PURPOSE
Selpercatinib, a first-in-class, highly selective, and potent CNS-active RET kinase inhibitor, is currently approved for the treatment of patients with RET fusion–positive non–small-cell lung cancer (NSCLC). We provide a registrational data set update in more than double (n = 316) of the original reported population (n = 144) and better characterization of long-term efficacy and safety.
METHODS
Patients were enrolled to LIBRETTO-001, a phase I/II, single-arm, open-label study of selpercatinib in patients with RET-altered cancers. An analysis of patients with RET fusion–positive NSCLC, including 69 treatment-naive and 247 with prior platinum-based chemotherapy, was performed. The primary end point was objective response rate (ORR; RECIST v1.1, independent review committee). Secondary end points included duration of response (DoR), progression-free survival (PFS), overall survival, and safety.
RESULTS
In treatment-naive patients, the ORR was 84% (95% CI, 73 to 92); 6% achieved complete responses (CRs). The median DoR was 20.2 months (95% CI, 13.0 to could not be evaluated); 40% of responses were ongoing at the data cutoff (median follow-up of 20.3 months). The median PFS was 22.0 months; 35% of patients were alive and progression-free at the data cutoff (median follow-up of 21.9 months). In platinum-based chemotherapy pretreated patients, the ORR was 61% (95% CI, 55 to 67); 7% achieved CRs. The median DoR was 28.6 months (95% CI, 20.4 to could not be evaluated); 49% of responses were ongoing (median follow-up of 21.2 months). The median PFS was 24.9 months; 38% of patients were alive and progression-free (median follow-up of 24.7 months). Of 26 patients with measurable baseline CNS metastasis by the independent review committee, the intracranial ORR was 85% (95% CI, 65 to 96); 27% were CRs. In the full safety population (n = 796), the median treatment duration was 36.1 months. The safety profile of selpercatinib was consistent with previous reports.
CONCLUSION
In a large cohort with extended follow-up, selpercatinib continued to demonstrate durable and robust responses, including intracranial activity, in previously treated and treatment-naive patients with RET fusion–positive NSCLC.
INTRODUCTION
RET fusions are identified in 1%-2% of patients with non–small-cell lung cancer (NSCLC).1-3 These fusions result in ligand-independent constitutive activation of the RET pathway and increased oncogenic signaling.4 Both selpercatinib4 and pralsetinib5 are selective RET inhibitors, which have demonstrated promising clinical activity in patients with RET fusion–positive NSCLCs. This underscores the benefit of including RET fusions as part of comprehensive oncogene driver testing in patients with NSCLCs.6,7
CONTEXT
Key Objective
Selpercatinib is a first-in-class, highly selective, and potent CNS-active RET kinase inhibitor. An updated assessment of the efficacy and safety of selpercatinib in patients with RET fusion–positive non–small-cell lung cancer treated in the phase I/II LIBRETTO-001 trial was performed. The data cutoff date was June 15, 2021.
Knowledge Generated
With longer follow-up and additional patients, selpercatinib continued to demonstrate marked efficacy, with a high objective response rate, continued durability of response, and substantial CNS activity, as well as a consistent safety profile.
Relevance
Given the durable efficacy observed in patients with non–small-cell lung cancer, broad-based genomic profiling should be considered to identify patients with RET fusions who may benefit from selpercatinib (Ann Oncol 32:337-350, 2021).
On the basis of compelling and durable responses observed in the largest clinical study in RET-altered cancers, selpercatinib emerged as a new standard of care for patients with RET-altered lung and thyroid cancers.4 The drug was first approved in May 2020 by the US Food and Drug Administration for adult patients with RET fusion–positive NSCLCs and subsequently has gained regulatory approval in multiple geographies. Additional data regarding the drug's activity and safety, with increased patient numbers and longer follow-up, are valuable as more providers treat patients with this first-in-class, highly selective, and potent RET inhibitor.
In LIBRETTO-001, a registrational, phase I/II, single-arm, open-label study, selpercatinib demonstrated durable antitumor activity, including intracranial efficacy.4,8,9 In the initial registrational analysis set (n = 144), high response rates and favorable tolerability were observed in both treatment-naive (n = 39) and platinum-based chemotherapy pretreated (n = 105) patients with RET fusion–positive advanced NSCLC.4 However, since the majority of patients were alive and progression-free at the time of initial approval, the median duration of response (DoR) and progression-free survival (PFS) could not be accurately estimated.
In this article, we provide an updated analysis of the activity of selpercatinib in RET fusion–positive NSCLC. This includes efficacy in a total of 316 patients (172 additional patients). Furthermore, we provide 18 more months of follow-up than that previously published.4
METHODS
Patients
The complete eligibility criteria are detailed in the Protocol (online only) as previously disclosed.4 Eligible patients were age ≥ 18 years or ≥ 12 years, if permitted by regulatory authorities, with measurable disease per RECIST Version 1.1. Local molecular testing in a certified laboratory was performed with next-generation sequencing, fluorescence in situ hybridization, or polymerase chain reaction to determine RET alteration status, and the result was reviewed and confirmed by the sponsor before enrollment. Patients were required to have an Eastern Cooperative Oncology Group Performance Score of 0-2 and adequate organ function. Patients with known brain metastases, either asymptomatic or neurologically stable for ≥ 2 weeks, were eligible. The trial was conducted in accordance with Good Clinical Practice guidelines, in line with principles of the Declaration of Helsinki, and all applicable country and local regulations. Protocol was approved by the institutional review board or independent ethics committee at each investigative site. All patients provided written informed consent.
Trial Design and Treatment
This open-label phase I/II trial was conducted at approximately 85 sites in 16 countries. Selpercatinib was orally administered in a continuous 28-day cycle until disease progression, death, unacceptable toxic effects, or withdrawal of consent. Patients enrolled in the phase I dose escalation portion received 20 mg once daily or 20-240 mg twice a day of selpercatinib. Intrapatient dose escalation was permitted with sponsor approval. All patients in phase II received the recommended phase II dose of 160 mg twice a day. Patients who dose-reduced were permitted to re-escalate once the adverse events (AEs) had been resolved. Patients with progressive disease could continue treatment with selpercatinib per investigator discretion with sponsor approval. The phase II primary end point was objective response rate (ORR) by the independent review committee (IRC) per RECIST version 1.1. The secondary end points included ORR by the investigator, PFS, DoR, overall survival (OS), and safety. All responses required a confirmation of radiologic assessment > 4 weeks after first assessment.
Assessments
Radiologic tumor assessments were performed at baseline, every 8 weeks for 1 year, and then every 12 weeks thereafter. Tumor evaluations were performed using RECIST version 1.1. During phase I, brain imaging was obtained at baseline only if clinically indicated. By contrast, all phase II patients underwent brain imaging at baseline. Intracranial responses were assessed by IRC using RECIST version 1.1. AEs were assessed from first dose of study drug until safety follow-up visit 28 days after last selpercatinib dose. Unresolved serious adverse events (SAEs) were continued to be assessed after the safety follow-up visit. AEs were graded according to Common Terminology Criteria for Adverse Events version 4.03.
Oversight
The trial was designed by both the sponsor and the investigators. All the authors contributed to the writing and/or revisions of the manuscript. A medical writer, paid by the sponsor, provided writing support. All the authors approved the final version for completeness and accuracy of clinical data and analysis and protocol adherence.
Statistical Analysis
For this analysis, the primary efficacy-evaluable analysis populations included all patients with documented RET fusion–positive NSCLC previously treated with platinum-based chemotherapy (n = 247) or no prior therapy (n = 69) who had at least a 6-month follow-up from the first dose. CNS efficacy was assessed in 106 patients with NSCLC treated with selpercatinib who had documented baseline CNS metastases per investigator assessment, regardless of disease measurability or prior therapy. The full safety population (N = 796) was defined as all patients who had received at least one dose of selpercatinib as of the data cutoff date of June 15, 2021. The NSCLC safety population (n = 356) was defined as all patients with RET fusion–positive NSCLC who had received at least one dose of selpercatinib as of the data cutoff date. Database lock was performed on August 6, 2021. CIs for response rates were calculated using the Clopper-Pearson method. DoR, PFS, and OS were estimated using the Kaplan-Meier method. As the time-to-event data remain immature, median follow-up times were provided for each efficacy end point to provide appropriate context. Follow-up times were estimated using the reverse Kaplan-Meier method.10 Cumulative incidence rates were calculated using a competing risk model with CNS/systemic disease progression or death as competing risks. An exploratory ad hoc intrapatient analysis was performed to compare (McNemar's exact test) retrospective physician-reported best overall response (BOR), on the basis of patients' medical records, from last systemic therapy received before enrollment with investigator-assessed BOR on selpercatinib treatment per RECIST version 1.1, assessed prospectively, with each patient serving as their own control.
RESULTS
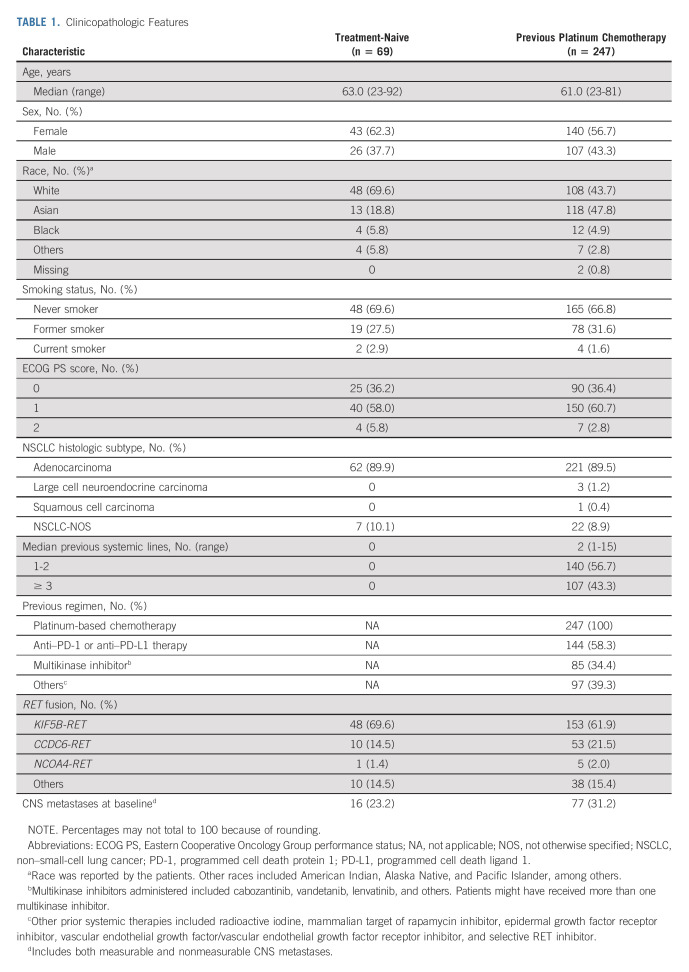
A total of 356 patients with RET fusion–positive advanced NSCLC were enrolled and treated with selpercatinib from May 2017 to May 2020. The baseline demographic characteristics of the primary efficacy-evaluable patients are displayed in Table 1. Additional information is provided in the Data Supplement (online only). Patients previously treated with platinum-based chemotherapy received a median of 2 previous lines of treatment (range, 1-15; ≥ 3: 42.3% of patients); 58.3% had received prior anti–programmed cell death protein 1 or anti–programmed cell death ligand 1 therapies, and 34.4% had prior multikinase inhibitors. With the exception of prior therapy, baseline characteristics were similar across patients who were previously treated or treatment-naive. The majority of RET fusions were identified with next-generation sequencing. The most common fusion partners identified included KIF5B and CCDC6 (Table 1).
TABLE 1.
Clinicopathologic Features
Treatment-Naive Patients
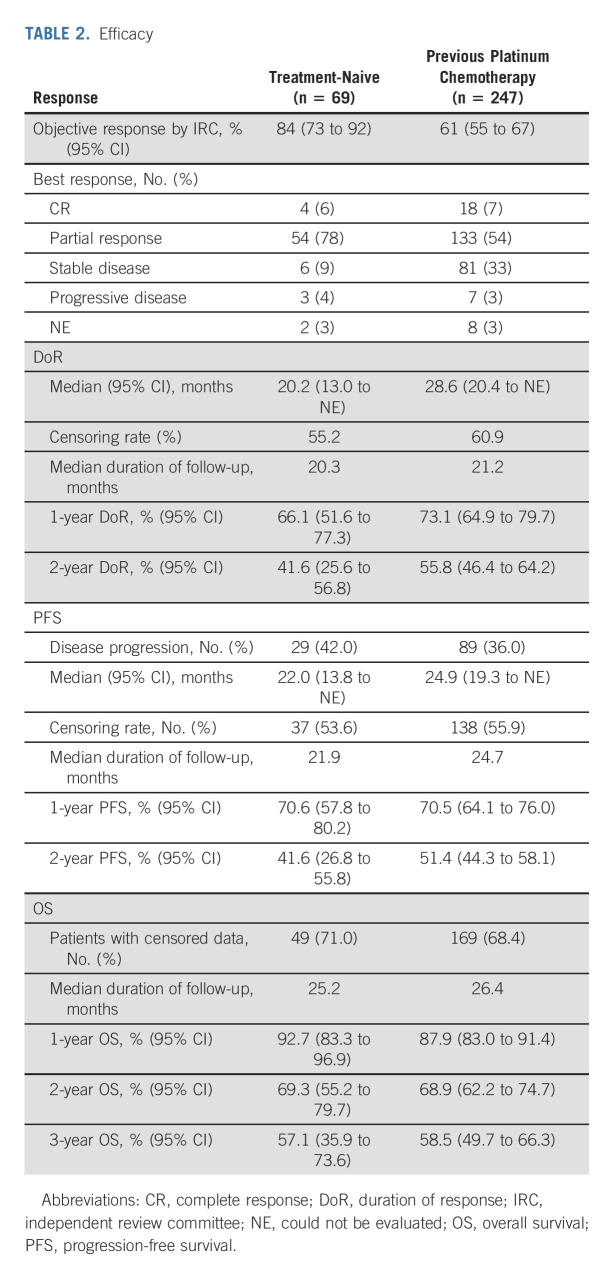
A total of 69 treatment-naive patients were analyzed. The ORR by IRC was 84% (95% CI, 73 to 92); 6% of patients achieved a complete response (CR; Table 2; Fig 1A). At a median follow-up of 20.3 months for 58 responders, the median DoR by IRC was 20.2 months (95% CI, 13.0 to could not be evaluated [NE]), with 40% of responses ongoing (Table 2; Fig 2A). The median time to response was 1.8 (range, 0.7-10.8) months, with the longest response ongoing at 39.3 months. The median PFS was 22.0 months (95% CI, 13.8 to NE), with 35% of patients alive and progression-free at a median follow-up of 21.9 months (Fig 2C). The estimated proportion of patients who were alive and progression-free at 1 and 2 years was 70.6% (95% CI, 57.8 to 80.2) and 41.6% (95% CI, 26.8 to 55.8; Table 2), respectively. At a median follow-up of 25.2 months, the median OS was not estimable (71% censoring rate). The estimated proportion of patients alive at 2 years was 69% (95% CI, 55 to 80; Data Supplement). At the time of data analysis, 46% of patients remained on selpercatinib treatment including 7% who remained on treatment beyond progression. Overall, 32% of patients received selpercatinib beyond progression on the basis of continuous clinical benefit per the investigator with sponsor approval.
TABLE 2.
Efficacy
FIG 1.
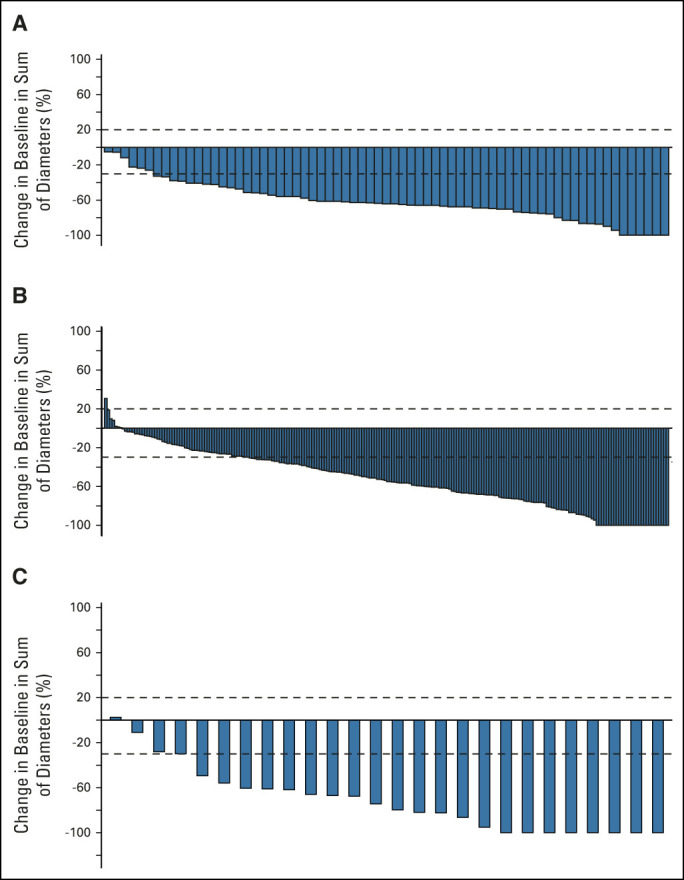
Response to selpercatinib. The waterfall plots of maximum change in tumor size are shown in (A) for the 69 patients who were treatment-naive and (B) for 226 with prior platinum chemotherapy and (C) the change in intracranial tumor size for the 26 patients with measurable CNS disease at baseline. Vertical bars represent the best percent change from baseline in the sum of diameters for all target lesions. Progressive disease (+20%) and partial response (–30%) are indicated with the dashed lines.
FIG 2.
Long-term outcomes with selpercatinib. Kaplan-Meier (KM) plots depict PFS for patients who (A) were treatment-naive or (B) had previous platinum chemotherapy. KM plots depict DoR for patients who (C) were treatment-naive or (D) had previous platinum chemotherapy. Median PFS is displayed in the Table inset. Tick marks indicate censored data. DoR, duration of response; IQR, interquartile range; PFS, progression-free survival.
Chemotherapy Pretreated Patients
A total of 247 patients previously received platinum-based chemotherapy. The ORR by IRC was 61% (95% CI, 55 to 67); a CR was achieved in 7% of patients (Table 2). At a median follow-up of 21.2 months, the median DoR by IRC was 28.6 months (95% CI, 20.4 to NE), with 49% of responses ongoing (Table 2 and Fig 2B). The median time to response was 1.9 (range, 0.7-21.9) months, with the longest response ongoing at 43.3 months.
The median PFS was 24.9 months (95% CI, 19.3 to NE), with 38% of patients alive and progression-free at a median follow-up of 24.7 months (Table 2). The estimated proportion of patients who were alive and progression-free at 1 and 2 years was 70.5% (95% CI, 64.1 to 76.0) and 51.4% (95% CI, 44.3 to 58.1; Fig 2D), respectively. At a median follow-up of 26.4 months, the median OS was not estimable (68% censoring rate). The estimated proportion of patients alive at 2 years was 69% (95% CI, 62 to 75; Data Supplement). The median duration of treatment was 24.9 months (95% CI, 20.5 to 32.2). At the time of data analysis, 47% of patients remained on selpercatinib treatment including 11% who remained on treatment beyond progression. Overall, 35% of patients received selpercatinib beyond progression with sponsor approval. Efficacy results were consistent with those observed in the registration analysis set (Data Supplement) and previous data cutoffs, December 2019 and March 2020 (Data Supplement).
An exploratory ad hoc analysis was performed in the patients who were previously treated to compare physician-reported BOR from last systemic therapy (received before enrollment) with selpercatinib BOR (RECIST version 1.1 per investigator). This analysis allowed each patient to serve as their own control (Data Supplement). Overall, responses were observed in 64% of patients with selpercatinib compared with a response rate of 15% to the last prior therapy received before enrollment (P < .0001, McNemar's exact test). The percentage of patients who responded to selpercatinib by response to prior therapy is shown in the Data Supplement. A similar analysis in a subgroup of patients who received selpercatinib in the second-line setting was conducted. This showed a response rate of 73% with second-line selpercatinib treatment compared with 18% with first-line platinum-based chemotherapy that these patients received before enrollment. Furthermore, improvements in ORR were observed regardless of the type of prior therapy received (Data Supplement).
Patients With CNS Metastases
In the 106 patients with brain metastases at baseline, the median intracranial PFS was 19.4 months (95% CI, 13.8 to NE) at a median follow-up of 22.1 months. In the subset of patients with measurable CNS metastasis at baseline (n = 26), the intracranial ORR by IRC was 85% (95% CI, 65 to 96), including 27% intracranial CRs (Data Supplement and Fig 1C). None of these 26 patients had primary intracranial progression as their best response. Objective responses were observed regardless of whether patients had received prior systemic therapy and/or radiotherapy. In the 22 responders with measurable CNS metastases, the median duration of CNS response was 9.4 months (95% CI, 7.4 to 15.3). In the 178 phase II patients with baseline confirmation that no CNS metastasis was present, the estimated probability of observing intracranial progression at 2 years was 0.7% (Data Supplement).
Safety
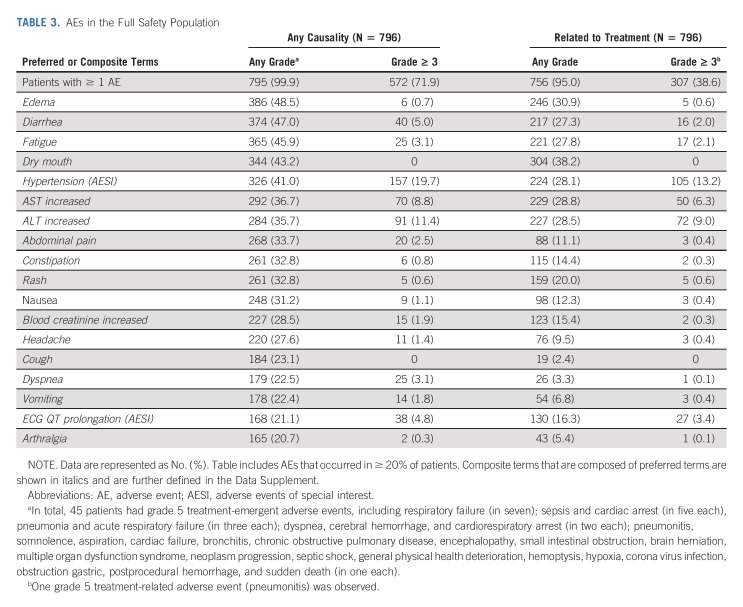
In the full safety population (n = 796), selpercatinib's AE profile was consistent with previous reports. With longer follow-up and larger patient number, this updated full safety population reflects a near doubling of total exposure time from data cutoff used in the previous report (16,098 months versus 8,692 months). Importantly, the profile observed in patients with NSCLC is consistent with that of the full safety patient population (Data Supplement).
Treatment-emergent AEs, regardless of causality, in addition to AEs that were deemed related to selpercatinib per the investigator, are shown in Table 3. The most common grade ≥ 3 treatment-emergent AEs were hypertension (19.7%), ALT increased (11.4%), AST increased (8.8%), diarrhea (5.0%), and electrocardiogram QT prolonged (4.8%). The most common grade ≥ 3 treatment-related AEs included hypertension (13.2%) and AST/ALT elevations (6.3%/9.0%).
TABLE 3.
AEs in the Full Safety Population
Treatment-emergent SAEs occurred in 44% of patients, including 11% related to selpercatinib. Pneumonia (4%) was the most common SAE, and drug hypersensitivity (1%) was the most common treatment-related SAE. There was one fatal AE that was considered related to selpercatinib per the investigator in a patient with RET-mutant medullary thyroid cancer who died because of acute respiratory failure. Dose reductions occurred in 41% of patients. In total, 64 of 796 patients (8%) discontinued treatment because of AEs, with 25 (3%) considered by the investigator to be related to selpercatinib.
DISCUSSION
Selpercatinib is currently approved for the treatment of patients with RET fusion–positive NSCLC in multiple countries. This update to the regulatory data set provided more than double the previously reported number of patients with an additional follow-up of 18 months.4 Several observations have emerged.
First, with increased patient numbers and longer follow-up, ORRs remained comparable with prior data cutoffs. The ORR was 84% (previously 85%4) for treatment-naive patients and 61% (previously 57%4) for platinum pretreated patients, many of whom received other systemic therapies including immunotherapy and multikinase inhibitor therapy. This substantial antitumor activity is further underscored in this article by our exploratory ad hoc analysis of response to systemic therapy received immediately before selpercatinib. In this intrapatient analysis, response to selpercatinib was significantly higher (P < .0001) than that to prior therapy including comparisons with response rates from prior treatment with chemotherapy or multikinase inhibitor therapy. Response rates were also higher with selpercatinib compared with immunotherapy or chemoimmunotherapy, consistent with previous reports, showing poor outcomes with immunomodulatory therapy in RET fusion–positive NSCLCs.11-13 Thus, as is supported by current guidelines,14 the administration of selpercatinib is recommended in patients with advanced NSCLC upon identification of a RET fusion, either in treatment-naive or systemic therapy pretreated patients.
Second, this article highlights the durability of selpercatinib benefit. In treatment-naive patients, our update demonstrates that the median DoR and median PFS are 20.2 months and 22.0 months, respectively (previously not estimable for both4). In platinum pretreated patients, the median DoR and median PFS were 28.6 months (previously 17.5 months4) and 24.9 months (previously 18.4 months4), respectively. Note that additional follow-up will be needed to characterize fully the durability of benefit for treatment-naive patients, as these medians remain immature. Regardless, these outcomes compare favorably with the durability of platinum-based therapy with or without the addition of checkpoint inhibitors (median PFS 7-9 months)15-17 or other standard-of-care therapies,18,19 recognizing the limits of cross-trial comparisons. We await the readout of an ongoing randomized trial of selpercatinib versus chemotherapy in RET fusion–positive NSCLCs (LIBRETTO-431, ClinicalTrials.gov identifier: NCT04194944).20
Third, compelling treatment outcomes were attained in both treatment-naive and previously treated patients with pre-existing measurable CNS metastases. A previous report highlighted how the lifetime prevalence of brain metastases in advanced RET fusion–positive lung cancers was 46%.21 In patients with measurable CNS disease, the intracranial ORR with selpercatinib was 85% and the median duration of intracranial response was 9.4 months. Furthermore, in the 106 patients with brain metastases at baseline, the median intracranial PFS was 19.4 months (95% CI, 13.8 to NE) at a median follow-up of 22.1 months. A response to selpercatinib in leptomeningeal metastases has also been reported in a patient with RET fusion–positive NSCLC.22 In addition to the activity observed in patients with brain metastases at baseline, in the 178 phase II patients with no CNS involvement at baseline, the estimated probability of observing intracranial progression at 2 years was 0.7%, suggesting that selpercatinib may also prevent the acquisition of metastases in the brain. As a brain-penetrant tyrosine kinase inhibitor,23 selpercatinib's capacity to reduce CNS tumor burden, as a poor prognostic factor and significant source of clinical morbidity, represents a significant advantage over many standard chemotherapy approaches.24
Finally, extended monitoring for safety analysis demonstrated consistency in the AE profile compared with previous data cutoffs. This profile was also similar between the full safety population (all cancers treated with selpercatinib) and the NSCLC safety population. Providers should carefully monitor notable AEs such as liver enzyme elevation, hypertension, QTc prolongation, and hypersensitivity. The risk of hypersensitivity has previously been observed with increased frequency in patients with prior immunotherapy,25 but has been manageable with the recommended guidance and supportive care.26 The median duration of treatment exceeded 3 years (36.1 months [95% CI, 30.9 to NE]) across the full safety population of 796 patients, the majority of whom were pretreated.
In conclusion, selpercatinib continues to demonstrate marked efficacy and a consistent safety profile in this global, multicenter data set with longer follow-up and additional patients. Data will continue to mature and be augmented by the ongoing phase III LIBRETTO-431 trial, which will assess the PFS of selpercatinib compared with pemetrexed-inclusive platinum-based chemotherapy and investigator's choice of pembrolizumab in treatment-naive patients with RET fusion–positive advanced or metastatic lung cancers (ClinicalTrials.gov identifier: NCT04194944).20,27 LIBRETTO-431 is designed to explore the ability of selpercatinib to not only treat existing CNS metastases but also to prevent or delay the occurrence of new CNS metastases.
The LIBRETTO-001 study (ClinicalTrials.gov identifier: NCT03157128) continues to enroll patients with RET-altered cancers. Selpercatinib has demonstrated potent and durable antitumor activity in RET-mutant cancers with medullary thyroid cancer, RET fusion–positive thyroid cancers,28 and a variety of cancer types other than lung or thyroid cancer (tissue-agnostic analyses).27 Given the durable efficacy observed in patients with lung cancer, broad-based genomic profiling should be considered across other cancer types to identify patients whose tumors harbor activating RET alterations.29
ACKNOWLEDGMENT
We thank the patients and their families and caregivers, as well as the investigators and their personnel for their participation in the study. We thank David Hyman and Boris Lin for their insights, guidance, and critical revisions of the manuscript files and Pavan ML and Rafael Heard for their data visualization assistance. Kristi Gruver, employee of Eli Lilly and Company, provided critical analysis, medical writing, and editorial assistance.
Alexander Drilon
Stock and Other Ownership Interests: Treeline Bio
Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, Med Learning, Imedex, Answers in CME, Clinical Care Options, EPG Health, JNCC/Harborside, Liberum, Remedica Ltd
Consulting or Advisory Role: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, ArcherDX, Monopteros, Novartis, EMD Serono, Medendi, Repare RX, Nuvalent, Merus, Chugai Pharmaceutical, Remedica Ltd, mBrace, AXIS, EPG Health, Harborside Nexus, Liberum, RV More, Ology, Amgen, TouchIME, Janssen, Entos, Treeline Bio, Prelude, Applied Pharmaceutical Science, Inc, AiCME, i3 Health, MonteRosa
Research Funding: Pfizer (Inst), Exelixis (Inst), GlaxoSmithKlein (Inst), Teva (Inst), Taiho (Inst), PharmaMar (Inst)
Patents, Royalties, Other Intellectual Property: Selpercatinib-Osimertinib (filed/pending), Wolters Kluwer, Merck, Puma, Merus, Boehringer Ingelheim
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharm, QED Therapeutics
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), AbbVie (Inst), MultiVir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Amgen (Inst), Turning Point Therapeutics (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Oliver Gautschi
Consulting or Advisory Role: Amgen (Inst), Lilly (Inst)
Other Relationship: Bayer, Pfizer, Roche, Merck, Lilly
Pascale Tomasini
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb Foundation, Takeda, Amgen, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb/Pfizer, AstraZeneca, Takeda
Filippo de Braud
Honoraria: Roche, Pfizer, BMS, Merck, MSD, Servier, Sanofi, Amgen Astellas BioPharma, Incyte
Consulting or Advisory Role: Roche, Incyte, EMD Serono, Bristol Myers Squibb, Nerviano Medical Sciences, Sanofi, Novartis Italy, NMS Medical Science, Menarini, AstraZeneca, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Merck Serono (Inst), Pfizer (Inst), Servier, Philogen (Inst), Loxo (Inst), Tesaro (Inst), Nerviano Medical Sciences (Inst), Kymab (Inst), Bristol Myers Squibb/Medarex, Merck KGaA, Ignyta, MedImmune, Exelixis, Bayer Health, Daiichi Sankyo Europe GmbH, Incyte, Basilea Pharmaceutical, Janssen Oncology
Benjamin J. Solomon
Honoraria: Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche/Genentech, Pfizer, Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pfizer (Inst), Roche/Genentech, Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Novartis (Inst), Janssen
Research Funding: Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Daniel Shao-Weng Tan
Honoraria: Bristol Myers Squibb, Takeda, Novartis, Roche, Pfizer
Consulting or Advisory Role: Novartis, Merck, Loxo, AstraZeneca, Roche, Pfizer, C4 Therapeutics
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Roche
Jürgen Wolf
Honoraria: AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, MSD, Novartis, Roche, Amgen, Bayer, Blueprint Medicines, Chugai Pharma Europe, Daiichi Sankyo Europe GmbH, Ignyta, Janssen, Lilly, Loxo, Loxo/Lilly, Pfizer, Seattle Genetics, Takeda
Consulting or Advisory Role: AbbVie, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai Pharma, Ignyta, Lilly, MSD Oncology, Novartis, Pfizer, Roche, Janssen, Loxo/Lilly, Blueprint Medicines, Amgen, Takeda, Bayer, Daiichi Sankyo Europe GmbH, Seattle Genetics
Research Funding: Bristol Myers Squibb, Novartis, Pfizer, Janssen
Keunchil Park
Consulting or Advisory Role: AstraZeneca, Lilly, Ono Pharmaceutical, Bristol Myers Squibb, MSD, Merck KGaA, AbbVie, Daiichi Sankyo, Boehringer Ingelheim, JNJ, IMBdx, Geninus
Speakers' Bureau: Boehringer Ingelheim
Research Funding: AstraZeneca, MSD Oncology
Koichi Goto
Honoraria: Guardant Health, Janssen, Daiichi Sankyo Co Ltd, Amgen, Eisai, Bristol Myers Squibb K.K., AstraZeneca Japan, Chugai Pharma, Ono Pharmaceutical, Novartis, Lilly Japan, Boehringer Ingelheim, Takeda, Otsuka, Amoy Diagnostics, Bayer, Mertck
Consulting or Advisory Role: Takeda, Bayer, Lilly Japan, Amgen, Medpace, Janssen
Research Funding: Medical & Biological Laboratories Co Ltd, Kyowa Kirin Co Ltd, Kissei Pharmaceutical, Merck, Merus, Spectrum Pharmaceuticals, Shanghai HaiHe Pharmaceutical, MSD K.K., AstraZeneca Japan, Taiho Pharmaceutical, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Sumitomo Dainippon Pharma Co Ltd, Takeda, Eisai, Lilly Japan, Pfizer, Bristol-Myers Squibb K.K., Ignyta, Janssen, Loxo, Sysmex, Amgen, Thermo Fisher Scientific, Daiichi Sankyo Co Ltd, NEC Corporation, Turning Point Therapeutics
Victoria Soldatenkova
Employment: Lilly
Leadership: Infinity Pharmaceuticals (I)
Stock and Other Ownership Interests: Lilly
Sylwia Szymczak
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Scott S. Barker
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Tarun Puri
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Aimee Bence Lin
Employment: Lilly (I)
Stock and Other Ownership Interests: Lilly (I)
Patents, Royalties, Other Intellectual Property: Co-inventor on a patent application related to selpercatinib activity
Herbert Loong
Consulting or Advisory Role: GlaxoSmithKline, Roche/Genentech, Novartis, Boehringer Ingelheim, Celgene, Lilly, Illumina, Guardant Health, Eisai, Takeda
Speakers' Bureau: Ignyta, Novartis, Guardant Health, Bayer
Research Funding: MSD Oncology (Inst)
Travel, Accommodations, Expenses: Roche, MSD Oncology, Bayer, Pfizer
Benjamin Besse
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Lilly (Inst), Onxeo (Inst), Inivata (Inst), AbbVie (Inst), Amgen (Inst), Blueprint Medicines (Inst), Celgene (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Takeda (Inst), Cristal Therapeutics (Inst), Daiichi Sankyo (Inst), Janssen Oncology (Inst), OSE Immunotherapeutics (Inst), BeiGene (Inst), Boehringer Ingelheim (Inst), Roche/Genentech (Inst), Tolero Pharmaceuticals (Inst), 4D Pharma (Inst), Aptitude Health (Inst), Cergentis (Inst), Chugai Pharma (Inst), Genzyme (Inst), Ipsen (Inst), Turning Point Therapeutics (Inst), Eisai (Inst)
No other potential conflicts of interest were reported.
See accompanying article on page 410
PRIOR PRESENTATION
Presented at the ESMO European Lung Cancer Conference, virtual, March 30-April 2, 2022.
SUPPORT
Supported by Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company. A.D. was supported in part by funding from the National Cancer Institute of the National Institutes of Health: 1R01CA251591-01A1 and P30 CA008748. Partial support was likewise provided by LUNGevity.
CLINICAL TRIAL INFORMATION
NCT03157128 (LIBRETTO-001)
A.D. and V.S. contributed equally as cofirst authors to this work.
DATA SHARING STATEMENT
Eli Lilly and Company provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic, genomic, or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set and will be set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
AUTHOR CONTRIBUTIONS
Conception and design: Vivek Subbiah, Jürgen Wolf, Keunchil Park, Victoria Soldatenkova
Administrative support: Vivek Subbiah, Filippo de Braud
Provision of study materials or patients: Vivek Subbiah, Oliver Gautschi, Pascale Tomasini, Filippo de Braud, Benjamin J. Solomon, Daniel Shao-Weng Tan, Jürgen Wolf, Keunchil Park, Koichi Goto, Herbert Loong
Collection and assembly of data: Alexander Drilon, Vivek Subbiah, Oliver Gautschi, Pascale Tomasini, Filippo de Braud, Benjamin J. Solomon, Daniel Shao-Weng Tan, Guzmán Alonso, Jürgen Wolf, Keunchil Park, Koichi Goto, Herbert Loong, Benjamin Besse
Data analysis and interpretation: Vivek Subbiah, Oliver Gautschi, Pascale Tomasini, Filippo de Braud, Benjamin J. Solomon, Daniel Shao-Weng Tan, Guzmán Alonso, Jürgen Wolf, Keunchil Park, Koichi Goto, Victoria Soldatenkova, Sylwia Szymczak, Scott S. Barker, Tarun Puri, Aimee Bence Lin, Benjamin Besse
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Selpercatinib in Patients With RET Fusion–Positive Non–Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alexander Drilon
Stock and Other Ownership Interests: Treeline Bio
Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, Med Learning, Imedex, Answers in CME, Clinical Care Options, EPG Health, JNCC/Harborside, Liberum, Remedica Ltd
Consulting or Advisory Role: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, ArcherDX, Monopteros, Novartis, EMD Serono, Medendi, Repare RX, Nuvalent, Merus, Chugai Pharmaceutical, Remedica Ltd, mBrace, AXIS, EPG Health, Harborside Nexus, Liberum, RV More, Ology, Amgen, TouchIME, Janssen, Entos, Treeline Bio, Prelude, Applied Pharmaceutical Science, Inc, AiCME, i3 Health, MonteRosa
Research Funding: Pfizer (Inst), Exelixis (Inst), GlaxoSmithKlein (Inst), Teva (Inst), Taiho (Inst), PharmaMar (Inst)
Patents, Royalties, Other Intellectual Property: Selpercatinib-Osimertinib (filed/pending), Wolters Kluwer, Merck, Puma, Merus, Boehringer Ingelheim
Vivek Subbiah
Consulting or Advisory Role: MedImmune, Helsinn Therapeutics, Loxo, R-Pharm, QED Therapeutics
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), AbbVie (Inst), MultiVir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Amgen (Inst), Turning Point Therapeutics (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer, Novartis, Helsinn Therapeutics
Other Relationship: Medscape
Oliver Gautschi
Consulting or Advisory Role: Amgen (Inst), Lilly (Inst)
Other Relationship: Bayer, Pfizer, Roche, Merck, Lilly
Pascale Tomasini
Consulting or Advisory Role: AstraZeneca, Roche, Bristol Myers Squibb Foundation, Takeda, Amgen, Janssen
Travel, Accommodations, Expenses: Bristol Myers Squibb/Pfizer, AstraZeneca, Takeda
Filippo de Braud
Honoraria: Roche, Pfizer, BMS, Merck, MSD, Servier, Sanofi, Amgen Astellas BioPharma, Incyte
Consulting or Advisory Role: Roche, Incyte, EMD Serono, Bristol Myers Squibb, Nerviano Medical Sciences, Sanofi, Novartis Italy, NMS Medical Science, Menarini, AstraZeneca, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Merck Serono (Inst), Pfizer (Inst), Servier, Philogen (Inst), Loxo (Inst), Tesaro (Inst), Nerviano Medical Sciences (Inst), Kymab (Inst), Bristol Myers Squibb/Medarex, Merck KGaA, Ignyta, MedImmune, Exelixis, Bayer Health, Daiichi Sankyo Europe GmbH, Incyte, Basilea Pharmaceutical, Janssen Oncology
Benjamin J. Solomon
Honoraria: Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Roche/Genentech, Pfizer, Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Pfizer (Inst), Roche/Genentech, Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Novartis (Inst), Janssen
Research Funding: Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Daniel Shao-Weng Tan
Honoraria: Bristol Myers Squibb, Takeda, Novartis, Roche, Pfizer
Consulting or Advisory Role: Novartis, Merck, Loxo, AstraZeneca, Roche, Pfizer, C4 Therapeutics
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, Boehringer Ingelheim, Roche
Jürgen Wolf
Honoraria: AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, MSD, Novartis, Roche, Amgen, Bayer, Blueprint Medicines, Chugai Pharma Europe, Daiichi Sankyo Europe GmbH, Ignyta, Janssen, Lilly, Loxo, Loxo/Lilly, Pfizer, Seattle Genetics, Takeda
Consulting or Advisory Role: AbbVie, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai Pharma, Ignyta, Lilly, MSD Oncology, Novartis, Pfizer, Roche, Janssen, Loxo/Lilly, Blueprint Medicines, Amgen, Takeda, Bayer, Daiichi Sankyo Europe GmbH, Seattle Genetics
Research Funding: Bristol Myers Squibb, Novartis, Pfizer, Janssen
Keunchil Park
Consulting or Advisory Role: AstraZeneca, Lilly, Ono Pharmaceutical, Bristol Myers Squibb, MSD, Merck KGaA, AbbVie, Daiichi Sankyo, Boehringer Ingelheim, JNJ, IMBdx, Geninus
Speakers' Bureau: Boehringer Ingelheim
Research Funding: AstraZeneca, MSD Oncology
Koichi Goto
Honoraria: Guardant Health, Janssen, Daiichi Sankyo Co Ltd, Amgen, Eisai, Bristol Myers Squibb K.K., AstraZeneca Japan, Chugai Pharma, Ono Pharmaceutical, Novartis, Lilly Japan, Boehringer Ingelheim, Takeda, Otsuka, Amoy Diagnostics, Bayer, Mertck
Consulting or Advisory Role: Takeda, Bayer, Lilly Japan, Amgen, Medpace, Janssen
Research Funding: Medical & Biological Laboratories Co Ltd, Kyowa Kirin Co Ltd, Kissei Pharmaceutical, Merck, Merus, Spectrum Pharmaceuticals, Shanghai HaiHe Pharmaceutical, MSD K.K., AstraZeneca Japan, Taiho Pharmaceutical, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Sumitomo Dainippon Pharma Co Ltd, Takeda, Eisai, Lilly Japan, Pfizer, Bristol-Myers Squibb K.K., Ignyta, Janssen, Loxo, Sysmex, Amgen, Thermo Fisher Scientific, Daiichi Sankyo Co Ltd, NEC Corporation, Turning Point Therapeutics
Victoria Soldatenkova
Employment: Lilly
Leadership: Infinity Pharmaceuticals (I)
Stock and Other Ownership Interests: Lilly
Sylwia Szymczak
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Scott S. Barker
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Tarun Puri
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Aimee Bence Lin
Employment: Lilly (I)
Stock and Other Ownership Interests: Lilly (I)
Patents, Royalties, Other Intellectual Property: Co-inventor on a patent application related to selpercatinib activity
Herbert Loong
Consulting or Advisory Role: GlaxoSmithKline, Roche/Genentech, Novartis, Boehringer Ingelheim, Celgene, Lilly, Illumina, Guardant Health, Eisai, Takeda
Speakers' Bureau: Ignyta, Novartis, Guardant Health, Bayer
Research Funding: MSD Oncology (Inst)
Travel, Accommodations, Expenses: Roche, MSD Oncology, Bayer, Pfizer
Benjamin Besse
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Lilly (Inst), Onxeo (Inst), Inivata (Inst), AbbVie (Inst), Amgen (Inst), Blueprint Medicines (Inst), Celgene (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Takeda (Inst), Cristal Therapeutics (Inst), Daiichi Sankyo (Inst), Janssen Oncology (Inst), OSE Immunotherapeutics (Inst), BeiGene (Inst), Boehringer Ingelheim (Inst), Roche/Genentech (Inst), Tolero Pharmaceuticals (Inst), 4D Pharma (Inst), Aptitude Health (Inst), Cergentis (Inst), Chugai Pharma (Inst), Genzyme (Inst), Ipsen (Inst), Turning Point Therapeutics (Inst), Eisai (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lipson D, Capelletti M, Yelensky R, et al. : Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 18:382-384, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuta K, Kohno T, Yoshida A, et al. : RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br J Cancer 110:1571-1578, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A, Hu ZI, Lai GGY, et al. : Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15:151-167, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drilon A, Oxnard GR, Tan DSW, et al. : Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med 383:813-824, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gainor JF, Curigliano G, Kim DW, et al. : Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol 22:959-969, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Belli C, Anand S, Gainor JF, et al. : Progresses toward precision medicine in RET-altered solid tumors. Clin Cancer Res 26:6102-6111, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Subbiah V, Yang D, Velcheti V, et al. : State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol 38:1209-1221, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subbiah V, Gainor JF, Oxnard GR, et al. : Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res 27:4160-4167, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besse B, Drilon AE, Solomon BJ, et al. : Updated overall efficacy and safety of selpercatinib in patients (pts) with RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 39, 2021. (abstr 9065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343-346, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Mazieres J, Drilon A, Lusque A, et al. : Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann Oncol 30:1321-1328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Offin M, Guo R, Wu SL, et al. : Immunophenotype and response to immunotherapy of RET-rearranged lung cancers. JCO Precis Oncol 10.1200/PO.18.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde A, Andreev-Drakhlin AY, Roszik J, et al. : Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open 5:e000799, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network : Non-small Cell Lung Cancer, Version 1.2022. www.nccn.org/patients [Google Scholar]
- 15.Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. : Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 38:1505-1517, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Abreu D, Powell SF, Hochmair MJ, et al. : Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann Oncol 32:881-895, 2021 [DOI] [PubMed] [Google Scholar]
- 17.West H, McCleod M, Hussein M, et al. : Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924-937, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Garon EB, Ciuleanu TE, Arrieta O, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 384:665-673, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Hanna N, Shepherd FA, Fossella FV, et al. : Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589-1597, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Solomon BJ, Zhou CC, Drilon A, et al. : Phase III study of selpercatinib versus chemotherapy +/- pembrolizumab in untreated RET positive non-small-cell lung cancer. Future Oncol 17:763-773, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Drilon A, Lin JJ, Filleron T, et al. : Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 13:1595-1601, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo R, Schreyer M, Chang JC, et al. : Response to selective RET inhibition with LOXO-292 in a patient with RET fusion-positive lung cancer with leptomeningeal metastases. JCO Precis Oncol 10.1200/PO.19.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drusbosky LM, Rodriguez E, Dawar R, et al. : Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J Hematol Oncol 14:50, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Illini O, Hochmair MJ, Fabikan H, et al. : Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): A retrospective analysis of patients treated through an access program. Ther Adv Med Oncol 13:17588359211019675, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, et al. : Hypersensitivity reactions to therapeutic monoclonal antibodies: Phenotypes and endotypes. J Allergy Clin Immunol 142:159-170.e2, 2018 [DOI] [PubMed] [Google Scholar]
- 26.McCoach CE, Rolfo C, Drilon A, et al. : Hypersensitivity reactions to selpercatinib treatment with or without prior immune checkpoint inhibitor therapy in patients with non-small-cell lung cancer in LIBRETTO-001. J Thorac Oncol 17:768-778, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subbiah V, Konda B, Bauer T, et al. : Efficacy and safety of selpercatinib in RET fusion-positive cancers other than lung or thyroid cancers. Cancer Res 81, 2021. (suppl 13; abstr CT011) [Google Scholar]
- 28.Wirth LJ, Sherman E, Robinson B, et al. : Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 383:825-835, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belli C, Penault-Llorca F, Ladanyi M, et al. : ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol 32:337-350, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Eli Lilly and Company provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic, genomic, or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set and will be set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.