Abstract
Aims
Biomarkers specifically related to atrial tissue may increase the understanding of the pathophysiology of atrial fibrillation (AF) and further improve risk prediction in this setting. Bone morphogenetic protein 10 (BMP10) is a protein expressed in the atrial myocardium. We evaluated the association between BMP10 and the risk of ischaemic stroke and other cardiovascular events in large cohorts of patients with AF, treated with and without oral anticoagulation (OAC).
Methods and results
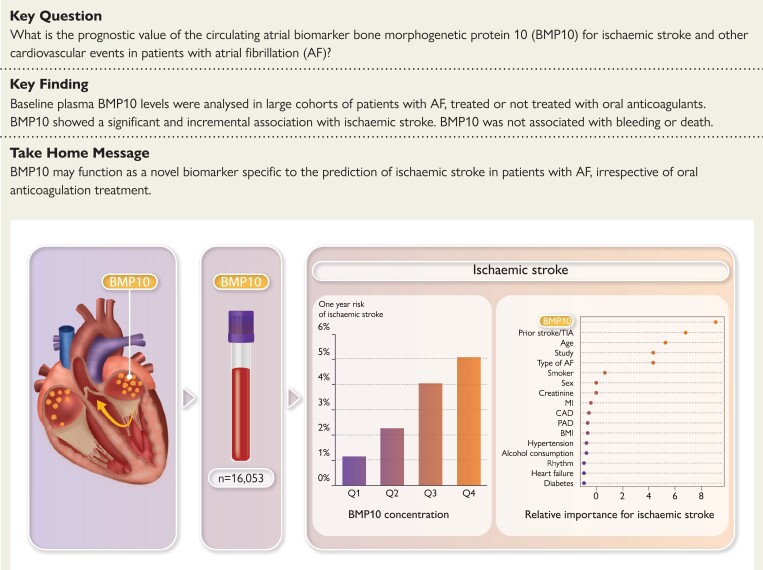
BMP10 was measured in plasma samples collected at randomisation in patients with AF without OAC in the ACTIVE A and AVERROES trials (n = 2974), and with OAC in the ARISTOTLE trial (n = 13 079). BMP10 was analysed with a prototype Elecsys immunoassay. Associations with outcomes were evaluated by Cox-regression models adjusted for clinical characteristics, kidney function, and N-terminal pro-B-type natriuretic peptide (NT-proBNP). Median concentrations of BMP10 were 2.47 and 2.44 ng/mL, in the non-OAC and OAC cohort, respectively. Increasing BMP10 was associated with lower body mass index, older age, female sex, kidney dysfunction, and AF rhythm. BMP10 was consistently associated with ischaemic stroke. In the non-OAC cohort, BMP10 increased the concordance index of the multivariable model from 0.713 to 0.733 (P = 0.004) and in the OAC cohort from 0.673 to 0.694 (P < 0.001). Additionally, BMP10 maintained a significant prognostic value after additionally adjusting for NT-proBNP. BMP10 was not independently associated with bleeding or with death.
Conclusion
The novel atrial biomarker BMP10 was independently associated with ischaemic stroke in patients with AF irrespective of OAC treatment. BMP10 seems to be more specifically related to the risk of ischaemic stroke in AF.
One-sentence Summary
In this study, BMP10 may be a novel specific biomarker of ischaemic stroke in patients with atrial fibrillation, irrespective of oral anticoagulation.
Keywords: Atrial fibrillation, Biomarker, BMP10, Risk stratification, Stroke, Heart failure
Structured Graphical Abstract
Structured Graphical Abstract.
See the editorial comment for this article ‘A specific new biomarker for atrial fibrillation and its sequelae?’, by M.F. Sinner and A.S. von Falkenhausen, https://doi.org/10.1093/eurheartj/ehac645.
Introduction
Atrial fibrillation (AF) is associated with increased risk of ischaemic stroke, heart failure, and mortality. The currently recommended preventive treatment with oral anticoagulation (OAC) substantially reduces the risk of stroke and stroke-related mortality. The risk of these complications is variable and related to the presence of clinical risk factors, e.g. older age, history of prior stroke, heart failure, and other cardiovascular (CV) comorbidities.1 In the past years, the circulating protein biomarkers N-terminal pro-B-type natriuretic peptide (NT-proBNP), reflecting cardiac stress and dysfunction, and cardiac troponin T, reflecting myocardial damage, have been shown to provide important incremental information on the risk of stroke, heart failure, and death in patients with AF and have subsequently led to the development of novel risk scores, such as the ABC-AF-stroke score (age, biomarkers, clinical history).2–4 These biomarkers are also associated with the aforementioned outcomes in patients with other CV conditions.5 The identification of circulating biomarkers specifically related to AF and its complications might improve the understanding and prognostication of the risk of ischaemic stroke and other clinical outcomes in patients with AF. Recently, bone morphogenetic protein 10 (BMP10) was identified as a biomarker mainly expressed by atrial tissue and positively associated with AF recurrence.6 Based on the availability of a prototype assay of BMP10, we investigated the associations between the circulating BMP10 level and the risk of ischaemic stroke and other outcomes in two large clinical trial cohorts of patients with AF without (n = 2974) and with OAC treatment (n = 13 079).
Methods
Study populations
Patients without oral anticoagulation (n = 2974)
The ACTIVE A trial7 randomised 7554 patients with AF and an increased risk of stroke who were deemed unsuitable for vitamin K antagonist treatment to long-term prevention with clopidogrel (75 mg) or placebo, in addition to aspirin (75–100 mg). Patients were followed for a median of 3.6 years. The study was conducted between June 2003 and November 2008. Baseline plasma samples for BMP10 analyses were available for 948 participants randomised to aspirin.
The AVERROES trial8 randomised 5599 patients with AF and an increased risk of stroke who were deemed unsuitable for vitamin K antagonist treatment to receive apixaban (5 mg twice daily) or aspirin (81–324 mg).8 The study was conducted between September 2007 and May 2010, when it was terminated early because of a superior ratio of benefit to risk of apixaban over aspirin. Of the 2791 patients randomised to aspirin, baseline plasma samples for BMP10 analyses were available for 2026 participants. The median follow-up time was 1.2 years.
Both aspirin cohorts have previously been shown to be rather similar in regard to baseline characteristics.9 Both cohorts were merged into one cohort with a total of 2974 patients to increase statistical power for the evaluation of BMP10 in patients without OAC.
Patients treated with oral anticoagulation (n = 13 079)
Between December 2006 and April 2010, the ARISTOTLE trial10,11 enrolled 18 201 patients with AF and an increased risk of stroke. Patients were randomised to double-blind treatment with either warfarin (n = 9081) or apixaban 5 mg twice daily (n = 9120). The median length of follow-up was 1.9 years for the participants in the biomarker cohort consisting of 14 798 included patients, out of whom 13 079 had plasma samples available for determination of BMP10.
The studies were conducted in accordance with the Declaration of Helsinki and were approved by the ethics committee at each participating site. All patients provided written informed consent before enrolment.
Outcomes
Outcome definitions (ischaemic stroke, hospitalisation for heart failure, major bleeding, CV, and all-cause death) and the adjudication process are listed in the Supplementary material online.7,8,11
Samples and biochemical analyses
Subsets of patients enrolled in all three trials provided blood samples at baseline. Blood was drawn from an antecubital vein into EDTA tubes and centrifuged within 2 h of collection. Plasma was aliquoted, frozen at −20°C, and within one week transferred to the long-term storage at −80°C, until shipment to a central laboratory.
BMP10 was analysed in plasma by a prototype Elecsys electrochemiluminescence immunoassay developed by Roche Diagnostics. The assay employs a quantitative sandwich principle, where the first monoclonal antibody specifically binds the BMP10 as a capture antibody and a ruthenylated second monoclonal antibody binds BMP10 as a detection antibody. Recombinant BMP10 is used to normalise the measurements across the runs with a high degree of accuracy. At the Uppsala Clinical Research Center (UCR) laboratory, Sweden, the coefficient of variation was 6.0% and 4.3% for BMP10 concentrations of 1.39 and 3.56 ng/mL, respectively. The levels of NT-proBNP were analysed in plasma by commercialized Elecsys electrochemiluminescence immunoassays (Roche Diagnostics) as previously published.12–14 The biochemical analysis of BMP10 was performed on an Elecsys e411 analyser at the UCR laboratory. NT-proBNP, cardiac troponin T and growth differentiation factor (GDF)-15 were analysed at the Clinical Research Laboratory and Biobank, Hamilton, Ontario, Canada (ACTIVE A and AVERROES), and in the UCR Laboratory, Uppsala. Sweden (ARISTOTLE).
Statistical methods
BMP10 was log-transformed using the natural logarithm in all analyses. The distribution of BMP10 was illustrated graphically by plotting both the estimated density and the empirical cumulative distribution function. Event rates were estimated as the number of events divided by the total follow-up time and corresponding approximate 95% confidence intervals (CI) were estimated using a gamma distribution. The median follow-up time was estimated by the Kaplan–Meier method using the observed time in the study while censoring for death. For descriptive purposes only, BMP10 was also categorised into quartile groups. Baseline characteristics were presented both within the quartile groups and combined. Cumulative event rates were estimated using the Kaplan-–Meier method and were plotted for the quartile groups.
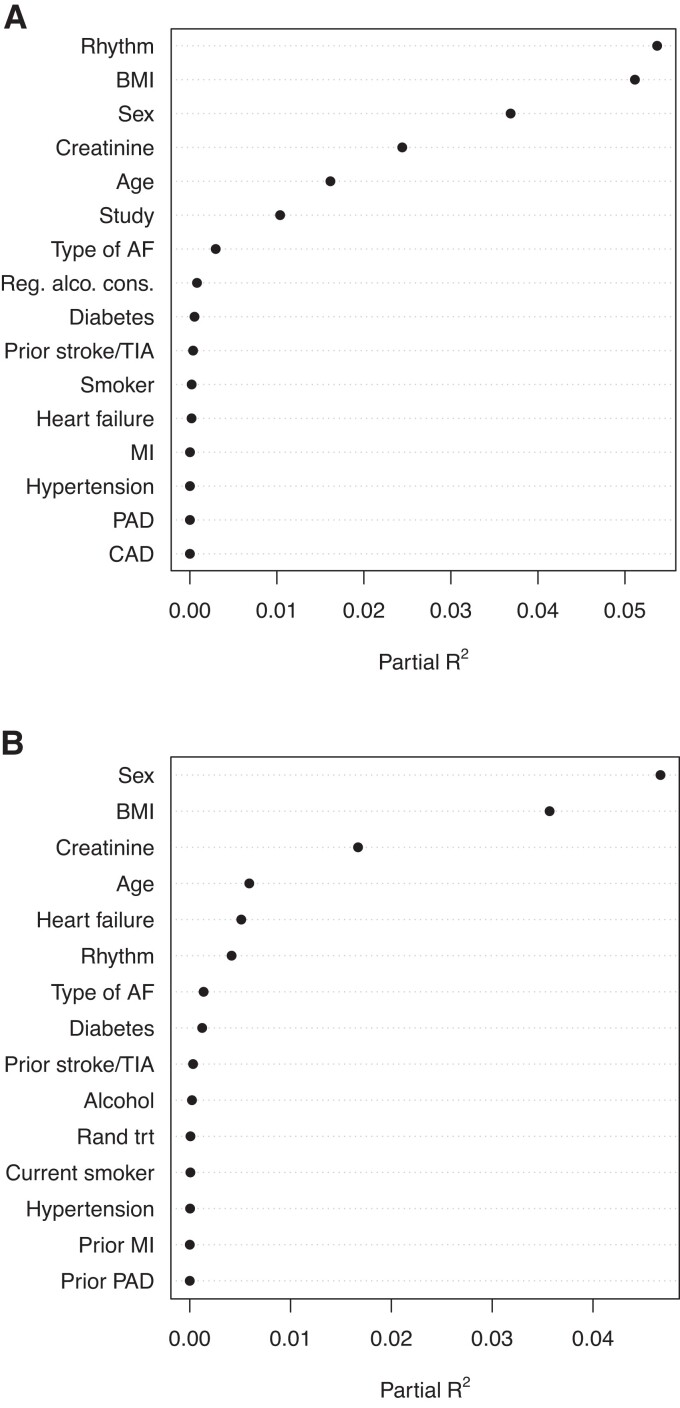
Marginal and conditional associations between BMP10 and baseline variables [age, sex, body mass index (BMI), smoking status, regular alcohol consumption, hypertension, diabetes, heart failure, type of AF, sinus rhythm at enrolment, prior myocardial infarction (MI), prior peripheral arterial disease (PAD), prior stroke/transient ischaemic attack (TIA), prior coronary artery disease, creatinine, and study] were evaluated by fitting linear regression models with BMP10 as the dependent variable and the baseline variables included one at a time and all together as independent variables. As BMP10 was log-transformed, the back-transformed parameter estimates can be interpreted as the ratio of geometric means of BMP10 on the original scale. To illustrate the amount each variable contributes to explaining the total variance of BMP10 in the conditional model, the partial R2 value for each variable was plotted.
Smooth associations between BMP10 and all outcomes were assessed by fitting Cox-regression models including BMP10 as a restricted cubic spline with three knots placed at the 10th, 50th, and 90th sample percentiles. As the association was allowed to be non-linear, it was not possible to give one single hazard ratio to summarise the association. Instead, a graphical representation gives the best illustration of the full association. Both unadjusted and conditional models, adjusting for relevant potential confounders, were fitted. The adjusted models included (Model 2): age, study, and creatinine; (Model 3): Model 2 + sex, BMI, smoking status, regular alcohol consumption, hypertension, diabetes, heart failure, type of AF, sinus rhythm at enrolment, prior MI, prior PAD, prior stroke/TIA, and prior coronary artery disease; (Model 4): Model 3 + NT-proBNP or, for major bleeding, Model 3 + GDF-15. In all models, all continuous variables were entered as restricted cubic splines. To save degrees of freedom in the full models, the number of knots for each spline representation was pre-specified to be three and were placed at the 10th, 50th, and 90th sample percentiles of the respective variable. In a sensitivity analysis, we increased the number of knots to the first four and then six for BMP10, but the results were materially unaltered (data not shown). Each predictor’s relative contribution in the models was measured as the partial χ2 statistic minus the predictor degrees of freedom. The discriminative ability of each model was evaluated using Harrell’s concordance index (C-index).15 In addition, the added predictive value of BMP10, beyond the discriminatory ability as expressed by the C-index, was evaluated by comparing the likelihood ratio , χ2 statistic for the model with BMP10 with the same model without BMP10, and by comparing the variance of the predictions from the models. The fraction of new information provided by BMP10 was defined as one minus the ratio of the respective statistic without BMP10 to the corresponding statistic with BMP10.16
Pre-specified interaction tests regarding cardiac rhythm and BMP10 were performed in the unadjusted model and Model 3. Although BMP10 was included as a restricted cubic spline, the interaction tests only included the linear part of BMP10. Thus, the functional form of the association was assumed to be the same but the slope of the association was allowed to be different for rhythm and study, respectively. BMP10 was also evaluated in relation to clinical risk models, such as the CHA2DS2-VASc risk score, and the newer biomarker-based ABC-AF-stroke score, which encompasses both NT-proBNP and high-sensitivity troponin T, to assess the additional value of BMP10 over these established stroke risk scores. Throughout, a statement of ‘significant’ means statistically significant at the 5% significance level. All analyses were done using R, version 4.0.517 and in particular the rms package.18,19
Results
BMP10 distribution and its association with baseline characteristics and other biomarkers
The median (25th–75th percentile) BMP10 level was similar in both cohorts, at 2.47 (2.10–2.95) and 2.44 (2.07–2.89) ng/mL, respectively, with the distribution shown in Supplementary material online, Figure S1. The baseline characteristics of the non-OAC cohort and the OAC cohort were similar except for a higher proportion of patients in sinus rhythm at entry in the non-OAC cohort (Table 1). The levels of established biomarkers were also similar in both cohorts. Baseline characteristics according to BMP10 quartile groups are presented in Supplementary material online, Table S1A and S1B. In both cohorts, in multivariable models, higher plasma levels of BMP10 were most strongly associated with, in falling order of importance: low BMI, female sex, impaired kidney function (increased creatinine), and older age. In the non-OAC cohort, with a higher proportion of patients in sinus rhythm at entry, AF rhythm was associated with higher BMP10 levels (Figure 1). BMP10 was positively correlated with NT-proBNP (r = 0.54 and 0.44) and with cTnT (r = 0.35 and 0.25) in the non-OAC and OAC cohorts, respectively. There was no marked difference between the subgroups defined by AF rhythm regarding the correlation.
Table 1.
Baseline characteristics, biomarker levels and outcomes in the ACTIVE A-AVERROES and ARISTOTLE cohorts
| Variable | ACTIVE A-AVERROES (n = 2974) | ARISTOTLE (n = 13 079) |
|---|---|---|
| Age (years) | 71.0 (63.0–77.0) | 70.0 (63.0–76.0) |
| Sex (male) | 58.1% (1729) | 63.8% (8349) |
| Height (cm) | 168.0 (160.0–175.3) [6] | 169.0 (161.0–176.0) [58] |
| Weight (kg) | 79.0 (68.0–92.0) [2] | 82.0 (70.0–95.7) [39] |
| BMI (kg/m2) | 27.8 (24.7–31.6) [6] | 28.6 (25.3–32.7) [60] |
| Current smoker | 7.4% (219) [1] | 7.8% (1023) [12] |
| Regular alcohol consumption | 26.2% (780) | 2.5% (331) |
| Permanent/persistent AF | 71.2% (2116) [2] | 84.6% (11 058) [3] |
| AF rhythm | 67.0% (1990) [2] | 82.7% (10 783) [35] |
| Sinus rhythm | 28.8% (857) | 12.9% (1673) [132] |
| Heart failure | 35.0% (1040) | 31.1% (4072) |
| Hypertension | 86.7% (2579) | 87.6% (11 462) |
| Diabetes | 19.8% (589) | 24.7% (3230) |
| Prior stroke/TIA | 12.8% (381) | 18.6% (2433) |
| Prior PAD | 3.5% (103) | 4.9% (640) [1] |
| Prior MI | 8.7% (257) [3] | 12.6% (1652) [1] |
| Creatinine (µmoL/L) | 89.0 (77.0–106.0) [146] | 89.3 (76.9–105.2) [5] |
| NT-proBNP (ng/L) | 704.0 (277.4–1379.1) | 702.0 (358.0–1235.5) |
| hs-cTnT (ng/L) | 13.2 (9.1–20.2) | 10.8 (7.4–16.4) |
| GDF-15 (ng/L) | 1593.0 (1094.0–2374.8) | 1358.0 (965.0–2025.0) |
| BMP10 (ng/mL) | 2.47 (2.10–2.95) | 2.44 (2.07–2.89) |
| Ischaemic strokea | 3.04 [2.59, 3.54] (n = 165) | 0.90 (0.79–1.03) (n = 226) |
| Deatha | 5.20 [4.62, 5.83] (n = 292) | 3.34 (3.12–3.57) (n = 853) |
| CV deatha | 3.67 [3.19, 4.21] (n = 206) | 1.69 (1.53–1.86) (n = 431) |
| Heart failurea | 4.11 [3.58, 4.69] (n = 220) | 2.01 (1.84–2.20) (n = 503) |
| Major bleedinga | 1.48 [1.18, 1.84] (n = 82) | 1.71 (1.54–1.89) (n = 391) |
per 100 patient-years.
(a—b) represents median (Q1—Q3).
P% (n) represent percentage (frequency). Percentages computed by group. [M] represents number of patients with missing data.
AF, atrial fibrillation; TIA, transient ischaemic attack; PAD, peripheral arterial disease; MI, myocardial infarction; NT-proBNP, N-terminal pro B-type natriuretic peptide; hs-cTnT, high-sensitivity cardiac troponin T; GDF-15, growth differentiation factor 15; BMP10, bone morphogenetic protein 10; CV, cardiovascular.
Figure 1.
Association between BMP10 and other variables in adjusted models. (A) non-OAC cohort and (B) OAC cohort
BMP10 association with ischaemic stroke
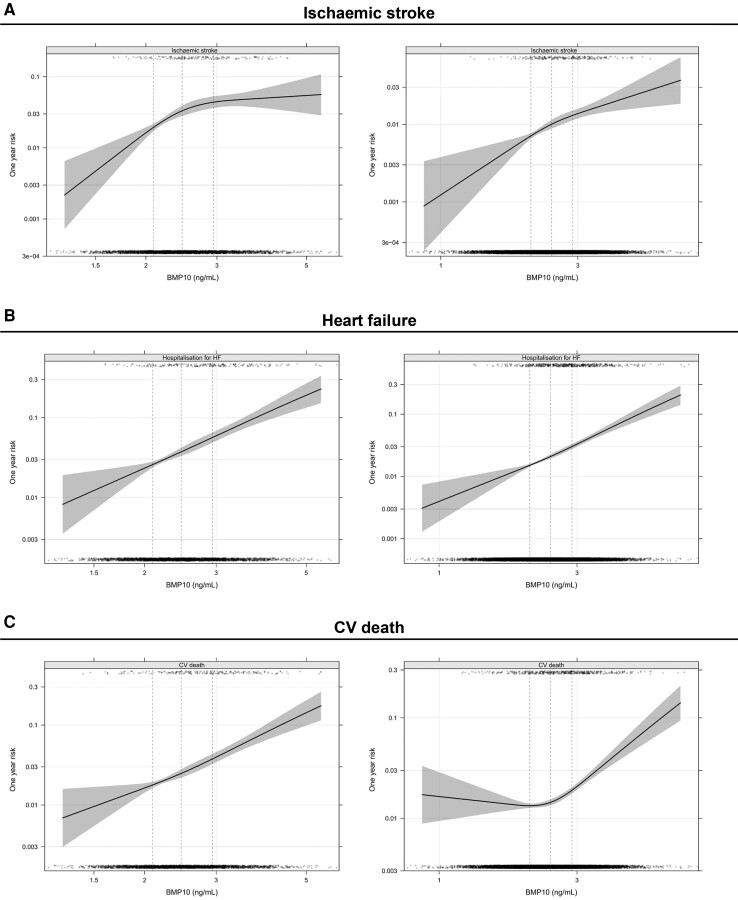
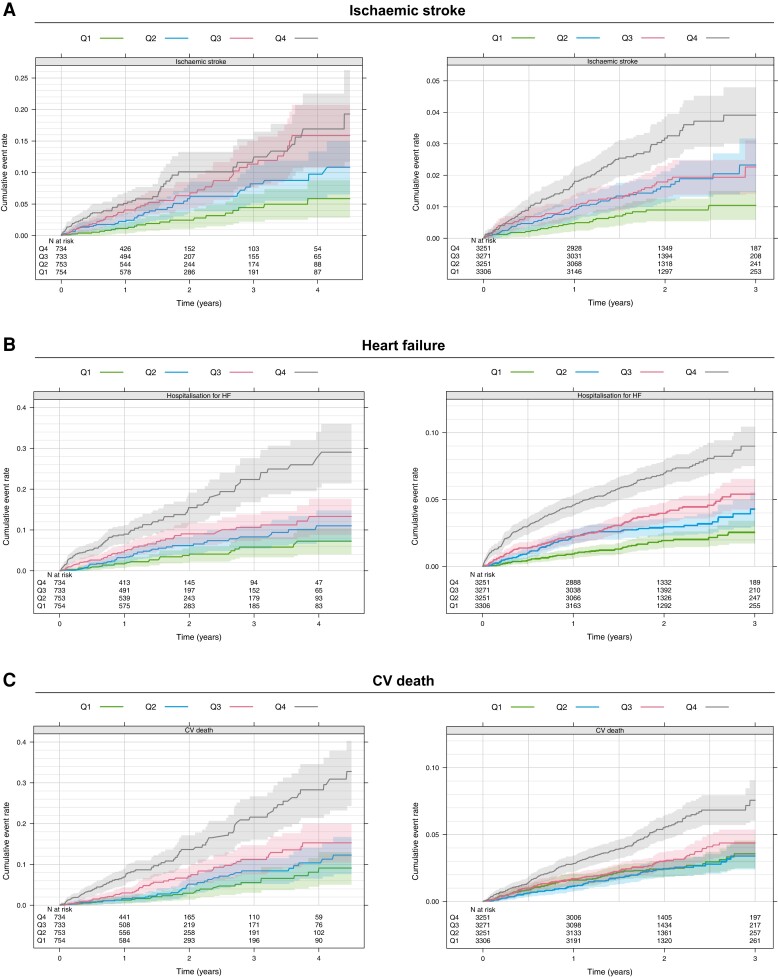
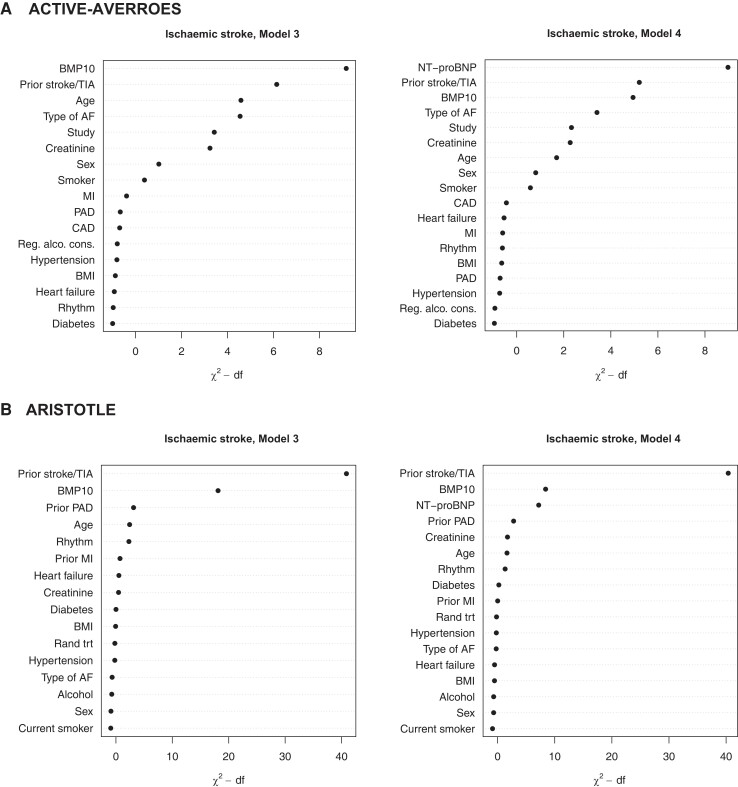
The number of events and annual event rates during follow-up in both cohorts are presented in Table 1. Overall, in the non-OAC cohort, the rates of ischaemic stroke were three times higher as compared with the OAC cohort (3.04%/year vs. 0.90%/year). The risk of ischaemic stroke increased continuously with increasing BMP10 level, in both the non-OAC cohort, with the higher event rate, as in the lower risk OAC cohort (Figure 2). In Kaplan–Meier estimates of cumulative event rates, there was a corresponding increase in the rate of ischaemic stroke by quartile group of BMP10 (Figure 3). In multivariable analyses in the non-OAC cohort, BMP10 was the variable most strongly associated with ischaemic stroke after adjusting for age, renal function, and all clinical characteristics (Figure 4), with a C-index increasing from 0.713 to 0.733 (P = 0.004), as compared with a model containing clinical variables (Table 2). In the OAC cohort, with a lower event rate, BMP10 was the second most important risk indicator for ischaemic stroke after prior stroke (Figure 4). The independent discriminatory value of BMP10 for ischaemic stroke was consistent in the OAC cohort, with a significant improvement of the C-index from 0.673 to 0.694 (P < 0.001) (Table 2). The incremental discriminatory value of BMP10 remained statistically significant after additional adjustment for the strongest prognostic biomarker, NT-proBNP, with increases in the C-indices of 0.008 (P = 0.031) and 0.011 (P = 0.006) in the non-OAC and OAC cohorts, respectively (Table 2). The addition of BMP10 to the clinical stroke risk scores, CHA2DS2-VASc and the ABC-AF-stroke score, resulted in significantly improved discrimination (Table 2). For all models, the addition of BMP10 provided new prognostic information according to the fraction of new information analysed (see Supplementary material online, Table S2). There were no significant interactions between heart rhythm at baseline and the associations between BMP10 and ischaemic stroke.
Figure 2.
Unadjusted, smoothed association between BMP10 and one-year risk of ischaemic stroke, heart failure hospitalisation, and CV death in the non-OAC cohort (left) and the OAC cohort (right)
Figure 3.
Kaplan–Meier estimate of the cumulative event rate of ischaemic stroke, hospitalisation for heart failure, and CV death by BMP10 quartile groups in the non-OAC cohort (left) and OAC cohort (right)
Figure 4.
Relative importance of BMP10 and other variables for different outcomes in multivariable models without (left panels) and with established biomarkers (right panels)
Table 2.
Discriminatory value of BMP10 for ischaemic stroke in multivariable models and comparisons with the CHA2DS2-VASc and ABC-AF-stroke scores within the non-OAC (ACTIVE A, AVERROES) and OAC (ARISTOTLE) cohorts
| Model | C-index (95% CI) no BMP10 | C-index (95% CI) incl. BMP10 | P |
|---|---|---|---|
| ACTIVE A-AVERROES | |||
| Model 2 | 0.666 [0.620, 0.711] | 0.709 [0.668, 0.749] | <0.001 |
| Model 3 | 0.713 [0.671, 0.755] | 0.733 [0.691, 0.774] | 0.004 |
| Model 4 | 0.745 [0.705, 0.785] | 0.753 [0.713, 0.793] | 0.031 |
| BMP10 only | 0.643 [0.601, 0.686] | NA | NA |
| CHA2DS2-VASc score | 0.634 [0.590, 0.679] | 0.677 [0.636, 0.718] | <0.001 |
| ABC-AF-stroke stroke | 0.707 [0.668, 0.746] | 0.715 [0.676, 0.753] | 0.024 |
| ARISTOTLE | |||
| Model 2 | 0.582 [0.545, 0.619] | 0.631 [0.595, 0.666] | <0.001 |
| Model 3 | 0.673 [0.637, 0.710] | 0.694 [0.660, 0.729] | <0.001 |
| Model 4 | 0.695 [0.660, 0.731] | 0.706 [0.672, 0.740] | 0.006 |
| BMP10 only | 0.618 [0.582, 0.655] | NA | NA |
| CHA2DS2-VASc score | 0.643 [0.609, 0.678] | 0.673 [0.640, 0.707] | <0.001 |
| ABC-AF-stroke score | 0.677 [0.642, 0.713] | 0.689 [0.655, 0.723] | 0.008 |
Model 2: Adjustment for age and creatinine.
Model 3: Adjustment for age and creatinine and all available clinical characteristics.
Model 4: Adjustment for age and creatinine and all available clinical characteristics and the level of NT-proBNP.
ABC-AF-stroke: Additive value of BMP in relation to the ABC-AF-stroke score, consisting of the variables age, prior stroke, and levels of NT-proBNP and troponin T.
All available clinical characteristics: sex, BMI, smoking status, regular alcohol consumption, hypertension, diabetes, heart failure, type of AF, sinus rhythm at enrolment, prior MI, prior PAD, prior stroke/TIA, and prior coronary artery disease.
BMP10 association with other clinical outcomes
The BMP10 levels were, in unadjusted analyses, associated with an increased risk of heart failure, CV death, and major bleeding in both the non-OAC and OAC cohorts. After adjusting for age and renal function, BMP10 did not remain significantly associated with major bleeding. After further adjustment for all clinical variables, BMP10 remained significantly associated with heart failure and CV death in both the non-OAC and OAC cohorts (all P ≤ 0.006) (Figure 2 and 3 and Supplementary material online, Figure S2). When also adjusting for the established risk biomarker NT-proBNP, BMP10 remained significantly associated with heart failure hospitalisations in both cohorts, although the improvement of C-index was modest, 0.002 (P = 0.021) and 0.005 (P < 0.001), in the non-OAC and OAC cohorts, respectively (see Supplementary material online, Figure S2).
Discussion
The identification of biomarkers specifically related to atrial tissue might improve the understanding of the pathophysiology and risk prediction of AF and its complications. BMP10 is a novel biomarker mainly expressed in the atrial myocardium. The present study is the first to evaluate the plasma concentrations of circulating BMP10 and its associations with clinical characteristics, other biomarkers, and the risk of ischaemic stroke and other outcomes in patients with AF. The study was performed in two very large cohorts consisting of 16 053 patients with AF—one cohort of 2974 patients without OAC, reflecting the natural course of the disease without effective stroke prevention, and a second cohort of 13 079 with effective stroke prevention by OAC treatment with apixaban or warfarin. A higher plasma concentration of BMP10 was consistently associated with lower BMI, female sex, older age, and kidney dysfunction. In this study, BMP10 was independently and consistently associated with the risk of ischaemic stroke in patients with AF, irrespective of OAC treatment. BMP10 also showed an incremental and consistent discriminatory value for the risk of ischaemic stroke beyond clinical characteristics and NT-proBNP, and improved discrimination when added to established clinical stroke risk scores (Structured Graphical Abstract). BMP10 was not significantly associated with major bleeding or death, and for heart failure the incremental value was small. Thus, BMP10 may be a novel and specific atrial biomarker indicating the risk of ischaemic stroke in patients with AF with an incremental prognostic value beyond currently used risk indicators. Another major finding in this analysis was the fact that the major risk markers of ischaemic stroke were largely similar in OAC-treated and untreated patients.
BMP10 and risk of stroke
In the past years, interest in biomarkers for risk stratification in AF has increased substantially.20 The cardiac biomarkers NT-proBNP and troponin have shown consistent associations with the risk of stroke in patients with AF. Their incorporation into biomarker-based risk scores in AF has improved discrimination and calibration as compared with traditional clinical risk scores.2–4,9 The identification of biomarkers specifically related to AF and its complications might expand the understanding and prognostication of the risk of ischaemic stroke in patients with AF. The current study showed that BMP10 may be a specific atrial biomarker related to AF and its most severe complications, as BMP10 was independently and consistently associated with ischaemic stroke events and provided incremental discrimination beyond clinical characteristics and NT-proBNP. This association was seen in both patients with AF at higher risk of stroke without OAC treatment and patients with a relatively lower risk of stroke due to effective OAC treatment. Therefore, BMP10 should be useful to evaluate the risk of stroke in both the OAC naïve patient as well as estimating the risk of stroke in the individual patient on OAC treatment. Although BMP10 was associated with higher levels of AF rhythm on the baseline electrocardiogram, there was no significant interaction between cardiac rhythm and the prognostic value of BMP10 as a risk marker. Accordingly, BMP10 should be a useful biomarker for stroke risk prediction irrespective of cardiac rhythm. The addition of BMP10 improved the performance of both the CHA2DS2-VASc score and the more precise biomarker-based ABC-AF-stroke score. Because BMP10 is not associated with the risk of bleeding, it has the potential to further improve risk stratification in AF. Importantly, such advancements may also provide novel opportunities to use more AF-specific risk markers in clinical stroke risk scores as they show less overlap between the risk of ischaemic stroke and the risk of major bleeding. Therefore, despite offering a relatively modest discriminatory improvement over the biomarker-based ABC-AF-stroke score, BMP10 could potentially provide a valuable improvement in stroke risk assessment in clinical practice by incorporating more specific markers.
Circulating BMP10 concentrations and associations with clinical characteristics
This study also, for the first time, presents the association between clinical characteristics and BMP10 levels. The finding that AF rhythm was independently associated with high levels of BMP10 is in accordance with an increased expression of BMP10 in patients with AF and the previously demonstrated association between higher BMP10 and AF recurrence after catheter ablation.6 BMP10 might therefore be a useful indicator of both the risk of AF development and the risk of stroke in patients with established AF. The finding that lower BMI was associated with higher levels, indicating more expression of BMP10, is interesting as several recent studies have demonstrated that patients with AF and low BMI have an increased risk of stroke.21 It might be hypothesised that the atrial tissue response to AF might vary by different degrees of body weight. Also, female sex has been suggested to be associated with ischaemic stroke, although results have been inconsistent and mechanisms are not fully explained or understood.1,22 The higher levels of BMP10 in females might reflect differences in the atrial tissue response to AF between men and women. Accordingly, it might be speculated that higher BMP10 levels and increased risk of stroke in AF rhythm, low BMI, and female sex might reflect a common pathophysiological origin in these conditions. Interestingly, this pattern also resembles that of natriuretic peptides, concerning the associations with age, female sex, kidney dysfunction, obesity, and risk of stroke, where part of the negative association with obesity might be related to uptake of NT-proBNP in fat cells.13,23–25 However, despite similarities between the distribution of BMP10 and NT-proBNP concentrations, it is noteworthy that BMP10 concentration was not associated with heart failure or other CV or structural comorbidities at baseline, such as hypertension, coronary artery disease, or prior MI, and was not independently associated with fatal outcomes. Therefore, the profile of the BMP10 associations with clinical characteristics and outcomes supports that BMP10 might be a more specific circulating biomarker of atrial stress and AF-specific complications, such as ischaemic stroke and hospitalisation for heart failure. The mechanisms behind the pattern of BMP10 concentrations should be further explored in experimental and clinical studies.
BMP10 origin and atrial fibrillation
Genome-wide association studies have consistently identified gene variants in a small region on chromosome 4q25 to be strongly associated with the development and recurrence of AF as well as cardioembolic strokes.6,26–28 PITX2 (paired like homeodomain-2 transcription factor) is a gene located close to this region that encodes for a transcriptional factor that regulates the development of thoracic organs. The cardiac isoform of PITX2 is expressed in the adult left atrium and regulates the expression of local ion channels.29,30 Since cardiac PITX2 concentrations cannot be easily measured in clinical practice, there has been a need to identify a surrogate marker measured from plasma. Studies have found that a genetic reduction of PITX2 results in the reciprocal upregulation of the BMP10 gene, which in turn increases the plasma concentration of BMP10.6 In clinical practice, recent subsequent analyses have shown that the use of plasma BMP10 as a surrogate for cardiac PITX2 was strongly associated with AF recurrence after catheter ablation and even outperformed other endothelial-, inflammatory-, and CV biomarkers.6 Plasma BMP10 has thus been proposed as a biomarker that reflects a more AF-specific pathophysiology. In the present study, we, for the first time, expanded on previous findings and showed that BMP10 was strongly and independently associated with ischaemic stroke in patients with AF, treated or not treated with OAC. This association of BMP10 with ischaemic stroke is potentially due to similar pathways reflecting a higher AF burden and genetic predisposition not captured by clinical characteristics or specifically by the currently established risk biomarkers.
Strengths and limitations
This study is one of the first and definitely the largest evaluations of circulating BMP10 in the clinical setting. The evaluation is based on very precise measurements of BMP10 using a novel prototype assay from Roche Diagnostics. Other strengths include the systematic evaluation of BMP10, the use of several models of adjustments, and the consistency of the findings in three independent, very large, well-characterised cohorts of patients with AF. We lack imaging characteristics of the left atrium and thus cannot correlate BMP10 levels, with atrial size measures. The absolute risk was estimated without accounting for the competing risk of death. This means that the risk should be interpreted as conditional on survival. However, with the relatively short follow-up time and with the relatively low proportion of patients dying before the event of interest, the impact of competing risk from death is negligible. Another limitation of the present study is that it is based on clinical trial populations at an increased risk of stroke and may therefore not be immediately extrapolated to a very low-risk AF population.
Conclusions
Plasma levels of the atrial biomarker BMP10 were independently associated with the risk of ischaemic stroke in patients with AF, irrespective of OAC treatment. BMP10 showed an incremental discriminatory value for the risk of ischaemic stroke beyond clinical characteristics and NT-proBNP, and it may be a novel specific biomarker for stroke in patients with AF.
Supplementary Material
Contributor Information
Ziad Hijazi, Uppsala Clinical Research Center, Uppsala University, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden; Department of Medical Sciences, Cardiology, Uppsala University, Ingång 40, 751 85 Uppsala, Sweden.
Alexander P Benz, Population Health Research Institute, McMaster University, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada; Department of Cardiology, Cardiology I, University Medical Center Mainz, Johannes Gutenberg-University, Langenbeckstraße 1, 55131 Mainz, Germany.
Johan Lindbäck, Uppsala Clinical Research Center, Uppsala University, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden.
John H Alexander, Duke Clinical Research Institute, Duke University School of Medicine, 300 W. Morgan Street Durham, NC 27701, USA.
Stuart J Connolly, Population Health Research Institute, McMaster University, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada.
John W Eikelboom, Population Health Research Institute, McMaster University, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada.
Christopher B Granger, Duke Clinical Research Institute, Duke University School of Medicine, 300 W. Morgan Street Durham, NC 27701, USA.
Peter Kastner, Roche Diagnostics GmbH, Nonnenwald 2, DE-82377 Penzberg, Germany.
Renato D Lopes, Duke Clinical Research Institute, Duke University School of Medicine, 300 W. Morgan Street Durham, NC 27701, USA.
André Ziegler, Roche Diagnostics GmbH, Nonnenwald 2, DE-82377 Penzberg, Germany.
Jonas Oldgren, Uppsala Clinical Research Center, Uppsala University, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden; Department of Medical Sciences, Cardiology, Uppsala University, Ingång 40, 751 85 Uppsala, Sweden.
Agneta Siegbahn, Uppsala Clinical Research Center, Uppsala University, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden; Department of Medical Sciences, Clinical Chemistry, Uppsala University, Ingång 40, 751 85 Uppsala, Sweden.
Lars Wallentin, Uppsala Clinical Research Center, Uppsala University, Dag Hammarskjölds väg 38, 751 85 Uppsala, Sweden; Department of Medical Sciences, Cardiology, Uppsala University, Ingång 40, 751 85 Uppsala, Sweden.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The ACTIVE A trial was supported by grants from Sanofi and Bristol Myers Squibb (BMS) Co., Princeton, NJ, USA. The AVERROES trial was funded by BMS and Pfizer Inc., New York, NY, USA, and coordinated by the Population Health Research Institute, Hamilton, Canada. The ARISTOTLE trial was funded by BMS and Pfizer Inc., New York, NY, USA, and coordinated by the Duke Clinical Research Institute, Durham, North Carolina, USA, and Uppsala Clinical Research Center, Uppsala, Sweden. Biomarker analyses were supported by Roche Diagnostics International, Rotkreuz, Switzerland. Dr Hijazi receives research support from The Swedish Society for Medical Research (S17-0133), Hjärt-Lungfonden (The Swedish Heart Lung Foundation, 20200722), and Uppsala University Hospital, Sweden.
Data availability
The data underlying this article are available from the corresponding author upon reasonable request.
Permissions information
The authors do hereby declare that all illustrations and figures in the manuscript are original and not require reprint permission.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
- 4. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al. A biomarker-based risk score to predict death in patients with atrial fibrillation: the ABC (age, biomarkers, clinical history) death risk score. Eur Heart J 2018;39:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J 2013;34:1475–1480. [DOI] [PubMed] [Google Scholar]
- 6. Reyat JS, Chua W, Cardoso VR, Witten A, Kastner PM, Kabir SN, et al. Reduced left atrial cardiomyocyte PITX2 and elevated circulating BMP10 predict atrial fibrillation after ablation. JCI Insight 2020;5:e139179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009;360:2066–2078. [DOI] [PubMed] [Google Scholar]
- 8. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 9. Benz AP, Hijazi Z, Lindbäck J, Connolly SJ, Eikelboom JW, Oldgren J, et al. Biomarker-based risk prediction with the ABC-AF scores in patients with atrial fibrillation not receiving oral anticoagulation. Circulation 2021;143:1863–1873. [DOI] [PubMed] [Google Scholar]
- 10. Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, et al. Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 2010;159:331–339. [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 12. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Alexander JH, Atar D, et al. High-sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin. J Am Coll Cardiol 2014;63:52–61. [DOI] [PubMed] [Google Scholar]
- 13. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, et al. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial (apixaban for the prevention of stroke in subjects with atrial fibrillation). J Am Coll Cardiol 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 14. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 15. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546. [PubMed] [Google Scholar]
- 16. Harrell FE Jr. Statistically Efficient Ways to Quantify Added Predictive Value of New Measurements. Statistical Thinking 2022. Available from: https://www.fharrell.com/post/addvalue/(accessed 30 Aug 2022).
- 17. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2021. [Google Scholar]
- 18. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer, 2015. [Google Scholar]
- 19. Harrell FE. rms: Regression Modeling Strategies. R package version 62-0 2021; https://CRAN.R-project.org/package=rms.
- 20. Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem 2017;63:152–164. [DOI] [PubMed] [Google Scholar]
- 21. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J 2016;37:2869–2878. [DOI] [PubMed] [Google Scholar]
- 22. Tomasdottir M, Friberg L, Hijazi Z, Lindbäck J, Oldgren J. Risk of ischemic stroke and utility of CHA(2) DS(2) -VASc score in women and men with atrial fibrillation. Clin Cardiol 2019;42:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandhu RK, Ezekowitz JA, Hijazi Z, Westerbergh J, Aulin J, Alexander JH, et al. Obesity paradox on outcome in atrial fibrillation maintained even considering the prognostic influence of biomarkers: insights from the ARISTOTLE trial. Open Heart 2018;5:e000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. St. Peter JV, Hartley GG, Murakami MM, Apple FS. B-type natriuretic peptide (BNP) and N-terminal pro-BNP in obese patients without heart failure: relationship to body mass index and gastric bypass surgery. Clin Chem 2006;52:680–685. [DOI] [PubMed] [Google Scholar]
- 25. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 26. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2010;55:747–753. [DOI] [PubMed] [Google Scholar]
- 28. Lemmens R, Buysschaert I, Geelen V, Fernandez-Cadenas I, Montaner J, Schmidt H, et al. The association of the 4q25 susceptibility variant for atrial fibrillation with stroke is limited to stroke of cardioembolic etiology. Stroke 2010;41:1850–1857. [DOI] [PubMed] [Google Scholar]
- 29. Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, Scheld HH, et al. PITX2c Is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet 2011;4:123–133. [DOI] [PubMed] [Google Scholar]
- 30. Syeda F, Holmes AP, Yu TY, Tull S, Kuhlmann SM, Pavlovic D, et al. PITX2 modulates atrial membrane potential and the antiarrhythmic effects of sodium-channel blockers. J Am Coll Cardiol 2016;68:1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from the corresponding author upon reasonable request.