Abstract
The fnbA and fnbB genes of Staphylococcus aureus 8325-4 encode fibronectin (Fn) binding proteins FnBPA and FnBPB, which promote adherence to host tissues. Each adhesin contains three copies of a repeated D motif that binds Fn and is a target for vaccine development. In this study, we assess variability within the Fn-binding domain of the FnBP adhesins and evaluate factors that promote variance in Fn binding among clinical isolates. Based on variation in the number of fnb genes or the number of D motifs, we identified five polymorphism groups. S. aureus 8325-4 and 91% of methicillin-resistant S. aureus (MRSA) isolates belong to polymorphism group I, with two fnb genes and three copies of the D motif. Polymorphism group II contained one fnb gene with only two D motifs and was associated with the epidemic CMRSA-4 strain, which exhibited high protease activity and low Fn binding. Polymorphism group III was unique to the epidemic CMRSA-1 strain, defined by the presence of a fourth D motif that exhibited antigenic variation within a conserved sequence that is essential for Fn binding. However, the sequence of the D motifs was otherwise highly conserved among the other polymorphism groups. Variation in Fn binding among MRSA isolates was inversely related to protease activity but not to the number of fnb genes or the number of D motifs. Therefore, the fnb locus is polymorphic in a small number of strains, but this does not contribute to variation in Fn binding. The antigenic variation that was observed only in the epidemic CMRSA-1 strain may have evolved in response to a host immune response encountered during successive cycles of colonization, transmission, and infection in the nosocomial environment.
Staphylococcus aureus is the overall leading cause of nosocomial infections (21) and is known for its ability to colonize multiple organs and tissues (38). Following successful colonization, it can quickly invade into deeper tissues, including bones and joints. The invasive process is facilitated by an accessory gene regulator locus, agr, which is activated at high cell density through a quorum-sensing mechanism, resulting in transcription of a regulatory RNA molecule named RNAIII (17). Commensurate with the induction of RNAIII transcription, expression of secreted toxins and tissue-degrading enzymes is induced, while expression of cell surface adhesion proteins is repressed (30). Adhesion to host tissue is the first critical step in the initiation of an infection. Therefore, adhesion proteins represent targets for vaccine development, and in recent years there has been increasing focus on understanding the function and mode of action of these proteins.
The fibronectin binding protein (FnBP) adhesins of S. aureus are members of the MSCRAMM family of microbial proteins, which promote adhesion to tissue extracellular matrix (32, 33). FnBP also promotes attachment to indwelling medical devices, keratinocytes, endothelial cells, and traumatized tissues and internalization by different cell types (8, 19, 26, 32, 35, 46). Although the ligand-binding domain of the FnBP adhesin can be employed to produce adhesion-blocking antibodies (3, 14), some studies have also shown that FnBP antigens may promote the formation of antibodies that stimulate Fn binding by stabilizing the ligand binding complex (4, 9, 13, 43). In contrast, Fn-binding adhesins of Streptococcus pyogenes elicit an immune response that confers protection from lethal challenge (12, 37). Therefore, Fn-binding adhesins are potential vaccine components, provided that the immune response can be directed towards the correct epitopes (3, 14, 44). However, another potential limitation to the use of FnBP as a vaccine component is the wide range of Fn binding that we have noted among clinical isolates of methicillin-resistant S. aureus (MRSA) (31), and factors that promote this heterogeneity have not been defined.
S. aureus 8325-4 possesses two tandem and homologous genes, fnbA and fnbB, which encode Fn-binding proteins FnBPA and FnBPB, respectively (39). While some strains of S. aureus possesses a single fnb gene (11, 34), these strains have not been characterized in any detail and their clinical significance is unknown. Each FnBP adhesin possesses three copies of a 37- or 38-amino-acid D motif, which individually bind Fn with low affinity and in tandem comprise a high-affinity Fn-binding domain designated D1-3 (15, 39). Although Fn-binding proteins of S. pyogenes may possess between two and seven copies of an Fn-binding motif (27), it is not known if such variability exists among the FnBP adhesins of S. aureus or if the sequence of the Fn-binding D motifs is conserved among genetically diverse clinical isolates. Our previous work also indicated that the FnBP adhesins are degraded by a secreted protease (25), suggesting an inverse relationship between protease and Fn binding, and this relationship would be influenced by the activity of the agr locus.
Herein, we present an analysis of these considerations among a diverse collection of MRSA and methicillin-susceptible S. aureus (MSSA) clinical isolates. Fn binding of MRSA isolates was inversely related to protease activity but did not vary in relation to the number of fnb genes or the number of Fn binding D motifs. Variation in the number of fnb genes or in the number of D motifs was unusual but was observed in two strains of epidemic MRSA. Epidemic strain CMRSA-4 possessed one fnb gene containing only two D motifs, while CMRSA-1 contained an additional D motif that exhibited antigenic variation within a sequence that is critical to Fn binding.
Nucleotide sequence accession number.
The nucleotide sequence of the variant D-motif domain of CMRSA-1 has been deposited GenBank (accession number AY029184).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus RN6390 (30) was obtained from R. Novick (Skirball Institute, New York, N.Y.), while S. aureus 879R4S (19), which possesses a single fnb gene (11), was obtained from Richard Proctor (University of Wisconsin). Clinical MRSA and MSSA isolates used in this study are listed in Table 1. MRSA (n = 40) and MSSA (n = 2) isolates were selected from a collection of isolates maintained by the Canadian Nosocomial Infection Surveillance Program, involving 23 sentinel hospitals across Canada and collected between 1995 and 1998 (41). All strains had been typed by pulsed-field gel electrophoresis (PFGE) of SmaI-digested genomic DNA, and determination of antibiotic resistance profiles was in accordance with National Committee for Clinical Laboratory Standards guidelines (28). Isolates were selected on the basis of PFGE profiles, including several isolates of the epidemic CMRSA-1, CMRSA-2, CMRSA-3, and CMRSA-4 strains which have been described previously (31, 40). MSSA clinical isolates (n = 14) and additional MRSA isolates (n = 4) were obtained from the University of Manitoba Health Sciences Clinical Microbiology Laboratory. Frozen stock cultures were streaked on brain heart infusion (BHI) agar (Difco, Detroit, Mich.) before selection of single colonies for growth in either BHI broth or protease culture medium (7). Escherichia coli DH5α was the host for recombinant plasmids and protein expression and was maintained in Luria-Bertani medium containing 15 g of agar liter−1 when necessary and 50 μg of ampicillin ml−1 when required for plasmid maintenance. All cultures were grown at 37°C.
TABLE 1.
Identities and descriptions of S. aureus isolates
| Strain no. | Geographic distributiona | PFGE identity |
|---|---|---|
| 21, 22, 34, 42, 46, 47, 72, 110, 170, 173, 174, 178, 182, 228, 287a, 300, 307, 329a, 364, 416 | ON, QUE, NS, AB | CNISP sporadic MRSA; 16 PFGE profiles |
| 171, 442 | ON, MB | CNISP MSSA; 2 PFGE profiles |
| 55, 64, 167, 355, 399, 433 | ON, MB | CMRSA-1 |
| 8, 56, 79, 98, 105, 111, 117, 148 | ON, QUE, MB | CMRSA-2 |
| 12, 24, 95 | ON, MB | CMRSA-3 |
| 81, 166, 349 | ON, MB | CMRSA-4 |
| 287b, 329b, 361, 1843 | MB | Sporadic MRSA |
| L48, L170, L218, L300, L397, L516, L528, L530, L600, L702, L768, L786, L800, L857 | MB | Clinical MSSA; PFGE not determined |
Location was classified by province in Canada: ON, Ontario; QUE, Quebec; NS, Nova Scotia; AB, Alberta; MB, Manitoba.
PCR and sequence analysis.
Primers D1 (5′-GGTTATGAAGGTGGCCAAAATAGC-3′) and D3 (5′-GCCGCTTACTTTTGGAAGTGTATC-3′) anneal to nucleotides 2338 to 2358 and 2697 to 2677, respectively, of the fnbA gene of S. aureus 8325-4 (39) and amplify a sequence that encodes the tandem D1, D2, and D3 motifs. Primers I1 (5′-AAGGTTAAAGCAGTGGCACC-3′) and I2 (5′-GCAGCTTCTTTTTCTTGTCCC-3′) anneal to nucleotides 3010 to 3029 of fnbA and nucleotides 107 to 188 of fnbB, respectively, flanking the intergenic region between the tandem fnb genes. Primers D1-F2 (5′-cccggatccGAAGGTGGCCAAAATActGGc-3′) and D4-R (5′-TACTTTTGGAAGTGTATCTTCTaCtttGTCAAtgCc-3′) are specific for the group III D motif polymorphism. The underlined sequences in D1-F2 and D4-R are identical to nucleotides 7 to 22 and 7 to 24 of primers D1 and D3, respectively. The 5′ lowercase nucleotides in D1-F2 incorporate an in-frame BamHI site for cloning in the pGEX2T vector (42), while the 3′ lowercase nucleotides in both D1-F2 and D4-R anneal to nucleotides that are specific to the group III polymorphism. Primers Du-F (5′-CGCTGATGTTGTTGAATATGAAGAAGATAC-3′) and Du-R (5′-TGTTGTATGATCGCTCACTG-3′) anneal to nucleotides 1986 to 2015 and 2118 to 2099 of fnbA from S. aureus 8325-4, respectively, and span a sequence representing an upstream Fn-binding domain that is N terminal to the D-motif domain (18). Primers for amplification of RNAIII have been described previously (31).
Template DNA for PCR was extracted from single colonies of S. aureus cultures grown on BHI agar plates as described previously (31), and 2.5 μl of this preparation or 1.24 ng of purified genomic DNA where indicated was used in PCR. The PCR mixtures contained 1.5 to 2.0 mM MgCl2, 37.5 pM concentrations of each forward and reverse primer, 0.2 mM deoxynucleoside triphosphate mix, 1.25 U of AmpliTaq DNA polymerase (Roche Diagnostics, Laval, Quebec, Canada), 2.5 μl of 10× PCR buffer as supplied with the polymerase, and sterile double-distilled H2O to bring the volume to 25 μl. The cycling conditions consisted of 30 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 2 min, and extension at 72°C for 1 min. After completion of PCR, aliquots were analyzed by agarose gel electrophoresis, and where indicated, PCR products were gel purified using the GeneClean II kit (BIO 101, La Jolla, Calif.) and submitted to the University of Toronto Biotechnology lab for sequencing.
Southern and Northern hybridization.
Probes for Southern and Northern blot analyses were generated by PCR and labeled with the ECL-direct nucleic acid labeling system (Amersham Pharmacia, Piscataway, N.J.). Genomic DNA was purified using the Qiagen genomic-tip 100/G (Qiagen Inc., Valencia, Calif.), following the protocol as recommended for gram-positive bacteria. Total RNA was prepared from stationary (18-h)-phase cultures of isolates grown in protease medium, using TriZol reagent (Gibco/BRL) and the FastPrep FP120 instrument (BIO 101) as described previously (31). The concentration and purity of each sample were determined by measuring the absorbance at 260 and 280 nm. RNA samples (10 μg) were separated by electrophoresis through a 1.0% (wt/vol) agarose gel containing 0.66 M formaldehyde, in morpholinepropanesulfonic (MOPS) acid running buffer (20 mM MOPS, 10 mM sodium acetate, 2 mM EDTA, pH 7.0). After electrophoresis, RNA was transferred to Hybond N+ membrane in 10× SSC buffer (0.15 M sodium citrate, 1.5 M NaCl, pH 7.0) by capillary transfer and baked at 80°C for 2 h (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). For Southern blotting, 1.0 μg of genomic DNA was digested with restriction enzyme DraI or HaeIII (Gibco), and the fragments were separated by electrophoresis in 0.8% (wt/vol) agarose. DNA was transferred to Hybond N+ membranes (Roche) by using the alkaline transfer method. For both Northern and Southern blottings, processing of the blotted membranes, including blocking, hybridization, and detection with chemiluminescent substrate, was performed using the protocols and reagents provided with the ECL-direct reagent system.
Assay of Fn binding and protease activity.
For Fn-binding assays, cultures were grown in BHI broth and harvested at 2 h. Preparation of heat-killed S. aureus cell suspensions standardized to a cell density of 1010 ml−1 was conducted as described previously (44). As described previously (31), each binding assay contained 50,000 cpm of 125I-Fn, 5 × 107 S. aureus cells, and 4.5 × 108 Staphylococcus simulans cells, which bind negligible levels of 125I-Fn as an inert carrier to assist in centrifugation. Results are expressed as the percentage of the total added Fn retained by the cell pellet. Each assay was conducted in duplicate, and all assays were performed within 24 h of the labeling reaction to ensure consistent results.
For determination of the relationship between Fn binding and protease activity, cultures were grown in protease medium for 18 h, starting from an inoculum density of 0.1 at 600 nm. Cells were harvested by centrifugation, and the cell-free supernatant was stored at −70°C until required for assay of protease activity. The cell pellets were washed, heat killed, and frozen at a cell density of 1010 ml−1 for assay of Fn binding. Fn binding assays were performed as described above on 5 × 108 CFU of heat-killed S. aureus cells ml−1. Total protease activity in culture supernatants was quantified with resorufin-labeled casein (Roche), as described by us previously (31).
Production and labeling of proteins and antibodies.
Glutathione S-transferase (GST) fusion proteins GSTD3 and GSTD1-3 possess the D3 motif and the tandem D1, D2, and D3 motifs (D1-3), respectively, of FnBPA from S. aureus 8325-4, as described previously (14, 15). To construct fusion protein GSTD1-4, PCR was conducted with D1-F2 and D4-R, which are specific for the group III fnb locus polymorphism. The 460-bp amplicon was cloned in the pCR2.1 vector (Invitrogen, Carlsbad, Calif.) and transformed into E. coli INVα cells by following the manufacturer's protocols. The insert was excised from pCR2.1 with BamHI and EcoRI and then was ligated to pGEX-2T and electroporated into E. coli DH5α cells. The resulting plasmid, pGEXD1-4, directed the expression in E. coli of the fusion protein GSTD1-4, which was purified by affinity chromatography on glutathione agarose (Sigma) as described previously for GSTD1-3 (15). A similar protocol was employed using primers D4-F (5′-gggggatccGGATTCAATAAGCACACTG-3′) and D4-R2 (5′-cccgaattcGCTTACTTTTGGAAGTG-3′) for the construction of GSTD4, which expresses a variant D3-like motif that is unique to the group III fnb locus polymorphism. Polyclonal antiserum and monoclonal antibody 9C3 (MAb 9C3) were both obtained by immunizing with synthetic peptide D320-33 spanning amino acids 20 to 33 of the D3 motif from FnBPA of S. aureus 8325-4, as previously described (14, 44). Human plasma Fn was purchased from Gibco/BRL and labeled with 125I by using the chloramine-T protocol (16) for quantification of Fn binding to S. aureus cell suspensions. For Western ligand blots and enzyme-linked immunosorbent assays (ELISAs), Fn was labeled with biotinamidocaproate N-hydroxysuccinimide ester (Sigma, St. Louis, Mo.) as described elsewhere (25).
ELISA protocols.
The affinity of biotinylated Fn or polyclonal and monoclonal antibody towards the recombinant GSTD1-3 and GSTD1-4 fusion proteins was measured by ELISA. Wells of a 96-well microtiter plate (Corning, Corning, N.Y.) were coated overnight at 4°C with 100 ng of either GST, GSTD1-3, or GSTD1-4 in carbonate-bicarbonate buffer. Wells were then blocked with phosphate-buffered saline (PBS) containing 3% (wt/vol) bovine serum albumin for 1 h and washed in PBS containing 0.05% (vol/vol) Tween 20. This was followed by incubation for 1 h at room temperature with increasing concentrations of either biotinylated Fn, polyclonal anti-D320-33 rabbit immunoglobulin G (IgG), or monoclonal anti-D320-33 mouse IgG (MAb 9C3), diluted in PBS containing 0.1% (wt/vol) bovine serum albumin and 0.05% (vol/vol) Tween 20. After extensive washing, wells were incubated with a 5,000-fold dilution of either alkaline phosphatase (AP)-conjugated streptavidin (Roche), AP-conjugated goat anti-rabbit IgG, or AP-conjugated goat anti-mouse IgG (Jackson Immunoresearch, West Grove, Pa.). After a 60-min incubation, the plates were washed extensively as described above and developed with 1 mg of para-nitrophenyl phosphatase substrate (Sigma) per ml in 0.1 M diethanolamine buffer (pH 9.8). After a 60-min incubation, the plates were quantified using a Bio-Rad model 3550 MicroPlate reader equipped with a 405-nm-pore-size filter.
RESULTS
Identification of fnb locus polymorphisms
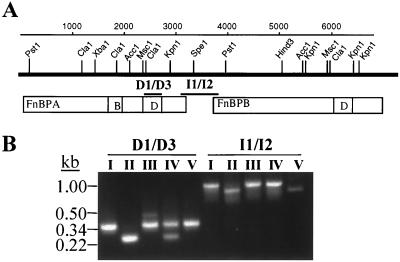
Within the fnb locus of S. aureus 8325-4, primer pair D1-D3 flanks the Fn binding D motifs, while primers I1/I2 flank the intergenic region between the two fnb genes (Fig. 1). PCR with these primer pairs divided the MRSA and MSSA isolates into five polymorphism groups (Fig. 1; Table 2). Both PCR amplicons of group I isolates (lane I) were identical in size to that predicted from the fnb locus of S. aureus 8325-4, while group II amplicons (lane II) were smaller than expected with both primer pairs. Three of the four group II MRSA isolates exhibited PFGE profiles corresponding to that of the CMRSA-4 strain, which is endemic in a number of Toronto hospitals but is also found in other geographic locations (40). The remaining isolates exhibited the predicted I1/I2 amplicon but displayed two amplicons of either 360 and 473 bp (group III; lane III) or 360 and 260 bp (group IV) with the D1 and D3 primers. The PFGE profiles of group III isolates all correspond to that of the epidemic CMRSA-1 strain that has been described previously (31, 40). S. aureus 879R4S, which possesses one fnb gene (11), displayed a unique polymorphism consisting of the expected size of amplicon with the D1 and D3 primers and a smaller I1/I2 amplicon similar in size to the group II isolates. As the I1 and I2 primers should yield an amplicon only in strains possessing two fnb genes, Southern hybridization was conducted to discriminate between isolates that possess one or two fnb genes (Fig. 2).
FIG. 1.
A representation of the fnb locus of S. aureus 8325-4 (A) and PCR of the fnb locus using primer pairs D1-D3 and I1-I2 (B). The scale of the fnb locus (in base pairs) is indicated by the top numbered line. The positions of restriction endonuclease restriction sites are shown above the thick black line, underneath which appear the location and diagram of the tandem FnBPA and FnBPB proteins. Region B of FnBPA represents a 30-amino-acid repeated motif unique to FnBPA, while region D in each adhesin represents the Fn-binding D repeat domain. The regions amplified by PCR are indicated by the labeled bars, D1-D3 and I1-I2. (B) Lane numbers correspond to each of polymorphism groups I to V.
TABLE 2.
Distribution of fnb locus polymorphisms among MRSA and MSSA isolates
| Polymorphism group | No. of MRSA isolates (n = 44) | No. of MSSA isolates (n = 18a) |
|---|---|---|
| I | 34 | 12 |
| II | 4 | 3 |
| III | 6 | 0 |
| IV | 0 | 2 |
| V | 0 | 1 |
MSSA isolates were comprised of 16 clinical isolates, as well as S. aureus strains RN4220 (group I) and 879R4S (group V).
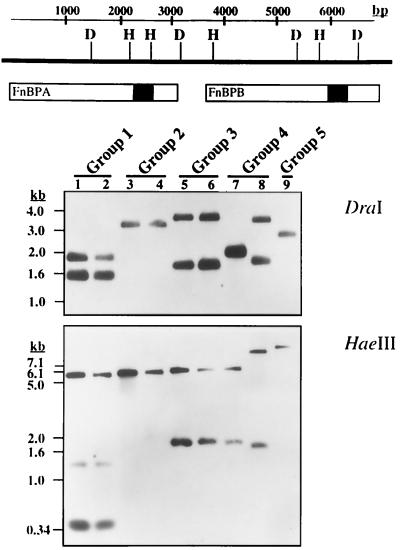
FIG. 2.
Southern blot hybridization of DraI- or HaeIII-digested genomic DNA (1.0 μg) from two different isolates of polymorphism groups I to IV and from S. aureus 879R4S, representing the single group V polymorphism. Lanes are numbered according the specific polymorphism group. The cut sites of DraI (D) and HaeIII (H) are indicated in the fnb locus diagram above the blots, and the regions hybridizing with the D1/D3 probe are shaded in black.
Based on the nucleotide sequence of the fnb locus of S. aureus 8325-4, digestion of genomic DNA with DraI should yield 1.8- and 1.4-kb fragments that hybridize with the D1 and D3 PCR amplicon. Two isolates from polymorphism group I exhibited this expected hybridization pattern (Fig. 2), indicating an fnb locus structure identical to S. aureus 8325-4. Group II isolates and S. aureus 879R4S exhibited single DraI fragments, consistent with a single fnb gene. Group III isolates exhibited fragments of 3.3 and 1.7 kb, indicative of two fnb genes. The two group IV isolates displayed two patterns, consisting of a single 1.9-kb fragment (lane 7) or two fragments of 3.2 and 1.8 kb (lane 8). However, when hybridization was conducted with HaeIII-digested DNA (Fig. 2), both group IV isolates exhibited two fragments, although their hybridization profiles were not identical. Group I and III isolates also exhibited HaeIII profiles indicative of two fnb genes, while group II and 879R4S again exhibited single fragments. Based on these results and the data in Table 2, 91% of MRSA and 78% of MSSA clinical isolates possessed fnbA and fnbB. When the variant I1/I2 amplicon of a group II isolate was sequenced, there was no significant homology to the fnb locus (data not shown). Therefore, the observed amplicon is a PCR artifact but was still characteristic of strains that possess one fnb gene.
Sequence analysis of variant D motif amplicons.
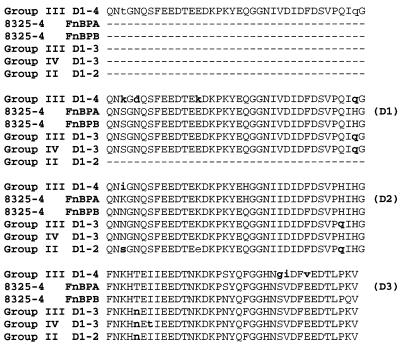
The D motif PCR amplicons from the different polymorphism groups correspond in size to either two (260 bp; D1-2), three (360 bp; D1-3), or four (473 bp; D1-4) copies of the D motif in the FnBP adhesins, and this was confirmed through sequence analysis (Fig. 3). In the FnBP adhesins of S. aureus 8325-4, the D1 and D2 motifs are nearly identical to one another while D3 is divergent, especially in the C-terminal 20 amino acids that confer Fn binding (39). This arrangement was maintained in each of the different polymorphism groups, where the terminal D motif encoded by each amplicon aligned to the D3 motif of the FnBP adhesins from S. aureus 8325-4, regardless of whether there were two, three, or four copies of the D motif. A common amino acid substitution was the presence of either histidine or glutamine in the center of the D2-like motif and in the C-terminal residues of the D1- and D2-like motifs in each of the polymorphism groups. However, the most significant changes occurred in the terminal D3-like motif of the 473-bp amplicon that defined the group III polymorphism. This motif was identical to the D3 motif of FnBPA from S. aureus 8325-4, with the exception of three amino acid changes near the C terminus that altered the sequence SVDFEED to GIDFVED. These changes were not present in the D3 motif encoded by the 360-bp amplicon from the same group III isolate. Therefore, group III isolates possess two FnBP adhesins, in which one contains a D motif structure similar to that of group I, while the second possesses an additional D1- or D2-like motif and a variant D3 motif. The D motif domain of S. aureus 879R4S and the 260-bp amplicon of the group IV polymorphism were not sequenced.
FIG. 3.
Amino acid sequences derived from the nucleotide sequences of PCR amplicons obtained with the D1-D3 primers in polymorphism groups II, III, and IV. The amino acid sequences are aligned to the corresponding sequences of the D1, D2, and D3 motifs (D1-3) of FnBPA and FnBPB from S. aureus 8325-4. Amino acids that differ from either the FnBPA or FnBPB proteins of S. aureus 8325-4 are designated in bold lowercase letters. The identities of the D1, D2, and D3 motifs of the FnBP adhesins from S. aureus 8325-4 are indicated in parentheses on the right.
Group III polymorphism is present in the epidemic CMRSA-1 strain of MRSA and represents functional antigenic variation.
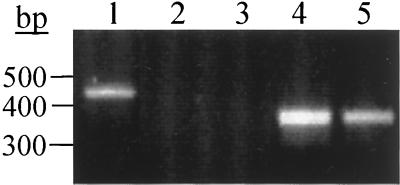
The six MRSA isolates comprising polymorphism group III exhibited PFGE profiles corresponding to that of the epidemic CMRSA-1 strain that we previously described (31, 40). To determine if this was a characteristic feature of CMRSA-1, we designed reverse PCR primer D4-R such that the 3′ end of the primer anneals to the variant nucleotides encoding the unique GIDFVED sequence. When combined with D1-F2, this primer pair yielded a single 460-bp amplicon in PCR with genomic DNA of a group III isolate (Fig. 4, lane 1) but did not produce an amplicon with genomic DNA from a polymorphism group I MRSA (lane 2) or MSSA (lane 3) isolate. In contrast, PCR with the D1 and D3 primer pair yielded a 360-bp amplicon with both the group III (lane 4) and group I (lane 5) MRSA isolates. Therefore, the D1-4R primer is specific for the group III polymorphism, in which one FnBP protein contains three copies of the D motif, while the other FnBP contains four D motifs. Although the CMRSA-1 strain exhibits several closely related subtypes, PCR with the D1-4R primer yielded the same 460-bp amplicon with five different subtypes (data not shown). Therefore, this unique polymorphism is a characteristic feature of CMRSA-1.
FIG. 4.
PCR of genomic DNA from a group III MRSA isolate (lanes 1 and 4), a group I MRSA strain (lanes 2 and 5), and a group I MSSA strain (lane 3) with primer pair D1-F2 and D4-R (lanes 1 to 3) or D1 and D3 (lanes 4 and 5). Size standards are indicated on the left.
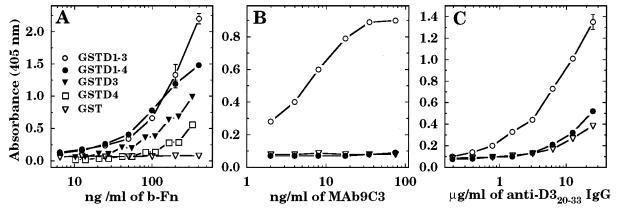
In a previous study, we showed that MAb 9C3 was specific for the epitope SVDFEED in the D3 motif of S. aureus FnBPA (14). This epitope contains conserved acidic and hydrophobic amino acids that are essential for Fn binding (23, 24), and MAb 9C3 abolished Fn binding by the D3 motif (14). In the four group III polymorphisms, this sequence is altered to GIDFVED in the D3-like motif, which is otherwise identical to D3 of FnBPA from S. aureus 8325-4. To determine the significance of these altered amino acids, the 460-bp amplicon from CMRSA-1 was cloned and expressed as a glutathione S-transferase fusion protein, GSTD1-4. This fusion protein showed binding of biotinylated Fn that was comparable to that of GSTD1-3 derived from S. aureus 8325-4 (Fig. 5A). However, fusion protein GSTD4 containing just the variant D motif of CMRSA-1 exhibited an approximate twofold reduction in Fn binding compared to GSTD3, which contains the D3 motif of FnBPA from S. aureus 8325-4 (Fig. 5A). Furthermore, MAb 9C3 recognized GSTD1-3, but not GSTD1-4 derived from CMRSA-1 (Fig. 5B). Polyclonal antibody specific for a synthetic peptide spanning amino acids 20 to 33 of the D3 motif of FnBPA from S. aureus 8325-4 also did not show appreciable recognition of GSTD1-4 (Fig. 5C). Therefore, the amino acid substitutions within the D3-like motif of the type III polymorphism group promote antigenic variation within a sequence that is critical to Fn binding but result in a reduced ability to bind Fn. However, within the context of the complete D1-to-D4 domain, the effect on Fn binding is less evident.
FIG. 5.
Fn-binding activity of GSTD1-4 and GSTD4 of CMRSA-1 compared to GSTD1-3 and GSTD3 from FnBPA of S. aureus 8325-4 (A) and recognition of GSTD1-4 by MAb 9C3 (B) or polyclonal antibody (C) specific for amino acids 20 to 33 of the D3 motif of S. aureus 8325-4. Wells of microtiter plates coated with 0.1 μg of fusion protein were incubated with various concentrations of biotinylated Fn (A), MAb 9C3 (B), or polyclonal antibody (C), which was obtained by immunizing with a synthetic peptide spanning amino acids 20 to 33 of the D3 motif. Each point represents the average (± standard deviation) of triplicate wells.
Sequence analysis of the Du upstream binding domain.
A recent study has demonstrated that the FnBPA adhesin of S. aureus possesses additional Fn-binding domains, including a partial D4 repeat, and an upstream nonrepetitive domain, designated Du, that is N terminal of the D-motif domain (18). To assess the potential for sequence variation in the Du region, PCR was employed to amplify this segment from three isolates representative of polymorphism groups II, III, and IV. Sequencing of the PCR amplicons after cloning in the pCR2.1 vector revealed both sequences to be identical to that of FnBPA from S. aureus 8325-4 (data not shown), which is the archetypal group I isolate.
Analysis of S. aureus isolates for binding of 125I-Fn.
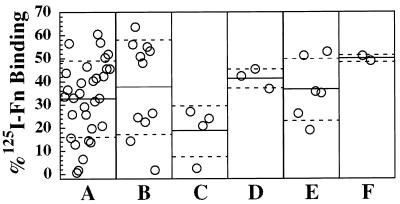
Forty-one MRSA and 16 MSSA isolates representing the four fnb locus polymorphisms were assayed for binding of 125I-Fn (Fig. 6). Collectively, these isolates were harvested from exponential-phase cultures binding (34.1% ± 16.6%) added Fn, ranging from a high of 60% to a low of <1% mean Fn binding. Group II MRSA isolates possessing only one fnb gene displayed the lowest mean Fn binding (n = 4; 18.5% ± 11.0%). However, this difference was not significant compared to all other MRSA isolates possessing two fnb genes (analysis of variance; P > 0.05). Furthermore, among MRSA isolates possessing two fnb genes, there was a wide variation in Fn binding, ranging from 0.5 to 65%. Therefore, factors other than the number of fnb genes or the number of Fn-binding D motifs promote variability in Fn binding.
FIG. 6.
Binding of 125I-Fn by MRSA and MSSA isolates from PCR polymorphism groups I to IV. Each point on the graph represents the Fn binding of a single isolate, expressed as the percentage of the total added 125I-Fn that was bound by the bacterial cell suspension. (A) Group I MRSA (32.5% ± 16.3%); (B) group I MSSA (37.7% ± 20.5%); (C) group II MRSA (18.5% ± 11.0%); (D) group II MSSA (41.4% ± 4.3%); (E) group III MRSA (36.6% ± 13.4%); and (F) group IV MSSA (48.7% ± 1.6%). The solid line on each panel of the graph represents the mean Fn binding for each group, and the broken lines represent the standard deviation.
Correlation between Fn binding, protease activity, and transcription of agr.
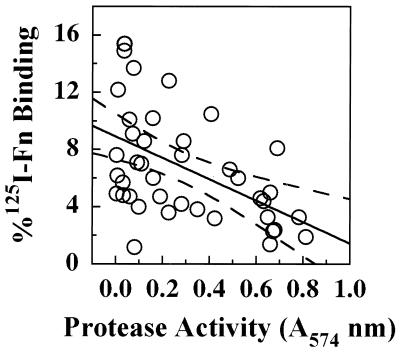
We previously showed that a clinical MSSA isolate with very low levels of Fn binding secreted high levels of a serine protease (V8 protease), and the purified protease promoted the loss of cell surface FnBP in exponential-phase cultures (25). To evaluate this relationship among MRSA isolates, the amount of Fn bound by cells from stationary-phase cultures was plotted as a function of protease activity in the culture supernatant (Fig. 7). Regression analysis revealed a significant negative linear relationship (P < 0.05) between Fn binding and protease activity. Of particular interest, four isolates comprising the group II polymorphism exhibited a tight cluster, with high protease activity and low Fn binding. As expression of protease activity is induced by the agr locus, while expression of fnb is repressed (36), Northern blottings were performed to assess transcription of agr among isolates that spanned a range of Fn binding and protease activities (Fig. 8). Three isolates with high Fn binding and low protease activity showed reduced transcription of RNAIII relative to that of S. aureus RN6390 (lanes 2 to 4), while three isolates with intermediate activity showed similar levels of RNAIII transcript (lanes 5 to 7). Surprisingly, three of four isolates exhibiting high protease activity and low Fn binding (lanes 8 to 11) showed less RNAIII transcript relative to RN6390, which itself produces high levels of protease (31). Two of these isolates (lanes 8 and 10) represent epidemic CMRSA-4, which possesses the type II polymorphism. Additional analysis established that this trait was also present in each of the group II MRSA isolates (data not shown). Therefore, this appears to be a characteristic feature of the group II polymorphism.
FIG. 7.
Protease activity and Fn binding of stationary-phase MRSA isolates from PCR polymorphism groups I, II, and III. Binding of 125I-Fn was plotted as a function of total protease activity (A405). Each point on the graph represents a single isolate. The solid line represents a significant linear regression line (P < 0.05), with 95% confidence intervals appearing as dashed lines.
FIG. 8.
Northern blotting for detection of the RNAIII transcript in MRSA isolates that differ in Fn binding and protease activity. Isolates shown in lanes 2 to 11 span a range of activities: high Fn binding and low protease activity (lanes 2 to 4), intermediate Fn binding and protease activity (lanes 5 to 7), and high protease and low Fn binding (lanes 8 to 11). The individual strains (and their polymorphism group designations) are as follows: lane 1, S. aureus RN6390, reference strain; lane 2, MRSA 98 (I); lane 3, MRSA 42 (I); lane 4, MRSA 21 (I); lane 5, MRSA 228 (I); lane 6, MRSA 55 (III); lane 7, MRSA 399 (III); lane 8, MRSA 166 (II); lane 9, MRSA 95 (I); lane 10, MRSA 349 (II); lane 11, MRSA 174 (I).
DISCUSSION
In agreement with a recent study where 77% of clinical S. aureus isolates in Europe were found to contain two fnb genes (34), we found that 91% of MRSA and 78% of MSSA isolates possess both fnbA and fnbB. These findings differ from a recent report in which six genetic lineages of S. aureus were found to account for approximately 66% of all clinical isolates, among which the presence of both fnbA and fnbB was exclusively associated with one lineage, designated Sal-2 (1). Furthermore, the most prevalent lineage, designated Sal-4, was comprised of MRSA isolates that possessed fnbA only. Although our own work suggests that MRSA isolates with just one fnb gene are unusual, this trait is associated with the epidemic CMRSA-4 strain that has recently emerged in Canadian hospitals (40). This strain has been identified primarily in a small number of Toronto hospitals but has also appeared in health care centers in Alberta, Quebec, Nova Scotia, and Newfoundland, Canada. Potentially, CMRSA-4 and Sal-4 are related to one another, and the latter strain could be more geographically dispersed in the United States.
Our present work has provided the first detailed genotypic and phenotypic characterization of strains that possess a single fnb gene. We find that group II MRSA and MSSA isolates with one fnb gene share another genotypic trait, defined by the presence of only two D motifs comprising the Fn-binding domain of the FnBP adhesin. Potentially, these isolates represent a progenitor strain from which isolates with two tandem fnb genes originated due to a gene duplication event. Gene duplication is thought to be the driving force behind the evolution of the emm locus of Streptococcus pyogenes. Some strains possess an emm gene encoding M protein, followed by a single gene encoding an emm-like protein, while other genotypes may possess several tandem genes encoding emm-like proteins (2). These emm-like proteins exhibit high homology towards M protein but differ in their N-terminal regions and ligand binding specificity, and many are able to bind more than one ligand. Recently, the N-terminal region of the S. aureus FnBPA adhesin has also been shown to bind fibrinogen (47). Therefore, it is possible that the fnb locus of S. aureus will evolve towards multiple ligand-binding specificity through recombination between the tandem fnb genes, as is believed to have occurred in the emm locus of S. pyogenes.
MRSA isolates that possessed the group II polymorphism also exhibited high protease activity, low Fn binding, and reduced transcription of RNAIII. This clonal behavior is consistent with the finding that three of the four MRSA isolates in polymorphism group II represent the CMRSA-4 strain. These findings add to our identification of unusual genotypic and phenotypic traits among strains of MRSA that are epidemic in Canada. CMRSA-1 and CMRSA-3 were found to exhibit either attenuated transcription of RNAIII and/or a pleiotropic defect in expression of secreted virulence factors (31). Although these phenotypic traits are suggestive of a reduced capacity for virulence in CMRSA-1 and CMRSA-3 cells, they appear to be partially reversed in CMRSA-4 cells, which demonstrates high protease activity. However, this was also combined with reduced transcription of RNAIII, which resembles a sar mutant phenotype. Inactivation of sar results in reduced transcription of RNAIII and increased expression of protease activity (5, 6) and also results in attenuated virulence in a septic arthritis model of infection (29). It has been suggested that enhanced opportunities for transmission of microbial pathogens are achieved at the expense of reduced virulence (20). Therefore, work is in progress to assess the virulence of these highly transmissible MRSA organisms.
In addition to the unusual traits of CMRSA-1 cells that we described previously (31), we now find that this strain possesses an additional D motif in the Fn binding domain of one of its FnBP adhesins, which defines the group III polymorphism. This fourth D motif exhibited variation within a sequence of acidic and hydrophobic amino acids that is essential for Fn binding (23, 24). This sequence, SVDFEED, was present in each polymorphism group except group III, which displayed the variant sequence GIDFVED. Amino acid substitutions within this conserved motif of acidic and hydrophobic amino acids that is present in several Fn-binding adhesins result in either reduced or abolished ability to bind Fn (18, 23). Consistent with these reports, we have demonstrated that the variant D3-like motif of CMRSA-1 exhibits a reduced ability to bind Fn compared to the D3 motif of FnBPA from S. aureus 8325-4. However, we previously showed that MAb 9C3 was specific for the SVDFEED sequence and could abolish Fn binding by the D3 motif (14). Our present finding that both polyclonal and monoclonal antibodies specific for the SVDFEED sequence did not recognize the variant D motif of CMRSA-1 provides evidence of antigenic variation. Therefore, the reduced ability of the variant D3-like motif of CMRSA-1 to bind Fn may represent the cost of immune evasion. Of interest in this respect, IgG recovered from plasma of patients with S. aureus infection preferentially binds the D repeat domain of FnBPA (4). Furthermore, most patients' IgG recognized an epitope in the C-terminal half of the D3 motif that contains the SVDFEED sequence but recognized D1 or D2 poorly. These observations establish that the D3 motif is highly immunogenic during in vivo infections. The D3 motif also binds Fn with much higher affinity than either D1 or D2 (15, 24). In view of these observations, the antigenic variation in the D3-like motif of CMRSA-1 may have evolved in response to a host immune response encountered during successive cycles of colonization, infection, and transmission. To our knowledge, this is the first report of antigenic variation within a cell surface protein of S. aureus, and this trait is restricted to the fnb locus polymorphism group III, which is represented by the epidemic CMRSA-1 strain of MRSA.
Although the high-affinity Fn-binding site in the D3 motif of FnBPA from S. aureus 8325-4 is localized to the C-terminal half of the motif (15, 18, 24), recent work has also established the presence of a weaker binding motif associated with the N-terminal portion. This N-terminal binding motif exhibits a different specificity for the N-terminal fragment of Fn compared to the C-terminal motif (18) and is defined by the sequence NKHTEIIEEDT. Of interest in this respect, fnb locus polymorphism groups II, III, and IV exhibited limited sequence variation in this segment (Fig. 3). The D3-like motif from the D1-3 amplicon of group IV isolates displayed the variant sequence NKHnEtIEEDT, while the D3-like motifs from the D1-2 and D1-3 amplicons of polymorphism groups II and III, respectively, displayed the same sequence, NKHnEIIEEDT. In each case, the sequence of each D3-like motif was otherwise identical to that of the D3 motifs from FnBPA and FnBPB of S. aureus 8325-4. However, the significance of these substitutions with respect to affinity and specificity for Fn and recognition by antibodies remains to be determined.
Our study also establishes that variation in the number of Fn-binding D motifs in the FnBP adhesin of clinical S. aureus isolates is much less than previously noted in the Fn-binding protein F (PrtF) of S. pyogenes. In the PrtF adhesin, the number of repeated R motifs, analogous in function to the D motifs of S. aureus, varies between two and seven complete copies, with five being the most common (27). In contrast, the loss or gain of a D motif in the FnBP adhesins of S. aureus is a relatively rare event, as two or four copies of the D motif were almost always associated with CMRSA-4 or CMRSA-1, respectively. It has been postulated that the diversity in the number of repeated motifs in the adhesins of S. pyogenes was due to intragenic recombination between different repeats (27), as is the case for the M protein. However, one study has shown that the sequence of each R motif is always the same, regardless of the number of motifs present or the origin of the strain (45). This differs from S. aureus, where the D3 motif is quite divergent from D1 and D2 (39). In addition, our present study has demonstrated the potential for antigenic variation within the high-affinity Fn-binding site of the D3 motif, which appears to contain the predominant epitopes recognized by antisera of convalescent patients with previous S. aureus infection (4).
In agreement with previous studies (10, 34), we find that S. aureus isolates which differ in the number of fnb genes did not exhibit a significant difference in Fn binding. However, there was a significant negative linear correlation (P < 0.05) between protease activity and Fn binding in stationary-phase cultures of MRSA. This is consistent with our finding that exogenous V8 protease of S. aureus promoted the loss of cell surface FnBP when added to exponential-phase cultures (25). Likewise, loss of protease expression in S. pyogenes cells results in increased amounts of cell surface proteins, including the Fn-binding PrtF adhesin (22), a finding consistent with a role for protease activity in controlling the stability of cell surface proteins. As expression of secreted proteases by S. aureus is induced by agr when cells approach stationary phase, we anticipated that the negative correlation between Fn binding and protease activity would also be reflected in the transcriptional activity of the agr locus. This general trend was observed among the MRSA isolates, with the exception of group II MRSA isolates as noted above, which exhibited low levels of Fn binding, high protease activity, and attenuated transcription of RNAIII. Transcription of RNAIII appears much earlier in the growth phase in some S. aureus isolates compared to others (17). This supports the notion that S. aureus strains exhibit heterogeneity in the growth-phase-dependent induction of agr, which could in turn promote variation in Fn binding and other phenotypic traits.
In summary, our findings suggest that although the fnb locus is polymorphic in a relatively small number of strains, it does not contribute to the variation in Fn binding observed among clinical S. aureus isolates. Rather, variance in Fn binding appears to be a function of transcriptional activity of the agr locus and total protease activity. The group III polymorphism containing four complete copies of the D motif in one of its FnBP adhesins was unique to CMRSA-1 isolates and was not detected among other MRSA or MSSA isolates. Furthermore, this additional D motif exhibited antigenic variation within a sequence that is critical to Fn binding. Therefore, epidemic MRSA may have a capacity to evolve in response to host defense mechanisms encountered during repeated cycles of colonization, infection, and transmission in the nosocomial environment.
ACKNOWLEDGMENTS
This work was supported by operating grant MOP12669 to M.J.M. from the Canadian Institutes of Health Research. K.R. received support from an Ontario Graduate Scholarship Award, and M.J.M. is a recipient of the Premiers Research Excellence Award.
We thank members of the Canadian Nosocomial Infection Surveillance Program for performing molecular typing of MRSA and for providing access to these isolates.
REFERENCES
- 1.Booth C M, Pence L M, Mahasreshti P, Callegan M C, Gilmore M S. Clonal associations among Staphylococcus aureus isolates from various sites of infection. Infect Immun. 2001;69:345–351. doi: 10.1128/IAI.69.1.345-352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle M D. Variation of multifunctional surface binding proteins—a virulence strategy for group A streptococci? J Theor Biol. 1995;173:415–426. doi: 10.1006/jtbi.1995.0073. [DOI] [PubMed] [Google Scholar]
- 3.Brennan F R, Jones T D, Longstaff M, Chapman S, Bellaby T, Smith H, Xu F, Hamilton W D, Flock J I. Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureus expressed on two different plant viruses. Vaccine. 1999;17:1846–1857. doi: 10.1016/s0264-410x(98)00485-x. [DOI] [PubMed] [Google Scholar]
- 4.Casolini F, Visai L, Joh D, Conaldi P G, Toniolo A, Hook M, Speziale P. Antibody response to fibronectin-binding adhesin FnbpA in patients with Staphylococcus aureus infections. Infect Immun. 1998;66:5433–5442. doi: 10.1128/iai.66.11.5433-5442.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan P F, Foster S J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Bayer M G, Heinrichs J H. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J Bacteriol. 1997;179:3963–3971. doi: 10.1128/jb.179.12.3963-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drapeau G R, Boily Y, Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972;247:6720–6726. [PubMed] [Google Scholar]
- 8.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster T J, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 10.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 11.Greene C, Vaudaux P E, Francois P, Proctor R A, McDevitt D, Foster T J. A low-fibronectin-binding mutant of Staphylococcus aureus 879R4S has Tn918 inserted into its single fnb gene. Microbiology. 1996;142:2153–2160. doi: 10.1099/13500872-142-8-2153. [DOI] [PubMed] [Google Scholar]
- 12.Guzman C A, Talay S R, Molinari G, Medina E, Chhatwal G S. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J Infect Dis. 1999;179:901–906. doi: 10.1086/314655. [DOI] [PubMed] [Google Scholar]
- 13.House-Pompeo K, Xu Y, Joh D, Speziale P, Hook M. Conformational changes in the fibronectin binding MSCRAMMs are induced by ligand binding. J Biol Chem. 1996;271:1379–1384. doi: 10.1074/jbc.271.3.1379. [DOI] [PubMed] [Google Scholar]
- 14.Huesca M, Sun Q, Peralta R, Shivji G M, Sauder D N, McGavin M J. Synthetic peptide immunogens elicit polyclonal and monoclonal antibodies specific for linear epitopes in the D motifs of Staphylococcus aureus fibronectin-binding protein, which are composed of amino acids that are essential for fibronectin binding. Infect Immun. 2000;68:1156–1163. doi: 10.1128/iai.68.3.1156-1163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huff S, Matsuka Y V, McGavin M J, Ingham K C. Interaction of N-terminal fragments of fibronectin with synthetic and recombinant D motifs from its binding protein on Staphylococcus aureus studied using fluorescence anisotropy. J Biol Chem. 1994;269:15563–15570. [PubMed] [Google Scholar]
- 16.Hunter W M. Radioimmunoassay. In: Weir D M, editor. Handbook of experimental immunology. Oxford, United Kingdom: Blackwell Scientific Publications; 1978. pp. 14.1–14.40. [Google Scholar]
- 17.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joh D, Speziale P, Gurusiddappa S, Manor J, Hook M. Multiple specificities of the staphylococcal and streptococcal fibronectin-binding microbial surface components recognizing adhesive matrix molecules. Eur J Biochem. 1998;258:897–905. doi: 10.1046/j.1432-1327.1998.2580897.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuypers J M, Proctor R A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsitch M, Moxon E R. Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol. 1997;5:31–37. doi: 10.1016/S0966-842X(97)81772-6. [DOI] [PubMed] [Google Scholar]
- 21.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 22.Lyon W R, Gibson C M, Caparon M G. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGavin M J, Gurusiddappa S, Lindgren P E, Lindberg M, Raucci G, Hook M. Fibronectin receptors from Streptococcus dysgalactiae and Staphylococcus aureus. Involvement of conserved residues in ligand binding. J Biol Chem. 1993;268:23946–23953. [PubMed] [Google Scholar]
- 24.McGavin M J, Raucci G, Gurusiddappa S, Hook M. Fibronectin binding determinants of the Staphylococcus aureus fibronectin receptor. J Biol Chem. 1991;266:8343–8347. [PubMed] [Google Scholar]
- 25.McGavin M J, Zahradka C, Rice K, Scott J E. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect Immun. 1997;65:2621–2628. doi: 10.1128/iai.65.7.2621-2628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mempel M, Schmidt T, Weidinger S, Schnopp C, Foster T, Ring J, Abeck D. Role of Staphylococcus aureus surface-associated proteins in the attachment to cultured HaCaT keratinocytes in a new adhesion assay. J Investig Dermatol. 1998;111:452–456. doi: 10.1046/j.1523-1747.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Natanson S, Sela S, Moses A E, Musser J M, Caparon M G, Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow anaerobically. Approved standard M7-A4. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 29.Nilsson I M, Bremell T, Ryden C, Cheung A L, Tarkowski A. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect Immun. 1996;64:4438–4443. doi: 10.1128/iai.64.11.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papakyriacou H, Vaz D, Simor A, Louie M, McGavin M J. Molecular analysis of the accessory gene regulator (agr) locus and balance of virulence factor expression in epidemic methicillin-resistant Staphylococcus aureus. J Infect Dis. 2000;181:990–1000. doi: 10.1086/315342. [DOI] [PubMed] [Google Scholar]
- 32.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 33.Patti J M, Hook M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol. 1994;6:752–758. doi: 10.1016/0955-0674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 34.Peacock S J, Day N P, Thomas M G, Berendt A R, Foster T J. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J Infect. 2000;41:23–31. doi: 10.1053/jinf.2000.0657. [DOI] [PubMed] [Google Scholar]
- 35.Peacock S J, Foster T J, Cameron B J, Berendt A R. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology. 1999;145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 36.Saravia-Otten P, Muller H P, Arvidson S. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol. 1997;179:5259–5263. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulze K, Medina E, Talay S R, Towers R J, Chhatwal G S, Guzman C A. Characterization of the domain of fibronectin-binding protein I of Streptococcus pyogenes responsible for elicitation of a protective immune response. Infect Immun. 2001;69:622–651. doi: 10.1128/IAI.69.1.622-625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheagren J N. Staphylococcus aureus. The persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- 39.Signas C, Raucci G, Jonsson K, Lindgren P E, Anantharamaiah G M, Hook M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simor A, Boyd D, Louie L, McGeer A, Mulvey M, Willey B. Characterization and proposed nomenclature of epidemic strains of methicillin-resistant Staphylococcus aureus in Canada. Can Commun Dis Rep. 1999;25:105–108. [PubMed] [Google Scholar]
- 41.Simor A, Ofner-Agostini M, Paton S. The Canadian Nosocomial Infection Surveillance Program: results of the first 18 months of surveillance for methicillin-resistant Staphylococcus aureus in Canadian hospitals. Can Commun Dis Rep. 1997;23:41–45. [PubMed] [Google Scholar]
- 42.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 43.Speziale P, Joh D, Visai L, Bozzini S, House-Pompeo K, Lindberg M, Hook M. A monoclonal antibody enhances ligand binding of fibronectin MSCRAMM (adhesin) from Streptococcus dysgalactiae. J Biol Chem. 1996;271:1371–1378. doi: 10.1074/jbc.271.3.1371. [DOI] [PubMed] [Google Scholar]
- 44.Sun Q, Smith G M, Zahradka C, McGavin M J. Identification of D motif epitopes in Staphylococcus aureus fibronectin-binding protein for the production of antibody inhibitors of fibronectin binding. Infect Immun. 1997;65:537–543. doi: 10.1128/iai.65.2.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talay S R, Valentin-Weigand P, Timmis K N, Chhatwal G S. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 46.Vaudaux P, Pittet D, Haeberli A, Lerch P G, Morgenthaler J J, Proctor R A, Waldvogel F A, Lew D P. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J Infect Dis. 1993;167:633–641. doi: 10.1093/infdis/167.3.633. [DOI] [PubMed] [Google Scholar]
- 47.Wann E R, Gurusiddappa S, Hook M. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J Biol Chem. 2000;275:13863–13871. doi: 10.1074/jbc.275.18.13863. [DOI] [PubMed] [Google Scholar]