Abstract
Background:
PMTCT programs serve women continuing and initiating ART in pregnancy, and follow-up schedules align to delivery rather than ART initiation, making conventional HIV retention measures (assessed from ART initiation) challenging to apply. We evaluated three measures of peripartum non-retention in Kenyan women living with HIV from pregnancy to 2-years postpartum.
Methods:
This longitudinal analysis used programmatic data from the Mobile WAChX trial (NCT02400671). Outcomes included loss to follow-up (LTFU) (no visit for ≥6 months), incomplete visit coverage (<80% of 3-month intervals with a visit), and late visits >2 weeks after scheduled date). Predictors of non-retention were determined using Cox proportional hazards, log-binomial, and generalized estimating equation models.
Results:
Among 813 women enrolled at a median of 24 weeks gestation, incidence of LTFU was 13.6/100 person-years; cumulative incidence of LTFU by 6, 12 and 24 months postpartum was 16.7%, 20.9% and 22.5%, respectively. Overall, 35.5% of women had incomplete visit coverage. Among 794 women with 12,437 scheduled visits, a median of 11.1% of visits per woman were late (IQR 4.3%-23.5%). Younger age, unsuppressed viral load, unemployment, ART initiation in pregnancy, and non-disclosure were associated with non-retention by all measures. Partner involvement was associated with better visit coverage and timely attendance. Women who became LTFU had higher frequency of previous late visits (16.7% vs. 7.7%, p<0.0001).
Conclusion:
Late visit attendance may be a sentinel indicator of LTFU. Identified cofactors of PMTCT programmatic retention may differ depending on retention measure assessed, highlighting need for standardized measures.
Keywords: Women, HIV care continuum, retention, Africa, loss-to follow-up, cohort studies
Background
Effective prevention of mother‐to‐child transmission (PMTCT) programs support women living with HIV (WLWH) to access care and antiretroviral therapy (ART) throughout pregnancy and postpartum1–3. Adherence to sequential steps of the PMTCT cascade4 is crucial, with one modeling study indicating that virtual elimination of vertical HIV transmission could be attained by 95% adherence to each step5. However, loss-to follow-up (LTFU) rates are often high in PMTCT programs with 16–22% of women LTFU during peripartum period6–9.
Despite recognition of the crucial need for high retention in PMTCT programs, there is no standard definition or methodology to evaluate retention in this context, and studies have used a wide range of definitions10–15. For surveillance purposes, the WHO consolidated guidelines define retention in general HIV care as attendance at a health facility at 12-months post-ART initiation10. In 2015, the Inter-Agency Task Team (IATT) on Children and HIV/ AIDS assessed maternal retention as continuous longitudinal engagement, defined as the proportion of women on ART per 3-month intervals until 12 months after ART initiation11. However, for pregnant and breastfeeding WLWH, the 12-months post-ART initiation timepoint is often challenging to align with routine PMTCT visits which are scheduled based on gestational age and time since delivery: most women initiate ART prior to pregnancy and for those who initiate ART in pregnancy, gestational age at ART initiation varies. Differences in outcome definitions have yielded variations in estimates of LTFU or retention within PMTCT programs6,8,16–25. A meta-analysis pooling six PMTCT intervention studies in Malawi, Nigeria and Zimbabwe observed marked variability in retention estimates ranging from 30% to 76% when different definitions were applied within the same dataset26. This variability makes charting progress and identifying effective interventions challenging across programs.
In the absence of a standard definition for non-retention in PMTCT programs, we evaluated three retention measures of potential utility to the peripartum period in a cohort of WLWH from pregnancy to 2-year postpartum. We also determined how these measures influenced identification of cofactors of non-retention.
Methods
Study design and population
This study leveraged data collected from a completed 3-armed randomized clinical trial (RCT) (Mobile WAChX study, ClinicalTrial.gov number NCT0240067127,28). The RCT assessed the effect of short messaging service (SMS) on ART adherence and retention among WLWH attending PMTCT programs. The RCT intervention did not have an impact on any non-retention measures; results have been previously reported28. Briefly, the trial enrolled pregnant WLWH from six public maternal-child health (MCH) clinics in Nairobi and Western Kenya if they were aged ≥14 years and had daily access to a mobile phone at the time of enrollment. Women were randomized to receive one-way SMS, two-way SMS or no SMS, and followed-up through 2 years postpartum28. This study aimed to determine effect of the intervention on programmatic retention and used medical record data from routine clinic visits for the retention outcomes; no additional sample size calculations were conducted. The RCT was approved by the UW Institutional Review Board and the Kenyatta National Hospital/University of Nairobi Ethical Review Committee.
Data Collection
At enrollment, a standardized survey was administered on a tablet using Open Data Kit (ODK). Data was collected on demographics, social support (using Medical Outcomes Study [MOS] survey29), stigma (using 4-item instrument adapted from the stigma scale for chronic illnesses [SSCI]30), depression (using Patient Health Questionnaire 9 [PHQ9]31), intimate partner violence (IPV) (using Abuse Assessment Screen [AAS]32), food security (using Household Food Insecurity Access Scale [HFIAS]33), disclosure of HIV status, and ART knowledge based on the Information–Motivation and Behavioural Skills (IMB) model, which was adapted from 15 items from the LifeWindows ART adherence questionnaire34. Data on ART use history was abstracted from the mother’s Mother Child Health (MCH) booklet. HIV VL testing results were obtained from the routine VL monitoring system of the Kenya National AIDS & STI Control Program (NASCOP). If programmatic VL results were not available, VL testing was done by the study on maternal plasma samples. Programmatic data of clinic visits was obtained from clinic paper records and electronic medical records (EMRs)35. Briefly, at in-person study visits at enrolment in pregnancy, 6 weeks postpartum and 6, 12, 18, and 24 months postpartum, study staff checked medical records and EMR for appointments, deliveries, clinic visits, and medication refills. For women with a recorded transfer-out status (based on self-report or official transfer report at the facility), data was extracted at other sites where participants received care after obtaining the approval from the sites.
Study outcomes
This study assessed three aspects of non-retention: programmatic LTFU, incomplete visit coverage, and late visits. Programmatic LTFU was defined as no clinic visit for at least 6 months, with no report of death or transfer. This was based on a study integrating data from 19 countries which proposed ≥6 months since the last clinic visit as a standard LTFU definition in general HIV care15. Visit coverage was defined for each participant as a proportion of 3-month intervals with at least one clinic visit over the entire follow-up period from pregnancy to 2 years postpartum. This approach aligns with the recommended schedule of maternal ART refills, follow-up visits for antenatal/postnatal care, and immunization windows for HIV exposed infants, according to the WHO guidelines of integrating PMTCT services into standard MCH services36 and the overall visit continuum37. The numerator is equal to the number of intervals since enrollment in which at least one clinic visit was attended, and the denominator is equal to the total number of intervals during follow-up (depending on gestational age at enrollment in this cohort)18,36. A late visit was defined for each participant as a scheduled visit that was not attended within 2 weeks of the expected date of clinic visit and was assessed for each scheduled visit.
In all analyses, participants who died, exited from the trial, or completed 2-year postpartum follow-up were censored at the time of corresponding events. In the analysis of LTFU and visit coverage, visit data abstracted both from the original study sites and from transfer sites was used. In the analysis of late visits, only data abstracted at the study sites was used. For women who transferred out, data after transfer was censored and subsequent scheduled visits were not included. In addition, women who stopped follow-up with dates known to be before their scheduled visit date + 14 days (defined attendance window for late visits) were also censored before the final scheduled visit, i.e. attendance of the final scheduled visit was marked as missing and not included in the analyses.
Statistical analysis
Kaplan-Meier survival curves were used to assess time to LTFU. Person-time at risk was calculated from enrollment to the last visit when participants were assessed for LTFU by definition. Cox proportional hazards regression was used to identify predictors of LTFU. Log-binomial regression was used to identify predictors of cumulative LTFU by 24-months postpartum and predictors of visit coverage <80%. Generalized estimating equation (GEE) models clustered by participant, with log-binomial link and exchangeable correlation structure were used to identify predictors of late visits. Two-sample t-tests were used to compare the proportion of late visits between women who were LTFU and not, excluding the last scheduled visit of women who were LTFU, because by definition LTFU involved no attendance of the last scheduled visit (and would be considered ‘late’). Study site, dichotomized as Nairobi (Mathare, Riruta) or Western Kenya (Ahero, Bondo, Siaya, Rachuonyo), was identified as an a priori confounder in all regression models to account for potential geographical differences in maternal characteristics and underlying retention in care. All models used robust standard errors. All analyses were conducted using RStudio Version 1.2.5042 (RStudio, Inc).
Results
Overall, 813 women from the Mobile WAChX trial had programmatic visit data available for inclusion in this analysis. At enrollment, median age was 27 years (interquartile range [IQR] 23–31) and median gestational age was 24.3 weeks (IQR 18.3–29.6). Over 1,349 person-years (py) of follow-up, the incidence rate of LTFU was 13.6/100 person-years (Table 1a, Figure 1a). The cumulative incidence of LTFU by delivery, 6, 12 and 24 months postpartum was 12.6%, 16.7%, 20.9% and 22.5%, respectively (Table 1c, Figure 1b). Depending on their gestational age at enrollment, women were expected to have a median of 10 (IQR 9–10) 3-month intervals during study follow-up (Table 1b), and a total of 7,771 expected intervals were included in the analysis. The proportion of intervals with ≥1 visit among all women was 74.9%, and each participant had a median of 88.9% (IQR 66.7%-90.9%) of quarterly intervals covered with at least 1 visit attended (Table 1b). Visit coverage per interval declined from 83.5% in the first 3 months after delivery, to 65.8% at 24 months postpartum (Figure 1c). Among 147 (18.1%) women documented as transferred out during study follow-up, 94 (63.9%) had data retrieved and abstracted from clinics they transferred to. In a secondary analysis not including visit data after transfer-out, the rate of LTFU by 24 months postpartum would be over-estimated as 28.8% (234/812), 6.3% higher than the 22.5% observed, and the overall proportion of quarterly intervals with at least 1 visit attended by 24 months postpartum would be 70.5%, 4.4% lower than the 74.9% observed.
Table 1.
Measures of non-retention
| a. LTFUa | ||||
|---|---|---|---|---|
| Postpartum period | Number of women | Event / person-year | Incidence rate / 100 py | Cumulative incidence (n/N)a |
| By delivery | 813 | 101 / 374.6 | 27.0 | 12.6% (101/813) |
| By 6 weeks | 813 | 109 / 452.5 | 24.1 | 13.5% (109/813) |
| By 6 months | 813 | 135 / 699.7 | 19.3 | 16.7% (135/813) |
| By 12 months | 813 | 170 / 996.7 | 17.0 | 20.9% (170/813) |
| By 18 months | 813 | 183 / 1,231.7 | 14.9 | 22.5% (183/813) |
| By 24 months | 813 | 183 / 1,349.0 | 13.6 | 22.5% (183/813) |
| b. Visit coverageb | |||||
|---|---|---|---|---|---|
| Postpartum period | Number of womenb | Among all women (n/N) | Per woman [median (IQR)] | ||
| Proportion of intervals with ≥1 visit | Number of expected intervals | Number of intervals with ≥1 visit | Proportion of intervals with ≥1 visit | ||
| By 24 months | 812 | 74.9% (5,822/7,771) | 10 (9–10) | 8 (6–9) | 88.9% (66.7–90.9%) |
| c. Late visitsc | |||||
| Postpartum period | Number of women† | Among all women (n/N) | Per woman [median (IQR)] | ||
| Proportion of late visits | Number of scheduled visits | Number of late visits | Proportion of late visits | ||
| By delivery | 569 | 14.4% (190/1,320) | 2 (1–3) | 0 (0–1) | 0% (0–33.3%) |
| By 6 weeks | 711 | 15.9% (324/2,041) | 3 (1–4) | 0 (0–1) | 0% (0–40.0%) |
| By 6 months | 783 | 13.0% (589/4,518) | 6 (3–8) | 1 (0–1) | 12.5% (0–33.3%) |
| By 12 months | 791 | 11.5% (891/7,776) | 11 (6–13) | 1 (0–2) | 10.0% (0–25.0%) |
| By 18 months | 793 | 11.0% (1158/10,535) | 14 (9–18) | 1 (1–2) | 10.5% (4.5%-23.1%) |
| By 24 months | 794 | 11.5% (1431/12,437) | 16 (10–22) | 2 (1–3) | 11.1% (4.3%-23.5%) |
LTFU was defined as no clinic visit for at least 6 months since the last clinic visit and not known to have died or transferred;
visit coverage was defined for each participant as a proportion of 3-month intervals with at least one clinic visit over the entire follow-up period from pregnancy to 2 years postpartum; excluding one woman who had a visit beyond 24-month postpartum;
late visit was defined for each participant as a scheduled visit that was not attended within 2 weeks of the expected date of clinic visit.
Figure 1. Retention over the follow-up period using the 3 measures (late visits, visit coverage, LTFU).
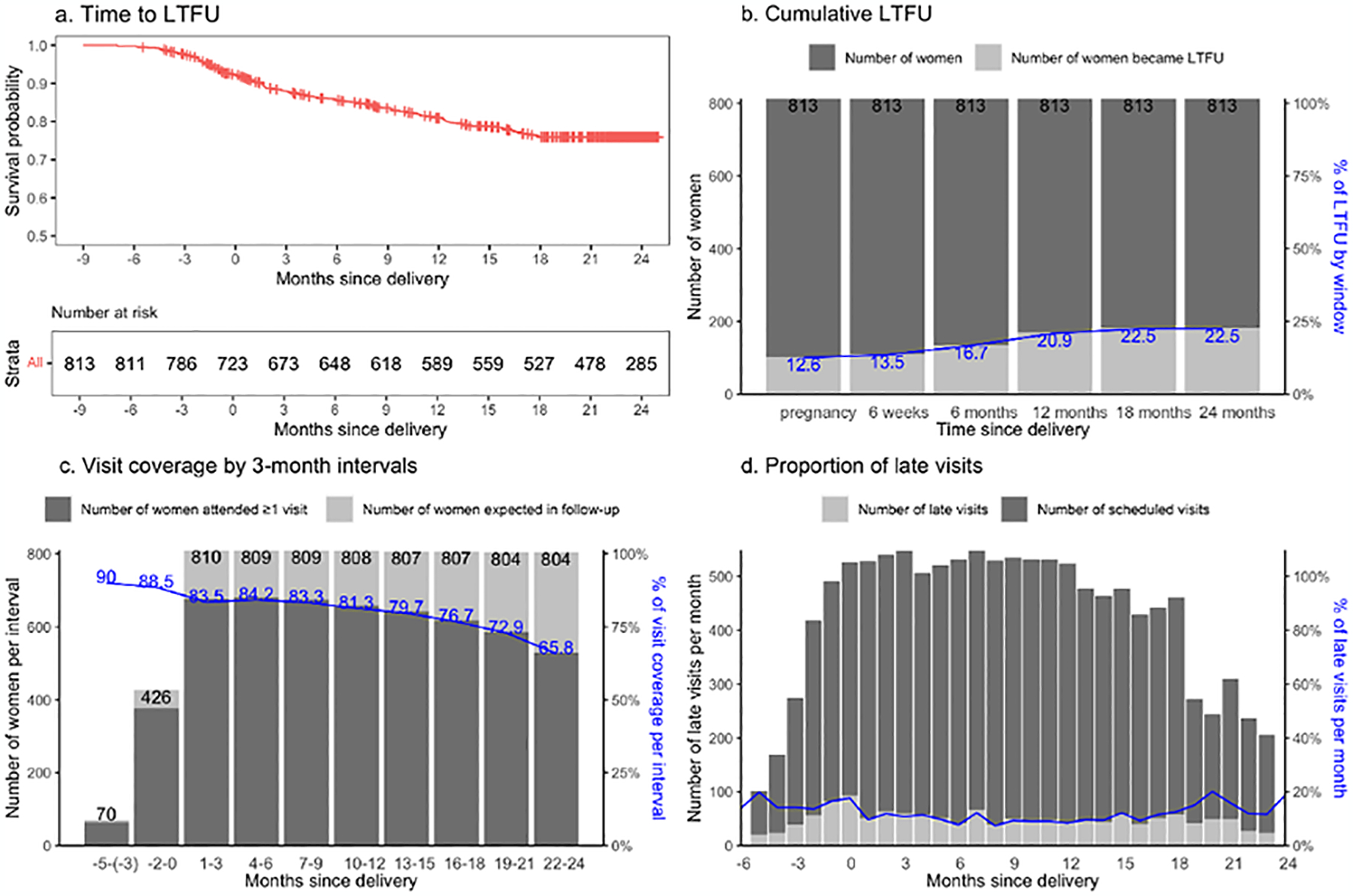
a. time-to-LTFU (no visit for ≥6 months since last visit) described by Kaplan-Meier survival curves; b. cumulative incidence of LTFU calculated as the proportion of women ever LTFU among women in follow-up; c. visit coverage calculated as the proportion of 3-month intervals with a visit; d. Late visit defined as no visit within 2 weeks of scheduled date.
A total of 794 women had ≥1 clinic appointment scheduled, with a median of 16 scheduled visits (IQR 10–22) per woman (Table 1c), for a total of 12,437 scheduled visits, of which 11.5% were late. Among late attended visits, the median duration from the scheduled date was 32 days later (IQR 27–59). The median proportion of late visits per woman was 11.1% (IQR 4.3%-23.5%) by 24-month postpartum (Table 1c). The proportion of late visits declined from 17.7% at 1-month postpartum, to 9.8% at 6 months postpartum, and remained around 11% until 18 months postpartum (Table 1c, Figure 1d). The proportion slightly increased to 20.0% at 20 months postpartum, potentially due to the total number of scheduled visits decreased after 20 months.
Most (85.0%, 675/794) women were late for at least 1 scheduled visit, and 61.2% (483/789) women had a late visit within 6 months after delivery. Women who were ever late for visits during the first 6 months postpartum, had a higher risk of subsequent LTFU (22.2% vs. 16.4%, p=0.05) (Figure 2a). Over follow-up through 2 years postpartum, women who became LTFU had a significantly higher proportion of previous late visits than those who did not (16.7% vs. 7.7%, p<0.0001) (Figure 2b). In a sensitivity analysis among 625 women who had at least 10 previous scheduled visits, women who became LTFU had higher frequency of prior late visits (13.4% vs. 7.7%, p<0.0001) (data not shown).
Figure 2. Comparison of any previous late visits and LTFU at 24-month postpartum*.
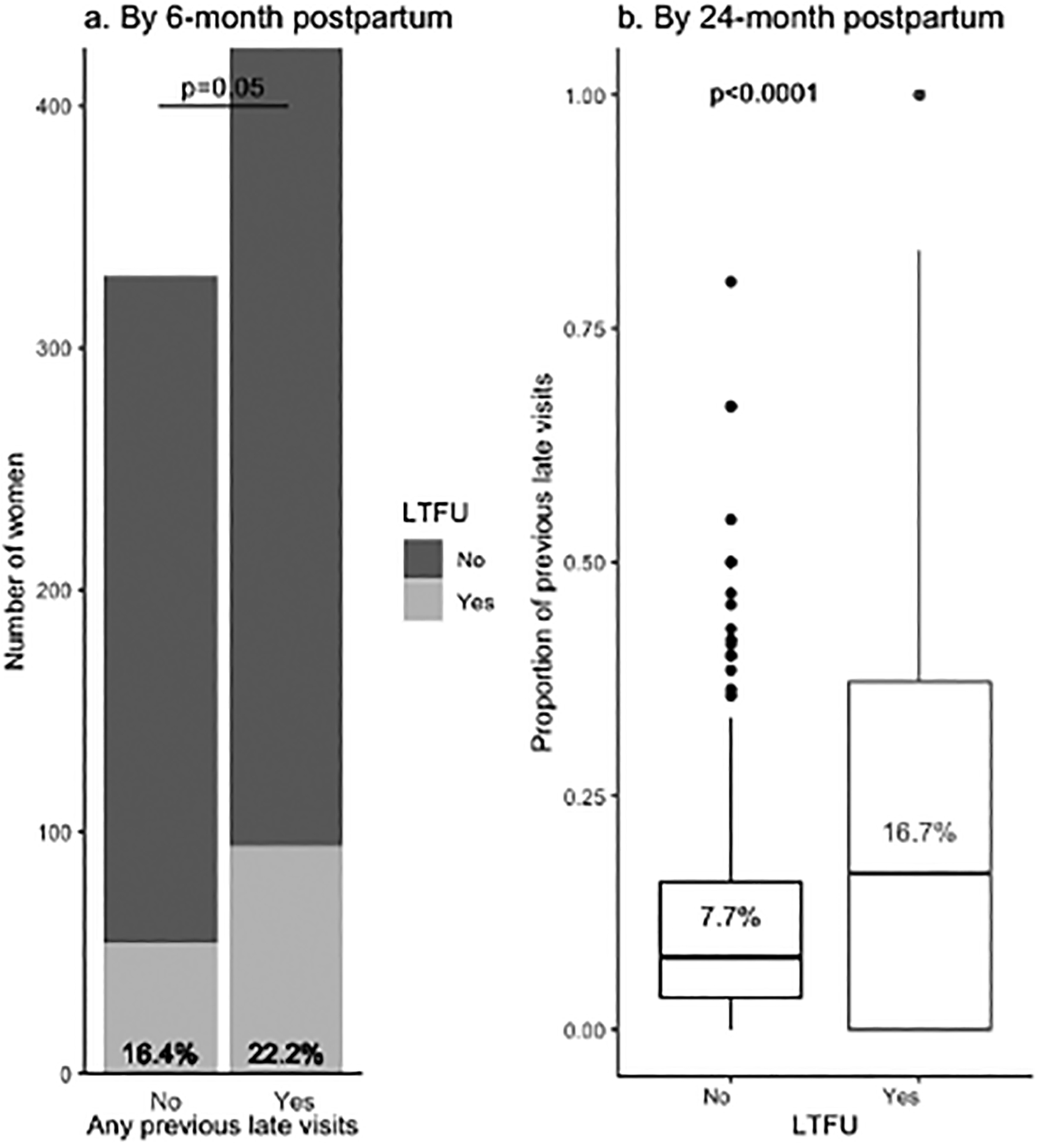
*Among 759 women with at least two visits during follow-up; a. risk of LTFU compared by chi-square test; b. proportion of last visits compared by two-sample t-test
In site-adjusted regression models, younger age was associated with higher risks of non-retention by all three measures (Figure 3). Women aged 15–24 years were more likely to be LTFU (adjusted hazard ratio [aHR] 2.13, 95%CI 1.59–2.86; p<0.0001), have a low visit coverage (<80%) (adjusted prevalence ratio [aPR] 1.60, 95%CI 1.35–1.90; p<0.0001), and be late for a scheduled visit (aPR 1.21, 95%CI 1.08–1.36; p=0.001) (Table 2). Unsuppressed VL at enrollment, being employed, HIV diagnosed before pregnancy, disclosure to others, and starting ART before pregnancy were significantly associated with better retention in all measures (Figure 3, Table 2).
Figure 3. Forest plot of cofactors for non-retention, by non-retention measure.
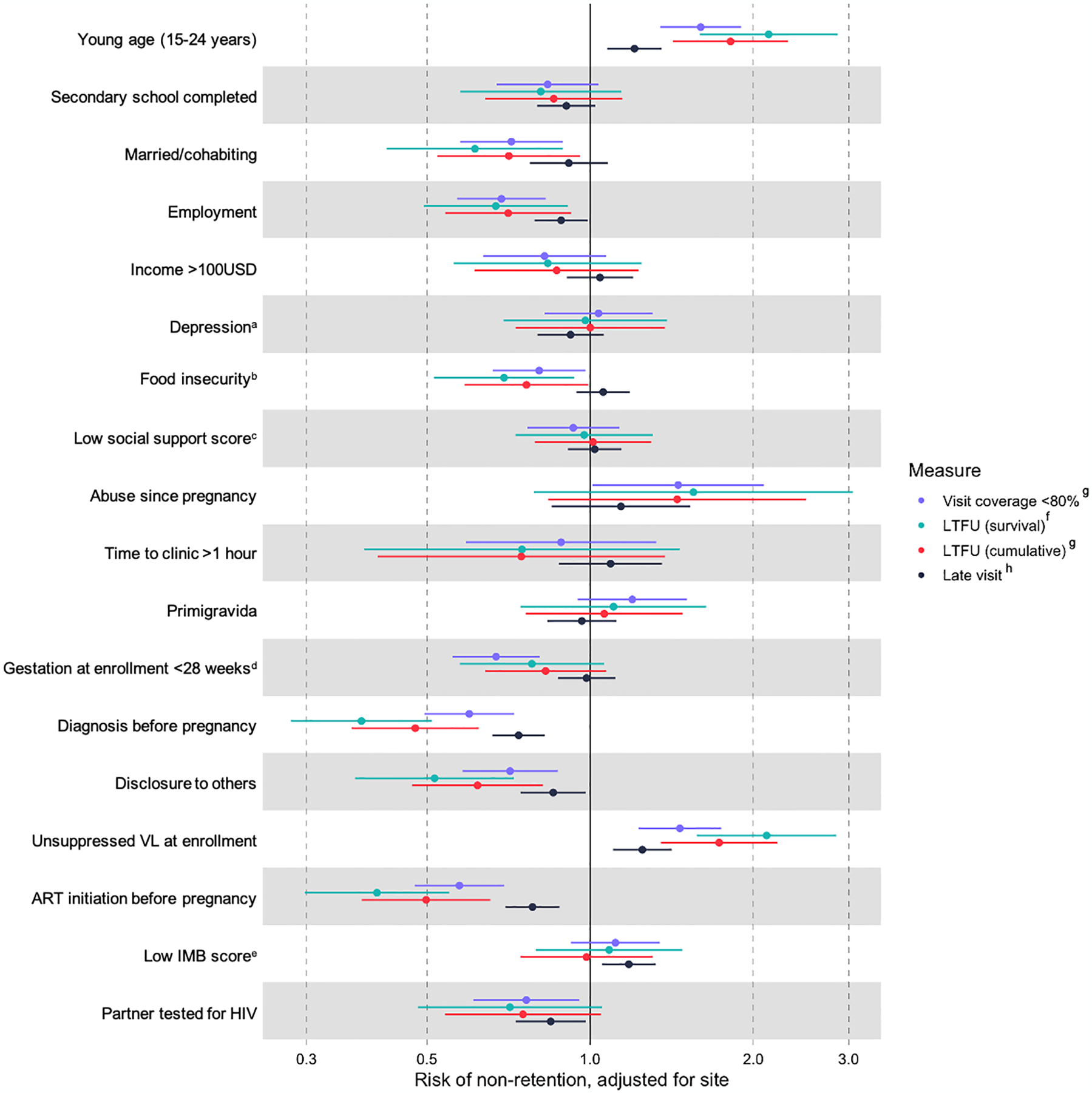
a evaluated by Patient Health Questionnaire 9 (PHQ9), a score >5 indicating at least moderate depressive symptoms; b evaluated by Household Food Insecurity Access Scale (HFIAS); c evaluated by Medical Outcomes Study (MOS) survey; d estimated by self-reported date of last menstruation period; e Information-Motivation-Behavioral (IMB) score evaluated by 15 items from LifeWindows ART adherence questionnaire; f HR estimated by cox proportional hazards regression calculating time-at-risk since enrollment and adjusting for site; g PR estimated by Log-binomial regression adjusting for site; h PR estimated by GEE regression adjusting for site.
Table 2.
Hazard ratio (HR) and prevalence ratio (PR) of loss-to follow-up (LTFU), and PRs of visit coverage <80% and late visits
| LTFU | Cumulative LTFU | Visit coverage<80% | Late visit | |
|---|---|---|---|---|
| aHRf (95%CI); p-value | aPRg (95%CI); p-value | aPRg (95%CI); p-value | aPRh (95%CI); p-value | |
| Young age (15–24 years) | 2.13 (1.59–2.86); <0.001 | 1.82 (1.42–2.32); <0.001 | 1.60 (1.35–1.90); <0.001 | 1.21 (1.08–1.36); 0.001 |
| Secondary school completed | 0.81 (0.58–1.14); 0.228 | 0.86 (0.64–1.15); 0.298 | 0.83 (0.67–1.04); 0.102 | 0.90 (0.8–1.02); 0.109 |
| Married/cohabiting | 0.61 (0.42–0.89); 0.01 | 0.71 (0.52–0.96); 0.026 | 0.72 (0.58–0.89); 0.003 | 0.91 (0.77–1.08); 0.283 |
| Employment | 0.67 (0.49–0.91); 0.01 | 0.71 (0.54–0.92); 0.011 | 0.69 (0.57–0.83); <0.001 | 0.88 (0.79–0.99); 0.032 |
| Income>100USD | 0.84 (0.56–1.24); 0.376 | 0.87 (0.61–1.23); 0.422 | 0.82 (0.63–1.07); 0.146 | 1.04 (0.91–1.20); 0.56 |
| Depressiona | 0.98 (0.69–1.39); 0.908 | 1.00 (0.73–1.37); 0.998 | 1.04 (0.82–1.30); 0.763 | 0.92 (0.8–1.06); 0.245 |
| Moderate/severe food insecurityb | 0.69 (0.51–0.93); 0.016 | 0.76 (0.59–0.99); 0.043 | 0.81 (0.66–0.98); 0.031 | 1.06 (0.94–1.18); 0.339 |
| Low social support score (<median)c | 0.97 (0.73–1.31); 0.863 | 1.01 (0.79–1.30); 0.926 | 0.93 (0.77–1.13); 0.471 | 1.02 (0.91–1.14); 0.74 |
| Abuse since pregnancy | 1.55 (0.79–3.05); 0.204 | 1.45 (0.84–2.50); 0.186 | 1.45 (1.01–2.09); 0.044 | 1.14 (0.85–1.53); 0.384 |
| Time to clinic >1 hour | 0.75 (0.38–1.46); 0.397 | 0.75 (0.41–1.37); 0.348 | 0.88 (0.59–1.32); 0.549 | 1.09 (0.88–1.36); 0.438 |
| Primigravida | 1.10 (0.74–1.64); 0.622 | 1.06 (0.76–1.48); 0.725 | 1.20 (0.95–1.51); 0.131 | 0.97 (0.83–1.12); 0.637 |
| Gestation at enrollment <28 weeksd | 0.78 (0.57–1.06); 0.113 | 0.83 (0.64–1.07); 0.149 | 0.67 (0.56–0.81); <0.001 | 0.98 (0.87–1.11); 0.801 |
| Diagnosis before pregnancy | 0.38 (0.28–0.51); <0.001 | 0.48 (0.36–0.62); <0.001 | 0.60 (0.49–0.72); <0.001 | 0.74 (0.66–0.83); <0.001 |
| Disclosure to others | 0.52 (0.37–0.72); <0.001 | 0.62 (0.47–0.82); 0.001 | 0.71 (0.58–0.87); 0.001 | 0.85 (0.74–0.98); 0.025 |
| Unsuppressed VL at enrollment | 2.12 (1.57–2.85); <0.001 | 1.73 (1.35–2.21); <0.001 | 1.46 (1.23–1.75); <0.001 | 1.25 (1.10–1.41); <0.001 |
| ART initiation before pregnancy | 0.40 (0.30–0.55); <0.001 | 0.50 (0.38–0.65); <0.001 | 0.57 (0.47–0.69); <0.001 | 0.78 (0.70–0.88); <0.001 |
| Low IMB scoree | 1.08 (0.79–1.48); 0.611 | 0.98 (0.74–1.30); 0.913 | 1.11 (0.92–1.35); 0.266 | 1.18 (1.05–1.32); 0.005 |
| Partner tested for HIV | 0.71 (0.48–1.05); 0.087 | 0.75 (0.54–1.05); 0.091 | 0.76 (0.61–0.96); 0.018 | 0.85 (0.73–0.98); 0.027 |
evaluated by Patient Health Questionnaire 9 (PHQ9), a score >5 indicating at least moderate depressive symptoms;
evaluated by Household Food Insecurity Access Scale (HFIAS);
evaluated by Medical Outcomes Study (MOS) survey;
estimated by self-reported date of last menstruation period;
Information-Motivation-Behavioral (IMB) score evaluated by 15 items from LifeWindows ART adherence questionnaire;
HR estimated by cox proportional hazards regression calculating time-at-risk since enrollment and adjusting for site;
PR estimated by Log-binomial regression adjusting for site;
PR estimated by GEE regression adjusting for sit
The relationship between some covariates and non-retention depended on which non-retention measure was used. Women who were married or cohabiting were less likely to be LTFU (aHR 0.61, 95%CI 0.42–0.89; p=0.01), and to have low visit coverage (aPR 0.72, 0.58–0.89; p=0.003), but not late visits (Table 2). Similarly, women with at least moderate food insecurity also had lower risk of LTFU (aHR 0.69, 95%CI 0.51–0.93; p=0.016) and low visit coverage (aPR 0.81, 95%CI 0.66–0.98; p=0.031) but not late visits. Having a male partner tested for HIV was associated with lower risk of low visit coverage (aPR 0.76, 95%CI 0.61–0.96; p=0.018) and late visits (aPR 0.85, 95%CI 0.73–0.98; p=0.027). Women with standardized IMB score <75% had a significantly higher risk of late visits (aPR 1.18, 95%CI 1.05–1.32; p=0.005) (Table 2). Women’s education level, depressive symptoms, or travel time from home to clinic were not associated with any non-retention measures (Table 2).
The parent trial found no significant impact of the SMS system on outcomes; thus, we pooled the results. Results from stratified analysis by each intervention arm yielded similar findings to the pooled data from the three arms in this study (data not shown).
Discussion
We evaluated three measures of non-retention in care among a cohort of women receiving PMTCT services in Kenya from pregnancy to 2 years postpartum. We found that among 813 participants, 22.5% were LTFU and the incidence rate was 13.6/100 person-year; 55.0% had low visit coverage per quarterly interval (<80%); and 85.0% were late for at least 1 scheduled visit. The incidence rate and cumulative incidence of LTFU in our study were similar to PMTCT studies in Malawi and Ethiopia. In Ethiopia, a LTFU incidence rate of 13.2 per 100 person-year was observed over the 2-year postpartum25. Cumulative incidence of LTFU at 24 months postpartum in Malawi and Ethiopia were 24.5% and 23.0%, respectively6,38. Studies with shorter follow-up periods have reported higher LTFU than our study16,17,39–41. When a one-time indicator of LTFU is used, estimates of retention have varied widely in similar population and settings13,39–41. Some patients thought to be LTFU may receive care at other facilities or return after a period of disengagement14,42–44. If transfer-out data was not obtained from other sites we would have over-estimated LTFU by 24-month postpartum as 28.8%, 6.3% higher than the 22.5% we observed.
Timely attendance of clinic visits in our study varied over pregnancy and postpartum, and we found a higher risk of late visits prior to delivery, consistent with a study in Nigeria45. We found that late visit attendance was associated with subsequent LTFU. Given the particular concern for LTFU during the postpartum period46,47, late visits may be a useful sentinel indicator to alert clinicians to initiate additional strategies to optimize retention.
We found that the proportion of women attending ≥1 clinic visit within each 3-month interval declined over time. In a large Nigerian study of WLWH conducted over a 10-year period, 66% of women had at least one visit during each of the antenatal, delivery, and 18 months postpartum periods9; however, aside from that study, studies analyzing visit coverage throughout the PMTCT care cascade including breastfeeding period is scarce. In our study, 22.5% of women were LTFU by 24 months’ postpartum, however, an additional 12.9% women had less than 80% visit coverage. Irregular PMTCT visits may lead to late drug refill and inadequate infant care, elevating risk of development of drug resistance in both mothers and infants48. Adding the frequency and regularity of visits into the concept of retention may enhance PMTCT program efficacy and evaluations, and HIV drug resistance surveillance should be planned alongside implementation, enabling a distinction to be made between women who attend all, or some care and treatment, and those who are late for scheduled visits and are less engaged in care.
Our study demonstrates variability of retention estimates and cofactors. As clinics seek to monitor retention, standardized definitions of late visits, LTFU and visit coverage will be important. Data sources may need to be combined or triangulated, with counseling registers, general MCH visits and pharmacy records. As women transfer to other clinics over the course of pregnancy and postpartum, obtaining data from multiple sites may necessary. Ideally, national unique identifiers and linked data systems could optimize assessment of transfer. Currently, capturing timeliness and regularity of women’s longitudinal visit data remains a complex task. Furthermore, as retention estimates are often used for the modeling of vertical transmission rates of HIV, a consensus on definitions is urgently needed. Lack of precision could lead to unclear interpretation of program achievements.
We also found that different non-retention measures yielded distinct cofactors of non-retention. Young maternal age and HIV non-disclosure were associated with all non-retention measures. There has been consistent evidence showing younger WLWH are at higher risk of non-retention6,19,20,24,49–52, late ART initiation, low service uptake and poor adherence5,40,53–55. Non-disclosure has also emerged as a consistent barrier to retention in PMTCT programs in sub-Sahara Africa24,56,57. We found that HIV diagnosis during pregnancy, late ART initiation and unsuppressed viral loads were associated with all three measures of non-retention, consistent with results from other studies6,8,28,58,59. Addressing disclosure, tailoring services for younger clients, those who are unsuppressed or starting ART late could improve all retention measures. Other cofactors (partner not HIV tested, low IMB score) were associated specifically with late visits, perhaps reflecting episodic lack of support or self-efficacy to navigate on-time clinic attendance. Marital support and need for food support were associated with lower likelihood of LTFU. Episodic lack of finances or support may delay ability to attend visits on-time, while other factors lead to disengagement and ultimately LTFU. Late visits reflect a less consequential outcome than LTFU; understanding what drives both late visits and LTFU is useful for programmatic improvements.
This study had several limitations. The RCT was conducted among six MCH clinics in two regions in Kenya, and among women who had daily access to a mobile phone, which may limit generalizability. We obtained clinic attendance data from routine medical records which can have data quality issues, and 11 (1.3%) women had no available medical records. EMR data were only available at two Nairobi sites, and although we adjusted for site in all models, there may be residual confounding. However, the combination of paper records at multiple sites and EMR data enabled us to monitor clinical visits for each participant, which added precision to our LTFU estimate. A significant strength of this study was direct comparisons of different retention measures using data from the same cohort. In addition, we identified late visits as a potential indicator of future LTFU in PMTCT programs. We also identified distinct risk factors associated with different retention measures.
Conclusion
This study provides a comprehensive analysis of non-retention rates in PMTCT programs using three different measures, including late visits, 3-month visit coverage and LTFU. We found late visits are an important sentinel indicator of subsequent LTFU. Adding visit timeliness into assessments may enhance PMTCT program evaluations. We also found that distinct risk factors may be associated with different aspects of retention. In summary, standardizing longitudinal definitions of visit timeliness, coverage and LTFU in PTMCT programs will be useful in comparing studies and programmatic interventions to optimize retention in long-term PMTCT programs.
Acknowledgements
We would like to acknowledge the significant contributions from the Mobile WAChX study participants and the team members. We would also like to acknowledge support from the University of Washington’s Global Center for Integrated Health of Women, Adolescents and Children (Global WACh).
Sources of support
This work was supported by grants NIH/NICHD R01HD080460, NIH/NIAID K01AI116298, NIH/NICHD K24HD054314, NIH/NICHD K12HD001264, NIH/NIAID P30AI027757, NIH/NIMH K18MH122978.
Footnotes
Competing interests
We report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Contributor Information
Wenwen Jiang, Department of Epidemiology, University of Washington, Seattle, Washington, USA..
Keshet Ronen, Department of Global Health, University of Washington, Seattle, Washington, USA..
Lusi Osborn, Department of Research and Programs, Kenyatta National Hospital, Nairobi, Kenya..
Alison L. Drake, Global Health, University of Washington, Seattle, Washington, USA..
Jennifer A. Unger, Department of Obstetrics and Gynecology, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA, Department of Global Health, University of Washington, Seattle, Washington, USA..
Daniel Matemo, Department of Research and Programs, Kenyatta National Hospital, Nairobi, Kenya..
Barbra A. Richardson, Departments of Biostatistics and Global Health, University of Washington, Division of Vaccine and Infectious Disease, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA..
John Kinuthia, Department of Research and Programs, Kenyatta National Hospital, Nairobi, Kenya..
Grace John-Stewart, Departments of Global Health, Medicine, Pediatrics, and Epidemiology, University of Washington, Seattle, Washington, USA..
References
- 1.World Health Organization. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Published online 2012. [PubMed]
- 2.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Published online 2015. [PubMed]
- 3.McNairy ML, Teasdale CA, El-Sadr WM, Mave V, Abrams EJ. Mother and child both matter: reconceptualizing the prevention of mother-to-child transmission care continuum. Curr Opin HIV AIDS. 2015;10(6):403–410. doi: 10.1097/COH.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.hIarlaithe MO, Grede N, de Pee S, Bloem M. Economic and social factors are some of the most common barriers preventing women from accessing maternal and newborn child health (MNCH) and prevention of mother-to-child transmission (PMTCT) services: a literature review. AIDS Behav. 2014;18 Suppl 5:S516–30. doi: 10.1007/s10461-014-0756-5 [DOI] [PubMed] [Google Scholar]
- 5.Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Tropical Medicine & International Health. 2018;23(2):136–148. doi: 10.1111/tmi.13014 [DOI] [PubMed] [Google Scholar]
- 6.Mitiku I, Arefayne M, Mesfin Y, Gizaw M. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc. 2016;19(1):20662. doi: 10.7448/IAS.19.1.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzangare J, Takarinda KC, Harries AD, et al. HIV testing uptake and retention in care of HIV-infected pregnant and breastfeeding women initiated on ‘Option B+’ in rural Zimbabwe. Tropical Medicine & International Health. 2016;21(2):202–209. doi: 10.1111/tmi.12637 [DOI] [PubMed] [Google Scholar]
- 8.Reece R, Norman B, Kwara A, Flanigan T, Rana A. Retention in Care of HIV-Positive Postpartum Females in Kumasi, Ghana. J Int Assoc Provid AIDS Care. 2016;15(5):406–411. doi: 10.1177/2325957415603507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawizza HE, Chang CA, Chaplin B, et al. Loss to Follow-Up within the Prevention of Mother-to-Child Transmission Care Cascade in a Large ART Program in Nigeria. Curr HIV Res. 2015;13(3):201–209. doi: 10.2174/1570162x1303150506183256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Consolidated Strategic Information Guidelines for HIV in the Health Sector.; 2015. [PubMed]
- 11.Inter-Agency Task Teams. B+ Monitoring & Evaluation Framework Dissemination and Country Consultation: Adapting Monitoring & Evaluation Systems for Cohort and Enhanced Monitoring as well as Outcome and Impact Evaluations/Assessments. Published online 2015. https://www.childrenandaids.org/sites/default/files/2017-04/IATT_B%2BMonitoring-and-Evaluation-Framework_2016.pdf
- 12.Rollins NC, Becquet R, Orne-Gliemann J, et al. Defining and analyzing retention-in-care among pregnant and breastfeeding HIV-infected women: unpacking the data to interpret and improve PMTCT outcomes. J Acquir Immune Defic Syndr. 2014;67 Suppl 2:S150–6. doi: 10.1097/QAI.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 13.Sibanda EL, Weller IVD, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013;27(17):2787–2797. doi: 10.1097/QAD.0000000000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–244. doi: 10.1007/s11904-010-0061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal Definition of Loss to Follow-Up in HIV Treatment Programs: A Statistical Analysis of 111 Facilities in Africa, Asia, and Latin America. PLOS Medicine. 2011;8(10):e1001111. 10.1371/journal.pmed.1001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yotebieng M, Thirumurthy H, Moracco KE, et al. Conditional Cash Transfers to Increase Retention in PMTCT Care, Antiretroviral Adherence, and Postpartum Virological Suppression: A Randomized Controlled Trial. J Acquir Immune Defic Syndr. 2016;72 Suppl 2(Suppl 2):S124–S129. doi: 10.1097/QAI.0000000000001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28(4). https://journals.lww.com/aidsonline/Fulltext/2014/02200/Retention_in_care_under_universal_antiretroviral.15.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woelk GB, Ndatimana D, Behan S, et al. Retention of mothers and infants in the prevention of mother-to-child transmission of HIV programme is associated with individual and facility-level factors in Rwanda. J Int AIDS Soc. 2016;19(5 Suppl 4):20837. doi: 10.7448/IAS.19.5.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman RM, Phiri K, Parent J, et al. Factors associated with retention in Option B+ in Malawi: a case control study. J Int AIDS Soc. 2017;20(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller K, Muyindike W, Matthews LT, Kanyesigye M, Siedner MJ. Program Implementation of Option B+ at a President’s Emergency Plan for AIDS Relief-Supported HIV Clinic Improves Clinical Indicators But Not Retention in Care in Mbarara, Uganda. AIDS Patient Care STDS. 2017;31(8):335–341. doi: 10.1089/apc.2017.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesevich A, Mtande T, Saidi F, et al. Role of male partner involvement in ART retention and adherence in Malawi’s Option B+ program. AIDS Care. 2017;29(11):1417–1425. doi: 10.1080/09540121.2017.1308464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etoori D, Kerschberger B, Staderini N, et al. Challenges and successes in the implementation of option B+ to prevent mother-to-child transmission of HIV in southern Swaziland. BMC Public Health. 2018;18(1):374. doi: 10.1186/s12889-018-5258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill MM, Ditekemena J, Loando A, et al. Addressing Early Retention in Antenatal Care Among HIV-Positive Women Through a Simple Intervention in Kinshasa, DRC: The Elombe “Champion” Standard Operating Procedure. AIDS Behav. 2018;22(3):860–866. doi: 10.1007/s10461-017-1770-1 [DOI] [PubMed] [Google Scholar]
- 24.Kiwanuka G, Kiwanuka N, Muneza F, et al. Retention of HIV infected pregnant and breastfeeding women on option B+ in Gomba District, Uganda: a retrospective cohort study. BMC Infect Dis. 2018;18(1):533. doi: 10.1186/s12879-018-3450-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alamdo AG, King EJ. Retention in Care and Health Outcomes of HIV-Exposed Infants in a Prevention of Mother-to-Child Transmission of HIV (PMTCT) Cohort in Addis Ababa, Ethiopia. HIV AIDS (Auckl). 2021;13:171–179. doi: 10.2147/HIV.S286347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Font H, Rollins N, Essajee S, et al. Retention-in-care in the PMTCT cascade: definitions matter! Analyses from the INSPIRE projects in Malawi, Nigeria and Zimbabwe. J Int AIDS Soc. 2020;23(10):e25609. doi: 10.1002/jia2.25609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drake AL, Unger JA, Ronen K, et al. Evaluation of mHealth strategies to optimize adherence and efficacy of Option B+ prevention of mother-to-child HIV transmission: Rationale, design and methods of a 3-armed randomized controlled trial. Contemp Clin Trials. 2017;57:44–50. doi: 10.1016/j.cct.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinuthia J, Ronen K, Unger ID JA, et al. SMS messaging to improve retention and viral suppression in prevention of mother-to-child HIV transmission (PMTCT) programs in Kenya: A 3-arm randomized clinical trial. Published online 2021. doi: 10.1371/journal.pmed.1003650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- 30.Rao D, Feldman BJ, Fredericksen RJ, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2012;16(3):711–716. doi: 10.1007/s10461-011-9915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soeken KL, McFarlane J, Parker B, Lominack MC. The Abuse Assessment Screen: A clinical instrument to measure frequency, severity, and perpetrator of abuse against women. In: Empowering Survivors of Abuse: Health Care for Battered Women and Their Children. Sage series on violence against women. Sage Publications, Inc; 1998:195–203. [Google Scholar]
- 33.Swindale A, Bilinsky P. Development of a universally applicable household food insecurity measurement tool: process, current status, and outstanding issues. J Nutr. 2006;136(5):1449S–1452S. doi: 10.1093/jn/136.5.1449S [DOI] [PubMed] [Google Scholar]
- 34.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462 [DOI] [PubMed] [Google Scholar]
- 35.Williams F, Boren SA. The role of the electronic medical record (EMR) in care delivery development in developing countries: a systematic review. Inform Prim Care. 2008;16(2):139–145. doi: 10.14236/jhi.v16i2.685 [DOI] [PubMed] [Google Scholar]
- 36.McDougal L, Moteetee MM, Mohai F, et al. Lesotho’s minimum PMTCT package: lessons learned for combating vertical HIV transmission using co-packaged medicines. J Int AIDS Soc. 2012;15(2):17326. doi: 10.7448/IAS.15.2.17326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabharwal CJ, Braunstein SL, Robbins RS, Shepard CW. Optimizing the Use of Surveillance Data for Monitoring the Care Status of Persons Recently Diagnosed With HIV in NYC. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;65(5). doi: 10.1097/QAI.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 38.Haas AD, van Oosterhout JJ, Tenthani L, et al. HIV transmission and retention in care among HIV-exposed children enrolled in Malawi’s prevention of mother-to-child transmission programme. J Int AIDS Soc. 2017;20(1):21947. doi: 10.7448/IAS.20.1.21947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gumede-Moyo S, Filteau S, Munthali T, Todd J, Musonda P. Implementation effectiveness of revised (post-2010) World Health Organization guidelines on prevention of mother-to-child transmission of HIV using routinely collected data in sub-Saharan Africa: A systematic literature review. Medicine. 2017;96(40):e8055–e8055. doi: 10.1097/MD.0000000000008055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wettstein C, Mugglin C, Egger M, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26(18):2361–2373. doi: 10.1097/QAD.0b013e328359ab0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcos Y, Phelps BR, Bachman G. Community strategies that improve care and retention along the prevention of mother-to-child transmission of HIV cascade: a review. J Int AIDS Soc. 2012;15 Suppl 2(Suppl 2):17394. doi: 10.7448/IAS.15.4.17394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan SR, Oosthuizen C, Stinson K, et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study. PLOS Medicine. 2017;14(11). doi: 10.1371/journal.pmed.1002407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiwanuka G, Kiwanuka N, Muneza F, et al. Retention of HIV infected pregnant and breastfeeding women on option B+ in Gomba District, Uganda: a retrospective cohort study. BMC Infectious Diseases. 2018;18(1):533. doi: 10.1186/s12879-018-3450-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sibanda EL, Cowan FM. Good news for retention of women on option B+ in Malawi. Lancet HIV. 2016;3(4):e151–2. doi: 10.1016/S2352-3018(16)00035-7 [DOI] [PubMed] [Google Scholar]
- 45.Rawizza HE, Chang CA, Chaplin B, et al. Loss to Follow-Up within the Prevention of Mother-to-Child Transmission Care Cascade in a Large ART Program in Nigeria. Current HIV Research. 2015;13(3). doi: 10.2174/1570162X1303150506183256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–2052. doi: 10.1097/QAD.0b013e328359590f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips T, Thebus E, Bekker LG, Mcintyre J, Abrams EJ, Myer L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J Int AIDS Soc. 2014;17(1):19242. doi: 10.7448/IAS.17.1.19242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. Impact of Antiretroviral Drugs in Pregnant Women and Their Children in Africa: HIV Resistance and Treatment Outcomes. Journal of Infectious Diseases. 2013;207(suppl 2):S93–S100. doi: 10.1093/infdis/jit110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atanga PN, Ndetan HT, Achidi EA, Meriki HD, Hoelscher M, Kroidl A. Retention in care and reasons for discontinuation of lifelong antiretroviral therapy in a cohort of Cameroonian pregnant and breastfeeding HIV-positive women initiating “Option B+” in the South West Region. Trop Med Int Health. 2017;22(2):161–170. doi: 10.1111/tmi.12816 [DOI] [PubMed] [Google Scholar]
- 50.Cichowitz C, Mazuguni F, Minja L, et al. Vulnerable at Each Step in the PMTCT Care Cascade: High Loss to Follow Up During Pregnancy and the Postpartum Period in Tanzania. AIDS Behav. 2019;23(7):1824–1832. doi: 10.1007/s10461-018-2298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahoua L, Tiendrebeogo T, Arikawa S, et al. PMTCT care cascade and factors associated with attrition in the first four years after Option B+ implementation in Mozambique. Trop Med Int Health. 2020;25(2):222–235. doi: 10.1111/tmi.13324 [DOI] [PubMed] [Google Scholar]
- 52.Alamdo AG, King EJ. Retention in Care and Health Outcomes of HIV-Exposed Infants in a Prevention of Mother-to-Child Transmission of HIV (PMTCT) Cohort in Addis Ababa, Ethiopia. HIV/AIDS - Research and Palliative Care. 2021;Volume 13. doi: 10.2147/HIV.S286347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nassali M, Nakanjako D, Kyabayinze D, Beyeza J, Okoth A, Mutyaba T. Access to HIV/AIDS care for mothers and children in sub-Saharan Africa: adherence to the postnatal PMTCT program. AIDS Care. 2009;21(9):1124–1131. doi: 10.1080/09540120802707467 [DOI] [PubMed] [Google Scholar]
- 54.Busza J, Walker D, Hairston A, et al. Community-based approaches for prevention of mother to child transmission in resource-poor settings: a social ecological review. J Int AIDS Soc. 2012;15 Suppl 2(Suppl 2):17373. doi: 10.7448/IAS.15.4.17373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong’ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17(5):564–580. doi: 10.1111/j.1365-3156.2012.02958.x [DOI] [PubMed] [Google Scholar]
- 56.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male Antenatal Attendance and HIV Testing Are Associated With Decreased Infant HIV Infection and Increased HIV-Free Survival. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalembo FW, Zgambo M, Mulaga AN, Yukai D, Ahmed NI. Association between Male Partner Involvement and the Uptake of Prevention of Mother-to-Child Transmission of HIV (PMTCT) Interventions in Mwanza District, Malawi: A Retrospective Cohort Study. PLOS ONE. 2013;8(6):e66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tweya H, Gugsa S, Hosseinipour M, et al. Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Trop Med Int Health. 2014;19(11):1360–1366. doi: 10.1111/tmi.12369 [DOI] [PubMed] [Google Scholar]
- 59.Ministry of Health. KENYA AIDS RESPONSE PROGRESS REPORT 2016.; 2016. https://nacc.or.ke/wp-content/uploads/2016/11/Kenya-AIDS-Progress-Report_web.pdf


