Abstract
Most efforts to understand the pathology of traumatic brain injury (TBI) have been centered on the brain, ignoring the role played by systemic physiology. Gut-derived serotonin is emerging as a major regulator of systemic homeostasis involving various organs and tissues throughout the body. Here, we shed light on the roles occupied by gut-derived serotonin and its downstream metabolic targets in the systemic pathogenesis of TBI. Male C57BL/6J mice were subjected to a fluid percussion injury (FPI) and RT-qPCR was used to examine mRNA levels in intestine, liver, and adipose tissue. In the intestinal tract, TBI transiently downregulated enteric neuronal markers Chat and Nos1 in the duodenum and colon, and altered colonic genes related to synthesis and degradation of serotonin, favoring an overall serotonin downregulation. There also was a decrease in serotonin fluorescence intensity in the colonic mucosa and reduced circulating blood serotonin levels, with concurrent alterations in serotonin-associated gene expression in downstream tissues after TBI (i.e., upregulation of serotonin receptor Htr2a and dysregulation of genes associated with lipid metabolism in liver and adipose). Levels of commensal bacterial species were also altered in the gut and were associated with TBI-mediated changes in the colonic serotonin system. Our findings suggest that TBI alters peripheral serotonin homeostasis, which in turn may impact gastrointestinal function, gut microbiota, and systemic energy balance. These data highlight the importance of building an integrative view of the role of systemic physiology in TBI pathogenesis to assist in the development of effective TBI treatments.
Keywords: Peripheral serotonin, Traumatic brain injury, Gut-brain axis, Microbiota, Liver, Adipose
1. Introduction
Traumatic brain injury (TBI) continues to be a major medical problem in the United States, with often long-term consequences that significantly reduce quality of life for affected individuals [1]. To date, most efforts to understand the pathology of TBI have been centered on the brain. The lack of information on the role played by systemic physiology in TBI pathogenesis has limited our understanding of the dimensionality of pathogenesis and hindered the development of efficacious treatments. Indeed, it is becoming increasingly apparent that the complex interplay between brain and body is an important modulator of normal brain function and neurological disease [2,3], and a thorough understanding of TBI pathogenesis in the context of both the brain and body is crucial for the development of effective treatments to cope with the long-term burden of TBI.
Communication between the gut and the brain and other organs plays a crucial role in the outcome of TBI pathogenesis. For example, preclinical and clinical studies indicate that TBI induces structural and functional damage to the gut and alters gut microbiome composition, likely as a result of communication along the gut-brain axis [4–10]. The gut-brain axis involves bidirectional communication between the brain and the enteric nervous system (ENS), an intricate network of neurons located in the gut that controls most major functions of the gastrointestinal (GI) system [11]. ENS nerve fibers innervate the intestinal mucosa where their activity is influenced by serotonin (5-hydroxytryptamine; 5-HT) produced by enterochromaffin (EC) cells [12–14]. Over 90 % of the body’s serotonin is synthesized by EC cells [15], which are a subtype of enteroendocrine cells located within the intestinal epithelium. These specialized cells, which facilitate communication between gut microbiota and afferent nerve terminals, play an important modulatory role in GI functions such as intestinal motility [12,13] and potential communication with peripheral organs. Importantly, the possibility that the gut responds to TBI by altering production and release of serotonin that can, in turn, affect the brain and other organs may have important implications for the treatment of TBI.
Gut-derived serotonin is involved in a variety of functions throughout the body, acting through 14 cell surface serotonin receptors (HTRs) belonging to seven distinct serotonin receptor families [16]. While all serotonin receptor subtypes can be found in the central nervous system, several receptors are also expressed in the periphery and are involved in serotonin-mediated regulation of processes such as haematopoiesis, bone metabolism and remodeling, and energy balance [16,17]. For instance, in liver and adipose tissues, gut-derived serotonin signals via serotonin receptors HTR2A, HTR2B, and HTR3 to regulate lipid and glucose metabolism [16–19]. While systemic dysfunction is common in TBI patients, it is not known how TBI affects the gut-derived serotonergic system and its associated functions, or how gut microbiota might interact with this system to inform the brain and periphery about the outcome of TBI pathogenesis occurring in the gut. Therefore, in the present study, we aimed to determine the action of TBI on the gut in connection with the modulatory role occupied by serotonin and microbiota in peripheral physiology.
2. Materials and methods
2.1. Animals and experimental design
Nine-week-old male C57BL/6J mice (Jackson Laboratory) weighing 20–25 g were used. The mice had access to fresh water and food ad libitum and were maintained under standard housing conditions (room temperature 22–24 °C) with a 12 h light/dark cycle. Animals were randomly assigned to sham or injury groups at each time point (n = 10/group). Groups were further subdivided to accommodate multiple tissue processing methods. One, three, or seven days following fluid percussion injury, mice were sacrificed by cervical dislocation. The brain, small intestine, cecum, colon, liver, blood, and various adipose depots (mesenteric, subcutaneous, gonadal, and intraperitoneal white adipose, and interscapular brown adipose) were collected, flash frozen, and stored at −70 °C until further use. Intestinal tissue was flushed with phosphate-buffered saline (PBS, pH 7.4) before freezing. For RT-qPCR and ELISA analyses, 6 mice/group were utilized. For immunofluorescence, 4 mice/group were used, and tissues from their proximal colonic regions were fixed and processed.
This study was performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Chancellor’s Animal Research Committee of the University of California at Los Angeles.
2.2. Fluid percussion injury
Mice were anesthetized with 4 % isoflurane and fixed to a stereotaxic device. During surgery, anesthesia was maintained at 2 % isoflurane with an air flow rate of 1 L/min and body temperature was maintained with a heating pad. Mice were randomly assigned to receive TBI or sham surgery. Surgery was performed under a dissecting microscope (Wild Heerbrugg, Switzerland). A 3-mm diameter craniotomy was made 2.5 mm posterior to bregma and 2.0 mm lateral (left) of the midline with a high-speed drill (Dremel, Racine, WI, USA), exposing the intact dura. A plastic cap was secured over the skull opening with super glue and sealed with dental cement. Once the cement had hardened, the cap was filled with 0.9 % sterile saline and attached to the fluid percussion injury device. At the first sign of hindlimb withdrawal in response to a paw pinch, a moderate fluid percussion injury (1.8–2.1 atm, wake up time > 5 min) was administered. In sham animals, a craniotomy was performed but no injury was administered.
After surgery, the mice were allowed to wake briefly, then were placed under light anesthesia while the plastic cap was removed and the skin was sutured. Triple antibiotic ointment was applied to the sutured skin and the animals recovered on a heating pad for approximately 1 h before returning to a clean cage.
2.3. RT-qPCR
Frozen tissue was transferred to RNAlater™-ICE (Invitrogen, Waltham, MA, USA) and stored at −20 °C. The E.Z.N.A.® Total RNA Kit II (Omega Bio-tek, Norcross, GA, USA) was used for RNA preparation according to manufacturer instructions. The concentration of RNA was determined by nanophotometer (IMPLEN, Westlake Village, CA, USA). A total of 500 ng - 2 μg total RNA was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA). Quantitative PCR was performed on the CFX96 qPCR system (Bio-Rad, Hercules, CA, USA) using standard cycling conditions and an annealing temperature of 60 °C (Bio-Rad, Hercules, CA, USA). Reactions of 10-μl volume contained 5 μl PowerUp™ SYBR™ Green Master Mix (Applied Biosystems, Waltham, MA, USA), 500 nM each forward and reverse primers, and template DNA. Samples were run in duplicate. Relative gene expression compared to sham groups was determined using the 2−ΔΔCt method and normalized to Gapdh expression levels. Mouse tissue primers used in this study are listed in Supplementary Table 1.
2.4. Microbial DNA extraction and qPCR
Approximately 200 mg frozen cecum contents were extracted from the cecum over wet ice and immediately processed for microbial DNA extraction using the E.Z.N.A.® Stool DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to manufacturer instructions. The concentration of genomic DNA was determined by nanophotometer and the isolated DNA samples were stored at −20 °C until use. Quantitative PCR was performed on the CFX96 qPCR system using standard 3-step cycling conditions with an annealing temperature of 58 °C and extension at 72 °C. Reactions of 10-μl volume contained 5 μl PowerUp™ SYBR™ Green Master Mix, 500 nM each forward and reverse primers, and template DNA. Samples were run in duplicate. Relative gene expression compared to sham groups was determined using the 2−ΔΔCt method and normalized to 16S rRNA. Primers used to identify bacterial species in this study are listed in Supplementary Table 2.
2.5. ELISA serotonin measurement
For measurement of serotonin in blood, mice were anesthetized and retro-orbital blood was collected in polypropylene microcentrifuge tubes. Blood was stored at 4 °C for 30–60 min, then centrifuged at 3900 g for 10 min with low deceleration. The supernatant was transferred to a new tube and serotonin was measured with the Serotonin ELISA Assay Kit according to manufacturer instructions (Eagle Biosciences, Amherst, NH, USA).
For measurement of serotonin in colon tissues, approximately 10 mg fresh frozen colon tissue from the proximal colonic region was sonicated in 100 μl cold standard buffer supplied with the Serotonin Sensitive ELISA Kit (Eagle Biosciences, Amherst, NH, USA) supplemented with 0.1 % ascorbic acid to prevent serotonin degradation. Homogenized tissue was centrifuged at 12,000 g for 15 min at 4 °C. The supernatant was collected and total protein concentration was determined by BCA assay. Prepared samples were stored at −20 °C until use. The ELISA assay was performed according to manufacturer instructions using 20 μl undiluted sample. Data were normalized to total protein.
2.6. Serotonin immunofluorescence
In 3 d and 7 d post-TBI experimental groups, n = 4 animals were selected from each group for immunofluorescent staining analyses. Colon tissues were dissected from the peritoneal cavity and flushed with cold PBS to remove lumenal contents. The colons were then cut open longitudinally and affixed to chilled agarose gels prepared in Petri dishes. Colons were fixed for 1 h at 4 °C with a neutral buffered 10 % formalin solution, then cryoprotected in a solution containing 20 % sucrose and 10 % glycerol for 72 h at 4 °C. The colons were then embedded in optimal cutting temperature compound and frozen at −70 °C. Frozen colon tissues were sectioned at 10-μm thickness in a cryostat (Leica CM1900) with cryochamber and specimen temperatures set to −20 °C. Tissue sections were immediately mounted onto positively charged glass slides (Fisherbrand, Fisher Scientific, Hampton, NH, USA) and frozen at −70 °C until use.
For immunofluorescent serotonin staining, slides were thawed briefly at room temperature and a hydrophobic barrier was applied around the tissue sections. Slides were then washed in cold PBS (pH 7.4), blocked for 1 h in PBS containing 5 % bovine serum albumin (BSA) and 0.3 % Triton-X 100, washed briefly in PBS, then incubated overnight at 4 °C with rabbit anti-serotonin antibody at 1:1000 dilution in PBS with 2 % BSA (Immunostar, Hudson, WI, USA; product ID: 20080) to visualize serotonin-expressing EC cells, which are the only cells producing serotonin in the crypts (epithelial layer) of the colon. The following day, sections were washed in cold PBS and incubated in the dark for 90 min with DyLight® 549 goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA) at 1:300 dilution in PBS with 2 % BSA. After secondary antibody incubation, colon sections were washed in PBS, then incubated with Hoechst nuclear counterstain (1 μg/mL) for 10 min, followed by a final PBS rinse. Slides were dried briefly in the dark, then were coverslipped with ProLong™ Gold antifade mounting medium (Invitrogen, Waltham, MA, USA). Slides were stored in the dark at 4 °C and imaged within 1 week.
2.7. Image acquisition and analysis
A blinded investigator acquired z-stack images in 1-μm z-steps through the entire colon tissue depth at 10× magnification on a Zeiss Axio Imager Z1 microscope equipped with an AxioCam MRm camera and AxioVision software (version 4.8.2). A total of 3–4 images were acquired per animal across adjacent sections of proximal colonic mucosa. Images were psuedo-colored and flattened using the Extended Focus function, then imported into ImageJ FIJI for analysis. Identical imaging parameters were maintained across all experimental groups.
Two endpoints were quantified in ImageJ FIJI by a blinded investigator: fluorescence intensity per signal area and number of serotonin immunoreactive (5-HT+) cells. Signal area represented serotonin confined to immunoreactive cell somas, as detected by the ImageJ threshold function. Cell counts were normalized to mucosa area (mm2). Averages across multiple adjacent tissue sections were determined for each animal. Data are represented as group averages ± SEM.
2.8. Statistics
Data were analyzed with unpaired two-tailed student’s t-test for comparison between sham and TBI groups at each time point. Pearson correlation was used to assess relationships between variables. Appropriate nonparametric alternatives, including Welch’s t-test and Mann-Whitney U test, were used when data did not meet test requirements. Outliers were detected using ROUT and Grubbs’ outlier tests, and statistically significant outliers (p < 0.05) were excluded from analyses. Excluded data points are indicated in figure captions where appropriate. All statistical analyses were performed in GraphPad Prism 9 for Windows.
3. Results
3.1. Effects of TBI in the gut
3.1.1. TBI reduces gene expression of Chat and Nos1 neuronal markers in the ENS at specific levels of the GI tract
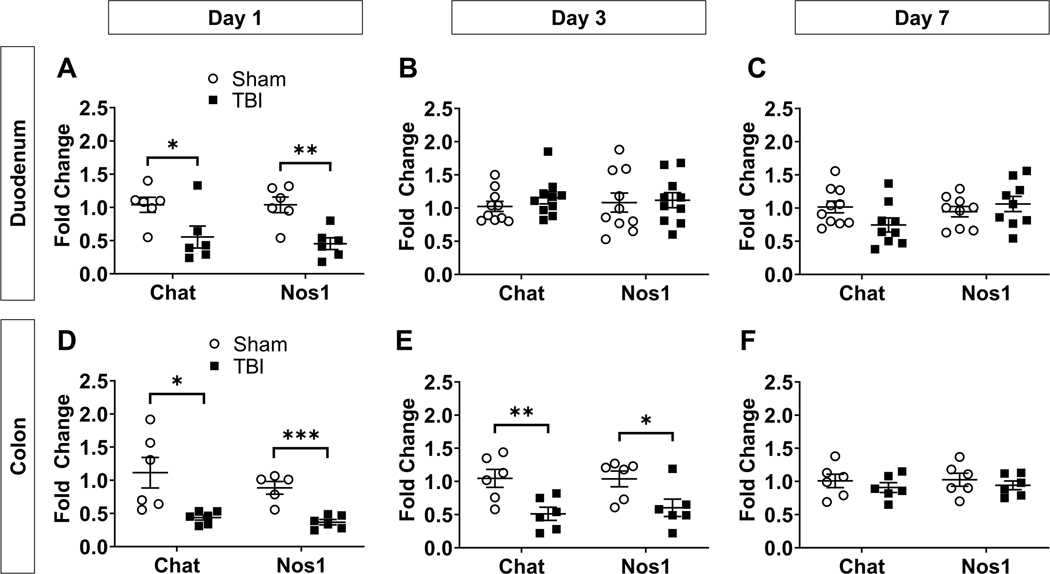
We first investigated the effects of TBI in the gut by examining regional gene expression of markers for cholinergic (choline acetyltransferase, Chat) and nitrergic (nitric oxide synthase, Nos1) neurons in the ENS. The duodenum, jejunum, ileum, and proximal colon were dissected from the GI tract and gene expression of Chat and Nos1 was measured in each region. In the duodenum, both Chat and Nos1 were significantly downregulated compared to sham at 1d post-TBI (0.5-fold change; Chat t(10) = 2.423, p = 0.0359; Nos1 t(10) = 3.991, p = 0.0026), then were restored to levels similar to sham at 3d and 7d post-TBI (p > 0.05 versus sham; Fig. 1A-C).
Fig. 1.
TBI transiently reduces gene expression of markers for cholinergic and nitrergic neurons in duodenum and colon. Chat and Nos1 gene expression were measured by qPCR at 1, 3, and 7 days post-TBI and fold change relative to sham was quantified in duodenum (A-C) and colon (D-F). Data are expressed as mean fold change (relative to sham) ± SEM and are normalized to Gapdh expression. Unpaired two-tailed t-test and Welch’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001 vs sham); n = 6–10/group.
Statistically significant outliers were excluded from Sham (n = 1) and TBI (n = 1) groups for Nos1 in panel (C), from the Sham group (n = 1) for Chat in Panel (C), and from the Sham group (n = 1) for Nos1 in panel (D). Abbreviations: Chat, choline acetyltransferase; Nos1, nitric oxide synthase 1.
A stronger TBI effect was observed in the colon, where both Chat and Nos1 remained downregulated for up to three days. Both neuronal markers were significantly decreased in the colon at 1d (0.4-fold change; Chat Welch-corrected t(5.283) = 2.894, p = 0.0319; Nos1 t(9) = 5.342, p = 0.0005) and 3d post-TBI (0.5 < fold change<0.6; Chat t(10) = 3.181, p = 0.0098; Nos1 t(10) = 2.460, p = 0.0337; Fig. 1D-E), returning to sham levels only at 7d post-TBI (p > 0.05; Fig. 1F).
No significant changes were found between sham and TBI groups in jejunum or ileum (Supplementary Fig. 1A). We also measured gene expression of the pan-neuronal marker, HuD (Elavl4), in the colon and observed a significant downregulation of Elavl4 at 3d post-TBI (0.6-fold change; Welch-corrected t(5.878) = 3.626, p = 0.0114), but not at 7d post-TBI (p > 0.05; Supplementary Fig. 1B). Based on this collective evidence, we focused primarily on the colon for our subsequent gut experiments.
3.1.2. TBI alters expression of genes associated with peripheral serotonin synthesis and degradation in the gut
Because peripheral serotonin is primarily produced in the gut and plays an important role regulating function of various organs and tissues, we examined the effects of TBI on transcriptomic alterations of genes involved in serotonin signaling in the duodenum and proximal region of the colon (Fig. 2). First, we examined the peripherally expressed rate-limiting enzyme of serotonin synthesis, tryptophan hydroxylase 1 (TPH1), which is enriched in EC cells of the intestinal mucosa. In TBI mice, duodenum Tph1 gene expression was decreased at 3 d post-TBI (0.9-fold change; t(18) = 2.673, p = 0.0155), but not at 1 d or 7 d post-TBI (p > 0.05; Fig. 2B). Similarly, in the colon, Tph1 expression was downregulated only at 3 d post-TBI (0.5-fold change; t(10) = 3.297, p = 0.0081; Fig. 2C).
Fig. 2.
TBI alters gene expression of serotonergic signaling components in the gut. (A) Simplified schematic depicting the serotonergic system in the intestinal epithelium. TPH1-mediated serotonin synthesis occurs in enterochromaffin cells of the intestinal epithelium. Dietary tryptophan is converted to 5-HTP by the rate-limiting enzyme, TPH1. 5-HTP is then processed into 5-HT (serotonin) by AADC. Serotonin is then packaged into vesicles by vesicular monoamine transporter 1 (VMAT1; indicated by purple square) and released in response to various stimuli. Serotonin transporter (SERT) located on adjacent enterocytes can sequester serotonin targeted for degradation by MAO-A. (B) Tph1 gene expression in duodenum at 1, 3, and 7 days post-TBI. (C) Tph1 gene expression in colon at 1, 3, and 7 days post-TBI. (D) Maoa and Slc6a4 gene expression in colon at 3 days post-TBI. (E) Maoa and Slc6a4 gene expression in colon at 7 days post-TBI. Data are expressed as mean fold change (relative to sham) ± SEM and are normalized to Gapdh expression. Unpaired two-tailed t-test (*p < 0.05, **p < 0.01 vs sham); n = 6–10/group. Statistically significant outliers were excluded from the Day 7 Sham group (n = 1) in panels (B) and (C). Abbreviations: AADC, aromatic l-amino acid decarboxylase; EC, enterochromaffin; MAO-A, monoamine oxidase A; SERT (gene name: Slc6a4), serotonin transporter; TPH1, tryptophan hydroxylase 1; Tryp, tryptophan; 5-HT, 5-hydroxytryptamine (serotonin); 5-HTP, 5-hydroxytryptophan.
We also quantified colonic expression of the genes for monoamine oxidase A (Maoa; main enzyme responsible for serotonin degradation) and serotonin transporter, SERT (Slc6a4), at 3 d and 7 d post-TBI. At both time points, Maoa expression was significantly upregulated, though the overall change was small (1.1 < fold change<1.2; Day 3 t(10) = 3.203, p = 0.0094; Day 7 t(10) = 4.386, p = 0.0014; Fig. 2D-E). Slc6a4 was significantly upregulated only at 7d post-TBI (1.5-fold change; t(10) = 3.884, p = 0.0030; Fig. 2D-E).
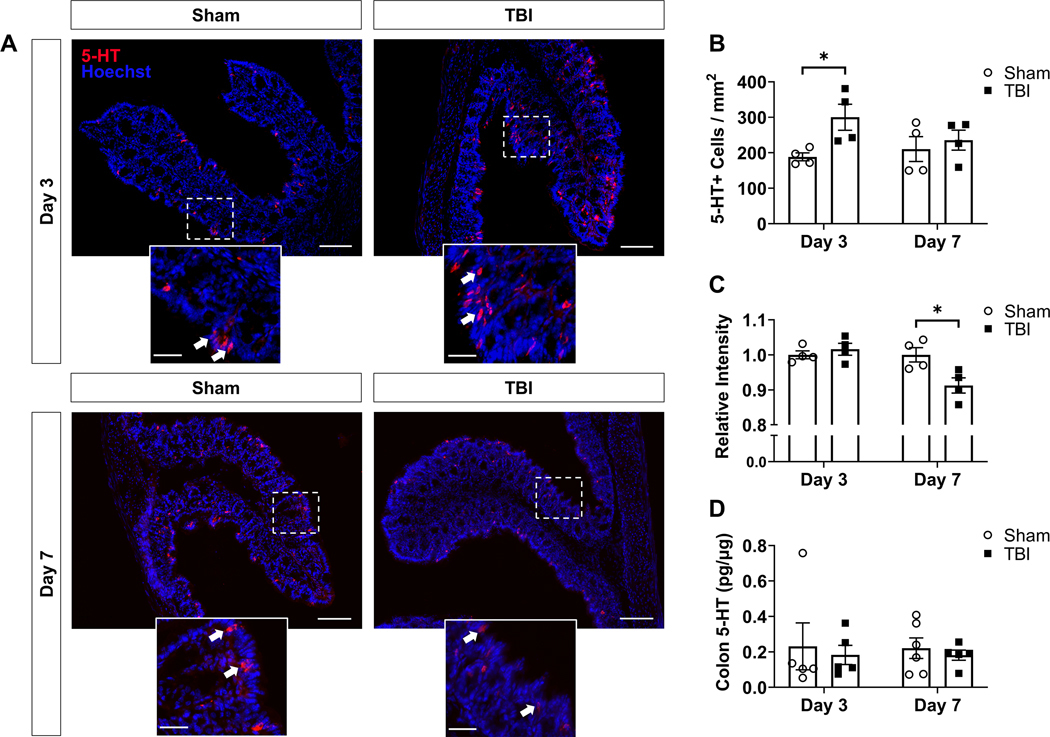
3.1.3. TBI promotes time-dependent changes in serotonin expression within the colonic mucosa
We next investigated the phenotypic effects of TBI on cells expressing serotonin in the mouse colon. Tissue sections of proximal mouse colon were immunolabeled for serotonin and visualized with a fluorophore-conjugated secondary antibody. The number and fluorescence intensity of serotonin-immunoreactive (5-HT+) cells were the primary endpoints. In 5-HT+ cells located in the colonic mucosa, several distinct changes in serotonin expression were observed in response to TBI (Fig. 3). At 3d post-TBI (acute post-injury period), the number of 5-HT+ cells per mucosa area was significantly increased in TBI animals compared to sham (t(6) = 2.912, p = 0.0269; Fig. 3A,B), while the mean fluorescence intensity per signal area (i.e., area of selected 5-HT+ somas) was not different between groups (p > 0.05; Fig. 3A,C). Conversely, at 7d post-TBI (post-acute period), the mean fluorescence intensity per signal area was significantly decreased in TBI mice compared to sham (t(6) = 2.924, p = 0.0265; Fig. 3A,C), while the number of 5-HT+ cells was statistically similar to sham (p > 0.05; Fig. 3A,B). In contrast to these cell specific findings, whole tissue serotonin content in proximal colon was quantified by ELISA in additional animals from the same experimental groups and no differences were found between sham and TBI mice at either time point (p > 0.05; Fig. 3D).
Fig. 3.
TBI promotes time-dependent changes in colonic serotonin expression without altering total tissue serotonin content. (A) Representative micrographs of serotonin-expressing cells (shown in red) within the proximal colonic mucosa of sham and TBI animals at 3 and 7 days post-TBI; n = 4/group; scale bar = 100 μm. The field of view within dashed boxes is shown with increased magnification below each image; scale bar = 30 μm. White arrows highlight representative examples of immunoreactive cells. (B) Number of serotonin-immunoreactive (5-HT+) cells within the field of view, normalized to mucosa area. (C) Fluorescence intensity per selection area (i.e., within 5-HT+ cell somas), relative to sham. (D) ELISA measurement of total tissue serotonin levels in proximal colon, normalized to total protein. Data are expressed as mean ± SEM. Unpaired two-tailed t-test (*p < 0.05 vs sham). Statistically significant outliers were excluded from Sham (n = 1) and TBI (n = 1) groups at Day 3 and the TBI group (n = 1) at Day 7 in panel (D). Abbreviations: 5-HT, 5-hydroxytryptamine (serotonin).
3.1.4. Bacterial species within the GI tract are differentially affected by TBI and levels are proportional to transcriptomic expression of markers within the host serotonergic system
Recent evidence indicates that there is bidirectional communication between gut microbiota and the host intestine in which spore-forming microbial species indigenous to the gut microbiome use serotonergic signaling to influence host physiology, and vice versa [21,22]. Therefore, we examined the effects of TBI on the abundance of bacterial species in the GI tract and their association with the serotonergic system in the mouse colon. Specifically, we measured the abundance of Clostridium cluster IV (Clostridium leptum subgroup) members with a group-specific primer as defined in [20], including the following species: Clostridium leptum, Clostridium viride, Eubacterium siraeum, Faecalibacterium prausnitzii, Ruminococcus albus, Ruminococcus bromii, and Ruminococcus callidus. We also quantified the abundance of a separate bacterium belonging to Clostridium cluster XIVa, Clostridium scindens. C. scindens and C. leptum display 7α-dehydroxylation activity, which is required for the production of deoxycholate, a secondary bile acid metabolite that has been shown to increase colonic serotonin levels and Tph1 expression [22]. Finally, we also measured the abundance of Turicibacter sanguinis, a microbial species that is sensitive to host serotonin, which has been shown to modulate T. sanguinis competitive colonization of the GI tract [21].
The abundance of bacterial species was measured by qPCR amplification of genomic DNA extracted from cecum contents. Following TBI, significant changes were observed in the abundance of the C. leptum subgroup and C. scindens (Fig. 4A-B). While no changes were detected in TBI animals compared to sham at 3d post-TBI, the abundance of C. leptum subgroup members was significantly decreased (t(17) = 3.034, p = 0.0075; Fig. 4A) and C. scindens was significantly increased (t(16) = 3.761, p = 0.0017; Fig. 4B) at 7d post-TBI. No changes were observed in T. sanguinis abundance at either time point (p > 0.05; Fig. 4C).
Fig. 4.
Commensal bacteria are differentially affected by TBI and correlate with transcriptomic changes in the host serotonergic system of TBI mice. (A-C) Relative abundance of commensal bacterial species: Clostridium leptum subgroup members (A), Clostridium scindens (B), and Turicibacter sanguinis (C) in the mouse cecum as determined by qPCR amplification of genomic DNA. Data are expressed as mean fold change (relative to sham) ± SEM at each time point and normalized to 16S rRNA. Unpaired two-tailed t-test (**p < 0.01 vs sham); n = 10/group. (D-F) Pearson correlations between bacterial species abundance and Tph1 gene expression in colons of TBI mice. (G-I) Pearson correlations between bacterial species abundance and serotonin transporter (Slc6a4) gene expression in colons of TBI mice. All correlation data shown indicate gene expression fold change relative to sham. Significant correlations are indicated by bolded p-values. *Note: “C. leptum” refers to the collective species included in the C. leptum subgroup per Matsuki et al. [20]. Statistically significant outliers were excluded from the Day 7 Sham group (n = 1) in panel (A), the Day 3 Sham group (n = 1) and the Day 7 Sham group (n = 2) in panel (B), and the Day 3 Sham (n = 1) and TBI (n = 2) groups in panel (C). Abbreviations: Slc6a4, solute carrier family 6 member 4 (serotonin transporter); Tph1, tryptophan hydroxylase 1; 3D, Day 3 post-TBI; 7D, Day 7 post-TBI.
Next, bacterial species abundance was correlated with markers of the host serotonergic system in the colon (Fig. 4D-I). In TBI animals, colonic Tph1 gene expression was positively associated with C. scindens abundance (r = 0.76, p = 0.0040; Fig. 4E), but not the C. leptum subgroup (r = −0.31, p = 0.33; Fig. 4D). Conversely, T. sanguinis abundance showed a strong, negative correlation with colonic Tph1 expression in TBI animals (r = −0.94, p < 0.0001; Fig. 4F). We also examined associations with SERT gene expression (Slc6a4; Fig. 4G-I) in the colon and found similar patterns of correlation. Specifically, while colonic Slc6a4 was positively associated with C. scindens abundance in TBI animals (r = 0.77, p = 0.0036), it was negatively associated with T. sanguinis abundance (r = −0.77, p = 0.0095) and not significantly associated with abundance of the C. leptum subgroup (r = −0.39, p = 021).
Correlations within sham groups were not statistically meaningful, except for T. sanguinis which showed a positive correlation with colonic Slc6a4 in sham mice (Supplementary Fig. 2A-F). Furthermore, significant negative correlations were observed between C. scindens abundance and both T. sanguinis and the C. leptum subgroup (Supplementary Fig. 2G-H). We also assessed correlations between the abundance of these bacterial species and Maoa expression in the colon, but no statistically significant correlations were detected (data not shown).
3.2. Effects of TBI on peripheral tissues involved in metabolic homeostasis
3.2.1. TBI decreases circulating serotonin levels and alters expression of genes associated with serotonergic signaling and lipid balance in liver
To investigate the potential action of TBI on downstream functions of gut-derived serotonin, we examined circulating levels of free serotonin in serum and gene expression of downstream targets of serotonin signaling in liver and adipose tissue. Consistent with the decrease in serotonin fluorescence observed in 5-HT+ cells located in colonic mucosa at 7d post-TBI, circulating serum serotonin levels were significantly reduced in TBI mice compared to sham at the same time point (Mann-Whitney U = 11, p = 0.0085; Fig. 5A). At acute post-injury time points (1d and 3d post-TBI), serum serotonin levels were unchanged (p > 0.05; Fig. 5A).
Fig. 5.
Effects of TBI in liver and adipose tissue. (A) ELISA serotonin concentration in serum extracted from retro-orbital blood at 1, 3, and 7 days post-TBI. (B-D) Transcriptomic expression of serotonin receptors (Htr), monoamine oxidase A (Maoa), and lipogenic genes in liver at 1, 3, and 7 days post-TBI. (E) Gene expression of markers related to adipogenesis, lipogenesis, lipolysis, and serotonin in interscapular brown adipose tissue at 3 and 7 days post-TBI. (F) Pearson correlation between Ucp1 gene expression in brown adipose tissue and Srebp1 gene expression in liver. UCP1 is responsible for thermogenesis in brown adipose tissue. (G) Gene expression of markers related to adipogenesis, lipogenesis, lipolysis, and serotonin in subcutaneous white adipose tissue at 3 and 7 days post-TBI. Tph1, Htr2a, and Htr2b are shown for both subcutaneous (SubQ) and gonadal (Gon) white adipose depots. (H) Pearson correlation between Pparg gene expression in subcutaneous white adipose tissue and Srebf1 gene expression in liver. PPARγ has numerous roles in adipose tissue, including regulation of adipogenesis and gene expression of adipokines such as adiponectin. SREBF1/SREBP1 is a master regulator of fatty acid biosynthesis in liver. Gene expression data are expressed as mean fold change (relative to sham) ± SEM and are normalized to Gapdh expression. Correlation data indicate gene expression fold change relative to sham for each gene shown. Significant correlations are indicated by bolded p-values. Unpaired two-tailed t-test, Welch’s t-test, and Mann-Whitney U test (*p < 0.05, **p < 0.01, ***p < 0.001 vs sham); n = 6–10/group. Statistically significant outliers were excluded from the Day 3 (n = 1) and Day 7 (n = 2) TBI groups in panel (A), the Sham (n = 1–2) and TBI (n = 1) groups in panel (C), the Sham (n = 1) and TBI (n = 1) groups in panel (D), and from all groups in panels (E) and (G) where appropriate (n = 1–2 outliers removed per group). Abbreviations: BAT, brown adipose tissue; WAT, white adipose tissue; 3D, Day 3 post-TBI; 7D, Day 7 post-TBI; 5-HT, 5-hydroxytryptamine (serotonin).
To investigate the effects of TBI on serotonin signaling in liver, we began by measuring TBI-associated changes in hepatic gene expression of serotonin receptors Htr2a and Htr2b, which are involved in serotonin-mediated regulation of glucose production and lipid accumulation in the liver [16]. Htr2a, but not Htr2b, was significantly downregulated at 1d post-TBI (t(12) = 2.935, p = 0.0125; Fig. 5B). No changes were observed at 3d post-TBI, while at 7d post-TBI a significant upregulation of Htr2a was detected (t(16) = 2.204, p = 0.0425; Fig. 5C-D). We also quantified Maoa expression in liver and found no significant changes at any time point (p > 0.05; Fig. 5B-D).
Hepatic lipid balance is an important component of whole-body energy homeostasis that is regulated by serotonin [16]. To determine whether TBI influences hepatic lipid balance, we quantified hepatic gene expression of the transcription factor, Srebf1 (encodes sterol regulatory element-binding protein 1 (SREBP1), regulator of fatty acid biosynthesis), and several lipogenic enzymes (fatty acid synthase (Fasn), stearoyl-Coenzyme A desaturase 1 (Scd1), and ELOVL fatty acid elongase 6 (Elovl6). At 1d post-TBI, Fasn and Srebf1 were significantly upregulated (Fasn t(12) = 3.005, p = 0.0110; Srebf1 Welch-corrected t(7.367) = 3.648, p = 0.0075) whereas Scd1 and Elovl6 were significantly downregulated in TBI mice compared to sham (Scd1 t(12) = 3.653, p = 0.0033; Elovl6 t(12) = 2.600, p = 0.0232; Fig. 5B). At 3d post-TBI, Srebf1 remained upregulated following TBI (Welch-corrected t(10.54) = 2.739, p = 0.0200; Fig. 5C). Elovl6 was also upregulated at this time point (Welch-corrected t(11.93) = 2.724, p = 0.0186), while Fasn and Scd1 were not different from the sham group (p > 0.05 versus sham). At 7d post-TBI, both Fasn and Srebf1 were upregulated compared to sham (Fasn t(17) = 2.394, p = 0.0285; Srebf1 Welch-corrected t(12.71) = 3.238, p = 0.0066), while Scd1 and Elovl6 were not different from sham (p > 0.05 versus sham; Fig. 5D).
3.2.2. TBI differentially alters expression of genes associated with serotonergic signaling and lipid metabolism in adipose tissue
Peripheral serotonin regulates key functions in adipose tissue, including adipogenesis, lipogenesis, and thermogenesis [16,23–26]. Therefore, we investigated changes induced by TBI in transcriptomic markers of serotonin signaling in association with adipogenesis and lipid balance in adipose tissues. In brown adipose tissue (BAT), we first measured gene expression of adipogenic markers for cellular proliferation and differentiation (Pparg (peroxisome proliferator-activated receptor gamma or PPARγ), Gadd45a (growth arrest and DNA-damage-inducible protein 45 alpha or GADD45α), and Mki67 (marker of proliferation Ki-67); per [27]). Each gene was significantly downregulated at 3d post-TBI (Pparg: Mann-Whitney U = 0, p = 0.0022; Gadd45a: t(9) = 3.591, p = 0.0058; Mki67: t(10) = 4.583, p = 0.0010). At 7d post-TBI, both Gadd45a and Mki67 remained downregulated (Pparg: p > 0.05; Gadd45a: t(10) = 2.917, p = 0.0154; Mki67: t(9) = 2.783, p = 0.0213; Fig. 5E). Next, we investigated the effects of TBI on adipose lipid balance by measuring gene expression of the lipogenic enzymes Fasn and Scd1, transcription factor Mlxipl (encodes carbohydrate response element binding protein (ChREBP), master regulator of lipogenesis in adipose), thermogenesis modulator uncoupling protein 1 (Ucp1), and lipases (patatin-like phospholipase domain containing 2 (Pnpla2), lipase E (Lipe), and monoacylglycerol lipase (Mgll). While Mlxipl was significantly downregulated compared to sham at 3d post-TBI (t(10) = 3.102, p = 0.0112), no changes were detected in Fasn or Scd1 expression compared to sham at either time point (p > 0.05; Fig. 5E). A stronger TBI effect was detected for lipases Pnpla2, Lipe, and Mgll. Each transcript was significantly downregulated at 3d post-TBI (Pnpla2: t(10) = 2.248, p = 0.0484; Lipe: Welch-corrected t(5.488) = 3.217, p = 0.0206; Mgll: Welch-corrected t(5.439) = 3.865, p = 0.0100), with Pnpla2 and Lipe remaining downregulated at 7d post-TBI (Pnpla2: t(10) = 3.710, p = 0.0040; Lipe: t(10) = 2.475, p = 0.0328; Mgll: p > 0.05). Similarly, gene expression of thermogenesis regulating protein Ucp1 was significantly downregulated at both 3d and 7d post-TBI (3d post-TBI: t(10) = 3.686, p = 0.0042; 7d post-TBI: Welch-corrected t(5.890) = 3.559, p = 0.0123). This was associated with increased Srebf1 expression in the liver of TBI mice (Fig. 5F). Finally, we quantified gene expression of serotonergic markers, Tph1 and Htr3a. Tph1, the rate-limiting enzyme in serotonin synthesis that is expressed by adipocytes, was downregulated at 7d post-TBI (Mann-Whitney U = 0; p = 0.0022), while Htr3a, the main serotonin receptor expressed in BAT, was downregulated at 3d post-TBI (t(9) = 5.476, p = 0.0004; Fig. 5E).
Subcutaneous white adipose tissue (WAT) was chosen for analysis of WAT lipid metabolism because a trending decrease in adipose tissue weight was observed in this particular fat depot collected from TBI animals at 3d post-TBI (expressed as % total bodyweight; t(10) = 2.135, p = 0.0586; data not shown), suggesting that lipid metabolism may be significantly altered in subcutaneous WAT following TBI. In subcutaneous WAT of TBI mice, we found a significant, nearly 7-fold upregulation of Pparg at 3d post-TBI (Welch-corrected t(5.528) = 6.984, p = 0.0006), which returned to sham levels by 7d post-TBI (p > 0.05; Fig. 5G). This finding was associated with decreased Srebf1 expression in the liver of TBI mice (Fig. 5H). Transcriptomic upregulation at 3d post-TBI was also detected for Mlxipl (t(9) = 2.396, p = 0.0402), Scd1 (t(9) = 2.377, p = 0.0414), and Mgll (t(8) = 4.262, p = 0.0028) in subcutaneous WAT.
TBI also differentially affected markers of WAT serotonergic signaling. While gene expression of serotonin receptors Htr2a and Htr2b was not different from sham in subcutaneous WAT (p > 0.05), both Htr2a and Htr2b were significantly upregulated compared to sham at 7d post-TBI in gonadal WAT (Htr2a: Mann-Whitney U = 0, p = 0.0022; Htr2b: Welch-corrected t(4.762) = 3.484, p = 0.0190; Fig. 5F). Tph1 expression was increased in both subcutaneous (Welch-corrected t (5.743), p = 0.0409) and gonadal WAT (t(8) = 2.441, p = 0.0405) at 7d post-TBI, while a significant downregulation was also observed at 3d post-TBI only in gonadal WAT (t(7) = 4.551, p = 0.0026; Fig. 5F).
4. Discussion
Autonomic failure and subsequent systemic dysfunction are common sequelae of TBI pathology [28], but the modulatory role played by the GI system is not yet well understood. Therefore, we sought to investigate the action of TBI on the ENS and peripheral serotonergic system as these systems are crucial regulators of gut function and systemic physiology. We found that TBI chiefly affects the colon and inflicts transcriptomic changes in metabolic tissues that are functionally regulated by gut-derived serotonin.
4.1. Effects of TBI in the gut: potential mechanisms underlying GI dysfunction
In recent years, researchers have begun to investigate the role of the gut as a mediator of TBI pathology [29–31], and conversely, how TBI alters homeostasis within the gut [5–7,10]. We used transcriptomic analyses to investigate which gut region(s) was/were the most impacted by TBI, and found that TBI has particularly strong effects in the colon, where it transiently reduced the abundance of mRNA for ENS neuronal markers Chat (cholinergic neurons) and Nos1 (nitrergic neurons) and altered the colonic serotonin system, favoring increased serotonin degradation and decreased serotonin production. Cholinergic and nitrergic neurons make up the majority of ENS neurons and, in concert with serotonin produced by EC cells, play central roles in the regulation of the peristaltic reflex in the gut [32,33]. Because the ENS and gut-derived serotonin are key regulators of gut function, it stands to reason that TBI-mediated dysfunction within these systems could negatively impact gut health. Indeed, GI autonomic dysregulation and overall GI dysfunction are commonly observed among TBI patients [34]. This dysfunction could be explained, at least in part, by a reduction in the main neurotransmitters produced by cholinergic and nitrergic neurons in the gut (acetylcholine and nitric oxide, respectively). Overall, our data suggest that TBI may impact gut function by disrupting neural signaling mechanisms controlling gut peristalsis.
4.2. Effects of TBI in the gut: commensal gut bacteria differentially interact with the colonic serotonin system
Recent evidence has indicated that TBI affects the composition of the gut microbiome [see [8] for review] and that therapeutic approaches targeting GI dysbiosis may improve neurological dysfunction associated with TBI and protect against secondary insults [35–38]. In turn, we show that TBI alters the abundance of microbial species that are thought to be associated with the host gut serotonin system, and that these changes correlate with TBI-mediated changes in the serotonin system of the colon. Specifically, we observed differential effects of TBI on the abundance of commensal Clostridia species extracted from the mouse cecum. C. leptum is a member of Clostridium cluster IV (C. leptum subgroup), the abundance and diversity of which has been shown to decline in cases of inflammatory bowel disease [39,40]. C. scindens, a member of Clostridium cluster XIVa, is known for its ability to inhibit the growth of Clostridioides difficile due to the production of secondary bile acids that inhibit C. difficile colonization [41–43], and has also recently been associated with resistance to obesity [44]. In the present study, we observed a decrease in the abundance of the C. leptum subgroup and an increase in C. scindens abundance following TBI. Further, the abundance of C. scindens was inversely associated with that of the C. leptum subgroup in TBI animals, indicating that direct competition for colonization of the GI tract may occur between these species and that TBI may disturb the balance between them.
Upon investigation of the relationships between microbiota and the host serotonin system, we found that increased abundance of C. scindens – but not C. leptum subgroup members – was associated with increased transcriptomic expression of Tph1 and Slc6a4 in the colon of TBI mice. The stronger connection that we observed between C. scindens abundance and serotonin-related genes in the colon may be due to the much higher 7α-dehydroxylation activity of this species compared to other secondary bile acid producing species such as C. leptum [45]. One particular bile acid, deoxycholate, has been shown to increase serotonin levels and Tph1 mRNA levels in the colon [22]. Therefore, increased secondary bile acid production resulting from increased C. scindens abundance could directly influence the serotonin system in the gut and disturb its downstream functions [3,12].
Colonization of the GI tract by another commensal bacterial species, T. sanguinis, is modulated by serotonin and the selective serotonin reuptake inhibitor fluoxetine [21]. Specifically, in the GI system of the mouse, gut T. sanguinis populations increase under conditions of elevated serotonin and decrease with fluoxetine treatment [21]. In contrast, we found that T. sanguinis abundance was negatively associated with Tph1 and Slc6a4 in the colon of TBI mice, indicating that under TBI conditions, colonic serotonin production may be decreased in animals with higher T. sanguinis colonization of the colon.
Overall, our evidence suggests that commensal bacterial species exhibit distinct patterns of interaction with the colonic serotonin system following brain injury. However, given the intrinsic variability in the bacterial species adaptation to injury, further studies are required to refine the association between serotonin and specific bacterial species. Indeed, considering the extensive interaction between gut microbiota and the gut-brain axis [3], and the numerous downstream systems that are modulated by gut-derived serotonin [17], it is feasible that the observed adaptations between these systems in the colon could have far-reaching consequences for systemic physiology and overall health of individuals affected by TBI.
4.3. Effects of TBI in peripheral metabolic tissues regulated by serotonin and implications for systemic energy balance
Due to its numerous physiological roles in the body, gut-derived serotonin is positioned such that it may serve as a conduit through which the effects of TBI on the gut may spread to other peripheral organs. Serotonin produced in the gut may enter the circulatory system where it is taken up by blood platelets or degraded by monoamine oxidase in the liver [13,16]. Once in general circulation, serotonin may be released by blood platelets in response to stimuli to induce vasoconstriction and blood coagulation, or alternatively, may be used in the regulation of processes such as haematopoiesis, bone metabolism and remodeling, and energy balance [16,17]. For example, in the liver, peripheral serotonin promotes gluconeogenesis, inhibits glucose uptake, and enhances lipid accumulation, a process which has been implicated in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) [16,19]. Peripheral serotonin also plays a role in the physiology of adipose tissue, where it promotes adipogenesis and lipogenesis in WAT, decreases WAT “browning”, and suppresses thermogenesis in BAT [16]. By inducing such effects in liver and adipose tissue, peripheral serotonin plays an integral role in the maintenance of systemic energy balance. Therefore, the effects of TBI on the serotonergic system of the gut have the potential to disrupt systemic energy homeostasis.
4.3.1. Transcriptomic effects of TBI on serotonin receptors and lipogenic genes in the liver
Consistent with our observations of decreased serotonin fluorescence in the colon, we found that circulating serotonin levels were significantly decreased in TBI animals in the post-acute period following brain injury. Our results also show that levels of serotonin were higher in the day 1 sham group, which could be related to possible batch effects due to surgery-related factors such as downstream response to the craniotomy procedure and potential stress experienced by the animals. As peripheral serotonin promotes an energy storage phenotype, these collective findings are consistent with clinical evidence indicating that persons with TBI often undergo an initial post-injury period of significant hypermetabolism and weight loss despite nutritional support [46,47]. We also detected an increase in hepatic gene expression of the serotonin receptor Htr2a in TBI animals, which may reflect a compensatory response to decreased serotonin levels in the circulation.
Hepatic lipogenesis and risk for hepatic steatosis may also be impacted by TBI, as various lipogenic genes were upregulated in response to TBI including Srebf1, an insulin-sensitive transcription factor that regulates the induction of lipogenesis. Indeed, we have shown previously that TBI promotes hepatic lipid accumulation [48]. It has been suggested that serotonin increases lipid concentrations in the liver [49,50]. Though we found evidence of decreased peripheral serotonin levels in response to TBI, this does not rule out a secondary action of serotonin in the liver, which is innervated by the ANS and functions under the action of hormones activated by TBI [51].
4.3.2. Does adipose tissue modulate hepatic lipid accumulation in TBI?
Interestingly, both Pparg expression in WAT and Ucp1 expression in BAT correlated with liver Srebf1 expression in TBI mice, suggesting a potential role for adipose tissues as modulators of hepatic lipid accumulation in TBI. The modulatory influence of adipose in the development of liver pathology has previously been demonstrated in NAFLD via secretion of adiponectin, an adipokine that regulates various metabolic processes and is itself modulated by PPARγ [52,53]. Furthermore, increased UCP1 activation in BAT is associated with resistance to hepatic lipid accumulation and NAFLD in rodents with high-fat diet-induced obesity [16] and has recently been shown to antagonize hepatic inflammation and pathology [54]. Collectively, this evidence supports our findings that both increased Ucp1 in BAT and increased Pparg in WAT are associated with a reduction in Srebf1 in the liver of TBI mice. Therefore, targeting adipose expression of UCP1 and/or PPARγ may be a useful approach to regulate hepatic lipid accumulation following TBI.
4.3.3. Transcriptomic effects of TBI in adipose tissue
The effects of TBI on lipid metabolism in adipose tissue are multifaceted. In interscapular BAT, we observed a sustained downregulation of genes associated with adipogenesis (Gadd45a, Mki67, Pparg), lipolysis (Pnpla2, Lipe, Mgll), and thermogenesis (Ucp1) in TBI animals, suggesting that TBI may disrupt BAT tissue growth and energy expenditure. Interestingly, Ucp1 downregulation in BAT occurred in TBI animals despite decreased circulating serotonin levels and decreased local expression of Tph1, indicating that TBI may alter Ucp1 expression and thermogenic activity through a serotonin-independent mechanism. Collectively, our data suggest that, at the transcriptional level, TBI alters lipid metabolism and energy expenditure in BAT, a consequence which could have downstream implications for systemic energy balance and the development of metabolic disorders such as obesity and type 2 diabetes [55,56].
In contrast to the persistent transcriptional downregulation that we identified in BAT, we found that TBI acutely increased the expression of genes involved in adipogenesis and fatty acid storage (Pparg) and lipogenesis (Mlxipl, Scd1) in subcutaneous WAT. In particular, the remarkable 7-fold upregulation of the nuclear receptor and adipogenesis modulator Pparg may be a compensatory response to the initial brain injury, as this molecule has been associated with beneficial effects in the injured brain including reduced inflammatory responses and oxidative stress, inhibition of apoptosis, and promotion of neurogenesis [57]. At the same acute time point after injury, we observed an upregulation of Mgll, the gene encoding monoacylglycerol lipase (MAGL). MAGL is responsible for catalyzing the final step of triglyceride lipolysis in adipose tissue, converting monoacylglycerols into free fatty acids and glycerol. Deficiency of this enzyme in MAGL knockout mice is associated with the prevention of hepatic steatosis by promoting lipid storage in adipose tissue [53]. Based on this study, it is reasonable to infer that increased production of adipose MAGL in response to brain injury may increase susceptibility to hepatic steatosis in TBI patients.
Serotonin has been shown to regulate de novo lipogenesis, lipolysis, and adipocyte differentiation in adipose tissue, primarily through the HTR2A receptor [16,23–26,58]. Here, we show differential effects of TBI on serotonin receptor expression in subcutaneous and gonadal WAT. Interestingly, while no TBI effect on Htr2a and Htr2b expression was detected in subcutaneous WAT, both genes were upregulated in gonadal WAT at 7 days post-TBI, possibly as a compensatory response to decreased circulating serotonin levels at this time point. Similarly, adipocyte Tph1 gene expression was differentially altered only at 7 days after injury in both BAT (decreased Tph1) and WAT (increased Tph1), which may reflect delayed responses to the cumulative changes occurring in the gut.
4.4. TBI: a multifaceted, systemic condition
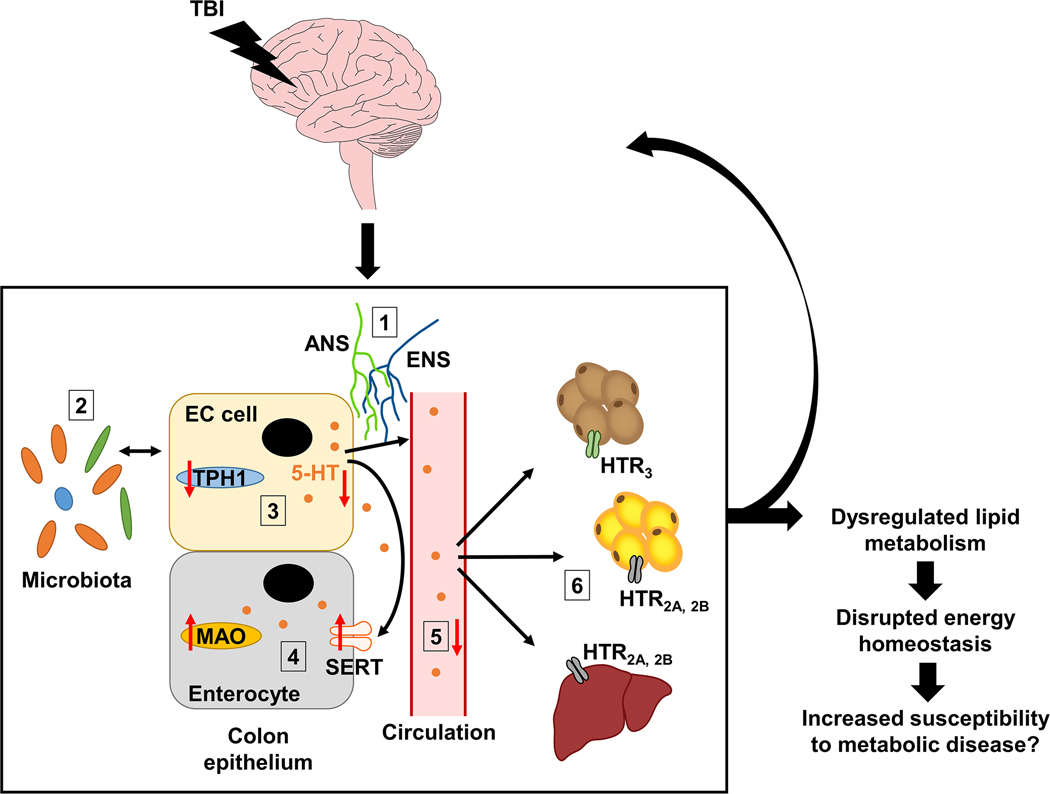
TBI is a serious disorder with chronic, far-reaching consequences and increasing global incidence [59], and yet treatment options remain limited. A significant barrier to the development of effective therapeutics for the management of TBI is our lack of understanding of how TBI alters peripheral physiology and the subsequent effects on recovery time and treatment efficacy. Here, we demonstrate in a mouse model that TBI affects the gut-derived serotonergic system and alters lipid homeostasis in downstream tissues that are regulated by peripheral serotonin (Fig. 6, Table 1). These changes to peripheral physiology appear to persist beyond the acute post-injury period and have the potential to alter energy homeostasis, which in turn may predispose individuals with TBI to metabolic dysfunction and reduced quality of life. Indeed, in one longitudinal study of 107 TBI patients, the incidence of new metabolic disorders during the follow-up period after injury was 16 % and included the onset of arterial hypertension, type 2 diabetes, and dyslipidemia [46]. In all, our findings support the growing body of evidence establishing TBI as a heterogeneous, multisystem condition [60] and will inform ongoing efforts to develop effective interventions for TBI treatment.
Fig. 6.
Effects of TBI on serotonin homeostasis in the colon and downstream effects in metabolic organs. Simplified summary schematic demonstrating the peripheral effects of TBI in the colon, liver, and adipose tissues. In TBI mice we observed: (1) a reduction in ENS neuronal markers Chat and Nos1 suggesting that ENS regulation of the gut may be disturbed; (2) altered abundance of serotonin-associated commensal bacterial species that correlated with TBI-mediated changes in serotonin production and signaling in the colon; (3) reduced serotonin synthesis as indicated by reduced Tph1 expression and subsequent reduction in colonic serotonin levels (serotonin is indicated by orange circles); (4) increased serotonin degradation as indicated by increased Slc6a4 (SERT) and Maoa (monoamine oxidase A) expression in colon; (5) decreased levels of circulating serotonin*; and (6) altered serotonin receptor (HTR) gene expression and lipid metabolism in brown adipose, white adipose, and liver. Overall, these changes suggest that TBI disrupts peripheral serotonin homeostasis, which may lead to negative consequences for normal gut function and whole-body energy balance and may even exacerbate the brain injury via bidirectional brain-gut communication. *Note that serotonin in circulation is primarily contained within blood platelets (omitted for simplicity). Abbreviations: ANS, autonomic nervous system; EC, enterochromaffin; ENS, enteric nervous system; HTR, 5-hydroxytryptamine (serotonin) receptor; MAO, monoamine oxidase; SERT, serotonin transporter; TBI, traumatic brain injury; TPH1, tryptophan hydroxylase 1; 5-HT, 5-hydroxytryptamine (serotonin).
Table 1.
Time course summary of observed gene expression changes in the periphery after TBI. Arrows indicate direction of change (increase or decrease) in TBI animals compared to sham and dashes indicate no significant change. Double arrows indicate a stronger TBI effect vs sham (p < 0.01). Abbreviations: ENS, enteric nervous system; N/A, data not available; 5-HT, 5-hydroxytryptamine (serotonin).
| Days post-TBI |
ENS markers & 5-HT system
|
Peripheral 5-HT levels
|
Peripheral 5-HT action: 5-HT receptors, lipid metabolism, & adipogenesis
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duodenum | Colon | Serum | Liver | Gonadal white adipose | Subcutaneous white adipose | Interscapular brown adipose | |||||
|
| |||||||||||
| Day 1 | Chat ↓ Nos ↓↓ Tph1 – |
Chat ↓ Nos ↓↓ Tph1 – |
5-HT – | Htr2a ↓ Htr2b – Maoa – |
Fasn ↑ Srebp1 ↑↑ Scd1 ↓↓ Elovl6 ↓ |
N/A | N/A | N/A | |||
| Day 3 | Chat – Nos – Tph1 ↓ |
Chat ↓↓ Nos ↓ Tph1 ↓↓ Maoa ↑↑ Slc6a4 – |
5-HT – | Htr2a – Htr2b – Maoa – |
Fasn – Srebp1 ↑ Scd1 – Elovl6 ↑ |
Tph1 ↓↓ Htr2a – Htr2b – |
Pparg ↑↑ Mlxipl ↑ Fasn – Scd1 ↑ Pnpla2 – |
Lipe – Mgll ↑↑ Tph1 – Htr2a – Htr2b – |
Gadd45a ↓↓ Ki67 ↓↓ Pparg ↓↓ Mlxipl ↓ Fasn – Scd1 – |
Ucp1 ↓↓ Pnpla2 ↓ Lipe ↓ Mgll ↓ Tph1 – Htr3a ↓↓ |
|
| Day 7 | Chat – Nos – Tph1 – |
Chat – Nos – Tph1 – Maoa ↑↑ Slc6a4 ↑↑ |
5-HT ↓↓ | Htr2a ↑ Htr2b – Maoa – |
Fasn ↑ Srebp1 ↑↑ Scd1 – Elovl6 – |
Tph1 ↑ Htr2a ↑↑ Htr2b ↑ |
Pparg – Mlxipl – Fasn – Scd1 – Pnpla2 – |
Lipe – Mgll – Tph1 ↑ Htr2a – Htr2b – |
Gadd45a ↓ Ki67 ↓ Pparg – Mlxipl – Fasn – Scd1 – |
Ucp1 ↓ Pnpla2 ↓↓ Lipe ↓ Mgll – Tph1 ↓↓ Htr3a – |
|
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers: NS111378; NS117148; NS050465; NS116838).
Data availability
Data will be made available on request.
Abbreviations:
- AADC
aromatic l-amino acid decarboxylase
- Chat
choline acetyltransferase
- EC
enterochromaffin
- Elavl4
ELAV-like protein4, also known as HuD
- Elovl6
ELOVL fatty acid elongase 6
- Fasn
fatty acid synthase
- Gadd45a
growth arrest and DNA-damage-inducible protein alpha
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Htr(2a,2b,3a)
5-hydroxytryptamine receptor (2a, 2b, 3a)
- Lipe
lipase E, also known as hormone-sensitive lipase (HSL)
- Maoa
monoamine oxidase A
- Mgll
monoacylglycerol lipase (MAGL
- Mki67
marker of proliferation Ki-67
- Mlxipl
MLX interacting protein like, also known as carbohydrate response element binding protein (ChREBP)
- Nos1
nitric oxide synthase 1
- Pnpla2
patatin-like phospholipase domain containing 2, also known as adipose triglyceride lipase (ATGL)
- Pparg
peroxisome proliferator activated receptor gamma
- Scd1
stearoyl-Coenzyme A desaturase 1
- Slc6a4
solute carrier family 6 member 4, also known as serotonin transporter (SERT)
- Srebf1
sterol regulatory element binding transcription factor 1, also known as sterol regulatory element binding protein 1 (SREBP1)
- TBI
traumatic brain injury
- Tph1
tryptophan hydroxylase
- Ucp1
uncoupling protein 1
Footnotes
CRediT authorship contribution statement
Natosha M. Mercado: Conceptualization, experimental work (histology, qpcr, and analysis of data), Preparation of the original and final draft of the manuscript.
Guanglin Zhang: Experimental work (surgeries, qpcr).
Zhe Ying: Experimental work (animal care, tissue collection), technical supervision.
Fernando Gomez-Pinilla: Conceptualization, design, writing (Review & Editing), Funding acquisition.
Declaration of competing interest
The authors declare that do not have direct or indirect competing financial interests. The authors are not aware of interests in the personal, academic competition, and intellectual passion categories.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbadis.2022.166491.
References
- [1].Stocchetti N, Zanier ER, Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review, Crit. Care 20 (2016) 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cryan JF, et al. , The microbiota-gut-brain axis, Physiol. Rev. 99 (2019) 1877–2013. [DOI] [PubMed] [Google Scholar]
- [3].Martin CR, et al. , The brain-gut-microbiome axis, Cell. Mol. Gastroenterol. Hepatol. 6 (2018) 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feighery L, et al. , Increased intestinal permeability in rats subjected to traumatic frontal lobe percussion brain injury, J. Trauma 64 (2008) 131–137, discussion 137–8. [DOI] [PubMed] [Google Scholar]
- [5].Hang CH, et al. , Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats, World J. Gastroenterol. 9 (2003) 2776–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ma EL, et al. , Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice, Brain Behav. Immun. 66 (2017) 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pan P, et al. , Intestinal barrier dysfunction following traumatic brain injury, Neurol. Sci. 40 (2019) 1105–1110. [DOI] [PubMed] [Google Scholar]
- [8].Pathare N, et al. , The impact of traumatic brain injury on microbiome composition: a systematic review, Biol. Res. Nurs. 22 (2020) 495–505. [DOI] [PubMed] [Google Scholar]
- [9].Urban RJ, et al. , Altered fecal microbiome years after traumatic brain injury, J. Neurotrauma 37 (2020) 1037–1051. [DOI] [PubMed] [Google Scholar]
- [10].You W, et al. , Traumatic brain injury induces gastrointestinal dysfunction and dysbiosis of gut microbiota accompanied by alterations of bile acid profile, J. Neurotrauma 39 (2022) 1–2. [DOI] [PubMed] [Google Scholar]
- [11].Spencer NJ, Hu H, Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility, Nat. Rev. Gastroenterol. Hepatol. 17 (2020) 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bellono NW, et al. , Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways, Cell 170 (185–198) (2017), e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bertrand PP, Bertrand RL, Serotonin release and uptake in the gastrointestinal tract, Auton. Neurosci. 153 (2010) 47–57. [DOI] [PubMed] [Google Scholar]
- [14].Mawe GM, Hoffman JM, Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets, Nat. Rev. Gastroenterol. Hepatol. 10 (2013) 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gershon MD, 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract, Curr. Opin. Endocrinol. Diabetes Obes. 20 (2013) 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yabut JM, et al. , Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule, Endocr. Rev. 40 (2019) 1092–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spohn SN, Mawe GM, Non-conventional features of peripheral serotonin signalling - the gut and beyond, Nat. Rev. Gastroenterol. Hepatol. 14 (2017) 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choi W, et al. , Serotonergic regulation of energy metabolism in peripheral tissues, J. Endocrinol. 245 (2020) R1–R10. [DOI] [PubMed] [Google Scholar]
- [19].Watanabe H, et al. , in: Energy Homeostasis by the Peripheral Serotonergic System, InTechOpen, 2017, pp. 185–201. [Google Scholar]
- [20].Matsuki T, et al. , Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces, Appl. Environ. Microbiol. 70 (2004) 7220–7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fung TC, et al. , Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut, Nat. Microbiol. 4 (2019) 2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yano JM, et al. , Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis, Cell 161 (2015) 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kinoshita M, et al. , Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5, Mol. Endocrinol. 24 (2010) 1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rozenblit-Susan S, et al. , Serotonin prevents differentiation into brown adipocytes and induces transdifferentiation into white adipocytes, Int. J. Obes. 42 (2018) 704–710. [DOI] [PubMed] [Google Scholar]
- [25].Shong KE, et al. , Serotonin regulates de novo lipogenesis in adipose tissues through serotonin receptor 2A, Endocrinol. Metab. (Seoul) 35 (2020) 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu B, et al. , Serotonin 5-HT2A receptor activity mediates adipocyte differentiation through control of adipogenic gene expression, Sci. Rep. 11 (2021) 19714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].You W, et al. , GADD45α drives brown adipose tissue formation through upregulating PPARγ in mice, Cell Death Dis. 11 (2020) 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baguley IJ, et al. , A critical review of the pathophysiology of dysautonomia following traumatic brain injury, Neurocrit. Care 8 (2008) 293–300. [DOI] [PubMed] [Google Scholar]
- [29].Celorrio M, et al. , Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis, Acta Neuropathol. Commun. 9 (2021) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shaito A, et al. , Western diet aggravates neuronal insult in post-traumatic brain injury: proposed pathways for interplay, EBioMedicine 57 (2020), 102829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sundman MH, et al. , The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease, Brain Behav. Immun. 66 (2017) 31–44. [DOI] [PubMed] [Google Scholar]
- [32].Grider JR, Neurotransmitters mediating the intestinal peristaltic reflex in the mouse, J. Pharmacol. Exp. Ther. 307 (2003) 460–467. [DOI] [PubMed] [Google Scholar]
- [33].Qu ZD, et al. , Immunohistochemical analysis of neuron types in the mouse small intestine, Cell Tissue Res. 334 (2008) 147–161. [DOI] [PubMed] [Google Scholar]
- [34].Kharrazian D, Traumatic brain injury and the effect on the brain-gut axis, Altern. Ther. Health Med. 21 (Suppl 3) (2015) 28–32. [PubMed] [Google Scholar]
- [35].Du D, et al. , Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neurological deficits after traumatic brain injury, Oxidative Med. Cell. Longev. 2021 (2021) 5816837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li H, et al. , Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis, Neurogastroenterol. Motil. 30 (2018), e13260. [DOI] [PubMed] [Google Scholar]
- [37].Ma Y, et al. , Lactobacillus acidophilus exerts neuroprotective effects in mice with traumatic brain injury, J. Nutr. 149 (2019) 1543–1552. [DOI] [PubMed] [Google Scholar]
- [38].Rice MW, et al. , Gut microbiota as a therapeutic target to ameliorate the biochemical, neuroanatomical, and behavioral effects of traumatic brain injuries, Front. Neurol. 10 (2019) 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kabeerdoss J, et al. , Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: a case-control study in India, BMC Gastroenterol. 13 (2013) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lopetuso LR, et al. , Commensal clostridia: leading players in the maintenance of gut homeostasis, Gut Pathog. 5 (2013) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Buffie CG, et al. , Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile, Nature 517 (2015) 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Greathouse KL, et al. , Dysfunctional families: Clostridium scindens and secondary bile acids inhibit the growth of Clostridium difficile, Cell Metab. 21 (2015) 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Studer N, et al. , Functional intestinal bile acid 7α-dehydroxylation by Clostridium scindens associated with protection from Clostridium difficile infection in a gnotobiotic mouse model, Front. Cell. Infect. Microbiol. 6 (2016) 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wei M, et al. , A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility, EBioMedicine 55 (2020), 102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Devendran S, et al. , Clostridium scindens ATCC 35704: integration of nutritional requirements, the complete genome sequence, and global transcriptional responses to bile acids, Appl. Environ. Microbiol. 85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Crenn P, et al. , Changes in weight after traumatic brain injury in adult patients: a longitudinal study, Clin. Nutr. 33 (2014) 348–353. [DOI] [PubMed] [Google Scholar]
- [47].Krakau K, et al. , Metabolism and nutrition in patients with moderate and severe traumatic brain injury: a systematic review, Brain Inj. 20 (2006) 345–367. [DOI] [PubMed] [Google Scholar]
- [48].Rege SD, et al. , Brain trauma disrupts hepatic lipid metabolism: blame it on fructose? Mol. Nutr. Food Res. 63 (2019), e1801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Haub S, et al. , Serotonin receptor type 3 antagonists improve obesity-associated fatty liver disease in mice, J. Pharmacol. Exp. Ther. 339 (2011) 790–798. [DOI] [PubMed] [Google Scholar]
- [50].Watanabe H, et al. , Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover, Endocrinology 151 (2010) 4776–4786. [DOI] [PubMed] [Google Scholar]
- [51].Palafox-Sanchez V, et al. , The interaction between brain and liver regulates lipid metabolism in the TBI pathology, Biochim. Biophys. Acta Mol. Basis Dis. 1867 (2021), 166078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Boutari C, et al. , Association of adipokines with development and progression of nonalcoholic fatty liver disease, Endocrinol. Metab. (Seoul) 33 (2018) 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tardelli M, et al. , Lack of monoacylglycerol lipase prevents hepatic steatosis by favoring lipid storage in adipose tissue and intestinal malabsorption, J. Lipid Res. 60 (2019) 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mills EL, et al. , UCP1 governs liver extracellular succinate and inflammatory pathogenesis, Nat. Metab. 3 (2021) 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cheng L, et al. , Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus, Adipocyte 10 (2021) 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tapia P, et al. , Biology and pathological implications of brown adipose tissue: promises and caveats for the control of obesity and its associated complications, Biol. Rev. Camb. Philos. Soc. 93 (2018) 1145–1164. [DOI] [PubMed] [Google Scholar]
- [57].Qi L, et al. , Peroxisome proliferator activated receptor-γ and traumatic brain injury, Int. J. Clin. Exp. Med. 3 (2010) 283–292. [PMC free article] [PubMed] [Google Scholar]
- [58].Hansson B, et al. , Serotonin (5-HT) and 5-HT2A receptor agonists suppress lipolysis in primary rat adipose cells, Biochem. Biophys. Res. Commun. 474 (2016) 357–363. [DOI] [PubMed] [Google Scholar]
- [59].GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators, Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet Neurol. 18 (2019) 56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McDonald SJ, et al. , Beyond the brain: peripheral interactions after traumatic brain injury, J. Neurotrauma 37 (2020) 770–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.