Abstract
Aims.
The goal of this study was to determine whether aging effects the expression of V1a and V2 vasopressin receptors in the urinary bladder mucosa (UBM) and kidney.
Methods.
UBM and kidneys were obtained from young (3 months-of-age) and old (25–30 months-of-age) female Fisher 344 rats. Tissue samples were analyzed by western blotting for V1a and V2 receptor expression, and rat plasma levels of vasopressin levels were measured by ELISA.
Results.
V1a and V2 receptors were detected in both the UBM and kidneys. Aging significantly (P<0.05) increased the expression of V2 receptors by 2.80 ± 0.52 and 6.52 ± 1.24 fold in the UBM and kidneys, respectively. Aging also increased V1a receptor expression in the kidneys (5.52 ± 1.05 fold; P<0.05), but not in the UBM. Because this is the first detection of V2 receptors in the mammalian bladder, we also probed human UBM for V2 receptors and observed high expression in human UBM. Unlike V1a and V2 receptors, aging had only a minor effect on plasma vasopressin levels (8% increase).
Conclusions.
V2 receptors are substantially increased in the aging UBM. This suggests that in the elderly the UBM is another site of V2 receptor-mediated free water reabsorption. The large age-related increase in the expression of V2 receptors in both the UBM and kidney may contribute to the effectiveness of desmopressin in age-related nocturia.
Keywords: Bladder mucosa, nocturia, aquaporins, older adults
Introduction
The urinary bladder mucosa (UBM), which lines the inner surface of the renal pelvis, ureters and bladder, forms a high-resistance barrier and functions as an integral part of a ‘sensory web’ which receives, amplifies and transmits information about its external milieu.1–5 There is increasing evidence that the UBM plays an active role in both solute and fluid reabsorption and is able to modify urine composition.5–10 While the underlying biochemical mechanisms have yet to be clarified, it is known that the UBM expresses a variety of receptors and channels including several types of aquaporin water channels and the renal epithelial sodium channel (ENaC), both of which play important roles in vasopressin-mediated water homeostasis in the kidney.1,4,11–15 An open question, however, is whether the mammalian UBM also expresses vasopressin receptors and whether expression of vasopressin receptors in the UBM is modified by aging. Accordingly the overall aim of this study was to examine the expression of vasopressin V1a and V2 receptors in the UBM, and for comparison the kidney, in both young and old rats. Here we report for the first time that the UBM indeed expresses both V1a and V2 receptors and that aging causes a profound increase in V2 receptor expression in both the UBM and kidney and substantially augments V1a receptor expression in the kidneys. As such, these findings may demonstrate that the UBM is possibly an active participant in modifying water homeostasis.
Materials and Methods
Animal Tissue.
Animal experiments were performed under the approval of the Animal Care and Use Committee of the University of Pittsburgh. Urinary bladders or kidneys were excised from Fisher F344 young adult rats (3 months-of-age; Envigo, East Millstone, NJ, n=8) or Fisher F344 aged rats (25–30 months-of-age; obtained from the National Institute on Aging, n=8) and sacrificed by CO2 inhalation.
Human Mucosal Bladder Tissue.
Samples were obtained from de-identified control subjects and study procedures were approved by the University of Pittsburgh Institutional Review Board.
Western Blotting for V1a and V2 Receptors.
Urinary mucosa was dissected form rat bladders and homogenized using Lysing Matrix D in a FastPrep 24 instrument (MP Biomedicals, Solon, OH) in HBSS (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHO3, 0.3 mM Na2HCO3, 0.3 mM Na2HPO4, 5.6 mM glucose and 10 mM HEPES, pH 7.4) containing protease and phosphatase inhibitors. We also homogenized whole rat kidney using the same procedure. After centrifugation (13,000 rpm; 15 min at 4ºC), the membrane protein fraction was prepared by suspending the membrane pellets in lysis buffer containing protease and phosphatase inhibitors. The protein concentrations of the samples were determined using the Pierce BCA protein assay (Thermo Scientific, Rockford, IL). After denaturation (100ºC for 5 min) lysate from each sample was separated on a 4–15% TGX Stain-Free SDS-PAGE gel (Bio-Rad Laboratories, Hercules, CA). As a loading control, total protein per sample was determined using Bio-Rad Stain Free SDS-PAGE gel technology. UV-activated protein fluorescence was imaged (ChemiDoc MP, Bio-Rad). Proteins were transferred to polyvinylidene fluoride membranes, incubated in 5% (w/v) dried milk dissolved in TBS-T and incubated overnight at 4ºC with primary antibodies to either V1a or V2 receptors. The V1a-receptor antibody was purchased from Alpha Diagnostics (San Antonio, TX; catalog number AVP1A11-A). The antigen used to generate this antibody was a 19 amino acid peptide that matches the corresponding sequence in the extracellular N-terminus of the rat V1a-receptor and has no significant similarity with V1b or V2 receptors. The V2-receptor antibody was also purchased from Alpha Diagnostics (catalog number AVPV21-A). The antigen used to generate this antibody was a 21 amino acid peptide that matches the corresponding sequence in the intracellular C-terminus of the rat V2-receptor and has no significant similarity with V1a or V1b receptors. The membranes were incubated with secondary antibody (donkey anti-rabbit HRP; GE Amersham, Pittsburgh, PA) for 1 hour and incubated in WesternBright Sirius (Advansta, Menlo Park, CA) and then imaged. For quantification, the optical density of each protein species was determined and normalized to total protein using Image Lab software (Bio-Rad). Both receptors were detected in both unmodified (50kDa) and glycosylated (65kDa) forms.
Primary bladder urothelial cell lysates:
Bladder urothelial cells (UTC) were prepared as previously described.16 The bladder was removed and placed in cold minimal essential medium (MEM; Invitrogen, Carlsbad, CA) supplemented with HEPES. The bladder was cut open to expose the urothelium, and incubated in dispase (2.5 mg/ml; Worthington Biochemical, Lakewood, NJ) overnight at 4°C. UTCs were gently scraped from the underlying tissue, placed in trypsin (0.25% wt/vol; Sigma) and dissociated by trituration. Single cell suspensions were washed in HBSS, pelleted, and proteins extracted in lysis buffer containing protease and phosphatase inhibitors.
Statistical Analysis:
Data were analyzed in GraphPad Prism 6 (GraphPad, La Jolla, CA) using Student’s t-test and one-way ANOVA followed by appropriate post-hoc tests. P<0.05 was considered significant. Results are expressed as means ± SEM.
Results
V1a And V2 Receptors Are Expressed In Bladder Mucosa And Increase With Age.
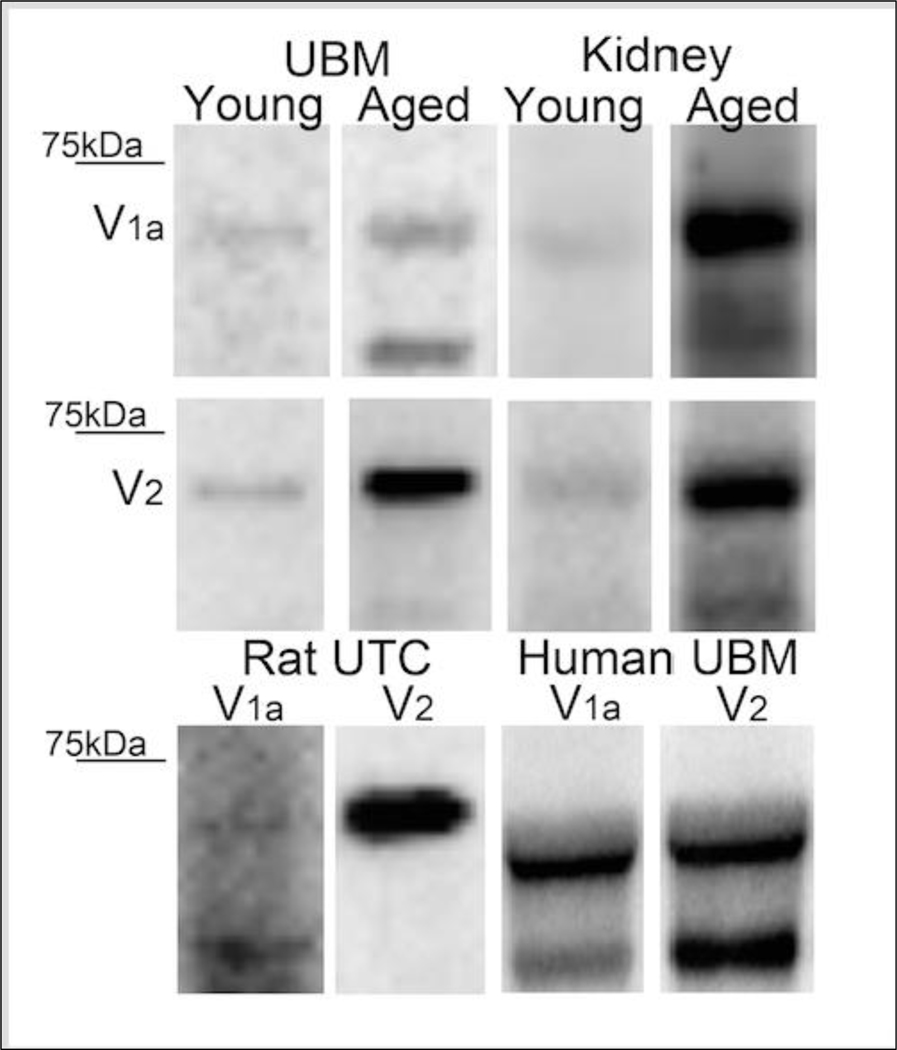
Western immunoblotting revealed that the rat UBM expressed both V1a and V2 receptors (Figure 1). Although age did not significantly affect V1a expression (Figure 1), V2-receptor expression was increased by 2.80 ± 0.52 fold (P<0.05; Student’s t-test) in UBM obtained from old versus young rats (Figure 1). Both V1a and V2 receptors were also detected in the kidney (Figure 1), and were more highly expressed in UBM from old versus young rats (Figure 1). In this regard, in the aged kidney V1a receptors were increased by 5.52 ± 1.05 fold and V2 receptors were increased by 6.52 ± 1.24 fold increase (P<0.05 for both receptor subtypes; Student’s t-test). As a comparison, we also found that human UBM expressed both V1a and V2 receptors (Figure 1). In order to definitively show that vasopressin receptors are expressed within the urothelium, we also performed western immunoblotting on urothelial cell lysates from rat bladders. Indeed, rat urothelial cells exhibited marked expression of V2 receptors (Figure 1). Although detectable, the V1a receptor signal was modest (Figure 1).
Figure 1. Representative immunoblots for V1a and V2 receptors.
Young versus aged rat UBM and young versus aged rat kidney representative blots are shown for V1a (top row) and V2 receptor (second row). Bottom row shows V1a and V2 receptor expression in rat UTC lysates and human UBM. In all panels, the upper band is glycosylated and lower band is unmodified form.
Vasopressin Can Be Detected In Both Plasma As Well As Bladder Mucosa.
There was a tendency toward increased vasopressin levels in the mucosa of aged rats; however the changes did not reach significance (P>0.05; Student’s t-test). Vasopressin levels in aged rat plasma were slightly, yet significantly, elevated (1.08 ± 0.02-fold increase; P<0.05; student’s t-test) as compared to younger controls. Vasopressin was also detected in human UBM and urothelial cell lysates (data not shown).
Discussion
The UBM not only plays an active role in sensory functions but also may participate in transporting water and electrolytes between the bladder lumen and bladder interstitium.1,4,5,7–10,14 In this regard, the presence of aquaporin-2 water channels in the UBM suggests, but does not prove, that vasopressin can elicit trafficking of these channels to the cell surface to increase water transport via a mechanism similar to that in the kidney epithelium. However, this hypothesis would only be viable if V2 receptors are expressed in the urothelium. The present study provides the first evidence that the UBM indeed expresses V2 receptors and that the expression of V2 receptors in the UBM is much greater in aged animals. These findings support the hypothesis that the bladder per se may regulate water homeostasis, particularly during old age.
The fact that V2 receptor expression in the UBM and kidneys increases with age has at least two important clinical implications. The first implication relates to the management of nocturia. A high percentage of elderly patients are affected by lower urinary tract dysfunction that generates lower urinary tract symptoms including urinary frequency, urgency and voiding difficulties including nocturia.17–19 Nocturia is a bothersome urologic symptom that significantly impacts quality of life in older adults with limited treatment options. A novel formulation of desmopressin has been recently approved for the treatment of nocturia or excessive nighttime urine production.20,21 Inasmuch as desmopressin acts via stimulation of V2 receptors, the current findings suggest that desmopressin may be particularly effective in the elderly due to increased expression of its intended target, i.e., the V2 receptor.
The second clinical implication of the current findings also relates to the management of another common condition in the elderly, i.e., hyponatremia. Hyponatremia is a prevalent, serious, and costly medical condition in the elderly.22,23 Approximately 8% of people over 55 years of age have hyponatremia, and by the age of 75 the prevalence increases to ~12%. The prevalence of hyponatremia is even greater in health care facilities, reaching 18% in the nursing home population and occurring in approximately 50% of hospitalized patients over the age of 65.24,25 Acute hyponatremia can be fatal; but of equal concern is the fact that chronic hyponatremia can result in gait deficits, cognitive impairment and loss of balance, thus predisposing the elderly to increased risk of falls leading to bone fractures. Indeed, hyponatremia is an independent risk factor for increased mortality in the elderly.23,26,27 The high prevalence of hyponatremia in the elderly remains unexplained. The present findings that the UBM expresses V2 receptors and that the expression of V2 receptors in both the UBM and kidneys increases substantially with age may explain the high prevalence of hyponatremia in the elderly. This new understanding could pave the way to effective treatments for this burdensome disorder.
Conclusions.
In short, while our findings may not provide a definitive link, it is tempting to speculate that the UBM may be an additional site of action for vasopressin and desmopressin and may impact both fluid and solute composition in a number of clinical conditions including nocturia and hyponatremia. Additional studies are underway to examine these exciting possibilities.
Acknowledgements:
This work was supported by the following grants: R01 AG056944 and R37 DK54824 (to LAB) and R01 DK091190 and P30 DK079307 (EKJ).
Footnotes
Institution where work was performed: University of Pittsburgh
References
- 1.Birder L, Andersson KE Urothelial Signaling. Physiol Rev. 2013;93:653–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birder LA. Urothelial Signaling. Auton Neurosci. 2010;153:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen DAW, Schalken JA, Heesakkers JPFA Urothelium update: how the bladder mucosa measures bladder filling. Acta Physiol. 2017;220:201–217. [DOI] [PubMed] [Google Scholar]
- 4.Apodaca G, Balestreire E, Birder L. The uroepithelial-sensory web. Kidney Int. 2007;72:1057–1064. [DOI] [PubMed] [Google Scholar]
- 5.Khanderwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297:F1477–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCloskey KD, Vahabi B, Fry CH Is electrolyte transfer across the urothelium important:. Neurourol Urodyn. 2017;36:863–868. [DOI] [PubMed] [Google Scholar]
- 7.Hohlbrugger G. Changes of hypo-and hypertonic sodium chloride induced by the rat urinary bladder at various filling stages. Evidence for an increased transurothelial access of urine to detrusor nerve and muscle cells with distension. Eur Urol. 1987;13:83–89. [DOI] [PubMed] [Google Scholar]
- 8.Eaton DC. Intracellular sodium ion activity and sodium transport in rabbit urinary bladder. J Physiol. 1981;316:527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis SA, Diamond JM Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol. 1976;28:1–40. [DOI] [PubMed] [Google Scholar]
- 10.Shafik A, Shafik I, El Sibai O, Shafik AA Changes in the urine composition during its passage through the ureter. A concept of urothelial function. Urol Res. 2005;33:426–428. [DOI] [PubMed] [Google Scholar]
- 11.Rubenwolf PC, Georgopoulos NT, Clements LA, Feather S, Holland P. Expression and localisation of aquaporin water channels in human urothelium in situ and in vitro. Eur Urol. 2009;56:1013–1023. [DOI] [PubMed] [Google Scholar]
- 12.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. [DOI] [PubMed] [Google Scholar]
- 13.Morizawa Y, Torimoto K, Hori S, Gotoh D, Nakai Y, Miyake M, Hirayama A, Tanaka N, Fujimoto K. Aquaporin-2 plays an important role in water transportation through the bladder wall in rats. Neurourol Urodyn. 2018;DOI: 10.1002/nau.23715. [DOI] [PubMed]
- 14.Smith PR, Mackler SA, Weiser PC, Brooker DR, Ahn YJ, Harte BJ, McNulty KA, Kleyman TR Expression and localization of epithelium sodium channel in mammalian urinary bladder. Am J Physiol Renal Physiol. 1998;274:F91–96. [DOI] [PubMed] [Google Scholar]
- 15.Brown D. The discovery of water channels (Aquaporins). Ann Nutr Metab. 2017;70:37–42. [DOI] [PubMed] [Google Scholar]
- 16.Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA Heterogeneity of muscarinic receptor-mediated calcium responses in cultured urothelial cells from rat. Am J Physiol 2008;294:F971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith A, Wein A. Current pharmacotherapy of nocturia. Expert Opin Pharmacother. 2013;14:885–894. [DOI] [PubMed] [Google Scholar]
- 18.Weiss JP. Nocturia: focus on etiology and consequences. Rev in Urol. 2012;14:48–55. [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalik CG, Cohn JA, Delpe S, Reynolds WS, Kaufman MR, Milam DF, Wein AJ, Dmochowski RR Nocturia: evaluation and current management strategies. Rev Urol. 2018;20:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminetsky J, Fein S, Dmochowski R, MacDiarmid S, Abrams S, Cheng M, Wein A. Efficacy and safety of SER120 nasal spray in patients with nocturia: pooled analysis of 2 randomized, double-blind, placebo controlled, phase 3 trials. J Urol. 2018;doi: 10.1016/j.uro.2018.04.050. [DOI] [PubMed]
- 21.Cohn JA, Kowalik CG, Reynolds WS, Kaufman MR, Milam DF, Dmochowski RR, Wein AJ Desmopressin acetate nasal spray for adults with nocturia. Expert Rev Clin Pharmacol. 2017;10:1281–1293. [DOI] [PubMed] [Google Scholar]
- 22.Filippatos TD, Makri A, Elisaf MS, Liamis G. Hyponatremia in the elderly: challenges and solutions. Clin Interv Aging. 12. 2017(1957–65). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch CA, Fulop T. Clinical aspects of changes in water and sodium homeostasis in the elderly. Rev Endocr Metabol Disor. 2017;18:49–66. [DOI] [PubMed] [Google Scholar]
- 24.Mannesse CK, Vondeling AM, van Marum RJ, van Solinge WW, Egberts TC, Jansen PA prevalence of hyponatremia on geriatric wards compared to other settings over four decades: a systemic review. Ageing Res Rev. 12. 2013(165–73). [DOI] [PubMed] [Google Scholar]
- 25.Arinzon Z, Feldman J, Peisakh A, Zuta A, Berner Y. Water and sodium disturbances predict prognosis of acute disease in long term care frail elderly. Arch Geront Geriatr. 2005;40:317–326. [DOI] [PubMed] [Google Scholar]
- 26.Kuo SCH, Kuo PJ, Rau CS, Wu SC, Hsu SY, Hsieh CH Hyponatremia is associated with worse outcomes from fall injuries in the elderly. Int J Environ Res Public Health. 2017;doi: 10.3390/lijerph14050460. [DOI] [PMC free article] [PubMed]
- 27.Hosseini SR, Baghitabar N, Mirzapour A, Oliaei F, Nooreddini H, Bijani A, Mouodi S. Hyponatremia,bone mineral density and falls in the elderly: results from AHAP study. Rom J Intern Med. 2018;56:41–46. [DOI] [PubMed] [Google Scholar]