Abstract
Background:
E3805 (CHAARTED) is a phase 3 trial demonstrating improved survival for men with metastatic hormone sensitive prostate cancer (mHSPC) randomized to treatment with docetaxel (D) and androgen deprivation therapy (ADT) vs ADT alone. We assessed the association of baseline body mass index (BMI) and metformin exposure with quality of life (QOL) and prostate cancer outcomes including survival in patients enrolled on the CHAARTED study.
Methods:
We performed a post hoc exploratory analysis of the CHAARTED trial of men with mHSPC randomized to treatment with ADT with or without D between 2006 and 2012. Cox proportional hazards models and Kruskal-Wallis test were used to evaluate the association between BMI with QOL and prostate cancer outcomes, and between metformin exposure and survival.
Results:
In 788 of 790 enrolled patients with prospectively recorded baseline BMI and metformin exposure status, lower BMI was not associated with survival, but was associated with high volume disease (p<0.0001) and poorer baseline QOL on FACT-P (p=0.008). Only 68 patients had prevalent metformin exposure at baseline in the CHAARTED trial. Four groups were identified: ADT+D+metformin (n=39); ADT+D (n=357); ADT+metformin (n=29); ADT alone (n=363). Baseline clinicopathologic characteristics were similar between groups. In this small exploratory multivariable analysis, metformin exposure was not associated with survival (HR 1.15; 95% CI 0.81–1.63, p=0.44).
Conclusions:
There was no link between baseline BMI and survival, but lower baseline BMI was associated with features of greater cancer burden and poorer QOL.
Keywords: metastatic prostate cancer, chemohormonal therapy, quality of life, metabolism
Introduction
The treatment of advanced prostate cancer has been revolutionized during the past decade with the development of multiple new therapies for castration-resistant disease, and improved survival when combining androgen deprivation therapy with docetaxel or androgen signaling inhibitors for men with mHSPC [1, 2]. Population-based studies suggest that patient-specific health indicators, such as exercise and body mass index, and exposure to metformin, may be associated with prostate cancer outcomes.[3–5] These factors have been less frequently studied in prospective clinical trials and analysis of data from these trials may provide further insight into the association of these factors with prostate cancer patient outcomes.
Obesity is a patient-specific factor that can be associated with metabolic dysregulation. A BMI that is too high is associated with being overweight or obese, and is a predictor of cardiovascular disease, type 2 diabetes and hypertension[6]. Numerous studies also suggest that increased BMI is associated with poorer prostate cancer outcomes, including increased prostate cancer incidence, recurrence and mortality [4, 5, 7–9]. Greater BMI has also been associated with poorer overall QOL after treatment of clinically localized prostate cancer, including worse urinary symptoms [10–15]. Whether BMI is associated with prostate cancer specific outcomes or QOL in men with mHSPC treated with hormonal therapy or chemohormonal therapy has not been defined.
The association between metformin use and cancer patient outcomes has also been a topic of debate for decades. Metformin is an oral anti-hyperglycemic drug commonly used to treat type 2 diabetes mellitus (DM). Evidence suggests that it may have putative antitumor activity through a variety of direct and indirect activities [16–18], including indirectly reducing cyclin D1 levels and pRb phosphorylation [19]. This reduction may cause antiproliferative and proapoptotic activities that can augment the in vitro and in vivo efficacy of selected chemotherapies. Clinical studies assessing metformin use in diabetic patients with breast, lung, and endometrial cancer demonstrate improved outcomes when compared with patients not receiving metformin [20] [21, 22]. The effect of metformin use on outcomes in men with prostate cancer remains uncertain. One recent population based study demonstrated improved survival in diabetic patients with vs without metformin exposure, while a separate cohort study of men receiving radiation for localized disease failed to find a benefit to metformin exposure in time to biochemical failure or overall survival [23, 24]. Further analyses to assess the association between metformin exposure and prostate cancer outcomes are needed.
To that end, we evaluated the association between BMI with clinically important prostate cancer outcomes, including disease burden, survival, and QOL during treatment of men with mHSPC with ADT chemohormonal therapy in the CHAARTED trial [25]. We also evaluated the association between metformin exposure at baseline and overall survival in the CHAARTED trial. Because metabolism in cancer patients is an interplay between cancer related cachexia and host related factors such as obesity, we also performed an integrated analysis linking metformin, BMI and QOL.
Materials and Methods
This multicenter, randomized, open-label, phase III NCI study (NCT00309985) led by ECOG (now part of ECOG-ACRIN) enrolled patients with mHSPC with a performance status suitable for treatment with docetaxel in addition to ADT as described previously [1]. Eligible patients were equally randomized to ADT alone versus ADT plus docetaxel chemotherapy at a dose of 75 mg/m2 every 3 weeks for up to 6 cycles without daily prednisone. Randomization was stratified according to age (<70 years versus ≥70 years), ECOG performance status (0–1 versus 2), duration of prior adjuvant therapy with ADT, combined androgen blockade for >30 days, disease volume (high volume defined as the presence of visceral metastases or ≥4 bone metastases with ≥1 beyond the vertebral bodies and pelvis) and use of agents to prevent skeletal-related events (such as zoledronic acid or denosumab). All patients provided written informed consent prior to study entry. The primary endpoint of the study was OS, defined as time from randomization until death due to any cause. Baseline metformin use, BMI and QOL data were identified as priority data points, and data was collected prospectively at enrollment via case report forms (metformin use, yes/no; BMI in kg/m2) or patient reported outcome survey instrument (QOL data). Specific medication use beyond metformin, including sulfonyurea and insulin use, and comorbidity information, including diagnosis of diabetes, were not collected as metformin use specifically, rather than glycemic control, was of interest.
Statistical analyses:
Descriptive statistics were used to characterize patients at study entry. Kaplan–Meier estimates were used for event-time distributions. Cox proportional-hazard models, stratified according to the stratification factors at randomization, were used to estimate hazard ratios for time-to-event end points. Variables with p<0.1 in the univariable analysis were included in the multivariable models. Stratified log-rank tests were used to compare event-time distributions between groups. Categorical variables and continuous variables were compared with the use of Fisher’s exact test and Kruskal-Wallis test, respectively. P values are two-sided, and confidence intervals are at the 95% level. This report represents data with a cutoff date for survival of April 2016 resulting in a median follow-up of 53.7 months.
Results
A total of 788 patients (of 790 in the primary analysis) were included in this analysis because they had data on baseline metformin exposure available for analysis (396 receiving ADT+D and 392 receiving ADT). Median follow-up was 53.7 mo for the population. In total 68 (8.6%) were receiving treatment with metformin at baseline (39 receiving ADT+D and 29 receiving ADT). Patient characteristics were generally balanced between treatment arms and metformin exposure groups, but fewer patients had ECOG 0, high burden of disease, Gleason <7 in the ADT + metformin use group than the others (Table 1). Median BMI was higher for patients on metformin (31 vs. 29; p=0.001 in patients receiving vs not-receiving metformin, respectively) (Table 1).
Table 1:
Patient Characteristics by Treatment Arm and Metformin Use
| Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADT+D+Metformin (n=39) | ADT+D (n=357) | ADT+Metformin (n=29) | ADT (n=363) | Total (n=788) | ||||||
| N | Percent | N | Percent | N | Percent | N | Percent | N | Percent | |
|
| ||||||||||
| Age | ||||||||||
| Median | 66 | 63 | 63 | 63 | 63 | |||||
| Range | 51–88 | 36–83 | 51–82 | 39–91 | 36–91 | |||||
| Race | ||||||||||
| White | 35 | 89.7 | 308 | 88.8 | 25 | 89.3 | 304 | 88.4 | 672 | 88.7 |
| Black | 4 | 10.3 | 35 | 10.1 | 3 | 10.7 | 34 | 9.9 | 76 | 10.0 |
| Orient | 0 | . | 4 | 1.2 | 0 | . | 6 | 1.7 | 10 | 1.3 |
| Missing/Unknown | 0 | . | 10 | 1 | 19 | 30 | ||||
| Weight (kg) | ||||||||||
| Median | 88.3 | 89.8 | 102.4 | 88.6 | 89.7 | |||||
| Range | 61.0–151.9 | 50.9–151.0 | 59.4–153.7 | 49.4–185.0 | 49.4–185.0 | |||||
| BMI | ||||||||||
| Median | 28.4 | 28.6 | 32.9 | 28.9 | 28.8 | |||||
| Range | 21.1–47.9 | 16.2–48.2 | 20.4–45.4 | 17.0–56.5 | 16.2–56.5 | |||||
| BMI | ||||||||||
| <25 | 4 | 10.3 | 60 | 16.9 | 2 | 6.9 | 66 | 18.2 | 132 | 16.8 |
| 25≤BMI<30 | 17 | 43.6 | 160 | 44.9 | 8 | 27.6 | 146 | 40.2 | 331 | 42.1 |
| ≥30 | 18 | 46.2 | 136 | 38.2 | 19 | 65.2 | 151 | 41.6 | 324 | 41.2 |
| Missing | 0 | 1 | 0 | 0 | 1 | |||||
| ECOG PS | ||||||||||
| 0 | 27 | 69.2 | 249 | 69.7 | 17 | 58.6 | 255 | 70.2 | 548 | 69.5 |
| 1 | 12 | 30.8 | 102 | 28.6 | 12 | 41.4 | 103 | 28.4 | 229 | 29.1 |
| 2 | 0 | . | 6 | 1.7 | 0 | . | 5 | 1.4 | 11 | 1.4 |
| Extent of Disease | ||||||||||
| High | 26 | 66.7 | 237 | 66.4 | 17 | 58.6 | 232 | 63.9 | 512 | 65.0 |
| Low | 13 | 33.3 | 120 | 33.6 | 12 | 41.4 | 131 | 36.1 | 276 | 35.0 |
| Visceral Disease (among high volume) | ||||||||||
| No | 20 | 76.9 | 186 | 78.5 | 11 | 64.7 | 172 | 74.1 | 389 | 76.0 |
| Yes | 6 | 23.1 | 51 | 21.5 | 6 | 35.3 | 60 | 25.9 | 123 | 24.0 |
| Gleason Score | ||||||||||
| <7 | 3 | 8.3 | 18 | 5.6 | 1 | 3.8 | 20 | 6.2 | 42 | 6.0 |
| 7 | 9 | 25.0 | 87 | 27.1 | 7 | 26.9 | 76 | 23.7 | 179 | 25.4 |
| 8–10 | 24 | 66.7 | 216 | 67.3 | 18 | 69.2 | 225 | 70.1 | 483 | 68.6 |
| Missing/Unknown | 3 | 36 | 3 | 42 | 84 | |||||
| Local Therapy Type | ||||||||||
| None | 29 | 74.4 | 260 | 72.8 | 22 | 75.9 | 264 | 72.7 | 575 | 73.0 |
| Prostatectomy | 9 | 23.1 | 71 | 19.9 | 6 | 20.7 | 67 | 18.5 | 153 | 19.4 |
| Definitive RT | 1 | 2.6 | 26 | 7.3 | 1 | 3.4 | 32 | 8.8 | 60 | 7.6 |
| Prior Adjuvant Hormone Therapy | ||||||||||
| No | 37 | 94.9 | 341 | 95.5 | 29 | 100.0 | 347 | 95.6 | 754 | 95.7 |
| Yes | 2 | 5.1 | 16 | 4.5 | . | . | 16 | 4.4 | 34 | 4.3 |
| Baseline PSA (ng/ml) | ||||||||||
| Median | 48.7 | 51.7 | 28.4 | 54.4 | 51.7 | |||||
| Range | 1.1–2377 | 0.2–8540 | 0.3–2646 | 0.1–8056 | 0.1–8540 | |||||
| ADT Prior to Randomization | ||||||||||
| No | 7 | 17.9 | 43 | 12.1 | 3 | 10.3 | 49 | 13.5 | 102 | 13.0% |
| Yes | 32 | 82.1% | 312 | 87.9% | 26 | 89.7% | 314 | 86.5% | 684 | 87.0% |
| Missing/Unknown | 0 | 2 | 0 | 0 | 2 | |||||
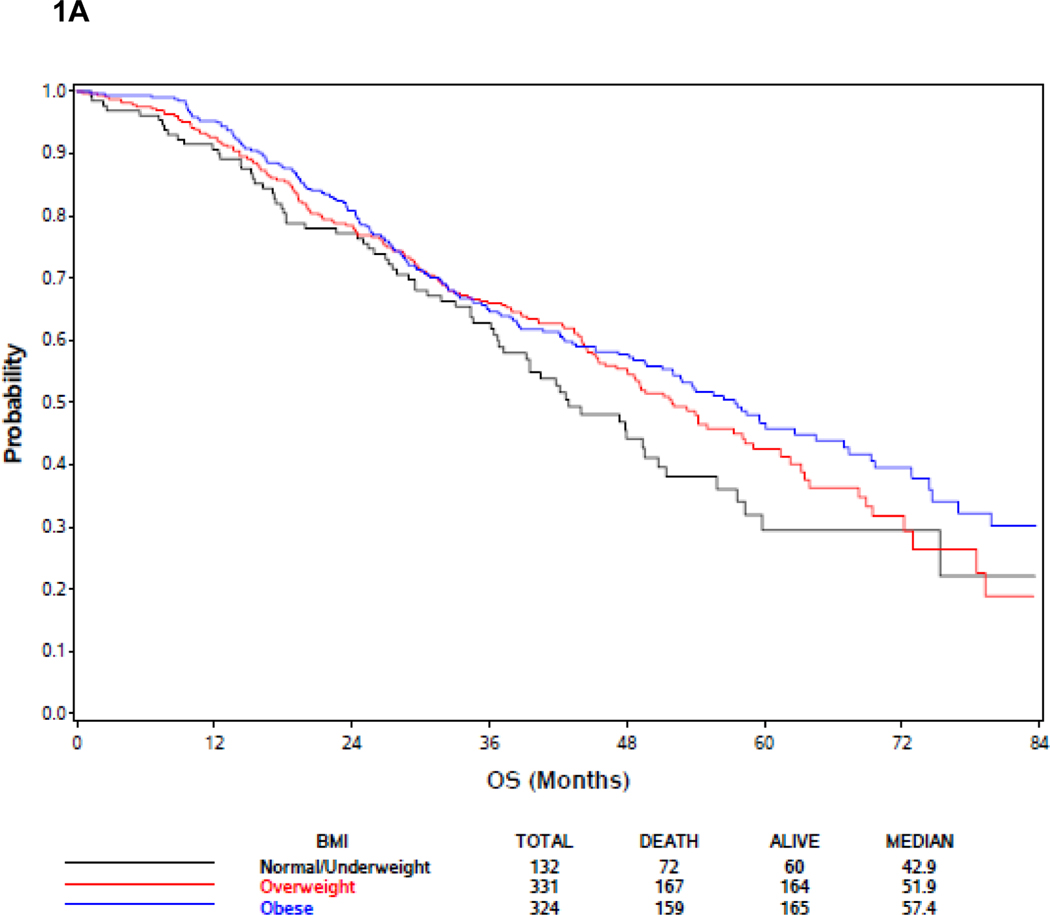
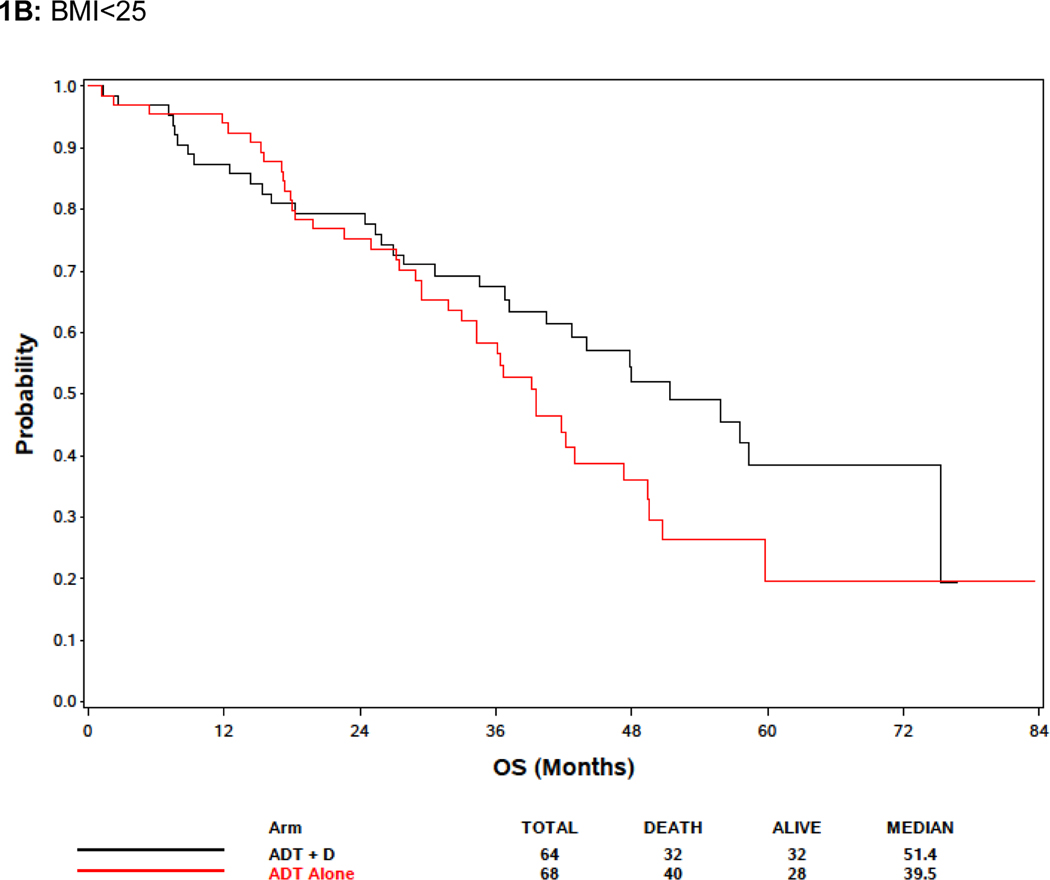
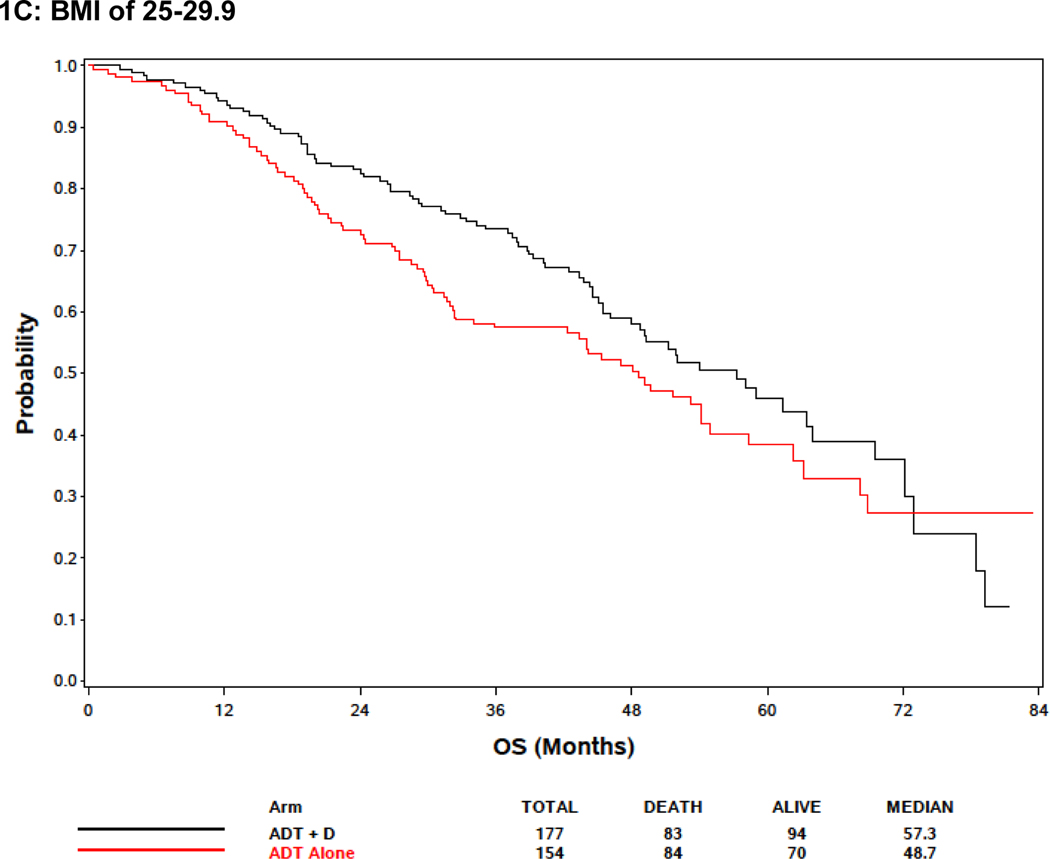
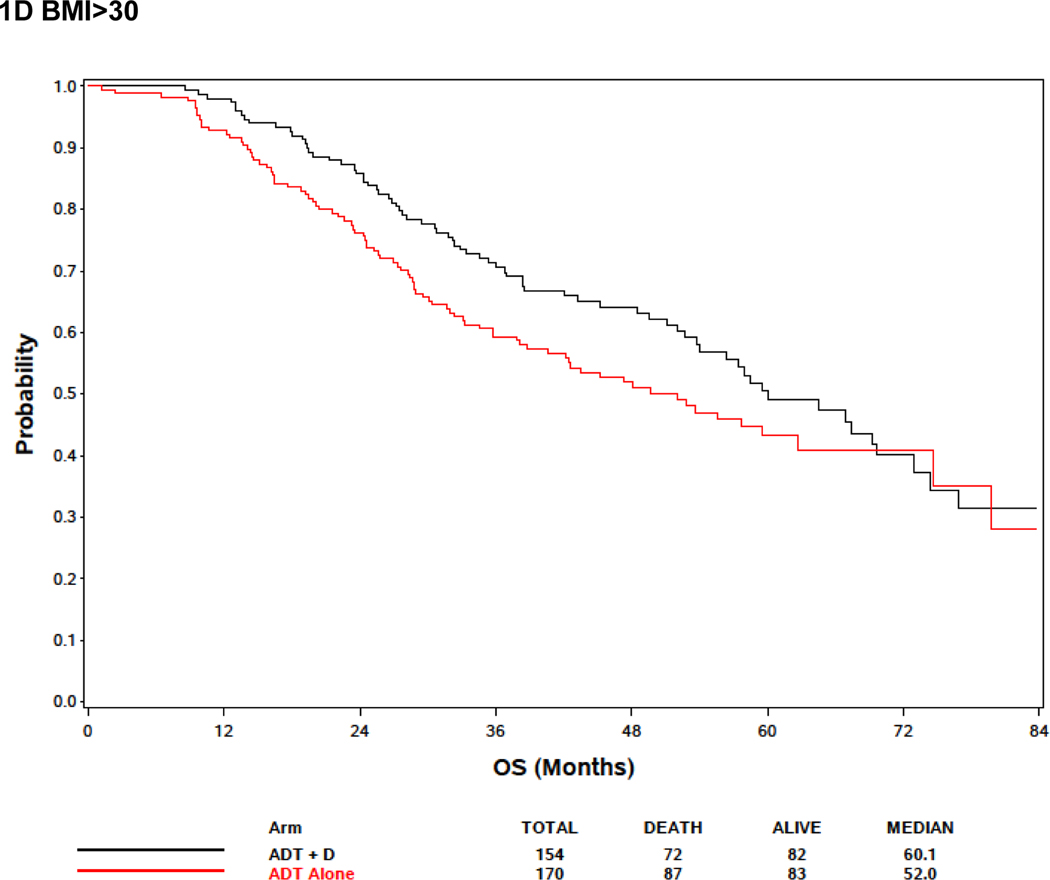
In the BMI analysis, baseline BMI was analyzed in clinical categories (BMI <25, 25≤BMI<30, and BMI ≥30 as normal and underweight, overweight, and obese, respectively). Baseline BMI was associated with extent of disease burden; men with low or normal BMI were more likely to have high volume disease than other groups. Specifically, 78.8%, 66.8% and 57.4% of men BMI<25, overweight, and obese men, respectively, had high volume disease (p<.0001) (Supplemental Table 1). There was no association between BMI and OS, but there was a trend towards improved survival in obese patients (Figure 1A; Table 2). Metformin exposure was included in the multivariable model because it was a prespecified exposure of interest. Although it was not included in the multivariable analysis initially because only variables with a p<0.10 in the univariable analysis were initially included, an analysis forcing BMI into the multivariable model despite the lack of significance in the univariable model was not significantly different from the model excluding BMI (Table 2). Response to treatment arm in these weight subgroups was assessed. In 132 patients with BMI<25, although there was no significant association between treatment arm and OS, the treatment effect was consistent with the overall and other subgroups with benefit from docetaxel (HR 0.65, 95% CI: [0.39, 1.09], p = 0.10) (Figure 1B). For 331 overweight (BMI >25 but < 30) men, there was improved OS associated with ADT+D as compared with ADT (HR 0.72, 95% CI: [0.53, 0.99], p=0.046), and for 324 obese men (BMI>30) ADT+D was associated with a consistent trend to improved OS vs ADT (HR 0.74, 95%: [0.53, 1.03], p=0.08) (Figure 1C and 1D).
Figure 1:
Effect of body mass index (BMI) on survival. (A) Including all E3805 treatment arms, no significant survival difference was noted between patients of different BMI at study presentation, but a trend seen towards improved survival in obese patients (BMI>30) was noted (see Table 3). (B) Effect of BMI on survival comparing ADT + D versus ADT alone for BMI<25). (C) Effect of BMI on survival comparing ADT + D versus ADT alone for BMI>25<30 (D) Effect of BMI on survival comparing ADT + D versus ADT alone for BMI>30.
Table 2:
Cox regression model1 for overall survival including BMI and metformin use
| UVA (n=7882) | MVA3 (n=7882) | MVA4 (n=7882) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Variable | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
|
| |||||||||
| Treatment arm | |||||||||
| ADT alone | 1.00 | - | 1.00 | - | 1.00 | - | |||
| ADT + D | 0.73 | (0.59, 0.89) | 0.002 | 0.70 | (0.58, 0.86) | 0.0007 | 0.70 | (0.57, 0.86) | 0.0006 |
| Metformin use | |||||||||
| No | 1.00 | - | 1.00 | - | 1.00 | - | |||
| Yes | 1.11 | (0.78, 1.58) | 0.55 | 1.15 | (0.81, 1.63) | 0.44 | 1.17 | (0.82, 1.67) | 0.37 |
| Volume of disease | |||||||||
| Low | 1.00 | - | 1.00 | - | 1.00 | - | |||
| High | 2.19 | (1.72, 2.79) | <.0001 | 1.91 | (1.49, 2.45) | <.0001 | 1.90 | (1.47, 2.44) | <.0001 |
| ECOG PS | |||||||||
| 0 | 1.00 | - | 1.00 | - | 1.00 | - | |||
| 1–2 | 1.45 | (1.17, 1.80) | 0.0007 | 1.51 | (1.22, 1.87) | 0.0002 | 1.50 | (1.21, 1.87) | 0.0003 |
| Gleason score | |||||||||
| ≤7 | 1.00 | - | 1.00 | - | 1.00 | - | |||
| 8–10 | 1.50 | (1.16, 1.93) | 0.002 | 1.36 | (1.04, 1.79) | 0.03 | 1.36 | (1.04, 1.79) | 0.03 |
| Missing/Unknown | 1.39 | (0.94, 2.06) | 0.10 | 1.12 | (0.75, 1.67) | 0.60 | 1.12 | (0.75, 1.68) | 0.58 |
| Prior local therapy | |||||||||
| No | 1.00 | - | 1.00 | - | 1.00 | - | |||
| Yes | 0.64 | (0.49, 0.84) | 0.001 | 0.67 | (0.50, 0.89) | 0.005 | 0.67 | (0.50, 0.89) | 0.006 |
| Baseline PSA | |||||||||
| <65 | 1.00 | - | - | - | - | - | - | - | |
| ≥65 | 1.14 | (0.92, 1.41) | 0.23 | - | - | - | - | - | - |
| BMI | |||||||||
| <25 | 1.00 | - | - | - | - | 1.00 | - | ||
| 25–29.9 | 0.92 | (0.69, 1.22) | 0.55 | - | - | - | 0.99 | (0.74, 1.32) | 0.94 |
| ≥30 | 0.83 | (0.62, 1.11) | 0.21 | - | - | - | 0.89 | (0.66, 1.19) | 0.42 |
| BMI | |||||||||
| <30 | 1.00 | - | - | - | - | - | - | - | |
| ≥30 | 0.88 | (0.72, 1.09) | 0.24 | - | - | - | - | - | - |
stratified by stratification factors at randomization, including age, ECOG PS, CAB use, duration of prior adjuvant hormonal therapy, use of FDA approved drugs for delaying skeletal related events and volume of disease; treatment arm by metformin use interaction was not statistically significant (p=0.86)
only patients with metformin data were included in the analysis
variables with p<0.10 in the univariable analysis were included in the multivariable analysis; metformin use was forced into the multivariable model regardless of significance.
BMI was included in the multivariable model regardless of significance per reviewers’ suggestion.
We also assessed the association between baseline BMI and QOL. QOL by median FACT-P score was poorer among patients with BMI<25 (118) when compared with overweight and obese patients (125 and 123, respectively, p=0.008) (Table 3). This difference met criteria for clinically meaningful difference (≥ 6–10) between BMI <25 and overweight, but not when other groups were directly compared [26]. Median FACIT-Fatigue score was lower (increased fatigue) among patients with BMI <25 when compared with overweight patients, but was similar to obese men (44.0 vs 46.0 vs 43.3 for BMI <25 vs overweight vs obese, respectively, p=0.004 for comparison of all 3 groups), indicating greater fatigue for underweight and obese patients. However, this small difference only met criteria for minimally important difference between overweight and obese patients (change by 3–4) [27], suggesting that fatigue levels are similar for BMI <25 and overweight patients, but may be greater in obese patients. Median pain score was similar between BMI <25 and obese patients (median BPI 1.25 in both groups), but was numerically lower in overweight patients (median BPI 1.0, p=0.07). (Table 3) This difference did not meet criteria for minimally important difference (difference by 2) [28]. Baseline QOL data by treatment arm was not substantially different.
Table 3:
Distribution of baseline QOL scores by BMI group
| Baseline QOL | Underweight/Normal (BMI <25) | Overweight (25≤BMI<30) | Obese (BMI ≥30) | p-value4 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | Median (Range) | n | Median (Range) | n | Median (Range) | ||
|
| |||||||
| Overall | |||||||
| FACT-P total1 | 116 | 117.8 (43.7–151.8) | 293 | 125.0 (47.0–156.0) | 294 | 122.9 (46.0–153.9) | 0.008 |
| Pain score2 | 113 | 1.25 (0–8.0) | 270 | 1.0 (0–7.5) | 277 | 1.25 (0–9.0) | 0.07 |
| FACIT-Fatigue3 | 116 | 44.0 (3.0–52.0) | 289 | 46.0 (6.0–52.0) | 299 | 43.3 (0–52.0) | 0.004 |
|
| |||||||
| ADT+D | |||||||
| FACT-P total1 | 56 | 116.3 (75.0–151.8) | 162 | 124.0 (47.0–156.0) | 140 | 122.9 (51.0–153.9) | 0.18 |
| Pain score2 | 55 | 1.25 (0–8.0) | 147 | 0.75 (0–7.0) | 133 | 1.0 (0–9.0) | 0.46 |
| FACIT-Fatigue3 | 56 | 44.5 (14.0–52.0) | 158 | 46.0 (9.0–52.0) | 145 | 44.0 (5.0–52.0) | 0.23 |
|
| |||||||
| ADT alone | |||||||
| FACT-P total1 | 60 | 120.0 (43.7–151.0) | 131 | 127.5 (54.0–153.9) | 154 | 122.8 (46.0–152.0) | 0.03 |
| Pain score2 | 58 | 1.13 (0–7.25) | 123 | 1.0 (0–7.5) | 144 | 1.25 (0–8.0) | 0.14 |
| FACIT-Fatigue3 | 60 | 43.0 (3.0–52.0) | 131 | 46.0 (6.0–52.0) | 154 | 43.0 (0–52.0) | 0.01 |
Higher scores indicate better QOL. Clinically meaningful change on the FACT-P total score is considered a change of 6–10 points.
Higher scores indicate greater pain.
Higher scores indicate improved fatigue.
Comparison among 3 BMI groups using Kruskal-Wallis test
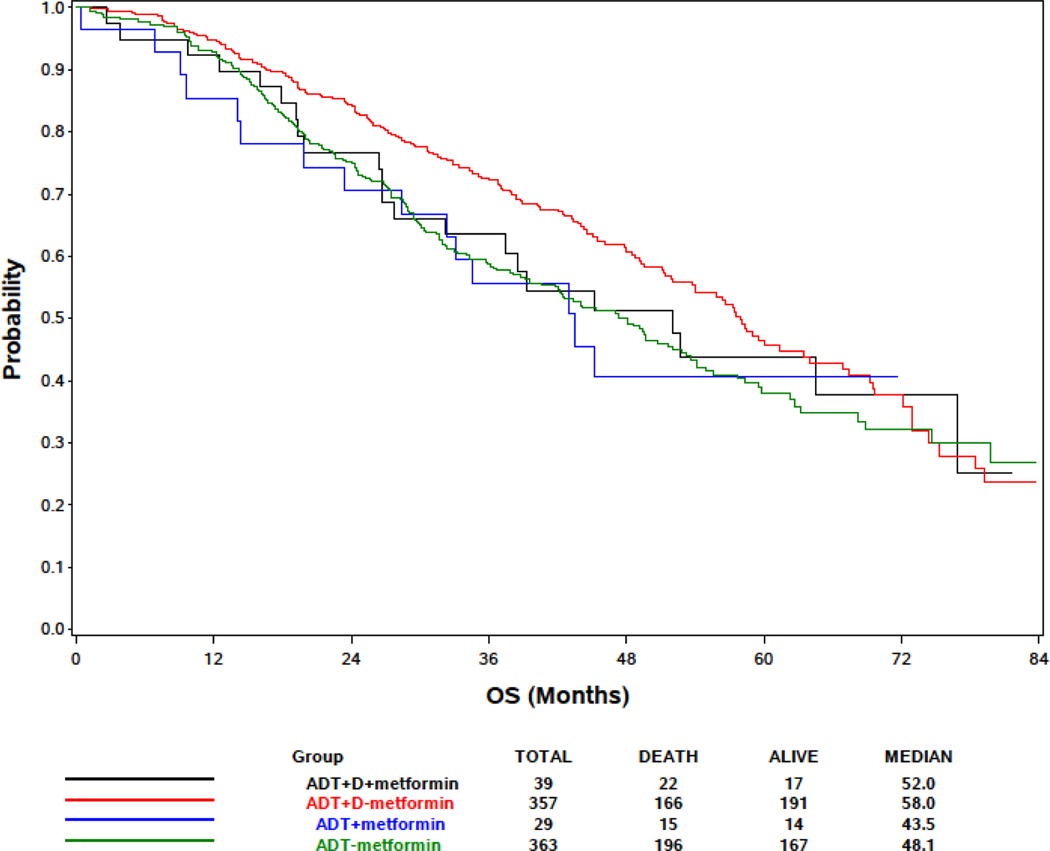
In the metformin analysis, prostate cancer was the cause of death in 15/22(68%) of ADT+D+metformin, 132/166(80%) ADT+D, 12/15(80%) ADT+metformin and 164/196(84%) ADT alone patients (Table 4). There was no significant difference in OS (Figure 2) by metformin exposure within the ADT+D treatment arm (p=0.31) and ADT alone arm (p=0.76) using the stratified logrank test (Table 4). Metformin exposure was not associated with survival on univariable analysis (HR 1.11 for metformin treated vs untreated patients, 95% CI 0.78–1.58, p=0.55) (Table 2) or with time to castration-resistant prostate cancer (CRPC) (HR 0.87 for metformin treated vs untreated patients, 95% CI 0.64 – 1.18, p=0.37) (Supplemental Table 2).
Table 4:
Distribution of overall survival by treatment arm and metformin use
| ADT+D+metformin (n=39) | ADT+D/No metformin (n=357) | ADT+metformin (n=29) | ADT/No metformin (n=363) | |
|---|---|---|---|---|
|
| ||||
| Overall survival (months) | ||||
| Median | 52.0 | 58.0 | 43.5 | 48.1 |
| 95% CI | 27.7, 76.9 | 52.1, 63.9 | 23.5, NA | 40.7, 53.3 |
| Number of deaths | 22 | 166 | 15 | 196 |
| Number of PC deaths | 15 | 132 | 12 | 164 |
| p-value (stratified* logrank) | 0.31 (compare metformin y/n in ADT+D arm) | 0.76 (compare metformin y/n in ADT alone arm) | ||
stratified on stratification factors at randomization
Figure 2:
Kaplan-Meier Estimates of Overall Survival Based on Metformin Use, Docetaxel (D) and Androgen Deprivation Therapy (ADT) for Patients with Metastatic Hormone Sensitive Prostate Cancer.
In a multivariable analysis adjusted for treatment arm, de novo metastatic presentation, volume of disease, Gleason score and ECOG PS and BMI, metformin exposure were not associated with survival (HR 1.17; 95% CI: 0.82–1.67, p=0.37) (Table 2). Metformin exposure was also not associated with time to clinical progression (HR: 1.02: 95%.CI 0.75–1.39, p=0.91) in a multivariable analysis adjusted for treatment arm, volume of disease, prior local therapy, baseline PSA and Gleason score (Supplemental Table 2). In addition, PSA treatment response < 0.2 ng/mL at 12 months was 161 of 720 (22.4%) without metformin and 15 of 68 (22.1%) with metformin. As in prior publications, factors in this population that were associated with overall survival include high volume disease, and ECOG PS ≥1 (HR 1.90, 95% CI 1.47–2.44, for high vs low volume; and HR 1.50, 95% CI 1.21–1.87, for ECOG PS ≥1 vs ECOG PS 0,), and recurrent disease after prior local therapy vs de novo metastatic status (HR 0.67, 95% 0.5–0.89 for prior local therapy vs de novo metastatic status). After adjustment for volume of disease and de novo metastatic presentation, Gleason score ≥ 8 was also associated with OS (HR=1.36; p=0.03, 95% CI 1.04–1.79) but a weaker predictor of survival and is not available for all patients, given some patients have metastatic biopsies or treated based on clinical features without a tissue diagnosis.
Discussion
Features associated with metabolic health, including BMI and metformin exposure, have been reported to play a role in outcomes of patients with advanced cancers. We evaluated the effect of baseline BMI on prostate cancer outcome and baseline QOL scores, and the association between metformin exposure at baseline and survival in men with mHSPC treated with ADT+D or ADT on E3805 (CHAARTED). This post-hoc analysis assesses timely questions that patients and clinicians have related to nutritional status and whether medications like metformin may impact prostate cancer outcomes.
In the BMI analysis, we assessed both prostate cancer specific outcomes and quality of life. We found an association between BMI <25 and poorer baseline QOL by the FACT-P. We hypothesize that this is because nearly 80% of patients with BMI <25 had high volume disease, while other BMI groups had significantly lower percentage of patients with high volume disease. Men in the BMI <25 group may have had a higher BMI prior to diagnosis with prostate cancer, but cancer induced cachexia due to advanced disease may have resulted in a decline in BMI prior to enrollment and normal or underweight BMI at study entry. Cancer-associated weight loss is commonly associated with symptomatic cancer syndromes including greater fatigue and pain. As such, it is not likely that a higher BMI portends a better prognosis, but that lower BMI is associated with a greater cancer burden and the findings are due to reverse-causation. Notably, weight loss after diagnosis in breast and other cancers is an independent predictor of worse survival independent of treatment and prognostic factors, suggesting a similar relationship in other solid tumors[34].
In the exploratory metformin analysis, there was no survival benefit associated with metformin exposure in the small number of men with mHSPC treated with ADT alone or chemohormonal therapy in the CHAARTED population. There were however few men receiving metformin at baseline in the study overall, making this analysis underpowered. When considered in the context of available literature on metformin in cancer, the lack of an association between metformin and survival in the mHSPC population in CHAARTED suggests that the anticancer activity of metformin may differ by cancer type, disease state, and treatment, and that the effect may be small and difficult to differentiate in the setting of effective disease directed treatment. Similar to these findings, a post hoc analysis of mCRPC patients in the docetaxel arm of TAX 327, a phase 3 randomized controlled trial, failed to find an association between metformin exposure and improved OS[29]. Additionally, a recent retrospective cohort study including 2,832 men with mCRPC receiving docetaxel chemotherapy found no association between metformin exposure and prostate-cancer specific or overall survival[30]. In a prospective single arm phase 2 study in men with mCRPC, minimal single agent activity was seen with metformin alone with a ≥50% PSA decline in 5% of patients and 36% of patients were progression-free at 12 weeks[31]. These studies suggest metformin has an effect on these cancer specific outcomes, it is minimal and likely difficult to detect in advanced mCRPC patients receiving chemotherapy.
Despite our findings, this does not refute the use of metformin in patients with diabetes initiating ADT alone for advanced androgen-sensitive prostate cancer. A recently published retrospective cohort study in a Veterans Administration population evaluated the impact of metformin on men initiating ADT, including 87,344 men treated with ADT >6 months and not receiving concurrent radiation[3]. This study found improved survival in men with diabetes mellitus on metformin (HR 0.82, 95% CI 0.78–0.86) compared to those with diabetes mellitus taking insulin who were not on metformin (HR 1.03, 95% CI 0.99–1.08). The referent group was men without diabetes. There are known antagonistic effects of metformin on cancer signaling pathways, and metformin use has been found to improve outcomes in other hormonally-mediated advanced cancers including breast and endometrial. [20, 22]. These results may be due in part to the adverse metabolic health effects of hormone ablation, which may be mitigated in diabetic men treated with metformin after ADT.[32] Metabolic syndrome has been associated with a shorter time to PSA progression and shorter OS in patients on ADT as compared with men without metabolic syndrome[33]. Nonetheless, its value as an adjunct in men receiving ADT and docetaxel is not clear, and use of metformin for men without diabetes is not supported by our data.
We acknowledge several limitations with this study as with any secondary exploratory analysis of phase 3 data. First, the absolute number of patients receiving metformin at baseline was low, possibly because patients included in clinical trials tend to have fewer comorbidities requiring treatment, including diabetes. Further, we do not have data describing specific comorbidities or concomitant diabetic or other medication use to include in the survival analysis or details describing whether patients treated with metformin had diabetes or were receiving metformin for other reasons. Having data on the indication for treatment with metformin, and information regarding comorbid cardiovascular disease, diabetes, and exposure to insulin, oral hypoglycemic or other diabetes medications, and statins could reduce confounding if this information were available. Additionally BMI and metformin exposure were calculated by a cross-sectional analysis performed only at baseline. Having data on BMI change prior to enrollment or over time during the study, and information describing metformin dose and duration of exposure over the course of the study would strengthen our analysis. In addition, we do not have data on patients taking insulin and other types of oral hypoglycemics at the time of docetaxel treatment. Finally, although metformin exposure at baseline was a pre-specified analysis, the study was not powered specifically to assess the effect of metformin exposure or BMI on OS or QOL due to the small number of exposed patients in the population, thus limiting our ability to definitively make conclusions about the effect of these factors on these outcomes despite the randomized prospective design of the study.
Conclusions
In this analysis of men with mHSPC treated on the CHAARTED study, men with low or normal BMI had evidence of more advanced cancer and a poorer baseline QOL when compared with overweight and obese men. Metformin exposure did not significantly affect prostate cancer outcomes, including survival, in men with mHSPC treated with ADT alone or ADT plus docetaxel. In the absence of a prospective randomized study designed to evaluate metformin exposure in men with mHSPC receiving ADT alone or as part of chemohormonal therapy irrespective of concurrent diabetes, there is no compelling reason to add metformin for men without diabetes. Definitive recommendations await the results of ongoing studies investigating metformin exposure regardless of underlying use for diabetes, including in the STAMPEDE MRC trial (Clinical registration number NCT00268476) that is powered to evaluate a difference in prostate cancer specific outcomes in men with mHSPC with or without metformin exposure.
Supplementary Material
Acknowledgments:
Funding & Research Support:
Sanofi provided docetaxel for early use and financial grant support NCI-CTEP and ECOG-ACRIN; Public Health Service Grants CA180794, CA180820, CA23318, CA66636, CA21115, CA49883, CA16116, CA21076, CA27525, CA13650, CA14548, CA35421, CA32102, CA31946, CA04919, CA107868, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: U10CA180820, U10CA180794, U10CA180821, U10CA180888, UG1CA189829, UG1CA232760, UG1CA233160, UG1CA233180, UG1CA233196, UG1CA233234, UG1CA233277, UG1CA233320, and UG1CA233339. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government. The authors wish to thank the late Dr. Linda Patrick-Miller for her tireless efforts and contribution to our assessment of quality of life in this study, and for ensuring the inclusion of the patient voice in cancer care.
References
- 1.Sweeney CJ, Chen YH, Carducci M et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015; 373: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James ND, Sydes MR, Clarke NW et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards KA, Liou JI, Cryns VL et al. Metformin Use Is Associated with Improved Survival in Patients with Advanced Prostate Cancer on Androgen Deprivation Therapy. J Urol 2018. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain C, Romundstad P, Vatten L et al. The association of weight gain during adulthood with prostate cancer incidence and survival: a population-based cohort. Int J Cancer 2011; 129: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 5.Wright ME, Chang SC, Schatzkin A et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 2007; 109: 675–684. [DOI] [PubMed] [Google Scholar]
- 6.Overweight and Obesity in Adults; Systematic Evidence Review from the Obesity Expert Panel, 2013. In Evidence Report. US Department of Health and Huma Services 2013; https://www.nhlbi.nih.gov/sites/default/files/media/docs/obesity-evidence-review.pdf. [Google Scholar]
- 7.Joshu CE, Mondul AM, Menke A et al. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 2011; 4: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011; 4: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renehan AG, Soerjomataram I, Tyson M et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer 2010; 126: 692–702. [DOI] [PubMed] [Google Scholar]
- 10.Dieperink KB, Hansen S, Wagner L et al. Living alone, obesity and smoking: important factors for quality of life after radiotherapy and androgen deprivation therapy for prostate cancer. Acta Oncol 2012; 51: 722–729. [DOI] [PubMed] [Google Scholar]
- 11.Anast JW, Sadetsky N, Pasta DJ et al. The impact of obesity on health related quality of life before and after radical prostatectomy (data from CaPSURE). J Urol 2005; 173: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 12.Mosher CE, Sloane R, Morey MC et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer 2009; 115: 4001–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard CM, Stein K, Courneya KS. Body mass index, physical activity, and health-related quality of life in cancer survivors. Med Sci Sports Exerc 2010; 42: 665–671. [DOI] [PubMed] [Google Scholar]
- 14.Wolin KY, Luly J, Sutcliffe S et al. Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol 2010; 183: 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin LL, Brown JC, Segal S, Schmitz KH. Quality of life, body mass index, and physical activity among uterine cancer patients. Int J Gynecol Cancer 2014; 24: 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012; 12: 159–169. [DOI] [PubMed] [Google Scholar]
- 17.Whitburn J, Edwards CM, Sooriakumaran P. Metformin and Prostate Cancer: a New Role for an Old Drug. Curr Urol Rep 2017; 18: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou G, Myers R, Li Y et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Sahra I, Laurent K, Loubat A et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27: 3576–3586. [DOI] [PubMed] [Google Scholar]
- 20.Jiralerspong S, Palla SL, Giordano SH et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009; 27: 3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan BX, Yao WX, Ge J et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer 2011; 117: 5103–5111. [DOI] [PubMed] [Google Scholar]
- 22.Ezewuiro O, Grushko TA, Kocherginsky M et al. Association of Metformin Use with Outcomes in Advanced Endometrial Cancer Treated with Chemotherapy. PLoS One 2016; 11: e0147145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards KA, Liou JI, Cryns VL et al. Metformin Use is Associated with Improved Survival for Patients with Advanced Prostate Cancer on Androgen Deprivation Therapy. J Urol 2018; 200: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 24.Ranasinghe WKB, Williams S, Ischia J et al. Metformin may offer no protective effect in men undergoing external beam radiation therapy for prostate cancer. BJU Int 2019; 123 Suppl 5: 36–42. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakopoulos CE, Chen YH, Carducci MA et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 2018; 36: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Nichol MB, Eton D et al. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy--Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009; 12: 124–129. [DOI] [PubMed] [Google Scholar]
- 27.Nordin A, Taft C, Lundgren-Nilsson A, Dencker A. Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med Res Methodol 2016; 16: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathias SD, Crosby RD, Qian Y et al. Estimating minimally important differences for the worst pain rating of the Brief Pain Inventory-Short Form. J Support Oncol 2011; 9: 72–78. [DOI] [PubMed] [Google Scholar]
- 29.Niraula S, Pond G, de Wit R et al. Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study. Can Urol Assoc J 2013; 7: E74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer MJ, Klotz LH, Venkateswaran V. The Effect of Metformin Use during Docetaxel Chemotherapy on Prostate Cancer Specific and Overall Survival of Diabetic Patients with Castration Resistant Prostate Cancer. J Urol 2017; 197: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 31.Rothermundt C, Hayoz S, Templeton AJ et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09). Eur Urol 2014; 66: 468–474. [DOI] [PubMed] [Google Scholar]
- 32.Zhu W, Xu H, Ma J et al. An Open-Label Pilot Study of Metformin as a Concomitant Therapy on Patients with Prostate Cancer Undergoing Androgen Deprivation Treatment. Urol Int 2017; 98: 79–84. [DOI] [PubMed] [Google Scholar]
- 33.Flanagan J, Gray PK, Hahn N et al. Presence of the metabolic syndrome is associated with shorter time to castration-resistant prostate cancer. Ann Oncol 2011; 22: 801–807. [DOI] [PubMed] [Google Scholar]
- 34.Cespedes Feliciano EM, Kroenke CH, Bradshaw PT et al. Postdiagnosis Weight Change and Survival Following a Diagnosis of Early-Stage Breast Cancer. Cancer Epidemiol Biomarkers Prev 2017; 26: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.