Abstract
Aging is an evolutionary paradox. Several hypotheses have been proposed to explain it, but none fully explains all the biochemical and ecologic data accumulated over decades of research. We suggest that senescence is a primitive immune strategy which acts to protect an individual’s kin from chronic infections. Older organisms are exposed to pathogens for a longer period of time and have a higher likelihood of acquiring infectious diseases. Accordingly, the parasitic load in aged individuals is higher than in younger ones. Given that the probability of pathogen transmission is higher within the kin, the inclusive fitness cost of infection might exceed the benefit of living longer. In this case, programmed lifespan termination might be an evolutionarily stable strategy. Here, we discuss the classical evolutionary hypotheses of aging and compare them with the pathogen control hypothesis, discuss the consistency of these hypotheses with existing empirical data, and present a revised conceptual framework to understand the evolution of aging.
Electronic supplementary material
The online version contains supplementary material available at 10.1134/S0006297922120021.
Keywords: aging, evolution, infection, immunity, phenoptosis
INTRODUCTION
Aging, defined in broad terms as an increase in mortality with age, is both an evolutionary paradox and the topic of heated biological and philosophical discussions. Rooted in religious concepts, the traditional view considers senescence as an entity, organically embedded into the structure of the Universe with the lifespan parameters predetermined: “...their days will be a hundred and twenty years.” (Genesis, 6:3), but with the ability of an individual to modify their longevity within a certain allowed interval: “Respect your father and your mother, and you will live a long time...” (Exodus, 20:12).
The first attempt to rationally address senescence was made at the very beginning of the Darwinian era. In 1881, August Weismann wrote that aging might be an evolutionary adaptation for clearing injured individuals who consume precious resources but do not contribute to reproduction [1]. This view was later dismissed as a group selection argument that cannot stand if long-lived defectors are invading the population of short-lived variants.
The next evolutionary concept came in 1930. Ronald A. Fisher argued that, since the replication capacity of an individual declines with age, so does the contribution to the future ancestry [2]. This work greatly influenced John B. S. Haldane and Peter Medawar, who proposed the “selection shadow” theory [3, 4]. They stated that, since individuals often die from non-natural, extrinsic causes (e.g., hunger or predation), the power of selection is reduced with age. If only a negligible proportion of individuals survive to older ages, senescence might have little effect on overall animal fitness. Medawar also hypothesized that aging traits might be linked to the adaptive traits that increase fitness at an early age, thus increasing the genetic stability of senescence.
The latter idea was explored by William D. Hamilton [5] and further developed by George C. Williams in his “antagonistic pleiotropy” theory [6]. He criticized selection shadow by pointing out that, regardless of selection decline with age, individuals survive to the age of senescence. Next, he proposed that aging can be favored by selection if the mechanisms of senescence result from the action of genes that are beneficial in early life and detrimental in older ages. This concept was further extended by Tom B. L. Kirkwood and Robin Holliday [7]. Their model, called the “disposable soma” theory, stated that “mortality may be due to an energy-saving strategy of reduced error regulation in somatic cells” to invest the saved resources into reproduction. This view was strongly supported by the well-documented negative correlation between fecundity and longevity [8, 9].
Independently, several mechanistic hypotheses were developed. In 1963, Leslie Orgel suggested that, because transcription and translation are error-prone processes, the mistakes made during the synthesis of macromolecules affect the synthetic machinery itself, creating a positive feedback loop of deterioration [10]. The field was dominated for decades by Denham Harman’s free radical hypothesis, which proposed that senescence is a consequence of oxidative damage [11]. Other models involved various damaging factors or even a cumulative, chaotic effect of multiple harmful processes [12-19]. A group of specifically elegant hypotheses suggested the selfish evolution of mitochondria [20], transposons [21], and cancer cells [22] as the primary drivers of senescence. These views formed a concept crystallized in a publication titled “hallmarks of aging” [23] that summarized molecular and cellular results and is frequently referred to as a paradigm of geroscience. However, this review, while presenting a comprehensive list of observations, fails to provide a mechanistic explanation of why we age [24]. A subset of mechanistic hypotheses proclaims the complexity of biological systems as the central problem of aging research by reasoning that aging is wired into the inability of complex systems to sustain homeostasis [18, 25].
A few hypotheses supported by a minority of researchers consider aging as an adaptation itself rather than an entropic process or a detrimental side effect of other beneficial functions. However, intuitively, senescence, which is highly variable in different species in terms of its developmental time and conserved within the species, bears a resemblance to other clearly adaptive characteristics such as body size or blood pressure. The adaptive senescence hypotheses claim aging either has an ecological role in preventing Malthusian catastrophes [1, 26-30], mediating faster generational change, thus accelerating evolution [31-33], or is a protective strategy to mitigate penalties of infectious diseases [34, 35]. More recently, a novel hypothesis involving pathogen exposure and the role of aging in the prevention of epidemics may provide a stronger and more comprehensive evolutionary hypothesis that overcomes the limitations of existing models [36, 37].
In the current article, we revisit the field to compare different evolutionary hypotheses and discuss their parsimony, explanatory power, and quality of predictions. Given that “nothing in biology makes sense except in the light of evolution” (Theodosius Dobzhansky), our discussion is an attempt to organize these ideas to provide a path to understanding the purpose of aging, and perhaps, to instruct development of anti-aging therapies.
ENTROPIC HYPOTHESES: IS IMMORTALITY IMPOSSIBLE?
Here, we do not use the term “immortal” to refer to an organism that exists in the environment for a continuous period of time. Instead, we use it in a sense of “biological immortality”, meaning that the probability of death does not increase with the age. The baseline mortality may still be substantial, but if it stays constant over time, an organism should be considered biologically immortal, or non-aging.
Several primitive metazoans, such as hydra [38], planarian flatworms [39], and jellyfishes [39] display no increase in mortality with age and are considered to be biologically immortal. Several vertebrates belonging to different taxa may also have negligible senescence [40-44]. However, the lack of senescence in these animals is not widely accepted since detection of an increase in mortality is difficult as it takes a very long time.
Yet, senescence may be an organic process rooted in some basic biochemistry of living organisms. Entropy in the form of molecular damage or selfish evolution may limit lifespan setpoint. Nelson and Masel [22] have suggested that the intracellular competition within proliferating tissues may be evolutionarily beneficial by removing low-fit and damaged cells. As an unavoidable side effect, competition favors the best replicators, often resulting in neoplastic transformation. Thus, they proposed that cancer-preventing mechanisms determine lifespan as an adverse side effect.
However, it is unclear how long-living species overcome the neoplastic problem, and why tissues with little proliferation and postmitotic organisms also present aging-related deterioration [45-47]. Two fundamental questions emerge from these considerations. First, what is the maximum lifespan possible in the context of the neoplastic problem, and second, how can this hypothesis explain the variability of aging across different species? Longevity positively correlates with body size and cancer resistance (Peto’s paradox [48]). A mouse lives around two years with a body mass of about 20 grams, and the blue whale lives up to 100 years with a body mass of ~100 tons. Accordingly, whales should have evolved effective mechanisms to protect themselves against cancer [49-51]. Conversely a small animal has the capacity to evolve cancer surveillance machinery as efficient as in whales and live a lot longer. It is unclear why this combination was not produced by evolution. Hence, while Nelson and Masel’s hypothesis might explain a theoretical lifespan limit, their work does not identify an obvious evolutionary determinant explaining the diverse lifespan setpoints in distinct species.
Nevertheless, hypotheses like this are prevalent, perhaps because they resonate with our culturally (or even pre-culturally) encoded stereotypes of frailty and senescence as an unavoidable part of being, a view broadly represented across the pre-Darwinian literature. Entropy or selfish evolution replace the transcendental powers while keeping the general perception intact. To overcome this cultural bias, we introduced a thought experiment we refer to as the “squirrel test”.
“THE SQUIRREL TEST”
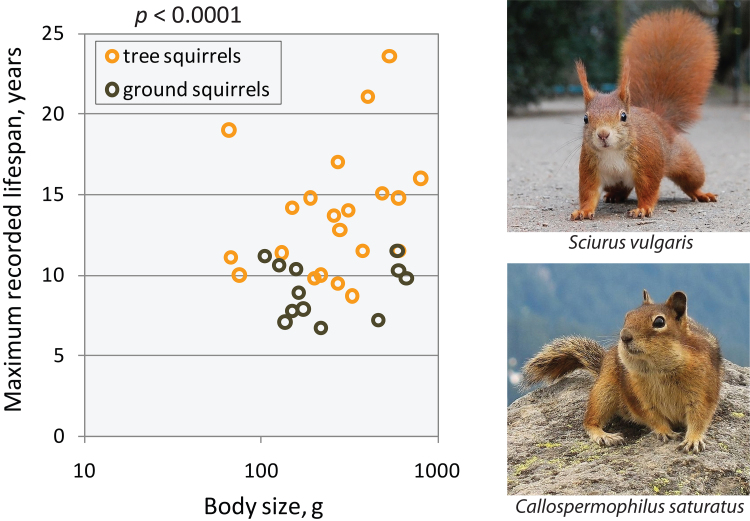
The squirrel test aims to clarify the hypotheses that seek to explain the primary evolutionary reasons for aging. The experiment is based on the maximum recorded lifespans of different squirrel species. Tree squirrels live 1.5-2 times longer than ground squirrels (Fig. 1) [52, 53]. Thus, genetically related, morphologically similar animals, having a close diet and lifestyle, have statistically distinguishable lifespan phenotypes. Every valid evolutionary hypothesis of aging should allow the difference in the lifespan of these closely related species. As a first example, we use “the squirrel test” to examine the senescence hypothesis that proposes that aging is determined by the balance between inevitable cancer and anti-cancer surveillance mechanisms. If tree squirrels control their neoplasia for many years, why can’t ground squirrels evolve similar mechanisms?
Fig. 1.
Tree squirrels live longer than ground squirrels. Maximum recorded lifespan data were collected from the AnAge database. p-Value is from the two-tailed t-test obtained on maximum lifespans without correction for body size.
The same problem can be found with other entropic hypotheses, such as selfish evolution of mitochondria [20] or transposable elements [21], developmental hyperfunction [54], accumulation of glycation [55], somatic mutations [14], oxidation [11], telomere shortening [56], DNA damage [57], complexity-centered ideas [18, 25] and others. Importantly, many of these processes may be relevant to senescence and are important to examine. However, they may not be the primary evolutionary forces driving the evolution of aging and lifespan setpoints. Below, we will focus on only the hypotheses passing the squirrel test, as this approach helps to provide a framework to address the evolutionary principles that determine lifespan setpoints.
NEUTRAL AGING: SELECTION SHADOW
The selection shadow hypothesis states that aging is a collection of detrimental traits that are expressed at the late age, irrelevant for selection. In his book “New Paths in Genetics,” Haldane discusses Huntington’s disease, an autosomal dominant inherited disorder that usually strikes between 30 and 50 years of age. By that time, most carriers have already had children and thus might have passed the pathologic mutation on [4]. The selection shadow hypothesis passes the squirrel test if we propose that ground squirrels are more prone to die than tree squirrels due to extrinsic reasons, not surviving to the age of senescence.
The selection shadow concept can find its immediate validation when focusing on species that are subject to die by time-determined reasons, such as evaporation of water pools in seasonal killifishes. Different species of killifishes can live in permanent water reservoirs or in ones that dry out during the summer. The species inhabiting the ephemeral waters have one of the shortest lifespans among vertebrates (about 3-9 months for Nothobranchius furzeri), but those that occupy permanent waters live much longer (2-3 years) [52, 58]. The same tendency was found in seasonal insects [59] and in Neurospora crassa exposed to regular anthropogenic fires [60]. Thus, a complete lack of selection in older ages results in the deterioration of residual lifespan. However, most animals are not exposed to deterministic lifespan termination. Probabilistic mortality cannot ensure the elimination of the entire age class, so the number of survivors cannot be zero. Since selection shadow proposes zero fitness loss due to aging, it requires that animals in the wild should never reach the age of senescence. This key requirement of the selection shadow hypothesis is likely to be incorrect. Thus, Williams argued that, according to the athletic records and life tables, a physical decline in humans starts in their thirties, and “surely this part of the human life-cycle concerns natural selection” [6].
An increase in mortality with age was observed in multiple natural populations of species from various taxa, including Mammalia, Aves, Reptilia, Amphibia, and even Arthropoda, showing that in many species, individuals survive to the age of senescence [61, 62]. This presumes the existence of some other evolutionary reason to evolve senescence, even if it is detrimental to fitness.
ADVERSE SIDE EFFECTS OF BENEFICIAL TRAITS: ANTAGONISTIC PLEIOTROPY
Antagonistic pleiotropy proposes that aging, even under negative selection, develops due to damaging side effects of genes with pleiotropic functions [6]. If some hypothetical gene is beneficial in younger ages and detrimental later in life, it will be favored by selection. Due to extrinsic mortality, the proportion of elderly individuals is lower than younger ones, so selection for beneficial traits expressed at late ages is reduced. Moreover, the selection gradients decline with age even without considering extrinsic mortality: younger individuals are usually the majority of animal populations just due to the basic laws of population growth [63]. Therefore, the pleiotropic alleles postulated by Williams as “...genes that have opposite effects on fitness at different ages…” can be selected for [6]. This hypothesis also passes the squirrel test if we assume that ground squirrels (but not the tree squirrels) have some fitness benefits in early ages that they are hindered by in their later years.
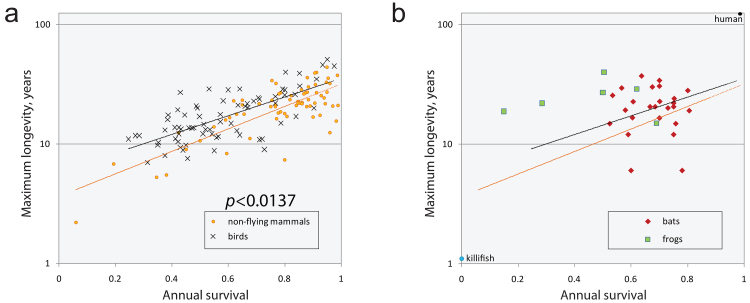
One of four fundamental assumptions of antagonistic pleiotropy, taken from the selection shadow hypothesis, is the idea of a declining selection gradient. It is also grounds for one of nine predictions formulated in the seminal paper: “low adult death rates should be associated with low rates of senescence, and high adult death rates with high rates of senescence” [6]. In support, Williams brought an example of birds and bats that have much longer lifespans than terrestrial mammals of comparable sizes, supposedly because of their ability to use flight to avoid predation. Correlation between senescence and extrinsic mortality attracted substantial attention in later years and, despite the ongoing debate on its mathematical consistency [64-66] and evidence that its effects on lifespan evolution depend on other ecological conditions [67, 68], it is still considered a key falsifiable prediction of the antagonistic pleiotropy hypothesis [69]. The ecological data on this correlation (excluding the above examples when death is strictly determined by the environment) are ambiguous. The classic result that seemingly supports the prediction was obtained in the Virginia opossum (Didelphis virginiana) by comparing a protected insular population and a continental population exposed to predation [70]. Insular opossums had slower senescence, longer lifespans, and a later onset of reproduction. However, this finding was not supported in other studied populations. In some cases, the correlation had the opposite trend. For example, guppies (Poecilia reticulata) and Daphnia from hunted populations had slower senescence than those inhabiting a protected environment [71-73]. The analysis of annual mortality and longevity in different species of mammals showed no strong correlation after correction for body size [74]. Moreover, the annual survival of long-lived animals, such as birds [75-77], bats [78], or frogs [79], seems to be lower than those of non-volant mammals with comparable lifespan setpoints (Fig. 2). Therefore, the data offer limited support for one of the main predictions of antagonistic pleiotropy in its classic formulation.
Fig. 2.
Extrinsic mortality does not define the rate of senescence. Maximum recorded lifespan data were collected from the AnAge database. No correction for body size was made. Data on annual survival are from the sources listed in Table S1, see Online Resource 1. Annual survival of adult individuals was collected. If data for females and males were present, the average value was calculated. If several options were available, age class-specific and sex-specific data were prioritized in the order mentioned. If several options with the same level of details were present, higher survival values were selected. Data presented here should be interpreted with care, as maximum lifespan can be biased by the size of the array analyzed, and the annual survival can vary in a broad range between seasons and different populations. Nevertheless, the evidence suggests some level of uncoupling of senescence from extrinsic mortality. a) Longevity of bird species seems to be higher than that of non-volant mammals with comparable annual survival. p-Value was produced from the two-tailed t-test made on the slopes. Regression lines were produced with an exponential model. b) Some species of bats and frogs demonstrate unexpectedly high longevity if compared to non-volant mammals and birds with comparable annual survival. Regression lines are from panel (a). Note that not all bats are long-lived [272]. The frog data are biased since only species with long recorded lifespans were selected for data collection.
While the failure of Williams’s key prediction does not entirely disprove his theory, the true Achilles heel of antagonistic pleiotropy resides in its discord with modern molecular genetics. The concept of pleiotropic genes was contemporary to the formation of the mechanistic views on mutation and recombination, the same year as the central dogma of molecular biology was proclaimed and almost a decade before the genetic code was deciphered. The variability and plasticity of genomes at that time were largely underestimated. For example, Hermann J. Muller wrote in 1950, “the average [human] individual is probably heterozygous for at least 8 genes” [80], instead of ~3 Mio of polymorphic sites. Hence, the antagonistic pleiotropy hypothesis clearly could not account for the consensus that was formed decades later. The main idea of the hypothesis, the existence of genes with opposite effects at different ages, indirectly implies non-evolvable genetic features. Indeed, the connection between two traits encoded by a single gene should be a genetic trait itself. If being under negative selection, this connection should be distorted by evolution, or, alternatively, the negative effect of the gene should be neutralized by mutations in other regions of the genome. While genes with the properties predicted by antagonistic pleiotropy were identified [81], explanations of their genetic stability were missing. The hypothetical mechanistic examples of senescence-promoting pleiotropic genes brought up by Williams and others in later literature are not very convincing. Here, we will discuss Williams’s example, which is still widely cited in the field [69]: “...mutation arising that has a favorable effect on the calcification of bone in the developmental period but which expresses itself in a subsequent somatic environment [age] in the calcification of the connective tissue of arteries. If the gene becomes established in the population and if this later effect is eventually deleterious, a selective premium would be placed on any gene that might suppress this arterial calcification. As the suppression approached completion, however, the selection pressure for further suppression would diminish. Complete suppression would probably never be realized.” [6]. This brings us to the situation, when, (i) according to the key premise of Williams’ paper, the trait of later artery calcification is under negative selection, (ii) an independent compensatory evolution of this trait is mechanistically possible, but (iii) paradoxically, the fixation of a beneficial trait does not proceed to completion. This scenario contradicts the core ideas of natural selection. The mechanistic connection between bone formation in youth and later calcification is also questionable: these two processes are well separated in time and space and can be regulated independently. Furthermore, cases where faster growth and calcification are combined with a longer lifespan are numerous. For example, relatively long-lived birds grow faster than mammals [82] and utilize calcification during their adulthood for eggshell formation. Thus, the effect of rigid pleiotropic genes may be inconsistent with the plasticity and adaptability of regulatory networks uncovered by modern genetics.
An elegant variation for the source of pleiotropic genes comes from host–pathogen interactions [83]. The pleiotropic properties of genes conferring pathogen resistance may have adverse effects on longevity. Infectious diseases are one of the most powerful selective forces in nature. Genes that interact with pathogens evolve much faster than the rest of the genome [84]. The evolution of immune genes induces adaptive responses in the pathogens and vice versa in an endless arms race [85, 86]. Some known genes associated with resistance to pathogens come with a substantial fitness cost. A classic example of such a trade-off is sickle cell anemia. The maladaptive phenotype only manifests in homozygotes who carry two copies of the pathological allele of hemoglobin [87]. Heterozygotes are healthy and possess partial resistance to malaria. Despite its detrimental effects, the pathologic allele occurs in relatively high frequencies in human populations historically exposed to malaria [88].
Can senescence be a maladaptive consequence of a trade-off between resistance and longevity? While modeling approaches suggest that it can [83], the empirical evidence suggests the contrary. First, several long-lived mutants of roundworms [89, 90] and fruit flies [91] display higher resistance to pathogens. Also, long-lived species are expected to have a better immune defense [92] together with improvements in other maintenance systems [50, 93-95]. Therefore, lifespan does not seem to correlate negatively with general immune robustness. One can argue that hypothetical pleiotropic genes might be stabilized only by a few exceptional pathogens, such as malaria, that impose very robust selection pressure and are not prevented by general immune mechanisms. This argument also is not compelling: malaria is a relatively recent pathogen, and sickle cell anemia may be a novel adaptation [96] with the potential for its maladaptive trait to be removed by evolution e.g., by duplication of the gene encoding hemoglobin that would allow for retention of both wild-type and pathological/resistant forms in every individual. Thus, the prevalence of sickle-shaped anemia is an evolutionary snapshot rather than a long-term steady state. Furthermore, similarly inherited pathogen-stabilized genetic disorders have entirely different symptoms. For example, cystic fibrosis might be associated with resistance to tuberculosis and other bacterial diseases and manifests in an altogether different symptomatic pattern [97-99]. Therefore, if aging is a result of such interactions, the manifestation of senescence would be expected to vary in different species and even different populations of the same species exposed to different pathogens. However, aging mechanisms are surprisingly conserved among various taxa [23], making this variant of antagonistic pleiotropy doubtful.
To rescue the antagonistic pleiotropy as a unifying hypothesis of aging without revisiting the basics of molecular genetics, one needs to suggest the existence of some fundamental trade-off defined by universal laws of nature. For example, the physical sizes of animals are defined by the trade-off between the benefits of being big and non-linearly increasing energetic demands to maintain and move a large body [100]. Maximum sizes of insects are believed to be limited by the rate of oxygen diffusion in their tracheal system and hemolymph [101]. If these types of trade-offs could be identified for lifespan, they would bring the antagonistic pleiotropy into an agreement with genetics. The most elaborated concept in this line, the “disposable soma” hypothesis, proposes that energy limitation is the basis of a trade-off between longevity and Darwinian fitness.
AGING AS AN ENERGY-SAVING STRATEGY: DISPOSABLE SOMA
The main focus of the disposable soma hypothesis [7] is the way organisms invest limited resources. It proposes that, since animals are restricted in nutrients, energy must be allocated either to reproduction or to body maintenance. With some parametric assumptions, a short-lived strain with faster reproduction might outcompete a long-lived variant with a slower reproduction rate. If an individual produces hundreds of progeny, and its successors are also replicating exponentially, the lifespan of the very first ancestral individual might appear to be unimportant for selection. On the contrary, the reproduction rate, which defines the base of the exponent in the equation describing population growth, is critically important. Therefore, evolution always favors reproduction over maintenance. Disposable soma passes the squirrel test if we assume that ground squirrels invest more in their reproduction and child care. Notably, fecundity and longevity are known to correlate inversely between different animal species [8, 9, 102], confirming the central postulate of this hypothesis.
However, disposable soma cannot fully explain aging [103]. First, population growth is not always limited by resource availability. In many instances, the elimination of predators by humans has resulted in explosive growth in prey numbers [104, 105]. Thus, it is easy to envision a situation in which resources are sufficient for both replication and maintenance.
Second, populations in nature do not grow exponentially but rather oscillate around some steady-state values. Faster reproduction at the expense of longevity might indeed be beneficial during population increase, but longer life and lower fecundity might be selected when the population numbers are declining or are stable. Consider animals with sexual reproduction and senescence in a population with stable numbers and a low rate of extrinsic mortality (e.g., elephants or whales). To retain the representation of its genes in the gene pool at the same level, an individual needs to produce at least two progeny that will survive to the age of reproduction. This can save energy only if the amount of nutrients spent on bringing the offspring from the zygote to adult is smaller than the resources required to maintain a parent’s body further. If the nutrients for parents and juveniles are coming from the same pool, the scenario runs into a contradiction with basic laws of energy conservation. In the case of exponentially growing populations, the disposable soma model runs into an internal contradiction regarding food abundance: the environment should be rich enough to maintain the growing progeny and, at the same time, be limited when it comes to investing in the body maintenance of the parent.
Third, resources are unevenly distributed in space and time. If available energy is a limiting factor for longevity, during a rich season when the food is sufficient for both maintenance and reproduction, some animals would activate a program of rejuvenation or at least delay their senescence. Mechanisms for slowing down aging do exist in nature but are activated under conditions of low resource abundance (caloric restriction) [106-108]. This is the opposite of the expectations of the disposable soma hypothesis. Kirkwood and Holliday have tried to adjust their theory by redefining severe caloric restriction as a “famine” that is different from regular resource shortage [109, 110] and arguing that many species do not reproduce when exposed to famine and invest in maintenance only. However, these arguments do not explain why we cannot see the activation of a senescence-delaying program under “better than optimal” conditions when food is in excess.
Also, disposable soma received some more specific criticism. (i) Although mammalian females invest more energy in reproduction than males, they senesce with substantially slower rates in most species [111]. (ii) Disposable soma proposes substantial investments in maintenance. However, organisms still need to spend large amounts of energy to continually regenerate proliferating tissues (e.g., skin, blood, gut epithelium). Thus, this hypothesis focuses only on the residual maintenance costs beyond day-to-day repair. The size of the budget was never calculated and it remains unclear, whether it contributes significantly to the overall lifetime expenditure. Moreover, some long-lived species evolved more energetically efficient systems for damage control than short-lived species, thus making long-lived body maintenance cheaper [95]. (iii) Longevity of the parent can be uncoupled from the number of offspring [112-114]. In some cases, simultaneous selection for a longer lifespan and higher fecundity is possible [115-117]. (iv) Energy intake decreases in older ages [118], but according to the hypothesis, higher investments in maintenance, if the food source is abundant, should be sufficient to extend lifespan.
Antagonistic pleiotropy and disposable soma are frequently grouped together as “pay later” hypotheses: young organisms “borrow” fitness from themselves in the future. While being internally non-contradictory, these hypotheses lack measurable factors controlling senescence. It is unclear what this means from a physiological point of view, as the currency for the borrowing transaction or the reasons for its evolutionary stability are unknown. Thus, the term “pay” is not clearly defined.
Yet another argument against “pay later” hypotheses is a requirement for a pre-set and rigid investment strategy. Hamilton’s [5] and Williams’ [6] concepts as well as disposable soma implicitly suggest that the individual distributes its hypothetical limited resources between early and late fitness and that this distribution strategy cannot change. For example, in the case of the disposable soma hypothesis [7], an aged individual cannot stop investing in reproduction and channeling the resources into rejuvenation.
Taking all in consideration, we believe that the arguments against the classical hypotheses, including those listed above and below, are at the moment stronger than the arguments in favor of them, and that the evolutionary theory of aging requires additional refinement [103, 119, 120].
CRITIQUE OF ADAPTIVE AGING HYPOTHESES
Historically, the very first evolutionary hypothesis claimed senescence as an adaptation evolved to release resources consumed by potentially maimed older organisms [1]. This idea was subsequently disproved as a group selection concept. The frequently cited arguments against adaptive aging were summarized by George C. Williams in 1957 [6]. These are:
“1) The fallacy of identifying senescence with mechanical wear [an argument related only to Wiesmann’s theory],
2) the extreme rarity, in natural populations, of individuals that would be old enough to die of the postulated death mechanism [disproved by Williams in the next pages],
3) the failure of several decades of gerontological research to uncover any death mechanism [nowadays, one can claim cellular senescence, cancer, and many other age-related disorders as a mechanistic basis of programmed death], and
4) the difficulties involved in visualizing how such a feature could be produced by natural selection...”
Thus, of the four arguments raised by Williams, only a single one (the fourth) stands the test of time. Indeed, we still have not found the evolutionary benefits and universal selection mechanisms that limit the animal’s lifespan. The general challenge faced by the adaptive aging hypotheses is the imbalance between the strong individual penalty of aging and the diffuse weak benefit it provides. If aging is an adaptation, it should be a strong altruistic trait that would fulfill Hamilton’s rule [121-123] for being selected by evolution: r*b>c, where r is relatedness between actor and recipient, b is the benefit for a recipient, and c – the cost for an actor. Since r ≤ 1, the hypothetical benefit b should exceed cost c, which in this case equals the life cost of an altruist. Thus, the shortening of the lifespan of an individual should generate a very robust and unambiguous benefit to its kin, that should exceed the cost of its residual lifespan. The nature of this benefit is unclear.
An additional argument often used to disprove the possibility for aging as an adaptation is the absence of aging-escape mutants [124]: adaptive senescence should be programmed somewhere in our genome, and these mechanisms could be destroyed by genetic mutations. However, biologically immortal mutants are not observed in nature or laboratory experiments. Mutations that prolong lifespan were usually associated with substantial adverse fitness effects in agreement with the predictions of antagonistic pleiotropy [81]. Thus, the criteria for a realistic adaptive aging hypothesis, in addition to stability in the squirrel test, should include the ability to explain a near-universal benefit of a shorter lifespan, the mechanisms of its selection, and the absence of biologically immortal mutants, a combination of conditions considered impossible by many scientists [112, 124, 125].
Nevertheless, evidence that death can be adaptive is readily found in the literature. Bacteria [126] and unicellular eukaryotes [127-129] commit adaptive suicide – phenoptosis [130] – when infected with pathogens or under other conditions. In the case of unicellular organisms, phenoptosis is functionally equivalent to bacterial programmed cell death or apoptosis. Among metazoans, a number of semelparous species likely commit phenoptosis shortly after reproduction [131]. Although semelparity was described in mollusks [132], mammals [133], insects [134], and other taxa [131], some scientists consider it as a secondary trait, resulting from exhaustion upon breeding. An exception is made for Salmonidae species (e.g., Pacific salmon, Oncorhynchus tshawytscha), the anadromous fishes that migrate from the ocean up to freshwater and die soon after reproduction [135]. Their death is accompanied by multiple organ failures, including immune deterioration and neurodegeneration, within two weeks and can hardly be considered a result of exhaustion [136-138]. The evolutionary hypotheses proposed so far suggest that salmon’s fry might feed on the decomposing corpses [139, 140], see below. Thus, under certain ecological conditions, lifespan termination might be favored by selection.
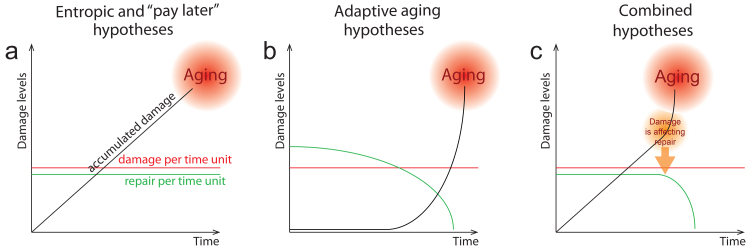
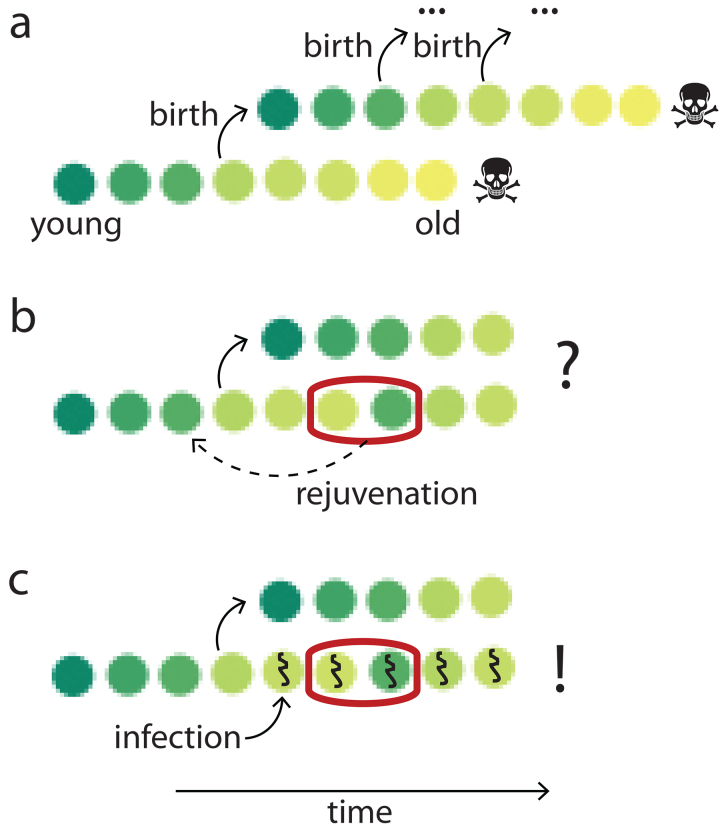
Yet another underappreciated argument in support of adaptive aging comes from cellular and molecular biology. In entropic and “pay later” hypotheses, the maintenance machinery is assumed to be imperfect and, thus, allow damage to accumulate (Fig. 3a). On the contrary, programmed aging hypotheses suggest that repair systems are robust in early ages and decline with time (Fig. 3b). These two opposite scenarios can be combined if the accumulated damage itself is detrimental to the repair systems (Fig. 3c) [10]. The observations made so far are likely to support the adaptive aging model (Fig. 3b): the activity of multiple molecular repair systems, such as DNA repair [141], autophagy [142], proteostasis [143], and RNA quality control [144] decline with age. At the same time, these repair systems can be reactivated at later ages by genetic, pharmacological, or environmental interventions, resulting in lifespan prolongation. Moreover, genes involved in repair are transcriptionally downregulated during aging [145, 146]. For example, the expression of Sirt1, a gene involved in DNA repair, is reduced in older mice [147]. These facts fit neither the model of a simple accumulation of damage (Fig. 3a) nor the damage-dependent deterioration of repair (Fig. 3c) and suggest aging to be an evolutionary adaptation (Fig. 3b).
Fig. 3.
Three models of damage management. a) Entropic and “pay later” hypotheses consider damage control mechanisms to be imperfect, and damage to accumulate across the lifespan, finally resulting in senescence. b) The adaptive aging hypotheses claim the developmentally encoded decline in the maintenance machinery leads to the accumulation of damage. c) A model explaining a decline in maintenance systems by a positive loop involving adverse effects of accumulated damage on the repair. Note that this model does not assume a developmental program of aging.
Regardless of the long history of neglect, the hypotheses of adaptive aging are still viable and developing. There are three types of adaptive aging hypotheses classified by the benefit aging might produce: (i) prevention of Malthusian catastrophes and resource release [1, 26-30], (ii) acceleration of evolution [31-33], and (iii) pathogen control [34-36].
RESOURCE RELEASE
The resource release models propose aging to be a mechanism that evolved to prevent overcrowding and resource exhaustion. However, the published models demand very strong assumptions: they require frequent group eliminations in the patches with long-lived variants due to local demographic collapses [29], a group selection framework, close to John Maynard Smith’s haystack model [148]; or impose unrealistically strong limitations on the dispersal of the individuals [27, 28, 30].
Furthermore, population control might be mediated by density-dependent inhibition in fecundity [149, 150], making aging redundant and expensive as a population-limiting strategy. Therefore, aging as a near-universal phenomenon cannot be explained based solely on resource release concepts.
ACCELERATION OF EVOLUTION
The concept of acceleration of evolution proposes that a shorter generation time might mediate faster turnover of genetic variants and fixation of the adaptive traits [31-33]. However, the benefits of such enhancement of evolution seem to be elusive. In fact, accelerated senescence may increase the effect of genetic drift in response to non-relevant evolutionary challenges and so reduce genetic variability essential for further evolution. Models of accelerated evolution are not convincing as they typically consider only a single selective factor [33, 124, 151]. In natural populations, individuals are exposed to multiple selective pressures. We believe that strong selective factors should be able to remove unfit individuals without assistance from the side of senescence. Enhancement of weak selection factors might result in increased genetic drift, reduced genetic variation, and failure to adapt to environmental changes. Moreover, while the models are built for asexual populations, recombination might complicate these models since the newly evolved adaptive traits will be readily combined with a high-fit non-aging allele. The models that strive to explain aging in sexually reproducing populations require genetic links between aging and some beneficial traits, bringing these works to the subclass of antagonistic pleiotropy hypotheses [151]. The acceleration of evolution concept also expects low aging rates in slowly evolving species, a prediction that fails in nature: slowly evolving sharks or tadpole shrimps are not the most long-lived animals if compared to other fish and arthropods, while the rapidly evolving taxa, such as primates or cetaceans, are among the longest-lived mammals [52, 152]. Also, this concept does not exclude immortality, and its near-absence in nature is not explained. Thus, at the current stage of development, acceleration of evolution hypotheses also fail to explain aging as a ubiquitous phenomenon.
LIFESPAN AND EPIDEMICS
A group of adaptive hypotheses claims that aging evolved to control epidemics of infectious diseases [34-36]. Indeed, pathogens are a universal and powerful selective factor [84, 86], and the intrinsic disease-independent mortality (lifespan setpoint) of the host is an important parameter in epidemiological models [153].
In a seminal study, limiting lifespan was suggested to reduce the burden of chronic infections [34]. If the probability of an individual becoming infected is equivalent across the lifespan and no recovery is presumed, older individuals are expected to be infected more often than younger ones. So, the removal of older individuals by aging obviously results in a decrease in chronic pathogen load. If one assumes that infection adversely affects reproduction [154], shortening the lifespan might paradoxically result in an increase in the population growth rate. Kirchner and Roy assumed a clustered metapopulation model with sporadic disease-independent elimination of clusters with their consequent recolonization from the survived ones (similar to the haystack model [148]), a scenario can be envisioned in which the shorter lifespan takes over the population [34].
Disease-mediated selection of a shorter lifespan was also suggested as a hybrid model involving elements of population density control, acceleration of evolution, and disease prevention with the emphasis on overcrowding as a reason for epidemic [35]. In spatially distributed populations with zero migration, death by aging was proposed to leave parts of the experimental lattice vacant. The model assumed epidemics of highly infective diseases with absolute mortality. Regions, with a proportion of vacant sites were incompletely eliminated by outbreaks due to percolation effects (similar to the glades limiting forest fires). Short-living variants were also more efficient in fixing the pathogen resistance alleles [35].
However, neither model is a universal explanation of aging. Both involve group selection, a condition believed to be rare in nature [148]. Both require constant severe epidemics to sustain the selective pressure against the longer lifespan, a premise that contradicts observations. Furthermore, such epidemics should promote a fast selection of host resistance. Kirchner and Roy [34] do not even claim the identification of a unifying hypothesis of aging as the effect was limited to clustered metapopulations. The future development of their model resulted in convergence with antagonistic pleiotropy discussed above [83]. The model of Mitteldorf and Pepper [35], in addition to the involvement of group selection and unrealistically severe pathogens (see also [155]), is very sensitive to host migration [124]. Both hypotheses, while passing the squirrel test and explaining the evolutionary benefits of aging, were unable to explain the absence of non-aging escape mutants. Thus, if analyzed carefully, both conclude that pathogens cannot be a unifying evolutionary driving force of aging, as the models’ requirements are unrealistically high.
A novel framework considering pathogen control as the driving force of aging.
Using realistic epidemiological and population dynamics models, we constructed a theoretical framework, supporting the idea that pathogens may be the force behind the evolutionary benefit of aging. The new model proposes that aging can be a unifying adaptation to limit the establishment and progression of infectious diseases [36].
First, we found that populations of short-lived individuals, in addition to reduced pathogen prevalence [34], bestow additional benefits when facing epidemics. Thus, novel pathogens infecting a new host species from another species or the environment might require substantial time to adapt to a new host. For example, some substrains of HIV-2 have been described only in single patients and are believed to be zoonotic viruses, not adapted to transmit efficiently from human to human [156, 157]. Our model shows that the shorter lifespan of a species might limit the time window available for such chronic pathogens to evolve better transmissibility, thus preventing zoonotic transmissions.
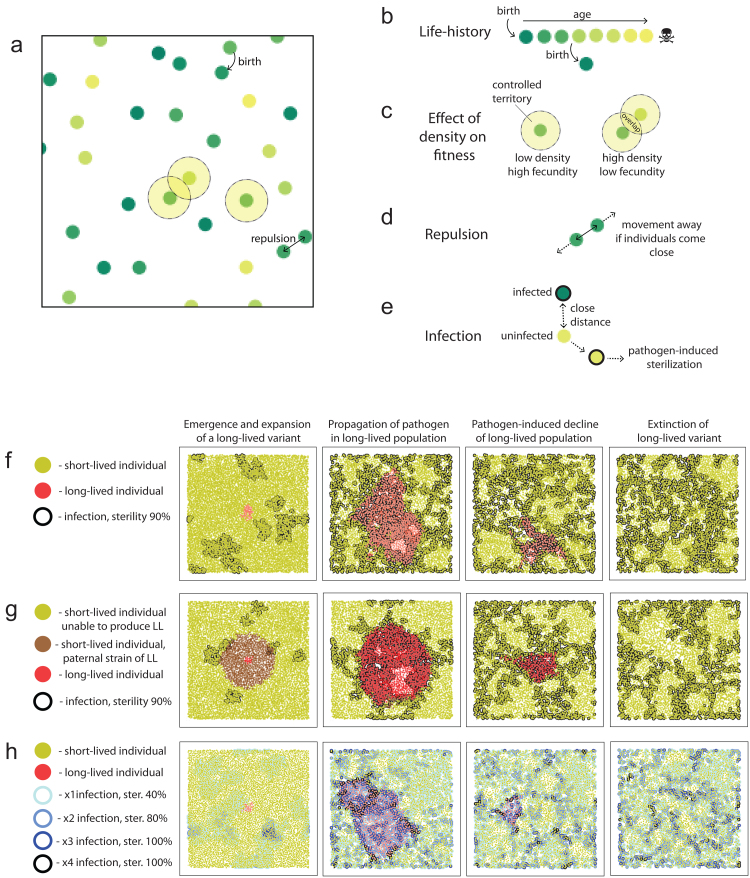
Next, we found that dramatic declines in infected population densities – bottlenecks [158] – during natural oscillations or migration into a new environment might be associated with pathogen clearance. If the last infected founders die before the density is reconstituted to levels permissive for epidemics, the pathogen will become extinct in that population. This effect depends directly on the species’ lifespan. Thus, a short lifespan has population-level benefits. Next, a simulation designed based on this hypothesis demonstrated the scenario of short lifespan selection. The selection in our model was based on several realistic and general assumptions concerning the host’s population structure and pathogen properties. We considered viscous animal populations [140, 159, 160], where limited dispersal makes infectious contact between relatives more likely [155]. The kin-specific bias of pathogen transmission might also be shaped by social and family structures, which should be considered in future simulations. We also assumed that dispersal is, to a certain extent, density-dependent [161, 162] as competition for resources in densely populated regions pushes animals into more sparsely inhabited areas. Both of these assumptions are entirely realistic, discussed in the literature, and can be widespread. We constructed a simple and intuitive stochastic model to investigate this scenario, recapitulating both presumptions (Fig. 4; Video 1, see Online Resource 2).
Fig. 4.
Pathogen control hypothesis: the model of selection. a) The spatially distributed agent-based model implies free movements of individuals (depicted as circles). b) Individuals die when reaching a critical age defined by the intrinsic strain-specific parameter: lifespan setpoint. Age in (a)-(e) is depicted with color. c) Higher density results in the overlapping territories of the individuals, thus reducing the available resources and decreasing the host’s fecundity. d) To maximize their available territories, the individuals are moving away from each other (see Video 1, Online Resource 2). e) The individuals may infect each other; the infection probability depends on the distance between individuals. f) The principal stages of stabilizing selection prevent the emergence of long-lived variants in the population. Colors correspond to the strains in (f) and (g). Sterilization by pathogen – 90%. g) The reasons for the absence of long-lived mutants in nature and the advantage of evolvability suppressors. In the initial stage of long-lived variant propagation, its paternal short-lived strain (brown) becomes displaced. Thus, not only long life per se but even the ability to produce a long-lived mutant is detrimental. h) Scenario of selection with several milder pathogens. Four pathogens, 40% sterilization.
Regarding the pathogen properties, the model assumes chronic pathogens with strong negative effects on reproductive fitness. Such pathogens are present in nature: evolutionary parasitology predicts pathogens to rather sterilize their hosts than shorten their lifespan to facilitate transmission [163]. Some pathogens use the host’s sterilization as a part of their reproductive strategies [164, 165]. Several human sexually transmitted diseases, such as gonorrhea, chlamydia, and syphilis, cause infertility [166, 167]. Reduced fecundity occurs in other chronic diseases, such as HIV [168], leprosy [169], HCV [170], tuberculosis [171], herpes [172], and many others. Furthermore, sterilization caused by coinfection with several mild chronic pathogens can also be envisioned. Thus, the pathogen-related conditions that satisfy the model’s criteria are also realistic.
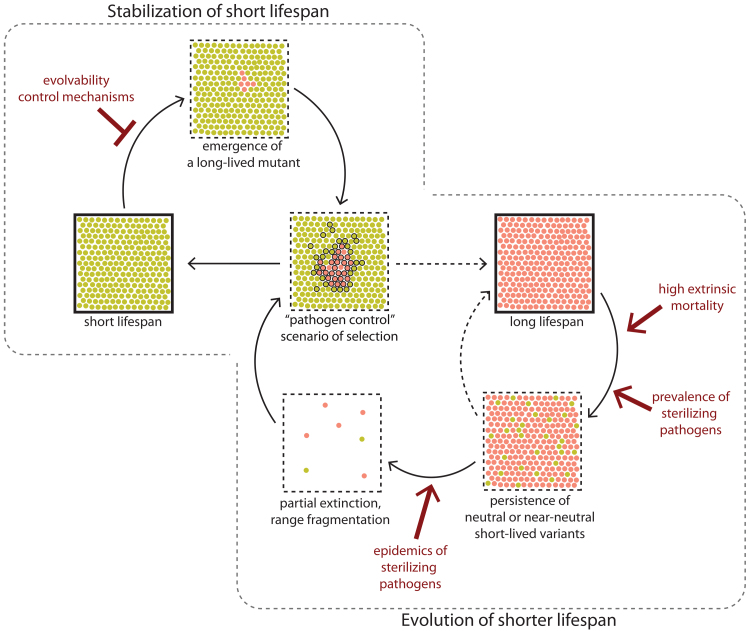
We modeled a population of short-lived individuals invaded by a long-lived mutant. With this model, we observed the stabilizing selection of shorter lifespans that occurred by the following mechanism: (i) in the early stages, the pathogens and long-lived mutants are spatially separated from each other, allowing mutants to expand due to their low mortality, (ii) the pathogens spread in the area occupied by long-lived hosts and, due to the reasons described above, becomes more prevalent than in short-lived hosts, (iii) higher pathogen prevalence results in higher sterilization and lineage-specific population decline that, in combination with population pressure of short-lived populations, results in a complete displacement of the long-lived individuals (Fig. 4f; Video 2, see Online Resource 3). Thus, in the presence of sterilizing chronic pathogens, a limited lifespan setpoint might be an evolutionarily stable strategy protected from the invasion of long-living defectors.
We found critical factors that affect the selection of lifespan setpoints in this model. In addition to population viscosity, the rate of pathogen transmission, degree of sterilization, and host birth rate are important factors to the outcome of the competition.
Population viscosity is a key conceptual parameter of the model. Suppose the population is well-mixed and interactions between its members are promiscuous. In that case, the infection is not biased toward the individual’s kin, and the benefit of shorter lifespan cannot be delivered specifically to relatives. Under such conditions, following the basic Tragedy of the Commons scenario [173], a longer lifespan is fixed by evolution.
We found that pathogens with too high or too low infectivity cannot mediate selection of shorter lifespan: if a pathogen is transmitted at a very high level, it infects short-lived and long-lived populations both at high levels, producing no selectivity. If transmission is too low, the prevalence of the pathogen and, therefore, its adverse effects on long-lived strain population growth is insufficient. Selection favoring short lifespans requires a highly (90%) sterilizing pathogen or a combination of mildly (10-40%) sterilizing diseases that can provide a strong cumulative penalty in coinfected hosts (Fig. 4h). All pathogens in the model were assumed to be chronic.
Thus, we conclude that only a subset of pathogens can affect the evolution of lifespan. However, considering the vast abundance of parasites in nature and their negative effects on the host’s fitness [154, 174], it is reasonable to assume that a sufficient number of pathogens will fulfill these criteria in most species. Furthermore, since we found populations of long-lived individuals to be a more suitable substrate for the adaptation of the novel “zoonotic” pathogens transmitted from different species, the spectrum of prospective pathogens is further expanded.
Unlike previous models of pathogen control type, our scenario does not require group selection or ongoing severe epidemics to explain a limited lifespan setpoint. Importantly, our hypothesis also mitigates the problems associated with the evolution of the host’s resistance to pathogens: selective pressure towards the shorter lifespan might be provided by zoonotic pathogens, the exposure to these pathogens is limited, and the likelihood for the evolution of resistance is reduced. In another scenario involving several milder pathogens, each of these pathogens confers only a little selective power to drive the evolution of resistance.
A negative association between host birth rate and evolution of lifespan in epidemiological models was reported in the previous group selection models [34, 35], and was stable in our kin-selection model as well (see below).
Importantly, we identified a selection mechanism that prevents the emergence of aging escape mutants. The scenario of selection described above presumes that the same trait (i.e., long lifespan) can be beneficial shortly after its emergence before the pathogen is established in the long-lived strain and detrimental after introducing a pathogen (Fig. 4f). We showed that this selection mechanism makes the evolutionary capacity of producing long-lived mutants a detrimental trait. A short-lived paternal strain is vulnerable to partial displacement by long-lived mutants, which are in turn, unstable due to infection penalties. The strain incapable of producing long-lived mutants might get a long-term selective advantage (Fig. 4g). These interactions might promote the fixation of gatekeeping mechanisms that limit the evolvability of lifespan. The concept of evolvability suppressors, selected to stabilize the far-sighted traits, was proposed by Lee Altenberg in 2005 [175] and was discussed afterward in the context of a resource release hypothesis of aging [29]. However, to our knowledge, our model is the first to show a potential implication of Altenberg’s selection in the evolution of lifespan without the involvement of group selection. Furthermore, the existence of evolvability suppressors predicted by our model is a potential explanation of mutations that prolong lifespan but reduce reproductive fitness, typically considered as evidence of “pleiotropic genes.” However, unlike classic hypotheses, linkages between senescence and vital functions in our model are favored by selection, and not fixed by chance [6, 81].
Thus, our hypothesis fulfills all four criteria critical for an adaptive hypothesis of aging. Our hypothesis passes the squirrel test if we assume ground squirrels are exposed to higher selective pressures from the pathogen’s side (see below). We can explain the benefits of a shorter lifespan and identify the mechanism of selection. The Hamilton’s inequality in the case of pathogen control hypothesis becomes Σr*b>c, where the left part represents the sum of all benefits of the altruist’s relatives coming from prevention or limitation of the epidemics. We can also explain the absence of non-aging or extremely long-lived mutants. We propose these factors as critical, enabling the pathogen control framework to be considered as a unifying hypothesis of the evolution of senescence.
Having revised the current evolutionary hypotheses of aging, in the next pages, we will examine a number of aging-related observations and discuss their agreement with the existing evolutionary hypotheses of senescence.
AGING-RELATED OBSERVATIONS AND THEIR FIT WITH AGING HYPOTHESES. UNIVERSALITY AND VARIABILITY OF AGING
Aging is nearly ubiquitous in nature [61], and this leads the entropic hypotheses to postulate that senescence is a fundamental property of living matter. Nevertheless, the variation in senescence rates in different species is significant [62]. For example, the lifespan setpoints of mammals range from about 2 years in the forest shrew to more than 200 years in the bowhead whale [52]. This plasticity does not fit the idea of aging as a basic biochemical feature of life. Furthermore, a handful of organisms display no detectable senescence (see above). Thus, aging does not seem to be a necessary property of life and is likely to be shaped by some ecological factors. The prevalence of aging suggests these ecological factors are universally present with few exceptions.
Classical “pay later” hypotheses (antagonistic pleiotropy and disposable soma) identify the high extrinsic mortality, the involvement of some lifespan-related trade-offs [6], and energetic limitations [7] as critical parameters that drive the evolution of senescence. However, none of these is sufficiently widespread to explain the universality of aging.
“Pay later” hypotheses do not formally exclude biological immortality to evolve (e.g., in an abundant and well-protected environment, not a rare combination of conditions). However, these assumptions poorly explain the exceptional lifespans that are found not in the predicted environments. For example, the naked mole rat is an example of an extremely long-lived species [44]. Nevertheless, mole rats are heavily hunted by snakes and are not living in a specifically rich environment compared to other rodents [176].
On the contrary, pathogens are universal companions of every free-living species and an exceptionally strong selective force [84, 86]. The epidemics control hypothesis relies on this key factor to explain the universality of aging and show a fair ability to explain the variations of lifespan in different species by involving parameters that modulate epidemic processes (see below).
IMMORTALITY IN PRIMITIVE METAZOANS
The reported lack of senescence in hydra (Hydra vulgaris) [38], planarian flatworms (Schmidtea mediterranea) [39], and jellyfishes (Turritopsis dohrnii)[39] is a paradox that classical hypotheses of aging cannot fully resolve. First, the evidence for the existence of biological immortality is a critical problem for entropic hypotheses. The “pay later” hypotheses and adaptive aging hypotheses claim that immortality in these species is caused by their inability to mechanistically disconnect reproduction and regeneration or, as it was pitched in the earlier manuscript, they are “...organisms in which the distinction between soma and germ-plasm may not exist” [6]. As soon as the disconnection is feasible, senescence evolves with the gains from trade-offs (“pay later” hypotheses) or the adaptive benefits specific to each adaptive hypothesis. In the case of the pathogen control model, it is a lower disease burden. A recent study demonstrated that upon stress, asexually reproducing hydras switch to a sexual lifecycle that includes senescence[177]. It is tempting to speculate that it might be associated with a reaction to infectious diseases.
INDIVIDUAL MUTATIONS CAN PROLONG LIFESPAN, BUT IMMORTAL MUTANTS ARE ABSENT
The plasticity of aging is generally not expected if aging is a general property of life somehow embedded into the basis of biochemistry. The “pay later” hypotheses propose that mutations may shift the balance towards a longer lifespan through the expense of detrimental side effects. The absence of immortal mutants results from the multiplicity of deterioration processes that are unlikely to be compensated by a few point mutations or from some hypothetical mechanistic constraint that makes the evolution of a longer lifespan difficult.
The pathogen control hypothesis agrees well with the evidence of aging plasticity since aging is proposed as a regulated adaptation. This is the first adaptive aging hypothesis that provides a model to explain the absence of immortal escapers by evolvability suppression (Fig. 4g) [36, 175]. The “pleiotropic” detrimental effects of mutations extending lifespan can be considered manifestations of these evolvability control mechanisms.
CORRELATION BETWEEN LONGEVITY AND BODY SIZE
The correlation between animal body size and longevity is a well-characterized covariance [8, 178-181]. Indeed, the obvious ecological constraint requires an animal to have enough time to grow big to utilize the benefits of its size, and this observation is stable in the context of every evolutionary hypothesis of aging [182].
However, this correlation is reversed in mutant variants and artificially selected breeds of animals. Thus, growth-defective mutants of mice and flies have prolonged lifespans [183, 184], a pattern opposite to the correlation observed when different species’ lifespans are compared. The most renowned example is the longevity of dogs: small breeds survive substantially longer than large ones [185]. In humans, Laron’s syndrome dwarfism is associated with a lower incidence of type II diabetes and certain types of cancer and might prolong lifespan [186, 187]. Growth seems to be detrimental to lifespan at the individual level. Growth hormones (GH/IGF-1) are believed to be involved in regulating the size and aging in model organisms [188], dog breeds [189], and humans with Laron syndrome [190], emphasizing the mechanistic conservation of the physiological connection between aging and growth.
These observations suggest that, while the ecological trade-off favors a longer lifespan in bigger animals, the physiological trade-off between growth and aging goes opposite. Based on these views, smaller long-lived mutants, if released into the wild, would be expected to evolve to either a bigger size or shorter lifespan.
This complex interaction of factors can be explained, to a greater or lesser extent, by all the non-entropic evolutionary hypotheses. However, in our opinion, the most parsimonious explanation comes from the concept of adaptive aging. It claims that, while longevity is a necessary prerequisite for larger body size, the process of growth is mechanistically connected to the aging timer to adjust aging rates with individual developmental progression. With this argument, one can explain the existence of lifespan prolonging potential and the reasons why this potential is not activated ubiquitously. Thus, deviations of aging rates in breeds of dogs and other artificial mutants are explained by a lack of exposure to the ecological niche to optimize their lifespan.
CORRELATION WITH METABOLIC RATE
Animals with faster metabolic rates are believed to age faster [191-193]. This correlation is often cited to support entropic hypotheses: increased damage levels occur at higher metabolic rates [194]. However, this correlation is not absolute. Birds and bats, the two groups of long-lived animals, have very high metabolic levels [195]. Moreover, body size inversely correlates with metabolic rates in mammals [196, 197], and a correction for body sizes makes the correlation between metabolic level and longevity statistically insignificant [182]. Thus, the damage accumulation hypotheses struggle to account for these observations.
Nevertheless, while the ecological connection between metabolic level and longevity is under debate, the physiological trade-off was clearly shown in multiple models. Mutants with lower metabolic speeds have a prolonged lifespan [198-200]. As in the case of body sizes, the adaptive hypotheses of aging interpret this as evidence of an interaction between metabolic speed and the aging timer.
FECUNDITY–LONGEVITY TRADE-OFF
A well-described ecological observation is a correlation between longevity and the number of offspring [8, 9, 102]. The longevity–fecundity trade-off forms the foundation of the disposable soma hypothesis [7] and the modern life-history theory [201, 202]. However, this trade-off is more obscure in individual life histories. The lifespan of a virgin Drosophila female is longer than that of mated ones [203]. However, more detailed analyses showed that this effect is not due to the exhaustion associated with egg-laying but rather a specific mating-associated phenomenon mediated by a sex-peptide in the male sperm. Females, mated with the fertile sex-peptide null mutants, have had a prolonged lifespan, whereas those crossed to the sterile sex-peptide positive partners have had shortened lifespans [204-206]. Fecundity-longevity trade-off can be uncoupled in Drosophila [207]. The correlation between post-reproductive longevity and women’s parity was minor or nonexistent, depending on the study [208]. Thus, the disposable soma-based predictions seem to work at the level of species and fail at the individual life history level.
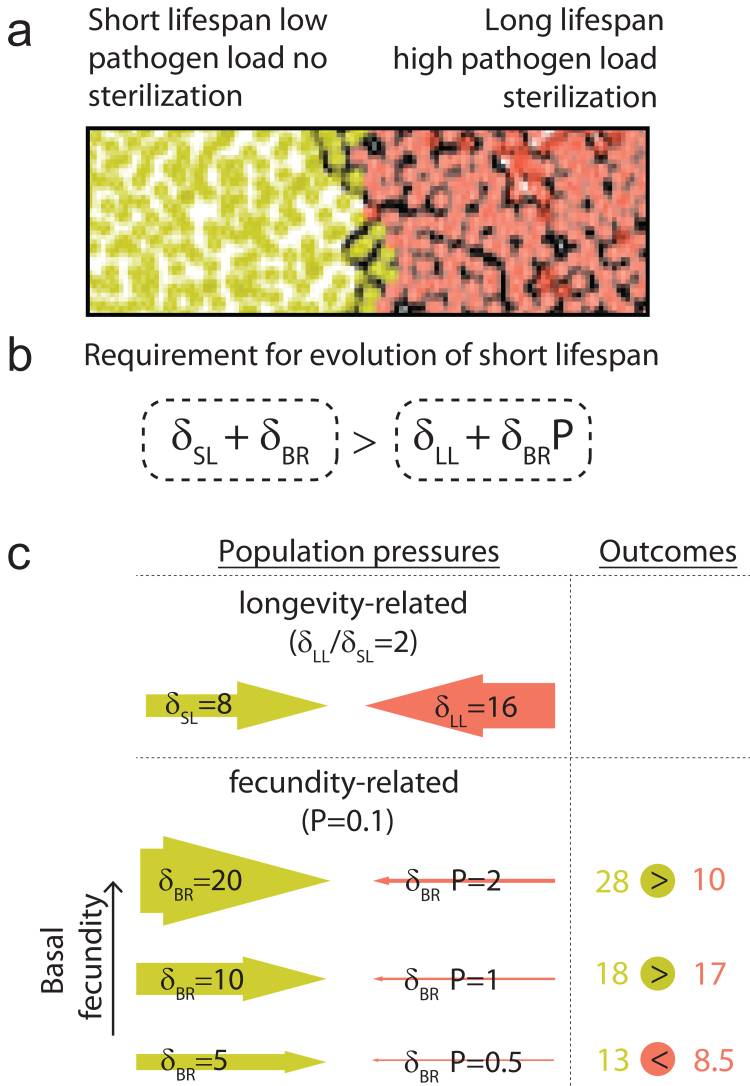
The pathogen control hypothesis identifies an ecological connection between longevity and fecundity in epidemiological models via effects on balance in population pressures between highly infected long-lived individuals and less infected short-lived individuals [36]. Both birth rate and mortality affect the population growth rate. Consider a population with very low fecundity. A tiny increase in longevity would affect the population growth more than in a highly fecund population. With an increase in birth rate, the relative role of longevity for maintaining the population pressures decreases, so that the longer lifespan of the highly infected long-lived individuals might become incapable of providing sufficient population pressure to balance the higher levels of reproduction of less infected short-lived strain (Fig. 5). Thus, the pathogen control hypothesis dissects the mechanism of this correlation, suggesting an ecological paradigm in which a higher birth rate should result in the evolution of a shorter lifespan and vice versa.
Fig. 5.
Longevity–fecundity trade-off explained by pathogen control hypothesis. a) The interface between short-lived and long-lived populations is in Fig. 4f. b) Required (but not sufficient) condition for selection towards shorter lifespan. The gradient of lifespan-dependent pressures obviously always favors the long-lived strain (since δSL < δLL), while the reproduction-dependent gradient - the short-lived strain (δBR > δBR P). Therefore, an increase in δBR results in an increase in selection power towards a shorter lifespan. c) The population pressures affecting the outcome of selection: mortality and fecundity both affect the population pressures. δSL and δLL are pressures produced due to the short and long lifespans, correspondingly. δBR is the pressure produced due to the reproduction of a healthy population. P is pathogen-dependent sterilization. δBR P – the pressure produced due to the reproduction in the infested population, P is a degree of sterilization; P < 1.
Notably, this mechanism does not contradict the life-history theory but provides a model alternative to the disposable soma. Yet, the predictions of the two hypotheses are slightly different. The disposable soma proposes senescence to be in a physiological trade-off with fecundity and to correlate better with the caloric investment in reproduction. The pathogen control hypothesis considers these two parameters to be in an ecological trade-off and, therefore, predicts the population growth rate to correlate better with the lifespan. The investigation of these two correlations might help discriminate between the two hypotheses.
CALORIC RESTRICTION AND HORMESIS
The disposable soma hypothesis proposes senescence results from energy allocation to reproduction at the expense of maintenance [7]. This view also influenced the modern life-history theory [201, 202]. However, this contradicts the observation that caloric restriction prolongs lifespan [106-108], whereas the ad libitum diet is not accompanied by extensive self-repair and extended longevity (see above). Interestingly, caloric restriction is not a unique stressor that induces a hormetic response. Some mild stressors, such as suboptimal temperature or oxidation, also extend lifespan [209-211]. This indicates the existence of pathways for conditional lifespan prolongation. The classical “pay later” hypotheses fail to explain why these lifespan-prolonging mechanisms are not active in optimal or “better than optimal” environments. Recent research demonstrated that lifespan extension by caloric restriction largely depends on olfactory cues rather than food consumption [212, 213]. Since smells should not affect metabolic balances, these observations point out that hormetic responses are ecological adaptations and not resulting from physiological trade-offs.
The pathogen control hypothesis may explain this phenomenon. Since the evolution of lifespan is driven by population pressures, the pause in reproduction induced by caloric restriction or other mild stressors also pauses the selection if applied to all population members. Therefore, the hormetic lifespan extension, in this case, can be adaptive as it allows replicating in more favorable conditions with only minor pathogen-related fitness penalties. In agreement with this hypothesis, chronic infections, and inflammation, unlike other environmental stressors, seem to shorten lifespan [214, 215], an observation pointing to the unique place of infections for the evolution of senescence.
LINKS TO EXTRINSIC MORTALITY
Higher senescence rates in species exposed to high extrinsic mortality (e.g., due to predation or hunger) are often considered the key prediction of the antagonistic pleiotropy hypothesis [6, 65, 69].
Empirically, the deterministic death of individuals due to seasonality or other periodic reasons results in the evolution of senescence, which is neutral under these conditions [58-60]. Thus, if death cannot be avoided, aging can evolve via genetic drift or even as an energy-saving strategy, for example, if accumulating damaged molecules is a cheaper strategy than recycling them [216]. However, data on non-deterministic mortality is controversial: in some populations, higher predation results in the evolution of a shorter lifespan [70, 217], but in others, a longer lifespan [68, 72, 73]. The correlation between annual mortality and longevity in different mammalian species is on the edge of statistical significance [74]. So, a consensus on the interaction between extrinsic mortality and senescence is still missing [71].
The pathogen control hypothesis suggests complex and indirect ecological interactions between extrinsic mortality and aging. On the one hand, high extrinsic mortality might remove old infected individuals to relax the selective pressure towards a shorter lifespan. This effect might be stronger if the infected animals are specifically vulnerable to the extrinsic causes of mortality. On the other hand, extrinsic mortality might favor the evolution of earlier maturation and higher birth rate [217], the traits favoring the evolution of a shorter lifespan in our model [36]. Therefore, faster senescence might indeed evolve due to the high level of extrinsic mortality as a secondary effect of an increase in fecundity. Future modeling and empirical work are required to elucidate the exact mode of interaction between extrinsic mortality and longevity in the frame of the pathogen control hypothesis of aging.
FLIGHT AND PROLONGED LIFESPAN
Flight correlates with longevity [53, 218]. Flying birds and bats have longer lifespans than terrestrial mammals of comparable sizes [8]. At the same time, flightless birds are believed to have shorter size-corrected lifespans [218]. The classic hypotheses claim this is due to the protective effects of flight as a strategy to avoid predation [6, 219]; however, the mortality of birds and bats often is higher than that of terrestrial mammals with a similar maximum recorded lifespan (Fig. 2 [77, 220, 221]). Curiously, a longer lifespan is recorded in gliding and arboreal animals than in relative terrestrial species [53, 218]. This explains the differences between ground and tree squirrels in Fig. 1.
The pathogen control hypothesis proposes flight decreases the population viscosity [RAP5]. Disease transmission between non-relative individuals is more likely if the population is not viscous. In the case of promiscuous interactions, the individual cannot allocate the benefits of its adaptive death to its own kin, driving the evolution of a longer lifespan. Future investigations are required to characterize the population in order to test predictions of the pathogen control hypothesis. Long-lived and short-lived bats are attractive objects for such research.
VARIABILITY OF SENESCENCE IN DEVELOPMENTAL ISOFORMS
Another example of aging plasticity is variability in lifespan between developmental isoforms of polyphenic species. The most studied example is the dauer larva of Caenorhabditis elegans, which develops in response to starvation and can survive for ~4 months instead of 2-3 weeks for the regular adult [222]. Another example comes from the queens in eusocial insects [223] and rodents [224, 225] that live longer than the non-breeding individuals (see below). A striking example of variability in lifespan is the migratory monarch butterfly (Danaus plexippus), that typically lives for less than two months as a resident adult; however, as a migrant adult, it can survive for more than six months [226]. Therefore, the same genetic material might instruct dramatically different lifespan setpoints. Entropic hypotheses fail to explain why the survival program encoded in the genome is not activated in the short-lived isoforms. The “pay later” hypotheses suggest that the trade-offs are different in developmental variants. Particularly, in the context of disposable soma, one can claim that the dauer of C. elegans is an energy-saving form that does not invest in reproduction and allocates all the resources towards longevity. In the case of queens in eusocial species, this hypothesis still can claim that the colony invests more resources into the queen than in worker maintenance [227]. These arguments seem to be weak, as it is unclear why sufficient amounts of resources are not available to the short-lived isoforms in some outstandingly favorable ecological niches or during rich seasons. Thus, why the survival program is not turned on in these “better than optimal” cases is unclear. The example of monarch butterflies is specifically contradictory in the context of disposable soma. While migratory monarchs are quiescent in reproduction (a state called diapause), unlike other insects in this state, they remain physically active while completing an extraordinarily long trip from Canada to Mexico. The amount of energy spent during this journey is obviously substantial. With these examples, we emphasize the general weakness of disposable soma that requires the cost of maintenance to be quite high from one side and, at the same time, within reach in the case of the long-lived developmental isoforms.
The pathogen control hypothesis considers lifespan to be defined as a trade-off between epidemic penalties associated with a long lifespan and the ecological benefits of longevity. Therefore, if a longer lifespan is essential for survival under certain circumstances (harsh environment, migration), it might evolve to be expressed specifically under these conditions. Pathogens are still present in the “better than optimal” environments, so the selection pressure toward a shorter lifespan is not mitigated.
EUSOCIALITY IN INSECTS AND MAMMALS
Eusociality is associated with an extreme variation in longevity between the individuals of the same species [223, 224] and specific social structures. These are worth discussing separately from longevity variations of other phenic isoforms. For example, honeybee (Apis mellifera) queens live 2-5 years, but the workers live 15-38 days during the summer. Young workers are first allocated to nursing jobs to take care of the eggs and larvae and do not leave the hive. A few weeks later, they switch to foraging and collecting nectar for a few more weeks until their death, which typically occurs outside the hive. Curiously, if prohibited from leaving the hive, the workers extend their lifespan to 75-135 days, indicating that the nurse-forager transition is the most important event in defining their lifespan. Furthermore, if forced to return back to nursing jobs, foragers prolong their lifespan. The overwinter stress-resistant variant of the worker honeybee can survive for 140-320 days [228-233]. Various classical hypotheses attempted to explain these patterns with differential hazard protection attributed to different jobs and with energy-saving strategies [231]. However, ~20% of the foragers survive to the age of senescence, thus contradicting the idea of evolutionary neutrality of honeybee aging [234, 235]. This example of extreme plasticity demonstrates the apparent absence of rigid mechanistic constraints that prevent the evolution of a long lifespan according to the antagonistic pleiotropy hypothesis [6]. The disposable soma model [236] also fails to explain these observations. The key element of this hypothesis, the nutrition level, does not seem to play a major role in the lifespan setpoint management of honeybees: it is difficult to envision a case when producing a new worker might be cheaper for the hive than investing in the maintenance of the old one.
The pathogen control hypothesis suggests that aging in different castes is determined by their exposure to pathogens, their risks of passing the pathogen further to the colony members, and by their relative cost to the hive’s fitness. The queen is residing within the hive, the cost of its fitness is very high, and the relative detrimental effect of the queen-to-worker pathogen transmission is probably minimal. The nurses are also residing inside the hive and are not exposed to pathogens. Foragers have higher risks of getting infected and can bring infection inside the colony, explaining why an allocation to the foraging work limits the lifespan. Intriguingly, tapeworms are able to extend the lifespan of infected workers, making them as long-lived as the queens [237].
Another example that links longevity and eusociality are mole rat species belonging to the Fukomys genus, a group of subterranean eusocial rodents. Breeders in most of the mole-rat species have substantially longer lifespans than non-breeders, similar to the eusocial insects [224, 225].
The puzzling exception is a mouse-sized naked mole rat (Heterocephalus glaber) that showed little if any detectable senescence after more than 30 years of laboratory observations [44]. Both breeder and non-breeder castes are believed to be extremely long-lived. This model is challenging all the classical evolutionary hypotheses of aging that fail to explain such extreme longevity with any ecological factors.
The pathogen control hypothesis provides a framework for explaining this phenomenon by linking the unique longevity of the naked mole-rat to the structure of the eusocial population, which is also infrequent in mammals. We propose that eusociality can allow adaptive suicide – phenoptosis – as a strategy for pathogen clearance within the population [155, 238, 239]. If the infected worker is immediately killed by behavioral or immune mechanisms, it may benefit its genes since the reproduction, in this case, is disconnected from the infection. Such an adaptive suicide strategy might keep the colony pathogen-free, therefore making aging redundant. Little is known about naked mole-rats immunity; however, these animals are hypersensitive to herpes simplex virus [240] and some bacterial infections [241, 242]. Furthermore, the queens of naked mole rats seem to be less susceptible to coronavirus than non-breeders [243] in agreement with the predictions of the pathogen control hypothesis. Sequencing of mole-rat immune cell samples revealed a loss of NK cells in these animals [244]. Interestingly, immune depression (decline in immune cell numbers) is also observed in bees upon transition to foraging and recuperates if the foragers return to nursing [245]. Future experimental research should explore a potential connection between eusociality, aging, and phenoptosis in response to infection.
REJUVENATION AND PROBLEM OF SYMMETRY
Aging is traditionally considered an irreversible unidirectional process [246, 247]. However, certain age-related parameters can be reversed by pharmacological interventions [248], blood transfusion[249, 250], or physical training [251]. A remarkable manifestation of physiological rejuvenation was observed in bees that shifted from foraging to nursing [245].
Do these observations reflect a systemic rejuvenation? The case when a “rejuvenated” older animal becomes functionally indistinguishable from a younger one is incompatible with the classic mainstream hypotheses. Consider a parental organism that produces a genetically equivalent daughter individual (assume clonal reproduction for simplicity) and then undergoes rejuvenation. Now both individuals are genetically and physiologically equivalent, share the same extrinsic risks and fertility, and are symmetrically valuable for selection. Clearly, senescence would be equally maladaptive in both individuals without any preferences for a parent (Fig. 6). The loss of a clear definition of time in such a model causes a major collision with classic evolutionary hypotheses of aging since the idea of damage accumulation and the formula “pay later” both require a unidirectional time arrow as a critical assumption.
Fig. 6.
Rejuvenation poses a critical controversy to the classical hypotheses. a) Senescence in the absence of rejuvenation. b) The opportunity to rejuvenate results in the symmetry between a parent and a daughter organism, making aging impossible. c) Infectious diseases (black worms) bring back asymmetry even in the systems with rejuvenation since the probability of being infected depends on chronological and not on the physiological age.
At the same time, rejuvenation itself contradicts neither the current knowledge in biochemistry (with an exception for the somatic mutations that we can consider negligible, for example, in short-living species) nor the pathogen control hypothesis. Indeed, the probability of being infected depends on the chronological and not the biological age and can be a parameter, discriminating between the rejuvenated parent from its offspring. Future experiments are required to determine if an overarching rejuvenation is possible or if the reported phenotypes are limited only to some particular tissues and can exist only on the sub-organismal level. If true systemic rejuvenation is found in any animal model, this fact alone would be sufficient to force a reconsideration of the whole framework of mainstream evolutionary concepts of aging.
SEMELPARITY AND ACCELERATED AGING
Semelparity can be interpreted as an extreme form of aging that develops rapidly after reproduction. Semelparous species can be found in many taxa (e.g., fish [135], mollusks [132], insects [252], mammals [253]). The ecological determinants of semelparity are not entirely clear. Some authors claim that the residual life of a parent might be less important than an increased number of offspring and that overinvestment in reproduction is beneficial even if it results in a deadly exhaust [131, 254]. However, this view does not seem to be persuasive as the progeny are usually exposed to higher juvenile mortality and might not survive to adulthood, while adults are typically better protected. Moreover, in some cases, the death of parental organisms seems to be caused by an adaptive developmental program rather than overinvestment in reproduction. For example, surgical removal of optic glands in female octopus prevents suicidal behavior and substantially prolongs lifespan [132]. Sterilization prolongs the lifespan of semelparous Pacific salmon [255]. In semelparous salmonids and marsupial species post-reproductive death is connected to elevated corticoid levels, indicating the programmed rather exhaust-based nature of accelerated death [256-258].
These observations clearly indicate that the death of an individual might be adaptive at least under certain conditions. Classic hypotheses usually neglect these facts (e.g., by declaring them to be not related to aging in the iteroparous species). The pathogen control hypothesis explains the variety of adaptive lifespan shortening. We hypothesize that semelparity is an adaptation that evolved to prevent the transgenerational transmission of pathogens. For example, pathogens might become a serious problem in overpopulated rivers, where the spawning of Pacific salmon occurs. In support of this concept, the effects of pathogens on the lifecycle of semelparous marsupials were also reported [259].
PREDICTIONS TO BE TESTED
Future modeling and empirical work should test the predictions of the pathogen control hypothesis. Here we delineate three main lines of the proposed research.
Interaction between aging and immunity. First, the models presented so far do not include the immune system and its interaction with senescence. However, if we assume aging is a part of the immune response, we would expect extensive crosstalk between these systems. Aging is associated with low-grade unresolved inflammation [260, 261], and chronic immune activation leads to lifespan shortening in experimental settings [215, 262] and to the acceleration of aging in patients [263, 264]. This sets chronic infection aside from other mild stressors that typically reduce the rate of senescence and prolong lifespan [209-211]. Anecdotal cases when pathogens prolong lifespan are described in eusocial [237] and semelparous species [265].
The evolutionary interpretation of accelerated aging by chronic infection can be twofold: the classic hypothesis would suggest this happens due to the allocation of resources towards the deployment of the immune response at the expense of maintenance[266]. The pathogen control hypothesis predicts a conditional lifespan shortening by immune activation to be an adaptive mechanism of lifespan management, protecting an individual’s kin (manuscript in preparation). The way to test these two predictions is to determine if the deployment of protective immunity in response to a pathogenic stimulus could be mechanistically uncoupled from the reduction in longevity (e.g., by genetic mutations or other interventions). If so, it would be a strong argument in favor of the pathogen control hypothesis. These experiments might also provide insight into the mechanisms of senescence and its connection with immunity.
Structure of infectious networks. The pathogen control hypothesis predicts that the evolution of lifespan depends on the structure of contact networks that define the routes of pathogen transmission. If networks are biased to interactions between kin individuals, it will favor the evolution of a shorter lifespan. In the case of more promiscuous chaotic interactions, a longer lifespan will be favored. Examining this prediction in long-lived and short-lived species should be a good test for the hypothesis. Species with unusual population structures and abnormal lifespans, such as eusocial insects and rodents, bats, and anadromous fish species, should be investigated with the means of modeling and ecological studies.
Pathogen abundance and infection-related penalties. The variability of pathogens is a critical assumption of the pathogen control hypothesis. Two general scenarios of selection were suggested. One scenario implies zoonotic transmission of a new pathogen. Therefore, animals from the rich biotopes, exposed to the pathogens from the multiple relative species, should experience a stronger selection pressure towards a shorter lifespan. Low pathogen abundance should relax the selection towards a shorter lifespan. In good agreement with these expectations, some well-known centenarian species (e.g., bowhead whale, Balaena mysticetus [267], Greenland shark, Somniosus microcephalus [268], mollusk, Arctica islandica [269], and the proteus, Proteus anguinus [40]) are found in the abiotic arctic or cave environments. However, this correlation might also be explained by ecological adaptation to low fecundity, resulting from low nutrition levels.
Our model suggests coinfection with several mild pathogens that results in sterilization by a cumulative effect (Fig. 4h) [36].
The feasibility of these two scenarios can be studied in natural or laboratory populations by investigating age-related pathogen prevalence, zoonotic transmissions, coinfection, and infection-induced sterilization.
Problems of the pathogen control hypothesis of aging. So far, we have compared the pathogen control hypothesis to classic theories. At the current stage of development, pathogen control explains most of the aging-related observations and is more parsimonious in terms of assumptions required.
Nevertheless, some general issues need to be clarified with this hypothesis. First are the numeric estimates of the epidemiological and population dynamics parameters that may affect the evolution of lifespan. The abundance of pathogens in nature and their ability to decrease the infected animal’s fecundity is well-established [154]; however additional investigations are needed to empirically evaluate these parameters in wild animal populations. The same is true for population viscosity: while it is clear that animal dispersal is limited by the laws of physics and territorial behavior is a near-universal feature [270], the exact numeric estimates and the effects of family and social structure in shaping the kin-specificity of infectious networks remain to be determined.
Second, the described scenario of selection accurately explains the prevention of the emergence of long-lived variants in the preexisting population of short-lived individuals [36]. However, the evolution of a shorter lifespan from the long-lived ancestors requires additional conditions. The key requirement is the necessity to nucleate a sub-population of “pure” short-lived individuals that can benefit from the low pathogen prevalence and provide sufficient populational pressure to expand further (Fig. 7).
Fig. 7.
Stabilizing selection of short lifespan setpoint and a potential scenario of lifespan shortening. The mechanism of stabilizing selection described in Fig. 4f is schematically depicted in the upper-left corner. In the lower-right corner is demonstrated a potential scenario of the shorter lifespan evolution in the population, where a longer lifespan setpoint initially prevails. Short-lived variants are persisting in such a population under conditions of high extrinsic mortality or prevalence of sterilizing pathogens. If such a population undergoes a dramatic decline (e.g., due to epidemics of sterilizing sexually transmitted diseases), the fragmentation of the range leads to the establishment of sub-populations with high founder effects. Thus, the pure populations of short-lived individuals can emerge, bringing the model to the scenario of competition described in Fig. 4f.
Several scenarios of shorter lifespan evolution can be envisioned, but the simplest one involves the accumulation of short-lived variants in a population with very high extrinsic mortality (neutral aging) with a consequent catastrophic reduction in population density, range fragmentation, and appearance of patches with high founder effects (Fig. 7). Thus, scenarios for establishing a limited lifespan setpoint is assumed to be more demanding (Fig. 7) than stabilizing scenario. However, longevity correlates with body size [8], and animal body size mostly evolves to increase, according to Cope’s rule [271]. Hence, the evolution of a shorter lifespan is expected to occur infrequently, and the imbalance between the requirements of two scenarios of selection is not critical for the explanatory power of the hypothesis (table).
Comparing the explanatory power of different hypotheses of aging
Notes. Reproduced with permission from [37]. Green cells contain supportive arguments, red – contradictions, yellow – predictions.
a Some species of invertebrates (e.g., flatworm Schmidtea mediterranea [273] and a cnidarian Hydra vulgaris [38]) are biologically immortal.
b Body size of a given species correlates with longevity: bigger animals live longer [182, 218]. Paradoxically, mutant variants with smaller body sizes often have a longer lifespan (or healthspan) in domestic dogs (Canis lupus familiaris) [185], humans [187], and model animals [183].
c Pathogen control hypothesis assumes population pressures to be a key element of lifespan selection. Famine simultaneously inhibits replication of all population members and might pause or delay selection progress. Thus, the deceleration of aging during famine might be a reasonable survival strategy.
d Longevity inversely correlates with fecundity among different species: highly productive animals are typically short-lived [182]. However, the lifespan of a particular individual in many cases does not depend on the history of its reproduction [274]; long-lived mutants can be highly fertile [207]; breeders of eusocial animals are long-lived and fecund [275]. Hence, this trade-off can be uncoupled, suggesting that ecological but not physiological factors shape it.
e Metabolic rate affects longevity when analyzed in model animals: mutant variants with lower metabolic rates age slower [200]. It also may correlate with the longevity of different species. However, this correlation disappeared when the body size effects were excluded [182]. Flying birds have very high metabolic rates and long lifespans. Non-flying birds are short-lived and have lower metabolic rates [182, 218]. Therefore, longevity and metabolism seem to evolve independently.
f The longer dispersal distances of flying animals may make their populations better intermixed than in terrestrial species[276]. If individuals interact promiscuously, the pathogen control hypothesis predicts the evolution of a longer lifespan.
g An example is reproductive diapause in monarch butterflies (Danaus plexippus): the longevity of overwinter migratory forms can be 5-6 times greater than that of summer resident adults [277].
h Pathogen control mediated by short worker lifespan is combined with the fitness advantage of a breeder’s extended reproduction period. Consider a poorly infectious pathogen with a transmission rate of β = 1 per month. It cannot propagate in a colony of eusocial animals with a lifespan A < 2 months since each infected host infects less than one susceptible animal: R0 < Aβ/2. Breeder-specific long lifespan provides selective advantages due to an extended period of reproduction, whereas the protection against diseases granted by a shorter non-breeders lifespan remains active. Breeders typically reside inside the colony and are isolated from the environmental contamination, thus reducing the probability of initial infection to attack the breeder itself.
i Both breeders and non-breeders of naked mole-rats seem to display negligible aging, thus challenging the general views on senescence [176]. The pathogen control hypothesis produces a potential explanation. If individuals die immediately in response to every pathogen, thus preventing its spread, the population might be kept virtually sterile of infections, making aging redundant. Eusociality might facilitate the evolution of this phenoptotic strategy: infected non-breeders may undergo programmed death to protect the reproductive capacity of the breeder. Indeed, some reports suggest these animals are hypersensitive to infections [66] and lack natural killer cells [244]. Further research is needed to test this prediction accurately.
j Some animals die immediately after reproduction. Semelparity can be considered as an extreme case of fecundity-longevity trade-off [131]. In some instances, the parent’s death seems to have a programmed component and does not happen solely due to exhaustion [278]. The ecological role of such programmed death is poorly understood. The pathogen control hypothesis proposes that semelparity evolved to prevent the transgenerational transmission of diseases.
k The work done so far can explain the evolutionary stability of the limited lifespan and inhibition of invading long-lived mutants [36]. However, the evolution of a shorter lifespan from a long-lived variant requires additional conditions: short-lived individuals should first accumulate locally to benefit from their altruism-based mechanism of infection control. Such scenarios might be feasible in epidemiological models but were not studied yet. However, this problem is minor: mammal’s body sizes predominantly increase with evolution (Cope’s rule) [271]. Since size correlates with longevity, lifespan should rarely evolve towards shortening.
SUMMARY
As discussed above, the mainstream evolutionary hypotheses of aging, despite a long history of investigation, remain controversial. These classic hypotheses are based on concepts of pleiotropic genes and underinvestment into maintenance as an energy-saving strategy – and do not agree well with modern molecular genetics and biochemistry. Aging is not universal and varies among different species, and this variability is only weakly explained by classic hypotheses. Key predictions of classical hypotheses failed: extrinsic mortality does not shape the evolution of senescence; fecundity-longevity trade-off can be uncoupled. Aging demonstrates unexpected plasticity, depending on the environment. Given that this plasticity exists, the responses predicted by the classic hypotheses are not observed.
The recently proposed pathogen control hypothesis is more parsimonious in terms of assumptions and makes a more compelling case than the mainstream hypotheses of aging. It is founded on elements (population viscosity and chronic sterilizing infections) well-accepted in the fields of population dynamics and parasitology. It provides focused and clear predictions. Specifically, this hypothesis provides a potential explanation for the apparent mechanistic connection between aging and immunity [261]. Moreover, the founding elements of the mainstream hypotheses, the pleiotropic genes and fecundity-longevity trade-off can be explained in the epidemiological models. We assert that pathogen control should be considered among the main concepts of the evolution of aging by experimentalists and evolutionary biologists.
Our model suggests that senescence can be an evolutionary adaptation, a view neglected by most researchers in the field. If true, this concept should significantly alter the approach to aging biology.
Electronic supplementary material
Video 1, Online Resourse 2. Modeling reproduction, death, and movement of individuals in a viscous population.
Video 2, Online Resourse 3. The basic scenario of stabilizing selection that suppresses the emergence.
Contributions
PVL – initial conceptualization; PVL, JY, JR, RA – discussion and refinement; JY – data collection for Table S1; PVL, JR, RA – writing, RA – funding.
Funding
The work was supported by NIH R01AI137471 and Impetus Longevity Grant for R.A.
Ethics declarations
The authors declare no conflicts of interest. This article does not contain description of studies with the involvement of humans or animal subjects.
Contributor Information
Peter V. Lidsky, Email: peter.lidsky@ucsf.edu
Raul Andino-Pavlovsky, Email: raul.andino@ucsf.edu.
References
- 1.Weismann, A. (1891) Essays Upon Heredity and Kindred Biological Problems, Clarendon Press, Oxford.
- 2.Fisher, R. A. (1930) The genetical theory of natural selection, 10.5962/bhl.title.27468.
- 3.Medawar, P. B. (1952) An Unsolved Problem of Biology: An Inaugural Lecture Delivered at University College, London, 6 December, 1951.
- 4.Haldane, J. B. S. (1942) New Paths in Genetics, Harper & Brothers, NY & London.
- 5.Hamilton W. D. The moulding of senescence by natural selection. J. Theor. Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 6.Williams G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398. doi: 10.2307/2406060. [DOI] [Google Scholar]
- 7.Kirkwood T. B., Holliday R. The evolution of ageing and longevity. Proc. R. Soc. Lond. B Biol. Sci. 1979;205:531–546. doi: 10.1098/rspb.1979.0083. [DOI] [PubMed] [Google Scholar]
- 8.Lindstedt S. L., Calder W. A. Body size, physiological time, and longevity of homeothermic animals. Quart. Rev. Biol. 1981;56:1–16. doi: 10.1086/412080. [DOI] [Google Scholar]
- 9.Ricklefs R. E. Life-history connections to rates of aging in terrestrial vertebrates. Proc. Natl. Acad. Sci. USA. 2010;107:10314–10319. doi: 10.1073/pnas.1005862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orgel L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl. Acad. Sci. USA. 1963;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harman D. Role of free radicals in mutation, cancer, aging, and the maintenance of life. Radiat. Res. 1962;16:753–763. doi: 10.2307/3571274. [DOI] [PubMed] [Google Scholar]
- 12.Bjorksten J. The crosslinkage theory of aging. J. Am. Geriatr. Soc. 1968;16:408–427. doi: 10.1111/j.1532-5415.1968.tb02821.x. [DOI] [PubMed] [Google Scholar]
- 13.Gavrilov L. A., Gavrilova N. S. The reliability theory of aging and longevity. J. Theor. Biol. 2001;213:527–545. doi: 10.1006/jtbi.2001.2430. [DOI] [PubMed] [Google Scholar]
- 14.Curtis, H. J. (1965) The somatic mutation theory of aging, Contribut. Psychobiol. Aging, 69-80, 10.1007/978-3-662-39847-0_6.
- 15.Gershon D. The mitochondrial theory of aging: is the culprit a faulty disposal system rather than indigenous mitochondrial alterations? Exp. Gerontol. 1999;34:613–619. doi: 10.1016/s0531-5565(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Kowald A. The mitochondrial theory of aging: do damaged mitochondria accumulate by delayed degradation? Exp. Gerontol. 1999;34:605–612. doi: 10.1016/s0531-5565(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 17.Terman A. Garbage catastrophe theory of aging: imperfect removal of oxidative damage? Redox Rep. 2001;6:15–26. doi: 10.1179/135100001101535996. [DOI] [PubMed] [Google Scholar]
- 18.Gladyshev V. N. Aging: progressive decline in fitness due to the rising deleteriome adjusted by genetic, environmental, and stochastic processes. Aging Cell. 2016;15:594–602. doi: 10.1111/acel.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijg J. Loss of gene coordination as a stochastic cause of ageing. Nat. Metab. 2020;2:1188–1189. doi: 10.1038/s42255-020-00295-2. [DOI] [PubMed] [Google Scholar]
- 20.Larsson N.-G. Somatic mitochondrial DNA mutations in mammalian aging. Annu. Rev. Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 21.Murray V. Are transposons a cause of ageing? Mutat. Res. 1990;237:59–63. doi: 10.1016/0921-8734(90)90011-f. [DOI] [PubMed] [Google Scholar]
- 22.Nelson P., Masel J. Intercellular competition and the inevitability of multicellular aging. Proc. Natl. Acad. Sci. USA. 2017;114:12982–12987. doi: 10.1073/pnas.1618854114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gems D., de Magalhães J. P. The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Res. Rev. 2021;70:101407. doi: 10.1016/j.arr.2021.101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen A. A., Ferrucci L., Fülöp T., Gravel D., Hao N., Kriete A., Levine M. E., Lipsitz L. A., Olde Rikkert M. G. M., Rutenberg A., Stroustrup N., Varadhan R. A complex systems approach to aging biology. Nat. Aging. 2022;2:580–591. doi: 10.1038/s43587-022-00252-6. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch, H. R. (1987) Why should senescence evolve? An answer based on a simple demographic model, Evol. Longev. Anim., 75-90, 10.1007/978-1-4613-1939-9_5. [DOI] [PubMed]
- 27.Travis J. M. J. The evolution of programmed death in a spatially structured population. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004;59:B301–B305. doi: 10.1093/gerona/59.4.b301. [DOI] [PubMed] [Google Scholar]
- 28.Mitteldorf J. J. Demographic evidence for adaptive theories of aging. Biochemistry (Moscow) 2012;77:726–728. doi: 10.1134/s0006297912070048. [DOI] [PubMed] [Google Scholar]
- 29.Mitteldorf, J. (2017) Aging is a Group-Selected Adaptation: Theory, Evidence, and Medical Implications, CRC Press.
- 30.Werfel J., Ingber D. E., Bar-Yam Y. Programed death is favored by natural selection in spatial systems. Phys. Rev. Lett. 2015;114:238103. doi: 10.1103/PhysRevLett.114.238103. [DOI] [PubMed] [Google Scholar]
- 31.Goldsmith T. C. Aging, evolvability, and the individual benefit requirement; medical implications of aging theory controversies. J. Theor. Biol. 2008;252:764–768. doi: 10.1016/j.jtbi.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Skulachev V. P. Aging is a specific biological function rather than the result of a disorder in complex living systems: biochemical evidence in support of Weismann’s hypothesis. Biochemistry. 1997;62:1191–1195. [PubMed] [Google Scholar]
- 33.Martins A. C. R. Change and aging senescence as an adaptation. PLoS One. 2011;6:e24328. doi: 10.1371/journal.pone.0024328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirchner J. W., Roy B. A. The evolutionary advantages of dying young: epidemiological implications of longevity in metapopulations. Am. Nat. 1999;154:140. doi: 10.2307/2463908. [DOI] [PubMed] [Google Scholar]
- 35.Mitteldorf J., Pepper J. Senescence as an adaptation to limit the spread of disease. J. Theor. Biol. 2009;260:186–195. doi: 10.1016/j.jtbi.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Lidsky P. V., Andino R. Epidemics as an adaptive driving force determining lifespan setpoints. Proc. Natl. Acad. Sci. USA. 2020;117:17937–17948. doi: 10.1073/pnas.1920988117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lidsky P. V., Andino R. Could aging evolve as a pathogen control strategy? Trends Ecol. Evol. 2022;37:1046–1057. doi: 10.1016/j.tree.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Martínez D. E. Mortality patterns suggest lack of senescence in hydra. Exp. Gerontol. 1998;33:217–225. doi: 10.1016/s0531-5565(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 39.Piraino S., Boero F., Aeschbach B., Schmid V. Reversing the life cycle: medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa) Biol. Bull. 1996;190:302–312. doi: 10.2307/1543022. [DOI] [PubMed] [Google Scholar]
- 40.Voituron Y., de Fraipont M., Issartel J., Guillaume O., Clobert J. Extreme lifespan of the human fish (Proteus anguinus): a challenge for ageing mechanisms. Biol. Lett. 2011;7:105–107. doi: 10.1098/rsbl.2010.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Congdon J. D., Nagle R. D., Kinney O. M., van Loben Sels R. C. Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii) Exp. Gerontol. 2001;36:813–827. doi: 10.1016/s0531-5565(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 42.Miller J. K. Escaping senescence: demographic data from the three-toed box turtle (Terrapene carolina triunguis) Exp. Gerontol. 2001;36:829–832. doi: 10.1016/s0531-5565(00)00243-6. [DOI] [PubMed] [Google Scholar]
- 43.Cailliet G. M., Andrews A. H., Burton E. J., Watters D. L., Kline D. E., Ferry-Graham L. A. Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 2001;36:739–764. doi: 10.1016/s0531-5565(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 44.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 45.Idda M. L., McClusky W. G., Lodde V., Munk R., Abdelmohsen K., Rossi M., Gorospe M. Survey of senescent cell markers with age in human tissues. Aging. 2020;12:4052–4066. doi: 10.18632/aging.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olivetti G., Melissari M., Capasso J. M., Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 47.Chien K. R., Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120:533–544. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Peto R. Epidemiology, multistage models, and short-term mutagenicity tests. Int. J. Epidemiol. 2016;45:621–637. doi: 10.1093/ije/dyv199. [DOI] [PubMed] [Google Scholar]
- 49.Tian X., Azpurua J., Ke Z., Augereau A., Zhang Z. D., Vijg J., Gladyshev V. N., Gorbunova V., Seluanov A. INK4 locus of the tumor-resistant rodent, the naked mole rat, expresses a functional p15/p16 hybrid isoform. Proc. Natl. Acad. Sci. USA. 2015;112:1053–1058. doi: 10.1073/pnas.1418203112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seluanov A., Gladyshev V. N., Vijg J., Gorbunova V. Mechanisms of cancer resistance in long-lived mammals. Nat. Rev. Cancer. 2018;18:433–441. doi: 10.1038/s41568-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiatt, H. H., Watson, J. D., and Winsten, J. A. (1977) Origins of Human Cancer, Cold Spring Harbor Laboratory, UK.
- 52.Tacutu R., Thornton D., Johnson E., Budovsky A., Barardo D., Craig T., Diana E., Lehmann G., Toren D., Wang J., Fraifeld V. E., de Magalhães J. P. Human Ageing Genomic Resources: new and updated databases. Nucleic Acids Res. 2018;46:D1083–D1090. doi: 10.1093/nar/gkx1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmes D. J., Austad S. N. Fly now, die later: life-history correlates of gliding and flying in mammals. J. Mammal. 1994;75:224–226. doi: 10.2307/1382255. [DOI] [Google Scholar]
- 54.Blagosklonny M. V. Aging is not programmed. Cell Cycle. 2013;12:3736–3742. doi: 10.4161/cc.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee A. T., Cerami A. Role of glycation in aging. Ann. NY Acad. Sci. 1992;663:63–70. doi: 10.1111/j.1749-6632.1992.tb38649.x. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn E. H., Greider C. W., Szostak J. W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 57.Gensler H. L., Bernstein H. DNA Damage as the primary cause of aging. Quart. Rev. Biol. 1981;56:279–303. doi: 10.1086/412317. [DOI] [PubMed] [Google Scholar]
- 58.Valdesalici S., Cellerino A. Extremely short lifespan in the annual fish Nothobranchius furzeri. Proc. Biol. Sci. 2003;270 Suppl 2:S189–191. doi: 10.1098/rsbl.2003.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tatar M., Gray D. W., Carey J. R. Altitudinal variation for senescence in Melanoplus grasshoppers. Oecologia. 1997;111:357–364. doi: 10.1007/s004420050246. [DOI] [PubMed] [Google Scholar]
- 60.Bertrand H., Chan B. S., Griffiths A. J. F. Insertion of a foreign nucleotide sequence into mitochrondrial DNA causes senescence in neurospora intermedia. Cell. 1985;41:877–884. doi: 10.1016/s0092-8674(85)80068-4. [DOI] [PubMed] [Google Scholar]
- 61.Nussey D. H., Froy H., Lemaitre J.-F., Gaillard J.-M., Austad S. N. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 2013;12:214–225. doi: 10.1016/j.arr.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones O. R., Scheuerlein A., Salguero-Gómez R., Camarda C. G., Schaible R., Casper B. B., Dahlgren J. P., Ehrlén J., García M. B., Menges E. S., Quintana-Ascencio P. F., Caswell H., Baudisch A., Vaupel J. W. Diversity of ageing across the tree of life. Nature. 2014;505:169–173. doi: 10.1038/nature12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caswell, H., and Shyu, E. (2017) Senescence, Selection Gradients and Mortality, in The Evolution of Senescence in the Tree of Life, Cambridge University Press, pp. 56-82, 10.1017/9781139939867.004.
- 64.Caswell H. Extrinsic mortality and the evolution of senescence. Trends Ecol. Evol. 2007;22:173–174. doi: 10.1016/j.tree.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Day T., Abrams P. A. Density dependence, senescence, and Williams’ hypothesis. Trends Ecol. Evol. 2020;35:300–302. doi: 10.1016/j.tree.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Moorad J., Promislow D., Silvertown J. Williams’ intuition about extrinsic mortality is irrelevant. Trends Ecol. Evol. 2020;35:379. doi: 10.1016/j.tree.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Abrams P. A. Does increased mortality favor the evolution of more rapid senescence? Evolution. 1993;47:877–887. doi: 10.1111/j.1558-5646.1993.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen H.-Y., Maklakov A. A. Longer life span evolves under high rates of condition-dependent mortality. Curr. Biol. 2012;22:2140–2143. doi: 10.1016/j.cub.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Gaillard J.-M., Lemaître J.-F. The Williams’ legacy: a critical reappraisal of his nine predictions about the evolution of senescence. Evolution. 2017;71:2768–2785. doi: 10.1111/evo.13379. [DOI] [PubMed] [Google Scholar]
- 70.Austad S. N. Retarded senescence in an insular population of Virginia opossums (Didelphis virginiana) J. Zool. 1993;229:695–708. doi: 10.1111/j.1469-7998.1993.tb02665.x. [DOI] [Google Scholar]
- 71.Furness, A. I., and Reznick, D. N. (2017) The Evolution of Senescence in Nature, in The Evolution of Senescence in the Tree of Life, Cambridge University Press, pp. 175-197, 10.1017/9781139939867.009.
- 72.Reznick D. N., Bryant M. J., Roff D., Ghalambor C. K., Ghalambor D. E. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature. 2004;431:1095–1099. doi: 10.1038/nature02936. [DOI] [PubMed] [Google Scholar]
- 73.Walsh M. R., Whittington D., Walsh M. J. Does variation in the intensity and duration of predation drive evolutionary changes in senescence? J. Anim. Ecol. 2014;83:1279–1288. doi: 10.1111/1365-2656.12247. [DOI] [PubMed] [Google Scholar]
- 74.Promislow D. E. L., Harvey P. H. Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. 1990;220:417–437. doi: 10.1111/j.1469-7998.1990.tb04316.x. [DOI] [Google Scholar]
- 75.Deevey, E. S. (1977) Life tables for natural populations of animals, Math. Demography, pp. 61-74, 10.1007/978-3-642-81046-6_9.
- 76.Botkin D. B., Miller R. S. Mortality rates and survival of birds. Am. Nat. 1974;108:181–192. doi: 10.1086/282898. [DOI] [Google Scholar]
- 77.Siriwardena G. M., Baillie S. R., Wilson J. D. Variation in the survival rates of some British passerines with respect to their population trends on farmland. Bird Study. 1998;45:276–292. doi: 10.1080/00063659809461099. [DOI] [Google Scholar]
- 78.Van Heerdt P. F., Sluiter J. W., Bezem J. J. Population statistics of five species of the bat genus myotis and one of the genus rhinolophus, hibernating in the caves of S. Limburg. Arch. Néerlandaises Zool. 1960;13:511–539. doi: 10.1163/036551660x00170. [DOI] [Google Scholar]
- 79.Gibbons M. M., McCarthy T. K. Growth, maturation and survival of frogs Rana temporaria L. Ecography. 1984;7:419–427. doi: 10.1111/j.1600-0587.1984.tb01143.x. [DOI] [Google Scholar]
- 80.Muller H. J. Our load of mutations. Am. J. Hum. Genet. 1950;2:111–176. [PMC free article] [PubMed] [Google Scholar]
- 81.Austad S. N., Hoffman J. M. Is antagonistic pleiotropy ubiquitous in aging biology? Evol. Med. Public Health. 2018;2018:287–294. doi: 10.1093/emph/eoy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Case T. J. On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Q. Rev. Biol. 1978;53:243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- 83.Kirchner J. W., Roy B. A. Evolutionary implications of host–pathogen specificity: the fitness consequences of host life history traits. Evol. Ecol. 2000;14:665–692. doi: 10.1023/a:1011647526731. [DOI] [Google Scholar]
- 84.Enard D., Cai L., Gwennap C., Petrov D. A. Viruses are a dominant driver of protein adaptation in mammals. Elife. 2016;5:e12469. doi: 10.7554/eLife.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duggal N. K., Emerman M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat. Rev. Immunol. 2012;12:687–695. doi: 10.1038/nri3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daugherty M. D., Malik H. S. Rules of engagement: molecular insights from host-virus arms races. Annu. Rev. Genet. 2012;46:677–700. doi: 10.1146/annurev-genet-110711-155522. [DOI] [PubMed] [Google Scholar]
- 87.Bunn H. F., Franklin Bunn H. Pathogenesis and treatment of sickle cell disease. New Engl. J. Med. 1997;337:762–769. doi: 10.1056/nejm199709113371107. [DOI] [PubMed] [Google Scholar]
- 88.Williams T. N., Thein S. L. Sickle cell anemia and its phenotypes. Annu. Rev. Genom. Hum. Genet. 2018;19:113–147. doi: 10.1146/annurev-genom-083117-021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans E. A., Chen W. C., Tan M.-W. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell. 2008;7:879–893. doi: 10.1111/j.1474-9726.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garsin D. A., Villanueva J. M., Begun J., Kim D. H., Sifri C. D., Calderwood S. B., Ruvkun G., Ausubel F. M. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 91.Fabian D. K., Garschall K., Klepsatel P., Santos-Matos G., Sucena É., Kapun M., Lemaitre B., Schlötterer C., Arking R., Flatt T. Evolution of longevity improves immunity in Drosophila. Evol. Lett. 2018;2:567–579. doi: 10.1002/evl3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donnelly R., White A., Boots M. Host lifespan and the evolution of resistance to multiple parasites. J. Evol. Biol. 2017;30:561–570. doi: 10.1111/jeb.13025. [DOI] [PubMed] [Google Scholar]
- 93.Huang Z., Whelan C. V., Dechmann D., Teeling E. C. Genetic variation between long-lived versus short-lived bats illuminates the molecular signatures of longevity. Aging. 2020;12:15962–15977. doi: 10.18632/aging.103725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorbunova V., Seluanov A., Zhang Z., Gladyshev V. N., Vijg J. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat. Rev. Genet. 2014;15:531–540. doi: 10.1038/nrg3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swovick K., Firsanov D., Welle K. A., Hryhorenko J. R., Wise J. P., George C., Sformo T. L., Seluanov A., Gorbunova V., Ghaemmaghami S. Interspecies differences in proteome turnover kinetics are correlated with lifespans and energetic demands. Mol. Cell. Proteomics. 2021;20:100041. doi: 10.1101/2020.04.25.061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carlton J. M. Evolution of human malaria. Nat. Microbiol. 2018;3:642–643. doi: 10.1038/s41564-018-0170-2. [DOI] [PubMed] [Google Scholar]
- 97.Pier G. B., Grout M., Zaidi T., Meluleni G., Mueschenborn S. S., Banting G., Ratcliff R., Evans M. J., Colledge W. H. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature. 1998;393:79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- 98.Cuthbert A. W., Halstead J., Ratcliff R., Colledge W. H., Evans M. J. The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study. J. Physiol. 1995;482 (Pt 2):449–454. doi: 10.1113/jphysiol.1995.sp020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poolman E. M., Galvani A. P. Evaluating candidate agents of selective pressure for cystic fibrosis. J. R. Soc. Interface. 2007;4:91–98. doi: 10.1098/rsif.2006.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill A. V. The dimensions of animals and their muscular dynamics. Nature. 1949;164:820. doi: 10.1038/164820b0. [DOI] [Google Scholar]
- 101.Harrison J. F., Kaiser A., VandenBrooks J. M. Atmospheric oxygen level and the evolution of insect body size. Proc. Biol. Sci. 2010;277:1937–1946. doi: 10.1098/rspb.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holliday, R. (1995) Understanding Ageing, Cambridge University Press, 10.1017/cbo9780511623233.
- 103.Trindade L. S., Aigaki T., Peixoto A. A., Balduino A., Mânica da Cruz I. B., Heddle J. G. A novel classification system for evolutionary aging theories. Front. Genet. 2013;4:25. doi: 10.3389/fgene.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gordon I. J., Hester A. J., Festa-Bianchet M. Review: The management of wild large herbivores to meet economic, conservation and environmental objectives. J. Appl. Ecol. 2004;41:1021–1031. doi: 10.1111/j.0021-8901.2004.00985.x. [DOI] [Google Scholar]
- 105.Nilsen E. B., Milner-Gulland E. J., Schofield L., Mysterud A., Stenseth N. C., Coulson T. Wolf reintroduction to Scotland: public attitudes and consequences for red deer management. Proc. R. Soc. B Biol. Sci. 2007;274:995–1003. doi: 10.1098/rspb.2006.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCay C. M., Crowell M. F., Maynard L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. doi: 10.1093/jn/10.1.63. [DOI] [PubMed] [Google Scholar]
- 107.Sohal R. S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masoro E. J. Caloric restriction and aging: an update. Exp. Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 109.Shanley D. P., Kirkwood T. B. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 110.Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- 111.Lemaître J.-F., Ronget V., Tidière M., Allainé D., Berger V., Cohas A., Colchero F., Conde D. A., Garratt M., Liker A., Marais G. A. B., Scheuerlein A., Székely T., Gaillard J.-M. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl. Acad. Sci. USA. 2020;117:8546–8553. doi: 10.1073/pnas.1911999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gavrilov L. A., Gavrilova N. S. Evolutionary theories of aging and longevity. Sci. World J. 2002;2:339–356. doi: 10.1100/tsw.2002.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flatt T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Grandison R. C., Piper M. D. W., Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leroi A. M., Chippindale A. K., Rose M. R. Long-term laboratory evolution of a genetic life-history trade-off in drosophila melanogaster. 1. The role of genotype-by-environment interaction. Evolution. 1994;48:1244–1257. doi: 10.1111/j.1558-5646.1994.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 116.Rose M. R. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 117.Zajitschek F., Georgolopoulos G., Vourlou A., Ericsson M., Zajitschek S. R. K., Friberg U., Maklakov A. A. Evolution under dietary restriction decouples survival from fecundity in Drosophila melanogaster females. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:1542–1548. doi: 10.1093/gerona/gly070. [DOI] [PubMed] [Google Scholar]
- 118.Morley J. E. Decreased food intake with aging. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56 Spec No 2:81–88. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- 119.Flatt T., Partridge L. Horizons in the evolution of aging. BMC Biol. 2018;16:93. doi: 10.1186/s12915-018-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cohen A. A., Coste C. F. D., Li X. y., Bourg S., Pavard S. Are trade‐offs really the key drivers of ageing and life span? Funct. Ecol. 2020;34:153–166. doi: 10.1111/1365-2435.13444. [DOI] [Google Scholar]
- 121.Hamilton W. D. The evolution of altruistic behavior. Am. Nat. 1963;97:354–356. doi: 10.1086/497114. [DOI] [Google Scholar]
- 122.Hamilton W. D. The genetical evolution of social behaviour. I. J. Theor. Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 123.Hamilton W. D. The genetical evolution of social behaviour. II. J. Theor. Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 124.Kowald A., Kirkwood T. B. L. Can aging be programmed? A critical literature review. Aging Cell. 2016;15:986–998. doi: 10.1111/acel.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cohen A. A., Kennedy B. K., Anglas U., Bronikowski A. M., Deelen J., Dufour F., Ferbeyre G., Ferrucci L., Franceschi C., Frasca D., Friguet B., Gaudreau P., Gladyshev V. N., Gonos E. S., Gorbunova V., Gut P., Ivanchenko M., Legault V., Lemaître J.-F., Liontis T., et al. Lack of consensus on an aging biology paradigm? A global survey reveals an agreement to disagree, and the need for an interdisciplinary framework. Mech. Ageing Dev. 2020;191:111316. doi: 10.1016/j.mad.2020.111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hampton H. G., Watson B. N. J., Fineran P. C. The arms race between bacteria and their phage foes. Nature. 2020;577:327–336. doi: 10.1038/s41586-019-1894-8. [DOI] [PubMed] [Google Scholar]
- 127.Bramucci A. R., Case R. J. Phaeobacter inhibens induces apoptosis-like programmed cell death in calcifying Emiliania huxleyi. Sci. Rep. 2019;9:5215. doi: 10.1038/s41598-018-36847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deponte M. Programmed cell death in protists. Biochim. Biophys. Acta. 2008;1783:1396–1405. doi: 10.1016/j.bbamcr.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 129.Feng Y., Hsiao Y.-H., Chen H.-L., Chu C., Tang P., Chiu C.-H. Apoptosis-like cell death induced by Salmonella in Acanthamoeba rhysodes. Genomics. 2009;94:132–137. doi: 10.1016/j.ygeno.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Skulachev V. P. Phenoptosis: programmed death of an organism. Biochemistry (Moscow) 1999;64:1418–1426. [PubMed] [Google Scholar]
- 131.Hughes P. W. Between semelparity and iteroparity: empirical evidence for a continuum of modes of parity. Ecol. Evol. 2017;7:8232–8261. doi: 10.1002/ece3.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wodinsky J. Hormonal inhibition of feeding and death in octopus: control by optic gland secretion. Science. 1977;198:948–951. doi: 10.1126/science.198.4320.948. [DOI] [PubMed] [Google Scholar]
- 133.Lopes G. P., Leiner N. O. Semelparity in a population of Gracilinanus agilis (Didelphimorphia: Didelphidae) inhabiting the Brazilian cerrado. Mammal. Biol. 2015;80:1–6. doi: 10.1016/j.mambio.2014.08.004. [DOI] [Google Scholar]
- 134.Fritz R. S., Stamp N. E., Halverson T. G. Iteroparity and semelparity in insects. Am. Nat. 1982;120:264–268. doi: 10.1086/283987. [DOI] [Google Scholar]
- 135.Quinn, T. P. (2011) The Behavior and Ecology of Pacific Salmon and Trout, UBC Press.
- 136.Dickhoff, W. W. (1989) Salmonids and annual fishes: death after sex, in Development, Maturation, and Senescence of Neuroendocrine Systems, pp. 253-266, 10.1016/b978-0-12-629060-8.50017-5.
- 137.Robertson O. H., Wexler B. C. Histological changes in the organs and tissues of migrating and spawning Pacific salmon (genus Oncorhynchus) Endocrinology. 1960;66:222–239. doi: 10.1210/endo-66-2-222. [DOI] [PubMed] [Google Scholar]
- 138.McBride J. R., van Overbeeke A. P. Effects of androgens, Estrogens, and cortisol on the skin, stomach, liver, pancreas, and kidney in gonadectomized adult Sockeye Salmon (Oncorhynchus nerka) J. Fish. Res. Board Can. 1971;28:485–490. doi: 10.1139/f71-068. [DOI] [Google Scholar]
- 139.Cederholm C. J., Jeff Cederholm C., Kunze M. D., Murota T., Sibatani A. Pacific salmon carcasses: essential contributions of nutrients and energy for aquatic and terrestrial ecosystems. Fisheries. 1999;24:6–15. doi: 10.1577/1548-8446(1999)024<0006:psc>2.0.co;2. [DOI] [Google Scholar]
- 140.Galimov E. R., Lohr J. N., Gems D. When and how can death be an adaptation? Biochemistry (Moscow) 2019;84:1433–1437. doi: 10.1134/s0006297919120010. [DOI] [PubMed] [Google Scholar]
- 141.Gorbunova V., Seluanov A., Mao Z., Hine C. Changes in DNA repair during aging. Nucleic Acids Res. 2007;35:7466–7474. doi: 10.1093/nar/gkm756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rubinsztein D. C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 143.Ben-Zvi A., Miller E. A., Morimoto R. I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. USA. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Son H. G., Seo M., Ham S., Hwang W., Lee D., An S. W. A., Artan M., Seo K., Kaletsky R., Arey R. N., Ryu Y., Ha C. M., Kim Y. K., Murphy C. T., Roh T.-Y., Nam H. G., Lee S.-J. V. RNA surveillance via nonsense-mediated mRNA decay is crucial for longevity in daf-2/insulin/IGF-1 mutant C. elegans. Nat. Commun. 2017;8:14749. doi: 10.1038/ncomms14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lipinski M. M., Zheng B., Lu T., Yan Z., Py B. F., Ng A., Xavier R. J., Li C., Yankner B. A., Scherzer C. R., Yuan J. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2010;107:14164–14169. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Collin G., Huna A., Warnier M., Flaman J.-M., Bernard D. Transcriptional repression of DNA repair genes is a hallmark and a cause of cellular senescence. Cell Death Dis. 2018;9:259. doi: 10.1038/s41419-018-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gong H., Pang J., Han Y., Dai Y., Dai D., Cai J., Zhang T.-M. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol. Med. Rep. 2014;10:3296–3302. doi: 10.3892/mmr.2014.2648. [DOI] [PubMed] [Google Scholar]
- 148.Smith J. M. Group selection and kin selection. Nature. 1964;201:1145–1147. doi: 10.1038/2011145a0. [DOI] [Google Scholar]
- 149.Lack, D. (1966) Population Studies of Birds, Oxford University Press, UK.
- 150.Andrewartha, H. G. (1954) The Distribution and Abundance of Animals, Univ Of Chicago Press, USA.
- 151.Markov A. V., Barg M. A., Yakovleva E. Y. Can aging develop as an adaptation to optimize natural selection? (application of computer modeling for searching conditions when the “Fable of hares” can explain the evolution of aging) Biochemistry. 2018;83:1504–1516. doi: 10.1134/S0006297918120088. [DOI] [PubMed] [Google Scholar]
- 152.Finch, C. E. (2010) The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans, Elsevier.
- 153.Anderson R. M., May R. M. Population biology of infectious diseases: Part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 154.Schmid-Hempel, P. (2011) Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics, OUP Oxford.
- 155.Débarre F., Lion S., van Baalen M., Gandon S. Evolution of host life-history traits in a spatially structured host-parasite system. Am. Nat. 2012;179:52–63. doi: 10.1086/663199. [DOI] [PubMed] [Google Scholar]
- 156.Santiago M. L., Range F., Keele B. F., Li Y., Bailes E., Bibollet-Ruche F., Fruteau C., Noë R., Peeters M., Brookfield J. F. Y., Shaw G. M., Sharp P. M., Hahn B. H. Simian immunodeficiency virus infection in free-ranging Sooty Mangabeys (Cercocebus atys atys) from the Taï Forest, Côte d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J. Virol. 2005;79:12515–12527. doi: 10.1128/JVI.79.19.12515-12527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marx P. A., Alcabes P. G., Drucker E. Serial human passage of simian immunodeficiency virus by unsterile injections and the emergence of epidemic human immunodeficiency virus in Africa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:911–920. doi: 10.1098/rstb.2001.0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mayr, E. (1999) Systematics and the Origin of Species, from the Viewpoint of a Zoologist, Harvard University Press.
- 159.Pollock G. B. Population viscosity and kin selection. Am. Nat. 1983;122:817–829. doi: 10.1086/284174. [DOI] [Google Scholar]
- 160.Mitteldorf J., Wilson D. S. Population viscosity and the evolution of altruism. J. Theor. Biol. 2000;204:481–496. doi: 10.1006/jtbi.2000.2007. [DOI] [PubMed] [Google Scholar]
- 161.Pulliam H. R., Ronald Pulliam H. Sources, sinks, and population regulation. Am. Nat. 1988;132:652–661. doi: 10.1086/284880. [DOI] [Google Scholar]
- 162.Pulliam H. R., Ronald Pulliam H., Danielson B. J. Sources, sinks, and habitat selection: a landscape perspective on population dynamics. Am. Nat. 1991;137:S50–S66. doi: 10.1086/285139. [DOI] [Google Scholar]
- 163.Schmid-Hempel, P. (2021) Evolutionary parasitology, 2 Edn., Oxford University Press, London, England.
- 164.Lafferty K. D., Kuris A. M. Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol. 2009;25:564–572. doi: 10.1016/j.pt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 165.Lima N. R. W., Azevedo J. d. S., Silva L. G. d., Dansa-Petretski M. Parasitic castration, growth, and sex steroids in the freshwater bonefish Cyphocharax gilbert (Curimatidae) infested by Riggia paranensis (Cymothoidea) Neotrop. Ichthyol. 2007;5:471–478. doi: 10.1590/s1679-62252007000400006. [DOI] [Google Scholar]
- 166.Sherman K. J., Daling J. R., Weiss N. S. Sexually transmitted diseases and tubal infertility. Sex. Transmitted Dis. 1987;14:12–16. doi: 10.1097/00007435-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 167.Schulz K. F., Cates W., Jr., O’Mara P. R. Pregnancy loss, infant death, and suffering: legacy of syphilis and gonorrhoea in Africa. Genitourin. Med. 1987;63:320–325. doi: 10.1136/sti.63.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zaba B., Gregson S. Measuring the impact of HIV on fertility in Africa. AIDS. 1998;12 Suppl 1:S41–50. [PubMed] [Google Scholar]
- 169.Smith D. G., Guinto R. S. Leprosy and fertility. Hum. Biol. 1978;50:451–460. [PubMed] [Google Scholar]
- 170.Villa E., Vukotic R., Cammà C., Petta S., Di Leo A., Gitto S., Turola E., Karampatou A., Losi L., Bernabucci V., Cenci A., Tagliavini S., Baraldi E., De Maria N., Gelmini R., Bertolini E., Rendina M., Francavilla A. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One. 2012;7:e44624. doi: 10.1371/journal.pone.0044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parikh F. R., Nadkarni S. G., Kamat S. A., Naik N., Soonawala S. B., Parikh R. M. Genital tuberculosis – a major pelvic factor causing infertility in Indian women. Fertil. Steril. 1997;67:497–500. doi: 10.1016/s0015-0282(97)80076-3. [DOI] [PubMed] [Google Scholar]
- 172.Kapranos N., Petrakou E., Anastasiadou C., Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil. Steril. 2003;79 Suppl 3:1566–1570. doi: 10.1016/s0015-0282(03)00370-4. [DOI] [PubMed] [Google Scholar]
- 173.Rankin D. J., Bargum K., Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol. Evol. 2007;22:643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 174.Anderson R. M., May R. M. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 175.Altenberg L. Evolvability suppression to stabilize far-sighted adaptations. Artif. Life. 2005;11:427–443. doi: 10.1162/106454605774270633. [DOI] [PubMed] [Google Scholar]
- 176.Sherman, P. W., Jarvis, J. U. M., and Alexander, R. D. (2017) The Biology of the Naked Mole-Rat, Princeton University Press.
- 177.Sun S., White R. R., Fischer K. E., Zhang Z., Austad S. N., Vijg J. Inducible aging in Hydra oligactis implicates sexual reproduction, loss of stem cells, and genome maintenance as major pathways. Geroscience. 2020;42:1119–1132. doi: 10.1007/s11357-020-00214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lindstedt S. L., Calder W. A. Body size and longevity in birds. Condor. 1976;78:91–94. doi: 10.2307/1366920. [DOI] [Google Scholar]
- 179.Sacher, G. A. (2008) Relation of Lifespan to Brain Weight and Body Weight in Mammals, Ciba Foundation Symposium – The Lifespan of Animals (Colloquia on Ageing, Vol. 5), 115-141, 10.1002/9780470715253.ch9.
- 180.Peters, R. H. (1983) The Ecological Implications of Body Size, Cambridge University Press, 10.1017/cbo9780511608551.
- 181.Read A. F., Harvey P. H. Life history differences among the eutherian radiations. J. Zool. 1989;219:329–353. doi: 10.1111/j.1469-7998.1989.tb02584.x. [DOI] [Google Scholar]
- 182.de Magalhães J. P., Costa J., Church G. M. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V., Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Flurkey K., Papaconstantinou J., Miller R. A., Harrison D. E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kraus C., Pavard S., Promislow D. E. L. The size-life span trade-off decomposed: why large dogs die young. Am. Nat. 2013;181:492–505. doi: 10.1086/669665. [DOI] [PubMed] [Google Scholar]
- 186.Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., Wei M., Madia F., Cheng C. W., Hwang D., Martin-Montalvo A., Saavedra J., Ingles S., de Cabo R., Cohen P., Longo V. D. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Laron Z., Kauli R., Lapkina L., Werner H. IGF-I deficiency, longevity and cancer protection of patients with Laron syndrome. Mutat. Res. Rev. Mutat. Res. 2017;772:123–133. doi: 10.1016/j.mrrev.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 188.Fontana L., Partridge L., Longo V. D. Extending healthy life span – from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Greer K. A., Hughes L. M., Masternak M. M. Connecting serum IGF-1, body size, and age in the domestic dog. Age. 2011;33:475–483. doi: 10.1007/s11357-010-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Laron Z. Lessons from 50 years of study of laron syndrome. Endocr. Pract. 2015;21:1395–1402. doi: 10.4158/EP15939.RA. [DOI] [PubMed] [Google Scholar]
- 191.Rubner, M. (1908) Das Problem der Lebensdauer und seine Beziehungen zu Wachstum und Ernährung, Oldenbourg Wissenschaftsverlag, 10.1515/9783486736380.
- 192.Pearl, R. (1928) The Rate of Living: Being an Account of Some Experimental Studies on the Biology of Life Duration, University of London press Ltd., London.
- 193.McCarter R., Masoro E. J., Yu B. P. Does food restriction retard aging by reducing the metabolic rate? Am. J. Physiol. 1985;248:E488–490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- 194.Beckman K. B., Ames B. N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 195.Munshi-South J., Wilkinson G. S. Bats and birds: exceptional longevity despite high metabolic rates. Ageing Res. Rev. 2010;9:12–19. doi: 10.1016/j.arr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 196.White C. R., Seymour R. S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl. Acad. Sci. USA. 2003;100:4046–4049. doi: 10.1073/pnas.0436428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bennett A. F. Structural and functional determinates of metabolic rate. Am. Zool. 1988;28:699–708. doi: 10.1093/icb/28.2.699. [DOI] [Google Scholar]
- 198.Van Voorhies W. A., Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc. Natl. Acad. Sci. USA. 1999;96:11399–11403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Trout W. E., Kaplan W. D. A relation between longevity, metabolic rate, and activity in shaker mutants of Drosophila melanogaster. Exp. Gerontol. 1970;5:83–92. doi: 10.1016/0531-5565(70)90033-1. [DOI] [PubMed] [Google Scholar]
- 200.Conti B., Sanchez-Alavez M., Winsky-Sommerer R., Morale M. C., Lucero J., Brownell S., Fabre V., Huitron-Resendiz S., Henriksen S., Zorrilla E. P., de Lecea L., Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 201.Flatt, T., and Heyland, A. (2011) Mechanisms of Life History Evolution: The Genetics and Physiology of Life History Traits and Trade-Offs, Oxford University Press.
- 202.Shefferson, R. P. J., Owen, R., and Salguero-Gómez, R. (2017) The Evolution of Senescence in the Tree of Life, Cambridge University Press.
- 203.Fowler K., Partridge L. A cost of mating in female fruitflies. Nature. 1989;338:760–761. doi: 10.1038/338760a0. [DOI] [Google Scholar]
- 204.Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 205.Liu H., Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chapman T., Bangham J., Vinti G., Seifried B., Lung O., Wolfner M. F., Smith H. K., Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Khazaeli A. A., Curtsinger J. W. Pleiotropy and life history evolution in Drosophila melanogaster: uncoupling life span and early fecundity. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:546–553. doi: 10.1093/gerona/gls226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gagnon A. Natural fertility and longevity. Fertil. Ster. 2015;103:1109–1116. doi: 10.1016/j.fertnstert.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 209.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 210.Hercus M. J., Loeschcke V., Rattan S. I. S. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4:149–156. doi: 10.1023/a:1024197806855. [DOI] [PubMed] [Google Scholar]
- 211.Cypser J. R., Johnson T. E. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:B109–114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- 212.Zhang B., Jun H., Wu J., Liu J., Xu X. Z. S. Olfactory perception of food abundance regulates dietary restriction-mediated longevity via a brain-to-gut signal. Nat. Aging. 2021;1:255–268. doi: 10.1038/s43587-021-00039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Alcedo J., Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41:45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 214.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D. W., Fasano A., Miller G. W., Miller A. H., Mantovani A., Weyand C. M., Barzilai N., Goronzy J. J., Rando T. A., Effros R. B., Lucia A., Kleinstreuer N., Slavich G. M. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Libert S., Chao Y., Chu X., Pletcher S. D. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 216.Vonk W. I. M., Rainbolt T. K., Dolan P. T., Webb A. E., Brunet A., Frydman J. Differentiation drives widespread rewiring of the neural stem cell chaperone network. Mol. Cell. 2020;78:329–345.e329. doi: 10.1016/j.molcel.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stearns S. C., Ackermann M., Doebeli M., Kaiser M. Experimental evolution of aging, growth, and reproduction in fruitflies. Proc. Natl. Acad. Sci. USA. 2000;97:3309–3313. doi: 10.1073/pnas.060289597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Healy K., Guillerme T., Finlay S., Kane A., Kelly S. B. A., McClean D., Kelly D. J., Donohue I., Jackson A. L., Cooper N. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. Biol. Sci. 2014;281:20140298. doi: 10.1098/rspb.2014.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ricklefs R. E. Evolutionary theories of aging: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am. Nat. 1998;152:24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- 220.Tidière M., Gaillard J.-M., Berger V., Müller D. W. H., Bingaman Lackey L., Gimenez O., Clauss M., Lemaître J.-F. Comparative analyses of longevity and senescence reveal variable survival benefits of living in zoos across mammals. Sci. Rep. 2016;6:36361. doi: 10.1038/srep36361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Thompson, W. (2013) Sampling Rare or Elusive Species: Concepts, Designs, and Techniques for Estimating Population Parameters, Island Press.
- 222.Klass M., Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- 223.Keller L., Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. doi: 10.1038/40130. [DOI] [Google Scholar]
- 224.Dammann P., Šumbera R., Maßmann C., Scherag A., Burda H. Extended longevity of reproductives appears to be common in Fukomys mole-rats (Rodentia, Bathyergidae) PLoS One. 2011;6:e18757. doi: 10.1371/journal.pone.0018757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schmidt C. M., Jarvis J. U. M., Bennett N. C. The long-lived queen: reproduction and longevity in female eusocial Damaraland mole-rats (Fukomys damarensis) Afr. Zool. 2013;48:193–196. doi: 10.3377/004.048.0116. [DOI] [Google Scholar]
- 226.Zhan S., Merlin C., Boore J. L., Reppert S. M. The monarch butterfly genome yields insights into long-distance migration. Cell. 2011;147:1171–1185. doi: 10.1016/j.cell.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Da Silva J. Plastic senescence in the honey bee and the disposable soma theory. Am. Nat. 2019;194:367–380. doi: 10.1086/704220. [DOI] [PubMed] [Google Scholar]
- 228.Omholt S. W. Epigenetic regulation of aging in honeybee workers. Sci. Aging Knowl. Environ. 2004;2004:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- 229.Winston, M. L. (1991) The Biology of the Honey Bee, Harvard University Press.
- 230.Lundie, A. E. (1925) The Flight Activities of the Honeybee, Washington, D.C. : U.S. Dept. of Agriculture, 10.5962/bhl.title.108871.
- 231.Münch D., Amdam G. V. The curious case of aging plasticity in honey bees. FEBS Lett. 2010;584:2496–2503. doi: 10.1016/j.febslet.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 232.Page R. E., Jr., Peng C. Y. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 2001;36:695–711. doi: 10.1016/s0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 233.Rueppell O., Bachelier C., Fondrk M. K., Page R. E., Jr. Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp. Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Visscher P. K., Dukas R. Survivorship of foraging honey bees. Insectes Sociaux. 1997;44:1–5. doi: 10.1007/s000400050017. [DOI] [Google Scholar]
- 235.Dukas R. Mortality rates of honey bees in the wild. Insectes Sociaux. 2008;55:252–255. doi: 10.1007/s00040-008-0995-4. [DOI] [Google Scholar]
- 236.Kramer B. H., Schaible R. Life span evolution in eusocial workers – a theoretical approach to understanding the effects of extrinsic mortality in a hierarchical system. PLoS One. 2013;8:e61813. doi: 10.1371/journal.pone.0061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Beros S., Lenhart A., Scharf I., Negroni M. A., Menzel F., Foitzik S. Extreme lifespan extension in tapeworm-infected ant workers. R Soc. Open Sci. 2021;8:202118. doi: 10.1098/rsos.202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Iranzo J., Lobkovsky A. E., Wolf Y. I., Koonin E. V. Immunity, suicide or both? Ecological determinants for the combined evolution of anti-pathogen defense systems. BMC Evol. Biol. 2015;15:43. doi: 10.1186/s12862-015-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Refardt D., Bergmiller T., Kümmerli R. Altruism can evolve when relatedness is low: evidence from bacteria committing suicide upon phage infection. Proc. Biol. Sci. 2013;280:20123035. doi: 10.1098/rspb.2012.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Artwohl J., Ball-Kell S., Valyi-Nagy T., Wilson S. P., Lu Y., Park T. J. Extreme susceptibility of African naked mole rats (Heterocephalus glaber) to experimental infection with herpes simplex virus type 1. Comp. Med. 2009;59:83–90. [PMC free article] [PubMed] [Google Scholar]
- 241.Githure J. I., Gardener P. J., Kinoti G. K. Experimental infection of the naked mole-rat, Heterocephalus glaber, with Leishmania donovani. Transact. R. Soc. Tropic. Med. Hygiene. 1988;82:563. doi: 10.1016/0035-9203(88)90507-x. [DOI] [PubMed] [Google Scholar]
- 242.Hill W. C. O., Osman Hill W. C., Porter A., Bloom R. T., Seago J., Southwick M. D. Field and laboratory studies on the naked mole rat, Heterocephalus glaber. Proc. Zool. Soc. Lond. 2009;128:455–514. doi: 10.1111/j.1096-3642.1957.tb00272.x. [DOI] [Google Scholar]
- 243.Ross-Gillespie A., Justin O'Riain M., Keller L. F. Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution. 2007;61:2268–2273. doi: 10.1111/j.1558-5646.2007.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hilton H. G., Rubinstein N. D., Janki P., Ireland A. T., Bernstein N., Fong N. L., Wright K. M., Smith M., Finkle D., Martin-McNulty B., Roy M., Imai D. M., Jojic V., Buffenstein R. Single-cell transcriptomics of the naked mole-rat reveals unexpected features of mammalian immunity. PLoS Biol. 2019;17:e3000528. doi: 10.1371/journal.pbio.3000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Amdam G. V., Aase A. L. T. O., Seehuus S.-C., Kim Fondrk M., Norberg K., Hartfelder K. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 2005;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Galkin F., Zhang B., Dmitriev S. E., Gladyshev V. N. Reversibility of irreversible aging. Ageing Res. Rev. 2019;49:104–114. doi: 10.1016/j.arr.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 247.Lemaitre J.-M. Reversibility of cellular aging by reprogramming through an embryonic-like state: a new paradigm for human cell rejuvenation. Cent. As. J. Glob. Health. 2013;2:88. doi: 10.5195/cajgh.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chang J., Wang Y., Shao L., Laberge R.-M., Demaria M., Campisi J., Janakiraman K., Sharpless N. E., Ding S., Feng W., Luo Y., Wang X., Aykin-Burns N., Krager K., Ponnappan U., Hauer-Jensen M., Meng A., Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Katsimpardi L., Litterman N. K., Schein P. A., Miller C. M., Loffredo F. S., Wojtkiewicz G. R., Chen J. W., Lee R. T., Wagers A. J., Rubin L. L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Conboy I. M., Conboy M. J., Wagers A. J., Girma E. R., Weissman I. L., Rando T. A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 251.Safdar A., Bourgeois J. M., Ogborn D. I., Little J. P., Hettinga B. P., Akhtar M., Thompson J. E., Melov S., Mocellin N. J., Kujoth G. C., Prolla T. A., Tarnopolsky M. A. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. USA. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gary N. E., Marston J. Mating behaviour of drone honey bees with queen models (Apis mellifera L.) Animal Behav. 1971;19:299–304. doi: 10.1016/s0003-3472(71)80010-6. [DOI] [Google Scholar]
- 253.Oakwood M., Bradley A. J., Cockburn A. Semelparity in a large marsupial. Proc. R. Soc. B Biol. Sci. 2001;268:407–411. doi: 10.1098/rspb.2000.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Andrade M. C. B. Sexual selection for male sacrifice in the australian redback spider. Science. 1996;271:70–72. doi: 10.1126/science.271.5245.70. [DOI] [Google Scholar]
- 255.Robertson O. H. prolongation of the life span of kokanee salmon (Oncorhynchus Nerka Kennerlyi) by castration before beginning of gonad development. Proc. Natl. Acad. Sci. USA. 1961;47:609–621. doi: 10.1073/pnas.47.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Stein-Behrens B. A., Sapolsky R. M. Stress, glucocorticoids, and aging. Aging. 1992;4:197–210. doi: 10.1007/BF03324092. [DOI] [PubMed] [Google Scholar]
- 257.McDonald I. R., Lee A. K., Bradley A. J., Than K. A. Endocrine changes in dasyurid marsupials with differing mortality patterns. Gen. Comp. Endocrinol. 1981;44:292–301. doi: 10.1016/0016-6480(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 258.Barry T. P., Unwin M. J., Malison J. A., Quinn T. P. Free and total cortisol levels in semelparous and iteroparous chinook salmon. J. Fish Biol. 2001;59:1673–1676. doi: 10.1111/j.1095-8649.2001.tb00230.x. [DOI] [Google Scholar]
- 259.Strona A. L. S., Levenhagem M., Leiner N. O. Reproductive effort and seasonality associated with male-biased parasitism in Gracilinanus agilis (Didelphimorphia: Didelphidae) infected by Eimeria spp. (Apicomplexa: Eimeriidae) in the Brazilian cerrado. Parasitology. 2015;142:1086–1094. doi: 10.1017/S0031182015000402. [DOI] [PubMed] [Google Scholar]
- 260.Chung H. Y., Cesari M., Anton S., Marzetti E., Giovannini S., Seo A. Y., Carter C., Yu B. P., Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69 Suppl 1:S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 262.Kounatidis I., Chtarbanova S., Cao Y., Hayne M., Jayanth D., Ganetzky B., Ligoxygakis P. NF-κB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Boulias K., Lieberman J., Greer E. L. An epigenetic clock measures accelerated aging in treated HIV infection. Mol. Cell. 2016;62:153–155. doi: 10.1016/j.molcel.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gindin Y., Gaggar A., Lok A. S., Janssen H. L. A., Ferrari C., Subramanian G. M., Jiang Z., Masur H., Emmanuel B., Poonia B., Kottilil S. DNA methylation and immune cell markers demonstrate evidence of accelerated aging in patients with chronic hepatitis B virus or hepatitis C virus, with or without human immunodeficienct virus co-infection. Clin. Infect. Dis. 2021;73:e184–e190. doi: 10.1093/cid/ciaa1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ziuganov V. V. A long-lived parasite extending the host life span: the pearl mussel Margaritifera margaritifera elongates host life by turns out the program of accelerated senescence in salmon Salmo salar. Dokl. Biol. Sci. 2005;403:291–294. doi: 10.1007/s10630-005-0115-9. [DOI] [PubMed] [Google Scholar]
- 266.Finch C. E., Morgan T. E., Longo V. D., de Magalhaes J. P. Cell resilience in species life spans: a link to inflammation? Aging Cell. 2010;9:519–526. doi: 10.1111/j.1474-9726.2010.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.George J. C., Bockstoce J. R. Two historical weapon fragments as an aid to estimating the longevity and movements of bowhead whales. Polar Biol. 2008;31:751–754. doi: 10.1007/s00300-008-0407-2. [DOI] [Google Scholar]
- 268.Nielsen J., Hedeholm R. B., Heinemeier J., Bushnell P. G., Christiansen J. S., Olsen J., Ramsey C. B., Brill R. W., Simon M., Steffensen K. F., Steffensen J. F. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus) Science. 2016;353:702–704. doi: 10.1126/science.aaf1703. [DOI] [PubMed] [Google Scholar]
- 269.Wanamaker A. D., Heinemeier J., Scourse J. D., Richardson C. A., Butler P. G., Eiríksson J., Knudsen K. L. Very long-lived mollusks confirm 17th century AD tephra-based radiocarbon reservoir ages for north Icelandic shelf waters. Radiocarbon. 2008;50:399–412. doi: 10.1017/s0033822200053510. [DOI] [Google Scholar]
- 270.Maher C. R., Lott D. F. A review of ecological determinants of territoriality within vertebrate species. Am. Midl. Nat. 2000;143:1–29. doi: 10.1674/0003-0031(2000)143[0001:aroedo]2.0.co;2. [DOI] [Google Scholar]
- 271.Brown J. H., Maurer B. A. Body size, ecological dominance and Cope’s rule. Nature. 1986;324:248–250. doi: 10.1038/324248a0. [DOI] [Google Scholar]
- 272.Wilkinson G. S., Adams D. M. Recurrent evolution of extreme longevity in bats. Biol. Lett. 2019;15:20180860. doi: 10.1098/rsbl.2018.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Tan T. C. J., Rahman R., Jaber-Hijazi F., Felix D. A., Chen C., Louis E. J., Aboobaker A. Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms. Proc. Natl. Acad. Sci. USA. 2012;109:4209–4214. doi: 10.1073/pnas.1118885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ricklefs R. E., Cadena C. D. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecol. Lett. 2007;10:867–872. doi: 10.1111/j.1461-0248.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 275.Rodrigues M. A., Flatt T. Endocrine uncoupling of the trade-off between reproduction and somatic maintenance in eusocial insects. Curr. Opin. Insect Sci. 2016;16:1–8. doi: 10.1016/j.cois.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 276.Sutherland G. D., Harestad A. S., Price K., Lertzman K. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000;4:16. doi: 10.5751/es-00184-040116. [DOI] [Google Scholar]
- 277.Herman W. S., Tatar M. Juvenile hormone regulation of longevity in the migratory monarch butterfly. Proc. Biol. Sci. 2001;268:2509–2514. doi: 10.1098/rspb.2001.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Quinn, T. P. (2018) The Behavior and Ecology of Pacific Salmon and Trout, 2 ed., University of Washington Press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1, Online Resourse 2. Modeling reproduction, death, and movement of individuals in a viscous population.
Video 2, Online Resourse 3. The basic scenario of stabilizing selection that suppresses the emergence.