PURPOSE
Nonresponse and relapse after CD19-chimeric antigen receptor (CAR) T-cell therapy continue to challenge survival outcomes. Phase II landmark data from the ELIANA trial demonstrated nonresponse and relapse rates of 14.5% and 28%, respectively, whereas use in the real-world setting showed nonresponse and relapse rates of 15% and 37%. Outcome analyses describing fate after post-CAR nonresponse and relapse remain limited. Here, we aim to establish survival outcomes after nonresponse and both CD19+ and CD19– relapses and explore treatment variables associated with inferior survival.
METHODS
We conducted a retrospective multi-institutional study of 80 children and young adults with B-cell acute lymphoblastic leukemia experiencing nonresponse (n = 23) or relapse (n = 57) after tisagenlecleucel. We analyze associations between baseline characteristics and these outcomes and establish survival rates and salvage approaches.
RESULTS
The overall survival (OS) at 12 months was 19% across nonresponders (n = 23; 95% CI, 7 to 50). Ninety-five percent of patients with nonresponse had high preinfusion disease burden. Among 156 morphologic responders, the cumulative incidence of relapse was 37% (95% CI, 30 to 47) at 12 months (CD19+; 21% [15 to 29], CD19–; 16% [11 to 24], median follow-up; 380 days). Across 57 patients experiencing relapse, the OS was 52% (95% CI, 38 to 71) at 12 months after time of relapse. Notably, CD19– relapse was associated with significantly decreased OS as compared with patients who relapsed with conserved CD19 expression (CD19– 12-month OS; 30% [14 to 66], CD19+ 12-month OS; 68% [49 to 92], P = .0068). Inotuzumab, CAR reinfusion, and chemotherapy were used as postrelapse salvage therapy with greatest frequency, yet high variability in treatment sequencing and responses limits efficacy analysis across salvage approaches.
CONCLUSION
We describe poor survival across patients experiencing nonresponse to tisagenlecleucel. In the post-tisagenlecleucel relapse setting, patients can be salvaged; however, CD19– relapse is distinctly associated with decreased survival outcomes.
BACKGROUND
Chimeric antigen receptor (CAR) therapy targeting CD19 is US Food and Drug Administration–approved for the treatment of patients age < 26 years with refractory B-cell acute lymphoblastic leukemia (B-ALL) or disease in second or greater relapse. Although response and relapse rates have been established across both the clinical trial and commercial settings,1-6 limited data exist describing the risk factors or the fate of tisagenlecleucel nonresponders or patients who relapse. We aimed to establish survival outcomes after post-tisagenlecleucel nonresponse and relapse. We interrogated if baseline characteristics are associated with risk of relapse and whether certain patterns of relapse confer survival advantage.
CONTEXT
Key Objective
Outcomes after post–chimeric antigen receptor (CAR) nonresponse and relapse are yet to be established in the pediatric B-cell acute lymphoblastic leukemia commercial setting. Our key objective was to describe overall survival (OS) and salvage approaches after post-tisagenlecleucel nonresponse and relapse, with distinct analysis across CD19+ and CD19– relapse patterns. To approach this, we extracted data across 80 patients experiencing nonresponse (n = 23) or relapse (n = 57) from the Pediatric Real-world CAR Consortium.
Knowledge Generated
We describe poor survival across patients experiencing post-tisagenlecleucel nonresponse (12 month OS; 19%). On relapse, we report a 12-month OS rate of 52%, yet CD19– disease is distinctly associated with inferior survival (12-month OS; 30%). Although salvage can be achieved after post-CAR relapse, salvage regimens remain highly heterogeneous.
Relevance
We identify patients with CD19– relapse post-tisagenlecleucel to be a high-risk patient population and highlight the need for the systematic study of salvage regimens in the post-tisagenlecleucel nonresponse and relapse setting.
The Pediatric Real-world CAR Consortium (PRWCC) previously described outcomes of 185 children and young adults (CAYAs) treated with commercial tisagenlecleucel. We reported a complete morphologic remission (CR) rate of 85% at day 28 (d28) postinfusion, with 37% of complete responders experiencing disease relapse.2 Center for International Blood and Marrow Transplant Research–generated data reported a similar nonresponse rate of 14.5% after tisagenlecleucel.6 These real-world reports compare with the phase II landmark trial studying tisagenlecleucel, ELIANA, where a nonresponse rate of 19% and a relapse rate of 28% were reported.1 Interestingly, although CD19– disease was the predominant relapse pattern in ELIANA, there was comparatively decreased CD19 downregulation on relapse in the real-world reports, possibly related to overall lower disease burden. Here, we describe characteristics and outcomes of CAYA patients who experienced nonresponse and relapse post-tisagenlecleucel and establish survival rates after post-tisagenlecleucel CD19+ and CD19– relapse.
METHODS
Study Design
We established the PRWCC and previously reported outcomes of 185 CAYA patients infused with tisagenlecleucel across 15 US institutions. Centers obtained independent institutional review board approval. Deidentified data were collected retrospectively and stored in a Health Insurance Portability and Accountability Act–compliant REDCap database. Here, we analyze baseline characteristics, salvage strategies, and survival outcomes across patients who experienced nonresponse or relapse after tisagenlecleucel between August 17, 2017, and March 6, 2020. Nonresponse is defined as detectable disease by morphology with > M1 marrow and/or CNS or non-CNS extramedullary (EM) disease at d28 post-tisagenlecleucel infusion. We additionally study patients who achieved morphological remission at d28, yet continued to have detectable minimal residual disease (MRD) by flow cytometry. Although these patients are not included in patients experiencing nonresponse, we independently describe baseline characteristics in this cohort. We study patients experiencing relapse after tisagenlecleucel, with relapse defined as detectable disease by morphology, CNS/non-CNS EM disease, and/or any level of MRD by flow cytometry or next-generation sequencing disease driving new antileukemia therapy. CD19 status at time of relapse was captured, as defined by institutional reporting. Because of lack of systematic quantification of antigen density, we use CD19 downregulation to include total or partial CD19 loss.
Study Aims, Clinical Outcomes Assessment, and Statistical Approach
Our primary aim was to establish the overall survival (OS) rate after post-tisagenlecleucel relapse. We first characterize relapse patterns using cumulative incidence curves for overall relapse and relapse with CD19+ and CD19– diseases. We measure OS from time of post-CAR relapse to occurrence of death by any cause or censored at last follow-up. Our secondary aim was to assess whether distinct relapse patterns are associated with improved OS. Postrelapse OS was measured for patients relapsing with CD19+ and CD19– disease. We additionally compare OS in patients experiencing post-CAR relapse with patients who remain relapse-free, using a 6-month landmark time point, with the 6-month time point chosen to capture the majority of relapses while minimizing sample attrition from postrelapse death. Our exploratory aims include analysis of associations between baseline patient and disease characteristics and nonresponse or relapse and characterization of salvage regimens used in the post-tisagenlecleucel relapse setting.
The cumulative incidence of relapse, CD19+ and CD19– relapse, was estimated using the Aalen-Johansen estimator. For overall relapse, death is treated as a competing event. For relapse with CD19+ and CD19– disease, relapse with the other disease type and death are treated as competing events. Baseline covariates investigated for association with nonresponse and relapse include the following: sex, age at diagnosis, age at infusion, race/ethnicity, cytogenetics, number of pre-CAR relapses, prior therapy lines, prior CD19-directed therapy, prior hematopoietic stem-cell transplantation (HSCT), disease burden at CAR T infusion (high burden defined as > 5% bone marrow [BM] lymphoblasts, CNS3/EM, or peripheral blood lymphoblasts before lymphodepleting chemotherapy; low burden includes patients with detectable disease, not meeting high burden criteria), CAR T-cell viability, percent CAR expression, time from diagnosis to CAR T infusion, and refractory/relapse status. We conducted univariate analyses examining these baseline characteristics to determine whether they are associated with relapse, CD19+ relapse and CD19– relapse, and OS postrelapse. Associations between patient and disease characteristics and relapse and death were assessed using Cox proportional hazards models. For the analysis of relapse, death was considered as a competing risk, and for CD19+ and CD19– relapses, the other type of relapse and death were treated as competing events, and cause-specific hazards were modeled using Cox models. Multivariate analysis of factors associated with OS after post-CAR relapse was performed using variables achieving significance in the univariate model.
OS for the overall relapse cohort and patients distinctly relapsing with CD19+ and CD19– diseases was estimated using Kaplan-Meier (KM) curves, with CD19+ and CD19– cohorts compared using the log-rank test. OS across patients receiving secondary CAR infusions because of relapse was estimated using KM curves. OS on the basis of timing from infusion to relapse was estimated using KM curves, analyzing 0-60, 60-180, and > 180 days to relapse as categorical variables, with cohorts compared using the log-rank test. Landmark analysis was used to compare OS in patients who relapse versus remained relapse-free at the 180-day landmark time point. Clinical course after nonresponse and relapse is descriptively illustrated. Characterization of lines of salvage therapies and respective responses is reported descriptively, using response by morphology and flow cytometry MRD, as reported by the treating physician. P values < .05 were considered significant.
RESULTS
Characteristics and Outcomes of Patients With Nonresponse Post-Tisagenlecleucel
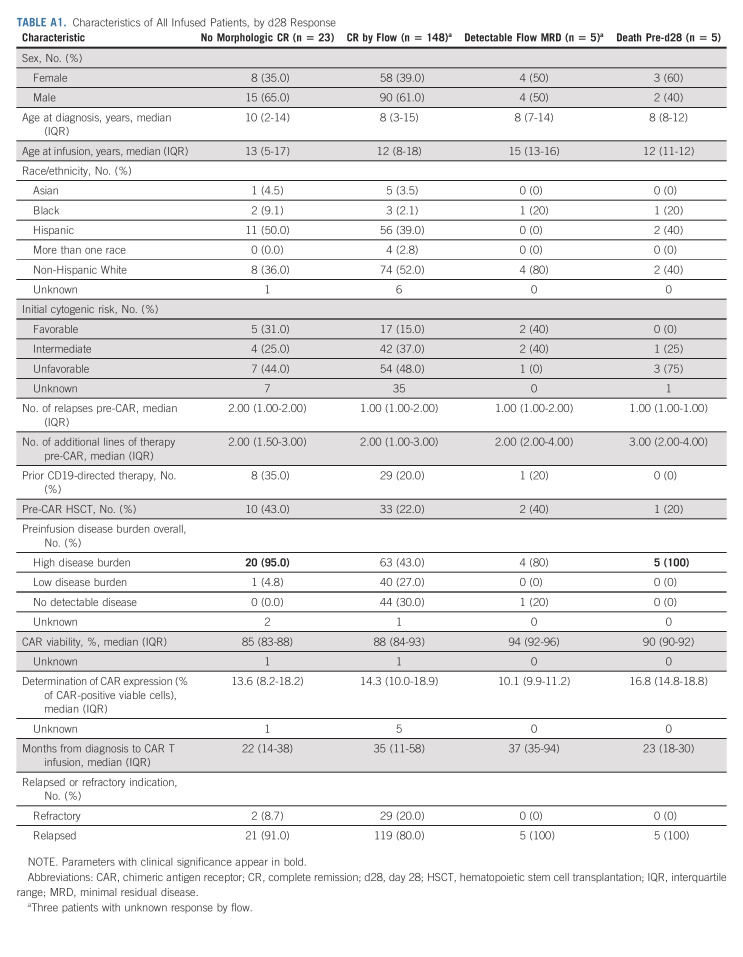
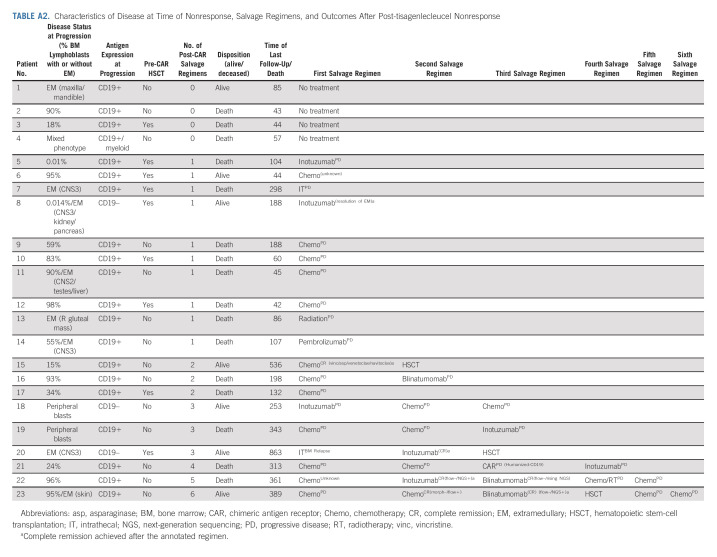
Across 184 patients evaluable for response, 23 (12.5%) did not achieve CR to tisagenlecleucel, as defined by morphologic or EM disease at d28. Five additional patients (3%) had detectable MRD by flow cytometry at this time, despite morphologic remission. Baseline characteristics of responders and nonresponders are reported in Appendix Table A1 (online only). Notably, 20 of 23 (95%) patients experiencing nonresponse had high baseline disease burden. At time of nonresponse, 22 of 23 (96%) patients had high disease burden with morphologic disease or EM (Appendix Table A2, online only). At time of nonresponse, three patients had CD19 antigen downregulation, one additional patient developed mixed phenotype acute leukemia (CD19+/myeloid), and remaining 19 patients had conserved CD19 expression. Details of salvage therapies after nonresponse are included in Appendix Table A2. Among nonresponders, 16 of 23 experienced death before last follow-up and the OS at 3, 6, and 12 months was 59% (95% CI, 41 to 84), 43% (95% CI, 26 to 71), and 19% (95% CI, 7 to 50), respectively. The median survival was 160 days (95% CI, 76 to 333).
Characteristics of Patients Experiencing Relapse Post-Tisagenlecleucel
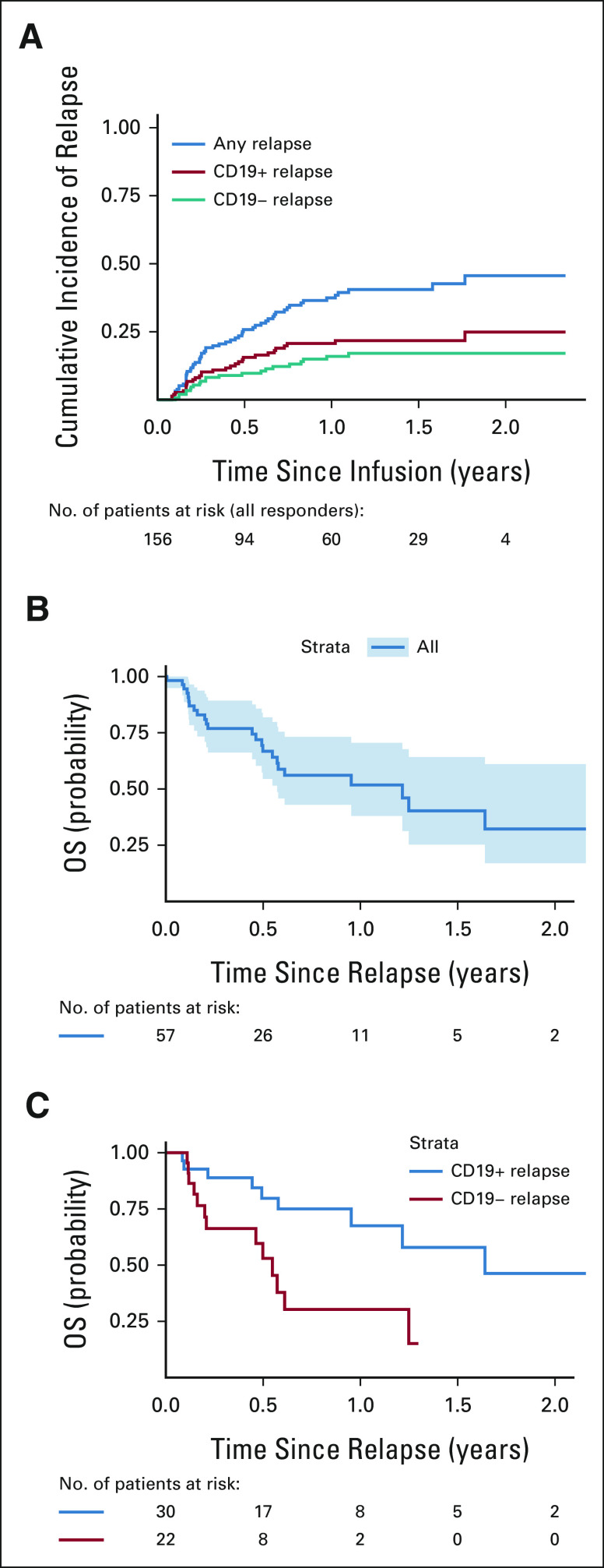
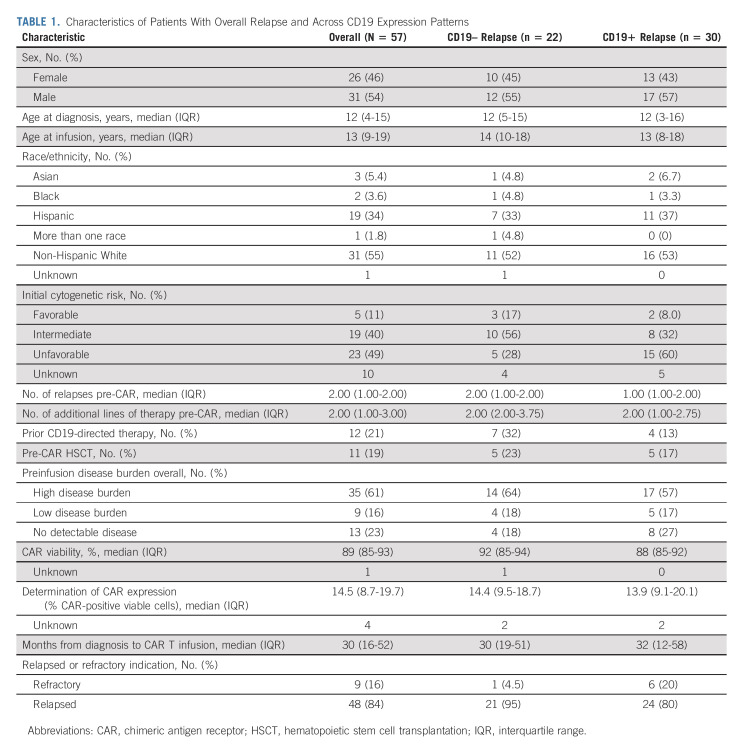
Of 156 patients achieving a CR, with a median follow-up of 380 days postinfusion, 57 experienced relapse disease post-tisagenlecleucel. The cumulative incidence of relapse among responders was 16% (95% CI, 11 to 23), 26% (95% CI, 20 to 34), and 37% (95% CI, 30 to 47) at 3, 6, and 12 months, respectively (Fig 1A). Baseline characteristics of patients who relapsed after tisagenlecleucel are included in Table 1. As previously reported, the median time from infusion to relapse among those who relapsed was 101 (range 30-645) days. The median time from relapse to last follow-up was 176 (range 2-806) days.
FIG 1.
(A) Cumulative incidence of relapse and OS after relapse in (B) the overall cohort and (C) patients with CD19+ and CD19– relapse. OS, overall survival.
TABLE 1.
Characteristics of Patients With Overall Relapse and Across CD19 Expression Patterns
Of 57 relapses, 48 (84%) had BM involvement (> M1morphologic marrow [n = 26]), flow-positive/morphology-negative (n = 14), flow-positive/morphology unknown (n = 3), and next-generation sequencing–positive/flow-negative (n = 5). Of 48 patients with BM involvement, 10 patients (21%) had combined BM and EM diseases (CNS: n = 3, testicular: n = 1, skin: n = 1, ocular: n = 1, and others: n = 4). Six (11%) had isolated EM disease (CNS: n = 5 and skin: n = 1). Relapse site was not reported in three patients.
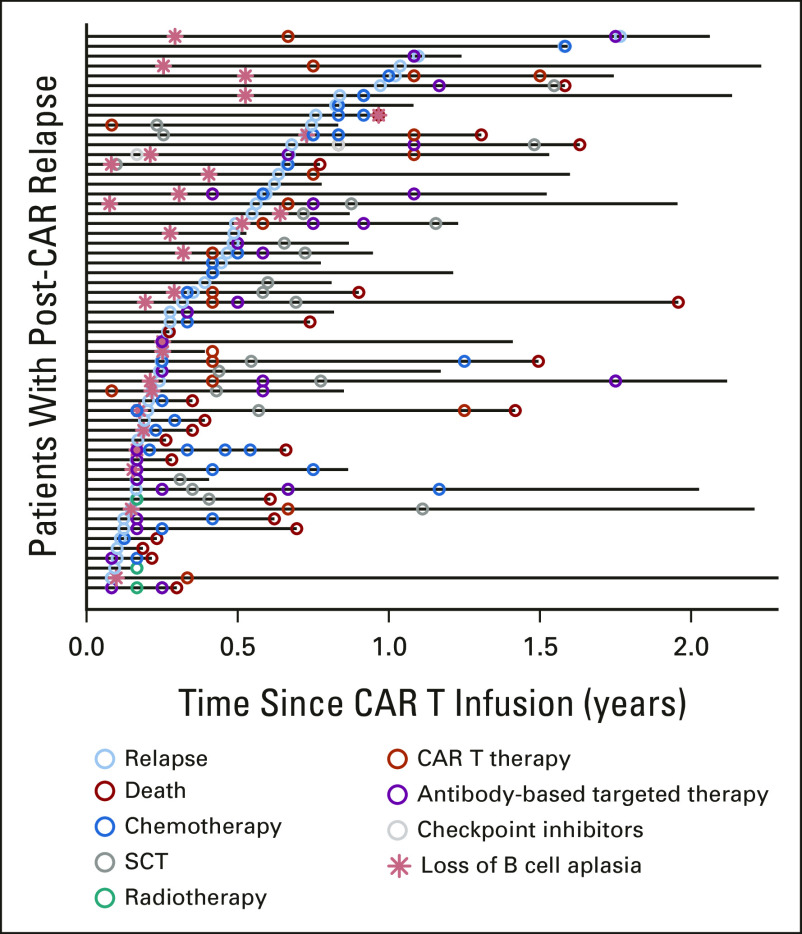
Relationship between loss of B-cell aplasia (BCA) and relapse was available for 25 relapsed patients (Fig 2). In 15 patients, loss of BCA preceded relapse with the median time between loss of BCA and relapse of 84 (range 4-538) days. Seven patients relapsed concurrently with loss of BCA, and three patients had ongoing documented BCA at time of relapse (CD19+: n = 2 and CD19–: n = 1).
FIG 2.
Interventions and outcomes across individual patients after relapse post-CAR T-cell therapy (swimmer's plot). CAR, chimeric antigen receptor.
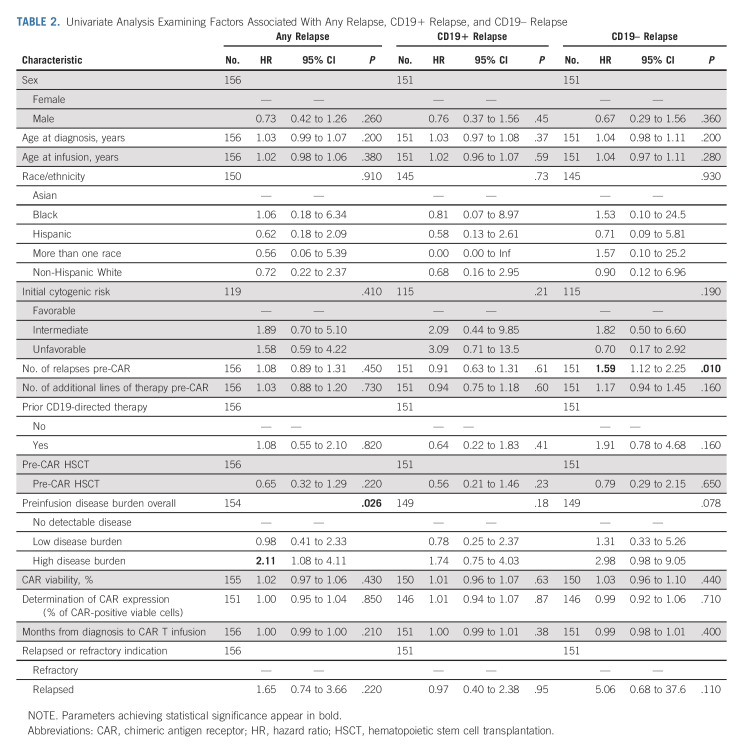
Fifty-two patients had CD19 status evaluable at relapse. Forty-one percent (22 of 52) relapsed with CD19 loss or downregulation, and 59% (30 of 52) relapsed with conserved CD19 expression. Cumulative incidence of CD19+ relapse was 9% (95% CI, 5 to 15), 16% (95% CI, 11 to 23), and 21% (95% CI, 15 to 29) at 3, 6, and 12 months, respectively. The cumulative incidence of CD19– relapse was 7% (95% CI, 4 to 12), 10% (95% CI, 6 to 16), and 16% (95% CI, 11 to 24) at 3, 6, and 12 months, respectively (Fig 1A). Median duration from infusion to relapse was similar across patients relapsing with CD19+ (median 104 days, range 30-645) and CD19– (median 101 day, range 37-401) diseases. Baseline characteristics across patients relapsing with CD19+ and CD19– diseases are included in Table 1. Univariate analysis studying risk of relapse across baseline characteristics identified high disease burden to associate with increased risk of overall relapse (hazard ratio [HR] 2.11 [95% CI, 1.08 to 4.11] for high burden versus no detectable disease, P = .026, Table 2). Univariate analysis studying baseline characteristics as predictors of CD19+ and CD19– relapse identified the number of relapses pre-CAR to associate with post-CAR CD19– relapse (HR 1.59 [95% CI, 1.12 to 2.25], P = .010). Preinfusion high disease burden (HR 2.98 [95% CI, 0.98 to 9.05], P = .078), relapse compared with refractory status (HR 5.06 [95% CI, 0.68 to 37.6]; P = .11), and prior CD19-targeted therapy (HR 1.91 [95% CI, 0.78 to 4.68]; P = .16) were estimated to have large HRs for CD19– relapse risk, yet do not achieve significance. Variables distinctly predictive of CD19+ relapse were not identified with significance.
TABLE 2.
Univariate Analysis Examining Factors Associated With Any Relapse, CD19+ Relapse, and CD19– Relapse
Outcomes After Post-Tisagenlecleucel Relapse
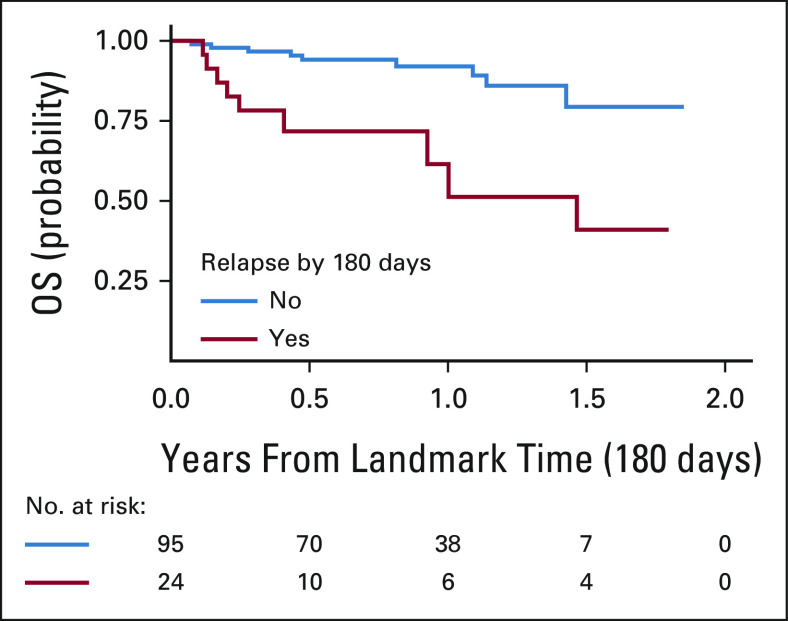
OS rates at 3, 6, and 12 months post-tisagenlecleucel relapse were 77% (95% CI, 66 to 89), 67% (95% CI, 55 to 82), and 52% (95% CI, 38 to 71), respectively (Fig 1B). Notably, CD19– post-tisagenlecleucel relapse is associated with significantly decreased OS rates, with 3-, 6-, and 12-month OS rates of 66% (95% CI, 49 to 90), 53% (95% CI, 34 to 82), and 30% (14 to 66), as compared with 3-, 6-, and 12-month OS rates of 90% (95% CI, 78 to 100), 80% (95% CI, 655 to 98), and 68% (95% CI, 49 to 92) after CD19+ post-tisagenlecleucel relapse (P = .0068; Fig 1C). Univariate analysis of factors associated with survival after post-tisagenlecleucel relapse identifies the number of additional lines of pre-CAR therapy (HR 128 [95% CI, 1.04 to 1.57]; P = .021) and relapse with CD19– disease (HR, 3.25 [95% CI, 1.32 to 7.99], P = .010) to significantly associate with decreased survival (Table 3). Multivariate analysis across variables with univariate significance confirms that CD19– relapse is associated with greater risk of death postrelapse (HR, 2.83 [95% CI, 1.11 to 7.20]; P = .03, Table 3). Timing of relapse after tisagenlecleucel (0-60, 60-180, or > 180 days) did not significantly affect OS after relapse (P = .37; Appendix Fig A1 [online only]).
TABLE 3.
Univariate and Multivariate Analyses of Factors Associated With Overall Survival After Relapse
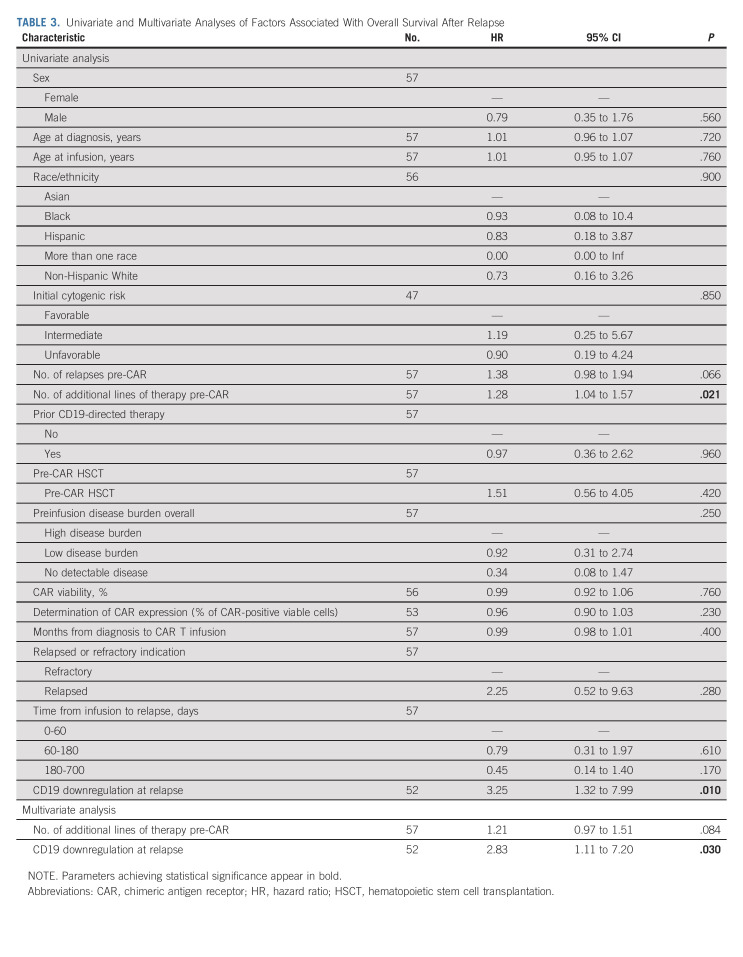
To evaluate the impact of relapse by 6 months on survival, we conducted a 6-month landmark OS analysis across 119 patients with a minimum follow-up of 6 months. Of these, 20% (n = 24) relapsed before 6 months and 80% (n = 95) were relapse-free at 6 months postinfusion. Nine of 24 (38%) and nine of 95 (9%) patients in respective relapse and nonrelapse cohorts experienced post-tisagenlecleucel death. We demonstrate that relapse before 6 months post-tisagenlecleucel is strongly associated with an increased risk of death (HR, 4.75 [95% CI, 1.86 to 12.1]; P = .001; Fig 3). Notably, in patients who survive to 6 months post-tisagenlecleucel without relapse, OS rates are remarkably high in this historically high-risk relapsed acute lymphoblastic leukemia cohort, with 6-month and 1-year survival rates of 94% (95% CI, 89 to 99) and 92% (95% CI, 86 to 99), respectively.
FIG 3.
One hundred eighty–day landmark overall survival analysis post-tisagenlecleucel.
Salvage Therapies After Relapse
Of 57 patients who relapsed post-tisagenlecleucel, 88% (n = 50) received salvage therapy (initial salvage therapy line: chemotherapy, n = 19; inotuzumab, n = 15; blinatumomab, n = 3; nivolumab, n = 1; CAR, n = 10; and HSCT, n = 2) and seven received supportive care (Appendix Fig A2, online only). Fifty-six percent (20 of 36) of evaluable patients achieved CR by flow cytometry with their first salvage therapy. When used as an initial salvage regimen, 9 of 14 (64%) evaluable patients treated with inotuzumab and 7 of 9 (78%) evaluable patients treated with CD19-CAR reinfusion (3 of 5 tisagenlecleucel reinfusion, 3 of 3 humanized CD19-CAR, and 1 of 1 tandem tisagenlecleucel/nivolumab/radiation) received CR by flow cytometry. Although chemotherapy was used as the most common salvage strategy, variable utilization and reporting limit analysis. Of nonchemotherapy-based salvage approaches, CAR reinfusion and inotuzumab emerge as most widely adopted initial salvage regimens. Expectedly, blinatumomab was less commonly used as post–CD19-CAR initial salvage therapy (n = 3, two progressive disease and one CR when combined with tyrosine kinase inhibitor).
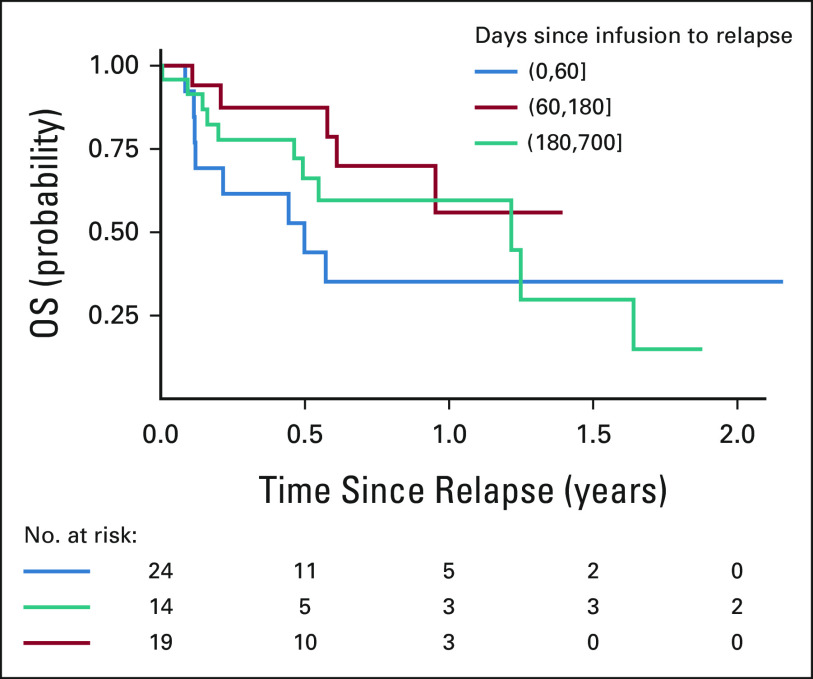
Across all salvage lines after nonresponse and post-tisagenlecleucel relapse, 28% of patients (16 of 57, one nonresponder and 15 relapses) received additional CAR infusions (seven same CAR construct, nine alternative CAR constructs [four, CD22; four, CD19 clinical trial; and one, CD19-bispecific/CD22-bispecific]) within reported follow-up. One patient received two subsequent CAR infusions. The single patient receiving CAR reinfusion after nonresponse had progressive morphologic disease. After relapse, 10, four, and two CAR products were infused as first, second, and fifth salvage lines, respectively. Of patients receiving CD22-targeting CARs (monospecific CD22, n = 3; bispecific CD19/CD22, n = 1), 2 of 4 achieved CR by flow cytometry. Overall, of 15 patients with post-tisagenlecleucel relapse receiving subsequent CAR, 67% (10 of 15) achieved CR by flow cytometry, demonstrating the utility of subsequent CAR infusions to reinstate remission. OS analysis after second CAR infusion used to salvage post-CAR nonresponse or relapse is demonstrated in Appendix Fig A3 (online only).
Of 57 patients who relapsed, 19 patients were successfully bridged to allogeneic HSCT, of which 37% (7 of 19) experienced death. Of 38 patients who did not go to HSCT, 45% (17) experienced death within reported follow-up. Longer follow-up is warranted to establish if HSCT in the post-tisagenlecleucel relapse setting is protective of death. Swimmer plot demonstrates individual patient clinical courses postrelapse (Fig 2). Details of sequenced salvage therapy lines and responses are shown across 57 relapse patients, with the varying sequencing and permutations highlighting that salvage approaches in post-CAR relapse are yet to be standardized (Appendix Fig A2).
DISCUSSION
We conduct a retrospective analysis across 80 pediatric and young adult patients who experienced nonresponse (n = 23) or relapse (n = 57) after tisagenlecleucel. Overall, we demonstrate that patients with nonresponse have poor survival outcomes (12 month OS; 19%) and patients who relapse post-tisagenlecleucel have an increased risk of death as compared with patients who sustain durable remissions (HR, 4.75; 95% CI, 1.86 to 12.1; P = .001). Furthermore, in a landmark analysis of patients who remain alive at 6 months post-tisagenlecleucel without relapse (n = 95), we report a remarkably high OS rate of 92% at 18 months post-CAR infusion.
Antigen downregulation has emerged as a major barrier challenging the durability of post-CAR remissions.1,7,8 Here, we conduct an outcome analysis across patients relapsing with distinct antigen expression patterns. We identify that relapse with CD19– disease is associated with poor OS (12 months; 30%), highlighting the need to address CD19– relapse as a major clinical gap. Therapeutics targeting alternative antigens to CD19 are under clinical development, with CD22-specific agents most advanced. Inotuzumab, a CD22-specific antibody-calicheamicin conjugate, achieved a 58% CR rate in CAYAs with B-ALL (phase II; n = 48).9 CD22-specific CAR T cells achieved a 70% CR rate (phase I; n = 58) in CAYAs with B-ALL.10 To our knowledge, this is the first CAR targeting an alternative antigen to CD19 to successfully achieve comparable efficacy. CD22 antigen density was, however, decreased upon nonresponse and relapse, highlighting that antigen downregulation is not a CD19-specific phenomenon.
Mitigation strategies to avert, rather than manage, antigen downregulation have driven CAR T-cell development with multispecificity. Phase I studies of bispecific CARs simultaneously targeting CD19 and CD22 are being clinically explored in pediatric B-ALL (ClinicalTrials.gov identifier: NCT03241940 and NCT03448393) and adult B-ALL and large B-cell lymphoma. The adult experience demonstrated an MRD-negative CR rate of 88% (n = 17). Relapse patterns, however, favored CD19– disease (50%), in the absence of CD22 antigen downregulation, supporting increased potency of the CD19 arm of this construct.11 Alternative approaches to bispecific targeting include cotransduction of distinct CD19 and CD22 CAR vectors to yield a mixed mono- and multispecific CAR cocktail product and bicistronic vector transduction.12,13 These respective efforts have been challenged by selective expansion of dominant monospecific CAR T-cell populations and limited persistence. These experiences showcase the challenges in developing equipotent, persisting, and multitargeting CARs.
Analysis of nonresponders highlights that 95% of nonresponders had high preinfusion disease burden. Disease burden is additionally associated with overall increased risk of relapse, supporting the growing body of literature identifying baseline high disease burden to associate with inferior survival outcomes.2,14,15 Although our data do not identify treatment with prior blinatumomab to be predictive of CD19– relapse, it has been shown that prior nonresponse to blinatumomab, rather than treatment itself, is a vital predictor of CD19-CAR outcomes.14 The PRWCC previously published outcomes using tisagenlecleucel for the treatment of EM disease and described that the presence of baseline EM disease versus BM-only disease was not a risk factor for nonresponse or relapse.16 Here, we identify that the number of preinfusion lines of therapy is associated with increased likelihood of relapse, possibly explained by more recalcitrant tumor biology, and compromised T-cell fitness or cumulative toxicity. Clinical studies evaluating CD19-CAR in the upfront MRD+ refractory setting are ongoing (ClinicalTrials.gov identifier: NCT03876769).
Analysis of individual patient's postrelapse treatment paths illustrates the heterogeneity of salvage strategies used. With the development of antibody-based therapies and cellular therapy, the therapeutic armamentarium for relapsed leukemia has expanded beyond traditional chemotherapy and HSCT. These agents have overlapping indications, yet optimized sequencing has not been standardized to date. Across 57 relapsed patients in our cohort, many distinct treatment permutations and response patterns are reported after post-CAR relapse. Chemotherapy, inotuzumab, and CAR reinfusion are used with greatest frequency as initial salvage regimens and can be used effectively to reinstate remission. The OS in this high-risk cohort of patients experiencing relapse post-tisagenlecleucel is 52% at 1 year and 32% at 2 years. This supports the need for relapse mitigation strategies, yet demonstrates the possibility of salvage in the relapse post-tisagenlecleucel setting and supports the systematic study in this window to establish treatment algorithms for post-tisagenlecleucel relapse.
ACKNOWLEDGMENT
We acknowledge the following individuals for their major roles in supporting successful execution of this multi-institutional study: administrative support: Anika Dove and Daisy Torres; legal counsel and contracting: Joshua Murphy; and data management: Michelle Fujimoto, Jennifer Sheppard, Jean Sosna, Victoria Koch, Katie Doherty, Emily Bakinowski, Elizabeth Klein, Daritzya Baraja, Courtney Newbold, Glenn McWillians, Maggie Dyer, Kasey Abrahamnson, Angie Peltz, Ahmed Tahoun, Mary Suarez, Megan Hanby, Stacy Cooper, and Brad Muller. The Stanford REDCap platform is developed and operated by Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidized by 1) Stanford School of Medicine Research Office, and 2) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant No. UL1 TR001085.
APPENDIX
FIG A1.
OS after relapse by time from infusion to relapse. OS, overall survival.
FIG A2.
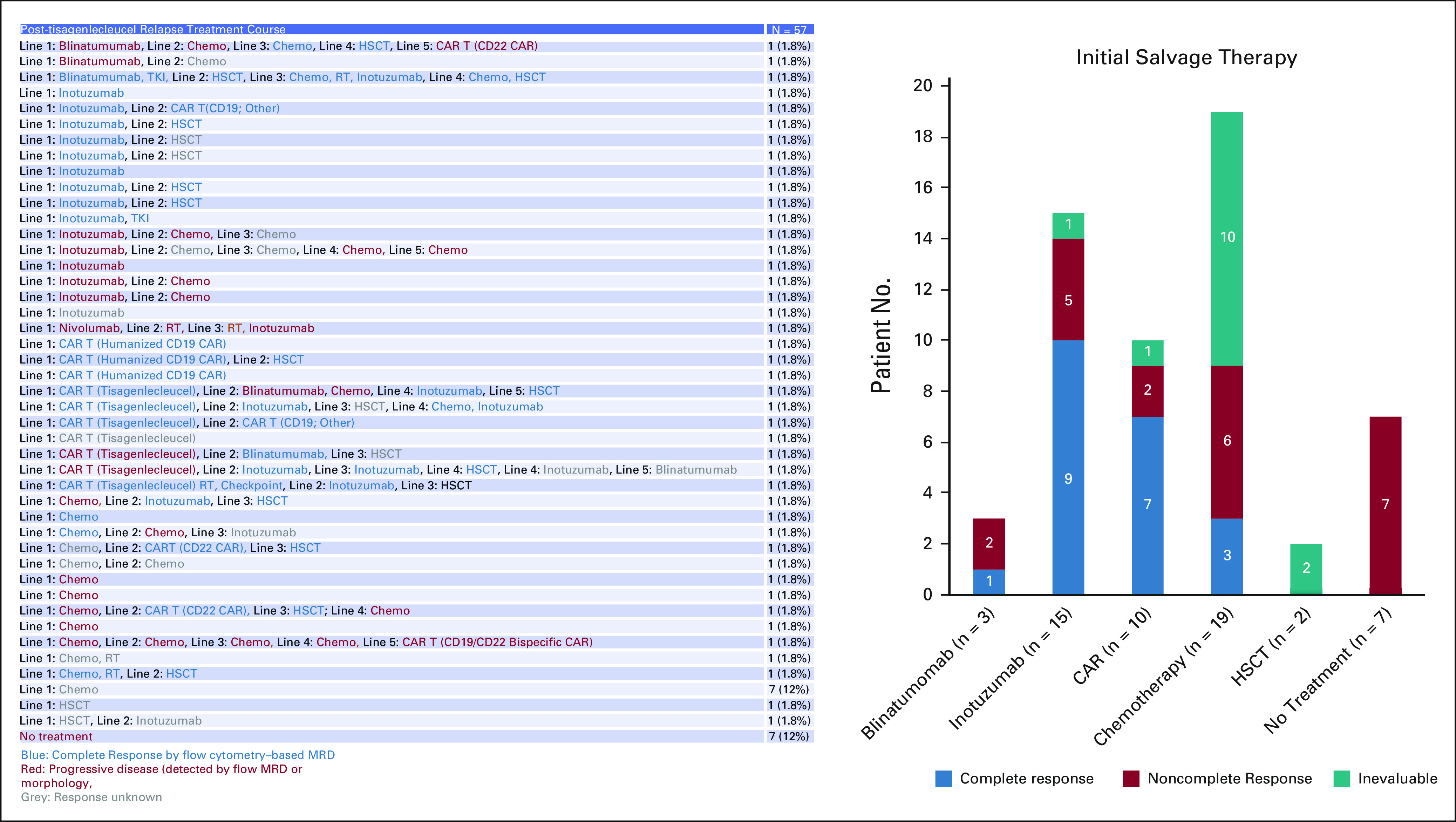
Salvage therapies and responses in the post-tisagenlecleucel relapse setting. CAR, chimeric antigen receptor; HSCT, hematopoietic stem cell transplantation; MRD, minimal residual disease; RT, radiotherapy; TKI, tyrosine kinase inhibitor.
FIG A3.
OS across patients who received secondary salvage CAR infusion after tisagenlecleucel nonresponse or relapse. Time 0 is the day of the second CAR infusion. CAR, chimeric antigen receptor; OS, overall survival.
TABLE A1.
Characteristics of All Infused Patients, by d28 Response
TABLE A2.
Characteristics of Disease at Time of Nonresponse, Salvage Regimens, and Outcomes After Post-tisagenlecleucel Nonresponse
Liora M. Schultz
Consulting or Advisory Role: Novartis
Jenna Rossoff
Consulting or Advisory Role: Novartis
Christa Krupski
Employment: US Acute Care Solutions (I)
Stock and Other Ownership Interests: US Acute Care Solutions (I)
Travel, Accommodations, Expenses: US Acute Care Solutions (I)
Christine L. Phillips
Consulting or Advisory Role: Novartis, Incyte
Julie-An Talano
Consulting or Advisory Role: Novartis
Susanne H.C. Baumeister
Employment: Takeda (I)
Stock and Other Ownership Interests: Takeda (I), AstraZeneca (I)
Gary Douglas Myers
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead Company, Amgen, Kura Oncology, Takeda
Muna Qayed
Honoraria: Novartis, Vertex
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Michelle Hermiston
Stock and Other Ownership Interests: Gladiator Biosciences (I), Coagulant Therapeutics (I)
Consulting or Advisory Role: Novartis, Sobi, KaliVir Immunotherapeutics (I)
Patents, Royalties, Other Intellectual Property: Spouse has patents pending for platform technology with application to oncology, diagnostics, and anti-infections and for antibleeding technology (I)
Prakash Satwani
Consulting or Advisory Role: Takeda
Speakers' Bureau: SOBI
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1047592
Rachel Wilcox
Research Funding: Novartis (Inst), AlloVir (Inst)
Travel, Accommodations, Expenses: Novartis
Vanessa A. Fabrizio
Consulting or Advisory Role: Adaptimmune
Crystal L. Mackall
Leadership: Syncopation Life Sciences
Stock and Other Ownership Interests: Lyell Immunopharma, Alimera Sciences, Vor Biopharma, Apricity Health, Syncopation Life Sciences, Ensoma, Mammoth Biosciences
Consulting or Advisory Role: Vor Biopharma, Apricity Health, Alimera Sciences, PACT Pharma, Nektar, Lyell Immunopharma, NeoImmuneTech, Syncopation Life Sciences, NeoImmuneTech, Bristol Myers Squibb, Immatics, GlaxoSmithKline, Ensoma, Mammoth Biosciences
Research Funding: Lyell Immunopharma
Patents, Royalties, Other Intellectual Property: I am an inventor on numerous patents related to chimeric antigen receptor therapeutics and received royalties from NIH for the CD22-CAR patent licensed to Juno therapeutics
Travel, Accommodations, Expenses: NeoImmuneTech, Roche, Nektar
Other Relationship: Lyell Immunopharma, Syncopation Life Sciences
Kevin J. Curran
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Mesoblast
Research Funding: Juno Therapeutics, Celgene (Inst), Novartis (Inst), Cellectis (Inst)
Michael R. Verneris
Stock and Other Ownership Interests: Fate Therapeutics
Honoraria: Novartis, Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: patent to make innate lymphoid cells. It is not licensed, patent for CAR T-cell homing
Theodore W. Laetsch
Stock and Other Ownership Interests: Advanced Microbubbles
Consulting or Advisory Role: Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicians' Education Resource, Y-mAbs Therapeutics, AI Therapeutics, Jazz Pharmaceuticals, GentiBio, Menarini, Pyramid Biosciences
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst), Turning Point Therapeutics (Inst)
Heather Stefanski
Honoraria: Novartis
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as oral abstract form at the American Society for Transplantation and Cellular Therapy Tandem Meeting, virtual, February 9, 2021.
SUPPORT
Supported by the Stanford Association of Auxiliaries for Children (L.M.S.) and a St Baldrick's/Stand Up 2 Cancer Pediatric Dream Team Translational Cancer Research Grant (C.L.M.). Stand Up 2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.L.M. is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. This work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research (C.L.M.). K.J.C. acknowledges support of the NIH/NCI Cancer Center Support Grant P30 CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: Liora M. Schultz, Anne Eaton, Christina Baggott, Amy K. Keating, Julie-An Talano, Michelle Hermiston, Prakash Satwani, Vasant Chinnabhandar, Sharon Mavroukakis, Crystal L. Mackall, Michael R. Verneris, Theodore W. Laetsch, Heather Stefanski
Financial support: Crystal L. Mackall
Administrative support: Sharon Mavroukakis, Emily Egeler
Provision of study materials or patients: Amy K. Keating, Susanne H.C. Baumeister, Nicole A. Karras, Muna Qayed, Michelle Hermiston, Vasant Chinnabhandar, Kevin J. Curran, Michael R. Verneris, Theodore W. Laetsch
Collection and assembly of data: Liora M. Schultz, Christina Baggott, Jenna Rossoff, Snehit Prabhu, Amy K. Keating, Holly Pacenta, Christine L. Philips, Julie-An Talano, Amy Moskop, Susanne H.C. Baumeister, Gary Douglas Myers, Patrick A. Brown, Muna Qayed, Michelle Hermiston, Prakash Satwani, Rachel Wilcox, Cara A. Rabik, Vanessa A. Fabrizio, Vasant Chinnabhandar, Michael Kunicki, Emily Egeler, Michael R. Verneris, Theodore W. Laetsch, Heather Stefanski
Data analysis and interpretation: Liora M. Schultz, Anne Eaton, Christina Baggott, Snehit Prabhu, Amy K. Keating, Christine L. Philips, Julie-An Talano, Amy Moskop, Nicole A. Karras, Patrick A. Brown, Muna Qayed, Michelle Hermiston, Prakash Satwani, Cara A. Rabik, Vasant Chinnabhandar, Yimei Li, Crystal L. Mackall, Kevin J. Curran, Michael R. Verneris, Theodore W. Laetsch, Heather Stefanski
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcomes After Nonresponse and Relapse Post-Tisagenlecleucel in Children, Adolescents, and Young Adults With B-Cell Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Liora M. Schultz
Consulting or Advisory Role: Novartis
Jenna Rossoff
Consulting or Advisory Role: Novartis
Christa Krupski
Employment: US Acute Care Solutions (I)
Stock and Other Ownership Interests: US Acute Care Solutions (I)
Travel, Accommodations, Expenses: US Acute Care Solutions (I)
Christine L. Phillips
Consulting or Advisory Role: Novartis, Incyte
Julie-An Talano
Consulting or Advisory Role: Novartis
Susanne H.C. Baumeister
Employment: Takeda (I)
Stock and Other Ownership Interests: Takeda (I), AstraZeneca (I)
Gary Douglas Myers
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis
Research Funding: Novartis (Inst)
Patrick A. Brown
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Servier, Kite, a Gilead Company, Amgen, Kura Oncology, Takeda
Muna Qayed
Honoraria: Novartis, Vertex
Consulting or Advisory Role: Novartis
Travel, Accommodations, Expenses: Novartis
Michelle Hermiston
Stock and Other Ownership Interests: Gladiator Biosciences (I), Coagulant Therapeutics (I)
Consulting or Advisory Role: Novartis, Sobi, KaliVir Immunotherapeutics (I)
Patents, Royalties, Other Intellectual Property: Spouse has patents pending for platform technology with application to oncology, diagnostics, and anti-infections and for antibleeding technology (I)
Prakash Satwani
Consulting or Advisory Role: Takeda
Speakers' Bureau: SOBI
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1047592
Rachel Wilcox
Research Funding: Novartis (Inst), AlloVir (Inst)
Travel, Accommodations, Expenses: Novartis
Vanessa A. Fabrizio
Consulting or Advisory Role: Adaptimmune
Crystal L. Mackall
Leadership: Syncopation Life Sciences
Stock and Other Ownership Interests: Lyell Immunopharma, Alimera Sciences, Vor Biopharma, Apricity Health, Syncopation Life Sciences, Ensoma, Mammoth Biosciences
Consulting or Advisory Role: Vor Biopharma, Apricity Health, Alimera Sciences, PACT Pharma, Nektar, Lyell Immunopharma, NeoImmuneTech, Syncopation Life Sciences, NeoImmuneTech, Bristol Myers Squibb, Immatics, GlaxoSmithKline, Ensoma, Mammoth Biosciences
Research Funding: Lyell Immunopharma
Patents, Royalties, Other Intellectual Property: I am an inventor on numerous patents related to chimeric antigen receptor therapeutics and received royalties from NIH for the CD22-CAR patent licensed to Juno therapeutics
Travel, Accommodations, Expenses: NeoImmuneTech, Roche, Nektar
Other Relationship: Lyell Immunopharma, Syncopation Life Sciences
Kevin J. Curran
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Mesoblast
Research Funding: Juno Therapeutics, Celgene (Inst), Novartis (Inst), Cellectis (Inst)
Michael R. Verneris
Stock and Other Ownership Interests: Fate Therapeutics
Honoraria: Novartis, Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: patent to make innate lymphoid cells. It is not licensed, patent for CAR T-cell homing
Theodore W. Laetsch
Stock and Other Ownership Interests: Advanced Microbubbles
Consulting or Advisory Role: Novartis, Bayer, Cellectis, Aptitude Health, Clinical Education Alliance, Deciphera, Jumo Health, Massive Bio, Med Learning Group, Medscape, Physicians' Education Resource, Y-mAbs Therapeutics, AI Therapeutics, Jazz Pharmaceuticals, GentiBio, Menarini, Pyramid Biosciences
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), AbbVie (Inst), Amgen (Inst), Atara Biotherapeutics (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), Epizyme (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Jubilant Pharmaceuticals (Inst), Novella Clinical (Inst), Servier (Inst), Foundation Medicine (Inst), Merck Sharp & Dohme (Inst), Turning Point Therapeutics (Inst)
Heather Stefanski
Honoraria: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz LM, Baggott C, Prabhu S, et al. : Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: A pediatric real-world chimeric antigen receptor Consortium report. J Clin Oncol 40:945-955, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curran KJ, Pegram HJ, Brentjens RJ: Chimeric antigen receptors for T cell immunotherapy: Current understanding and future directions. J Gene Med 14:405-415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner RA, Finney O, Annesley C, et al. : Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129:3322-3331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquini MC, Hu ZH, Curran K, et al. : Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4:5414-5424, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majzner RG, Mackall CL: Tumor antigen escape from CAR T-cell therapy. Cancer Discov 8:1219-1226, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Sotillo E, Barrett DM, Black KL, et al. : Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 5:1282-1295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien MM, Ji L, Shah NN, et al. : Phase II trial of inotuzumab ozogamicin in children and adolescents with relapsed or refractory B-cell acute lymphoblastic leukemia: Children's Oncology Group protocol AALL1621. J Clin Oncol 40:956-967, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah NN, Highfill SL, Shalabi H, et al. : CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: Updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol 38:1938-1950, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel JY, Patel S, Muffly L, et al. : CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat Med 27:1419-1431, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordoba S, Onuoha S, Thomas S, et al. : CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: A phase 1 trial. Nat Med 27:1797-1805, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner RA, Annesley C, Wilson A, et al. : Efficacy of SCRI-CAR19x22 T cell product in B-ALL and persistence of anti-CD22 activity. J Clin Oncol 38, 2020. (15 suppl; abstr 3035) [Google Scholar]
- 14.Myers RM, Taraseviciute A, Steinberg SM, et al. : Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol 40:932-944, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadauke S, Myers RM, Li Y, et al. : Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: A prospective clinical trial. J Clin Oncol 39:920-930, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabrizio VA, Phillips CL, Lane A, et al. : Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: A pediatric real world CAR Consortium report. Blood Adv 6:600-610, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]