, respectively) were the highest among survivors of nonhematologic malignancy (Table 3, Fig 3).
TABLE 3.
IRR of Infections in Cancer Survivors > 5 Years From Diagnosis Relative to Comparators
FIG 3.
IRRs (Poisson regression) of infections by organism and organ system for survivors versus comparators. aNo influenza hospitalizations in nonheme survivors. IRR, incidence rate ratio.
Both survivors and comparators experienced relatively low rates of inpatient vaccine-preventable infections associated with hospitalization (Table 2); however, rates were significantly higher in cancer survivors versus comparator subjects. Specifically, survivors of both hematologic malignancy and nonhematologic malignancy have an increased risk of varicella zoster virus infections relative to comparator subjects (IRR, 69.7; 95% CI, 24.8 to 195.4; and IRR, 6.7; 95% CI, 1.3 to 34.5, respectively). Survivors of hematologic malignancy also had a significantly increased risk of influenza A and B infection associated with hospitalization (IRR, 32.9; 95% CI, 8.8 to 122.7; Table 3).
DISCUSSION
In this observational comparative study, infections diagnosed in the inpatient setting were consistently and significantly higher in cancer survivors versus age- and sex-matched comparators. Although higher rates of infections diagnosed during a hospitalization were documented in the 5- to 10-year period, the increased risk for such infections persisted for survivors of hematologic malignancy and nonhematologic malignancy > 10 years from cancer diagnosis. These results are consistent with prior literature. In 2011, Lorenzi et al found that pediatric cancer survivors in a British Columbia registry had an increased risk of infections diagnosed during hospitalization for up to 5 years after their diagnosis. This risk was particularly evident among the leukemia survivor subgroup.15 A separate Canadian study from Pelland-Marcotte et al6 leveraged administrative data from the Pediatric Oncology Group of Ontario Networked Information System to show that leukemia survivors had an increased relative risk of infections diagnosed in the inpatient and outpatient settings at 5 years and > 5 years after the completion of chemotherapy compared with a control group. Data from our US-based study, in conjunction with these Canadian reports, highlight the potential long-lasting impact that cancer therapy has on infection risk.
Although survivors of hematologic malignancy tended to have higher risk of infection than survivors of nonhematologic malignancy relative to comparators, both groups had significantly increased relative risks for inpatient infections across most of the organ systems and pathogen groups assessed. Given that the increased risk for infections associated with hospitalization existed across most infection subtypes, clinicians caring for pediatric cancer survivors need to remain vigilant for a wide range of infections in these patients compared with their healthy counterparts. This is particularly true for bacterial infections, which were the most numerous. However, it is also important to maintain awareness for fungal infections, which yielded the highest IRR of all infection types, as they were far less common in the comparators. Among organ systems, the increased risk of infections of the respiratory tract, nervous system, and genitourinary system, and sepsis were particularly profound. This higher index of suspicion may warrant lower thresholds for bringing patients into the clinic for evaluation, pursuing diagnostic testing, or referring to subspecialists for additional evaluation. Furthermore, it is imperative that patients and their caregivers are appropriately counseled about this long-term risk so that they may seek timely medical attention for infectious symptoms, rather than assuming that their infectious symptoms can be managed with a similar approach used for otherwise healthy children and adolescents.
Survivors of both hematologic and nonhematologic malignancy also had significantly increased risk for vaccine preventable illnesses diagnosed during a hospitalization. The magnitude of risk relative to comparators was noted be higher among the survivors of hematologic malignancy. Varicella zoster virus was the most common vaccine-preventable illness reported followed by influenza. It is important to note, however, that many of these patients (approximately 70%) were born before incorporation of the varicella vaccine into the routine childhood vaccination series, and thus may not represent a failure of prior vaccine receipt. The increased risk of influenza infections in the survivors of hematologic malignancy underscores the importance of annual immunization for this population. Further investigation is necessary to determine whether revaccination after completion of chemotherapy would prevent some of these vaccine-preventable illnesses that present in the immediate years after completing chemotherapy. Importantly, there were few diagnosed vaccine-preventable infections associated with hospitalization in the period beyond 10 years from cancer diagnosis suggesting that the increased risk of infection from these pathogens declines with time. At present, international guidelines from the Children's Oncology Group recommend that all survivors of childhood cancer make up missed doses of regularly scheduled vaccines.9 For survivors who have received all scheduled vaccinations, it is recommended that either (1) boosters be given or (2) titers be measured (if available) and a booster be given if a survivor lacks seroprotection for a given vaccine.9 These guidelines generally align with practice in the United Kingdom and published recommendations from the European Conference on Infection in Leukemia, where boosters are recommended.16-18
Major strengths of this study include a large cohort of pediatric cancer survivors with a variety of cancer diagnoses and a large comparator group with a 10:1 ratio of age- and sex-matched subjects. Additionally, data came from robust and substantiated population-based data sources that are prospectively coded, thus helping to circumvent response bias.19 Furthermore, this study contributes to a knowledge gap regarding long-term risk of infections diagnosed during hospitalizations in survivors of nonhematologic malignancy, specifically the high rates of fungal and genitourinary infections and significantly increased risk of sepsis relative to comparators, even > 10 years from cancer diagnosis. Overall, there is a paucity of published data reporting on late-onset (> 5 years and 10 years after diagnosis) inpatient infections in cancer survivors and a lack of results specific to certain organ systems and pathogen types, notably vaccine-preventable illnesses. The results from this study contribute to our knowledge regarding the extent of these types of late effects of treatment.
There are several limitations of this study. First, non-Hispanic Whites accounted for > 80% of the subjects in both groups. Although the population of Washington state is reported to have higher percentages of non-Hispanic White and Asian and a slightly lower percentage of Hispanic and Black people than the US population as a whole,20 this cohort spans a diagnosis period dating back to the 1980s when the US population, especially children, was less diverse than today. Second, the data source for patients with cancer in this study did not identify whether additional treatment for relapse, secondary malignancy, or allogeneic hematopoietic cell transplantation occurred. It is possible that some of the identified infections were attributable to this additional immune suppression rather than residual effect of initial cancer therapy. However, this should be less of a concern among survivors analyzed > 10 years from initial cancer diagnosis, when significantly increased rates of infection were still observed. Third, given that this analysis only assessed inpatient infections and was dependent on diagnostic codes for infection event detection, we likely underestimated the burden of infections in the observed follow-up period. However, by restricting outcomes to those associated with hospitalization, our results should have captured the most clinically relevant serious infections.
Advancement in treatment protocols has yielded survival rates of > 80% at 5 years from diagnosis of childhood cancer; however, these patients are still at risk of significant long-term risk from infection secondary to their cancer diagnosis and therapy.21,22 Our data show that the risk of an infection associated with hospitalization continues to be significantly increased in survivors of childhood cancer compared with age-matched controls many years to decades after initial diagnosis, with an even higher burden on survivors of hematologic malignancies. It is vital for patients, caregivers, and their providers to have an awareness of this longer-term infection risk to allow for better counseling by physicians and recommendations of care for survivors that will reduce the morbidity of infection during the survivorship period. Additional investigation is necessary to optimize strategies aimed at preventing these infections altogether.
ACKNOWLEDGMENT
The authors would like to acknowledge the Washington State Department of Health for data access and Mr William O'Brien for programming and file management.
APPENDIX
TABLE A1.
Outcome Categories Derived From the Washington State Hospital Discharge and Death Registries
TABLE A2.
Incidence Rates per 1,000 Person-Years with 95% CI of Inpatient Infections Overall and by Pathogen and Organ System of Cancer Survivors 5 to <10 Years From Diagnosis and Comparators
TABLE A3.
Incidence Rates per 1,000 Person-Years with 95% CI of Inpatient Infections Overall and by Pathogen and Organ System of Cancer Survivors >10 Years From Diagnosis and Comparators
Adam J. Esbenshade
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Gregory M.T. Guilcher
Research Funding: Bluebird Bio
Christopher C. Dvorak
Consulting or Advisory Role: Alexion Pharmaceuticals, Omeros, Jazz Pharmaceuticals
Research Funding: Jasper Therapeutics
Brian T. Fisher
Consulting or Advisory Role: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst)
Beth A. Mueller
Stock and Other Ownership Interests: AstraZeneca
Eric J. Chow
Research Funding: Abbott
Jenna Rossoff
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
SUPPORT
Supported by Alex's Lemonade Stand Foundation for Childhood Cancer (grant to B.A.M. and E.J.C.). Cancer registry support: National Cancer Institute #HHSN261201300012I; Centers for Disease Control and Prevention #DP12-1205 DP003899-02.
AUTHOR CONTRIBUTIONS
Conception and design: Gregory M.T. Guilcher, Christopher C. Dvorak, Brian T. Fisher, Beth A. Mueller, Eric J. Chow
Financial support: Eric J. Chow, Beth A. Mueller
Administrative support: Eric J. Chow
Provision of study materials or patients: Beth A. Mueller, Eric J. Chow
Collection and assembly of data: Beth A. Mueller, Eric J. Chow
Data analysis and interpretation: Leena Chehab, David R. Doody, Adam J. Esbenshade, Gregory M.T. Guilcher, Christopher C. Dvorak, Brian T. Fisher, Eric J. Chow, Jenna Rossoff
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
A Population-Based Study of the Long-Term Risk of Infections Associated With Hospitalization in Childhood Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Adam J. Esbenshade
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Gregory M.T. Guilcher
Research Funding: Bluebird Bio
Christopher C. Dvorak
Consulting or Advisory Role: Alexion Pharmaceuticals, Omeros, Jazz Pharmaceuticals
Research Funding: Jasper Therapeutics
Brian T. Fisher
Consulting or Advisory Role: Astellas Pharma
Research Funding: Pfizer (Inst), Merck (Inst)
Beth A. Mueller
Stock and Other Ownership Interests: AstraZeneca
Eric J. Chow
Research Funding: Abbott
Jenna Rossoff
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. (eds): SEER Cancer Statistics Review, 1975-2018. Bethesda, MD, National Cancer Institute, 2021. https://seer.cancer.gov/csr/1975_2018/ [Google Scholar]
- 2.Frobisher C, Glaser A, Levitt GA, et al. : Risk stratification of childhood cancer survivors necessary for evidence-based clinical long-term follow-up. Br J Cancer 117:1723-1731, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeffen EAH, Kremer LCM, Mulder RL, et al. : The importance of evidence-based supportive care practice guidelines in childhood cancer-a plea for their development and implementation. Support Care Cancer 25:1121-1125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Tilburg CM, van Gent R, Bierings MB, et al. : Immune reconstitution in children following chemotherapy for haematological malignancies: A long-term follow-up. Br J Haematol 152:201-210, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Perkins JL, Chen Y, Harris A, et al. : Infections among long-term survivors of childhood and adolescent cancer: A report from the Childhood Cancer Survivor Study. Cancer 120:2514-2521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelland-Marcotte MC, Pole JD, Hwee J, et al. : Long-term risk of infections after treatment of childhood leukemia: A population-based cohort study using administrative health data. J Clin Oncol 37:2651-2660, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Weil BR, Madenci AL, Liu Q, et al. : Late infection-related mortality in asplenic survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol 36:1571-1578, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller BA, Doody DR, Weiss NS, et al. : Hospitalization and mortality among pediatric cancer survivors: A population-based study. Cancer Causes Control 29:1047-1057, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Guilcher GMT, Rivard L, Huang JT, et al. : Immune function in childhood cancer survivors: A Children's Oncology Group review. Lancet Child Adolesc Health 5:284-294, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343-346, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Foord AM, Cushing-Haugen KL, Boeckh MJ, et al. : Late infectious complications in hematopoietic cell transplantation survivors: A population-based study. Blood Adv 14:1232-1241, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steliarova-Foucher E, Stiller C, Lacour B, et al. : International Classification of childhood cancer, third edition. Cancer 103:1457-1467, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cummings P: Analysis of Incidence Rates. Boca Raton, FL, CRC Press, Taylor & Francis Group, 2019. pp 145-171 [Google Scholar]
- 14.StataCorp : Stata Statistical Software: Release 17. College Station, TX, StataCorp LLC, 2021 [Google Scholar]
- 15.Lorenzi MF, Xie L, Rogers PC, et al. : Hospital-related morbidity among childhood cancer survivors in British Columbia, Canada: Report of the childhood, adolescent, young adult cancer survivors (CAYACS) program. Int J Cancer 128:1624-1631, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Mikulska M, Cesaro S, de Lavallade H, et al. : Vaccination of patients with haematological malignancies who did not have transplantations: Guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19:e188-e199, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Patel SR, Heath PT, Skinner R: Guidelines for the Immunisation of Children Following Treatment With Standard-Dose Chemotherapy Department of Haematology & Oncology Birmingham Children’s Hospital Adapted From Vaccinations for Paediatric Patients Treated With Standard-Dose Chemotherapy and Hematopoietic Stem Cell Transplantation (HSCT) Recipients, 2014. https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/04/immunisation-schedule-in-children-following-standard-risk-chemotherapy-v2.pdf [Google Scholar]
- 18.Patel SR, Ortín M, Cohen BJ, et al. : Revaccination of children after completion of standard chemotherapy for acute leukemia. Clin Infect Dis 44:635-642, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Williams DJ, Shah SS, Myers A, et al. : Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr 167:851-858, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Census Bureau : Quick Facts: United States, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219 [Google Scholar]
- 21.Suh E, Stratton KL, Leisenring WM, et al. : Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: A retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol 21:421-435, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellblom AV, Kiserud CE, Rueegg CS, et al. : Self-reported late effects and long-term follow-up care among 1889 long-term Norwegian Childhood, Adolescent, and Young Adult Cancer Survivors (the NOR-CAYACS study). Support Care Cancer 29:2947-2957, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]