PURPOSE
To determine the specific types, durations, and intensities of recreational physical activity associated with the greatest improvements in disease-free survival (DFS) of patients with colon cancer.
METHODS
We conducted a prospective cohort study nested within a randomized multicenter trial of stage III colon cancer that compared 3 versus 6 months of fluorouracil, leucovorin, and oxaliplatin with or without celecoxib. We measured recreational physical activity in the first 3 months of chemotherapy and again 6 months after completion of chemotherapy. The primary end point was DFS.
RESULTS
During a median follow-up of 5.9 years, 457 of 1,696 patients experienced disease recurrence or death. For total recreational physical activity volume, the 3-year DFS was 76.5% with < 3.0 metabolic equivalent task hours per week (MET-h/wk) and 87.1% with ≥ 18.0 MET-h/wk (risk difference [RD], 10.6%; 95% CI, 4.7 to 19.4; P < .001). For light-intensity to moderate-intensity activities, the 3-year DFS was 65.7% with 0.0 h/wk and 87.1% with ≥ 1.5 h/wk (RD, 21.4%; 95% CI, 9.2 to 37.1; P < .001). For vigorous-intensity activity, the 3-year DFS was 76.0% with 0.0 h/wk and 86.0% with ≥ 1.0 h/wk (RD, 10.0%; 95% CI, 4.5 to 18.9; P < .001). For brisk walking, the 3-year DFS was 81.7% with < 1.0 h/wk and 88.4% with ≥ 3.0 h/wk (RD, 6.7%; 95% CI, 3.0 to 13.8; P < .001). For muscle strengthening activity, the 3-year DFS was 81.8% with 0.0 h/wk and 88.8% for ≥ 0.5 h/wk (RD, 7.0%; 95% CI, 3.1 to 14.2; P = .003).
CONCLUSION
Among patients with stage III colon cancer enrolled in a trial of postoperative treatment, larger volumes of recreational physical activity, longer durations of light- to moderate-intensity aerobic physical activity, or any vigorous-intensity aerobic physical activity were associated with the greatest improvements in DFS.
INTRODUCTION
One in three patients with colon cancer has regional lymph node metastases at diagnosis.1 Since the introduction of postoperative chemotherapy in the 1990s, the disease-free survival (DFS) of patients with stage III colon cancer has improved by 25 absolute percentage points.2,3 However, there remains substantial variability in patient outcomes on the basis of multiple prognostic factors.4 Additional strategies are needed to improve the cure rate for this common cancer.
Higher postdiagnosis physical activity energy expenditure is associated with improved DFS in colon cancer survivors.5,6 However, it is uncertain if this is due to light-intensity and moderate-intensity physical activity, vigorous-intensity physical activity, or expending more energy by physical activity through any means possible (eg, a combination of greater frequency, longer duration, or higher intensity). Muscle-strengthening activity is recommended for all adults7,8 and may confer general health benefits to cancer survivors,9 but how this type of activity affects prognosis independent of aerobic activity in colon cancer is unknown.10 These uncertainties may affect the success of the straightforward-sounding advice avoid inactivity11 for health care providers and patients whose primary motivation for physical activity is to prevent disease recurrence and death.12,13
We examined the association of different physical activity characteristics with disease recurrence and death in a cohort of patients with stage III colon cancer who were enrolled in a randomized multicenter trial of postoperative treatment that was sponsored by the National Cancer Institute.14 Within this trial, we prospectively collected measures of recreational physical activity, body mass index, and dietary patterns that were updated during the conduct of the trial. Moreover, because data on pathologic tumor stage, performance status, treatment, and follow-up were carefully measured in this trial, the simultaneous effect of disease characteristics and the use of postoperative therapy could be assessed. We hypothesized that larger volumes of recreational physical activity, longer durations of light-intensity to moderate-intensity aerobic physical activity, and any vigorous-intensity aerobic physical activity would be associated with the greatest improvements in DFS.
METHODS
Study Design
The Cancer and Leukemia Group B (now part of the Alliance for Clinical Trials in Oncology) and SWOG trial 80702 was designed in collaboration with the National Cancer Institute. The trial used a 2 × 2 factorial design to test the primary hypothesis of the superiority of celecoxib compared with placebo and the secondary hypothesis of the noninferiority of 3 months compared with 6 months of chemotherapy as part of an international pooling project.15 The superiority of celecoxib versus placebo to improve DFS was not proven.14 The results of the secondary hypotheses are reported.4,16 At the time of trial enrollment, patients were offered the option to participate in a nested cohort study of lifestyle factors that included completing standardized assessments at specified time intervals. Institutional review board approval was obtained at all participating centers, and patients provided written informed consent.
Study Population
Patients were enrolled at community and academic centers across the National Cancer Trials Network in the United States and Canada. Eligible patients had curatively resected (margin negative), histologically documented colonic adenocarcinoma. Tumors had at least one pathologically confirmed metastatic lymph node or N1c designation, defined as tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic tissue without regional lymph node metastases. Patients were age at least 18 years, with an Eastern Cooperative Oncology Group performance status of 0-2, and had normal hepatic, renal, and hematologic values.14
Physical Activity Assessment
Patients reported their average weekly time spent on a range of recreational physical activities during the past 2 months using a validated questionnaire (Data Supplement, online only).17 Each physical activity was assigned a metabolic equivalent (MET) value according to standardized criteria (Data Supplement, online only).18,19 We calculated the MET-hours per week (MET-h/wk) for each activity by multiplying the MET value with the patient's reported number of hours of physical activity each week. Total recreational physical activity volume was quantified as the sum of the MET-h/wk for all aerobic and muscle-strengthening activities. Light-intensity to moderate-intensity physical activities were < 6 METs, and vigorous-intensity activities were ≥ 6 METs.19 Patients reported their physical activity midway through postoperative chemotherapy (4 months after surgical resection) and 6 months after completing postoperative chemotherapy (14 months after surgical resection). Considering the potential for declining health to bias the physical activity assessment, we prespecified that the patients who experienced disease recurrence or death within 60 days after completing the first physical activity assessment would be excluded from the analysis.
Study End Points
The primary end point was DFS, defined as the time from completing the first physical activity assessment to the date of documented disease recurrence or death from any cause. Patients were assessed for disease recurrence by history, physical examination, and carcinoembryonic antigen measures every 3 months for 3 years after commencement of therapy and subsequently every 6 months for 6 years after random assignment or until disease recurrence, whichever came first. All patients had surveillance imaging of the chest, abdomen, and pelvis every 6 months for at least 3 years and then yearly for 3 years or until disease recurrence. Patients without an event were censored at their last disease evaluation date. The secondary end point was overall survival (OS), defined as the time from completing the first physical activity assessment to the date of death from any cause. The exploratory end point was colon cancer–specific survival, defined as the time from completing the first physical activity assessment to the date of death attributed to colon cancer.
Covariates
Data for patient demographic factors, including age, sex, race, and ethnicity, were self-reported. Clinical factors including the extent of tumor invasion through the bowel wall (T-stage), the extent of lymph node metastases (N-stage), pathologic risk group (low [T1, T2, or T3, N1] or high [T4, N2, or both]), tumor location, performance status, and low-dose aspirin use were obtained from a combination of physician assessment and the medical record. Smoking history was self-reported. Body mass index was abstracted from a combination of the electronic medical record and self-report. Diet was assessed using a 131-item food frequency questionnaire20; prudent and western dietary patterns were defined using previously validated factor loadings in this population.21 Body mass index and diet were updated when physical activity was reassessed.
Statistical Analysis
To test for differences in baseline patient characteristics by categories of total recreational physical activity volume, χ2 was used for categorical variables and analysis of covariance was used for continuous variables. Physical activity was modeled using cumulative averaging, which quantifies the time-weighted average of all reported physical activities.21,22 We updated physical activity on the basis of the results of the second questionnaire that was weighted proportional to the time between the first and the second questionnaires and the time between the second questionnaire and the DFS period. Repeatedly measured variables (eg, body mass index and dietary patterns) were included as time-varying covariates. Flexible parametric proportional hazards survival models were used to quantify the association between physical activity characteristics and study end points.23 Parametric survival models allow for smooth predictions and the estimation of absolute and relative effects while permitting flexibility in the shape of the baseline hazard function.24,25 Absolute effects are presented as risk differences (RDs) at 3 years for DFS and 5 years for OS, as these time horizons are clinically meaningful.26 CIs for the RD were calculated using the bootstrap method with 1,000 replicates.27 Relative effects are presented as the hazard ratio (HR) using all observed data in a time-to-event framework.
After testing the primary hypothesis of total recreational physical activity volume, we tested four hypotheses (each nested within total recreational physical activity volume), including (1) light-intensity and moderate-intensity activities, (2) vigorous-intensity physical activity, (3) brisk walking, and (4) muscle-strengthening activity. A two-sided P < .05 was considered statistically significant. Our statistical analysis plan did not prespecify any adjustment for multiple comparisons; reported P values are therefore not adjusted for multiplicity.
The population-attributable risk was estimated for total recreational physical activity volume to quantify the proportion of DFS and OS events that could be theoretically prevented if all patients with colon cancer increased their physical activity by 3-MET-h/wk (approximately 1 hour of brisk walking per week).28 The population-attributable risk is a function of the prevalence of physical activity and corresponding effect size.28 Effect modification of aerobic physical activity volume by muscle-strengthening activity was examined with a multiplicative interaction term and the likelihood ratio test.29 Planned subgroup analyses of total recreational physical activity volume included sex, body mass index, risk group, and chemotherapy length. Subgroup analyses were conducted by including a multiplicative interaction term of the subgroup factor with the total recreational physical activity volume in regression models. Sensitivity analyses were conducted to quantify the strength that an unmeasured confounder must have to explain the observed associations.30 To test for differences in baseline patient characteristics by inclusion or exclusion from the nested cohort, χ2 was used for categorical variables and the t-test was used for continuous variables. Multivariable-adjusted generalized linear models were used to quantify the association between physical activity and chemotherapy relative dose intensity. Data were collected by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. Data analysis was conducted by the Alliance Statistics and Data Management Center using SAS (Version 9.4) and R (Version 4.1.0) on a data set locked on August 10, 2020.
RESULTS
From June 2010 to November 2015, 2,524 patients from 654 centers were eligible and randomly assigned; 1,696 (67.2%) were enrolled in the nested cohort of diet and lifestyle study (Fig 1). Patients enrolled in the cohort were more often White and non-Hispanic and more likely to use low-dose aspirin (Data Supplement).
FIG 1.
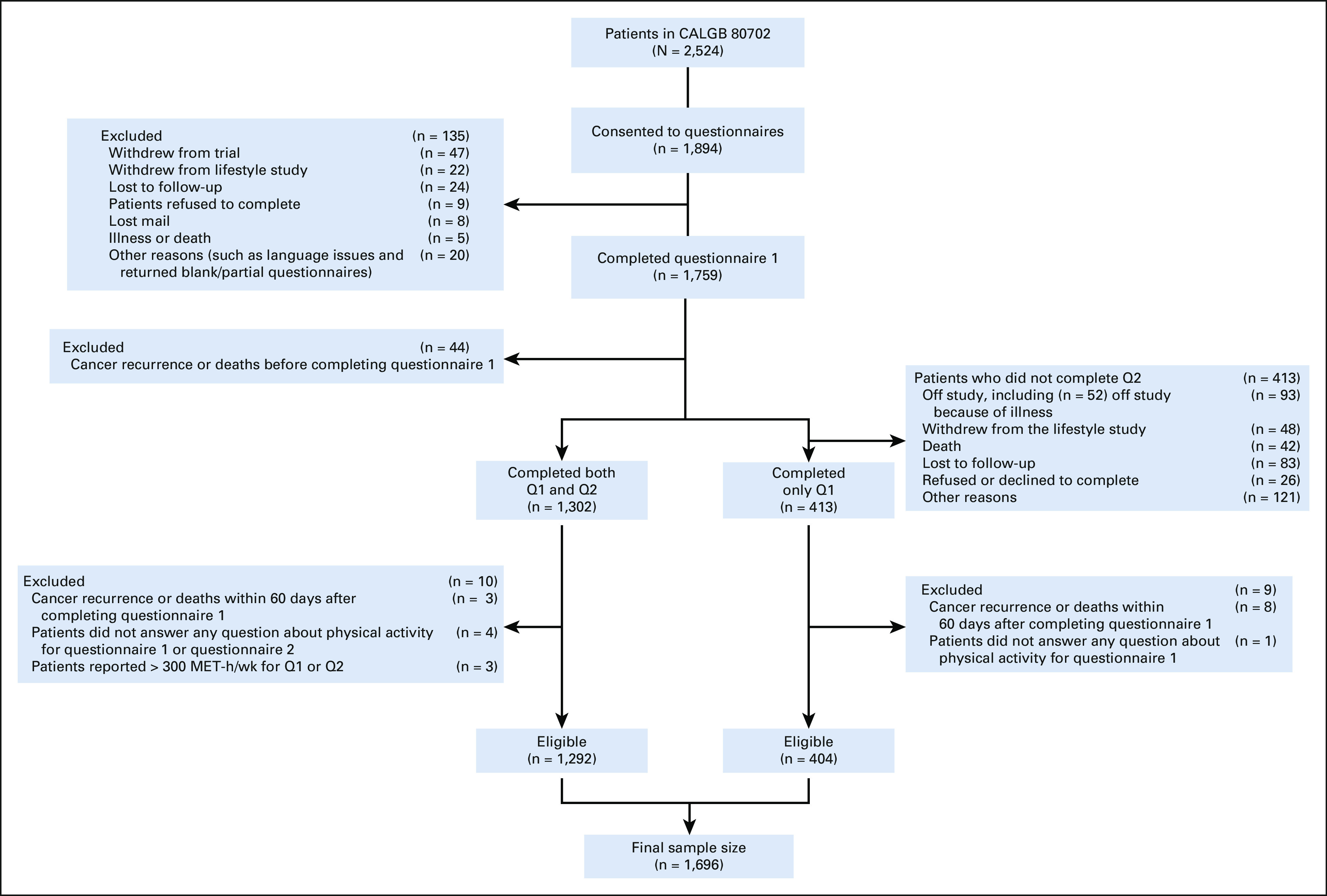
Derivation of the study cohort. MET-h/wk, metabolic equivalent total physical activity energy expenditure; Q1, questionnaire 1; Q2, questionnaire 2.
Patients with higher volumes of total recreational physical activity were younger, more likely to be male, White, non-Hispanic, with lower-risk pathologic staging and a lower body mass index, and more likely to consume a more prudent dietary pattern (Table 1). Patients with larger volumes of total recreational physical activity during treatment had a higher relative dose intensity of chemotherapy (Data Supplement).
TABLE 1.
Baseline Patient Characteristics, Overall and by Category of Total Recreational Physical Activity Volume
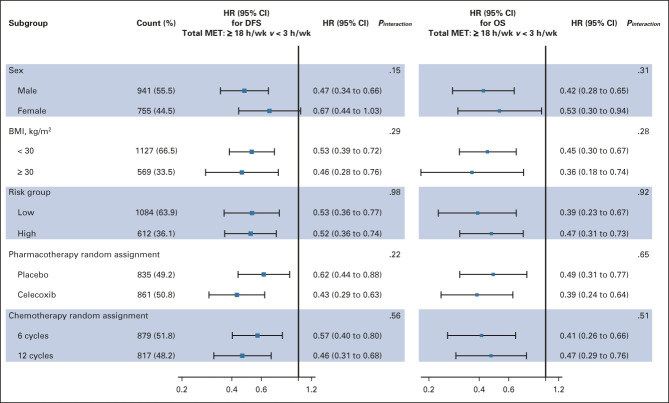
During a median follow-up of 5.9 years, 457 patients experienced disease recurrence or death; the 3-year DFS was 83.9% (95% CI, 78.9 to 87.6). For total recreational physical activity volume, the 3-year DFS was 76.5% with < 3.0 MET-h/wk and 87.1% with ≥ 18.0 MET-h/wk (RD, 10.6%, 95% CI, 4.7 to 19.4; HR, 0.52, 95% CI, 0.36 to 0.70; P < .001; Table 2). The population-attributable risk for each 3-MET-h/wk increase in total recreational physical activity volume was 22%. No significant effect modification was observed for any planned subgroups, including sex, body mass index, risk group, and chemotherapy length (Fig 2). During follow-up, 281 patients died; the 5-year OS rate was 89.4% (95% CI, 84.9 to 92.7); 255 deaths (90.7%) were attributed to colon cancer. Associations with physical activity were comparable for OS and colon cancer–specific survival (Data Supplement).
TABLE 2.
Association of DFS and OS End Points With Category of Total Recreational Physical Activity Volume
FIG 2.
Subgroup analyses of the association of DFS and OS end points with total recreational physical activity volume. Adjusted for age, sex, race, extent of invasion through the bowel wall, nodal stage, tumor location, ECOG performance status, low-dose aspirin use, smoking history, body mass index (time-varying), western dietary pattern (time-varying), prudent dietary pattern (time-varying), chemotherapy random assignment, and pharmacotherapy random assignment. BMI, body mass index; DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; MET-h/wk, metabolic equivalent total physical activity energy expenditure; OS, overall survival.
For light-intensity and moderate-intensity activities, the 3-year DFS was 65.7% with 0.0 h/wk and 87.1% with ≥ 1.5 h/wk (RD, 21.4%, 95% CI, 9.2 to 37.1; HR, 0.33, 95% CI, 0.20 to 0.51; P < .001; Table 3). For vigorous-intensity activity, the 3-year DFS was 76.0% with 0.0 h/wk and 86.0% with ≥ 1.0 h/wk (RD, 10.0%, 95% CI, 4.5 to 18.9; HR, 0.55, 95% CI, 0.40 to 0.73; P < .001). For brisk walking, the 3-year DFS was 81.7% with < 1.0 h/wk and 88.4% with ≥ 3.0 h/wk (RD, 6.7%, 95% CI, 3.0 to 13.8; HR, 0.61, 95% CI, 0.43 to 0.82; P < .001). For muscle strengthening activity, the 3-year DFS was 81.8% with 0.0 h/wk and 88.8% for ≥ 0.5 h/wk (RD, 7.0%, 95% CI, 3.1 to 14.2; HR, 0.59, 95% CI, 0.39 to 0.85; P = .003). Associations with specific physical activity types were comparable for OS and colon cancer–specific survival (Data Supplement). The association of light-intensity and moderate-intensity activities with DFS was independent of vigorous-intensity activity and vice versa (Data Supplement).
TABLE 3.
Association of DFS and OS End Points by Type of Physical Activity
Muscle-strengthening activity did not modify the association of aerobic activity with DFS (Pinteraction = .64). Compared with no resistance activity and 0 MET-h/wk of aerobic activity (sedentary), patients who reported resistance activity but no aerobic activity had a lower risk of disease recurrence or death (HR, 0.68; 95% CI, 0.49 to 0.94); patients who engaged in 18 MET-h/wk of aerobic activity and did not report resistance activity (HR, 0.60; 95% CI, 0.47 to 0.77) or who did report resistance activity (HR, 0.41; 95% CI, 0.28 to 0.59) had a lower risk of disease recurrence or death (Data Supplement). Muscle-strengthening activity was not associated with DFS after adjustment for aerobic activity (Data Supplement).
Sensitivity analyses indicated that unmeasured confounders would need to have a HR larger than 2.15 to explain away a RD of 10% and larger than 1.74 to explain away a HR of 0.75, independent of all the other variables included in the regression models.
DISCUSSION
In this nested cohort study of 1,696 patients with stage III colon cancer enrolled in a randomized multicenter trial of postoperative treatment, postdiagnosis physical activity was associated with meaningful improvements in DFS. The greatest absolute improvements in 3-year DFS were with larger volumes of recreational physical activity, longer durations of light-intensity to moderate-intensity aerobic physical activities, or vigorous-intensity aerobic physical activity. Patients who engaged in ≥ 18 MET-h/wk of recreational physical activity had a 10.6 absolute percentage point improvement in 3-year DFS compared with patients who engaged in < 3.0 MET-h/wk of physical activity (87.1% v 76.5% were alive and disease-free at 3 years). Patients who engaged in ≥ 1.5 h/wk light-intensity to moderate-intensity physical activities had a 21.4 absolute percentage point improvement in 3-year DFS, compared with patients who engaged in no light-intensity to moderate-intensity physical activity (87.1% v 65.7% were alive and disease-free at 3 years). Patients who engaged in ≥ 1 h/wk vigorous-intensity physical activity had a 10.0 absolute percentage point improvement in 3-year DFS compared with patients who engaged in no vigorous-intensity physical activity (86.0% v 76.0% were alive and disease-free at 3 years). These findings clarify how different physical activity characteristics among stage III colon cancer survivors have distinct associations with DFS.
It was previously unknown how different physical activity characteristics associate with disease recurrence and death in colon cancer survivors.31 Consequently, clinical guidelines encouraged patients to avoid inactivity.11 Our data indicate that participation in physical activity may be associated with clinically meaningful improvements in prognosis, which may be informative for health care providers and patients who seek additional details regarding the benefits of physical activity to reduce disease recurrence and death. In randomized clinical trials of patients with colorectal cancer, longer-duration or higher-intensity aerobic exercise improves cardiovascular fitness and body composition to a greater extent than shorter-duration or lower-intensity aerobic exercise.32,33 Changes in cardiovascular fitness and body composition are hypothesized mediators of the relationship between physical activity and clinical outcomes in cancer survivors.34,35
The hypothesis-generating observation that patients with larger volumes of total recreational physical activity during treatment had a higher relative dose intensity of chemotherapy may partly explain the benefit of physical activity on DFS. The National Cancer Institute has launched a randomized clinical trial (U01-CA271279) to test the hypothesis that aerobic exercise training improves chemotherapy relative dose intensity in patients with colon cancer. This trial is part of the Exercise and Nutrition Interventions to Improve Cancer Treatment-Related Outcomes (ENICTO) in Cancer Survivors Consortium.
Engaging in ≥ 0.5 h/wk of muscle-strengthening exercise was associated with a 7.0 absolute percentage point improvement in 3-year DFS (88.8% v 81.8% were alive and disease-free at 3 years). This observation contributes to a growing evidence base that muscle strengthening may improve prognosis in cancer survivors.10 In the general population, engaging in muscle-strengthening exercise is associated with a lower risk of all-cause and cancer-specific death.36 Adults who engage in sufficient aerobic and muscle-strengthening activity have a lower risk of death than adults who engage in either activity modality alone.37
The observation that a higher total recreational physical activity volume is associated with improved DFS can be compared with a similar nested cohort study conducted in 832 patients with stage III colon cancer recruited between 1999 and 2001 (CALGB 89803).5 The proportion of patients engaged in ≥ 18 MET-h/wk of total physical activity was 28.2% in CALGB 89803, which is comparable with 28.5% in the current study. In CALGB 89803, compared with patients engaged in < 3.0 MET-h/wk of physical activity, patients who engaged in 18.0-26.9 MET-h/wk had a 49% relative risk reduction in disease recurrence or death, which is comparable with the 48% relative risk reduction in the current study. Patients in CALGB 89803 were not treated with oxaliplatin-containing chemotherapy. Until the current study, it was unclear if physical activity remained an independent correlate of clinical outcomes for patients treated with contemporary chemotherapy.
The association of total recreational physical activity volume with DFS was not statistically significantly different among any planned subgroups, including pathologic risk group and randomized chemotherapy length. This finding indicates that postdiagnosis physical activity is associated with a clinical benefit for patients with pathologic features that are predictive of high recurrence risk, including T4-stage primary tumors and N2-stage regional lymph node involvement.
There are several limitations to the current study. This was not a randomized trial, and because of the observational design of our study, we cannot rule out the possibility of residual confounding. Our sensitivity analyses indicated that an unmeasured confounder would need to be of a moderate magnitude to shift the absolute and relative effect size estimates to the null.
Patients who enroll in randomized trials differ from the underlying population,38 which may reduce the generalizability of our findings. This ancillary study was optional, and patients who enrolled in this ancillary study were more likely to be White, non-Hispanic, and use low-dose aspirin, which may influence our findings. However, because our study sample was recruited throughout the United States and Canada in academic and community cancer centers, we believe that our findings can be generalized to a large proportion of patients with stage III colon cancer.
Physical activity was self-reported in this study. The questionnaire was restricted to specific recreational physical activities and not a comprehensive assessment of all recreational activities. Muscle-strengthening activity was assessed using a single question. The physical activity questionnaire is validated17 and was completed before any knowledge of clinical events, such as disease recurrence, reducing the likelihood of reporting bias. Our results parallel data from the general population that larger volumes or higher intensities of accelerometer-measured physical activity are associated with a lower risk of all-cause death.39
Patients who are more physically active after a cancer diagnosis might have been similarly active before diagnosis; thus, we cannot exclude the possibility that patients who engaged in higher physical activity develop biologically less aggressive tumors. In colorectal cancer survivors, meta-analyses of cohort studies indicate that postdiagnosis physical activity may be more strongly associated with cancer-specific death than prediagnosis physical activity.40,41
There are several important strengths in the current study. Nesting a cohort study within a randomized clinical trial to examine the association of physical activity with clinical outcomes offers several advantages over other data sources. First, because of uniform eligibility criteria, the disease status of study participants was extensively characterized, thereby improving patient homogeneity. Second, because this analysis was conducted within the context of a therapeutic trial, treatment, follow-up care, and end point ascertainment were standardized. Finally, detailed information on confounding variables, such as smoking status, body mass index, and dietary patterns, allowed for extensive covariate adjustment.
Among patients with stage III colon cancer enrolled in a trial of postoperative treatment, larger volumes of recreational physical activity, longer durations of light-intensity to moderate-intensity aerobic physical activity, or any vigorous-intensity aerobic physical activity were associated with the greatest improvements in DFS.
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Charles S. Fuchs
Employment: Genentech/Roche
Leadership: CytomX Therapeutics, EvolveImmune Therapeutics
Stock and Other Ownership Interests: CytomX Therapeutics, Entrinsic Health, EvolveImmune Therapeutics, Roche/Genentech
Consulting or Advisory Role: Sanofi, Merck, Entrinsic Health, Agios, Taiho Pharmaceutical, Genentech/Roche, CytomX Therapeutics, Unum Therapeutics, Bain Capital, Lilly, Amylin, Daiichi-Sankyo, EvolveImmune Therapeutics, AstraZeneca
Expert Testimony: Lilly, Amylin
Jeffrey Meyer
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Felix Couture
Consulting or Advisory Role: Bristol Myers Squibb, Novartis Canada Pharmaceuticals Inc
DeQuincy Lewis
Stock and Other Ownership Interests: Cellular Therapeutics, Verastem, Veru, Pieris Pharmaceuticals, Oncternal Therapeutics, Inc, Spectrum Pharmaceuticals, Curis, Precision Biosciences
Benjamin Tan
Research Funding: Roche/Genentech (Inst), Eisai (Inst), Exelixis (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Adaptimmune (Inst), TYME (Inst), Agios (Inst)
Smitha Krishnamurthi
Research Funding: Bristol Myers Squibb (Inst), Aravive (Inst)
Eileen M. O'Reilly
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Adicet Bio (I), AstraZeneca (I), Alnylam (I), Autem Medical (I), BeiGene (I), Berry Genomics (I), CytomX Therapeutics, Eisai (I), Exelixis (I), Genentech/Roche (I), Genoscience Pharma (I), Helio Health (I), Incyte (I), Ipsen (I), Legend Biotech (I), Merck, Nerviano Medical Sciences (I), QED Therapeutics (I), RedHill Biopharma (I), Yiviva (I), Novartis, Rafael Pharmaceuticals, Seattle Genetics, Boehringer Ingelheim, IDEAYA Biosciences, Noxxon Pharma, BioSapien, Thetis Pharma, Cend Therapeutics, Flatiron Health (I)
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Silenseed (Inst), Arcus Ventures (Inst), BioNTech (Inst), Elicio Therapeutics (Inst), Parker Institute for Cancer Immunotherapy (Inst)
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences, Cogent Biosciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst), Gritstone Bio (Inst), SQZ Biotechnology (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Merck
Research Funding: Boston Biomedical (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported by grants U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology, https://acknowledgments.alliancefound.org; U10CA180794, UG1CA233163, UG1CA233253, UG1CA233290, UG1CA233320, UG1CA233337, UG1CA233339, UG1CA189954, and U10CA180863 to the Canadian Cancer Trials Group; UG1CA233196 and U10CA180820 to the Eastern Cooperative Oncology Group–Alliance for Clinical Trials in Oncology; U10CA180868 to NRG Oncology; U10CA180888 to the Southwest Oncology Group from the National Cancer Institute of the National Institutes of Health; and R00CA218603 to J.C.B.. J.A.M. was supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, and the George Stone Family Foundation. The National Cancer Institute was involved in the design of the study and review and approval of the manuscript; it was not involved in the conduct of the study; collection, management, analysis, and interpretation of the data; and decision to submit the manuscript for publication. Pfizer participated in initial protocol development and review and approval of the final manuscript. Pfizer provided celecoxib and placebo tablets. Pfizer was not involved in collection, management, analysis, and interpretation of the data. Neither Pfizer nor the National Cancer Institute had neither the right to veto publication nor control the decision to which journal the article was submitted.
AUTHOR CONTRIBUTIONS
Conception and design: Justin C. Brown, Chao Ma, Charles S. Fuchs, Donna Niedzwiecki, Anthony F. Shields, Jeffrey A. Meyerhardt
Financial support: Justin C. Brown, Jeffrey A. Meyerhardt
Administrative support: Justin C. Brown, Charles S. Fuchs, Eileen M. O'Reilly, Jeffrey A. Meyerhardt
Provision of study materials or patients: Justin C. Brown, Qian Shi, Charles S. Fuchs, Felix Couture, Philip Kuebler, Smitha Krishnamurthi, Anthony F. Shields, Jeffrey A. Meyerhardt
Collection and assembly of data: Justin C. Brown, Chao Ma, Qian Shi, Jeffrey Meyer, Felix Couture, Philip Kuebler, DeQuincy Lewis, Benjamin Tan, Anthony F. Shields, Jeffrey A. Meyerhardt
Data analysis and interpretation: Justin C. Brown, Chao Ma, Qian Shi, Charles S. Fuchs, Jeffrey Meyer, Tyler Zemla, Pankaj Kumar, Smitha Krishnamurthi, Eileen M. O'Reilly, Anthony F. Shields, Jeffrey A. Meyerhardt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Physical Activity in Stage III Colon Cancer: CALGB/SWOG 80702 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Qian Shi
Honoraria: Chugai Pharma
Consulting or Advisory Role: Yiviva, Boehringer Ingelheim, Regeneron, Hoosier Cancer Research Network
Research Funding: Celgene (Inst), Roche/Genentech (Inst), Janssen (Inst), BMS (Inst), Novartis (Inst)
Charles S. Fuchs
Employment: Genentech/Roche
Leadership: CytomX Therapeutics, EvolveImmune Therapeutics
Stock and Other Ownership Interests: CytomX Therapeutics, Entrinsic Health, EvolveImmune Therapeutics, Roche/Genentech
Consulting or Advisory Role: Sanofi, Merck, Entrinsic Health, Agios, Taiho Pharmaceutical, Genentech/Roche, CytomX Therapeutics, Unum Therapeutics, Bain Capital, Lilly, Amylin, Daiichi-Sankyo, EvolveImmune Therapeutics, AstraZeneca
Expert Testimony: Lilly, Amylin
Jeffrey Meyer
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Felix Couture
Consulting or Advisory Role: Bristol Myers Squibb, Novartis Canada Pharmaceuticals Inc
DeQuincy Lewis
Stock and Other Ownership Interests: Cellular Therapeutics, Verastem, Veru, Pieris Pharmaceuticals, Oncternal Therapeutics, Inc, Spectrum Pharmaceuticals, Curis, Precision Biosciences
Benjamin Tan
Research Funding: Roche/Genentech (Inst), Eisai (Inst), Exelixis (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Adaptimmune (Inst), TYME (Inst), Agios (Inst)
Smitha Krishnamurthi
Research Funding: Bristol Myers Squibb (Inst), Aravive (Inst)
Eileen M. O'Reilly
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Adicet Bio (I), AstraZeneca (I), Alnylam (I), Autem Medical (I), BeiGene (I), Berry Genomics (I), CytomX Therapeutics, Eisai (I), Exelixis (I), Genentech/Roche (I), Genoscience Pharma (I), Helio Health (I), Incyte (I), Ipsen (I), Legend Biotech (I), Merck, Nerviano Medical Sciences (I), QED Therapeutics (I), RedHill Biopharma (I), Yiviva (I), Novartis, Rafael Pharmaceuticals, Seattle Genetics, Boehringer Ingelheim, IDEAYA Biosciences, Noxxon Pharma, BioSapien, Thetis Pharma, Cend Therapeutics, Flatiron Health (I)
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Silenseed (Inst), Arcus Ventures (Inst), BioNTech (Inst), Elicio Therapeutics (Inst), Parker Institute for Cancer Immunotherapy (Inst)
Anthony F. Shields
Consulting or Advisory Role: ImaginAb, Caris Life Sciences, Cogent Biosciences
Speakers' Bureau: Caris Life Sciences
Research Funding: Taiho Pharmaceutical, Bayer, Boehringer Ingelheim, Plexxikon, Eisai, Inovio Pharmaceuticals, H3 Biomedicine, Caris Life Sciences, ImaginAb, Exelixis, Xencor, Lexicon, Daiichi Sankyo, Halozyme, Incyte, LSK BioPharma, Esperas Pharma, Nouscom, Boston Biomedical, Astellas Pharma, AstraZeneca, Five Prime Therapeutics, MSK Pharma, Alkermes, Repertoire Immune Medicines, Telix Pharmaceuticals, Hutchison China Meditech, Seattle Genetics, Jiangsu Alphamab Biopharmaceuticals, Shanghai HaiHe Pharmaceutical, TopAlliance BioSciences Inc (Inst), Gritstone Bio (Inst), SQZ Biotechnology (Inst)
Travel, Accommodations, Expenses: GE Healthcare, Caris Life Sciences, TransTarget, ImaginAb, Inovio Pharmaceuticals
Jeffrey A. Meyerhardt
Honoraria: Cota Healthcare, Merck
Research Funding: Boston Biomedical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. : Colorectal cancer statistics, 2020. CA Cancer J Clin 70:145-164, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Fleming TR, Macdonald JS, et al. : Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352-358, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Mounedji-Boudiaf L, et al. : Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343-2351, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Sobrero AF, Shields AF, et al. : Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378:1177-1188, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. : Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol 24:3535-3541, 2006 [DOI] [PubMed] [Google Scholar]
- 6.McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. : Physical activity in cancer prevention and survival: A systematic review. Med Sci Sports Exerc 51:1252-1261, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piercy KL, Troiano RP, Ballard RM, et al. : The physical activity guidelines for Americans. JAMA 320:2020-2028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bull FC, Al-Ansari SS, Biddle S, et al. : World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54:1451-1462, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz KH, Courneya KS, Matthews C, et al. : American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42:1409-1426, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Hardee JP, Porter RR, Sui X, et al. : The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc 89:1108-1115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell KL, Winters-Stone KM, Wiskemann J, et al. : Exercise guidelines for cancer survivors: Consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 51:2375-2390, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher A, Beeken RJ, Heinrich M, et al. : Health behaviours and fear of cancer recurrence in 10 969 colorectal cancer (CRC) patients. Psychooncology 25:1434-1440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Mangu PB, Flynn PJ, et al. : Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 31:4465-4470, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Shi Q, Fuchs CS, et al. : Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: The CALGB/SWOG 80702 (Alliance) randomized clinical trial. JAMA 325:1277-1286, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andre T, Iveson T, Labianca R, et al. : The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: Prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: Trial design and current status. Curr Colorectal Cancer Rep 9:261-269, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andre T, Meyerhardt J, Iveson T, et al. : Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): Final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol 21:1620-1629, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf AM, Hunter DJ, Colditz GA, et al. : Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 23:991-999, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Whitt MC, et al. : Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 32:S498-S504, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Glass S, Dwyer GB, Medicine ACoS : ACSM's Metabolic Calculations Handbook. Philadelphia, PA, Lippincott Williams & Wilkins, 2007 [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. : Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122:51-65, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. : Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298:754-764, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer MJ, Rimm E, et al. : Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 149:531-540, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Royston P, Parmar MK: Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 21:2175-2197, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Rutherford MJ, Crowther MJ, Lambert PC: The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: A simulation study. J Stat Comput Simulation 85:777-793, 2013 [Google Scholar]
- 25.Jackson CH: Flexsurv: A platform for parametric survival modeling in R. J Stat Softw 70:i08, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sargent DJ, Wieand HS, Haller DG, et al. : Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23:8664-8670, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lloyd CJ: Bootstrap and second-order tests of risk difference. Biometrics 66:975-982, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Spiegelman D, Hertzmark E, Wand HC: Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control 18:571-579, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Harrell F: Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY, Springer, 2015 [Google Scholar]
- 30.VanderWeele TJ, Ding P: Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med 167:268-274, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Patel AV, Friedenreich CM, Moore SC, et al. : American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 51:2391-2402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devin JL, Sax AT, Hughes GI, et al. : The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: A randomised controlled trial. J Cancer Surviv 10:467-479, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Devin JL, Jenkins DG, Sax AT, et al. : Cardiorespiratory fitness and body composition responses to different intensities and frequencies of exercise training in colorectal cancer survivors. Clin Colorectal Cancer 17:e269-e279, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Brown JC, Gilmore LA: Physical activity reduces the risk of recurrence and mortality in cancer patients. Exerc Sport Sci Rev 48:67-73, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JC, Meyerhardt JA, Cespedes Feliciano EM, et al. : The association of abdominal adiposity with premature discontinuation of postoperative chemotherapy in colon cancer. Clin Nutr 41:1600-1604, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatakis E, Lee IM, Bennie J, et al. : Does strength-promoting exercise confer unique health benefits? A pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am J Epidemiol 187:1102-1112, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Zhao M, Veeranki SP, Magnussen CG, et al. : Recommended physical activity and all cause and cause specific mortality in US adults: Prospective cohort study. BMJ 370:m2031, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murthy VH, Krumholz HM, Gross CP: Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 291:2720-2726, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Strain T, Wijndaele K, Dempsey PC, et al. : Wearable-device-measured physical activity and future health risk. Nat Med 26:1385-1391, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedenreich CM, Stone CR, Cheung WY, et al. : Physical activity and mortality in cancer survivors: A systematic review and meta-analysis. JNCI Cancer Spectr 4:pkz080, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid D, Leitzmann MF: Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol 25:1293-1311, 2014 [DOI] [PubMed] [Google Scholar]