Abstract
Cell-mediated immune (CMI) responses defined by delayed-type hypersensitivity (DTH) reactivity to cryptococcal culture filtrate antigen (CneF) can be either protective or nonprotective against an infection with Cryptococcus neoformans. The protective and nonprotective anticryptococcal DTH responses are induced by different immunogens and have differing activated-T-cell profiles. This study examined the effects of blockade of the interaction between cytotoxic T lymphocyte antigen 4 (CTLA-4) and its ligands B7-1 (CD80) and B7-2 (CD86) on the anticryptococcal DTH responses and protection. We found that CTLA-4 blockade at the time of immunization with the immunogen that induces the protective response, CneF, in complete Freund's adjuvant (CFA) or the immunogen that induces the nonprotective response, heat-killed cryptococcal cells (HKC), enhanced anticryptococcal DTH reactivity. In contrast, blocking CTLA-4 after the immune response was induced failed to enhance responses. Blockade of CTLA-4 in an infection model resulted in earlier development of the anticryptococcal CMI response than in control mice. Concomitant with increases in DTH reactivity in mice treated with anti-CTLA-4 Fab fragments at the time of immunization, there were decreases in cryptococcal CFU in lungs, spleens, and brains compared to controls. Blockade of CTLA-4 resulted in long-term protection, as measured by significantly increased survival times, only in mice given the protective immunogen, CneF-CFA. Anti-CTLA-4 treatment did not shift the response induced by the nonprotective immunogen, HKC, to a long-term protective one. Our data indicate that blockade of CTLA-4 interactions with its ligands may be useful in enhancing host defenses against C. neoformans.
Cell-mediated immunity is an essential protective mechanism of the host against an infection with the basidiomycetous yeast-like organism Cryptococcus neoformans (reviewed in reference 26). Using two different nonreplicating immunogens, we have shown that not all anticryptococcal cell-mediated immune (CMI) responses, as detected by positive anticryptococcal delayed-type hypersensitivity (DTH) reactions, are indicative of protection (29). For instance, subcutaneous immunization with cryptococcal culture filtrate antigen (CneF) in complete Freund's adjuvant (CFA) induces an anticryptococcal CMI response that is protective, whereas a similar immunization with heat-killed C. neoformans yeast cells (HKC) either alone or in CFA induces anticryptococcal DTH reactivity accompanied by no protection (29). Differing activated-T-lymphocyte profiles exist in the mice undergoing the two different responses. The protective response is associated with a typical Th1-type response, i.e., activated CD4+ T cells that produce gamma interferon and interleukin 2 (IL-2) when stimulated in vitro with CneF (27, 29). These activated CD4+ T cells will transfer anticryptococcal DTH reactivity to naïve mice and will cause amplified DTH reactivity when transferred to naïve recipient mice at the time of immunization of the recipient with CneF-CFA (11, 12, 17). The nonprotective anticryptococcal DTH response has an activated-T-cell profile consisting of CD4+ and CD8+ T cells and an unconventional T-cell population that will directly bind to C. neoformans cells and kill the organism (25, 29, 31). Our laboratory has been interested in gaining an understanding of the host components involved in these two divergent responses with the idea that we might be able to heighten protection or that components in the nonprotective response might be manipulated to provide protection to the host.
A coinhibitory receptor that could be influencing the nature of an anticryptococcal immune response is cytotoxic T lymphocyte antigen 4 (CTLA-4 or CD152). This coinhibitory receptor is structurally similar to the well-characterized costimulatory molecule CD28, which provides the needed secondary signal for effective T-cell activation (14). Both CD28 and CTLA-4 engage the same ligands, B7-1 (CD80) and B7-2 (CD86), on antigen-presenting cells; however, unlike that of CD28, CTLA-4 ligation to B7 results in down-regulation of the adaptive immune response, i.e., inhibition of IL-2 production, IL-2R expression, and T-cell proliferation (6, 19, 34). Expression of CTLA-4 is undetectable on resting T cells, but increased expression occurs on the surfaces of T cells within 24 to 48 h after in vitro stimulation with a mitogen or nominal antigen (2, 13, 32) or is detectable on T cells from draining lymph nodes by 2 days after intranasal stimulation with peptide (24). Blockade of the signal delivered by CTLA-4 has been shown to result in increased severity of autoimmune diseases (15), improved clearance of infectious agents (23, 30, 33), increased adaptive immune responses to infectious agents without improved clearance (18), and prevention of the induction of peripheral tolerance (35). It is not altogether clear whether CTLA-4 functions during the induction or the expression phase of an immune response. However, based on data from in vitro studies in which CTLA-4 ligation has been shown to inhibit induction of mRNA for the T-cell growth factor, IL-2, as well as interfere with production of components critical to cell cycle progression in T cells (6), it might be predicted that CTLA-4 plays a role in induction rather than expression of the immune response. Another unresolved issue is whether blockade of CTLA-4 can skew the immune response. Saha et al. (33) have reported that CTLA-4 blockade biases an immune response towards a Th1 response; however, there are reports that show little to no effect of CTLA-4 blockade on the characteristics of the immune response, with the only effect of the blockade being augmentation of the typical response induced by the immunogen (30).
The purpose of this study was to investigate the effects of CTLA-4 blockade on the induction and expression phases of protective and nonprotective anticryptococcal CMI responses and to determine if the blockade would change the nonprotective response against C. neoformans into a protective response. Our data illustrate that CTLA-4 plays an inhibitory role during the induction phase of both protective and nonprotective anticryptococcal CMI responses. CTLA-4 engagement does not affect the expression of an ongoing anticryptococcal CMI response. Only mice immunized with the protection-inducing immunogen and treated with anti-CTLA-4 show significantly lengthened survival times when infected intravenously (i.v.) with a weakly virulent isolate of C. neoformans. The nonprotective response could not be converted to a protective response by blocking CTLA-4 engagement with its ligand. Blockade of CTLA-4 during an infection with a highly virulent isolate given by the intratracheal (i.t.) route did increase survival times. These findings lead to the speculation that blockade of CTLA-4 might be useful under certain defined conditions as a therapeutic measure in cryptococcosis and possibly other infectious diseases in which a protective immune response is induced by the organism.
MATERIALS AND METHODS
Mice.
Female CBA/J mice (Jackson Laboratory, Bar Harbor, Maine) from 7 to 10 weeks of age were used in all experiments.
Organisms.
C. neoformans serotype A isolate 184A was used to prepare the HKC, to prepare the culture filtrate antigen, CneF, for the immunization procedures, and for i.v. infection studies. C. neoformans isolate NU-2 (serotype A) was used for the i.t.-infection experiments. Isolate 184A has a small capsule and is weakly virulent, whereas NU-2 has a large capsule and is highly virulent for mice (3).
Maintenance of endotoxin-free conditions.
To prevent endotoxin from influencing experimental outcomes, all experiments were done under conditions to minimize endotoxin contamination. This included the use of endotoxin-free plasticware, glassware that had been heated for 3 h at 180°C, and reagents that contained less than 8 pg of endotoxin per ml (minimal detectable level of the assay used) when tested with the Limulus amebocyte lysate assay (Whittaker Bioproducts Inc., Walkersville, Md.).
Antigens.
HKC preparations were made by growing the 184A C. neoformans isolate on modified Sabouraud dextrose agar for 3 days before harvesting cells in endotoxin-free sterile physiological saline solution (saline). The cryptococcal cells were harvested from Sabouraud dextrose agar slants, heated at 60°C for 1 h, and then washed three times in saline. After being washed, the cryptococcal cells were counted using a hemocytometer. The lack of viability of the cryptococcal cells was confirmed by plating the suspension on Sabouraud dextrose agar. Appropriate dilutions of HKC were made to give mice 107 HKC in 0.2 ml of saline.
Cryptococcal culture filtrate antigen CneF used in the immunization and footpad challenge procedures was prepared with C. neoformans isolate 184A as described previously (7, 28). Briefly, a defined medium was inoculated with 109 C. neoformans cells per liter of medium and incubated for 5 days at 30°C before the cryptococcal cells were killed with 2% Formalin. At 24 h after addition of the Formalin, the cells were removed from the culture supernatant with a OM-141 Pelicon tangential-flow system and a 0.45-μm-pore-size cassette (Millipore, Bedford, Mass.). The culture supernatant was washed extensively with saline and concentrated 10-fold with a 30,000-molecular-weight-cutoff cassette in the Pelicon system. The concentrated CneF was filter sterilized and stored at −20°C until used. The CneF had a protein concentration of 0.268 mg/ml as determined by the bicinchoninic acid assay (BCA protein assay; Pierce Chemical Co., Rockford, Ill.) and a carbohydrate concentration of 5.8 mg/ml as determined by the phenol-sulfuric acid assay (10). CneF-CFA was prepared by emulsifying 1 part CneF with 1 part CFA (Difco Laboratories, Detroit, Mich.) (vol/vol).
Generation of Fab fragments.
Hamster anti-mouse CTLA-4 antibody (Ab) was purified from the hybridoma UC10-4F10-11 (a gift from Jeffrey Bluestone, University of Chicago) supernatant using a protein G affinity column (Pharmicia Biotech Inc., Uppsala, Sweden). After purification, the anti-CTLA-4 Ab was dialyzed extensively against phosphate-buffered saline (PBS) to remove residual acid from the solution. To generate Fab fragments, the purified anti-CTLA-4 Ab was placed in an equal volume of digestion buffer (PBS, 0.02 M EDTA [Sigma Aldrich, St. Louis, Mo.], 0.02 M cysteine [Sigma Aldrich]). Papain was added to the solution until an enzyme/antibody ratio of 1:20 (wt/wt) was achieved. The digestion reaction mixture was incubated at 37°C overnight. The Fab fragments were then purified using a protein G column (Pharmicia Biotech Inc.), and the purity of the fragments was determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The fragments were tested for functionality using a previously defined system (35). Briefly, the functionality test consisted of showing the ability of the Fab fragments to prevent the deletion of Vβ8 T-cell receptor-positive (TCR+) T cells after injection with the superantigen staphylococcal enterotoxin B (SEB). CBA/J mice were injected intraperitoneally (i.p.) with 100 μg of SEB (Sigma Aldrich) or saline. Each day for the next 7 days the mice were injected with 100 μg of anti-CTLA-4 Fab fragments, hamster control antibody, or saline. At the end of the treatment period, the mice were killed and their spleens were removed for analysis. The splenocytes were stained with anti-CD4 (Cy-Chrome; rat immunoglobulin G2a (IgG2a); Pharmingen, San Diego, Calif.) and anti-Vβ8 TCR (fluorescein isothiocyanate, mouse IgG2a, Pharmingen) Abs. The splenocytes were then examined for the presence of Vβ8+ CD4+ T cells using flow cytometric methods as described by Walunas and Bluestone (35). We found that treatment with the anti-CTLA-4 Fab fragments resulted in the persistence of CD4+ Vβ8+ T cells in the spleens of mice treated with SEB, whereas mice given control antibody or SPSS injections had CD4+ Vβ8+ T-cell levels significantly lower than those of untreated control animals (data not shown). These results indicated that CTLA-4 signaling had been blocked effectively by the anti-CTLA-4 Fab fragments, allowing the persistence of a T-cell population which would otherwise be deleted in animals treated with SEB (35). Consequently, the anti-CTLA-4 Fab fragment preparation was deemed active and was used throughout the studies.
Ab treatment.
Mice were injected i.p. with 100 μg of anti-CTLA-4 Fab fragments (clone UC10-4F10-11), anti-CTLA-4 IgG, or hamster IgG control Ab (Cappel, Weschester, Pa.) in 0.4 ml of saline on the day before immunization or infection. The anti-CTLA-4 Fab fragment, anti-CTLA-4 IgG, or control IgG treatment was continued every day afterward until 0.7 mg of Fab fragments, anti-CTLA-4 IgG, or control IgG had been administered. On the second day of anti-CTLA-4 or control Ab treatment, the mice were immunized with either HKC or CneF-CFA. The immunizations were performed by injecting mice subcutaneously (s.c.) at each of two sites near the base of the tail with 0.1 ml of a 1:1 emulsion of CneF-CFA or with 107 HKC in saline. Control mice were injected with saline or saline-CFA in a similar manner. On day 7 after immunization or control treatment, mice either had their footpads tested or were infected i.v. with 105 viable C. neoformans cells.
In one set of experiments we examined the effect of CTLA-4 blockade during the expression phase of the anticryptococcal CMI response. In this, mice were immunized as described above with HKC or CneF-CFA s.c. at each of two sites near the base of the tail. As before, control mice were injected with saline-CFA in a similar manner. On days 6 and 7 after immunization, the mice were given i.p. injections of 350 μg of anti-CTLA-4 IgG, anti-CTLA-4 Fab fragments, or control hamster IgG (Cappel) in 0.4 ml of saline. On day 7, the animals were assessed for DTH reactivity.
Having observed that mice treated with anti-CTLA-4 IgG responded in the same manner as mice treated with anti-CTLA-4 Fab fragments, for the i.t.-infection experiments mice were given 100 μg of anti-CTLA-4 IgG or control IgG in 0.4 ml of saline i.p. twice a week throughout the course of the experiment beginning at the time of infection.
Assessment of DTH reactivity.
Seven days after immunization or infection, the mice from each treatment group were subjected to measurements of hind footpads prior to injecting the right hind footpad with 0.03 ml of CneF and the left hind footpad with 0.03 ml of saline. The footpads were measured at 24 h after antigen or saline injection to determine the level of swelling. The DTH reaction, which is characterized as a significant increase in footpad swelling in the antigen-injected pad over the saline-injected pad at 24 h after injection, is indicative of the level of CMI reactivity of the animal to the footpad test antigen (8, 11, 12, 29, 31).
Infection with C. neoformans.
Mice were injected i.v. with 105 viable 184A C. neoformans cells in 0.2 ml of saline. Viability of the cryptococcal cells was determined by plating dilutions of the cryptococcal suspension after infection on Sabouraud dextrose agar. For determination of CFU, lungs, livers, spleens, and brains of the mice were removed at 7 days after infection and the fungal burden of each organ was determined as described previously (9). Five mice per group were used for CFU and DTH reactivity experiments, whereas 10 mice per group were used for survival studies.
For i.t. infections, animals were anesthetized with ketamine (50 mg/kg of body weight) and xylazine (5 mg/kg). The trachea was exposed surgically, a 22-gauge catheter tube was placed in the trachea of the animal, and 25 μl of saline containing 105 viable C. neoformans isolate NU-2 cells was injected into the trachea with a Hamilton syringe followed by 50 μl of air to flush the liquid into the lungs. The incision was then closed with wound clips, and the animals were observed daily for survival.
Statistical analysis.
Means, standard errors of the means, and unpaired Student t test results were used to analyze the data from DTH and CFU studies. When comparing two groups, a P value of ≤0.05 was considered to be significant. Survival data were analyzed with Kaplan-Meier survival plots followed by the log-rank test (Prism; GraphPad Software, Inc., San Diego, Calif.) on a personal computer.
RESULTS
Blocking CTLA-4 during induction of an anticryptococcal CMI response induced by immunization with CneF-CFA or HKC boosts the response.
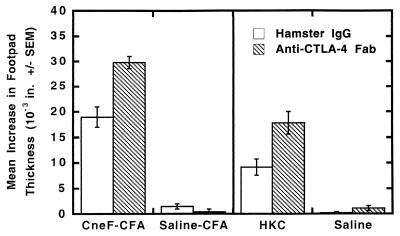
CTLA-4 blockade has been shown to enhance the immune response in both Th1- and Th2-mediated reactivity (16). Therefore, to examine the effect of CTLA-4 blockade on protective and nonprotective anticryptococcal CMI responses, mice were given Fab fragments of anti-CTLA-4 antibody at the time of immunization with either CneF-CFA (induces a protective anticryptococcal CMI response) or HKC (induces a nonprotective CMI response). As expected, we found that blocking CTLA-4 at the time of immunization resulted in significant increases in the anticryptococcal DTH reactivity as measured by footpad swelling (Fig. 1; P was <0.01 for the comparison of control Ab-treated and anti-CTLA-4 Fab-treated groups of HKC- or CneF-CFA-immunized mice). In mice immunized with CneF-CFA and treated with anti-CTLA-4 IgG, the anticryptococcal DTH responses were significantly elevated over the DTH responses of the control Ab-treated group as early as 5 days after immunization (data not shown). These results indicate that CTLA-4 blockade augments the anticryptococcal CMI response early in induction and irrespective of the immunogen. HKC-immunized mice treated with anti-CTLA-4 Fab fragments displayed almost twice the level of anticryptococcal DTH reactivity as did the hamster IgG-treated, HKC-immunized animals (mean increase in footpad thickness of 17.8 × 10−3 ± 2.3 × 10−3 in. compared to 9.2 × 10−3 ± 1.7 × 10−3 in., respectively). In fact, blockade of CTLA-4 at the time of immunization with HKC resulted in a level of DTH reactivity similar to that induced by CneF-CFA in mice treated with the control Ab (Fig. 1).
FIG. 1.
Blockade of CTLA-4 at the time of induction of an immune response to cryptococcal antigens significantly augments anticryptococcal DTH. Mice were treated i.p. with either hamster IgG, as a control, or anti-CTLA-4 Fab to block CTLA-4 1 day before, the day of, and days 1 through 5 after immunization with cryptococcal culture filtrate antigen in CFA (CneF-CFA) or HKC or treatment with saline or saline-CFA on day 0. On day 7 after immunization, the mouse footpads were challenged with CneF or saline, and footpad swelling was measured on day 8. Five mice were used per group, and the experiment was repeated four times with similar results. Error bars show standard errors of the means.
CTLA-4 blockade during the expression phase of an anticryptococcal CMI response has no effect on the response.
Having found that CTLA-4 blockade enhanced the anticryptococcal CMI response when the blocking reagent (CTLA-4 Fab fragments) was given during the induction phase of the response, we wanted to know if the same blocking agent would alter the anticryptococcal DTH response when given after the immune T cells had been induced. For this, we immunized groups of mice with HKC or CneF-CFA and injected a group with saline-CFA as a control. The mice were given anti-CTLA-4 IgG, anti-CTLA-4 Fab fragments, or control hamster IgG i.p. as before to show the efficacy of the treatments or on days 6 and 7 after immunization. The footpads of animals were injected on day 7 with CneF or saline, and footpad swelling was measured on day 8. Immunized mice treated with hamster IgG had the expected levels of DTH reactivity (29, 31), and the CTLA-4 blocking Ab preparations enhanced the anticryptococcal DTH responses when given during induction of the response (Table 1). In contrast, CTLA-4 blockade during the expression phase had no effect on the DTH reactivity of any treatment group (Table 1). In the immune groups given hamster IgG, anti-CTLA-4 Fab fragments, or anti-CTLA-4 Ab, the mean increases in the thickness of the CneF-injected footpads were significantly elevated over the mean increases in footpad thickness in the antigen-challenged pads in the respective treatment groups of saline-CFA-injected mice (P < 0.05).
TABLE 1.
Blockade of CTLA-4 Fab with either anti-CTLA-4 IgG or anti-CTLA-4 Fab augments the anticryptococcal DTH response if the blocking reagent is given during the induction period (days −1, 0, and +1 through +3) but not if the blocking reagent is given after the sensitized T cells have been induced (days 6 and 7)
| Treatment days and immunization group | Mean increase in footpad thickness (10−3 in.) ± SEM of mice treated with:
|
||
|---|---|---|---|
| Hamster IgG control | Anti-CTLA-4 IgG | Anti-CTLA-4 Fab | |
| Days −1, 0, 1, 2, and 3a | |||
| CneF-CFA | 19.0 ± 0.9 | 33.0 ± 3.3 | 29.7 ± 0.3 |
| HKC | 11.5 ± 1.2 | 19.5 ± 1.3 | 19.0 ± 0.6 |
| Saline-CFA | 0.3 ± 0.3 | 0.3 ± 0.5 | 0.5 ± 0.3 |
| Days 6 and 7a | |||
| CneF-CFA | 25.2 ± 1.5 | 21.0 ± 1.0 | 23.0 ± 1.9 |
| HKC | 15.0 ± 1.9 | 15.6 ± 1.3 | 14.0 ± 0.9 |
| Saline-CFA | 0.7 ± 0.3 | 0.5 ± 0.3 | 0.8 ± 0.5 |
Treatment days relative to day of immunization (day 0).
Treatment with anti-CTLA-4 Fab fragments reduces the numbers of cryptococcal CFU in tissues of infected mice.
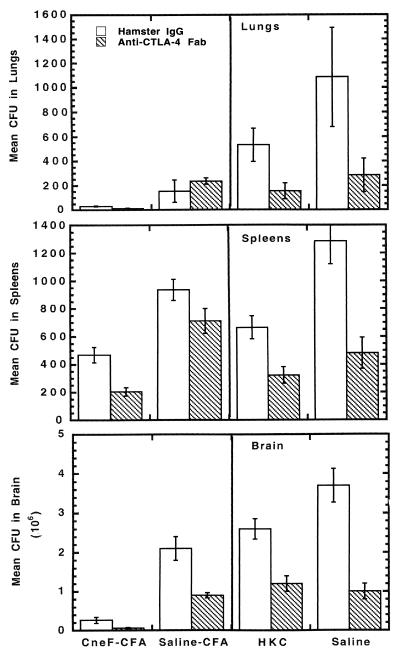
To assess whether or not treatment with anti-CTLA-4 would also affect clearance of the organisms from tissues after infection, we evaluated the numbers of cryptococcal CFU in lungs, livers, spleens, and brains of C. neoformans-infected mice that had been immunized with CneF-CFA or HKC or treated with saline-CFA or saline 7 days prior to infection. As expected based on our previous work (29), CneF-CFA-immunized mice were found to have significantly lower numbers of CFU in lungs, spleens, and brains than did the saline-CFA-treated group when the respective tissues were compared (P < 0.05; Fig. 2). The HKC-immunized group also had lower numbers of CFU in lungs, spleens, and brains than the saline-treated control mice in the respective tissues (P < 0.05; Fig. 2). The results from the hamster IgG-treated animals were consistent with our previous findings (29).
FIG. 2.
Blockade of CTLA-4 during induction of the immune response results in increased early clearance of C. neoformans from lungs, spleens, and brains. Mice were treated with hamster IgG or anti-CTLA-4 Fab and immunized as described in the Fig. 1 legend, but instead of footpad challenge on day 7 after immunization, the mice were infected i.v. with 105 C. neoformans 184A cells. Seven days after infection, the numbers of cryptococcal CFU were determined in the designated tissues. Five mice were used per group, and the experiment was repeated twice with similar results. Error bars show standard errors of the means.
Blockade of CTLA-4 with anti-CTLA-4 resulted in significantly (P < 0.05) reduced numbers of cryptococcal CFU in lungs, spleens, and brains of immunized and control treatment groups of infected mice compared to CFU in the respective organs from mice treated with hamster control IgG with one exception (Fig. 2). The exception was in mice initially treated with saline-CFA, where the lung CFU of the anti-CTLA-4-treated group were not reduced below those of the hamster IgG-treated control group (Fig. 2). CTLA-4 blockade did not result in a reduction in cryptococcal CFU in the livers of mice from any of the treatment groups (data not shown).
Blocking CTLA-4 enhances DTH reactivity at 7 days after infection.
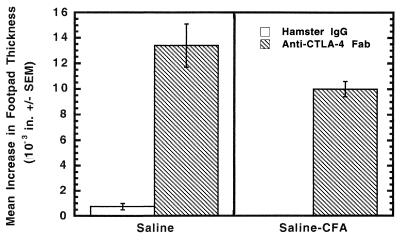
Having observed that blockade of CTLA-4 resulted in significantly (P < 0.01) lower numbers of cryptococcal CFU in the spleens and brains of saline-treated and saline-CFA-treated mice than in saline-treated or saline-CFA-treated mice given hamster IgG, we surmised that the CTLA-4 blockade improved clearance of the organism because it augmented the development of the anticryptococcal CMI response in response to infection. To test this prediction, we blocked CTLA-4 at the time we treated mice with saline or saline-CFA (anti-CTLA-4 Fab was given on days −1, 0, and +1 through +5). In addition, five mice from each treatment group were given hamster IgG as a control in place of anti-CTLA-4 Fab. All the mice were infected with C. neoformans 184A i.v. on day 7 after saline or saline-CFA treatment. The level of anticryptococcal DTH reactivity induced by the infection was measured on day 8 after infection. Mice typically do not develop measurable levels of anticryptococcal DTH reactivity until 14 to 21 days after i.v. infection with C. neoformans isolate 184A (unpublished observations). Here we found that mice given saline or saline-CFA and treated with hamster IgG prior to infection did not develop positive (>5 × 10−3 in.) anticryptococcal DTH responses by day 7 of the infection (Fig. 3). In contrast, infected animals treated with anti-CTLA-4 Fab displayed significantly positive anticryptococcal DTH reactions compared to the hamster IgG-treated groups (P < 0.0004) (Fig. 3). These results indicate that CTLA-4 blockade allows the mice to develop an anticryptococcal CMI response earlier than expected during an infection.
FIG. 3.
Blockade of CTLA-4 results in mice developing anticryptococcal DTH reactivity as early as 7 days after infection. Mice were treated with hamster IgG or anti-CTLA-4 Fab and injected with saline or saline-CFA as indicated in the Fig. 1 legend. On day 7 after treatment with saline or saline-CFA, the mice were infected i.v. with 105 C. neoformans 184A cells. Seven days after infection, the mouse footpads were challenged and the footpad swelling was measured the following day. Five mice were used per experimental group, and the experiment was repeated twice with similar results. Error bars show standard errors of the means.
Blockade of CTLA-4 enhances survival of mice immunized with CneF-CFA but not of mice immunized with HKC.
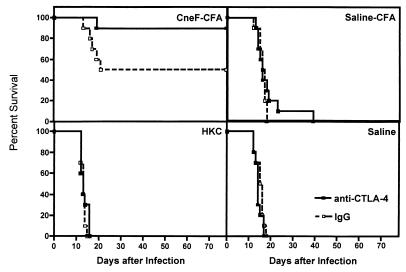
Reduction in cryptococcal CFU in tissues early after infection does not always translate into increased survival of the mice (29). Consequently, we decided to determine if CTLA-4 blockade in mice undergoing a protective anticryptococcal CMI response induced by immunization with CneF-CFA and in mice undergoing a nonprotective anticryptococcal CMI response induced with HKC would survive longer than mock-immunized (saline-CFA- or saline-treated) mice. Given the observed increased anticryptococcal CMI response and decreased CFU in mice that had been treated with anti-CTLA-4 Fab, we expected to see an extension of survival times in the immune groups treated with anti-CTLA-4 Fab compared to mice treated with hamster IgG. Contrary to our expectations, only mice immunized with the protective immunogen, CneF-CFA, and given anti-CTLA-4 Fab had significantly extended survival times (P < 0.01) over the control IgG-treated mice (Fig. 4; CneF-CFA). CTLA-4 blockade in HKC-immunized mice did not result in improved survival times beyond survival times of control IgG-treated mice (Fig. 4; HKC). Neither the saline-treated nor the saline-CFA-treated control groups that were given anti-CTLA-4 Fab survived significantly longer than the normal IgG-treated controls (Fig. 4; saline-CFA and saline).
FIG. 4.
Increased protection against C. neoformans as measured by increased survival times is seen only in mice in which CTLA-4 is blocked during immunization with the protective immunogen, CneF-CFA. Mice were immunized and treated with hamster IgG or anti-CTLA-4 Fab as described in the Fig. 1 legend. The animals were infected i.v. with 105 C. neoformans 184A cells on day 7 after immunization or treatment with control reagents. Survival of the mice was monitored for 80 days before the experiment was terminated. Ten mice were used per experimental group, and the experiment was repeated twice with similar results.
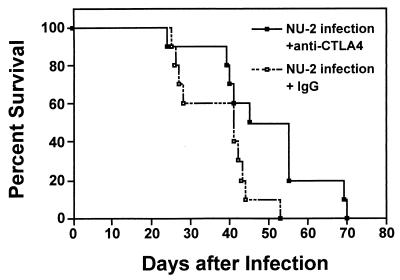
CTLA-4 blockade at the onset of infection with a highly virulent isolate of C. neoformans enhances resistance.
The natural route of acquiring cryptococcosis is by inhalation of the organism, so to assess whether blockade of CTLA-4 would extend the survival time of mice under conditions similar to a natural infection, we infected the animals i.t. with a highly virulent isolate of C. neoformans, NU-2. The weakly virulent isolate 184A used for the i.v. infection studies described above has an approximately 30% mortality rate in mice over a 100-day period when 104 organisms are given i.t., whereas the highly virulent isolate NU-2 kills 100% of the mice within 65 days after i.t. infection with 104 organisms (4). Consequently in the present study, we infected mice i.t. with 105 NU-2 cells and treated half of the animals with anti-CTLA-4 IgG and the other half with hamster IgG as a control. All of the animals in the control group were dead by day 52 (mean survival time = 41 days) (Fig. 5). Mice given anti-CTLA-4 IgG survived significantly longer (P = 0.02), with a mean survival time of 50 days.
FIG. 5.
Blockade of CTLA-4 during a C. neoformans infection with a highly virulent isolate results in significantly increased survival times for the mice. Mice were infected i.t. with 105 C. neoformans cells of the highly virulent isolate NU-2 on day 0. The animals were injected i.p. with 100 μg of hamster IgG or anti-CTLA-4 IgG on day 0 and day 3 and then twice weekly for the duration of the experiment. Ten mice were used per group.
DISCUSSION
Blockade of coinhibitory molecule CTLA-4 ligation has been shown to be an effective means of enhancing the T-cell immune responses (5, 19, 23). Our data show that inhibiting CTLA-4-mediated signaling during immunization with cryptococcal antigens or during infection also results in augmented CMI responses as characterized by increased anticryptococcal DTH responses. These results show that CTLA-4 typically plays an inhibitory role in anticryptococcal CMI responses as measured by DTH reactivity to the cryptococcal antigen in mice immunized with the nonreplicating immunogens, i.e., a soluble cryptococcal antigen (CneF) preparation in CFA or HKC, or in mice infected with C. neoformans. Surprisingly, the enhanced anticryptococcal CMI responses mediated by blocking CTLA-4 only translated into improved protection for animals immunized with CneF-CFA. The explanation for this seemingly contradictory result may lie in the types of immune responses induced by the different immunogens. CneF-CFA induces the strongest responses of any of the antigens used in the assays employed. CneF-CFA immunization resulted in superior anticryptococcal DTH reactivity, clearance of the organism, and survival times after infection. HKC, on the other hand, induces CMI reactivity, but the reactivity is not protective and can, under some circumstances, exacerbate the cryptococcal infection (29; unpublished data). Although blockade of CTLA-4 augments the anticryptococcal CMI response induced by HKC, augmentation of this immune response, which is ineffective in protection, does not lead to protection but rather to an augmented ineffective response. In the case of immunization with CneF-CFA, the anticryptococcal CMI response is protective, and CTLA-4 blockade leads to the amplification of that already-protective response resulting in further protection. Considering that CneF-CFA induces CD4+ T cells that produce relatively high levels of gamma interferon that can activate macrophages to kill C. neoformans whereas HKC induces a different array of activated T cells including both CD4+ and CD8+ T cells and T cells that can directly inhibit the growth of C. neoformans, it is possible that mixed populations of activated T cells are either ineffective or insufficient to effect sufficient clearance of the organism to increase the survival time of the mice. Even when the populations of activated T cells are elevated sufficiently to affect the DTH reactivity after blockade of CTLA-4, their activity may either be inappropriate or insufficient to mediate long-term protection.
Augmentation of anticryptococcal immune responses was achieved when the blocking anti-CTLA-4 Ab (either anti-CTLA-4 Fab fragments or whole anti-CTLA-4 Abs) was given during the induction phase of the CMI response (first 5 or 6 days after immunization or infection) and not when the blocking Ab was given during the expression phase (at 6 days after immunization). This outcome was anticipated because expansion of T-cell populations would be greatest during the induction rather than the expression phase of anticryptococcal CMI responses. It is well established that CTLA-4 interactions with B7 ligands block production of the T-cell growth factor, IL-2, and inhibit expression of the IL-2 receptor α-chain, which is needed for IL-2 signaling (19, 34). Furthermore, CTLA-4 ligation blocks the activation of proteins such as cyclin D3 and cyclin-dependent kinases 4 and 6 involved in cell cycling (5, 6, 19, 34). Consequently, these inhibitory activities induced by CTLA-4 binding to B7 would result in a significant reduction in T-cell proliferation, an essential process in the induction of a CMI response. Our observations are consistent with those of Saha et al. (33), who found that anti-CTLA-4 affected DTH reactivity against Leishmania major only when given at the onset of infection.
CTLA-4 is undetectable on naïve T cells but is up-regulated on the T-cell surface within 48 h after stimulation, and inhibitory activity of CTLA-4 in other models has been reported to occur during the first 48 to 72 h after antigenic stimulation (1, 20, 22). Our findings with the cryptococcal model are in accord with these previously described characteristics of CTLA-4 expression and activity. All of the cryptococcal immunogens must stimulate the up-regulation of CTLA-4 on T cells because blockade of CTLA-4 has a significant effect on the level of the anticryptococcal CMI response induced.
Protection due to immune responses against infectious agents can be assessed in two different ways in experimental models. Protection can be related to the numbers of CFU of the organism in tissues of animals at given times after infection. If the numbers of CFU are significantly reduced in the treated or immunized groups compared to the control group, then one might term this protection. Another means of expressing protection is to measure mean survival times after infection. If the mean survival time for animals is significantly extended over control levels, then this could be termed protection. In chronic infectious disease models, it is more meaningful to express protection in terms of extended life expectancy of the animals. Indeed, we have shown in the murine cryptococcosis model that a reduction in cryptococcal CFU counts in tissues assessed after the first week of infection does not always indicate protection, if one uses increased survival time as the ultimate definition of protection. In the studies presented here we measured protection by both parameters and found that reduction in cryptococcal CFU in lungs, spleens, and brains at 7 days after infection did not necessarily translate to long-term protection as assessed by increased survival time. Blockade of CTLA-4 ligation enhanced the ability of the mice to kill C. neoformans in three of the four tissues that were assessed for cryptococcal CFU. Despite this early evidence of clearing of the organism from tissues of mice treated with anti-CTLA-4 and given saline or immunized with HKC, the animals were unable to survive significantly longer than the animals in which CTLA-4 was not blocked. Consequently our criterion for showing enhanced protection is the demonstration of significantly extended survival times. With this definition, it is clear that nonprotective immune responses such as those induced by HKC cannot be skewed to a protective response by the blockade of CTLA-4. In contrast, blocking CTLA-4 during immunization with the immunogen that induces protection, i.e., CneF-CFA, amplifies protection. Our findings add support to the concept that blockade of CTLA-4 does not change the character of the immune response induced by an immunogen but only amplifies the response typically induced by that immunogen.
Infection with C. neoformans by the more natural route of i.t. instillation induces an anticryptococcal CMI response that can be detected by measuring DTH reactivity (3). There appears to be a protective component in the immune response induced during the pulmonary phase of the disease process. We draw this conclusion because when we blocked CTLA-4 during a pulmonary infection with the highly virulent C. neoformans isolate NU-2, we observed that the animals lived significantly longer than did the hamster IgG-treated control infected mice. Considering that we were unable to convert a nonprotective immune response induced by HKC into a protective immune response when we treated mice with anti-CTLA-4 antibody but that we were able to augment a protective immune response by CTLA-4 blockade, we interpret our findings with the i.t. infection model to indicate that the infection itself is inducing a protective immune component that can be augmented by CTLA-4 blockade. The natural route of infection is more likely to induce a stronger CMI response than injection of the organism directly into the bloodstream or into the peritoneum (21; unpublished data). Lungs, the early site of C. neoformans deposition under natural conditions, are known to be populated with effective antigen-presenting cells. Those antigen-presenting cells take up the organism and then migrate to the draining lymph nodes where they lodge and activate T cells. Our findings indicate that blockade of CTLA-4 in the cryptococcosis model should be further investigated with the idea of gaining sufficient information to develop CTLA-4 blockade therapeutic protocols to enhance T-cell-mediated protection in individuals with cryptococcosis.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants AI-15716 and AI-18895 from the National Institute of Allergy and Infectious Diseases.
We thank Fredda Schafer for excellent technical assistance.
REFERENCES
- 1.Alegre M-L, Noel P J, Eisfelder B J, Chuang E, Clark M R R, Reiner S L, Thompson C B. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 2.Allison J P, Krummel M F. The yin and yang of T cell costimulation. Science. 1995;270:932–933. doi: 10.1126/science.270.5238.932. [DOI] [PubMed] [Google Scholar]
- 3.Blackstock R, Buchanan K L, Adesina A M, Murphy J W. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–3609. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackstock R, Murphy J W. Secretion of the C3 component of complement by peritoneal cells cultured with encapsulated Cryptococcus neoformans. Infect Immun. 1997;65:4114–4121. doi: 10.1128/iai.65.10.4114-4121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair P J, Riley J L, Levine B L, Lee K P, Craighead N, Francomano T, Perfetto S J, Gray G S, Carreno B M, June C H. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-XL induction. J Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- 6.Brunner M C, Chambers C A, Chan F K, Hanke J, Winoto A, Allison J P. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 7.Buchanan K L, Murphy J W. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1993;61:2854–2865. doi: 10.1128/iai.61.7.2854-2865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan K L, Murphy J W. Regulation of cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1994;62:2930–2939. doi: 10.1128/iai.62.7.2930-2939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle H A, Murphy J W. MIP-1α contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J Leukoc Biol. 1997;61:147–155. doi: 10.1002/jlb.61.2.147. [DOI] [PubMed] [Google Scholar]
- 10.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 11.Fidel P L, Jr, Murphy J W. Characterization of a cell population which amplifies the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1990;58:393–398. doi: 10.1128/iai.58.2.393-398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidel P L, Jr, Murphy J W. Effects of cyclosporin A on the cells responsible for the anticryptococcal cell-mediated immune response and its regulation. Infect Immun. 1989;57:1158–1164. doi: 10.1128/iai.57.4.1158-1164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn P W, He H, Wang Y, Wang Z, Guan G, Listman J, Perkins D L. Synergistic induction of CTLA-4 expression by costimulation with TCR ligation plus CD28 signals mediated by increased transcription and messenger ribonucleic acid stability. J Immunol. 1997;158:4073–4081. [PubMed] [Google Scholar]
- 14.Harper K, Balzano C, Roouvier E, Mattei M G, Luciani M F, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147:1037–1044. [PubMed] [Google Scholar]
- 15.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney E R, Walunas T L, Karr R W, Morton P A, Loh D Y, Bluestone J A, Jenkins M K. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and is inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 17.Khakpour F R, Murphy J W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s) Infect Immun. 1987;55:1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirman J, McCoy K, Hook S, Prout M, Delahunt B, Orme I, Frank A, LeGros G. CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection. Infect Immun. 1999;67:3786–3792. doi: 10.1128/iai.67.8.3786-3792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummel M D, Allison J P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 21.Lim T S, Murphy J W, Cauley L K. Host-etiological agent interactions in intranasally and intraperitoneally induced cryptococcosis in mice. Infect Immun. 1980;29:633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linsley P S. Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule-4 receptors during T cell activation. J Exp Med. 1995;182:289–292. doi: 10.1084/jem.182.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCoy K, LeGros G. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzler B, Burkhart C, Wraith D C. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. Int Immunol. 1999;11:667–675. doi: 10.1093/intimm/11.5.667. [DOI] [PubMed] [Google Scholar]
- 25.Mody C H, Paine III R, Jackson C, Chen G H, Toews G B. CD8 cells play a critical role in delayed type hypersensitivity to intact Cryptococcus neoformans. J Immunol. 1994;152:3970–3979. [PubMed] [Google Scholar]
- 26.Murphy J W. Cell-mediated immunity and medically related fungi. In: Cunningham M W, Fujinami R S, editors. Effects of microbes on the immune system. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. pp. 593–621. [Google Scholar]
- 27.Murphy J W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993;61:4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy J W, Schafer F, Casadevall A, Adesina A. Antigen-induced protective and nonprotective cell-mediated immune components against Cryptococcus neoformans. Infect Immun. 1998;66:2632–2639. doi: 10.1128/iai.66.6.2632-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy M L, Cotterell S E J, Gorak P M A, Engwerda C R, Kaye P M. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen Leishmania donovani. J Immunol. 1998;161:4153–4160. [PubMed] [Google Scholar]
- 31.Muth S M, Murphy J W. Effects of immunization with Cryptococcus neoformans cells or cryptococcal culture filtrate antigen on the direct anticryptococcal activities of murine T lymphocytes. Infect Immun. 1995;63:1645–1651. doi: 10.1128/iai.63.5.1645-1651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins D, Wang Z, Donovan C, He H, Mark D, Guan G, Wang Y, Walunas T, Bluestone J, Listman J, Finn P W. Regulation of CTLA-4 expression during T cell activation. J Immunol. 1996;156:4154–4159. [PubMed] [Google Scholar]
- 33.Saha B, Chattopadhyay S, Germond R, Harlan D M, Perrin P J. CTLA4 (CD152) modulates the Th subset response and alters the course of experimental Leishmania major infection. Eur J Immunol. 1998;28:4213–4220. doi: 10.1002/(SICI)1521-4141(199812)28:12<4213::AID-IMMU4213>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Walunas T L, Bakker C Y, Bluestone J A. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walunas T L, Bluestone J A. CTLA-4 regulates tolerance and T cell differentiation in vivo. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]