Abstract
Background:
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators, especially (elexacaftor/tezacaftor/ivacaftor), have positively impacted the cystic fibrosis (CF) population and quickly decreased lung transplant (LTx) numbers. However, no study has investigated if this reduction is universal across all races/ethnicities.
Methods:
Using the United Network for Organ Sharing (UNOS) Registry, we explored the frequency/proportions of LTx in White non-Hispanic (WNH) and non-White (NW) (Black, Non-Hispanic/Hispanic-Latino/Asian-Non Hispanic/American Indian-Alaskan Native-Non-Hispanic/Native Hawaiian/Other Pacific Islander-Non-Hispanic/Multiracial) in children and adults with CF in the United States (US).
Results:
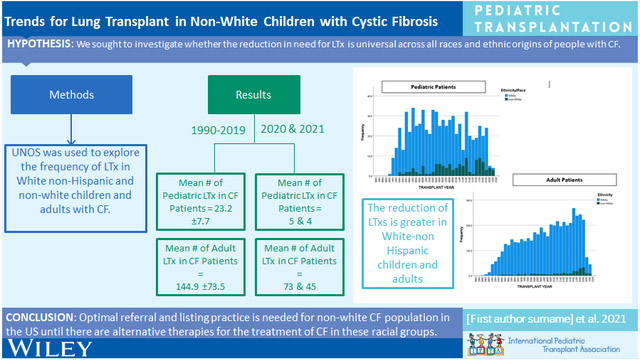
Between 1990 and 2019, the annual mean (±SD) number of LTxs for children with CF was 23.2 (±7.7) compared to 5 in 2020 (p<0.001) and in 2021 (p<0.001). In adults from 1990 to 2019, the mean (±SD) number of LTxs performed was 144.9 (±73.5), which was significantly higher than 2020 (n=73; p<0.001) and 2021 (n=45; p<0.001). Comparing 1990-2019 to post-2019, the proportion of LTxs performed in both children and adults with CF has decreased from 50.5% (696/1378) to 16.4% (9/55) and from 12.1% (4773/39,542) to 2.4% (118/5004), respectively. In WNH pediatric patients, the difference in the percentage of all LTx made up by CF patients between the two eras was 41.2% compared to NW patients where the difference was 11%. Similarly in adults, the difference between the two eras was 10.4% in WNH and 2.4% in NW patients.
Conclusions:
Recent reduction in LTx for the CF population has had less impact on the NW population in the US, so continuation of optimal referrals for this group is needed.
Keywords: adults, children, cystic fibrosis, diversity, gap, lung transplant, pediatrics
Graphical Abstract
The advent of cystic fibrosis transmembrane conductance regulator (CFTR) modulators has caused a paradigm shift in the treatment of the cystic fibrosis (CF) population, starting with ivacaftor in 2012 and then elexacaftor/tezacaftor/ivacaftor (E/T/I) in November 2019. [1] Because of its efficacy in patients with at least one copy of the F508del mutation, E/T/I has expanded CTFR modulator treatment for a larger proportion of people with CF (PwCF). Early evidence with ivacaftor therapy found a positive impact of FEV1 decline, [2] thus preserving higher pulmonary function for a longer time period. A population-based study found that ivacaftor seemed to decrease mortality risk and possible reduced the need for solid organ transplant in PwCF, [3] but this study was limited by a small number of deaths and transplants. More recently, Avdimiretz et al. reported a downward trend over time for absolute number of LTxs in children with CF and proportion of total pediatric LTxs in children attributed to CF. [4] A prospective, observational study found a twofold decrease in the number of LTxs performed in PwCF over 12 years of age in 2020 compared to the 2 previous years. [5] Further work by others has confirmed that the need for LTx is dropping in PwCF. [6, 7] Notably, none of this work has addressed whether these changes in LTx in PwCF differ by race and/or ethnicity. Therefore, we explored trends in LTx for PwCF in the United States (US) using the (UNOS) Registry given the release of E/T/I therapy to determine if LTx in PwCF differ by race and ethnicity, comparing White non-Hispanic (WNH) and non-White (NW) (Black, Non-Hispanic/Hispanic-Latino/Asian-Non Hispanic/American Indian-Alaska Native-Non-Hispanic/Native Hawaiian/Other Pacific Islander-Non-Hispanic) groups. For our analysis, we included the recently updated Organ Procurement and Transplantation Network descriptions for race/ethnicity. [8] For those patients listed as multiracial which is collected in the UNOS Registry, we included that population in the NW cohort.
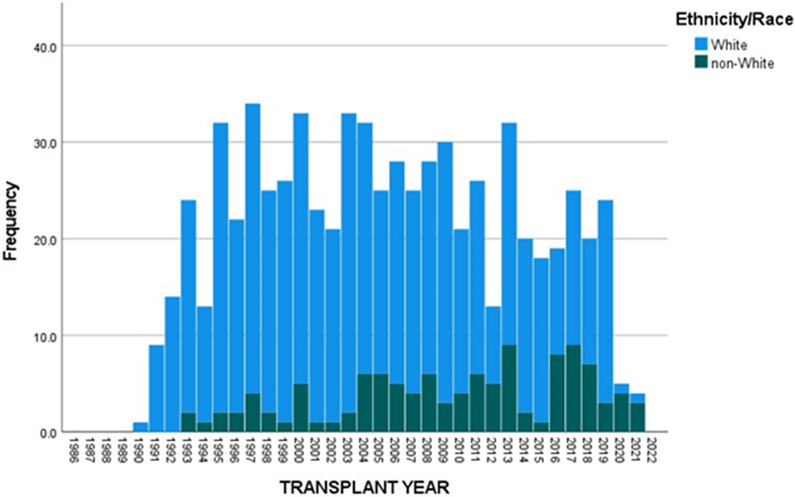
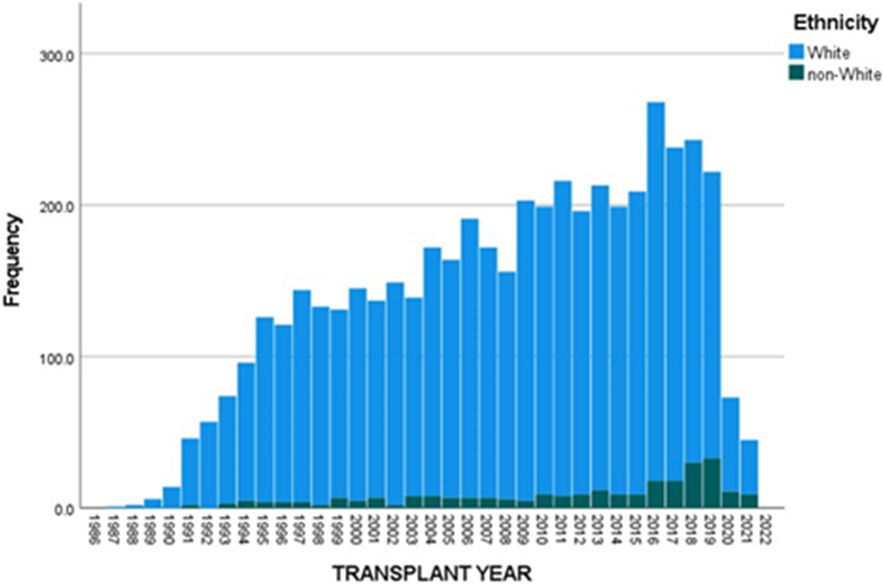
The UNOS Registry was queried to identify both children (<18-years-of-age) and adults (≥18-years-of-age) with a diagnosis of CF who underwent LTx in the US between October 1987 and December 2021. Figures 1 and 2 show the annual number of LTxs performed in children and adults with CF. There has been a significant reduction in the number of annual LTxs for both age cohorts in 2020 and 2021. For children with CF from 1990-2019 the annual mean (±SD) number of LTxs was 23.2 (±7.7), whereas this number was significantly higher than 2020 (n=5) (p<0.001) and 2021 (n=4) (p<0.001). For non-CF children, the annual mean number of LTxs (20.8 (±9)) was significantly lower than the number for 2020 (n=27) (p<0.001) but similar to 2021 (n=19) (p=0.3). In adults from 1990 to 2019, the mean (±SD) number of LTxs performed in CF recipients was 144.9 (±73.5); this number was significantly higher than 2020 (n=73; p<0.001) and 2021 (n=45; p<0.001).
Figure 1.
Annual number of lung transplants performed in children (<18-years-of-age) with cystic fibrosis in the United States.
Figure 2.
Annual number of lung transplants performed in adults (≥18-years-of-age) with cystic fibrosis in the United States.
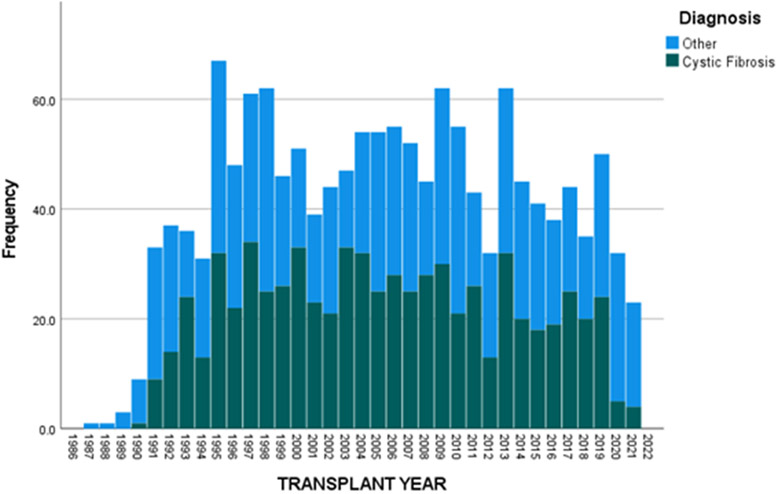
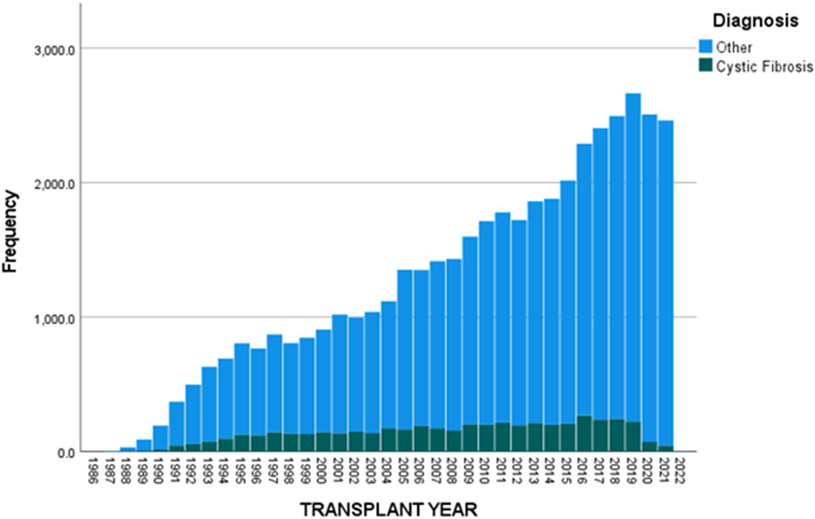
Figures 3 and 4 show the decreasing proportions of LTx occurring in both pediatric and adult CF populations. Between 1990 and 2019, the number of children with CF undergoing LTx was 696 with a total of 1378 being performed, making up 50.5% of the pediatric LTx population. After 2019, 9 of 55 children who underwent LTx had CF or 16.4%. Looking at adults, 4773 of the total 39542 LTx performed between 1990 and 2019 were in PwCF, so a total of 12.1% of all LTxs performed were in PwCF. After 2019, 118 of 5004 LTxs have been performed in PwCF which is 2.4%, thus further substantiating a significant reduction of LTx in the CF population. Further exploring this, we found differences based on racial and ethnic differences. Comparing 1990-2019 to post-2019, the proportion of LTxs performed in WNH and NW children with CF has decreased from 49.5% to 8.3% and 33.5% to 22.5% of all pediatric LTxs, respectively. Similarly, when comparing 1990-2019 to post-2019, we found the proportion of LTxs performed in WNH and NW adults with CF has decreased from 13% to 2.6% and 3.9% to 1.5% of all adult LTxs, respectively.
Figure 3.
Proportion of lung transplants occurring in children (<18-years-of-age) with cystic fibrosis compared to all lung transplants in the United States.
Figure 4.
Proportion of lung transplants occurring in adults (≥18-years-of-age) with cystic fibrosis compared to all lung transplants in the United States.
Although CFTR modulator treatment is not collected by the UNOS Registry, the timing of this change in the number of LTxs in CF suggests that E/T/I therapy is a major contributing factor. [4] The reduction of LTxs is greater in the WNH population, indicative of the benefit of E/T/I targeting F508del mutation as the higher prevalence in WNH patients. Previous studies have identified the various categories of CFTR mutations present in the NW CF population. [9] Based on CFTR mutations alone innate to respective races and ethnicities, 92.4% of NWH patients, 69.7% of Black/African American patients, 75.6% of Hispanic patients, and 80.5% of other race patients are eligible for CFTR modulators. [10]
While we cannot directly examine if the SARS-CoV-2 pandemic influenced these results, it is known that LTx volume decreased in programs in the US. [11] Although there is no direct evidence that E/T/I was the cause of the reduction in LTx for CF, our findings are consistent with this hypothesis as the LTx volume has returned to pre-SARS-CoV-2 pandemic volume and, the reduction in LTx volume for both children and adults with CF in the US persists, supporting the introduction of E/T/I therapy as the primary cause of our findings.
In addition to confirming previous findings by other investigators that need for LTx in CF is rapidly decreasing, [5-7] this analysis has identified a persistent need for LTx in NW children with CF. With the F508del mutation being far less common in NW individuals at 15-25% of the population, [12] E/T/I therapy will be less impactful for this patient population. Due to minorities representing a proportion of the CF population having worse disease burden and increased mortality, [13] there is a need to optimize referral and listing practices for LTx for this patient population until there are alternative therapies for treatment of their chronic lung disease.
Acknowledgements
The authors have no relevant disclosures or conflicts of interest. The funding for this work included the following NIH grants: R01HL142210 (AZ), R01HL151588 (AZ-MPI), NIH R01HL147957-01 (AD, AGG, FZ, DLSM).
Financial Support:
NIH 1R01HL147957-01 (AD, AGG, FZ, DLSM), NIH R01HL142210 (AZ), NIH R01HL151588 (AZ)
Abbreviations:
- (CF)
cystic fibrosis
- (CFTR)
cystic fibrosis transmembrane conductance regulator
- (E/T/I)
elexacaftor/tezacaftor/ivacaftor
- (LTx)
lung transplant
- (NW)
non-White
- (PwCF)
people with CF
- (WNH)
White non-Hispanic
- (UNOS)
United Network for Organ Sharing
- (US)
United States
Footnotes
Conflicts of Interest: The authors report no conficts of interest and have no relevant disclosures regarding this manuscript.
Data: Data available upon request.
References
- 1.Ramsey BW and Bell SC, Cystic Fibrosis: A Disease in Transformation, yet More Work to Be Done! Am J Respir Crit Care Med, 2022. 205(5): p. 487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkova N, et al. , Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J Cyst Fibros, 2020. 19(1): p. 68–79. [DOI] [PubMed] [Google Scholar]
- 3.Bessonova L, et al. , Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax, 2018. 73(8): p. 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avdimiretz N and Benden C, The changing landscape of pediatric lung transplantation. Clin Transplant, 2022. 36(4): p. e14634. [DOI] [PubMed] [Google Scholar]
- 5.Burgel PR, et al. , Rapid Improvement after Starting Elexacaftor-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and Advanced Pulmonary Disease. Am J Respir Crit Care Med, 2021. 204(1): p. 64–73. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, et al. , Major Decrease in Lung Transplantation for Patients with Cystic Fibrosis in France. Am J Respir Crit Care Med, 2022. 205(5): p. 584–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin C, et al. , Sustained effectiveness of elexacaftor-tezacaftor-ivacaftor in lung transplant candidates with cystic fibrosis. J Cyst Fibros, 2022. 21(3): p. 489–496. [DOI] [PubMed] [Google Scholar]
- 8.Updated Race and Ethnicity Labeling Coming to OPTN Data Reports (Organ Procurement & Transplantation Network; ) 2022. [cited 2022 July 27 2022]; Available from: https://optn.transplant.hrsa.gov/news/updated-race-and-ethnicity-labeling-coming-to-optn-data-reports/. [Google Scholar]
- 9.Sugarman EA, et al. , CFTR mutation distribution among U.S. Hispanic and African American individuals: evaluation in cystic fibrosis patient and carrier screening populations. Genet Med, 2004. 6(5): p. 392–9. [DOI] [PubMed] [Google Scholar]
- 10.McGarry ME and McColley SA, Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol, 2021. 56(6): p. 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan EG, et al. , Trends in Lung Transplantation Practices Across the United States During the COVID-19 Pandemic. Transplantation, 2021. 105(1): p. 187–192. [DOI] [PubMed] [Google Scholar]
- 12.Bobadilla JL, et al. , Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat, 2002. 19(6): p. 575–606. [DOI] [PubMed] [Google Scholar]
- 13.Rho J, et al. , Disparities in Mortality of Hispanic Patients with Cystic Fibrosis in the United States. A National and Regional Cohort Study. Am J Respir Crit Care Med, 2018. 198(8): p. 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]