PURPOSE
To examine longitudinal relationships between levels of C-reactive protein (CRP) and cognition in older breast cancer survivors and noncancer controls.
METHODS
English-speaking women age ≥ 60 years, newly diagnosed with primary breast cancer (stage 0-III), and frequency-matched controls were enrolled from September 2010 to March 2020; women with dementia, neurologic disorders, and other cancers were excluded. Assessments occurred presystemic therapy/enrollment and at annual visits up to 60 months. Cognition was measured using the Functional Assessment of Cancer Therapy-Cognitive Function and neuropsychological testing. Mixed linear effect models tested for survivor-control differences in natural log (ln)-transformed CRP at each visit. Random effect–lagged fluctuation models tested directional effects of ln-CRP on subsequent cognition. All models controlled for age, race, study site, cognitive reserve, obesity, and comorbidities; secondary analyses evaluated if depression or anxiety affected results.
RESULTS
There were 400 survivors and 329 controls with CRP specimens and follow-up data (average age of 67.7 years; range, 60-90 years). The majority of survivors had stage I (60.9%), estrogen receptor–positive (87.6%) tumors. Survivors had significantly higher adjusted mean ln-CRP than controls at baseline and 12-, 24-, and 60-month visits (all P < .05). Higher adjusted ln-CRP predicted lower participant-reported cognition on subsequent visits among survivors, but not controls (P interaction = .008); effects were unchanged by depression or anxiety. Overall, survivors had adjusted Functional Assessment of Cancer Therapy-Cognitive Function scores that were 9.5 and 14.2 points lower than controls at CRP levels of 3.0 and 10.0 mg/L. Survivors had poorer neuropsychological test performance (v controls), with significant interactions with CRP only for the Trails B test.
CONCLUSION
Longitudinal relationships between CRP and cognition in older breast cancer survivors suggest that chronic inflammation may play a role in development of cognitive problems. CRP testing could be clinically useful in survivorship care.
INTRODUCTION
The majority of 3.9 million US breast cancer survivors are age 60 years and older.1-3 Many of these older survivors live with long-term symptoms after treatment.4 Cognitive problems are among the most concerning of these symptoms, potentially leading to decrements in functioning and social and emotional well-being.5-14
CONTEXT
Key Objective
To determine if higher inflammation predicts later cognitive function in a large, prospective national cohort of older breast cancer survivors and matched noncancer controls followed for up to 60 months.
Knowledge Generated
Older breast cancer survivors had persistently higher C-reactive protein (CRP) levels than controls over time. Survivors with high CRP levels were significantly more likely to report clinically meaningful levels of cognitive problems at later points in time, but this relationship was not seen in controls.
Relevance
Longitudinal relationships between CRP and cognition in older breast cancer survivors suggest that chronic inflammation plays a mechanistic role in development of cognitive problems. CRP testing could be clinically useful in survivorship care to identify survivors needing intervention to prevent and/or long-term surveillance for cognitive decline.
Despite decades of recognition of cognitive problems after breast cancer and its therapy, underlying mechanisms remain elusive.15-17 One candidate mechanism is inflammation driven by cellular damage occurring with cancer and its therapies.7,15,18-21 Preclinical models of peripheral inflammatory activation document neuroinflammation and impaired cognition, raising the possibility that peripheral indicators of increased inflammation may precede the development of cognitive decline in cancer survivors.15,22
Higher levels of inflammatory markers have been associated with cognition in noncancer populations.23-31 C-Reactive protein (CRP)32-34 is a measure of chronic inflammation signaling risk for cardiovascular disease32,35 and mortality,32,36 and higher levels have been associated with cognitive problems in patients with cancer.26,37,38 However, previous studies have been cross-sectional or focused on largely younger patients with cancer pre- and postchemotherapy, limiting inference about the potential casual role of CRP in longer-term cognitive problems in cancer survivors.26,37,38
We used longitudinal data from the Thinking and Living with Cancer (TLC) study to evaluate CRP as an inflammatory signal predicting subsequent changes to cancer-related cognitive problems. TLC is a large, multisite cohort study that enrolled survivors before systemic therapy and followed them and frequency-matched noncancer controls for up to 60 months. We describe long-term CRP levels and evaluate directional relationships by testing if higher CRP levels predict later cognitive problems and explore if effects of higher CRP on cognition are stronger in survivors than controls.21,39 The results are intended to build the evidence base about biologic pathways involved in cancer-related cognitive problems and determine whether CRP could be useful to identify older breast cancer survivors at risk for cognitive problems.
METHODS
TLC enrolled participants from five cancer centers and affiliated community hospitals and practices.8,40 We report a planned analysis among participants enrolled from September 1, 2010, to March 1, 2020. All institutional review boards approved the study protocol (ClinicalTrials.gov identifier: NCT03451383).
Population
English-speaking women age 60 years and older, newly diagnosed with primary breast cancer (stage 0-III), were eligible. Noncancer controls were frequency-matched at enrollment within each study site to survivors on the basis of age (within 5 years), education level, and race (White v non-White). Exclusion criteria for survivors and controls were non–English-speaking, history of stroke, head injury, major psychiatric or neurodegenerative disorder, treatment for another cancer within 5 years (except nonmelanoma skin cancers) or receipt of past systemic cancer treatment at any time, and a Mini-Mental State Examination score of < 24 or less than a third-grade reading level on the Word Reading subtest of the Wide Range Achievement Test (WRAT4).
In 2016, the protocol was amended (with reconsent) to extend follow-ups and add blood collection. Thus, reconsenting participants enrolled before 2016 could only provide samples at follow-up visits, whereas those entering in 2016 provided enrollment and follow-up samples.
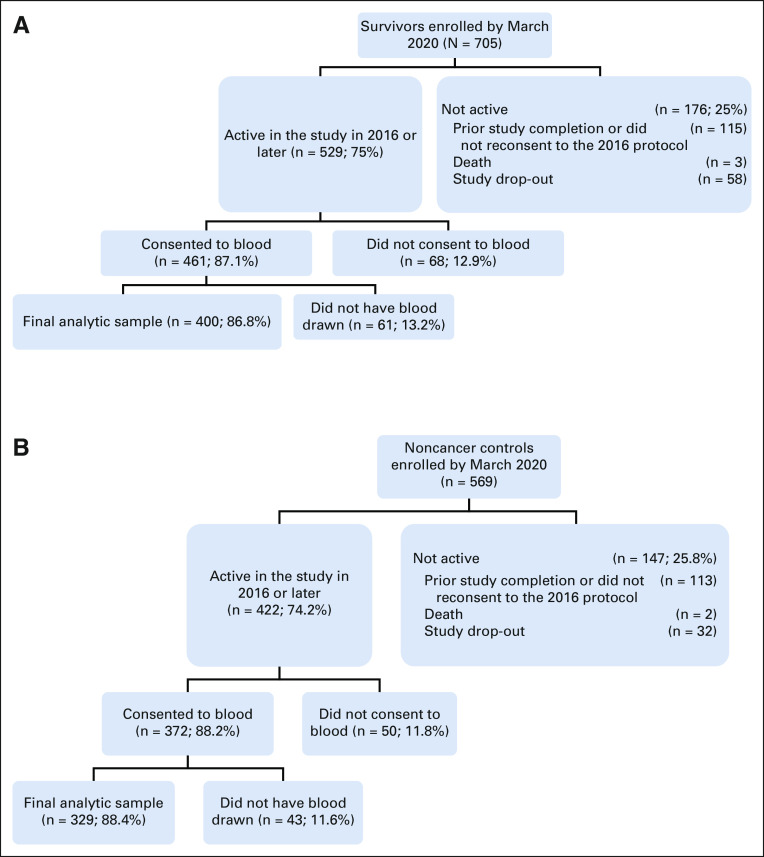
There were 705 survivors and 569 controls enrolled by March 2020, and 529 survivors and 422 controls were active in the study in 2016 (Fig 1). Reasons for no longer being active in the study included completing the study before 2016 or declining consent for the 2016 protocol (115 survivors and 113 controls), death (three survivors and two controls), or study dropout (58 survivors and 32 controls). Among active women, 87.1% of survivors and 88.2% of controls consented to blood collection, and 400 survivors and 329 noncancer controls provided one or more specimens for CRP assays, constituting the analytic sample. Data from survivors who experienced cancer recurrence (n = 6) or developed exclusion conditions during follow-up (n = 2) were removed from that point forward. The analytic sample was similar to the remainder of the overall TLC sample except for higher percentages of White participants (83.1% v 77.2%, P = .008) and more women with > 2 comorbidities (49.1% v 42.3%, P = .021). Survivors in the analytic sample had breast-conserving surgery more often than those in the remainder of the overall survivor sample (71.5% v 61.7%, P < .001).
FIG 1.
Flow diagram of older breast cancer survivors and noncancer controls included in analyses of the relationships between CRP and cognition. The final analytic sample had one or more CRP result. Reasons for not having blood drawn among those consenting to blood collection included being unable to obtain a specimen and participants choosing to skip the blood draw. Since blood specimens for CRP were not obtained until 2016 under a protocol revision, participants enrolled from 2010 to 2015 might have already completed the study, decided not to continue, or have died or dropped out before blood collection. (A) Survivors. (B) Noncancer controls. CRP, C-reactive protein.
Data and Sample Collection
Neuropsychological tests and questionnaires were completed at each visit. The baseline visit occurred after cancer-related surgery but before initiation of systemic therapies and/or neoadjuvant therapy; controls were assessed contemporaneously. Questionnaires ascertained sociodemographic, clinical (eg, comorbidities, height, and weight), and psychosocial (eg, anxiety and depression) factors, and participant-reported cognition and medical record data were abstracted for survivors.
Venous blood specimens were chilled and processed within 8 hours. Platelet-poor EDTA plasma was obtained by centrifugation at 4°C (2,000g for 15 minutes or 3,000g for 10 minutes), frozen immediately at −80°C, and later shipped on dry ice to the UCLA Cousins Center for Psychoneuroimmunology for storage at –80°C until being assayed.
CRP was assayed on a single kit lot, using the Human CRP Quantikine ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol with minor modifications, including using a 500-fold sample dilution and extension of the standard curve to obtain a lower limit of detection of 0.2 mg/L.23 Samples falling below the lower limit of detection (15 samples, 1%) were assigned a value of 0.1 mg/L to retain these samples in our analyses, per standard practice.23 Three samples were above the upper limit of detection (75 mg/L) and were assigned a value of 75 mg/L to remove outlier effects. All samples from the same participant at different study visits were run on the same ELISA plate, with a balance of samples from survivors and controls, from at least three recruitment sites, on each plate. All assays were performed in duplicate, with an interassay coefficient of variation of < 6% and a mean intra-assay coefficient of variation of < 4%.
Measures
CRP was the primary predictor of cognition outcomes. The Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 341 measured participant-reported cognition. The FACT-Cog Total score was the primary cognition outcome, as specified in the study protocol, and captured perceived ability and self-reported difficulties, which can precede decrements on neuropsychological tests.42 The FACT-Cog Total score (higher scores indicate better cognition) has been used in previous work from our group and others,8,43 has established thresholds for a clinically significant decline (decrease of 7-10 points), and can be compared across populations with a high degree of reliability in our sample (Cronbach's alpha .95).44,45 Two FACT-Cog subscales, the Perceived Cognitive Impairments (PCI; Cronbach's alpha = .93) and Perceived Cognitive Abilities (PCA; Cronbach's alpha .84), were used in supplemental analyses.
Secondary cognitive outcomes were based on performance in 11 neuropsychological tests46-48 of two domains (Attention, Processing speed, and Executive function [APE], and Learning and Memory [LM]).8,49 Individual tests were also examined.6,50,51 Scores were standardized (z-scores) to the control means at baseline by the age group and education level.
Variables were examined as potential confounders of CRP-cognition relationships, including age, study site, race (White v non-White), cognitive reserve (WRAT4 Word Reading score), number of comorbidities (≤ 2 v > 2), and obesity (body mass index ≥ 30 v < 30 kg/m2). We also considered depression (CES-D scores52) and state anxiety (STAI scores53) at each study visit. Among survivors, we considered cancer stage, molecular subtype, and types of therapy.
Analyses
Natural log-transformed CRP (ln-CRP) values were used in all analyses since values were not normally distributed. Results are shown for both ln-CRP and back-transformed non-log CRP mg/L for ease of clinical interpretation: CRP levels are generally categorized as normal/low risk (< 1 mg/L), moderately elevated (1 to < 3 mg/L), and high (≥ 3 mg/L).54 T-tests, analysis of variance, and chi-square tests were used to test bivariate differences in characteristics of survivors and controls and whether covariates were associated with both CRP and cognition (ie, potential confounders). We tested the stability of a woman's CRP values on repeated assessments using intraclass and median pairwise correlations.
Mixed linear effect models were used to test for differences in adjusted ln-CRP levels for survivors and controls at each study visit. Covariates included in all models were age, race, site, WRAT score, obesity, and comorbidities.
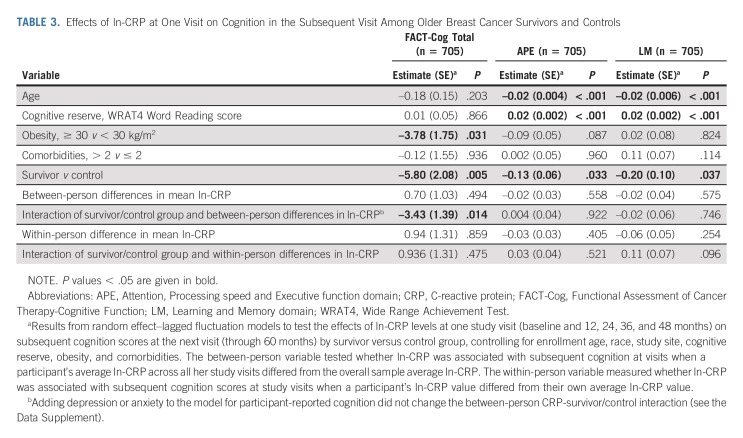
To evaluate the relationship between CRP and cognition, we used a random effect–lagged fluctuation model to test effects of ln-CRP levels at one study visit (baseline and 12, 24, 36, and 48 months) on subsequent cognition (ie, FACT-Cog, APE, and LM) score at the next visit (through 60 months) among survivors versus controls.55,56 All models include the following covariates: enrollment age, race, site, WRAT score, obesity, and comorbidities. Because of the time-varying nature of the ln-CRP variable, we created between-person and within-person predictors. The between-person predictor tested whether participant's average ln-CRP differed from others and was associated with subsequent FACT-Cog scores. The within-person variable measured whether ln-CRP was associated with subsequent FACT-Cog scores at visits when a participant's ln-CRP value differed from their own average ln-CRP value. We included interactions of survivor versus control status and within-person and between-person ln-CRP values. Significant interactions were decomposed by stratifying by survivor-control status. Finally, exploratory analyses evaluated Fact-Cog PCA and PCI scores as outcomes.
Secondary random effect-lagged mixed fluctuation model analyses tested (1) if the interaction between the survivor-control group and ln-CRP effects on subsequent cognition changed if depression or anxiety at each visit was considered, (2) effects of ln-CRP on FACT-Cog subscales (PCI and PCA) and neuropsychological test performance domain Z-scores and individual test Z-scores, and (3) if CRP interacted with treatments in effects on cognition in survivor-only analyses. Since stage and molecular subtype were strongly colinear with therapy, we considered therapies only. All models were conducted using SAS Version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
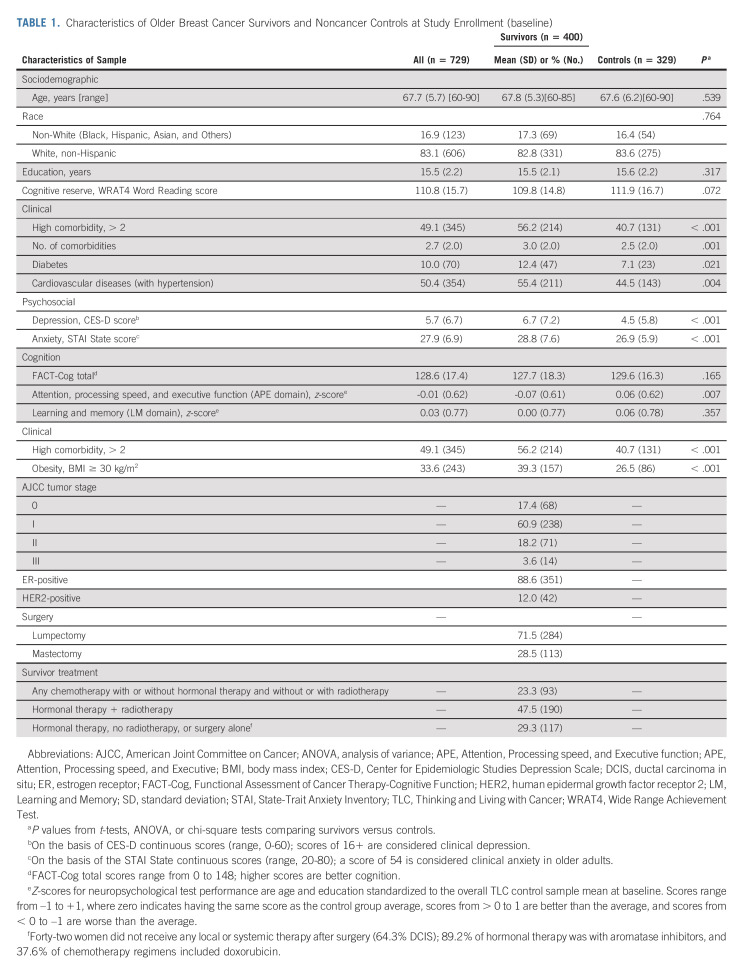
The study participants ranged in age from 60 to 90 years (average 67.7, standard deviation 5.7) and were largely White and well-educated (Table 1). The survivors were comparable with frequency-matched noncancer controls at enrollment in demographics, but survivors were more likely to have > 2 comorbidities (56.2% v 40.7%, P = .001) and be obese (39.3% v 26.5%, P < .001); subsequent analyses controlled for these imbalances. Most survivors had stage I disease (60.9%), with estrogen receptor–positive (87.6%) and human epidermal growth factor receptor 2–negative (88.0%) tumors.
TABLE 1.
Characteristics of Older Breast Cancer Survivors and Noncancer Controls at Study Enrollment (baseline)
CRP Levels
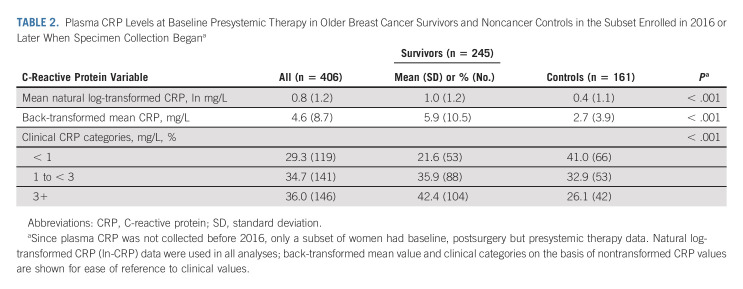
CRP levels were obtained from 1,550 specimens (819 among 400 survivors; 731 among 326 controls); 62.5% and 70.5% of survivors and controls, respectively, provided two or more specimens (Data Supplement, online only). Among participants with a baseline sample, survivors had a significantly greater percentage of unadjusted, nontransformed baseline CRP values ≥ 3 mg/L than controls (42.4% v 26.1%, P < .001; Table 2). Baseline CRP levels were significantly associated with obesity in survivors and controls (P < .001), but not with other covariates, so obesity was included in subsequent models.
TABLE 2.
Plasma CRP Levels at Baseline Presystemic Therapy in Older Breast Cancer Survivors and Noncancer Controls in the Subset Enrolled in 2016 or Later When Specimen Collection Begana
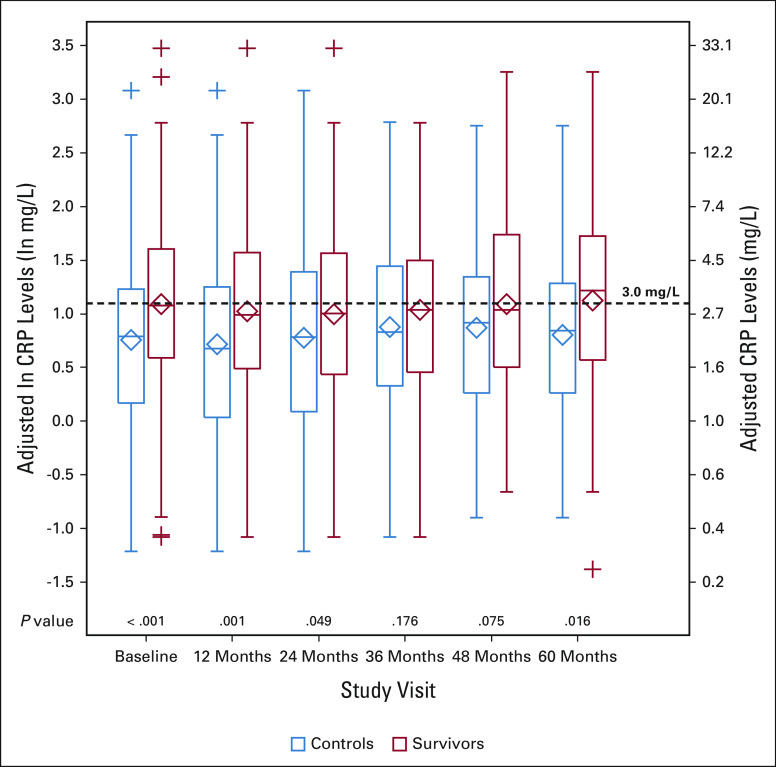
Women's ln-CRP values were stable over time (0.74 and 0.76 for intraclass correlation and median pairwise correlations). Survivors had higher adjusted mean ln-CRP levels than controls at all time points, and these were statistically significantly higher at baseline and 12-, 24- and 60-month study visits (all P < .05; Fig 2 and Data Supplement).
FIG 2.
Adjusted CRP levels by study visit for older breast cancer survivors and noncancer controls. Results of mixed model analyses in survivors (red, n = 380 total) and noncancer controls (blue, n = 318 total) at each study visit (baseline and 12, 24, 36, 48, and 60 months) using natural log-transformed CRP data (ln-CRP, left axis), adjusted for age, race (White v Others), cognitive reserve (WRAT4 Word Reading score), study site, obesity (≥ 30 v < 30 kg/m2), and comorbidities (> 2 v ≤ 2). The results for adjusted ln-CRP values were also back-transformed to mg/L (right axis), and a horizontal dotted line at 3 mg/L (considered high CRP) has been added for ease of interpretation. The boxes indicate the interquartile range (ie, 25th-75th percentiles) of adjusted ln-CRP values, the whiskers above and below the boxes indicate the 5th and 95th percentiles, and + signs indicate values below the fifth or above the 95th percentile. Diamonds represent the mean CRP values; heavy lines inside the box are the median. P values from the mixed models for survivor versus control differences in adjusted ln-CRP at each study visit are shown along the x axis. See the Data Supplement for detailed data at each study visit. CRP, C-reactive protein; WRAT4, Wide Range Achievement Test.
Relationships of CRP Levels at One Visit to Cognition at Later Visits
Self-reported cognition.
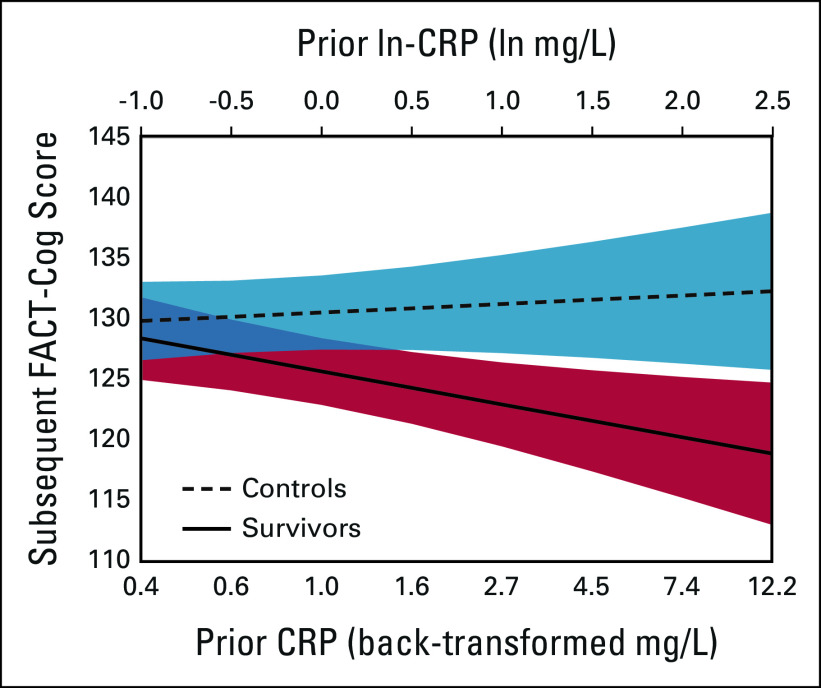
In longitudinal analyses testing directionality of effects, there was a differential impact of adjusted ln-CRP levels on subsequent participant-reported cognition among survivors versus controls, after controlling for age, race, study site, cognitive reserve, obesity, and comorbidities. When survivors had higher ln-CRP values than others (ie, between-person differences), they reported statistically worse cognition on the next study visit (P = .040); this relationship was not seen in controls (P = .795; P for interaction = .014, Table 3 and Fig 3). Notably, as survivor back-transformed CRP levels increased from 1.0 to 3.0 to 10.0 mg/L, adjusted FACT-Cog scores were 5.2, 9.5, and 14.2 points lower, respectively, than controls (Fig 3). The interaction of CRP with survivor/control status was unchanged after considering depression or anxiety (Data Supplement). Similar results were observed examining relationships with FACT-Cog PCI and PCA subscales (Data Supplement).
TABLE 3.
Effects of ln-CRP at One Visit on Cognition in the Subsequent Visit Among Older Breast Cancer Survivors and Controls
FIG 3.
Relationship between CRP levels and participant-reported cognition score on the subsequent visit among survivors and controls (n = 641). The graph illustrates subsequent FACT-Cog Total scores on the basis of the CRP level at the prior visit from fluctuation model analyses using adjusted natural log-transformed CRP (ln-CRP) values (upper axis); corresponding back-transformed CRP values shown on the lower axis for ease of interpretation. The model adjusted ln-CRP for age, race (White v Others), cognitive reserve (WRAT4 Word Reading score), study site, obesity (≥ 30 v < 30 kg/m2), and comorbidities (> 2 v ≤ 2). Possible FACT-Cog scores range from 0 to 148, with higher scores indicating better cognition; analyses include the full FACT-Cog score range, but the graph shows a truncated scale. The interaction of between-person differences in ln-CRP and the survivor/control group in effects on participant-reported cognition was significant at P = .008. CRP, C-reactive protein; FACT-Cog, Functional Assessment of Cancer Therapy-Cognitive Function; WRAT4, Wide Range Achievement Test.
Neuropsychological test performance.
Adjusted ln-CRP levels did not predict subsequent scores in either survivors or controls on overall neuropsychological test performance for the APE or LM domains (Table 3). Survivors did have small decreases in neuropsychological test performance (v controls) with significant interactions with CRP for the Trails B test (a component of the APE domain) and trends for other tests (Data Supplement) although after considering multiple testing, these did not remain significant.
Survivors only.
Among survivors, only 23.3% received chemotherapy, and different combinations of systemic and radiotherapy did not interact with ln-CRP levels in effects on participant-reported cognition or neuropsychological test performance (Data Supplement). The majority of women received aromatase inhibitors (Data Supplement), so we were unable to test differences by specific hormonal treatment.
DISCUSSION
To our knowledge, this is one of the first studies examining the long-term longitudinal relationship between chronic inflammation and cognition in older breast cancer survivors and comparing these effects with those seen in noncancer controls. We found that higher CRP levels predicted having lower participant-reported cognition on later visits among survivors, but not controls. There were also suggestive but nonsignificant trends in the relationship of CRP to subsequent performance on standardized neuropsychological tests. Interestingly, older survivors had significantly higher circulating CRP levels than controls even before systemic therapy, and survivors' CRP levels remained consistently higher at visits up to five years postsurgery.
Our results add to the body of evidence linking inflammation to cognition in cancer survivors57-59 by determining longitudinal relationships between CRP and cognition over a period of up to 60 months. We found that having higher-than-average CRP on one study visit significantly predicted decrements in participant-reported cognition at the next visit in older survivors but not controls. This effect was unchanged by depression or tanxiety. The effects of higher CRP on participant-reported cognition were clinically meaningful, with survivors having adjusted FACT-Cog scores that were 9.5 points lower than controls at CRP levels of ≥ 3 mg/L.
Although suggestive, our results were less robust for the impact of CRP on subsequent neuropsychological test performance. Others have reported declines in neuropsychological test performance in short-term investigations mainly in patients receiving chemotherapy.57,58 Our results for neuropsychological test performance may differ from previous work because the TLC sample has low chemotherapy rates. It is also possible that well-educated women like those enrolled in TLC noticed cognitive problems, but had sufficient cognitive reserve to maintain neuropsychological test performance. Alternatively, neuropsychological testing might have low ecological validity since it is performed in a highly structured environment60 although subjective measures can capture challenges that individuals experience in their everyday lives and be more sensitive to change.60 In addition, it is also plausible that the effects of chronic systemic inflammation on the brain manifest years after self-reported problems and only become evident on objective measures with declines in compensatory capacity as individuals age or accumulate greater comorbidity. Longitudinal neuroimaging studies will be useful to understand these longitudinal relationships.
Our observation that older breast cancer survivors had significantly higher CRP levels compared with controls before beginning any systemic therapy suggests that having cancer may be related to or cause higher inflammation. Baseline elevations in CRP in survivors versus noncancer controls also persisted over time at most visits, independent of covariates. This observation suggests that CRP remains higher well after surgical removal of the primary cancer and is independent of some of the most common risk factors for cancer and inflammation, including medical comorbidities and obesity.
Taken together, our prospective results suggest that inflammation may be involved in mechanistic pathways leading to cancer-related cognitive problems. This idea is biologically plausible since peripheral inflammation results in subsequent impairments in cognitive performance in preclinical models,22 has a known relationship with cognitive disorders, can increase brain inflammation seen with neurodegeneration,59,61 and can further promote inflammation in a feed forward loop.61 In the case of cancer and its treatments, compromised integrity of the blood brain barrier because of chemotherapy exposure38 may also elevate risk for subsequent inflammation-mediated cancer-related cognitive problems.62 Although we did not observe an effect of chemotherapy in these analyses, only a small proportion of our survivors had this treatment modality.
Our data support the need for studies to test the hypothesis that behavioral and/or pharmacological interventions targeting inflammation may prevent or reduce cancer-related cognitive problems in older breast cancer survivors.17,63 Potential interventions targeting inflammation include increasing physical activity, improving sleep, reducing stress, and administering drugs that block inflammatory pathways.64-72
This study has many strengths, including the large cohort, long follow-up, and inclusion of matched controls. However, there are several limitations that should be considered in evaluating our findings. First, although women in our sample were representative of the communities served by our tertiary academic medical centers and their community affiliates, they were predominantly White and well-educated, limiting external generalizability. It will be critical to replicate our results in more diverse samples, especially groups with lifetime experiences associated with increased chronic inflammation.39 Second, there were insufficient numbers receiving chemotherapy and limited variability in types of regimens to determine if the relationship between CRP and cognition varied by specific regimens. Others have found that radiotherapy and/or chemotherapy induce cellular damage that can, in turn, increase peripheral inflammation73,74 and cognitive problems.23,25-28,58,75-77 Although our sample of survivors were predominantly prescribed aromatase inhibitors, future research will need to investigate whether differences in hormonal treatments (eg, tamoxifen v aromatase inhibitors) differentially relate to hepatic production of CRP. This is an important future direction given existing data linking hormonal therapy to perceived impairments in cognition78 and CRP levels.79,80 Third, since blood collection was added to an established cohort, not all survivors had plasma CRP data before systemic therapy. Fourth, it will be important to replicate results with other inflammatory markers. Finally, there may be critical windows during and immediately after active treatment when inflammation is particularly higher and drives changes in cognition that we might have missed with having our first follow-up visit at 12 months.
Overall, this large longitudinal multisite study demonstrated that older breast cancer survivors had higher inflammation as measured by circulating levels of CRP starting before systemic therapy and continuing over time. This higher inflammation was predictive of clinically meaningful participant-reported cognitive problems at later time points in survivors but not controls. The results underscore the importance of asking about survivors' perceptions of their cognitive function and suggest that CRP data may be useful to oncology providers to identify older breast cancer survivors at risk for cognitive problems.
ACKNOWLEDGMENT
The work of Paul B. Jacobsen was performed while he was at the Moffitt Cancer Center. We would like to thank the participants in the TLC study for their sharing of their time and experiences; without their generosity, this study would not have been possible. We are also indebted to Sherri Stahl, Naomi Greenwood, Margery London, and Sue Winarsky, who serve as patient advocates from the Georgetown Breast Cancer Advocates, for their insights and suggestions on study design and methods to recruit and retain participants. We thank the TLC study staff who contributed by ascertaining, enrolling, and interviewing participants.
Martine Extermann
Honoraria: OncLive Clinical Congress Consultants
Consulting or Advisory Role: Alnylam, Aileron Therapeutics
Deena Graham
Stock and Other Ownership Interests: Cota Healthcare
Consulting or Advisory Role: Eisai
Claudine Isaacs
Consulting or Advisory Role: Pfizer, Genentech/Roche, Novartis, Puma Biotechnology, Seattle Genetics, Sanofi/Aventis, Eisai, Ion Solutions, Biotheranostics, AstraZeneca/MedImmune, Gilead Sciences
Research Funding: Tesaro (Inst), Merck (Inst), Seattle Genetics (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Novartis (Inst), Genentech/Roche (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing, UpToDate—Wolters Kluwer—Author of chapters, Elsevier—Editor of Book
Other Relationship: Side-Out Foundation
Heather S.L. Jim
Consulting or Advisory Role: Janssen Medical Affairs, Merck
Research Funding: Kite, a Gilead company (Inst)
Patents, Royalties, Other Intellectual Property: Methods of Treating Cognitive Impairment, United States Letters Patent No. 10806772, October 20, 2020
Andrew J. Saykin
Consulting or Advisory Role: Bayer
Research Funding: Lilly (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health through grant Nos R01CA129769 and R35CA197289 to J.S.M. This study was also supported in part by the National Institutes of Health through grant No. P30CA51008 to the Georgetown-Lombardi Comprehensive Cancer Center with the Biostatistics and Bioinformatics Resource and the Non-Therapeutic Shared Resource, R56AG068086 (J.E.C. and J.S.M.), R01AG068193 (J.S.M. and A.J.S.), R01CA237535 (J.E.C.), K01AG065485 (K.E.R.), P30AG028716 (H.J.C.), K01CA212056 (T.N.B.), K08CA241337 (K.V.D.), K12HD001441 (Z.M.N.), R01CA172119 (T.A.A., J.R.), U54CA137788 (T.A.A.), P30CA008748 (T.A.A.), T32CA090314 (D.B.T.), R01CA244673 (B.C.M.), and P30AG010133 and P30AG072976 (A.J.S.); American Cancer Society through grant No. 17-023-01-CPPB (S.K.P.); and UCLA Cousins Center for Psychoneuroimmunology (K.E.R. and J.E.C.).
E.C.B. and J.S.M. share cosenior authorship.
AUTHOR CONTRIBUTIONS
Conception and design: Judith E. Carroll, Harvey J. Cohen, Tim A. Ahles, Jaeil Ahn, Deena Graham, Andrew J. Saykin, Elizabeth C. Breen, Jeanne S. Mandelblatt
Financial support: Judith E. Carroll, Tim A. Ahles, Andrew J. Saykin
Administrative support: Tim A. Ahles, Andrew J. Saykin, Elizabeth C. Breen, Jeanne S. Mandelblatt
Provision of study materials or patients: Tim A. Ahles, Brenna C. McDonald, Andrew J. Saykin
Collection and assembly of data: Xingtao Zhou, Tim A. Ahles, Heather S.L. Jim, Brenna C. McDonald, Sunita K. Patel, Andrew J. Saykin, Elizabeth C. Breen, Jeanne S. Mandelblatt
Data analysis and interpretation: Judith E. Carroll, Zev M. Nakamura, Brent J. Small, Xingtao Zhou, Harvey J. Cohen, Tim A. Ahles, Jaeil Ahn, Traci N. Bethea, Martine Extermann, Claudine Isaacs, Paul B. Jacobsen, Brenna C. McDonald, Kelly Rentscher, James Root, Andrew J. Saykin, Danielle B. Tometich, Kathleen Van Dyk, Wanting Zhai, Elizabeth C. Breen, Jeanne S. Mandelblatt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Elevated C-Reactive Protein and Subsequent Patient-Reported Cognitive Problems in Older Breast Cancer Survivors: The Thinking and Living With Cancer Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Martine Extermann
Honoraria: OncLive Clinical Congress Consultants
Consulting or Advisory Role: Alnylam, Aileron Therapeutics
Deena Graham
Stock and Other Ownership Interests: Cota Healthcare
Consulting or Advisory Role: Eisai
Claudine Isaacs
Consulting or Advisory Role: Pfizer, Genentech/Roche, Novartis, Puma Biotechnology, Seattle Genetics, Sanofi/Aventis, Eisai, Ion Solutions, Biotheranostics, AstraZeneca/MedImmune, Gilead Sciences
Research Funding: Tesaro (Inst), Merck (Inst), Seattle Genetics (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Novartis (Inst), Genentech/Roche (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing, UpToDate—Wolters Kluwer—Author of chapters, Elsevier—Editor of Book
Other Relationship: Side-Out Foundation
Heather S.L. Jim
Consulting or Advisory Role: Janssen Medical Affairs, Merck
Research Funding: Kite, a Gilead company (Inst)
Patents, Royalties, Other Intellectual Property: Methods of Treating Cognitive Impairment, United States Letters Patent No. 10806772, October 20, 2020
Andrew J. Saykin
Consulting or Advisory Role: Bayer
Research Funding: Lilly (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bluethmann SM, Mariotto AB, Rowland JH: Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 25:1029-1036, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parry C, Kent EE, Mariotto AB, et al. : Cancer survivors: A booming population. Cancer Epidemiol Biomarkers Prev 20:1996-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surveillance Epidemiology and End Results (SEER) Program: SEER*Stat Database: Incidence—SEER 21 Regs Research Data, Nov 2018 Sub (2000-2016) <Katrina/Rita Population Adjustment>—Linked to County Attributes—Total U.S., 1969-2017 Counties, Bethesda, MD, National Cancer Institute; 2019 [Google Scholar]
- 4.Mandelblatt JS, Zhai W, Ahn J, et al. : Symptom burden among older breast cancer survivors: The Thinking and Living With Cancer (TLC) study. Cancer 126:1183-1192, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandelblatt JS, Jacobsen PB, Ahles T: Cognitive effects of cancer systemic therapy: Implications for the care of older patients and survivors. J Clin Oncol 32:2617-2626, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin AJ, McDonald BC, et al. : Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol 28:4434-4440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janelsins MC, Kesler SR, Ahles TA, Morrow GR: Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 26:102-113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelblatt JS, Small BJ, Luta G, et al. : Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol 36:3211-3222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura ZM, Deal AM, Nyrop KA, et al. : Associations of functional, psychosocial, and medical factors with cognitive impairment in older, chemotherapy naïve patients with early breast cancer. Psychooncology 29:1366-1369, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvio L, Peugeot M, Bruns GL, et al. : Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med 52:219-227, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Von Ah D, Tallman EF: Perceived cognitive function in breast cancer survivors: Evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy-cognitive function instrument. J Pain Symptom Manage 49:697-706, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Von Ah D, Crouch A: Relationship of perceived everyday cognitive function and work engagement in breast cancer survivors. Support Care Cancer 29:4303-4309, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Klaver KM, Duijts SFA, Engelhardt EG, et al. : Cancer-related cognitive problems at work: Experiences of survivors and professionals. J Cancer Surviv 14:168-178, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duijts SFA, van der Beek AJ, Boelhouwer IG, Schagen SB: Cancer-related cognitive impairment and patients’ ability to work: A current perspective. Curr Opin Support Palliat Care 11:19-23, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Ahles TA, Saykin AJ: Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7:192-201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnuson A, Ahles T, Chen BT, et al. : Cognitive function in older adults with cancer: Assessment, management, and research opportunities. J Clin Oncol 39:2138-2149, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schagen SB, Tsvetkov AS, Compter A, Wefel JS: Cognitive adverse effects of chemotherapy and immunotherapy: Are interventions within reach? Nat Rev Neurol 18:173-185, 2022 [DOI] [PubMed] [Google Scholar]
- 18.Gorelick PB: Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 1207:155-162, 2010 [DOI] [PubMed] [Google Scholar]
- 19.McAfoose J, Baune BT: Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 33:355-366, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Ahles TA, Root JC, Ryan EL: Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol 30:3675-3686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodes RJ, Sierra F, Austad SN, et al. : Disease drivers of aging. Ann N Y Acad Sci 1386:45-68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AH, Maletic V, Raison CL: Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732-741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogland AI, Nelson AM, Gonzalez BD, et al. : Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain Behav Immun 80:308-314, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conole ELS, Stevenson AJ, Muñoz Maniega S, et al. : DNA methylation and protein markers of chronic inflammation and their associations with brain and cognitive aging. Neurology 97:e2340-e2352, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganz PA, Bower JE, Kwan L, et al. : Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun 30:S99-S108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams AM, Shah R, Shayne M, et al. : Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol 314:17-23, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyon DE, Cohen R, Chen H, et al. : Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol 301:74-82, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesler S, Janelsins M, Koovakkattu D, et al. : Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun 30:S109-S116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SK, Wong AL, Wong FL, et al. : Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. JNCI J Natl Cancer Inst 107:131, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Willik KD, Koppelmans V, Hauptmann M, et al. : Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res 20:1335, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung YT, Ng T, Shwe M, et al. : Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann Oncol 26:1446-1451, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaptoge S, Di Angelantonio E, Lowe G, et al. : C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 375:132-140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko Y-J, Kwon Y-M, Kim KH, et al. : High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev 21:2076-2086, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Reuben DB, Cheh AI, Harris TB, et al. : Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 50:638-644, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pearson T, Mensah G, Alexander R, et al. : Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499-511, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Pierce BL, Ballard-Barbash R, Bernstein L, et al. : Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27:3437-3444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belcher EK, Culakova E, Gilmore NJ, et al. : Inflammation, attention, and processing speed in patients with breast cancer before and after chemotherapy. J Natl Cancer Inst 114:712-721, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pomykala KL, Ganz PA, Bower JE, et al. : The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav 7:511-523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandelblatt JS, Ahles TA, Lippman ME, et al. : Applying a life course biological age framework to improving the care of individuals with adult cancers. JAMA Oncol 7:1692, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandelblatt JS, Zhou X, Small BJ, et al. : Deficit accumulation frailty trajectories of older breast cancer survivors and non-cancer controls: The thinking and living with cancer study. J Natl Cancer Inst 113:1053-1064, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner LI, Sweet J, Butt Z, et al. : Measuring patient self-reported cognitive function: Development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol 7:W32-W39, 2009 [Google Scholar]
- 42.Magnuson A, Ahles T, Chen BT, et al. : Cognitive function in older adults with cancer: Assessment, management, and research opportunities. J Clin Oncol 39:2138-2149, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janelsins MC, Heckler CE, Peppone LJ, et al. : Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 35:506-514, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung YT, Foo YL, Shwe M, et al. : Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol 67:811-820, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Bell ML, Dhillon HM, Bray VJ, Vardy JL: Important differences and meaningful changes for the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog). J Patient Rep Outcomes 2:48, 2018 [Google Scholar]
- 46.Wefel JS, Vardy J, Ahles T, Schagen SB: International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12:703-708, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Weintraub S, Salmon D, Mercaldo N, et al. : The Alzheimer’s Disease Centers’ Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 23:91-101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern R, White T: Neuropsychological Assessment Battery (NAB). Psychological Assessment Resources, 2003. https://www.parinc.com/Products/Pkey/4527 [Google Scholar]
- 49.Clapp JD, Luta G, Small BJ, et al. : The impact of using different reference populations on measurement of breast cancer-related cognitive impairment rates. Arch Clin Neuropsychol 33:956-963, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vega JN, Dumas J, Newhouse PA: Cognitive effects of chemotherapy and cancer-related treatments in older adults. Am J Geriatr Psychiatry 25:1415-1426, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahles TA, Saykin AJ, Noll WW, et al. : The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 12:612-619, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Radloff LS: The CES-D Scale: A self report depression scale for research in the general population. Appl Psychol Meas 1:385-401, 1977 [Google Scholar]
- 53.Spielberger C, Gorsuch R: STAI Manual for the State-Trait Anxiety Inventory (STAI). Palo Alto, CA, Consulting Psychologists Press, 1970 [Google Scholar]
- 54.Sabatine MS, Morrow DA, Jablonski KA, et al. : Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 115:1528-1536, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Carroll JE, Small BJ, Tometich DB, et al. : Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: Interaction with genotype. Cancer 125:4516-4524, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman L: Longitudinal Analysis: Modeling Within-Person Fluctuation and Change. New York, NY, Routledge, 2015 [Google Scholar]
- 57.Laird BJ, McMillan DC, Fayers P, et al. : The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 18:1050-1055, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Starkweather A, Kelly DL, Thacker L, et al. : Relationships among psychoneurological symptoms and levels of C-reactive protein over 2 years in women with early-stage breast cancer. Support Care Cancer 25:167-176, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroyen G, Vissers J, Smeets A, et al. : Blood and neuroimaging biomarkers of cognitive sequelae in breast cancer patients throughout chemotherapy: A systematic review. Transl Oncol 16:101297, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savard J, Ganz PA: Subjective or objective measures of cognitive functioning-what’s more important? JAMA Oncol 2:1263-1264, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Chitnis T, Weiner HL: CNS inflammation and neurodegeneration. J Clin Invest 127:3577, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandez HR, Varma A, Flowers SA, Rebeck GW: Cancer chemotherapy related cognitive impairment and the impact of the Alzheimer’s disease risk factor APOE. Cancers (Basel) 12:1-25, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carroll JE, Bower JE, Ganz PA: Cancer-related accelerated ageing and biobehavioural modifiers: A framework for research and clinical care. Nat Rev Clin Oncol 19:173-187, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antoni MH, Lutgendorf SK, Blomberg B, et al. : Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry 71:366-372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bower JE, Greendale G, Crosswell AD, et al. : Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology 43:20-29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irwin MR, Olmstead R, Breen EC, et al. : Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: A randomized controlled trial. JNCI Monogr 2014:295-301, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bower JE, Irwin MR: Mind-body therapies and control of inflammatory biology: A descriptive review. Brain Behav Immun 51:1-11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irwin MR, Olmstead R, Carrillo C, et al. : Cognitive behavioral therapy vs. Tai chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep 37:1543-1552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palesh O, Scheiber C, Kesler S, et al. : Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: Effects on insomnia and circadian rhythm during chemotherapy: A phase II randomised multicentre controlled trial. Br J Cancer 119:274-281, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown JC, Zhang S, Ligibel JA, et al. : Effect of exercise or metformin on biomarkers of inflammation in breast and colorectal cancer: A randomized trial. Cancer Prev Res 13:1055-1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loh KP, Pandya C, Zittel J, et al. : Associations of sleep disturbance with physical function and cognition in older adults with cancer. Support Care Cancer 25:3161-3169, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker AK, Chang A, Ziegler AI, et al. : Low dose aspirin blocks breast cancer-induced cognitive impairment in mice. PLoS One 13:e0208593, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JH, Jenrow KA, Brown SL: Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J 32:103-115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vyas D, Laput G, Vyas AK: Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther 7:1015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marsland AL, Gianaros PJ, Kuan DC-H, et al. : Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun 48:195-204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santos JC, Pyter LM: Neuroimmunology of behavioral comorbidities associated with cancer and cancer treatments. Front Immunol 9:1195, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janelsins MC, Lei L, Netherby-Winslow C, et al. : Relationships between cytokines and cognitive function from pre- to post-chemotherapy in patients with breast cancer. J Neuroimmunol 362:577769, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner LI, Gray RJ, Sparano JA, et al. : Patient-reported cognitive impairment among women with early breast cancer randomly assigned to endocrine therapy alone versus chemoendocrine therapy: Results from TAILORx. J Clin Oncol 38:1875-1886, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pierce BL, Neuhouser ML, Wener MH, et al. : Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat 114:155-167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Decensi A, Robertson C, Viale G, et al. : A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. JNCI J Natl Cancer Inst 95:779-790, 2003 [DOI] [PubMed] [Google Scholar]