Abstract
Background and study aims Gastric outlet obstruction (GOO) is traditionally managed with surgical gastroenterostomy (surgical-GE) and enteral stenting (ES). Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) is now a third option. Large studies assessing their relative risks and benefits with adequate follow-up are lacking. We conducted a comparative analysis of patients who underwent EUS-GE, ES, or surgical-GE for GOO.
Patients and methods In this retrospective comparative cohort study, consecutive patients presenting with GOO who underwent EUS-GE, ES, or surgical-GE at two academic institutions were reviewed and independently cross-edited to ensure accurate reporting. The primary outcome was need for reintervention. Secondary outcomes were technical and clinical success, length of hospital stay (LOS), and adverse events (AEs).
Results A total of 436 patients (232 EUS-GE, 131 ES, 73 surgical-GE) were included. The median duration of follow-up of the entire cohort was 185.5 days (interquartile range 55.25–454.25 days). The rate of reintervention in the EUS-GE group was lower than in the ES and surgical-GE groups (0.9 %, 12.2 %, and 13.7 %, P < 0.0001). Technical success was achieved in 98.3 %, 99.2 %, and 100 % ( P = 0.58), and clinical success was achieved in 98.3 %, 91.6 %, and 90.4 % ( P < 0.0001) in the EUS-GE, ES, and surgical-GE groups, respectively. The EUS-GE group had a shorter LOS (2 days vs. 3 days vs. 5 days, P < 0.0001) and a lower AE rate than the ES and surgical-GE groups (8.6 % vs. 38.9 % vs. 27.4 %, P < 0.0001).
Conclusion This large cohort study demonstrates the safety and palliation durability of EUS-GE as an alternative strategy for GOO palliation in select patients.
Introduction
Gastric outlet obstruction (GOO) is a clinical syndrome characterized by abdominal pain and postprandial emesis because of different benign and malignant etiologies 1 2 . GOO can significantly impair quality of life and require interventions to palliate debilitating symptoms 3 . Surgical gastroenterostomy (surgical-GE) and enteral stenting (ES) are the historical gold standard therapies offered for management of GOO. Several prior studies have compared these two approaches 4 5 6 7 8 9 . Surgical-GE and ES offer comparable technical and clinical success; however, ES is limited by stent patency duration, higher rate of reintervention, and limiting access to the major papilla for concurrent pancreaticobiliary interventions often required in the palliative setting. Surgical-GE has a higher rate of adverse events (AEs) and is more invasive.
Endoscopic ultrasound-guided gastroenterostomy (EUS-GE) has emerged as a third option. This approach allows endoscopists to create a gastrointestinal anastomosis, which bypasses the obstruction using a lumen-apposing metal stent (LAMS) inserted and deployed under EUS guidance. It has been increasingly utilized in recent years, and several studies have demonstrated its favorable outcomes and safety risk profile 10 11 12 13 14 . Previous studies have directly compared the relative risks and benefits of EUS-GE to surgical-GE and ES 15 16 17 18 19 20 21 22 . Most studies had a small sample size and they reported only short-term follow-up from heterogeneous data compiled from large number of centers each contributing a small number of patients, a potential confounding factor. We hypothesize that EUS-GE can provide similar outcomes to surgical-GE in terms of luminal patency, but with lower procedural-associated morbidity and can maintain a longer luminal patency period with no excess risks compared to ES. Therefore, we conducted a large international comparative study assessing treatment outcomes and AE of EUS-GE vs. ES and surgical-GE for management of GOO.
Patients and methods
This was an international comparative retrospective cohort study of EUS-GE vs. ES and surgical-GE for the treatment of GOO. Our cohort consisted of GOO cases from two academic institutions (Mayo Clinic Rochester, n = 265, Universitair Ziekenhuis Brussel, n = 171) between January 2002 and June 2021. This study was approved by the Mayo Clinic Institutional Review Board (IRB 19-006760) and was conducted following the guidelines outlined in the Declaration of Helsinki.
Patients were identified through the prospectively maintained clinical data repository that allows case identification through its search functionality. Consecutive patients were adults aged 18 years or older with GOO from either benign or malignant etiology who underwent ES, EUS-GE, or surgical-GE. Excluded patients were those with surgically altered upper gastrointestinal tract anatomy, insufficient medical records, or inadequate follow-up to determine technical or clinical success. GOO was diagnosed by imaging and/or endoscopy. Information on patient baseline characteristics, previous treatment attempts (endoscopic balloon dilation or self-expandable stent placement [SEMS]), GOO characteristics, treatment outcomes, and AEs was collected in a web-based secured database. The functional status of patients with malignant GOO was determined using Eastern Cooperative Oncology Group (ECOG) Performance Status. The severity of GOO was determined according to the GOO symptom score system (GOOSS; 0 = no oral intake, 1 = liquid diet only, 2 = soft diet, and 3 = low-residue or full diet) 23 . Data were extracted up to the last date available in the medical record or the date of death. Auditing of both centers’ databases was performed to ensure quality and integrity.
EUS-GE procedure
All procedures were performed under general anesthesia. Different techniques of EUS-GE were used at the discretion of the performing endoscopist, using 15-mm or 20-mm electrocautery-enhanced LAMS (Hot AXIOS, Boston Scientific Corporation Inc., Marlborough, Massachusetts, United States) for the creation of anastomoses. Technical details of these techniques have been described in previously published studies 11 14 24 . In principle, a curved linear-array echoendoscope (EUS) was introduced into the stomach. A segment of small bowel was identified by EUS, typically proximal jejunum, and a LAMS was then advanced through the gastric and enteric wall into the small bowel, where it was deployed. Advancement was facilitated by the electrocautery-enhanced tip of the device and can be directly, without use of any guidewire or over-the-wire, which was inserted through and following previous puncture by 19-gauge EUS needle.
In the vast majority of cases, EUS-coupling was enhanced by irrigation of the small bowel behind the obstruction with saline, often mixed with contrast agent and thus facilitating safe identification of an enteral segment suitable for creation of EUS-GE. Such intralumenal injection of saline also allows sufficient distention of the small bowel by creating a bigger target to advance the LAMS introducer into and it provides enough space to deploy enteral flange of the LAMS. Irrigation was performed by using various devices, including nasoenteral and oroenteral tubes, a double-balloon occlusion tube in the so-called EPASS technique or a pediatric gastroscope inserted beyond the obstruction in which this particular technique allows both irrigation of the small bowel as well as retrograde visualization of the puncture and with the possibility of grasping wire and thus stabilizing and securing the guidewire position. The procedure was then performed with both the echoendoscope and pediatric gastroscope inserted simultaneously. Less frequently, a balloon catheter technique was used, in which the balloon expanded by saline represents the target for puncture and guidewire insertion, whereas no saline is directly injected into the enteric lumen.
Surgical-GE procedure
Patients underwent surgery under general anesthesia in the standard surgical setting of an operating room. Laparoscopic surgery was a preferred approach. If patients were not eligible for a laparoscopic approach, they underwent exploratory laparotomy with creation of gastroenterostomy.
ES procedure
The procedure was performed with a therapeutic gastroscope under general anesthesia. The site of stenosis was traversed by a guidewire. Contrast was injected via the gastroscope or balloon catheter to assess the length of stenosis and aid in selecting an appropriate stent length. An uncovered SEMS was then deployed across the obstruction under endoscopic and fluoroscopic guidance, respectively.
Definitions and outcome assessment
The primary outcome was the rate of reintervention for recurrent GOO or inadequate palliation. Secondary outcomes were technical success, clinical success, length of hospital stay (LOS), and AEs. Technical success was defined as successful creation of an anastomosis in the EUS-GE and surgical-GE groups, and a successful stent deployment and placement across the obstruction in the ES group. Clinical success was defined as the ability to tolerate at least a full liquid diet within 2 weeks. LOS was the time from procedure to discharge. During follow-up, if patients developed symptoms concerning for recurrent GOO, an endoscopic and or radiographic evaluation was then pursued.
Statistical analysis
Data were expressed as mean and standard deviation (SD) for continuous variables with normal distribution or median and interquartile range (IQR) for skewed data and proportions for categorical variables. Continuous data were compared using a one-way analysis of variance (ANOVA) with Student’s t -test of each pair if the ANOVA test was significant (F-test < 0.05). Nonparametric continuous data were compared using the Wilcoxon rank-sum test with the Wilcoxon rank-sum test of each pair if the overall P value was significant. Categorical data were analyzed using a Chi-square test with a pairwise Chi-square test if the overall P value was significant. A subgroup analysis of patients with malignant GOO was also performed. Multivariate logistic regression analysis was performed to determine the independent variables influencing the need for reintervention. Time-to-event analysis based on the Kaplan-Meier method and the log-rank test was conducted to determine the differences in the time to reintervention in each treatment arm. The analysis was performed using SPSS version 28.0 (SPSS Inc., Chicago, Illinois, United States). P < 0.05 was considered significant. A Bonferroni correction was applied to each set of analyses to control the family-wise error rate. The Bonferroni adjusted P of 0.0167 (0.05 /3 variables) was used to determine statistical significance for a comparison of each pair.
Results
A total of 436 patients (232 EUS-GE, 131 ES, and 73 surgical-GE) were included from two academic institutions. The mean age was 64.8 ± 12.6 years, and 40.2 % were women. Of the patients, 97.9 % were symptomatic and 82.6 % had malignant etiology. The most common malignant etiologies were pancreatic cancer (n = 175, 48.6 %), metastatic cancer involving the duodenum (n = 67, 18.6 %), and biliary/gallbladder cancer (n = 46, 12.8 %). The most common benign etiologies were chronic pancreatitis (n = 24, 31.5 %), followed by acute or recurrent pancreatitis (n = 13, 17.1 %). The median duration of follow-up of the entire cohort was 185.5 days (IQR 55.25–454.25 days). The median follow-up for the EUS-GE and the surgical-GE cohorts was longer at 233 days (IQR 95.3–498.5 days) and 331 days (IQR 66–1071.5 days), respectively, compared to the ES cohort 56 days (IQR 22–208 days). Table 1 outlines patient baseline and GOO characteristics stratified by each treatment modality. Baseline characteristics among the three groups were comparable except for race, etiology of GOO, symptoms of GOO, GOOSS, site of GOO, prior treatment attempts, and ECOG performance status (all P < 0.05).
Table 1. Patient and GOO characteristics.
| EUS-GE (n = 232) | ES (n = 131) | Surgical-GE (n = 73) | Overall P value | P-value EUS-GE vs. ES † | P-value EUS-GE vs. surgical-GE 1 | |
| Age (years, mean ± SD) | 64.5 ± 12.3 | 66.9 ± 11.8 | 62.1 ± 14.4 | 0.03* | 0.07 | 0.16 |
| Male gender (N, %) | 135 (58.2) | 67 (51.1) | 36 (49.3) | 0.26 | 0.19 | 0.18 |
| Race (N, %) | 0.02* | 0.06 | 0.02 | |||
|
228 (98.3) | 124 (94.7) | 67 (91.8) | |||
|
4 (1.7) | 7 (5.3) | 6 (8.2) | |||
| Prior treatment attempts (N, %) | ||||||
|
15 (6.5) | 39 (29.8) | 15 (20.5) | < 0.0001* | < 0.0001* | < 0.0001* |
|
12 (5.2) | 31 (23.7) | 22 (30.1) | < 0.0001* | < 0.0001* | < 0.0001* |
| ECOG status (mean ± SD) | 1.2 ± 0.6 | 1.5 ± 1.2 | 0.4 ± 0.6 | < 0.0001* | 0.03 | 0.005* |
| Presence of ascites (N, %) | 57 (24.6) | 36 (27.5) | 11 (15.1) | 0.13 | 0.20 | 0.02 |
| Presence of peritoneal carcinomatosis (N, %) | 27 (11.6) | 26 (19.8) | 7 (9.6) | 0.05* | 0.03 | 0.63 |
| Etiology of GOO (N, %) | < 0.0001* | < 0.0001* | < 0.0001* | |||
|
191 (82.3) | 126 (96.2) | 43 (58.9) | |||
|
41 (17.7) | 5 (3.8) | 30 (41.1) | |||
| Symptomatic GOO (N, %) | 231 (99.6) | 130 (99.2) | 66 (90.4) | < 0.0001* | 1.00 | < 0.0001* |
|
194 (83.6) | 113 (86.3) | 53 (72.6) | 0.04* | 0.50 | 0.04 |
|
113 (48.7) | 94 (71.8) | 34 (46.6) | < 0.0001* | < 0.0001* | 0.75 |
|
115 (49.6) | 87 (66.4) | 22 (30.1) | < 0.0001* | 0.002* | 0.004* |
| Severity of GOO (mean ± SD) | 1.3 ± 0.8 | 0.8 ± 1.1 | 1.3 ± 1.1 | < 0.0001* | < 0.0001* | 0.80 |
| Site of GOO (N, %) | ||||||
|
11 (4.7) | 13 (9.9) | 20 (27.4) | < 0.0001* | < 0.0001* | < 0.0001* |
|
27 (11.6) | 44 (33.6) | 20 (27.4) | |||
|
109 (47.0) | 54 (41.2) | 20 (27.4) | |||
|
84 (36.2) | 18 (13.7) | 9 (12.3) | |||
|
1 (0.4) | 2 (1.5) | 4 (5.5) | |||
| Duration of follow-up (days, median [IQR]) | 233 (95.3–498.5) | 56 (22–208) | 331 (66–1071.5) | < 0.0001* | < 0.0001* | 0.18 |
* statistically significant
ECOG, Eastern Cooperative Oncology Group performance status; ES, enteral stenting; EUS-GE, endoscopic ultrasound-guided gastroenterostomy; GOO, gastric outlet obstruction; IQR, interquartile range; surgical-GE, surgical gastroenterostomy.
P < 0.0167 indicates statistical significance based on a Bonferroni correction
The techniques of EUS-GE used were as follows: direct approach with no assisting devices (n = 22), enteral catheter-assisted method (n = 186), balloon catheter-assisted method (n = 11), pediatric gastroscope-assisted method (n = 10), and double-balloon enteric tube-assisted method (n = 3). Two patients had initial stent maldeployment, but subsequently achieved a successful LAMS placement with an intervention continued in the same endoscopic session and modified by using a pediatric gastroscope-assisted method. These two patients, therefore, were not considered as technical failures based on our study definition. For the ES cohort, 127 patients had an uncovered stent and four patients had a covered stent. In the surgical-GE cohort, the anastomotic creation techniques were antecolic gastrojejunostomy in 32 patients, retrocolic gastrojejunostomy in 10 patients, and no data available in 31 patients.
Clinical outcomes
The treatment outcomes of the three groups are summarized in Table 2 . Technical success was achieved in 98.3 %, 99.2 %, and 100 % in the EUS-GE, ES, and surgical-GE groups, respectively ( P = 0.58). Of four EUS-GE patients with technical failures, three were due to stent maldeployment. Following attempted EUS-GE, one patient underwent surgical-GE, the family of the second patient elected comfort care measures only, and the third patient underwent nasojejunal feeding tube placement. The fourth patient had a long-segment intramural metastasis to the small bowel; thus the EUS-GE was not completed and the patient later underwent surgical-GE.
Table 2. Treatment outcomes and adverse events.
| EUS-GE (n = 232) | ES (n = 131) | Surgical-GE (n = 73) | Overall P value | P value EUS-GE vs. ES † | P value EUS-GE vs. surgical-GE 1 | |
| Technical success (N, %) | 228 (98.3) | 130 (99.2) | 73 (100.0) | 0.58 | 0.66 | 0.58 |
| Clinical success (N, %) | 228 (98.3) | 120 (91.6) | 66 (90.4) | 0.002* | 0.002* | 0.005* |
| Length of hospital stay (days, median [IQR]) | 2 (1–3) | 3 (1–10) | 5 (2–9) | < 0.0001* | < 0.0001* | 0.18 |
| Rate of re-intervention (N, %) | 2 (0.9) | 16 (12.2) | 10 (13.7) | < 0.0001* | < 0.0001* | < 0.0001* |
| AEs, N (%) | 20 (8.6) | 51 (38.9) | 20 (27.4) | < 0.0001* | < 0.0001* | < 0.0001* |
|
2 (0.9) | 1 (0.8) | 2 (2.7) | |||
|
4 (1.7) | 0 | 0 | |||
|
0 | 1 (0.8) | 8 (10.9) | |||
|
8 (3.4) | 9 (6.9) | 4 (5.5) | |||
|
1 (0.4) | 0 | 2 (2.7) | |||
|
0 | 3 (2.3) | 1 (1.4) | |||
|
4 (1.7) | 4 (3.1) | 2 (2.7) | |||
|
1 (0.4) | 4 (3.1) | 0 | |||
|
0 | 9 (6.9) | 0 | |||
|
1 (0.4) | 12 (9.2) | 0 | |||
|
1 (0.4) | 0 | 0 | |||
|
1 (0.4) | 2 (1.5) | 1 (1.4) | |||
|
5 (2.2) | 12 (9.2) | 6 (4.6) |
* statistically significant
AE, adverse event; ES, enteral stenting; EUS-GE, endoscopic ultrasound-guided gastroenterostomy; surgical-GE, surgical gastroenterostomy.
P < 0.0167 indicates statistical significance based on a Bonferroni correction
The clinical success rate for EUS-GE was significantly higher than in the ES and surgical-GE groups (98.3 % vs. 91.6 %, and 90.4 %, respectively, P = 0.002). Furthermore, the rate of reintervention in the EUS-GE group was significantly lower than in the ES and surgical GE groups (0.9 %, 12.2 %, and 13.7 %, respectively, P < 0.0001). The length of post-procedural hospital stay in the EUS-GE group was shorter than in the ES and surgical GE groups (median LOS: 2 days [IQR 1–3 days], 3 days [IQR 1–10 days], and 5 days [IQR 2–9 days], respectively, P < 0.0001). A subgroup analysis of treatment outcomes in patients with malignant GOO demonstrated similar results to the principal analysis (Supplementary Table 1). Multivariable logistic regression analysis adjusting for etiology, severity and site of GOO, prior enteral stenting and endoscopic dilation, and ECOG status showed that EUS-GE (odds ratio [OR] = 0.10 [95 % CI 0.01–0.94] P = 0.04) was a negative predictor of reintervention relative to ES ( Table 3 ). Compared to EUS-GE, Kaplan-Meier curves for time to reintervention demonstrated a shorter interval in the ES group (log-rank test, P < 0.0001) and surgical-GE group (log-rank test, P < 0.0001) ( Fig. 1 ).
Table 3. Multivariate logistic regression analysis of predictors of need for reintervention.
| Reintervention | ||
| Type of treatment | ||
| ES | 1 | Ref |
| EUS-GE | 0.10 (0.01–0.94) | 0.04* |
| Surgical-GE | 1.29 (0.24–6.79) | 0.77 |
| Type of GOO (malignant vs. benign) | – | 0.99 |
| GOO severity | 0.76 (0.35–1.64) | 0.48 |
| Site of GOO (distal duodenum vs. others) | 0.46 (0.08–2.48) | 0.37 |
| Prior enteral stenting | 1.63 (0.35–7.48) | 0.53 |
| Prior endoscopic dilation | 1.30 (0.24–7.16) | 0.76 |
| ECOG status | 1.00 (0.51–1.98) | 0.76 |
ECOG, Eastern Cooperative Oncology Group performance status; ES, enteral stenting; EUS-GE, endoscopic ultrasound-guided gastroenterostomy; GOO, gastric outlet obstruction; surgical-GE, surgical gastroenterostomy.* Indicates statistical significance
Fig. 1.
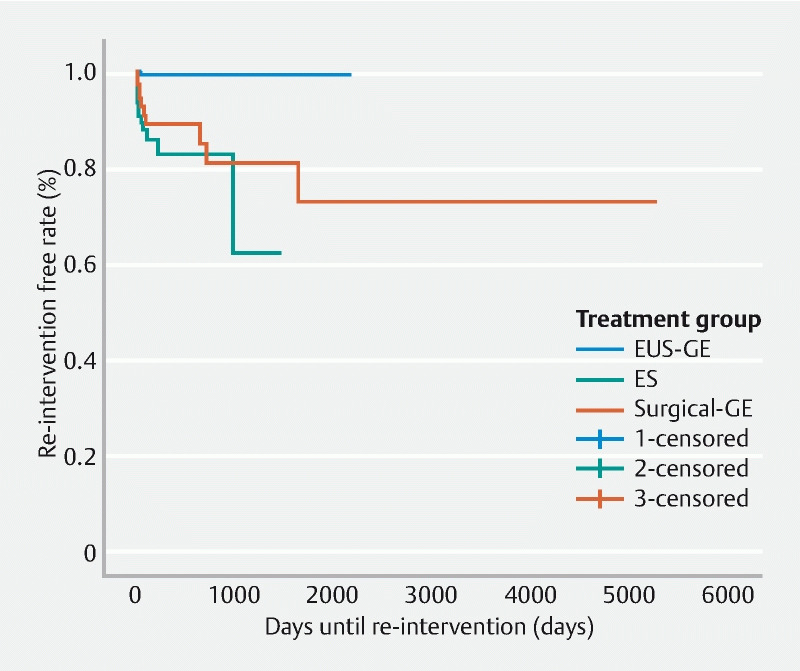
Kaplan-Meier curves of time to re-intervention: EUS-GE, ES, and Surgical-GE. Log-rank test: < 0.0001.
Adverse events
Compared to EUS-GE, the overall rates of AE were higher in both the ES (38.9 % vs. 8.6 %, P < 0.0001) and surgical-GE groups (27.4 % vs. 8.6 %, P < 0.0001). Regarding stent-related AEs, stent obstruction, tumor ingrowth, and stent migration occurred in 12, 9, and 4 patients in the ES group, respectively, whereas stent obstruction, stent migration and inadequate stent expansion occurred in one patient each in the EUS-GE group. In the surgical-GE group, the most common AEs were ileus/gastroparesis, infection, and anastomotic leak in eight, four, and two patients, respectively. None of the EUS-GE patients had ileus/gastroparesis or wound infection. In one patient with delayed stent migration within 1 week after EUS-GE, an anastomotic leak and abdominal infection with peritonitis developed requiring open surgical intervention. Of note, a 15-mm AXIOS stent was used with no immediate complications and no ascites pre-procedure.
Discussion
Given the limitations of ES and surgical-GE, the advent of EUS-GE represents an appealing alternative for management of GOO, as it is a minimally invasive option with improved palliation 18 20 . In this study, we performed a large, international two-center analysis of all three techniques and demonstrated a decreased rate of reintervention in the EUS-GE cohort compared to surgical-GE and ES with superior clinical resolution of the GOO and safety over an extended period of follow-up. These findings persisted in a subgroup analysis of malignant GOO and in a multivariate logistic regression after adjustment for potential confounders.
Our study reported a technical success rate of 98.3 % that was comparable to ES (99.2 %) and surgical-GE (100 %) ( P = 0.58). EUS-GE is considered technically challenging, with a reported failure rate of close to 10 % in the literature 12 13 17 18 20 . It should be noted that all EUS-GE procedures in our study were performed by experienced endoscopists. Two patients who had initial stent maldeployment subsequently achieved successful LAMS placement with a rescued intervention in the same endoscopic session. Etiologies of stent maldeployment during EUS-GE include lack of appropriate endoscopic accessories to stabilize the targeted small bowel during stent deployment, misidentification of the transverse colon as small bowel, and premature expansion of the distal LAMS flange in the peritoneal cavity 13 24 . Although we have demonstrated that a reattempt at EUS-GE during the same session can be successful, we do advocate for caution and careful assessment of risksw, benefits, and alternative approaches in the setting of sent maldeployment. In addition, the consequences and need for endoscopic closure of the maldeployed LAMS tract is still not clear.
The EUS-GE technique is still in early phases of development and will continue to evolve with the advent of purpose-built accessories to improve the efficiency and safety of this technique. Several EUS-GE techniques have been described; however, the optimal approach has not yet been established 10 11 24 . In our study, four technically unsuccessful EUS-GE cases were from different techniques, including direct method with no assisting devices (n = 2) and enteral catheter-assisted method (n = 2). Further studies are needed to address the optimal technique to maximize a safe and successful LAMS placement.
Clinical success was significantly higher in the EUS-GE (98.3%) compared to the ES (91.6 %) and surgical-GE groups (90.4 %). In our surgical-GE cohort, eight patients (10.9 %) had post-surgical gastroparesis prohibiting them from tolerating diet. This AE is not uncommon after surgical-GE, reportedly 2% to 10 % in previous studies 18 20 25 . Abdominal surgeries, especially pylorus-preserving operations, are a common trigger for postoperative gastroparesis. This AE usually occurs when a patient starts on solid diet. Importantly, gastroparesis was not observed after EUS-GE in our cohort or in the published literature, possibly contributing to the higher clinical success rate observed with EUS-GE 12 14 16 17 18 20 .
We hypothesized that EUS-GE has a lower rate of procedure-associated morbidity than surgical-GE. Our study demonstrated a significantly lower AE rate in the EUS-GE group than the other two modalities. The most common AEs of EUS-GE were infection (3.4 %), followed by stent maldeployment (2.2 %) and bleeding (1.7 %). Stent obstruction was the most common AE of ES (n = 12, 9.2 %). This AE was much less common in the EUS-GE group and occurred in one case (0.4 %). This could arise from the loss of the silicone coating covering the LAMS, resulting in ingrowth of surrounding tissue into the stent lumen. Although rare, this situation could be salvaged by a stent-in-stent placement endoscopically as previously described 26 . About 25 % of the EUS group had ascites. A previous study 27 showed comparable clinical success and survival rates between patients with and without ascites undergoing EUS-GE. The rate of peritonitis/sepsis was 8.3 % in the ascites group, compared with none in the no-ascites group. Further study is needed to determine the appropriate candidates for EUS-GE with ascites. Nonetheless, the risk-benefit discussion with a patient for shared decision-making in the setting of risk mitigation with prophylactic antibiotic and paracentesis should be exercised.
The significantly lower reintervention rate in EUS-GE group can be attributed to permanently indwelling coated LAMS, with only rarely observed overgrowth by mucosal hyperplasia and almost absent observations of stent ingrowth. Regardless of the stent designs (covered or uncovered), stent dysfunction due to ingrowth and overgrowth was frequently observed in the ES group, which limits stent durability. In the EUS-GE group, in contrast, stent ingrowth and overgrowth were rarely observed. Stent occlusions in the EUS-GE group are also rarely seen, both in 15-mm and 20-mm diameter stent size. Low-fiber diet was the standard in all stented patients and short length of LAMS is likely to contribute favorably to the statistically significant lower reintervention rate. In the surgical group, most reinterventions were related to disease progression, anastomotic stricture, adhesions, and jejunogastric intussusception.
This study has several limitations inherent in its retrospective methodology. These include residual confounders that we could not adjust for in the analysis, selection bias based on the non-randomized nature of the study, heterogeneity introduced by inclusion of multiple endoscopists and surgeons, missing data for some patients, limited follow-up duration, and changes in practice patterns over time. However, we attempted to adjust for confounders using statistical modeling and we limited the study to two centers to minimize heterogeneity and control data quality by cross auditing the data. Second, it also should be noted that candidates who underwent ES or EUS-GE might be too ill to undergo surgery, which could lead to selection bias. Third, most of our cohort (69.7 %) died during the study period secondary to underlying malignancy.
Conclusions
In conclusion, EUS-GE could offer a lower rate of reintervention, longer patency duration compared to ES, and a lower rate of AEs compared to ES and surgical-GE with comparable technical success and higher clinical success rates. However, further studies are needed to confirm these findings and address the optimal EUS-GE technique and patient selection to maximize technical success and clinical benefits.
Footnotes
Competing interests Dr. Abu Dayyeh is a consultant for Endogenex, Endo-TAGSS, Metamodix, and BFKW; is a consultant for and receives grant/research support from USGI, Boston Scientific; receives grant/research support from Cairn Diagnostics, Aspire Bariatrics; has speaker roles with Olympus, Johnson and Johnson; has speaker and grant/research support from Medtronic, Endogastric solutions; and has research support from Apollo Endosurgery, and Spatz Medical. Dr. Law is a consultant for ConMed and Medtronic. Dr. Chandrasekhara is a consultant for Boston Scientific, Covidien, and LP; a shareholder at Nevakar Corporation; and on the medical advisory board for Interpace Diagnostics. Dr. Storm is a consultant for Apollo Endosurgery, ERBE, GI Dynamics, and Olympus and received research grant support from Apollo Endosurgery, Boston Scientific, Endo-TAGSS, Endogenex, and Enterasense. Dr. Marya is a consultant for AnX Robotica. Dr. Kunda is a consultant of Boston Scientific, Omega Medical Imaging, Apollo Endosurgery, and Olympus, Ambu, M.I.Tech, Medconsgroup, EndiaTx, Q3 Medical – AMG International and Tigen Pharma.
Supplementary material :
References
- 1.Chowdhury A, Dhali G K, Banerjee P K. Etiology of gastric outlet obstruction. Am J Gastroenterol. 1996;91:1679. [PubMed] [Google Scholar]
- 2.Koop A H, Palmer W C, Stancampiano F F. Gastric outlet obstruction: A red flag, potentially manageable. Cleve Clin J Med. 2019;86:345–353. doi: 10.3949/ccjm.86a.18035. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt C, Gerdes H, Hawkins W et al. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg. 2009;198:92–99. doi: 10.1016/j.amjsurg.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasegaram M D, Eslick G D, Mansfield C O et al. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction. Surg Endosc. 2012;26:323–329. doi: 10.1007/s00464-011-1870-3. [DOI] [PubMed] [Google Scholar]
- 5.Jang S H, Lee H, Min B H et al. Palliative gastrojejunostomy versus endoscopic stent placement for gastric outlet obstruction in patients with unresectable gastric cancer: a propensity score-matched analysis. Surg Endosc. 2017;31:4217–4223. doi: 10.1007/s00464-017-5480-6. [DOI] [PubMed] [Google Scholar]
- 6.Khashab M, Alawad A S, Shin E J et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27:2068–2075. doi: 10.1007/s00464-012-2712-7. [DOI] [PubMed] [Google Scholar]
- 7.Mintziras I, Miligkos M, Wachter S et al. Palliative surgical bypass is superior to palliative endoscopic stenting in patients with malignant gastric outlet obstruction: systematic review and meta-analysis. Surg Endosc. 2019;33:3153–3164. doi: 10.1007/s00464-019-06955-z. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraja V, Eslick G D, Cox M R. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction-a systematic review and meta-analysis of randomized and non-randomized trials. J Gastrointest Oncol. 2014;5:92–98. doi: 10.3978/j.issn.2078-6891.2014.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy A, Kim M, Christein J et al. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc. 2012;26:3114–3119. doi: 10.1007/s00464-012-2301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irani S, Baron T H, Itoi T et al. Endoscopic gastroenterostomy: techniques and review. Curr Opin Gastroenterol. 2017;33:320–329. doi: 10.1097/MOG.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 11.Itoi T, Baron T H, Khashab M A et al. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig Endosc. 2017;29:495–502. doi: 10.1111/den.12794. [DOI] [PubMed] [Google Scholar]
- 12.Kerdsirichairat T, Irani S, Yang J et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019;7:E144–E150. doi: 10.1055/a-0799-9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty T R, Garg R, Thompson C C et al. Efficacy and safety of EUS-guided gastroenterostomy for benign and malignant gastric outlet obstruction: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E1474–E1482. doi: 10.1055/a-0996-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyberg A, Perez-Miranda M, Sanchez-Ocana R et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4:E276–E281. doi: 10.1055/s-0042-101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas A, Dolan R D, Bazarbashi A N et al. Endoscopic ultrasound-guided gastroenterostomy versus surgical gastrojejunostomy for the palliation of gastric outlet obstruction in patients with peritoneal carcinomatosis. Endoscopy. 2022;54:671–679. doi: 10.1055/a-1708-0037. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y I, Itoi T, Baron T H et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946–2952. doi: 10.1007/s00464-016-5311-1. [DOI] [PubMed] [Google Scholar]
- 17.Ge P S, Young J Y, Dong W et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33:3404–3411. doi: 10.1007/s00464-018-06636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khashab M A, Bukhari M, Baron T H et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–E281. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouanda A, Binmoeller K, Hamerski C et al. Endoscopic ultrasound-guided gastroenterostomy versus open surgical gastrojejunostomy: clinical outcomes and cost effectiveness analysis. Surg Endosc. 2021;35:7058–7067. doi: 10.1007/s00464-020-08221-z. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Miranda M, Tyberg A, Poletto D et al. EUS-guided Gastrojejunostomy Versus Laparoscopic Gastrojejunostomy: An International Collaborative Study. J Clin Gastroenterol. 2017;51:896–899. doi: 10.1097/MCG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 21.van Wanrooij R LJ, Vanella G, Bronswijk M et al. Endoscopic ultrasound-guided gastroenterostomy versus duodenal stenting for malignant gastric outlet obstruction: an international, multicenter, propensity score-matched comparison. Endoscopy. 2022;54:1023–1031. doi: 10.1055/a-1782-7568. [DOI] [PubMed] [Google Scholar]
- 22.Widmer J L, Winner M, Allendorf J et al. Single center comparative study of endoscopic gastrojejunostomy versus surgical gastrojejunostomy for malignant gastric outlet obstruction. Gastrointest Endosc. 2019;89:AB291. [Google Scholar]
- 23.Adler D G, Baron T H. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72–78. doi: 10.1111/j.1572-0241.2002.05423.x. [DOI] [PubMed] [Google Scholar]
- 24.Itoi T, Ishii K, Ikeuchi N et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016;65:193–195. doi: 10.1136/gutjnl-2015-310348. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L P, Tabrizian P, Nguyen S et al. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS. 2011;15:169–173. doi: 10.4293/108680811X13022985132074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyberg A, Zerbo S, Barthet M et al. A novel technique for salvaging a dislodged lumen-apposing metal stent during creation of an endoscopic gastrojejunostomy. Gastrointest Endosc. 2016;83:254. doi: 10.1016/j.gie.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud T, Storm A C, Law R J et al. Efficacy and safety of endoscopic ultrasound-guided gastrojejunostomy in patients with malignant gastric outlet obstruction and ascites. Endosc Int Open. 2022;10:E670–E678. doi: 10.1055/a-1797-9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


