Abstract
Enterococci are important nosocomial pathogens that are increasingly difficult to treat due to intrinsic and acquired resistance to antibiotics, including vancomycin. A recently described capsular polysaccharide (CP) isolated from Enterococcus faecalis 12030 was used to evaluate the potential efficacy of active or passive immunotherapy regimens as adjunctive treatments. Evaluation of protective efficacy was carried out in immunocompetent mice challenged intravenously (i.v.) with live enterococci. In nonimmune mice, i.v. inoculations resulted in high levels of bacteria in kidneys, spleens, and livers 5 days after challenge. Mice immunized with four 10-μg doses of CP antigen/mouse were protected against challenge with the homologous E. faecalis strain. High-titer opsonic immunoglobulin G was also induced by immunizing rabbits with the purified CP, and passive transfer of this antiserum to mice produced significantly lower bacterial counts in organs than did normal rabbit serum or sterile saline. Antibodies to the polysaccharide isolated from E. faecalis 12030 were protective against Enterococcus faecalis OG1RF and against two serologically related, vancomycin-resistant Enterococcus faecium clinical isolates. Antibodies to this CP antigen were also effective as a therapeutic reagent in mice when passive therapy was initiated 48 h after live bacterial challenge. These data indicate that CP antigens from enterococci are potential targets of protective antibodies and that these antibodies may be useful for prophylaxis and treatment of enterococcal infections.
Enterococcal infections are an increasing threat in modern medicine and especially in intensive care unit (ICU) patients and immunocompromised patients such as neonates, oncology patients, and transplant recipients. In some medical ICUs, enterococci are the second most common bloodstream isolate, more common even than Staphylococcus aureus (31). The National Nosocomial Infection Surveillance System reported in 1998 that Enterococcus spp. were responsible for 10% of bloodstream infections, 14% of urinary tract infections, and 20% of cardiovascular infections in coronary care units (23).
The increasing occurrence of multidrug-resistant enterococcal infections, including vancomycin-resistant strains, has resulted in some cases of infection that are difficult to treat with routinely used antimicrobials. Current rates of about 14 to 25% vancomycin resistance in enterococcal isolates in ICUs raise the prospect of a “postantibiotic era” of untreatable bacterial infections (7, 8, 24). Recently, we identified an enterococcal surface antigen that is a target of opsonic killing (13), a hallmark of antibodies protective against bacterial pathogens (5). Purification and chemical characterization of this antigen revealed a glycerol-teichoic acid-like molecule with a backbone structure of -6-alpha-d-glucose-1-2-glycerol-3-PO4- substituted on carbon 2 of the glucose molecule with an alpha-2-1-linked molecule of -d-glucose (38). The purified antigen was immunogenic in rabbits, and the resulting high-titer antibodies bound to an extracellular capsule layer as observed by electron microscopy (13). The present study was performed to evaluate the protective efficacy of antibodies to this enterococcal capsule in an in vivo model of enterococcal infection.
MATERIALS AND METHODS
Antigen.
Enterococcal capsular polysaccharide (CP) antigen and antibodies to this antigen were prepared as described earlier (13). In brief, a high-molecular-weight carbohydrate-rich fraction was isolated from Enterococcus faecalis 12030 bacteria grown in Columbia broth. The bacterial cells were recovered by centrifugation, suspended in phosphate-buffered saline, and digested with mutanolysin and lysozyme (0.1 mg/ml) for 16 to 18 h at 37°C. Treatment of the cell suspension with nucleases (100 μg/ml) at 37°C for 4 h was followed by addition of proteinase K (100 μg/ml) and further incubation at 56°C overnight. The insoluble cell wall fragments and cell bodies were removed by centrifugation. The supernatant was collected, filtered, and size fractionated on a Sephacryl S-500 column, with 0.4 M ammonium carbonate buffers. Material that eluted in the void volume was pooled, dialyzed, and lyophilized. This material was further purified by dissolution in 50 mM bicarbonate buffer (pH 8.0) and application to an anion-exchange Q-column. Bound antigen was eluted from the column with increasing concentrations of NaCl (0 to 1 M, linear gradient), and fractions containing polysaccharides were identified with immuno-dot blots and rabbit antiserum to E. faecalis 12030. Fractions were pooled, dialyzed, and lyophilized. Gas chromatography-mass spectrometry and nuclear magnetic resonance spectroscopy were used for structural analysis as described elsewhere (13, 38).
Antibodies.
Antibodies to purified enterococcal polysaccharides were elicited in New Zealand White rabbits by subcutaneous immunization with two 100-μg doses of polysaccharide emulsified in 0.5 ml of complete Freund adjuvant followed by three intravenous (I.V.) injections of 10 μg of antigen in saline spaced 3 days apart. After the final injection the animals were bled periodically for determination of antibody opsonic activity and boosted monthly by i.v. injection of 10 μg of CP antigen in saline.
Swiss-Webster mice were injected intraperitoneally with 10 μg of purified polysaccharide (dry weight) dissolved in 0.2 ml of sterile saline at 5-day intervals for 15 days (i.e., four injections). A control group of animals received an identical amount of P. aeruginosa mucoid exopolysaccharide (MEP) as an unrelated antigen (28, 29).
ELISA and opsonophagocytic assay.
Immunologic analysis of antibody titers and determination of the effectiveness of serum absorption with bacterial cells were carried out by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (13). Opsonic killing of bacteria was measured in vitro as described elsewhere (13); the percentage of CFU killed by immune serum was calculated respective to the absolute number of CFU of enterococci surviving in normal rabbit serum using the following formula: percent killed = 100 − [(CFU surviving in immune serum/CFU surviving in normal serum) × 100].
Animal model.
Female Swiss-Webster mice (6 to 8 weeks old) were used to evaluate the protective efficacy of antibody to the enterococcal CP. Outbred mice were used to mimic the genetic situation of humans and to minimize artifactual contributions from host factors related to inbreeding that could exacerbate or reduce innate susceptibility to infection. The challenge inoculum of the enterococcal strains was suspended in saline and injected i.v. into the tail vain of actively or passively immunized mice. The actual inoculum was verified by viable counts. For evaluation of passive protection, the mice received 0.2 ml of rabbit serum adsorbed at 4°C for 60 min with 109 CFU of E. faecalis 12107 (heterologous strain) per ml. Bacterial cells were removed by centrifugation and filter sterilization. Serum was given i.v. via the tail vein 24 h before challenge and again 4 and 24 h after bacterial challenge. Mice were sacrificed after 5 days unless otherwise indicated; livers, spleens, and kidneys were removed under sterile conditions, weighed, homogenized, and cultured quantitatively on enterococcal selective agar medium (Enterococcosel agar; Becton Dickinson).
Statistical analysis.
ELISA titers were calculated by linear regression plotting of optical density (OD) values versus the log10 of the serum dilutions. The reciprocal of the calculated dilution giving a reading of 0.2 OD was arbitrarily defined as the endpoint titer. The significance of the percentage of organisms killed using immune sera in the opsonophagocytic assay was determined by a t test. Statistical analysis of the bacterial counts in the animal experiments employed the Mann-Whitney U test (two-group comparison) or the Kruskal-Wallis nonparametric analysis of variance (multigroup comparison) with the Dunn procedure used for pairwise comparisons. Comparisons of rate of infection were carried out with the Fisher exact test. Calculations were done with either the Statview statistical software program (Abacus Concepts, Berkeley, Calif.) or in an Excel spreadsheet (Microsoft Corp., Redmond, Wash.) on a Macintosh computer.
RESULTS
Expression of glycerol-teichoic acid capsule by enterococcal strains.
The bacterial strains used in this study are listed in Table 1. We have previously reported (13, 38) that the CP of E. faecalis 12030 is also made by a vancomycin-resistant Enterococcus faecium strain, 838970VRE, as shown by immunologic and structural analysis of the isolated CP antigen. Serologic studies with specific antibodies raised to purified CP antigen from strain 12030 identified additional E. faecalis strains that expressed an antigen seroreactive with antibodies raised to this purified CP (i.e., E. faecalis OG1RF and a vancomycin-resistant clinical isolate from a patient, E. faecium 805370VRE). E. faecalis OG1RF is commonly studied and has been extensively characterized by a number of investigators (12, 22, 30, 32, 35, 39, 40). Another clinical isolate, E. faecalis 12107, has been shown to be only minimally opsonized and killed by serum against strain 12030 CP (13) and served as a negative control for the strain 12030 CP antigen.
TABLE 1.
Bacterial strains used in the present study and their susceptibilities to opsonic killing by antibodies specific to E. faecalis 12030 CP
| Bacterial strain | Origin | % Killeda | Pb |
|---|---|---|---|
| E. faecalis | |||
| 12030 | Clinical isolate; prototype strain for CP antigen | 81 | <0.01 |
| 12107 | Clinical isolate | 25 | NS |
| OG1RF | G. Dunneyc | 71 | <0.01 |
| Vancomycin-resistant E. faecium | |||
| 838970 | Clinical isolate | 64 | <0.01 |
| 805370 | Clinical isolate | 78 | <0.01 |
Opsonophagocytic assay using immune rabbit serum raised against polysaccharide of E. faecalis 12030. Percent killed = 100 − [(CFU surviving in immune serum/CFU surviving in normal serum) × 100].
Calculated by t test, with a hypothesized mean difference from CFU surviving in normal serum of zero.
University of Minnesota.
Active immunization studies.
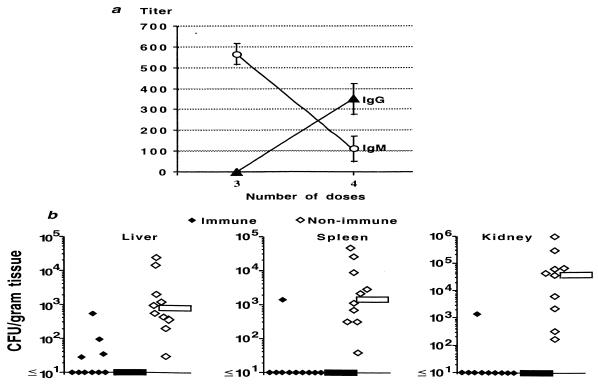
Ten outbred Swiss-Webster mice were injected with purified enterococcal CP antigen, and an additional 10 animals were injected with the MEP antigen of P. aeruginosa (28, 29) as a control immunogen. Antibody titers were measured by ELISA after three or four injections with 10 μg of either purified CP or MEP that were given i.v. and spaced 5 days apart. Sera from mice injected with the P. aeruginosa MEP antigen were no more reactive with the enterococcal CP antigen than were preimmunization sera (data not shown). Five days after the third injection of enterococcal CP antigen, the mean immunoglobulin M (IgM) titer was >500, but IgG antibodies were not detected (Fig. 1a). Five days after the fourth injection of CP antigen, the mean IgG titer was 550, while the IgM antibody titers had dropped to about 100 (Fig. 1a). Five days after i.v. challenge of these immunized mice with 6 × 106 CFU of the homologous E. faecalis 12030, the median CFU of bacteria/g of tissue was <10 (lower limit of detection) in livers, spleens, and kidneys compared with medians of 771, 1,420, and 42,790 CFU of bacteria/g of tissue, respectively, in the group of animals receiving P. aeruginosa MEP (Fig. 1b; P < 0.001 for all three comparisons, Mann-Whitney U test). Kidneys and spleens from only 1 of 10 immune mice contained ≥10 bacteria/g of tissue compared with all 10 of the kidneys and spleens from control animals (P = 0.00006, Fisher's exact test for both comparisons). Four of ten livers from CP-immunized mice had detectable bacteria compared with all 10 livers from controls (P = 0.005, Fisher's exact test).
FIG. 1.
Immune response and protective efficacy of E. faecalis 12030 CP antigen. (a) Mean and standard deviation (error bars) of antibody titer to the CP antigen after three or four immunizations of 10 mice with purified antigen. (b) CFU of E. faecalis 12030 per gram of liver, spleen, or kidney of mice immunized with enterococcal CP antigen (immune) or P. aeruginosa MEP (nonimmune) after challenge with 6 × 106 CFU of E. faecalis 12030. Each point represents the bacterial counts from a single mouse. Bars indicate the median CFU/gram of tissue for the group. P was <0.001 for comparisons in all three tissues between immune and control animals as determined by the Mann-Whitney U test.
Passive immunotherapy studies.
Prophylactic immunotherapy for at-risk patients with antibody to the CP antigen is a potential approach to prevent enterococcal infections. To evaluate this strategy, we prepared an antiserum to purified CP antigen from E. faecalis 12030. Comparable to previous results (13), a 1:500 dilution of this serum mediated killing of >65% of the E. faecalis strains 12030 and OG1RF and the E. faecium strains 838970VRE and 805370VRE (Table 1). Adsorption of this immune rabbit serum (IRS) with heterologous E. faecalis 12107 whole bacterial cells did not alter the opsonic killing activity against any of the strains susceptible to these antibodies (data not shown). Adsorption with cells of the homologous E. faecalis 12030 or inhibition with purified CP reduced the opsonic killing activity of the specific antiserum to <10% against all of the E. faecalis 12030 CP-positive enterococcal isolates (data not shown).
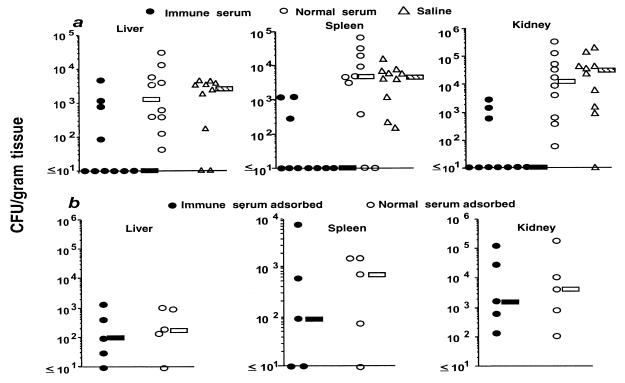
We then used this CP-specific antiserum to evaluate passive protective efficacy in comparison with normal rabbit serum (NRS) and normal saline. Sera or saline were given to mice 24 h before infection and again 4 and 24 h after infection. To ensure that serum antibodies were not directed at enterococcal antigens other than the CP, we chose to routinely adsorb all sera with heterologous E. faecalis 12107 (13). There was no difference in the CFU of E. faecalis/g of tissue 5 days after challenge with 107 CFU of E. faecalis 12030/mouse in animals given the adsorbed NRS or saline (Fig. 2a). In contrast, immune serum to the CP antigen, similarly adsorbed with the heterologous E. faecalis 12107 strain, drastically reduced tissue levels of E. faecalis 12030 (Fig. 2a, P <0.004 in the liver, spleen, and kidney; Kruskal-Wallis test and Dunn procedure for pair-wise comparisons with normal serum or saline). However, if the specific immune serum was adsorbed with whole cells of the homologous E. faecalis strain 12030 the protective efficacy was removed following challenge of mice with 6 × 106 CFU of E. faecalis 12030 (Fig. 2b, P > 0.7 for CFU/g of tissue in the liver, spleen, and kidney). An ELISA of the adsorbed serum evaluating antibody levels against the purified CP antigen confirmed the removal of specific antibody (not shown).
FIG. 2.
Passive protective efficacy of rabbit antibodies to E. faecalis 12030 CP antigen. (a) CFU of E. faecalis strain 12030/gram of liver, spleen, or kidney of mice receiving either IRS, NRS, or saline and challenged with 107 CFU of E. faecalis 12030 per mouse. Sera were adsorbed with whole cells of heterologous E. faecalis 12107 before administration to the mice. Each point represents the bacterial counts from a single mouse. Bars indicate the median CFU/gram of tissue for the group. P was ≤0.004 for reductions in CFU/gram of tissue in the liver, spleen, and kidney for IRS-treated mice versus the other two treatments as determined by the Kruskal-Wallis test and Dunn procedure for pairwise comparisons with NRS or saline. (b) CFU of E. faecalis per gram of liver, spleen, or kidney of mice after prophylactic administration of IRS or NRS adsorbed with E. faecalis 12030 and challenge with 6 × 106 CFU of E. faecalis 12030 per mouse. Each point represents the bacterial counts from a single mouse. Bars indicate the median CFU/gram of tissue for the group. P was >0.7 for all comparisons as determined by the Mann-Whitney U test.
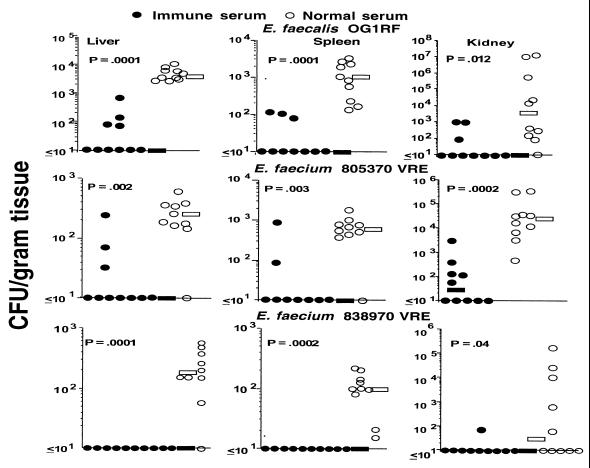
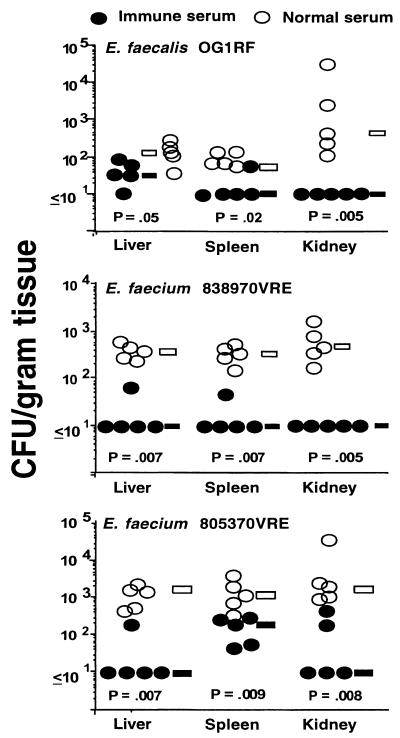
Additional groups of Swiss-Webster mice given IRS or NRS adsorbed with E. faecalis 12107 were then challenged with either E. faecalis OG1RF, E. faecium 805370VRE, or E. faecium 838970VRE. These three strains express a CP antigen structurally or serologically related to that from E. faecalis 12030. Significant reductions in CFU/gram of tissue with all three of the additional enterococcal isolates were achieved in the organs of animals receiving IRS compared to animals receiving NRS (Fig. 3). Further confirmation of the specificity of the antibodies to the CP antigen from strain 12030 was documented in an experiment using passive administration of antibodies to the strain 12030 CP antigen and challenge with the heterologous E. faecalis strain 12107. Two groups of mice were given either unabsorbed NRS or unabsorbed IRS to E. faecalis 12030 CP antigen, followed by challenge with strain 12107. No protection was observed (not shown; P > 0.7, Mann-Whitney U test for comparison of IRS versus NRS for the CFU/gram of tissue in the liver, kidney, and spleen).
FIG. 3.
Passive protective efficacy of rabbit antibodies to E. faecalis 12030 CP antigen against challenge with heterologous enterococcal strains that express this antigen. Bars indicate the median CFU/gram of tissue for the group. P values were determined by the Mann-Whitney U test for comparisons between immune and normal serum. Challenge doses: E. faecalis OG1RF, 1 × 107 CFU/mouse; E. faecium 805370VRE, 7.6 × 107 CFU/mouse; E. faecium 838970VRE, 1.1 × 107 CFU/mouse.
Therapy of established infection.
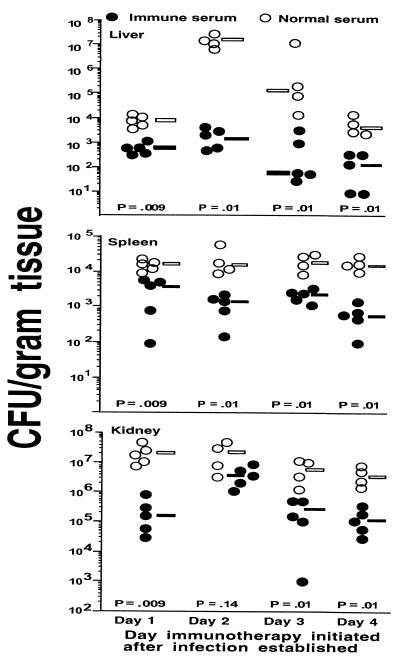
Since therapy for established infection would be another important potential use of antibodies to enterococcal capsules, we infected Swiss-Webster mice with 3 × 107 CFU of E. faecalis 12030 and initiated immunotherapy with normal or immune serum starting 1, 2, 3, or 4 days after infection. Three injections of antiserum in 24-h intervals were given per mouse, and infected animals were sacrificed 8 days after infection. The bacterial counts in the tissues of animals in all groups were statistically significantly lower (P < 0.01, Mann-Whitney U test) when immune serum was given, the only exception being the CFU/gram of kidney in the group starting therapy on day 2 after infection (Fig. 4).
FIG. 4.
Passive protective efficacy of immune serum to E. faecalis 12030 CP antigen administered after establishment of infection with 3 × 107 CFU of E. faecalis 12030 per mouse. Serum was administered three times at 24-h intervals starting 1, 2, 3, or 4 days after bacterial challenge with E. faecalis 12030. Bars indicate the median values for the group; P values were determined by the Mann-Whitney U test for comparisons between immune and normal serum.
We next evaluated the therapeutic potential of the enterococcal-CP-specific immune serum using the panel of strains against which there was protection in the prophylaxis trial. Serum was given three times at 24-h intervals, with the first dose administered 48 h after infection; animals were sacrificed 9 days after infection. There was a significant reduction in the CFU/gram of tissue for all three of the enterococcal strains in mice treated with IRS compared with those given NRS (Fig. 5), and 30 of the 45 cultures of infected organs had <10 CFU/g of tissue (lower limit of detection) compared with none of the 45 cultures from NRS-treated animals (P = 5.1 × 10−13, Fisher's exact test).
FIG. 5.
Passive protective efficacy of immune serum administered after establishment of infection with three different enterococcal strains. IRS or NRS were administered three times at 24-h intervals starting 2 days after bacterial challenge with the indicated strains, all of which express the E. faecalis 12030 CP antigen. Bars indicate the median values for the group, and P values were determined by the Mann-Whitney U test for comparisons between immune and normal serum. Challenge doses: E. faecalis OG1RF, 107 CFU/mouse; E. faecium 805370VRE, 107 CFU/mouse; E. faecium 838970VRE, 107 CFU/mouse.
DISCUSSION
Enterococci are among the most important nosocomial pathogens, especially in patients in ICUs and in severely immunocompromised individuals. Because of their intrinsic and acquired resistance to multiple antibiotics, the effective treatment options available for enterococcal infections are quite limited (36). Thus, immunotherapy, particularly prophylactic and passive therapies, offer an alternative for treatment of these infections. We have previously reported on the structure, surface occurrence, and ability to elicit opsonic antibodies of a glucose-glycerol-teichoic acid molecule from E. faecalis 12030 (13, 38). In this report we demonstrate that this CP antigen also elicited protective antibodies in a mouse model of systemic enterococcal infection. Rabbit antibodies made in response to the CP antigen passively protected mice against serologically related enterococcal strains that were susceptible to the opsonic killing activity of this serum. The specificity of the activity for the CP antigen was shown by the inability of an E. faecalis strain that did not express the same CP antigen to remove protective activity from the IRS, whereas adsorption of the serum with the homologous E. faecalis strain 12030 removed protective antibody. All sera were routinely adsorbed with the heterologous E. faecalis strain to ensure the specificity of the protective activity for the CP antigen. Of critical importance is the fact that the antibodies to this CP antigen also reduced tissue levels of enterococcal strains if given therapeutically after initiation of an infection, a situation analogous to that routinely encountered with human patients.
Effective immunotherapies for bacterial pathogens are usually directed at microbial virulence factors, and a number of such factors, including a hemolysin-bacteriocin (6, 14, 16, 17, 37) and aggregation and binding substances involved in bacterial conjugation (6, 18, 19, 32), have been described for enterococci. However, only limited information is available on the specific role of polysaccharide antigens in the pathogenicity of enterococcal infections (1, 2, 13, 39, 40). CP antigens and teichoic acids from enterococci have been identified and partially characterized by a number of investigators in the past (3, 4, 9, 25–27), but the host immune response to these antigens and their potential usefulness as vaccines have not previously been elucidated. Only one attempt at a systematic seroepidemiologic approach to classifying enterococci based on CP structures has been reported (33, 34). Sharpe (34) defined 11 different type strains but, because of recent taxonomic changes, it is not clear that all of these strains belong to the genus Enterococcus. Importantly, we found that the glucose-glycerol-teichoic acid CP isolated from E. faecalis 12030 is also made by E. faecium. While infections with E. faecalis predominate in humans, multiple resistances to antibiotics such as vancomycin and penicillin are more commonly found in E. faecium strains.
Numerous attempts to demonstrate a capsule on enterococci by conventional electron microscopy have been inconclusive (1, 19, 21). However, we recently were able to show a capsule-like structure on a number of enterococcal strains by immunoelectron microscopy using antibodies raised against the CP antigen from E. faecalis 12030 to stabilize this surface polysaccharide (13). Ongoing studies by our group indicate there are several more serologically distinct enterococcal capsules that may prove to be targets for immunotherapy. However, the extent of structural and serologic variability among enterococcal capsular antigens is not known.
Most antibodies that mediate elimination of live bacterial pathogens in vivo also mediate opsonic killing. There was complete correlation in our study between the ability of an antiserum raised to the E. faecalis 12030 CP antigen to mediate opsonic killing in vitro and protect mice from enterococcal challenge. Previous studies investigating the potential immunologic mechanisms associated with resistance to enterococcal infections concluded that neutrophil killing of enterococci is mediated primarily by complement, with antibodies playing a less-important role (1, 2, 11). However, one more recent study found that E. faecalis-specific antibodies promoted neutrophil killing; the authors speculated that an adjunctive therapy using antibodies specific to enterococcal antigens could augment the host response to enterococcal infections (10). Our results on the opsonic and protective efficacy of antibodies to CP antigens support the findings of Gaglani et al. (10) on a role for antibody in promoting killing of enterococci.
The data presented here demonstrate that, in addition to eliciting opsonic killing by antibodies in animals, the CP antigen from E. faecalis 12030 also induces protective immunity in mice. The animal model used in the present study was chosen to mimic the clinical condition of systemic enterococcal infections in hospital patients. Significantly more bacteria were isolated from the livers, spleens, and kidneys of mice receiving NRS than from those receiving IRS against the purified CP antigen from the homologous strain. Furthermore, serum raised against E. faecalis 12030 CP also protected mice against challenge with E. faecalis OG1RF and against two clinical isolates of vancomycin-resistant E. faecium (13). These data suggest that CP antigens from enterococci (E. faecalis and E. faecium) are targets of opsonic antibodies and that these antibodies are potentially useful for immunotherapy of systemic enterococcal infections.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grants AI 23335 and AI 42261 from the NIAID.
REFERENCES
- 1.Arduino R C, Jacques-Palaz K, Murray B E, Rakita R M. Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect Immun. 1994;62:5587–5594. doi: 10.1128/iai.62.12.5587-5594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arduino R C, Murray B E, Rakita R M. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun. 1994;62:987–993. doi: 10.1128/iai.62.3.987-993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleiweis A, Krause R. The cell walls of group D streptococci. I. The immunochemistry of the type 1 carbohydrate. J Exp Med. 1965;122:237–249. doi: 10.1084/jem.122.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleiweis A S, Young F E, Krause R M. Cell walls of group D streptococci. II. Chemical studies on the type 1 antigen purified from the autolytic digest of cell walls. J Bacteriol. 1967;94:1381–1387. doi: 10.1128/jb.94.5.1381-1387.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A, Scharff M D. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38:1695–1702. doi: 10.1128/aac.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen M L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 8.Edmond M B, Wallace S E, McClish D K, Pfaller M A, Jones R N, Wenzel R P. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 9.Elliott S. Type and group polysaccharides of group D streptococci. J Exp Med. 1960;11:621–630. doi: 10.1084/jem.111.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaglani M J, Baker C J, Edwards M S. Contribution of antibody to neutrophil-mediated killing of Enterococcus faecalis. J Clin Immunol. 1997;17:478–484. doi: 10.1023/a:1027371727225. [DOI] [PubMed] [Google Scholar]
- 11.Harvey B S, Baker C J, Edwards M S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992;60:3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hols P, de Halleux S, Delcour J. A strategy to construct vector-free amylolytic strains through nondisruptive homologous recombination: application to Enterococcus faecalis. Gene. 1992;118:31–38. doi: 10.1016/0378-1119(92)90245-k. [DOI] [PubMed] [Google Scholar]
- 13.Huebner J, Wang Y, Krueger W A, Madoff L C, Martirosian G, Boisot S, Goldmann D A, Kasper D L, Tzianabos A O, Pier G B. Isolation and chemical characterization of a capsular polysaccharide antigen shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Infect Immun. 1999;67:1213–1219. doi: 10.1128/iai.67.3.1213-1219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ike Y, Hashimoto H, Clewell D B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987;25:1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jett B D, Gilmore M S. The growth-inhibitory effect of the Enterococcus faecalis bacteriocin encoded by pAD1 extends to the oral streptococci. J Dent Res. 1990;69:1640–1645. doi: 10.1177/00220345900690100301. [DOI] [PubMed] [Google Scholar]
- 16.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lounatmaa K, Meurman J H. Electron microscopic visualization of extracellular polysaccharides on the cell wall of some streptococci. J Dent Res. 1980;59:729–735. doi: 10.1177/00220345800590041501. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa S, Yoshioka M, Kumamoto Y. Proposal of a new scheme for the serological typing of Enterococcus faecalis strains. Microbiol Immunol. 1992;36:671–681. doi: 10.1111/j.1348-0421.1992.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 21.Matthews K R, Oliver S P. Encapsulation of streptococci isolated from bovine milk. Zentbl Veterinarmed B. 1993;40:597–602. doi: 10.1111/j.1439-0450.1993.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 22.Murray B F, Singh K V, Ross R P, Heath J D, Dunny G M, Weinstock G M. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993;175:5216–5223. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Nosocomial Infections Surveillance. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from October 1986–April 1998, issued June 1998. Am J Infect Control. 1998;26:522–533. doi: 10.1016/s0196-6553(98)70026-4. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowsky B E, Venkataraman L, D'Agata E M, Gold H S, DeGirolami P C, Samore M H. Vancomycin-resistant enterococci in intensive care units: high frequency of stool carriage during a non-outbreak period. Arch Intern Med. 1999;159:1467–1472. doi: 10.1001/archinte.159.13.1467. [DOI] [PubMed] [Google Scholar]
- 25.Pazur J H. β-d-Glucose 1-phosphate: a structural unit and an immunological determinant of a glycan from streptococcal cell walls. J Biol Chem. 1982;257:589–591. [PubMed] [Google Scholar]
- 26.Pazur J H, Anderson J S, Karakawa W W. Glycans from streptococcal cell walls. Immunological and chemical properties of a new diheteroglycan from Streptococcus faecalis. J Biol Chem. 1971;246:1793–1798. [PubMed] [Google Scholar]
- 27.Pazur J H, Cepure A, Kane J A, Hellerquist C G. Glycans from streptococcal cell walls. The molecular structure of an antigenic diheteroglycan of glucose and galactose from Streptococcus faecalis. J Biol Chem. 1973;248:279–284. [PubMed] [Google Scholar]
- 28.Pier G B, Small G J, Warren H B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infection. Science. 1990;249:537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- 29.Pier, G. B., D. DesJardins, M. Grout, C. Garner, S. E. Bennett, G. Pekoe, S. A. Fuller, M. O. Thornton, W. S. Harkonen, and H. C. Miller. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect. Immun. 62:3972–3979. [DOI] [PMC free article] [PubMed]
- 30.Qin X, Singh K V, Xu Y, Weinstock G M, Murray B F. Effect of disruption of a gene encoding an autolysin of Enterococcus faecalis OG1RF. Antimicrob Agents Chemother. 1998;42:2883–2888. doi: 10.1128/aac.42.11.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards M J, Edwards J R, Culver D H, Gaynes R P. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Schlievert P M, Gahr P, Assimacopoulos A P, Dinges M M, Stoehr J A, Harmala J W, Hirt H, Dunny G M. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpe M, Shattok P. The serological typing of group D streptococci associated with outbreaks of neonatal diarrhoea. J Gen Microbiol. 1952;6:150–165. doi: 10.1099/00221287-6-1-2-150. [DOI] [PubMed] [Google Scholar]
- 34.Sharpe M. Serological types of Streptococcus faecalis and its varieties and their cell wall type antigen. J Gen Microbiol. 1964;36:151–160. doi: 10.1099/00221287-36-1-151. [DOI] [PubMed] [Google Scholar]
- 35.Singh K V, Qin X, Weinstock G M, Murray B F. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J Infect Dis. 1998;178:1416–1420. doi: 10.1086/314453. [DOI] [PubMed] [Google Scholar]
- 36.Spera R V, Jr, Farber B F. Multidrug-resistant Enterococcus faecium. An untreatable nosocomial pathogen. Drugs. 1994;48:678–688. doi: 10.2165/00003495-199448050-00003. [DOI] [PubMed] [Google Scholar]
- 37.Stevens S X, Jensen H G, Jett B D, Gilmore M S. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophthalmitis. Investig Ophthalmol Vis Sci. 1992;33:1650–1656. [PubMed] [Google Scholar]
- 38.Wang Y, Huebner J, Tzianabos A O, Martirosian G, Kasper D L, Pier G B. Structure of an antigenic teichoic acid shared by clinical isolates of Enterococcus faecalis and vancomycin-resistant Enterococcus faecium. Carbohydr Res. 1999;316:155–160. doi: 10.1016/s0008-6215(99)00046-4. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Jiang L, Murray B E, Weinstock G M. Enterococcus faecalis antigens in human infections. Infect Immun. 1997;65:4207–4215. doi: 10.1128/iai.65.10.4207-4215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Murray B E, Weinstock G M. A cluster of genes involved in polysaccharide biosynthesis from Enterococcus faecalis OG1RF. Infect Immun. 1998;66:4313–4323. doi: 10.1128/iai.66.9.4313-4323.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]