Abstract
Solutions containing Ag0 nanoclusters, Ag+1, and higher oxidation state silver, generated from nanocrystalline silver dressings, were anti-inflammatory against porcine skin inflammation. The dressings have clinically-demonstrated broad-spectrum antimicrobial activity, suggesting application of nanosilver solutions in treating pulmonary infection.
Nanosilver solutions were tested for antimicrobial efficacy; against HSV-1 and SARS-CoV-2; and nebulized in rats with acute pneumonia. Patients with pneumonia (ventilated), fungal sinusitis, burns plus COVID-19, and two non-hospitalized patients with COVID-19 received nebulized nanosilver solution.
Nanosilver solutions demonstrated pH-dependent antimicrobial efficacy; reduced infection and inflammation without evidence of lung toxicity in the rat model; and inactivated HSV-1 and SARS-CoV-2. Pneumonia patients had rapidly reduced pulmonary symptoms, recovering pre-illness respiratory function. Fungal sinusitis-related inflammation decreased immediately with infection clearance within 21 days. Non-hospitalized patients with COVID-19 experienced rapid symptom remission.
Nanosilver solutions, due to anti-inflammatory, antiviral, and antimicrobial activity, may be effective for treating respiratory inflammation and infections caused by viruses and/or microbes.
Abbreviations: aMMP, active matrix metalloproteinase; ANOVA, analysis of variance; BCS, bovine calf serum; COVID-19, coronavirus disease of 2019; CT, computerized tomography; DNA, deoxyribonucleic acid; DNCB, dinitrochlorobenzene; EGF, epidermal growth factor; FDA, U.S. Food and Drug Administration; HSV, herpes simplex virus; IL, interleukin; IV, intravenous; KGF, keratinocyte growth factor; MEM, minimum essential media; MMP, matrix metalloproteinase; MDR, multi-drug resistant; pMMP, pro matrix metalloproteinase; PMN, polymorphonuclear leukocytes; PFU, plaque forming units; RNA, ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TGF, transforming growth factor; TNF, tumor necrosis factor; TUNEL, terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling
Keywords: Anti-inflammatory, Antiviral, Antimicrobial, SARS-CoV-2, Nanocrystalline silver
Graphical abstract
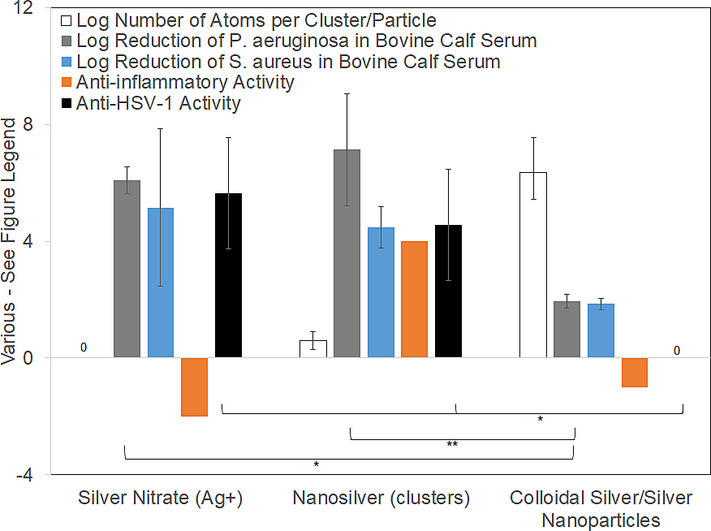
Antibacterial efficacy against P. aeruginosa and S. aureus in bovine calf serum, anti-HSV-1 activity (3 h challenge), anti-inflammatory activity based on literature review, and quantities of atoms per unit (cluster or particle) are compared when silver is delivered via various treatments. Only nanosilver solutions containing silver nanoclusters (Ag02–6), Ag+, and higher oxidation state silver generate antiviral, anti-inflammatory, and antimicrobial activity. For AgNO3, only Ag+(aq) is delivered. Testing demonstrated antibacterial and antiviral efficacy, but the treatment was moderately pro-inflammatory. Colloidal/nanoparticle silver, which contains larger quantities of atoms per particle/colloid, did not demonstrate antimicrobial or antiviral efficacy, and was mildly pro-inflammatory.
Introduction
History of silver in medicine
Silver has been used as an antimicrobial for centuries. In the 1800s, Crede's method of preventing neonatal conjunctivitis using 2 % AgNO3(aq) (followed by a saline rinse) proved effective because AgNO3 is surface-active and facilitates agglutination and inactivation of gonococci.1 Since then, the safety of silver, including nanosilver, has been established through >120 years of use worldwide. Stable aggregates of silver atoms (“colloidal silver” or “silver nanoparticles”) were introduced in the early 1920s,2., 3. but the initial U.S. Food and Drug Administration (FDA) support received was withdrawn due to lack of efficacy.4 The advent of more directed antimicrobials and discovery of antibiotics reduced silver use. In the 1960s, George Winter demonstrated that moist wound healing was better than dry healing, but moist environments promoted infection if no antimicrobials were present. Treatment of burn patients with 0.5 % AgNO3(aq), followed by 1 % silver sulfadiazine, led to a near-universal adoption of silver in burn units.5 The primary concern with silver use to treat large burns was electrolyte imbalance caused by chloride depletion, which was monitored and adjusted for by electrolyte administration. Patients were not exposed to enough silver to cause argyria (a cosmetic darkening of epithelial and other tissues, typically due to excessive silver consumption, causing reaction products of silver with proteins, unsaturated lipids, deoxyribonucleic acid (DNA), or ribonucleic acid (RNA) to deposit in epithelial tissues with no known pathology). In the 1990s, wound dressings, incorporating various forms of silver, including metallic silver, AgCl, and various silver salts, were introduced.6 Technological advancements in physical vapor deposition led to the development of nanocrystalline silver (nanosilver) dressings with an unstable crystalline silver lattice allowing for sustained release of Ag(0) clusters of 2–6 atoms, Ag+, and higher oxidation state silver species.7 Nanosilver dressings are currently used in burn units worldwide. Many studies examining nanosilver safety have been published (Table 1 ), showing that there is minimal risk to nanosilver use in infection treatment, with no significant safety concerns in >20 years of literature, including that it is safe to use in the treatment of fragile neonates.8 There have been cases of patients with silver allergies, but with few literature reports. Nanosilver is not safe for systemic or intravenous (IV) use due to hemolytic effects, but shows no signs of toxicity towards epithelial or fibroblast cells.
Table 1.
Literature describing healing, anti-inflammatory activity, toxicity in vivo, and antimicrobial activity of nanosilver dressings and solutions.
| Authors | Reference | Healing | Anti-inflammatory | Toxicity in vivo | Anti-microbial |
|---|---|---|---|---|---|
| Bhol et al. | 9 | – | Yes | – | – |
| Brouillard et al. | 10 | – | – | None | – |
| Burrell et al. | 11 | – | – | None | Yes |
| Demling, DeSanti | 12 | Accelerated | – | – | – |
| Drake, Hazelwood | 13 | – | – | Low | – |
| Dunn, Edwards-Jones | 14 | Accelerated | Yes | None | Yes |
| Fong, Wood | 15 | Accelerated | Yes | None | – |
| Hadrup et al. | 16 | – | – | Limited | – |
| Khansa et al. | 17 | – | – | Low | – |
| Khundkar et al. | 18 | Accelerated | Yes | Low | Yes |
| Lansdown | 19 | – | – | None | – |
| Mazurak et al. | 20 | – | – | Innate immunity improvement | – |
| Mullally et al. | 21 | Accelerated | – | – | – |
| Nadworny, Burrell | 22 | Accelerated | Yes | – | – |
| Olson et al. | 23 | Accelerated | – | – | – |
| Nadworny et al. | 24 | Accelerated | Yes | – | – |
| Nadworny et al. | 7 | – | Yes | – | – |
| Nadworny et al. | 25 | Accelerated | Yes | – | – |
| Rustogi et al. | 8 | – | – | None | – |
| Stryjska et al. | 26 | – | – | Low | Yes |
| Varas et al. | 27 | – | – | None | Yes |
| Vlachou et al. | 28 | – | – | None | – |
| Wang et al. | 29 | – | – | Limited | – |
| Wright et al. | 30 | Accelerated | Yes | – | Yes |
Relationship between nanosilver, inflammation, and COVID-19
Severe cases of COVID-19 (coronavirus disease of 2019) are marked by inflammation and secondary microbial infections of patients' lungs. This triggers cytokine or bradykinin storms31., 32. by overactivating the immune system, which causes the damage resulting in high mortality rates if left untreated. This response is driven by the body's need to attack the infecting organism. If the inflammation is controlled without viral or microbial control, the patient may become worse. Equally, if infectious agents are controlled without inflammation control, the ongoing storm may cause irreversible harm.
Various treatments have been tried with limited success, including Remdesivir and cortical steroids.33 A moderate dose of dexamethasone (6 mg daily for 10 days) reduced mortality in hospitalized patients with COVID-19 who required therapy with supplemental oxygen or mechanical ventilation.34 Antiviral drugs under development have undesirable side effects and interactions with other medications.35 Currently, there are few options for early treatment of less severe COVID-19 cases. New variants emerge rapidly, and vaccination, particularly when not recently boosted, has not consistently prevented infection by variants such as omicron,36 indicating more effective treatments are needed.
In severe burns, cytokine storms are a threat to patient survival.37 These storms have been less severe in patients treated with nanosilver dressings, based on clinical observations. The impact of these dressings, and solutions derived from them, on the inflammatory process has been studied extensively.
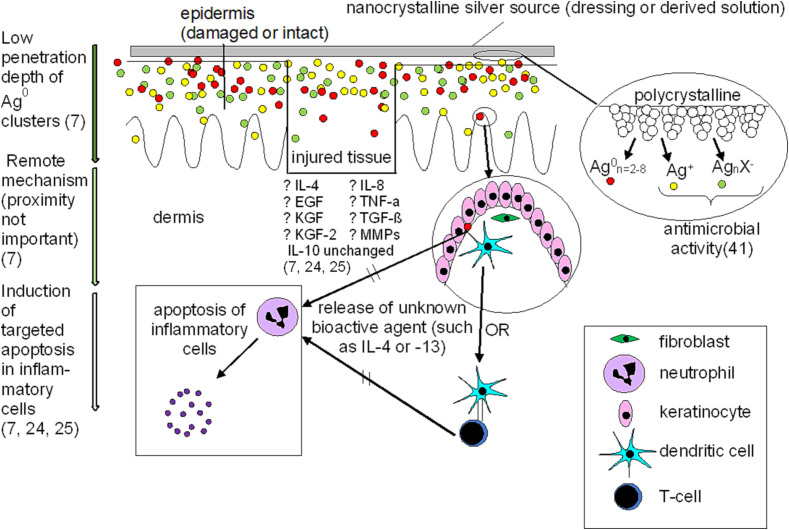
Studies had shown that nanosilver treatments are bactericidal with improved healing outcomes.9., 30., 38. However, it was unclear if outcomes were due solely to microbial clearance, or also to triggering of an anti-inflammatory/pro-healing host response. Porcine contact dermatitis models were generated using dinitrochlorobenzene to examine the anti-inflammatory activity of nanosilver independent from its antimicrobial activity.7., 24., 25. Animals displayed severe edema, erythema, and scabbing, with histology demonstrating infiltration of red blood cells and leukocytes, and disrupted epidermis.7., 24., 25.
Nanosilver-treated pigs showed significantly reduced edema and erythema after 24 h and 48 h, respectively, with no significant difference from the negative controls after 72 h,24 while saline and AgNO3-treated pigs showed no improvement. Histologically, after 72 h, nanosilver-treated pigs had an intact but immature epidermal layer with very few inflammatory cells present within the dermis or epidermis, while tissue damage and inflammatory cell populations were still significant in saline and AgNO3 groups.24 With nanosilver treatment, inflammatory cells in the dermis underwent apoptosis while epidermal cells were unaffected.24 AgNO3 treatment displayed apoptosis in epidermal cells but not inflammatory cells,24 suggesting Ag+ may be toxic to epithelial cells. Saline controls displayed low levels of apoptosis in both layers.24 Nanosilver treatment decreased matrix metalloproteinases (MMPs) and proinflammatory cytokines (tumor necrosis factor (TNF)-α, interleukin (IL)-8, transforming growth factor (TGF)-β), unlike other treatments,24 suggesting nanosilver has anti-inflammatory/pro-healing activity independent of antimicrobial activity, and releases species producing different effects than Ag+.24
Silver depositions in skin following treatment were examined, to determine whether effects seen were due to direct silver-cell interactions or indirect mechanisms, using X-ray photoelectron spectroscopy and secondary ion mass spectrometry to measure silver species deposited and tissue penetration depth.7 No silver was detected in the mid-dermis or subcutaneous fat layer from either silver-based treatment.7 AgNO3-treated animals had AgNO3, Ag2O, AgCl, and Ag deposited throughout the epidermis with ~100 μm dermal penetration.7 The majority of silver deposited during nanosilver treatment was in Ag0 clusters (2–6 silver atoms) in a layer on the epidermal surface.7 The remaining silver was deposited throughout the epidermis as AgCl and Ag2O, with ~150 μm dermal penetration.7 TUNEL (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling) staining on AgNO3-treated samples showed apoptosis patterns very similar to the silver species concentration gradient deposited in the skin – apoptosis occurred primarily in the epidermis, with little in the dermis. This suggests that Ag+ elicited direct toxic effects on cells contacted, without stimulating indirect mechanisms.7 With nanosilver treatment, minimal epidermal apoptosis occurred, while a large proportion of the inflammatory cells in the dermis underwent apoptosis. Given that a large proportion of the silver was deposited in the epidermis, this suggests that silver clusters stimulated remote mechanisms leading to inflammatory cell apoptosis, without harming epidermal cells directly contacted.7
To explore whether nanosilver produced a transferable effect, nanosilver dressings were placed on the opposite side of pigs from where dermatitis was induced.7 With two days remote treatment, edema and erythema significantly decreased, there were histological signs of tissue repair and reduced inflammation, and there was significant apoptosis of inflammatory cells in the injured dermis.7 These results were not seen in the controls; thus, nanosilver can elicit remote effects.7 Fig. 1 suggests mechanisms of action regarding nanosilver interactions with biological systems, particularly at sites of injury. In the porcine contact dermatitis models, the skin before treatment had significant damage to the epidermis and dermis with a large infiltration of inflammatory cells.7., 24., 25. Skin after 48-72 h treatment with nanocrystalline silver wound dressings showed significant tissue recovery, including a newly formed epidermal layer attached to the dermis, either when the treatment was applied directly to the damaged tissue or applied remotely to healthy tissue.7., 24., 25. Apoptosis of inflammatory cells occurred in the dermis, while silver was mostly deposited as Ag0 clusters in the epidermis, suggesting a remote mechanism for apoptosis induction. MMP and pro-inflammatory cytokine expression were also reduced. Nanocrystalline silver did not demonstrate further penetration of species into the tissues than silver nitrate treatments (Ag+),7 despite very different impacts on the tissues. Silver cluster weights showed poor penetration, even relative to such species as AgCl and Ag-O within the nanocrystalline silver treated animals, again suggesting a remote mechanism of action.7
Fig. 1.
Interactions between nanocrystalline silver and biological systems, including impact on signaling molecules at sites of injury.
Nanosilver solution-saturated gauze dressings (starting pH 4, 5.6, 7, or 9) were applied directly to contact dermatitis.25 pH 7 and 9 solution treatments showed the most visual improvement with significant decreases in TNF-α by 72 h compared to positive controls.25 pH 9 treatments showed the most significant histological signs of tissue repair.25 There was significantly reduced expression of pro matrix metalloproteinase (pMMP)-2 and active matrix metalloproteinase (aMMP)-2 measured, particularly with treatments generated at a starting pH of 9, but also in some instances with pH 4 and 5.6. IL-8 expression was found to decrease significantly with solutions generated at a starting pH of 5.6, with a similar trend, not reaching significance, at other starting pHs. IL-4 expression was increased with silver solutions generated at starting pHs of 4, 5.6, 7, and 9. Epidermal growth factor (EGF) expression was increased early on with the silver solutions generated at a starting pH of 9. A similar pattern was observed for keratinocyte growth factor (KGF) expression, which was also significantly increased using the silver solution generated at a starting pH of 5.6. KGF-2 expression was increased early on in the healing process using the silver solution generated at a starting pH of 9, and later on for pHs 5.6 and 7. No significant differences were measured in IL-10 expression.
Anti-inflammatory activity did not correlate with total silver in solution – due to differences in pH and buffers utilized, each solution likely produced different forms of silver with different total quantities of Ag+ and Ag0 clusters, suggesting that which silver species are present is critical for silver-based medical treatments.25 With correct dissolution conditions, nanosilver solutions have anti-inflammatory activity.25 Nanosilver solutions also contain ~1/32 the silver used in AgNO3 and silver sulfadiazine treatments, increasing their safety, as described above in Table 1.
This study examined the antimicrobial and viral inactivation activity of nanosilver solutions, compared to ionic and colloidal silver. Clinical effectiveness using nebulized nanosilver solutions was assessed in select patients.
Some of the results in this study have been previously reported in the form of abstracts.39., 40.
Methods
Solution preparation
Nanosilver dressings (Smith&Nephew, London, UK) were soaked in sterile or distilled water to create solutions used below (details in Supplementary Material).
Nanosilver dissolution profile
1 mL samples collected over time from solutions generated under various conditions (n = 3 per condition: pH 4, 5.6, 7, or 9; stirred or unstirred; different dressing-to-solution ratios) were submitted to Sherritt Technologies Analytical Laboratory (Fort Saskatchewan, AB, Canada) for total silver analysis by atomic absorption (details in Supplementary Material).
In vitro antimicrobial efficacy against Pseudomonas aeruginosa (ATCC 27317) and Staphylococcus aureus (ATCC 25923)
In Bovine Calf Serum (BCS): Nanosilver solutions, silver nitrate (0.5 %) and colloidal silver (20 ppm, Purest Colloids, Westampton, NJ, USA) were tested at 1.8 mL solution per 0.2 mL inoculum using one-hour challenges, followed by neutralization, serial dilution, and spot plating to determine CFU counts to be used in the calculation of the log reductions41 in BCS.
In pH-Adjusted Water: 30-min challenges, followed by neutralization, serial dilution, and spot plating to determine CFU counts to be used in the calculation of the log reductions were performed following standard methods41 (details in Supplementary Material).
Murine lung infection
The protocol was approved by the University of Calgary Animal Care Committee and conducted following Canadian Council on Animal Care guidelines with humane care of the animals.
Pseudomonas aeruginosa strain 5588 was inoculated at ~109 CFU/animal. Rats were anaesthetized by 2 % halothane inhalation. 400 μL inoculum was intratracheally administered non-surgically on intubated animals. Positive controls were infected but not treated; negative controls were challenged and treated with sterile water; tobramycin treated animals received 300 mg/5 mL (final dose = 30 mg/kg; 12 mg/rat); vehicle treated animals received water as prepared for the nanosilver-treated group, but without nanosilver (n = 12/treatment group; treatments performed 2× daily). Lungs were scored based on gross observation and histopathology. See Supplementary Material for treatment process, nanosilver treatment details, and scoring methods.
Viral inactivation efficacy
Nanosilver, 0.1 mM AgNO3, and colloidal silver (20 ppm) solutions were tested for inactivation of HSV-1 strain 17syn+ (low passage) and SARS-CoV-2 strain UF142 using ASTM E1052-20.43 For HSV-1, 7 × 107 or 7 × 105 plaque forming units (PFU) in 5 μL Minimum Essential Media (MEM) with 5 % BCS were incubated with 50 μL of test solutions, media-only controls, inactivated solutions, or water, at room temperature with rotation (12 rpm) for various times. For SARS-CoV-2, 5 × 105 PFU in 100 μL Eagle's MEM with supplements (see Supplementary Material), were incubated with 100 μL of the above test and control solutions without rotation at 37 °C. Sodium thioglycolate solution was added to inactivate the silver, followed by standard plaque enumeration assays to determine the level of viral inactivation achieved (see Supplementary Material).
Clinical evaluation
In off-label use, with informed written consent of the patient or surrogate, nanosilver was used to treat multi-drug resistant (MDR) pneumonia, upper respiratory infections, and fungal sinusitis. Nanosilver dissolved in 15 mL water was nebulized twice daily for 7–21 days for treatment of the conditions. For fungal sinusitis, solution was also irrigated daily. A patient with a 45 % total body surface area burn was found to have COVID-19 and MDR bacterial pneumonia (samples obtained from bronchoscopy). The MDR pneumonia was treated as above for 14 days and the response of the COVID-19 infection was observed. Two nurses (outpatients) developed COVID-19 and utilized nebulized nanosilver solutions as above.
Statistics
One-way analysis of variance (ANOVAs) with Tukey-Kramer post-tests were performed using GraphPad InStat® v3.10 ©2009 (Dotmatics, San Diego, CA, US). Averages are reported with standard deviations.
Results
Nanosilver dissolution profile
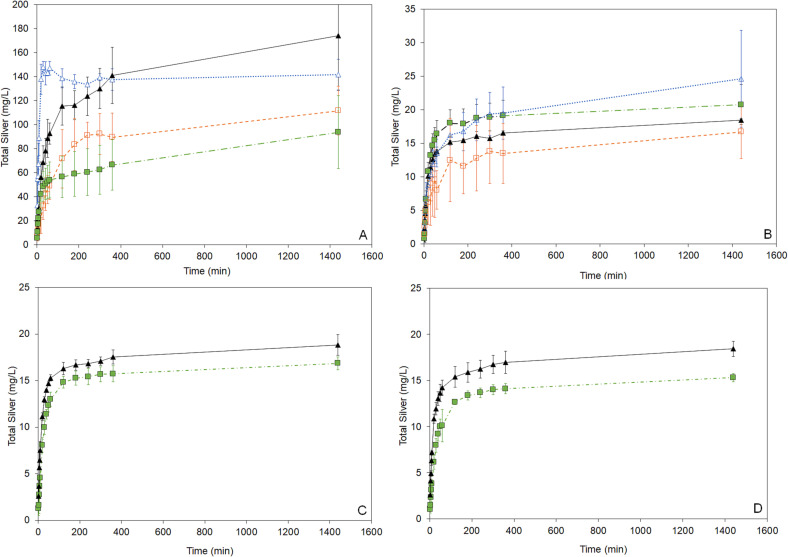
Fig. 2 shows total silver released over time from nanosilver dressings into solution under various conditions. Lower pH solutions released higher total silver. All conditions were semi-equilibrating.
Fig. 2.
Total silver profiles over time for nanocrystalline silver-derived solutions generated at a starting pH of 4 (A), approximately 5.6 (distilled water) (B), 7 (C), and 9 (D) under various conditions. Solutions were generated by adding nanocrystalline silver to water with or without pH adjustment at 6.5 cm2/mL (data with triangular symbols) or 1.3 cm2/mL (data with square symbols) of nanocrystalline silver dressing. The silver was allowed to dissolve either stirred (symbols filled in) or unstirred (no fill for symbols), and samples were collected for total silver analysis by AAS at set times. Error bars = standard deviations (n = 3).
In vitro antimicrobial efficacy
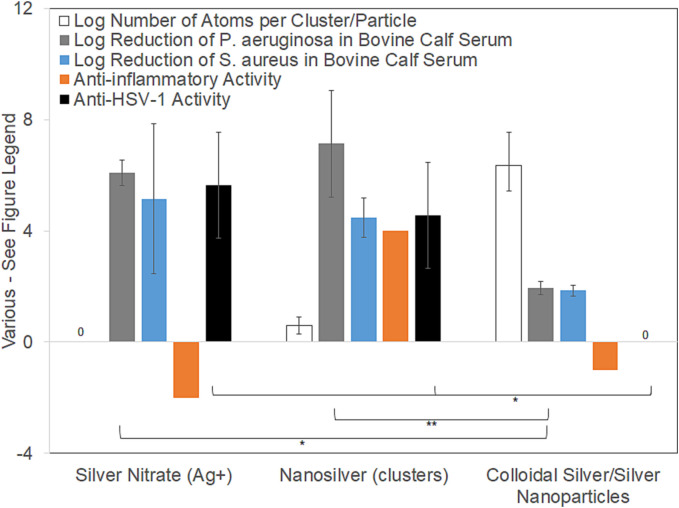
Fig. 3 compares antibacterial efficacy in BCS, anti-HSV-1 activity (see below), anti-inflammatory activity, and quantities of atoms per unit (cluster or particle) delivered via various silver treatments. Only nanosilver solutions containing silver nanoclusters (Ag0 2 – 6), Ag+, and higher oxidation state silver generate combined antiviral, anti-inflammatory, and antimicrobial activity. For AgNO3 (typically used as a 0.5 % solution = 3176 μg/mL Ag), only Ag+ (aq) is delivered, so the number of atoms grouped together is one (for a logarithmic value of zero). Nanosilver releases metallic silver nanoclusters containing 2–8 atoms7 (size ~6–32 Å), as well as Ag+ and a higher oxidation state silver, at pH-dependent concentrations in water as shown in Fig. 1. Colloidal silver or silver nanoparticles contain larger quantities of atoms per particle of colloid, which varies with synthesis method. The logarithmic number of atoms per particle shown here is for a particle size of 20 nm (calculated assuming spherical particles, using the density of silver (10.49 g/cm3), Avogadro's number (6.02 × 1023 atoms/mol), and the atomic mass of silver (107.87 g/mol)), with error bars representing colloids of 10 and 50 nm. Data for the log reduction against P. aeruginosa and S. aureus in BSC was obtained as described in the methods and Supplementary Material. For P. aeruginosa, there were very significant differences between groups, as determined by one-way ANOVA with Tukey-Kramer Multiple Comparisons Testing (p = 0.0034). Individual significant differences determined via post-testing are indicated on the figure (* indicates p < 0.05; ** indicates p < 0.01). For S. aureus, there were no significant differences (p = 0.1003). Overall anti-inflammatory activity scores were assigned based on previously published data. AgNO3 was assigned a score of −2 (moderately pro-inflammatory) because treatment of dinitrochlorobenzene (DNCB)-induced porcine contact dermatitis with AgNO3 induces apoptosis of epithelial cells, without reducing erythema, edema, matrix metalloproteinase (MMP)-2 or -9, or proinflammatory cytokine expression.7., 24., 25. Nanosilver clusters were assigned a score of +4 (strongly anti-inflammatory), as treatment of DNCB-induced porcine contact dermatitis with nanosilver resulted in apoptosis of inflammatory cells, while reducing erythema, edema, MMPs, and pro-inflammatory cytokines (see Fig. 1).7., 24., 25. As well, treatment of allergic contact dermatitis in a guinea pig model demonstrated that a nanosilver-containing cream had a stronger anti-inflammatory effect than high potency steroids.9 Colloidal silver and silver nanoparticles were assigned a score of −1 (mildly pro-inflammatory), based on reports indicating that they caused lesions, mixed inflammatory cell infiltration, and chronic alveolar inflammation in a rat inhalation model.44., 45. Antiviral data against herpes simplex virus 1 (HSV-1) was measured at 3 h, as described in the methods (also see Supplementary Materials for further data). There were significant differences between groups, as determined by one-way ANOVA with Tukey-Kramer Multiple Comparisons Testing (p = 0.0097). Individual significant differences determined via post-testing are indicated on the figure. Note that bulk silver, not shown here, is insoluble in water, and has no known biological activity.
Fig. 3.
A comparison of various types of silver and their biological activity.
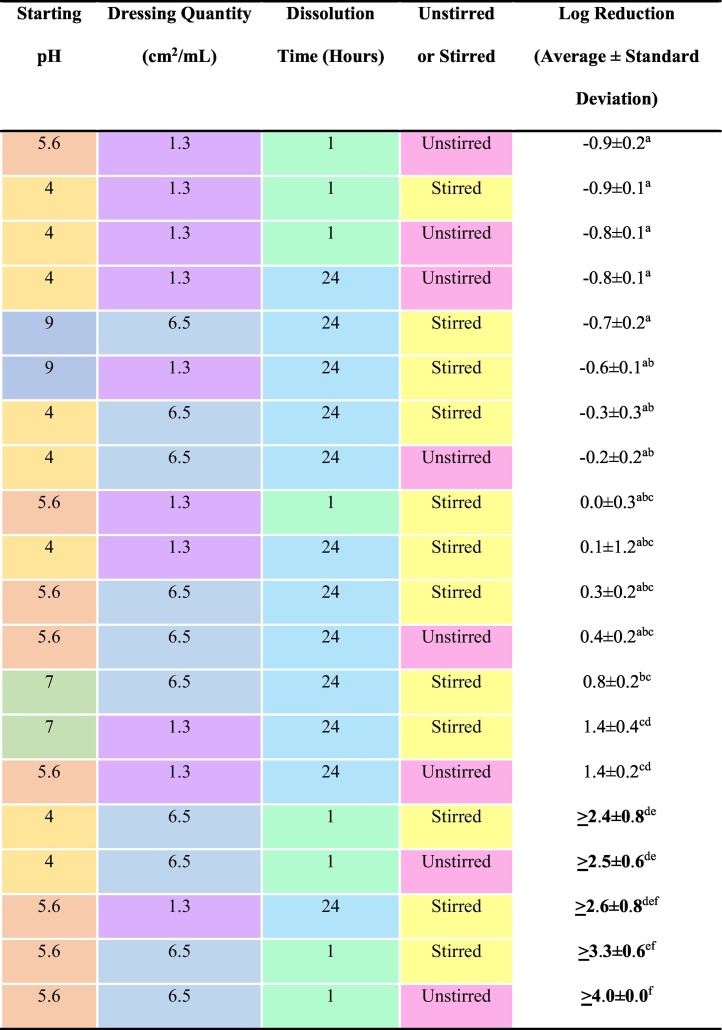
30-minute log reductions against S. aureus in water are shown in Table 2 . At pH 4, only higher quantities of nanosilver dissolved for 1 h resulted in total kill, regardless of stirring. At pH 5.6, these same conditions resulted in total kill, as did 24 h dissolution at low concentration with stirring. pH 7 and 9 solutions did not demonstrate bactericidal activity, but perhaps bactericidal activity might have been generated with shorter dissolution times. At mid-range pHs (i.e., in distilled or reverse osmosis water), more antimicrobial species appear to be released than at lower or higher pHs. Based on Fig. E1 (see Supplementary Material), and because P. aeruginosa is more silver sensitive than S. aureus,41 solutions would likely prove more effective against P. aeruginosa in equivalent testing. In preliminary tests with P. aeruginosa, solutions generated at 1.3 cm2/mL, unstirred for 24 h, and starting pHs of 4.0, 4.5, and 5.6 all resulted in total kill (with a log reduction of >5.5 ± 0.0). Under the same conditions, a one-hour dissolution resulted in total kill at pH 4.0 (with a log reduction of >3.6 ± 0.2), but not at pH 4.5 (with a log reduction of 1.7 ± 0.3, which was significantly lower than the log reduction produced at pH 4, p = 0.0036).
Table 2.
Log reductions generated using nanosilver-derived solutions generated under a variety of conditions against S. aureus. Note that for bolded results, total kill was achieved. A statistical analysis comparing the different conditions was performed using a one-way Analysis of Variance (ANOVA), which showed there were extremely significant differences between groups (p < 0.0001). Means that do not share a letter in common are statistically significantly different (p < 0.05 to p < 0.001) according to Tukey-Kramer Multiple Comparisons post-testing.
Murine lung infection
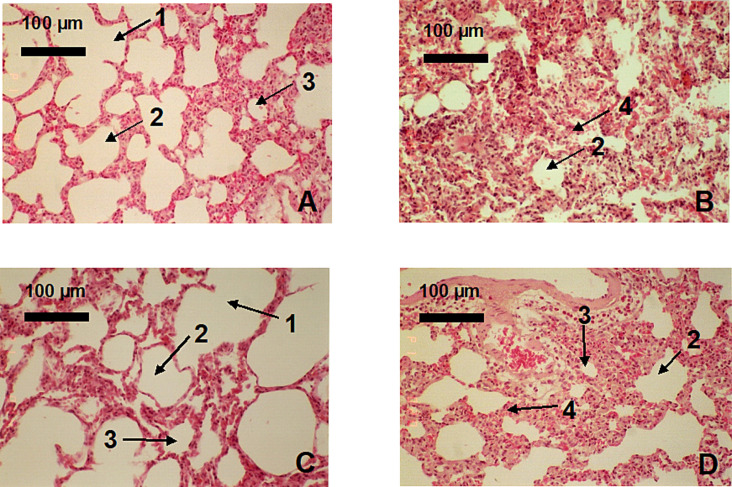
Gross pathology showed that at 48 h, negative control (uninfected) lungs were normal, while vehicle and positive controls had 60–80 % interstitial pneumonia. Tobramycin-treated animals had 70 % interstitial pneumonia, while nanosilver-treated animals showed no pneumonia (see Supplementary Material for details). Histopathologically, positive control rats, vehicle-treated rats, and tobramycin-treated rats had similar pathology – at 24 and 48 h, severe infiltration of polymorphonuclear leukocytes (PMNs) into lung interstitial spaces was observed, and was observed to a lesser degree in the alveolar and bronchiolar spaces (Fig. 4 ). In these animals, pulmonary vessels were dilated and alveolar spaces were filled with proteinaceous material. Nanosilver-treated rats had occasional PMN infiltration, no fluid accumulation in alveolar or bronchiolar spaces, and no evidence of a toxic response. The anti-inflammatory activity observed in dermal models7., 9., 24., 25. was thus also observed in this lung model, via reduced PMNs and reduced tissue destruction.
Fig. 4.
Comparison of negative control rat lung (uninfected, nebulized water – A) to the effects of vehicle control (infected, nebulized water – B), nebulized nanosilver solution (C), and nebulized tobramycin (D) on Pseudomonas aeruginosa infected rat lungs at 48 h treatment. In the figure, 1 indicates a terminal bronchiole, 2 indicates the alveolar duct, 3 indicates an alveoli, and 4 indicates an interstitial space infiltrated with polymorphonuclear cells (PMNs).
Viral inactivation efficacy
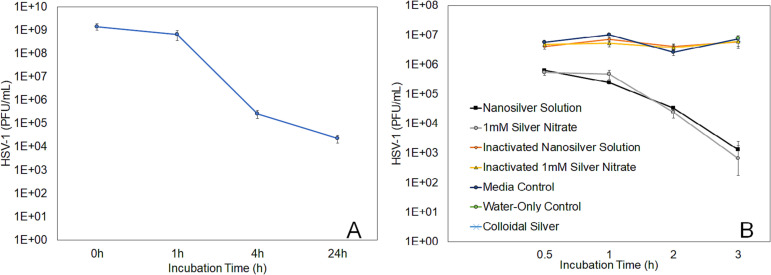
The nanosilver solution resulted in a >4.0-log reduction of infectious HSV-1 within four hours of treatment, and almost a five-log reduction by 24 h post-treatment (Fig. 5 ). This was not immediate, starting between one and four hours post-treatment. AgNO3 results were similar, while colloidal silver showed no activity. The nanosilver and AgNO3 solutions showed similar kinetics in reducing HSV-1 infectious titre – a one-log reduction was seen as early as 30 min, with a two-log reduction by 1 h – while their inactivated forms showed no log reduction. The colloidal silver solution and water-only control (data shown only at 3 h) showed no log reduction during the three-hour test period. The inactivated form of the colloidal silver solution was not tested, given that there was no activity measured when it was used in its original form. Statistical analysis was performed at each timepoint using one-way ANOVAs. At all timepoints, there were extremely significant differences between groups (p < 0.0001). At all timepoints, the nanosilver and silver nitrate solutions' viral inactivation activity were not significantly different from each other (p > 0.05). The nanosilver and silver nitrate solutions were significantly better than the inactivated solutions and media control at all-time points (p < 0.001, except for p < 0.01 for both solutions versus media at 2 h and inactivated solutions at 3 h). The inactivated solutions and media controls were not significantly different from each other (p > 0.05), except for the inactivated nanosilver solution versus the media control at 0.5 h (p < 0.05), and the inactivated nanosilver solution (p < 0.01) and the inactivated silver nitrate solution (p < 0.001) versus the media control at 1 h. At 3 h, the nanosilver and silver nitrate solutions' performances were significantly better than the colloidal silver and water control (p < 0.001 in each case). The water control, media control, colloidal silver, and inactivated solutions were not significantly different from each other (p > 0.05).
Fig. 5.
Silver solutions reduce infectious titre of HSV-1 in a time-dependent manner. Incubation of 7 × 107 plaque forming units (PFU) with nanosilver solution reduced infectious titre by 54 % at 1 h, four-fold at 4 h, and five-fold by 24 h (A). Comparison of nanosilver, AgNO3, and colloidal silver solutions, along with their inactivated controls, a water control, and a media control, is shown in Panel B, starting with a lower PFU (7 × 105).
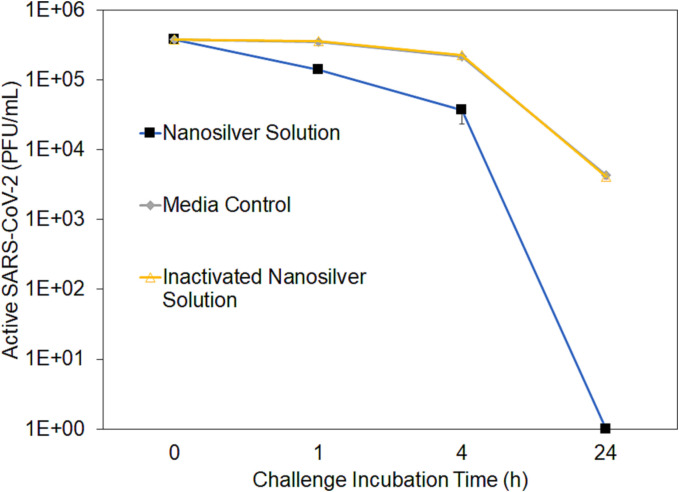
Similar results were observed for nanosilver solutions with SARS-CoV-2 (Fig. 6 ). There were undetectable levels of infectious virus following 24 h of incubation with the nanosilver solution (detection limit 10 PFU/mL). At all timepoints, the levels of infectious virus showed significant differences between groups (p < 0.0001), as determined by one-way ANOVAs with Tukey Kramer multiple comparisons post-testing. The post testing showed that at all time points, the nanosilver solution produced significantly reduced infectious virus titres compared to the media control and inactivated nanosilver solution (p < 0.001).
Fig. 6.
Nanosilver solution reduced infectious titre of SARS-CoV-2 in a time-dependent manner. 5 × 105 PFU (plaque forming units) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) strain UF-1 was incubated with the nanosilver solution or controls (media or inactivated nanosilver solution) for times indicated on the figure and enumerated following inactivation. All timepoints were tested in triplicate.
Clinical evaluation
During treatment of MDR pneumonia, patients demonstrated significant improvement, stabilizing within 36–48 h and eradicating infection within 14 days. Fungal sinusitis patients demonstrated immediate inflammation reduction and infection clearance within 21 days, compared to six weeks required for previous treatment regimens. The burn patient with COVID-19 had noted improvement on chest X-rays within 72 h, with significantly decreased ventilated oxygen requirements based on arterial blood gases, although the patient did require ventilator assistance while continuing burn care. Physical signs of COVID-19 infection disappeared, and the patient tested negative within 45 days. Nurse A with COVID-19 developed mild symptoms of headache and fatigue for six days before developing fever. On Day Seven she tested positive for COVID-19, and developed chills and nausea. By Day 11, she had developed anosmia, dysgeusia, kidney pain, and dyspnea. At Day 16, she was disoriented, febrile (>40 °C), borderline hypoxic via O2 saturation testing at home (90–92 %), and overall appeared and felt worse, so she started nebulized nanosilver solution treatment twice daily, and within 48 h, dyspnea was improved, O2 saturation was increased (94–97 %), and fever was spiking at a lower degree. At Day 21, all symptoms were resolved, apart from minor cough. Nurse B developed headache, body aches, and fever (~40 °C) for two days and tested positive on Day Three when she developed dysgeusia and anosmia. Nurse B immediately initiated nebulized nanosilver treatments twice daily. Nurse B's symptoms did not worsen and she was symptom free by Day Seven.
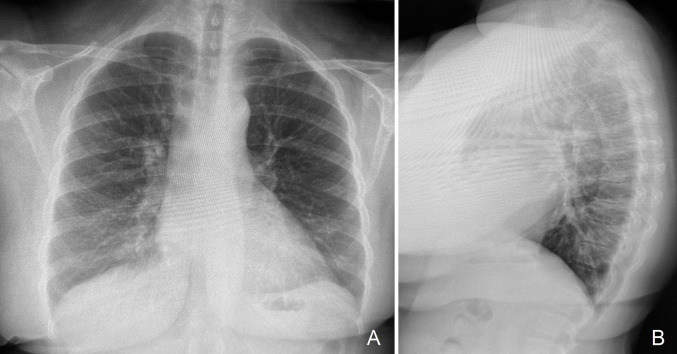
X-rays (Fig. 7 ) and computerized tomography (CT) scans post-treatment of MDR pneumonia, fungal sinusitis, and COVID-19 with nanosilver solution showed no evidence of silver in the lungs or sinuses.
Fig. 7.
Chest X-ray image of a patient after three weeks of treatment with nanocrystalline silver solutions prepared as described in the Methods section (with further details in the Supplementary Materials). The chest X-ray is of a 41 year old renal transplant patient on chronic immunosuppressive treatment. This patient had used inhaled nanocrystalline silver solutions for pulmonary issues on several prior occasions over the previous two years to prevent the development of resistance to antibiotics, should the need arise. The patient developed an upper respiratory infection and had been treated for three weeks at the time of the chest X-rays shown. Purulent secretions resolved within 72 h of treatment, but a persistent cough lingered initially. By the time of the X-ray, the cough had also ceased. There is no evidence of silver deposition in the lungs.
Discussion
Inhalation, nebulization, and irrigation of nanosilver solution were clinically beneficial for treatment of fungal sinusitis, MDR pneumonia, and COVID-19. The treatment appears to act on epithelial tissues, stopping the immune system from initiating/continuing a cytokine or bradykinin storm by dampening the immune response, thus preventing the further tissue destruction that causes significant morbidity and mortality. This suggests a mechanism for broader treatment applications of nanosilver solutions, including in severe acute autoimmune diseases such as Toxic Epidermal Necrolysis.46 Due to the high oxidation power of silver(I) and higher states, the treatment further protects patients by preventing or eliminating life-threatening infections, including viral infection, such as SARS-CoV-2, and microbial primary, secondary or co-infections, at lung epithelia.
In vitro testing demonstrated that nanosilver is efficacious against a range of viruses, including both HSV-1 and SARS-CoV-2. Nanosilver may have broad antiviral activity due to oxidation of essential viral proteins, as ionic silver reacts strongly with various proteins.47 It is thus anticipated to be effective against current and future variants of COVID-19, along with other epithelium-based viral infections.
Nanosilver dissolved into sterile, distilled, or reverse osmosis water (not tap water), aerosolized and inhaled by the patient, directly accesses sinuses and lungs. This provides targeted epithelial tissue treatment, avoiding any systemic safety issues. Nebulization can be performed on ventilated patients. It can also be used proactively upon developing COVID-19, or other infections, to eliminate early symptoms, using, for example, a nebulizer sold for asthma treatment. This may reduce the likelihood of serious outcomes and infection risk to others. Further, decreasing the inflammatory response to COVID-19 via this treatment, and thus reducing related damage, may lessen the risk of developing long COVID symptoms, which are significantly tied to inflammatory responses.48
Clinical results suggest that twice-daily treatments with patients breathing normally are effective. Treatment of the sinuses can be achieved by nasal inhalation of nebulized solution, potentially combined with irrigation of the solution, for example, with a neti pot. During nebulization, droplet size impacts treatment deposition, with larger droplets (>6 μm) mainly depositing in upper airways or nasal cavities, small droplets (<2 μm) depositing mainly in alveoli, and intermediate droplets depositing in central and small airways.49
Controlling pH during dissolution can tune for antimicrobial and antiviral or anti-inflammatory activity,25 but use of distilled water appears to provide a balance of both (see Table 2 and in vivo anti-inflammatory testing25).
Six hours dissolution is recommended to bring nanosilver levels close to equilibrium. The dose delivered depends on this concentration (which at pH 4 was <170 μg/mL, and at higher pHs was <30 μg/mL – see Fig. 2) and the nebulized volume that is inhaled. For a nebulizer with a 15 mL charge, the maximum possible dose per treatment would be 2.55 mg at pH 4 and 0.45 mg at higher pHs – a very low dose when compared, for example, with AgNO3 burn treatments.5 The actual dose would be lower still, because not all of the nebulized silver would be inhaled. Although Ag+ precipitates with chloride, Ag2 – 6 clusters and higher oxidation state silver, which may be complexed with other elements resulting in a negative charge, would not interact with bodily fluid components in this way, making nanosilver a safer treatment option than AgNO3.
Nanosilver treatment is likely compatible with systemic COVID-19 treatments including steroids, monoclonal antibodies, convalescent serum, and drugs reducing virus synthesis, as silver serum levels would not be sufficiently high to negatively interact with the treatments. It may have a synergistic effect with treatments that address different aspects of infection and subsequent symptoms.
Based on the data in Fig. 3, it is critically important that only nanocrystalline silver dressing-derived solutions be used for treatment (NOT treatments that only release Ag+, silver nanoparticles or colloidal silver), because only the nanosilver solutions provide anti-inflammatory, antiviral, and antimicrobial activity, while the pro-inflammatory response to Ag+ 7., 24., 25. or colloidal silver/silver nanoparticles44., 45. could worsen the patient's immune response, increasing the risk of cytokine storms. Nanosilver dressings are FDA-cleared for use in a related medical application, unlike commercially available colloidal silver and silver nanoparticle products touted for applications with no FDA-approved data.4., 50.
Overall, nanosilver solutions appear to provide a novel, highly effective, broad acting, inexpensive, easily accessible treatment for severe pulmonary microbial and/or viral infections and inflammatory conditions.
Financial support
This work was supported by the University of Alberta, Edmonton, AB to RB; Canada Foundation for Innovation, Ottawa, ON to RB; University Hospital Foundation, Edmonton, AB to RB; National Research Council –Women in Engineering and Science, Ottawa, ON to PN; Natural Sciences and Engineering Research Council of Canada (NSERC) – Canada Graduate Scholarship (CGS) – Master's program, Ottawa, ON to PN; NSERC CGS – 2-year Doctoral program (D2), Ottawa, ON to PN; Alberta Innovates Technology Futures (AITF) Studentship, Edmonton, AB to PN; AITF Graduate Scholarship in Nanotechnology, Edmonton, AB to PN; Honorary Izaak Walton Killam Memorial Scholarship, Edmonton, AB to PN; and University of Florida training grant, Gainesville, FL [grant number T32AI007110] to TG. Funding sources had no role in study design, data collection/analysis/interpretation, writing the paper, or deciding to submit the paper for publication.
Disclosures
The work has been presented in part, with abstracts at the 44th Annual John A. Boswick Burn and Wound Care Symposium (January 22–27, 2022) and the Wound Healing Society Annual Meeting (April 6–10, 2022).
CRediT authorship contribution statement
Patricia Nadworny – conceptualization (nanosilver dissolution profile, in vitro antimicrobial efficacy of solutions), data curation (nanosilver dissolution profile, in vitro antimicrobial efficacy of solutions), formal analysis, visualization, writing - original draft.
William Hickerson – data curation, formal analysis, supervision (clinical applications), writing - review and editing.
Holly Denise Holley-Harrison – data curation, formal analysis (clinical applications), writing - original draft, review, and editing.
David Bloom – conceptualization, supervision, methodology (viral inactivation study), data curation, formal analysis, writing - review and editing.
Tristan Grams – methodology, data curation, formal analysis (viral inactivation study), writing - review and editing.
Terri Edwards - methodology, data curation, formal analysis (viral inactivation study), writing - review and editing.
Gregory Schultz - conceptualization, supervision, formal analysis, writing - original draft.
Robert Burrell - conceptualization, project administration, resources, funding acquisition, supervision, methodology (in vitro antimicrobial efficacy in bovine calf serum, in vivo rat pulmonary model), data curation (in vitro antimicrobial efficacy in bovine calf serum, in vivo rat pulmonary model), formal analysis, writing - original draft.
Conflict of interest
RB was a consultant for Smith&Nephew from 2011 to 2013 and lectured for the same on wound care until 2017. RB is a founder of Kheprion, Inc., a University of Alberta start-up for biomedical devices. The remaining authors do not have a commercial or other association that might pose a conflict of interest.
Acknowledgements
We would like to acknowledge Andrew P. Bluhm and Michael H. Norris for their support in developing the protocols, obtaining the permissions, and training related to the BSL-3 experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nano.2023.102654.
Appendix A. Supplementary data
Supplementary material
Data availability
Data presented in this study is available from the corresponding author upon request.
References
- 1.Grier N. In: Disinfection, Sterilization and Preservation. Third ed. Block S., editor. Lea & Febiger; Philadelphia: 1983. Silver and its compounds; pp. 375–389. [Google Scholar]
- 2.Demling R.H., DeSanti L. Effects of silver on wound management. Wounds. 2001;13:4–15. [Google Scholar]
- 3.Tomaselli N. The role of topical silver preparations in wound healing. J Wound Ostomy Continence Nurs. 2006;33:367–378. doi: 10.1097/00152192-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services (HHS) Public Health Service (PHS) Food and Drug Administration (FDA) Over-the-counter drug products containing colloidal silver ingredients or silver salts. Final rule. Fed Regist. 1999;64:44653–44658. [PubMed] [Google Scholar]
- 5.Monafo W.W., West M.A. Current treatment recommendations for topical burn therapy. Drugs. 1990;40:364–373. doi: 10.2165/00003495-199040030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Wright J.B., Lam K., Olson M.E., Burrell R.E. Is antimicrobial efficacy sufficient? A question concerning the benefits of new dressings. Wounds. 2003;15:133–141. [Google Scholar]
- 7.Nadworny P.L., Landry B.K., Wang J., Tredget E.E., Burrell R.E. Does nanocrystalline silver have a transferable effect? Wound Repair Regen. 2010;18:254–265. doi: 10.1111/j.1524-475X.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 8.Rustogi R., Mill J., Fraser J.F., Kimble R.M. The use of Acticoat in neonatal burns. Burns. 2005;31:878–882. doi: 10.1016/j.burns.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Bhol K.C., Alroy J., Schechter P.J. Anti-inflammatory effect of topical nanocrystalline silver cream on allergic contact dermatitis in a guinea pig model. Clin Exp Dermatol. 2004;29:282–287. doi: 10.1111/j.1365-2230.2004.01515.x. [DOI] [PubMed] [Google Scholar]
- 10.Brouillard C., Bursztejn A.C., Latarche C., Cuny J.F., Truchetet F., Goulle J.P., et al. Silver absorption and toxicity evaluation of silver wound dressings in 40 patients with chronic wounds. J Eur Acad Dermatol Venereol. 2018;32:2295–2299. doi: 10.1111/jdv.15055. [DOI] [PubMed] [Google Scholar]
- 11.Burrell R.E., Heggers J.P., Davis G.J., Wright J.B. Efficacy of silver-coated dressings as bacterial barriers in a rodent burn sepsis model. Wounds. 1999;11:64–71. [Google Scholar]
- 12.Demling R.H., DeSanti L. The rate of re-epithelialization across meshed skin grafts is increased with exposure to silver. Burns. 2002;28:264–266. doi: 10.1016/s0305-4179(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 13.Drake P.L., Hazelwood K.J. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005;49:575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- 14.Dunn K., Edwards-Jones V. The role of Acticoat with nanocrystalline silver in the management of burns. Burns. 2004;30:S1–S9. doi: 10.1016/s0305-4179(04)90000-9. [DOI] [PubMed] [Google Scholar]
- 15.Fong J., Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine. 2006;1:441–449. doi: 10.2147/nano.2006.1.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadrup N., Sharma A.K., Loeschner K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: a review. Regul Toxicol Pharmacol. 2018;98:257–267. doi: 10.1016/j.yrtph.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Khansa I., Schoenbrunner A.R., Kraft C.T., Janis J.E. Silver in wound care-friend or foe?: a comprehensive review. Plast Reconstr Surg Glob Open. 2019;7 doi: 10.1097/GOX.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khundkar R., Malic C., Burge T. Use of Acticoat dressings in burns: what is the evidence? Burns. 2010;36:751–758. doi: 10.1016/j.burns.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Silver Lansdown A.B. I: its antibacterial properties and mechanism of action. J Wound Care. 2002;11(4):125–130. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 20.Mazurak V.C., Burrell R.E., Tredget E.E., Clandinin M.T., Field C.J. The effect of treating infected skin grafts with Acticoat on immune cells. Burns. 2007;33:52–58. doi: 10.1016/j.burns.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Mullally C., Carey K., Seshadri R. Use of a nanocrystalline silver dressing and vacuum-assisted closure in a severely burned dog. J Vet Emerg Crit Care (San Antonio) 2010;20:456–463. doi: 10.1111/j.1476-4431.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 22.Nadworny P.L., Burrell R.E. A review of assessment techniques for silver technology in wound care. Part 2: tissue culture and in vivo methods for determining antimicrobial and anti-inflammatory activity. J Wound Tech. 2008;1:13–22. [Google Scholar]
- 23.Olson M.E., Wright J.B., Lam K., Burrell R.E. Healing of porcine donor sites covered with silver-coated dressings. Eur J Surg. 2000;166:486–489. doi: 10.1080/110241500750008817. [DOI] [PubMed] [Google Scholar]
- 24.Nadworny P.L., Wang J.F., Tredget E.E., Burrell R.E. Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomed: NBM. 2008;4:241–251. doi: 10.1016/j.nano.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Nadworny P.L., Wang J., Tredget E.E., Burrell R.E. Anti-inflammatory activity of nanocrystalline silver-derived solutions in porcine contact dermatitis. J Inflamm (Lond) 2010;7:13. doi: 10.1186/1476-9255-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stryjska K., Radko L., Checinska L., Kusz J., Posyniak A., Ochocki J. Synthesis, spectroscopy, light stability, single-crystal analysis, and in vitro cytotoxic activity on HepG2 liver cancer of two novel silver(I) complexes of miconazole. Int J Mol Sci. 2020;21:3629. doi: 10.3390/ijms21103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varas R.P., O’Keeffe T., Namias N., Pizano L.R., Quintana O.D., Herrero Tellachea M., et al. A prospective, randomized trial of Acticoat versus silver sulfadiazine in the treatment of partial-thickness burns: which method is less painful? J Burn Care Rehabil. 2005;26:344–347. doi: 10.1097/01.bcr.0000170119.87879.ca. [DOI] [PubMed] [Google Scholar]
- 28.Vlachou E., Chipp E., Shale E., Wilson Y.T., Papini R., Moiemen N.S. The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns. 2007;33:979–985. doi: 10.1016/j.burns.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Wang X.Q., Kempf M., Mott J., Chang H.E., Francis R., Liu P.Y., et al. Silver absorption on burns after the application of Acticoat: data from pediatric patients and a porcine burn model. J Burn Care Res. 2009;30:341–348. doi: 10.1097/BCR.0b013e318198a64c. [DOI] [PubMed] [Google Scholar]
- 30.Wright J.B., Lam K., Buret A.G., Olson M.E., Burrell R.E. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141–151. doi: 10.1046/j.1524-475x.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 31.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche J.A., Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020;34:7265–7269. doi: 10.1096/fj.202000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthay M.A., Thompson B.T. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med. 2020;8:1170–1172. doi: 10.1016/S2213-2600(20)30503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S Food, Drug Administration . 2021. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid™ 2021. Available from: https://www.fda.gov/media/155050/download. [Google Scholar]
- 36.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell. 2022;185:457–466. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanojcic M., Chen P., Xiu F., Jeschke M.G. Impaired immune response in elderly burn patients: new insights into the immune-senescence phenotype. Ann Surg. 2016;264:195–202. doi: 10.1097/SLA.0000000000001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirsner R.S., Orsted H., Wright J.B. Matrix metalloproteinases in normal and impaired wound healing: a potential role of nanocrystalline silver. Wounds. 2002;13:4–12. [Google Scholar]
- 39.Hickerson W.S., Burrell R., Harrison D., Grams T., Edwards T., Nadworny P., et al. John A. Boswick Burn and Wound Care Symposium. 2022. Nebulized nanocrystalline silver as a treatment for MDR pneumonia and COVID-19. January 22-27. Wailea, Hawaii. [Google Scholar]
- 40.Schultz G.H., Burrell R., Grams T., Edwards T., Nadworny P., Bloom D. Wound Healing Society Annual Meeting April 6-10. 2022. Nebulized inhalation of a unique solution from a nanocrystalline silver wound dressing has powerful antiviral activity and has successfully treated patients with MDR pneumonia, TENS, and COVID-19. Phoenix, Arizona. [Google Scholar]
- 41.Taylor P.L., Ussher A.L., Burrell R.E. Impact of heat on nanocrystalline silver dressings. Part I: chemical and biological properties. Biomaterials. 2005;26:7221–7229. doi: 10.1016/j.biomaterials.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 42.Lednicky J., Salemi M., Subramaniam K., Waltzek T.B., Sabo-Attwood T., Loeb J.C., et al. Earliest detection to date of SARS-CoV-2 in Florida: identification together with influenza virus on the main entry door of a university building, February 2020. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Standard practice to assess the activity of microbicides against viruses in suspension. ASTM; West Conshohocken, Pennsylvania, United States: 2020. p. 4. [Google Scholar]
- 44.Sung J.H., Ji J.H., Park J.D., Yoon J.U., Kim D.S., Jeon K.S., et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicol Sci. 2009;108:452–461. doi: 10.1093/toxsci/kfn246. [DOI] [PubMed] [Google Scholar]
- 45.Johnston H.J., Hutchison G., Christensen F.M., Peters S., Hankin S., Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 46.Guerrero R., Menezes J., Reilly D.A., Garner W.L. Wound care with Acticoat in patients with non-burn skin loss. J Invest Med. 2000;48:59A. [Google Scholar]
- 47.Russell A.D., Hugo W.B. Antimicrobial activity and action of silver. Prog Med Chem. 1994;31:351–370. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 48.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–147. doi: 10.1089/jamp.2011.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institutes of Health (NIH) 2017. Colloidal Silver. Available from https://www.nccih.nih.gov/health/colloidal-silver. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data presented in this study is available from the corresponding author upon request.