Abstract
Background –
Surgical ablation for atrial fibrillation (AF) can be effective, yet has mixed results. It is important to improve the success of AF surgery, yet unclear which endocardial lesions will best augment surgical lesion sets in individual patients. We addressed this question by systematically mapping AF endocardially after surgical ablation and relating findings to early recurrence.
Methods –
We studied 81 consecutive patients undergoing epicardial surgical ablation (stage 1 hybrid), of whom 64 proceeded to endocardial catheter mapping and ablation (Stage 2). Stage 2 comprised high-density mapping of pulmonary vein (PV) and/or posterior wall (PW) reconnections, low voltage zones, and potential localized AF drivers. We related findings to post-surgical recurrence of AF (SRAF).
Results –
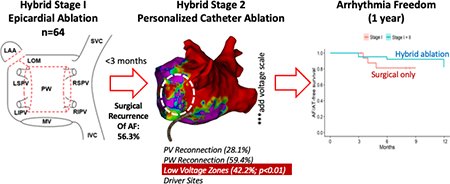
Mapping at stage 2 revealed PWI reconnection in 59.4%, PVI reconnection in 28.1% and LVZ in 42.2% of patients. SRAF occurred in 36 patients (56.3%), particularly those with long-standing persistent AF (LsPeAF, p=0.017), but had no relationship to reconnection of PVs (p=0.53) or PWI (p=0.75) when compared to those without SRAF. LVZ were more common in patients with SRAF (p=0.002), LsPeAF (p=0.002), advanced age (p=0.03), and elevated CHA2DS2-VASc (p=0.046). AF mapping revealed 4.4 ± 2.7 localized focal/rotational sites near and also remote from PV or PW reconnection. After ablation at patient-specific targets, arrhythmia freedom at 1 year was 81.0% including and 73.0% excluding previously ineffective antiarrhythmic medications.
Conclusions –
After surgical ablation, AF may recur by several modes including recovery of posterior wall or pulmonary vein isolation, mechanisms related to localized LVZ or other sustaining mechanisms. LVZ are more common in patients at high clinical risk for recurrence. Patient-specific targeting of these mechanisms yields excellent long-term outcomes from hybrid ablation.
Journal Subject Terms: Atrial Fibrillation, Electrophysiology, Surgery, Ablation
Keywords: Atrial Fibrillation, Hybrid Surgery, Atrial Fibrillation Mapping
Graphical Abstract
Introduction
Surgical therapy for atrial fibrillation (AF) is increasingly used, with success varying from 63% in multicenter experiences1 to ~85% at single centers.2, 3 Combining surgical epicardial ablation with endocardial catheter ablation (hybrid therapy) is also increasingly used, with one year success also varying from 40%4 to 80%5, 6. While it is essential to optimize hybrid ablation, it is unclear which components of surgical therapy are most successful and which are best complemented by endocardial ablation.
Candidate mechanisms for early post-stage I recurrence of AF (SRAF) include, first, reconnection of pulmonary veins (PVs)7 or posterior wall lesion sets or, second, sites not targeted anatomically (including low voltage zones, LVZ, or localized electrical mechanisms). The latter patient-specific sites may be critical when AF recurs despite intact pulmonary vein isolation (PVI) and posterior wall isolation (PWI).
We studied modes for recurrence after surgical AF ablation by systematic mapping for PVI and PWI reconnection and other mechanisms at the endocardial portion of hybrid ablation. Our approach to surgical AF ablation was standardized, enabling mapping to be compared between patients. We related these findings to the presence or absence of SRAF, which strongly predicts long-term recurrence.6–8
Methods
The data that support the findings of this study are available from the correspond author upon reasonable request.
Patient flow
Our study was approved by the institutional review board at Stanford University, and all patients provided written informed consent. Between October 2015 and August 2019, a total of 85 consecutive patients were prospectively approached for hybrid ablation for symptomatic AF refractory to at least one anti-arrhythmic drug.8 Figure 1A details patient flow.
Figure 1. Mapping AF after Hybrid 1 Surgical Ablation: Patient Flow and Study Design.
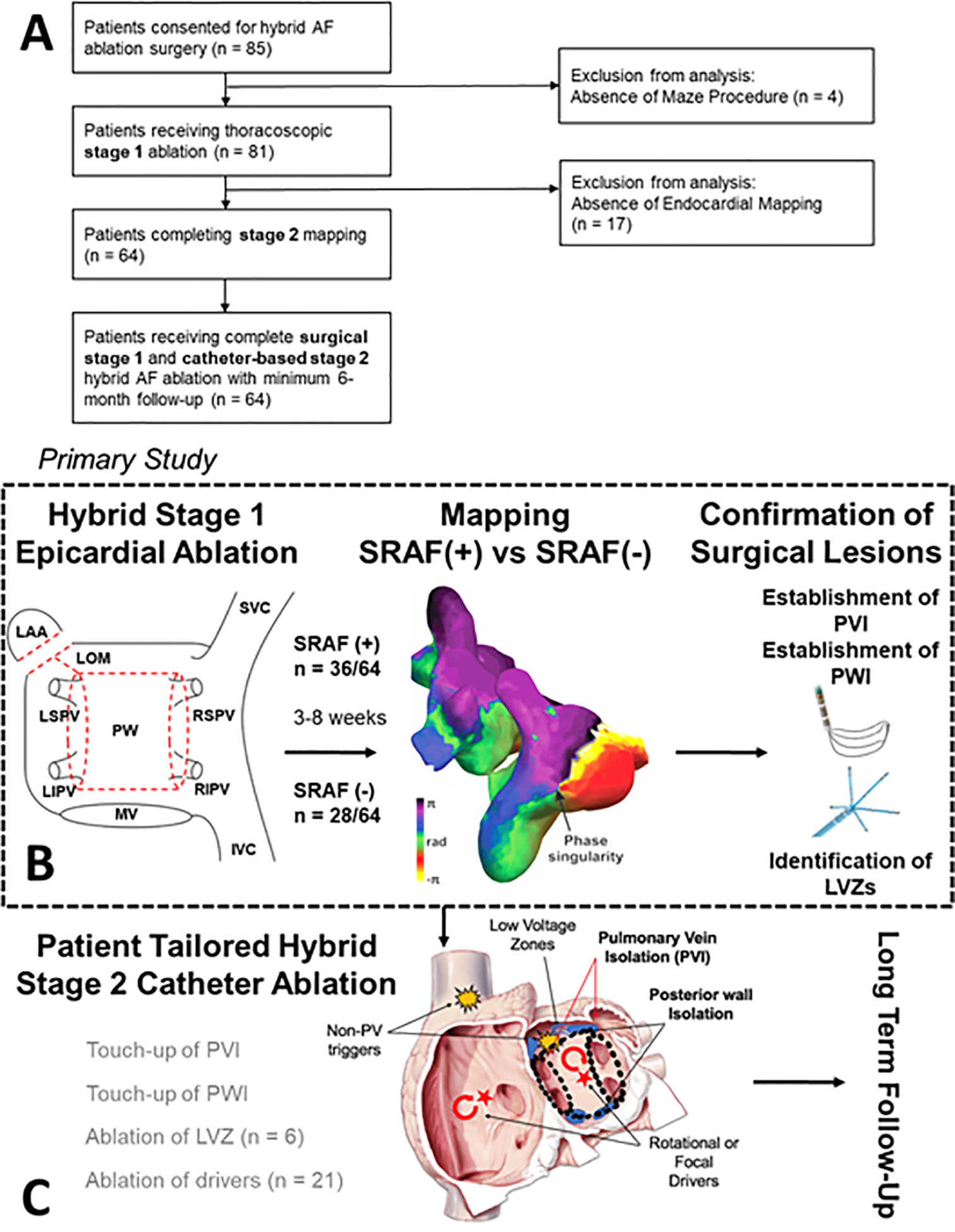
Panel A shows the study design. Panel B shows details of endocardial mapping to compare cases with SRAF (+) versus SRAF (−). Panel C shows that tailored endocardial ablation.
Four patients did not undergo surgical ablation (2 declined and 2 relocated). Eighty one patients underwent surgical ablation (stage I), of whom N=17 did not proceed to endocardial ablation for varied reasons (see Adverse events below) and N=64 completed stages I and II.
Surgical Epicardial Procedure (Stage I)
Patients underwent standardized video-assisted thoracoscopic (VATs) epicardial ablation by a single experienced surgeon (A.M.L.). Transesophageal echocardiography (TEE) was performed to rule out left atrial thrombus or severe valvular heart disease. Under general anesthesia with selective lung ventilation, three thoracoscopic ports were inserted: 1) a 5mm port at the fifth intercostal space (midaxillary); 2) a 5 mm port at third intercostal space (anterior axillary); and 3) a 12 mm port in the 7th intercostal space (midaxillary). After protecting the phrenic nerve, the pericardium was accessed. A lighted dissector (Lumitip, AtriCure, Mason, OH) was inserted through the inferior port to guide the bipolar radiofrequency clamp (Isolator Synergy Clamps, AtriCure) to isolate the left PVs, then right PVs with multiple applications. The posterior wall (PW) was isolated using lines of ablation across the roof and floor. PV and PW entrance and exit block were confirmed (Isolator Transpolar Pen, AtriCure). The ligament of Marshall (LOM) was identified and excised. Finally, the left atrial appendage (LAA) was excluded (AtriClip, AtriCure). Figure 1B summarizes these stereotypical lesion sets.
Follow-up after stage 1
Patients had at least one clinic visit and monitoring with a 48-hour Holter monitor or 7–14 day monitor (Zio, iRhythm, San Francisco, CA) to assess SRAF between Stages 1 and 2. SRAF was defined by ECG as AF>30 seconds, regardless of symptoms.
Endocardial Mapping and Ablation (Stage 2)
Procedures were performed by one of 4 operators. All patients underwent TEE or cardiac-gated computed tomography with contrast to exclude left atrial thrombus and assess left atrial appendage closure. 3D reconstruction of the atria and PVs was performed using CARTO3 (Biosense Webster, Irvine, CA) or EnSite Precision (Abbott, St. Paul, MN). Bipolar voltage maps were created for >2,000 points using the PENTARAY® (Biosense Webster) or Advisor™ HD Grid (Abbott) catheter. Gaps in PVI or PWI box lesion sets were recorded. Left atrial voltage maps were examined for low voltage zones (LVZ), defined as bipolar voltage < 0.45 mV (sinus rhythm) or <0.31 mV (AF) adjacent or remote from surgical lesions. Patients in sinus rhythm underwent burst pacing from the coronary sinus to cycle length 180 ms to induce sustained AF (> 30 seconds).
Endocardial ablation was individualized (figure 1C), using 3.5 mm irrigated-tip catheters (SmartTouch®, Biosense Webster; FlexAbility™, Abbott) targeting 30–35 watts at 10–20 g force. The first goal was to close detected gaps within the epicardial lesion set. A complete lesion set was defined by isolation of all PVs and the PW. Additional lesions were patient-tailored. LVZ ablation was performed in 6 patients by creating a line of block to the nearest non-conducting obstacle. In patients in whom AF was sustained, panoramic mapping was performed (N=21). A 64-pole basket was advanced to the right and left atria (RhythmView™, Abbott), and AF mapped to identify (a) focal impulses, with centrifugal activation, or (b) rotational activation (reentrant drivers), in localized regions for >50% of the duration of mapped AF.9 Selected sites were ablated to cover 2–3 cm regions, to an endpoint of voltage <0.5 mV (Focal Impulse and Rotational Modulation, FIRM). Any ablation was abandoned at sites that heated the esophagus >1.5 °C rise despite reduced power or high power short-duration lesions (50W, 6 seconds), or that overlay regions of phrenic nerve capture.9
Patients in AF at the end of ablation underwent synchronized cardioversion. High-density voltage maps were repeated in sinus rhythm to confirm the complete lesion sets and entrance and exit block from each PV and the PW.
Follow up after stage 2
A blanking period was defined as 90 days from stage 2. All patients were followed at 6- and 12- months by office visit and continuous ECG monitoring as part of our registry. N=15 patients had implantable cardiac devices, and others received ambulatory monitoring (Zio, iRhythm). Efficacy events were defined as atrial arrhythmia > 30 seconds beyond the blanking period. Safety endpoints included all-cause mortality, stroke or transient ischemic attack, atrioesophageal fistula, phrenic nerve paralysis, unsuccessful appendage ligation.
Statistics
Continuous normally distributed variables are summarized by means and standard deviations and evaluated by Student’s t-tests for independent samples. Where the Kologorov-Smirnov test indicated non-normal distributions, variables are summarized by medians and inter-quartile ranges (IQR) and evaluated with Mann-Whitney U tests. Categorical variables are summarized with counts and evaluated with chi-square tests or Fisher exact tests as appropriate. A Kaplan-Meier plot summarizes AT/AF free survival during follow-up and generates survival estimates at follow-up observation time points. As comparisons are primarily descriptive no adjustments for multiple testing were made. A p-value of 0.05 was considered statistically significant
Results
Baseline Characteristics
Table 1 summarizes demographics. Patients presented for endocardial ablation at 29.5 days (IQR 5, 86). SRAF occurred in 36/64 patients (group 1, 56.3%) and did not occur in 28/64 patients (group 2). Patients in group 1 had a higher prevalence of long-standing persistent AF than in group 2. The N=17 patients who did not present for endocardial mapping had a similar rate of SRAF (10/17 patients; 58.8%) at a 45.0 days (IQR 27, 81) after surgical ablation.
Table 1.
Patient Characteristics of the entire population
| All Patients n = 64 |
SRAF (+) n = 36 |
SRAF (−) n = 28 |
p- value | |
|---|---|---|---|---|
| Age (year) | 67.5 ± 8.6 | 66.0 ± 9.0 | 67.0 ± 10 | 0.64 |
| Time of SRAF, median days (IQR) | 11.0 (4.75, 93.0) | 32.5 (5.0, 83.5) | *** | |
| Female sex | 12 (18.8%) | 4 (11.1%) | 8 (28.6%) | 0.11 |
| Race | 0.76 | |||
| White | 50 (78.1%) | 29 (80.6%) | 21 (75.0%) | |
| Non-white | 14 (21.9%) | 7 (19.4%) | 7 (25.0%) | |
| BMI kg/m2 | 31.8 ± 6.4 | 31.5 ± 6.2 | 32.1 ± 6.6 | 0.72 |
| Hemoglobin A1c | 5.6 [5.3, 5.8] | 5.6 [5.4, 6.0] | 5.5 [5.3, 5.7] | 0.29 |
| Creatinine | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.96 ± 0.23 | 0.31 |
| CHA2DS2-VASc | 3 [2, 4] | 3 [2, 4] | 2 [2, 3.75] | 0.54 |
| Arrhythmia Type | 0.017 | |||
| Paroxysmal | 8 (12.5%) | 3 (8.3%) | 5 (17.9%) | |
| Persistent | 16 (25%) | 6 (16.7%) | 10 (35.7%) | |
| Long-Standing Persistent | 40 (62.5%) | 27 (75.0%) | 13 (46.4%) | |
| Previous Catheter Ablation | 27 (42.2%) | 16 (44.4%) | 10 (35.7%) | 0.61 |
| Comorbidities | ||||
| Cerebral Vascular Accident | 2 (3.1%) | 2 (5.6%) | 0 | 0.50 |
| Chronic Renal Disease | 54 (84.4%) | 32 (89.9%) | 22 (78.6%) | 0.31 |
| Congestive Heart Failure | 39 (60.9%) | 23 (63.9%) | 16 (57.1%) | 0.62 |
| Coronary Artery Disease | 14 (21.9%) | 8 (22.2%) | 6 (21.4%) | 1.00 |
| Diabetes Mellitus | 8 (12.5%) | 6 (16.7%) | 2 (7.1%) | 0.45 |
| Hypertension | 45 (70.3) | 28 (77.8%) | 17 (60.7%) | 0.17 |
| Obstructive Sleep Apnea | 28 (43.8%) | 17 (47.2%) | 11 (39.3%) | 0.62 |
| Tobacco Use | 24 (37.5%) | 13 (36.1%) | 11 (39.3%) | 0.80 |
| Pre-operative Echocardiogram | ||||
| LV Ejection Fraction (%) | 51.6 ± 11.9 | 50.1 ± 12.6 | 53.5 ± 11.0 | 0.27 |
| LA Volume (mL) | 88.7 ± 30.5 | 91.5 ± 33.2 | 85.3 ± 26.7 | 0.48 |
| LA Volume Index (mL/m2) | 37.9 [31.5, 50.8] | 39.1 [33.3, 51.8] | 36.3 [30.5, 46.5] | 0.44 |
Table represents n (%), median [Q1, Q3] or mean (±SD); SRAF indicates Post-Stage I recurrence of AF; LV indicates left ventricle; and LA indicates left atrial.
Stage I Procedure Characteristics
All patients (n=81) had acute isolation of PVs and PW during the surgical procedure, without ablation beyond the stereotypical lesion set. All patients had successful ligation of the left atrial appendage.
Stage II Endocardial Mapping. Durability of Surgical PVI
PVs had reconnected in 18/64 patients (28.1%). There was no relationship between PV reconnection and the presence or absence of SRAF (25.0% vs 32.1%, p=0.53). Compared to those without PV reconnection, patients with PV reconnection were more likely to be of non-white race (p=0.04), with non-significant trends for a higher prevalence of coronary artery disease, elevated creatinine, lower BMI and lower frequency of prior catheter ablation. There were no differences in age nor type of AF (Supplementary Table I).
Stage II Endocardial Mapping: Durability of Surgical PWI
Figure 2 shows longstanding persistent AF in a 57-year-old man with AF 7 weeks after surgical ablation. Mapping confirmed PV isolation but revealed multiple sites of PWI reconnection. Ablation of these gaps acutely terminated AF. No other ablation was performed, and the patient remains free of atrial arrhythmias at 1 year off medications.
Figure 2. Early Reconnection of Posterior Wall after Surgical Ablation, Associated with Post-Surgical recurrence of AF, Treated with Endocardial Ablation.
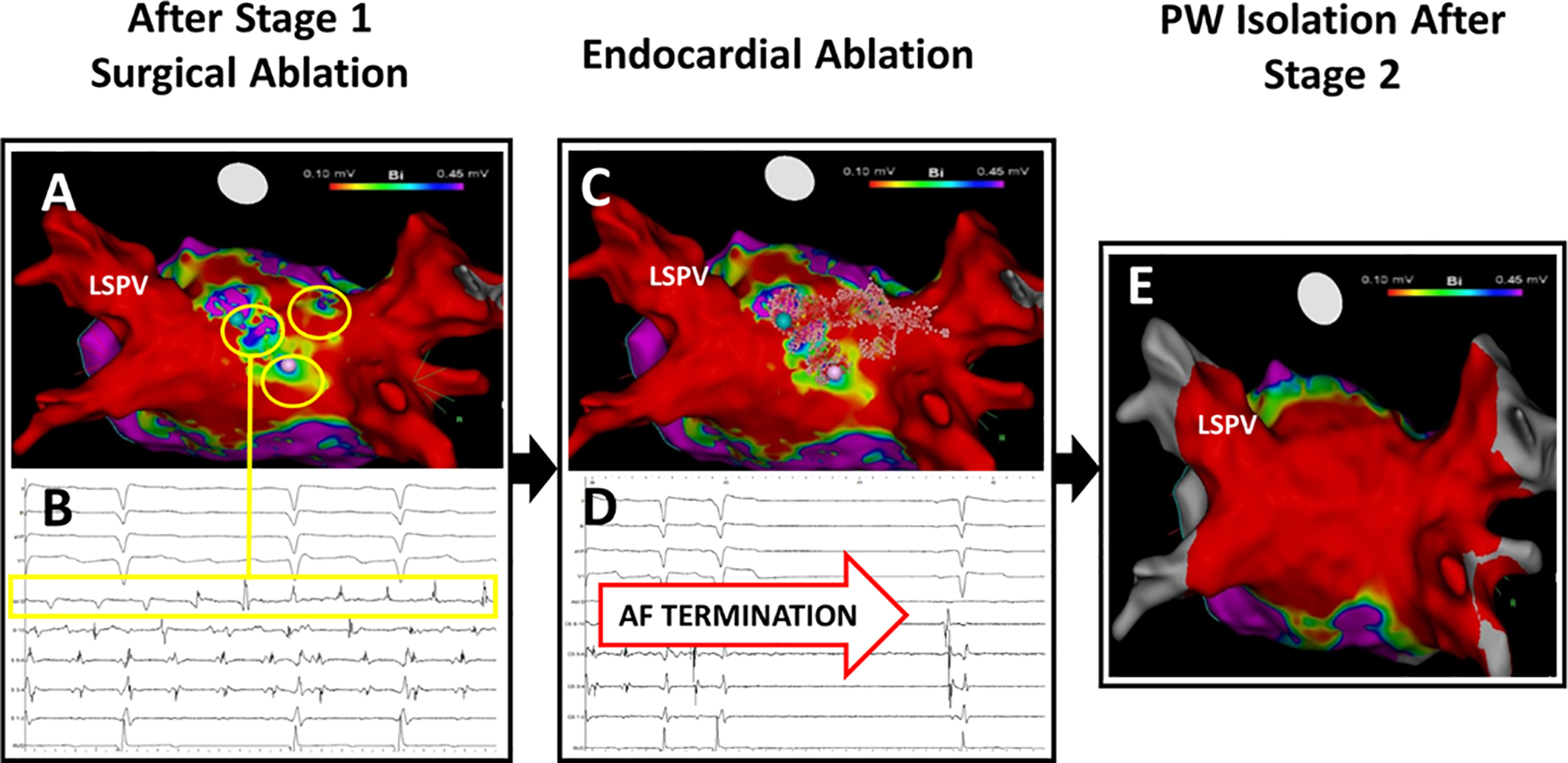
(A) High density mapping (3000 points) at 23 weeks after surgical ablation shows durable PVI (red; < 0.1 mV), yet PW reconnection (yellow circles). (B) Gaps (yellow circles; >0.1 mV). (C) Radiofrequency lesions (pink tags) within the PW box and the left atrial roof. (D) AF termination upon closing this PW box. (E) Post-ablation voltage map with complete isolation of PVI and PWI, confirmed by entrance/exit block. The patient is free of AF or AT at 1 year off anti-arrhythmic medications.
Overall, the PWI box set reconnected in 38/64 (59.4%) of patients, particularly in patients without prior ablation (p=0.04). There was no relationship between PWI reconnection and the presence or absence of SRAF nor other clinical features (Supplementary Table II). The most common PWI reconnection sites were the atrial roof (43.8%) followed by the floor (31.3%).
Stage II: Endocardial Mapping of low voltage regions
Figure 3 shows persistent AF in a 69-year-old woman with AF 2 weeks after surgical ablation. Mapping revealed intact PWI and PVI, but LVZ in the lateral LA outside the PWI box. AF was induced and focal radiofrequency ablation was applied to this LVZ, with no change in AF. Panoramic AF mapping was not performed. The patient underwent direct current cardioversion and remains free of atrial arrhythmias at 1 year off medications.
Figure 3. Low Voltage Zone outside Posterior Wall and Pulmonary Veins, Associated with Post-Surgical AF recurrence, Treated with Endocardial Ablation.
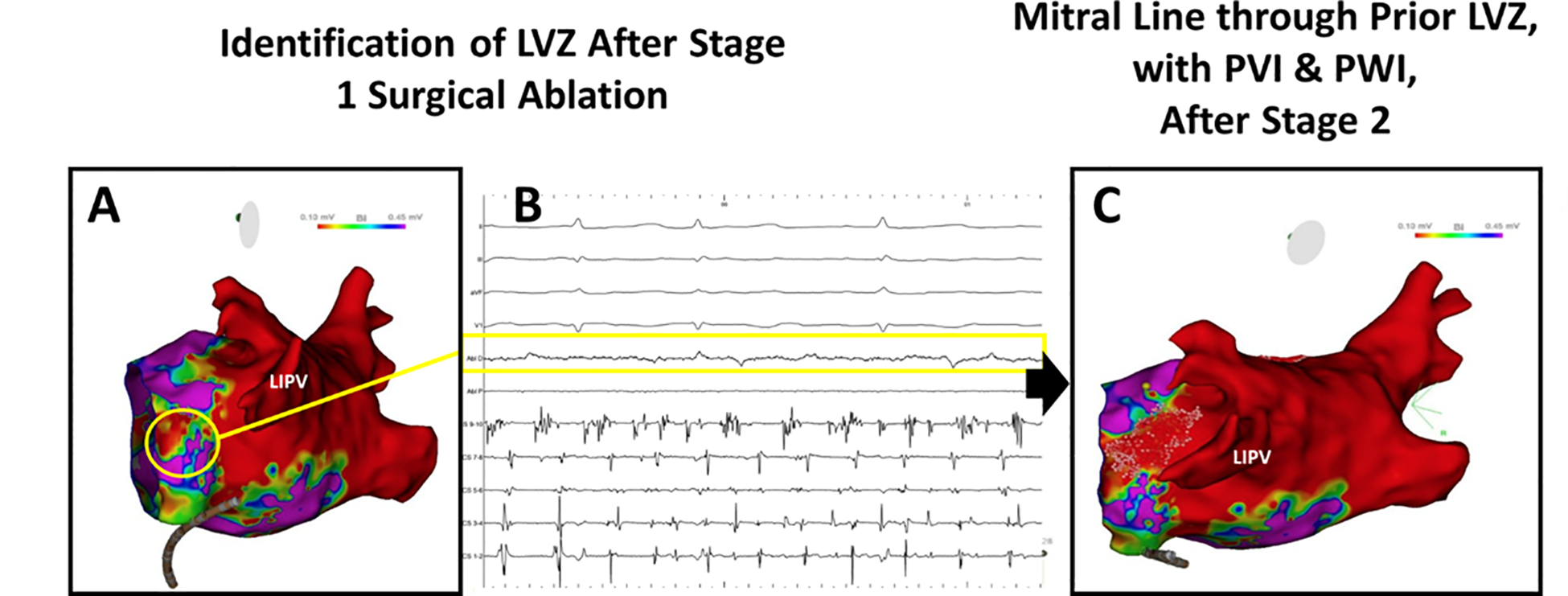
. (A) High density mapping (3,400 points) shows isolated PW box and PVs (red; < 0.1 mV). LVZ in yellow circles (0.11–0.3 mV) along the mitral isthmus and lateral LA, showing (B) LVZ (>0.1 mV; yellow box). (C) Ablation (pink tags) completed by a line from the mitral isthmus to the left inferior PV. AF did not terminate. (C) Post-cardioversion voltage map in sinus rhythm confirmed absence of prior LVZ, PV and PW isolation. This patient was free of AF and AT 1 year off anti-arrhythmic medications.
The presence of LVZ was more likely in patients with than without SRAF (p=0.002; Table 2). In patients with SRAF, 35 LVZ were identified in 27 patients for 1.0 ± 0.7 zones per patient. In patients without SRAF, 11 LVZ were identified in 8 patients: 0.4 ± 0.7 zones per patient (p=0.72). The most common location for LVZ outside the PW box was the anterior LA. Figure 4 shows the anatomical locations of LVZ in patients with and without SRAF. Of 6 patients who underwent ablation of LVZ outside of isolated PW and PVs, none had AF termination during ablation.
Table 2.
Patient Characteristics: Presence of Low Voltage Zones versus Absence of Low Voltage Zones following Hybrid Stage 1 Surgical Ablation (Beyond PV and PW Lesion Set)
| Patient Characteristics n = 64 |
LVZ Present n = 35 |
LVZ Absent n = 29 |
p-value |
|---|---|---|---|
| Age (year) | 68.4 ± 8.7 | 62.5 ± 10.1 | 0.03 |
| Female sex | 7 (20.0%) | 5 (17.2%) | 1.00 |
| Presence of SRAF | 27 (77.1%) | 9 (31.0%) | 0.002 |
| Race | 0.14 | ||
| White | 30 (85.7%) | 20 (69.0%) | |
| Non-white | 5 (14.3%) | 9 (31.0%) | |
| BMI kg/m2 | 31.8 ± 6.0 | 31.8 ± 6.9 | 0.99 |
| Hemoglobin A1c | 5.6[5.3, 5.9] | 5.5 [5.1, 5.8] | 0.306 |
| Creatinine | 1.0 ± 0.3 | 1.0 ± 0.2 | 0.70 |
| CHA2DS2-VASc | 3 [2, 4] | 2 [2, 3] | 0.07 |
| Arrhythmia Type | 0.007 | ||
| Paroxysmal | 1 (2.9%) | 7 (25.0%) | |
| Persistent | 7 (20.0%) | 9 (32.1%) | |
| Long-Standing Persistent | 27 (77.1%) | 12 (42.9%) | |
| Previous Catheter Ablation | 16 (45.7%) | 19 (34.5%) | 0.45 |
| Comorbidities | |||
| Cerebral Vascular Accident | 2 (5.9%) | 0 | 0.50 |
| Chronic Renal Disease | 33 (94.3%) | 21 (72.4%) | 0.04 |
| Congestive Heart Failure | 21 (60.0%) | 18 (62.1%) | 0.87 |
| Coronary Artery Disease | 10 (29.4%) | 4 (13.8%) | 0.14 |
| Diabetes Mellitus | 6 (19.4%) | 2 (7.1%) | 0.17 |
| Hypertension | 20 (82.9%) | 16 (55.2%) | 0.03 |
| Obstructive Sleep Apnea | 15 (42.9%) | 13 (46.4%) | 0.80 |
| Tobacco Use | 12 (36.4%) | 12 (42.9%) | 0.79 |
| Pre-operative Echocardiogram | |||
| LV Ejection Fraction (%) | 50.1 ± 32.8 | 53.4 ± 10.9 | 0.28 |
| LA Volume (mL) | 93.0 ± 32.8 | 82.5 ± 26.5 | 0.21 |
| LA Volume Index (mL/m2) | 39.5 [34.2, 52.6] | 36.0 [30.0, 40.2] | 0.10 |
Table represents n (%), median [Q1, Q3] or mean (±SD); LVZ indicates low voltage zones; LV indicates left ventricle; and LA indicates left atrial.
Figure 4. Anatomical distribution of LVZ, classified by presence/absence of SRAF.
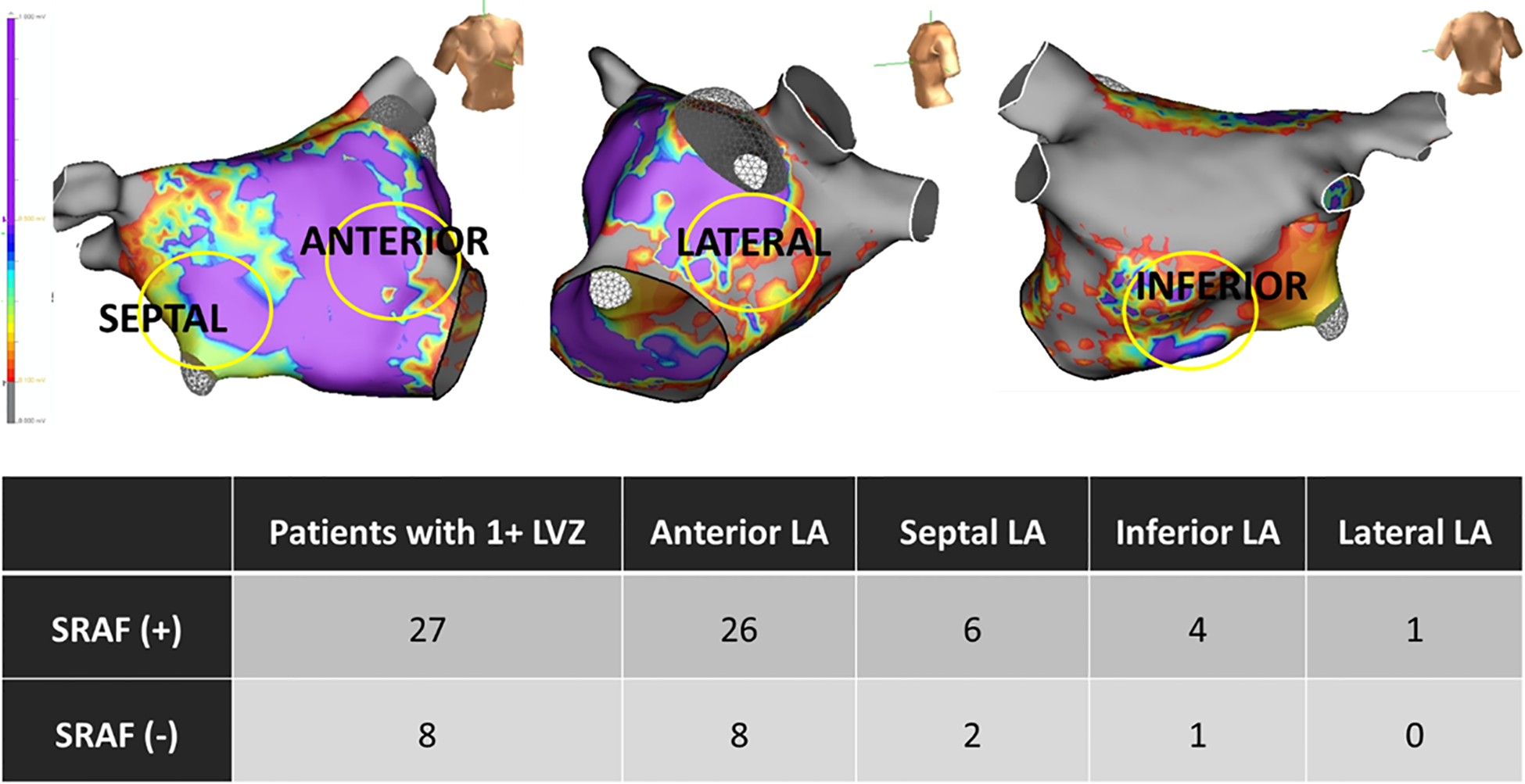
LVZ were observed throughout the left atrium outside the PW box, and were most common in the anterior wall (largest segment).
Compared to patients without LVZ (Table 2), those with LVZ had a higher prevalence of longstanding persistent AF (p=0.007), advanced age (p=0.03), renal disease (p=0.04), hypertension (p=0.03), and a trend for elevated CHA2DS2-VASc (p=0.07).
Stage II: Endocardial Mapping of AF
Twenty-five of the 64 patients presented in AF. Panoramic mapping was performed in n=21 (N=14 presenting in AF, N=7 induced). PVs remained isolated in 15/21 (71.4%) and PW remained isolated in 7/21 (33.3%) of this cohort.
Figure 5A shows longstanding persistent AF in a 69-year-old man with SRAF at 6 weeks, showing a small region of preserved voltage at the edge of the posterior box lesion set, with rotational activity at this site. Ablation at this site terminated AF and closed the gap. At 1 year the patient remains free of atrial arrhythmias off medications. Figure 5B shows longstanding persistent AF in a 51-year-old man with SRAF at 7 weeks, revealing a site of rotational activity remote from intact surgical PWI and PVI. Ablation at this site terminated AF and the patient remains free of atrial arrhythmias at 1 year.
Figure 5. AF Driver Associated Post-Surgical AF recurrence, Treated with Catheter Ablation (A) Near Posterior Box set.
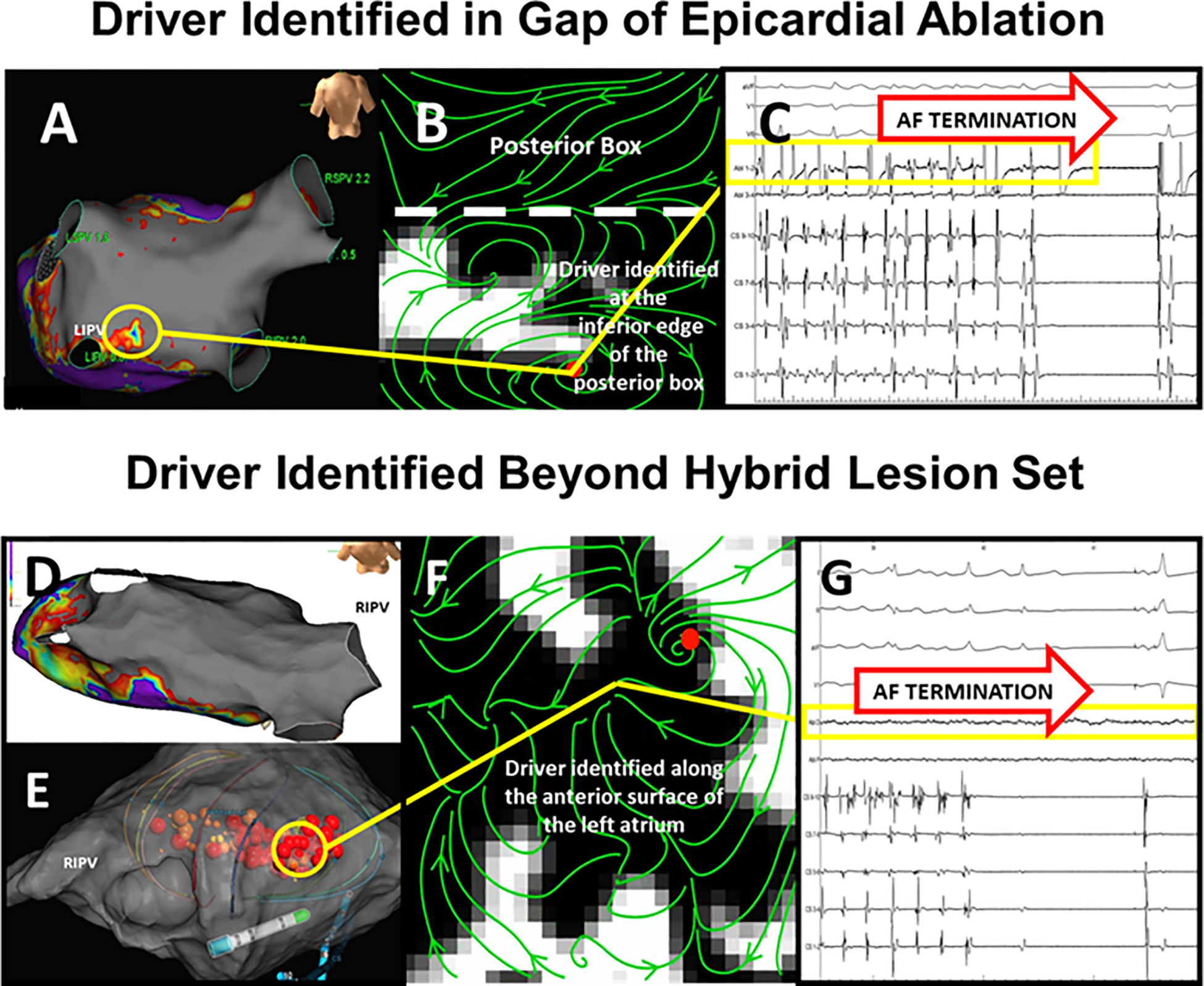
. High density voltage mapping (5,000 points) reveals a nearly complete posterior wall box (gray; < 0.1 mV) with a small low voltage zone (yellow circle) at the inferior border. (B) AF mapping revealed a driver at this site. (C) Radiofrequency ablation closed the gap and terminated AF (yellow box). This patient was free of AF and AT 1 year after ablation off anti-arrhythmic medications D. AF Driver Associated with SRAF, with Durably Isolated Posterior Wall and Pulmonary Veins. High density map (4,000 points) shows isolated PW and PVs (gray; <0.1 mV). (E) 64-pole basket catheter. (F) AF driver (correlating with yellow circle) on anterior left atrium outside surgical lesion sets, where (G) Ablation (red tags) terminated AF. This patient was free of AF and AT 1 year after ablation off anti-arrhythmic medications.
Overall, mapping revealed 4.4 ± 2.7 sites of focal or rotational activity (51% left, 49% right atrial). Driver locations were not consistent relative to surgical lesions. In the 14 patients with spontaneous AF, 11 had AF termination to sinus rhythm when ablating driver sites, and 1 during touch-up of PV. Of the n=7 with induced AF, 1 patient experienced AF termination during PVI touch up but none during driver ablation.
Follow-up after Endocardial Ablation
Acutely, all patients had complete PVI after endocardial ablation (stage 2). PW isolation was complete in 57/64 (89.1%) but could not be completed in 7 patients due to esophageal temperature rises.
All patients were followed for 1 year (N=64/64). In patients who underwent both Stage I and Stage II, compliance with monitoring was 75.0% and 63.0% at 6 and 12 months, respectively.
Figure 6 shows a Kaplan-Meier curve of AF/AT freedom for all 81 patients. In patients who underwent Stage 1 and II, freedom from AF/AT was 92.2% at 6 months and 81.0% at 12 months. Freedom from AF/AT at 12 months without antiarrhythmic medications was 73.0%. In patients who received only Stage I ablation, freedom from AF/AT was 76.5% at 6 months and 64.7% at 9 months (shorter due to low monitor compliance beyond 6 months) on or off previously ineffective antiarrhythmic medication.
Figure 6.
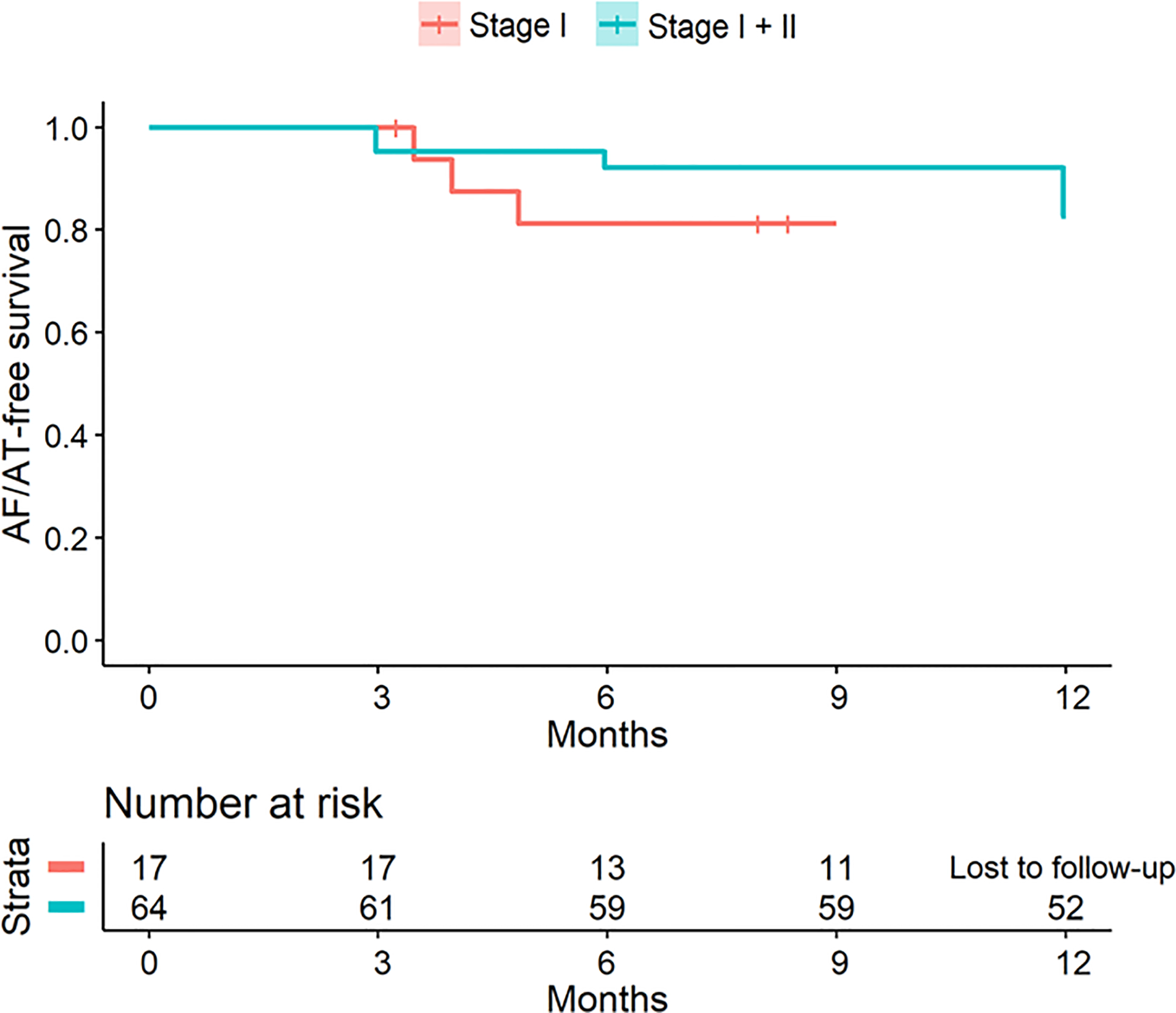
Kaplan-Meier curve, Freedom from AF/AT at 12-months after hybrid ablation (stage I and II) with shorter follow-up for surgical maze alone (Stage I) patients.
AT/AF recurrence was more prevalent in patients in whom PWI could not be achieved (50%) after stages 1 and 2, compared with those with a complete lesion set (15.8%; p=0.042). Freedom from AF/AT was similar for patients with versus without AF termination during ablation (10/13;76.9 % vs 42/50; 84.0%; p=0.55), or with versus without LVZ outside the epicardial lesion set (26/34, 76.5% versus 25/29; 86.2%; p=0.33).
Adverse Events and Complications
Of the 64 patients undergoing both Stage I and Stage II, one patient died from cancer at 9 months. One patient who underwent surgical ablation died of respiratory arrest after several months. Endocardial ablation was not performed in N=17 patients after surgical ablation due to intracranial hemorrhage (n=2), gastrointestinal bleeding (n=1), cardiac thrombus (n=2), heparin-induced thrombocytopenia (n=1), decompensated heart failure (n=1), perioperative stroke (n=1), pulmonary embolism (n=1), phrenic nerve injury (n=2) and constrictive pericarditis (n=1). Thus, for the entire N=81 cohort, the complication rate was 12/81 (14.8%). There were no unsuccessful appendage ligations.
Discussion
The major findings from this study are that:
Surgical ablation for AF followed by patient-tailored endocardial ablation resulted in a 12 month freedom from atrial arrhythmia on ambulatory monitoring of 81.0% on or off previously unsuccessful medications, and 73.0% off medications.
Anatomically-based, surgical epicardial ablations show distinct modes of failure including A) PVI reconnection; B) Posterior wall reconnection, and C) the presence of localized LVZ and putative drivers outside anatomical PV and PW isolation where ablation could be acutely successful.
SRAF was associated with the presence of LVZ outside the surgical lesion set and high-risk demographics, but was not related to PV or PWI reconnection.
Early Recurrence of AF
The incidence and modes of recurrent AF after epicardial surgery have been difficult to compare in prior studies that applied diverse procedures. The prevalence of SRAF in our study agrees with Choi et al10, who reported SRAF in 49% of 259 patients. SRAF may share mechanisms for long-term AF recurrence, which has been the rationale for repeat ablation within the blanking period in AF ablation trials.8, 11 Recent analysis from the ADVICE trial and from CIRCA-DOSE showed that early recurrence of AF within the blanking period strongly associated with 1 year AF recurrence.7, 12
Mechanisms of SRAF
Mechanisms for SRAF after surgical ablation are difficult to define in studies that did not systematically remap patients. In theory, SRAF could reflect inflammation13, lack of durable lesions, or missed ablation targets. In our cohort, AF recurrence after surgical ablation related to LVZ, but not reconnection of PVI or PWI. A recent meta-analysis14 showed PV reconnection in only 58% of patients with AF recurrence, supporting the identification of targets beyond PVI. LVZs represent a potential ablation target, although their mechanistic basis is unclear. Recently, Masuda et al15 randomized patients to PVI with or without LVZ ablation and found that LVZ predicted poor outcome, yet ablation of LVZ sites did not improve results.
Reconnections involving the PVs
PV reconnection rates in our study are in line with the CONVERGENT trial16, which motivates the need for improved surgical PVI in parallel with efforts to improve endocardial PVI. PVI durability from endocardial ablation is improving17 due to high power short duration energy delivery18, force sensing, and technologies such as pulsed field ablation19. The durability of surgical PVI could be overestimated by prior catheter ablation, yet underestimated by excluding patients who did not return for endocardial ablation (with a similar SRAF rate).
Posterior Wall Isolation
The PWI box reconnected in 59.4% of patients, with reconnection most commonly at the left atrial roof and floor as in prior surgical studies.20–22 The LA roof harbors relatively thick atrial muscle which may hinder transmural lesions. The outcomes from adding endocardial PWI to PVI are mixed, and it is unclear how randomized studies showing no improvement23, 24 differ from studies showing improvement.25
A recent European multicenter registry22 of hybrid ablation comprising a single box lesion with endocardial ablation at gaps, mitral or cavotricuspid isthmus lines showed gaps in 75% of patients, slightly higher than our rate (28.1% PV reconnection, 59% PW reconnection; Supplementary Table III), with a 66% freedom from AF at 18 months (off and on AAD). Those studies did not map other features such as LVZ or drivers.
Patient-specific targets to Enhance Hybrid Ablation
SRAF was associated with the presence of LVZ, which may represent fibrosis or scar that associate with worse outcomes26 and AF recurrence after ablation.27 LVZ may be sites where putative AF drivers may anchor, revealed by optical mapping of human AF28 and simultaneous mapping with the system used in this study (FIRM, RhythmView).9 Several emerging AF mapping systems (Ablacon, CardioNXT, Acutus, Cartofinder) could thus complement LVZ mapping or structural imaging by delayed enhancement MRI imaging.26
Some studies mapped other AF features. Bisleri et al29 utilized a staged surgical approach with additional targeting of CFAE and RA isthmus lesions. Bulava et al30 used sequential hybrid ablation with PVI, PWI, LOM, ganglionic plexi, and LAA ablation, followed by endocardial ablation of gaps, empiric mitral and CTI line. In our study, LAA was successfully ligated in all patients, although the recent aMAZE study showed that LAA exclusion did not improve AF outcomes over PVI.31 De Asmundis et al21 performed same day surgical/endocardial ablation targeting PV, PW, ganglionic plexi, LAA, and ligament of Marshall, with a freedom from AF of 68.6% at 24 months. The CONVERGE trial16 randomized 153 patients with persistent AF in a 2:1 fashion to Hybrid convergent versus catheter ablation. The hybrid arm targeted PVI, PWI, and CTI. The catheter ablation arm targeted PVs, atrial roof line, and CFAE. Freedom from AF with or without AAD was 71% the Hybrid convergent arm, compared to 51% in the catheter ablation arm.
Lastly, we observed no adverse complications from endocardial ablation, but an 14.8% complication rate from surgical ablation in line with 2.9% in the CONVERGENT trial to 20.8%.16, 30, 32, 33 Further studies are thus needed to reduce surgical related complications.
Limitations
Our study design was not identical to other hybrid studies. We did not compare ablation versus non-ablation of any specific target. Implanted loop recorders were not used to document long-term follow-up. AF was not induced nor mapped in all patients, which would have provided a better survey of sustaining mechanisms including potential drivers outside surgical lesion sets. These are goals of future studies. It would have been useful to know modes of recurrence in the 17 patients who did not have endocardial ablation. Although this was nonrandomized single center experience, our results are in line with similar hybrid approaches and the recent CONVERGE trial16.
Conclusion
Early recurrence of AF occurs by several modes after surgical ablation, including recovery of pulmonary veins and/or posterior left atrial wall lesions, but was related significantly only to sites of low voltage outside surgical lesions sets. Patient specific endocardial mapping may improve hybrid procedures and further improve long term outcomes.
Supplementary Material
What Is Known:
Success rates of hybrid therapy for atrial fibrillation vary greatly between centers;
It is unclear which components of surgical ablation are most likely to require support or should be the focus of additional endocardial ablation.
What This Study Adds:
In this registry of hybrid AF ablation, reconnection of surgical PVI and posterior wall isolation were 28.1% and 59.4% at 1 month, respectively, but did not predict early recurrence of AF;
On endocardial mapping, early recurrence of AF after surgical ablation related to the presence of low voltage zones, some of which overlapped with potential AF driver sites
Surgical followed by patient tailored endocardial ablation for persistent AF resulted in 1 year freedom from any atrial arrhythmia of 81.0% (on/off medications) and 73.0% (off medications).
Sources of Funding:
This work was funded in part by grants to SMN by the National Institutes of Health (R01 HL 83359, R01 HL149134).
Nonstandard Abbreviation and Acronyms
- AF
atrial fibrillation
- PVs
pulmonary veins
- PWI
posterior wall isolation
- PVI
pulmonary vein isolation
- LVZ
low voltage zones
- TEE
transesophageal echocardiogram
- CL
cycle length
- SRAF
Post-Stage I recurrence of AF
Footnotes
Disclosures: Drs. Bhatia and Kapoor report no disclosures. Drs Shah reports consulting reimbursement from Abbott Laboratories. Dr. Narayan reports consulting from Life Signals.ai, TDK Inc., Up to Date, Abbott Laboratories, and American College of Cardiology Foundation; Intellectual Property Rights from University of California Regents and Stanford University.
Supplemental Materials:
References:
- 1.Gillinov AM, Gelijns AC, Parides MK, DeRose JJ Jr., Moskowitz AJ, Voisine P, Ailawadi G, Bouchard D, Smith PK, Mack MJ, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weimar T, Bailey MS, Watanabe Y, Marin D, Maniar HS, Schuessler RB and Damiano RJ Jr. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox JL. Surgical treatment of atrial fibrillation: a review. EP Europace. 2003;5:S20–S29. [DOI] [PubMed] [Google Scholar]
- 4.Maclean E, Yap J, Saberwal B, Kolvekar S, Lim W, Wijesuriya N, Papageorgiou N, Dhillon G, Hunter RJ, Lowe M, et al. The convergent procedure versus catheter ablation alone in longstanding persistent atrial fibrillation: A single centre, propensity-matched cohort study. Int J Cardiol 2020;303:49–53. [DOI] [PubMed] [Google Scholar]
- 5.Maesen B, Pison L, Vroomen M, Luermans JG, Vernooy K, Maessen JG, Crijns HJ and La Meir M. Three-year follow-up of hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg 2018;53:i26–i32. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Gache L, Frame D, Boo LM, Ghaly N, Schilling R, Deering T, Duytschaever M and Packer DL. Predictive value of atrial fibrillation during the postradiofrequency ablation blanking period. Heart rhythm. 2021;18:366–373. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg C, Champagne J, Deyell MW, Dubuc M, Leong-Sit P, Calkins H, Sterns L, Badra-Verdu M, Sapp J, Macle L, et al. Prevalence and outcome of early recurrence of atrial tachyarrhythmias in the Cryoballoon vs Irrigated Radiofrequency Catheter Ablation (CIRCA-DOSE) study. Heart Rhythm. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients With Paroxysmal Atrial Fibrillation: A Randomized Controlled Trial. JAMA. 2010;303:333–340. [DOI] [PubMed] [Google Scholar]
- 9.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K and Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 2014;63:1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi JH, Hwang KW, Jung SM, Lee SY, Lee SH, Chon MK, Kim JS, Je HG, Park YH, Kim JH, et al. Incidence and clinical impact of early recurrence of atrial tachyarrhythmia after surgical ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:2898–2906. [DOI] [PubMed] [Google Scholar]
- 11.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S and Dello Russo A. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 12.Willems S, Khairy P, Andrade JG, Hoffmann BA, Levesque S, Verma A, Weerasooriya R, Novak P, Arentz T, Deisenhofer I, et al. Redefining the Blanking Period After Catheter Ablation for Paroxysmal Atrial Fibrillation: Insights From the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ Arrhythm Electrophysiol 2016;9. [DOI] [PubMed] [Google Scholar]
- 13.Mariani MA, Pozzoli A, Maat G, Alfieri OR and Benussi S. What Does The Blanking Period Blank? J Atr Fibrillation. 2015;8:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nery PB, Belliveau D, Nair GM, Bernick J, Redpath CJ, Szczotka A, Sadek MM, Green MS, Wells G and Birnie DH. Relationship Between Pulmonary Vein Reconnection and Arial Fibrillation Recurrence. JACC Clin Electrophysiol 2016;2:474–483. [DOI] [PubMed] [Google Scholar]
- 15.Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y and Hata Y. Low-Voltage-Area Ablation in Paroxysmal Atrial Fibrillation: Extended Follow-Up Results of the Volcano Trial. Circulation Journal. 2021;CJ–21. [DOI] [PubMed] [Google Scholar]
- 16.DeLurgio DB, Crossen KJ, Gill J, Blauth C, Oza SR, Magnano AR, Mostovych MA, Halkos ME, Tschopp DR, Kerendi F, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020. [DOI] [PubMed] [Google Scholar]
- 17.Buist TJ, Adiyaman A, Smit JJJ, Ramdat Misier AR and Elvan A. Arrhythmia-free survival and pulmonary vein reconnection patterns after second-generation cryoballoon and contact-force radiofrequency pulmonary vein isolation. Clin Res Cardiol 2018;107:498–506. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VY, Grimaldi M, De Potter T, Vijgen JM, Bulava A, Duytschaever MF, Martinek M, Natale A, Knecht S, Neuzil P, et al. Pulmonary Vein Isolation With Very High Power, Short Duration, Temperature-Controlled Lesions: The QDOT-FAST Trial. JACC Clin Electrophysiol 2019;5:778–786. [DOI] [PubMed] [Google Scholar]
- 19.Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami K, Breskovic T, Sikiric I, Dukkipati SR, Kawamura I, et al. Pulsed Field Ablation in Patients With Persistent Atrial Fibrillation. J Am Coll Cardiol 2020;76:1068–1080. [DOI] [PubMed] [Google Scholar]
- 20.Markman TM, Hyman MC, Kumareswaran R, Arkles JS, Santangeli P, Schaller RD, Supple GE, Frankel DS, Riley MP, Lin D, et al. Durability of posterior wall isolation after catheter ablation among patients with recurrent atrial fibrillation. Heart Rhythm. 2020;17:1740–1744. [DOI] [PubMed] [Google Scholar]
- 21.de Asmundis C, Varnavas V, Sieira J, Stroker E, Coutino HE, Terasawa M, Abugattas JP, Salghetti F, Maj R, Guimaraes OT, et al. Two-year follow-up of one-stage left unilateral thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation. J Interv Card Electrophysiol 2020;58:333–343. [DOI] [PubMed] [Google Scholar]
- 22.Haywood GA, Varini R, Osmancik P, Cireddu M, Caldwell J, Chaudhry MA, Loubani M, Della Bella P, Lapenna E, Budera P, et al. European multicentre experience of staged hybrid atrial fibrillation ablation for the treatment of persistent and longstanding persistent atrial fibrillation. Int J Cardiol Heart Vasc 2020;26:100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamborero D, Mont L, Berruezo A, Matiello M, Benito B, Sitges M, Vidal B, de Caralt TM, Perea RJ, Vatasescu R, et al. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol 2009;2:35–40. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Shim J, Park J, Yu HT, Kim TH, Park JK, Uhm JS, Kim JB, Joung B, Lee MH, et al. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin Electrophysiol 2019;5:1253–1261. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Yu HT, Kim T-H, Jae-Sun U, Joung B, Lee M-H and Pak H-N. Electrical Posterior Box Isolation in Repeat Ablation for Atrial Fibrillation: A Prospective Randomized Clinical Study. JACC Clin Electrophysiol 2022;in press. [DOI] [PubMed] [Google Scholar]
- 26.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 27.Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- 28.Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin S, Wang Y, Atwal J, Kalyanasundaram A, Abudulwahed SH, Helfrich KM, et al. Human Atrial Fibrillation Drivers Resolved With Integrated Functional and Structural Imaging to Benefit Clinical Mapping. JACC Clin Electrophysiol 2018;4:1501–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bisleri G, Rosati F, Bontempi L, Curnis A and Muneretto C. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg 2013;44:919–23. [DOI] [PubMed] [Google Scholar]
- 30.Bulava A, Mokracek A, Hanis J, Kurfirst V, Eisenberger M and Pesl L. Sequential hybrid procedure for persistent atrial fibrillation. J Am Heart Assoc 2015;4:e001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilber D Outcomes of adjunctive left atrial appendage ligation utilizing the Lariat compared to pulmonary vein antral isolation alone: the aMAZE trial. Paper presented at: American Heart Association 2021; 2021. [Google Scholar]
- 32.Edgerton Z, Perini AP, Horton R, Trivedi C, Santangeli P, Bai R, Gianni C, Mohanty S, Burkhardt JD, Gallinghouse GJ, et al. Hybrid Procedure (Endo/Epicardial) versus Standard Manual Ablation in Patients Undergoing Ablation of Longstanding Persistent Atrial Fibrillation: Results from a Single Center. J Cardiovasc Electrophysiol 2016;27:524–30. [DOI] [PubMed] [Google Scholar]
- 33.JACC Clin ElectrophysiolKurfirst V, Mokráček A, Bulava A, Čanádyová J, Haniš J and Pešl L. Two-staged hybrid treatment of persistent atrial fibrillation: short-term single-centre results. Interactive cardiovascular and thoracic surgery. 2014;18:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


