Abstract
Objective:
Quantify the independent and combined effects of abdominal muscle quantity and lumbar bone mineral density (BMD) on injury risk and in-hospital outcomes in severely injured motor vehicle crash (MVC) occupants ages 50 and older.
Methods:
Skeletal muscle area measurements of MVC occupants were obtained through semi-automated segmentation of an axial computed tomography (CT) slice at the L3 vertebra. An occupant height-normalized Skeletal Muscle Index (SMI) was calculated - a defining metric of sarcopenia and low muscle mass (sarcopenia thresholds: <38.5 cm2/m2 females; <52.4 cm2/m2 males). Lumbar BMD was obtained using a validated, phantomless CT calibration method (osteopenia threshold: <145 mg/cm3). SMI and BMD values were used to categorize occupants, and logistic regression was used to associate sarcopenia, osteopenia, and osteosarcopenia predictors to injury outcomes (e.g., Injury Severity Score (ISS), maximum Abbreviated Injury Scale (MAIS) score, fractures) and hospital outcomes (e.g., length of stay, ICU days).
Results:
Of the 336 occupants, 210 (63%) were female (mean±SD: age 66.3±10.6). SMI was 41.7±8.0 cm2/m2 in females and 51.2±10.8 cm2/m2 in males. Based on SMI, 40% of females and 55% of males were classified as sarcopenic. BMD was 163.2±38.3 mg/cm3 in females and 164.1±35.4 mg/cm3 in males, with 41% of females and 33% of males classified as osteopenic. Prevalence of both conditions (osteosarcopenia) was similar between females (21%) and males (22%). Incidence of low SMI and BMD increased with age. Sarcopenic individuals were less likely to sustain a MAIS 2+ thorax injury and had longer ICU stays. Osteopenic individuals were more likely to sustain upper extremity injuries and fractures, and were less likely to be discharged to a rehabilitation facility. Osteosarcopenic individuals were less likely to be ventilated or admitted to the ICU but tended to spend more time on the ventilator if placed on one.
Conclusions:
Osteosarcopenia was not associated with any injury outcomes, but sarcopenia was associated with thoracic injury and osteopenia was associated with upper extremity injury incidence. Sarcopenia was only associated with ICU length of stay, while osteopenia was only associated with discharge destination. Osteosarcopenia was associated with likelihood of being ventilated, being admitted to the ICU, and with increased length of ventilation.
Keywords: Medical Imaging, Osteopenia, Skeletal Muscle Mass, Injury Risk, Hospital Outcomes, Crash Injury Research and Engineering Network (CIREN)
INTRODUCTION
Patients with diminished musculoskeletal quality carry a significant health burden. Examples of this include sarcopenia, which involves a loss of skeletal muscle mass, muscle strength, and/or physical function (Cruz-Jentoft, et al., 2019; Fielding, et al., 2011), and osteopenia and osteoporosis, which involve low bone mineral density (BMD) and higher risk of fracture (Weaver, et al., 2015). Patients with diminished skeletal muscle mass cost the United States healthcare system an estimated $40.4 billion annually (Goates, et al., 2019) and conditions involving decreased BMD cost at least $22 billion in 2008 (Blume, et al., 2011). Adults over the age of 50 comprise 55% of all licensed U.S. drivers (OHPI, 2018) and the prevalence of low skeletal muscle mass and bone mineral density are estimated to be 19% (Liu, et al., 2020) and 54% (Wright, et al., 2014) in this group, respectively. Low skeletal muscle mass and BMD are independent risk factors for fracture incidence (Weaver, et al., 2015), poor in-hospital outcomes (Looijaard, et al., 2016), functional decline (Cawthon, et al., 2015), and mortality (Cawthon, et al., 2015; Looijaard, et al., 2016). Despite this information, the independent effects of skeletal muscle mass and BMD on injuries and hospital outcomes have been minimally studied in motor vehicle crash (MVC) occupants. Furthermore, osteosarcopenia, a combination disorder involving both low skeletal muscle mass and BMD, has only been studied in a sample of 61 MVC occupants (Weaver, et al., 2019).
Though the association between low BMD and increased fracture risk has been well established (Riggs, et al., 1995), associations between skeletal muscle mass and fracture risk, as well as the interactions between skeletal muscle mass and BMD as they pertain to injury risk, are still unclear. Three plausible mechanisms have been proposed for this potential association. The first involves interactions between the muscle and bone that lead to low bone quality. Low skeletal muscle mass and low BMD commonly occur together, and it remains unclear if low skeletal muscle mass predisposes to fractures independently of low BMD (Hars, et al., 2016). The combination of the two, known as osteosarcopenia, intensifies the risk for negative outcomes, including falls (Huo, et al., 2015), fractures (Huo, et al., 2015), impaired physical function (Frisoli, et al., 2018), and mortality (Yoo, et al., 2018). It is associated with a higher risk of 1-year mortality than sarcopenia alone, osteopenia alone, and non-sarcopenia/non-osteopenia (Yoo, et al., 2018). The second mechanism involves greater predisposition to falls due to decreased strength, function, and balance, which often accompanies low skeletal muscle mass (Cawthon, et al., 2015). It is also unclear whether low skeletal muscle mass carries a greater risk of fractures independent of fall incidence. The third proposed mechanism for greater fracture incidence in trauma is decreased soft-tissue cushioning due to low skeletal muscle mass (Cederholm, et al., 2013).
Previous investigations of associations between skeletal muscle mass and in-hospital outcomes have seen increased length of stay (Looijaard, et al., 2016), increased frequency of infectious complications, lower likelihood of being discharged to rehab/home (Malekpour, et al., 2017), and an increase in in-hospital mortality (Malekpour, et al., 2017; see Appendix A for additional references). Osteopenia/osteoporosis increases fracture risk, and osteoporotic fractures themselves have shown to be a predictor of mortality (Appendix A). The combined condition of osteosarcopenia is also associated with increased mortality and longer length of stay in the hospital (Appendix A).
The objective of the current study was to quantify the individual and combined effects of skeletal muscle mass and BMD on injury risk and in-hospital outcomes in severely injured MVC occupants aged 50 and older. As the population ages and older adult drivers become more prevalent, information about the association between skeletal muscle mass and injury incidence in MVC will allow for the determination of injury risk independent of low BMD. To optimize the treatment and injury causation analysis of MVC occupants, it is imperative to quantify the impact of skeletal muscle mass and BMD on injuries and outcomes. Additionally, since skeletal muscle mass and BMD can be quantified from a single L3 vertebral computed tomography (CT) scan image (Weaver, et al., 2015), they may be able to assist in the prediction of injuries and outcomes of patients that receive abdominal CT scans on admission and improve the management of MVC trauma occupants.
METHODS
Study Population
The Crash Injury Research and Engineering Network (CIREN) is a multi-center prospective cohort study, active since 1997, involving detailed medical and engineering analyses to determine injury causations and mechanisms of seriously injured occupants who have been in a MVC (Schneider, et al., 2011). CIREN eligibility criteria include MVC occupants of a modern vehicle (≤7 years old) who were seriously injured (at least one Abbreviated Injury Scale (AIS) 3+ injury or some specific AIS 2+ injuries). For the current study, information concerning occupants 50 years and older with abdominal CT scans that were enrolled between 2005 and 2015 were extracted from the CIREN database (Saffarzadeh, et al., 2016). Injuries were coded in CIREN using the version of the AIS lexicon that was current at the time of case enrollment (versions 1998 or 2008; see additional references in Appendix A). Subjects provided informed consent per IRB protocols at each enrolling CIREN center.
Image Analysis
A single axial CT slice at the L3 mid-vertebral level was extracted from each abdominal CT scan and all visualized skeletal muscle was segmented using a semi-automated approach in Mimics (Materialise, Leuven, Belgium; Figure 1). All tissue with attenuation between −29 and +150 Hounsfield Units (HU) was segmented and non-muscle tissues were manually removed. Skeletal cross-sectional muscle area measurements obtained from the segmentations were used to calculate the skeletal muscle index (SMI) in Equation 1. Since absolute muscle mass is correlated strongly with height, it is necessary to normalize the skeletal muscle area to the patient height (Baumgartner, et al., 1998). SMI thresholds for sarcopenia are < 38.5 cm2/m2 for females and < 52.4 cm2/m2 for males.
Figure 1.
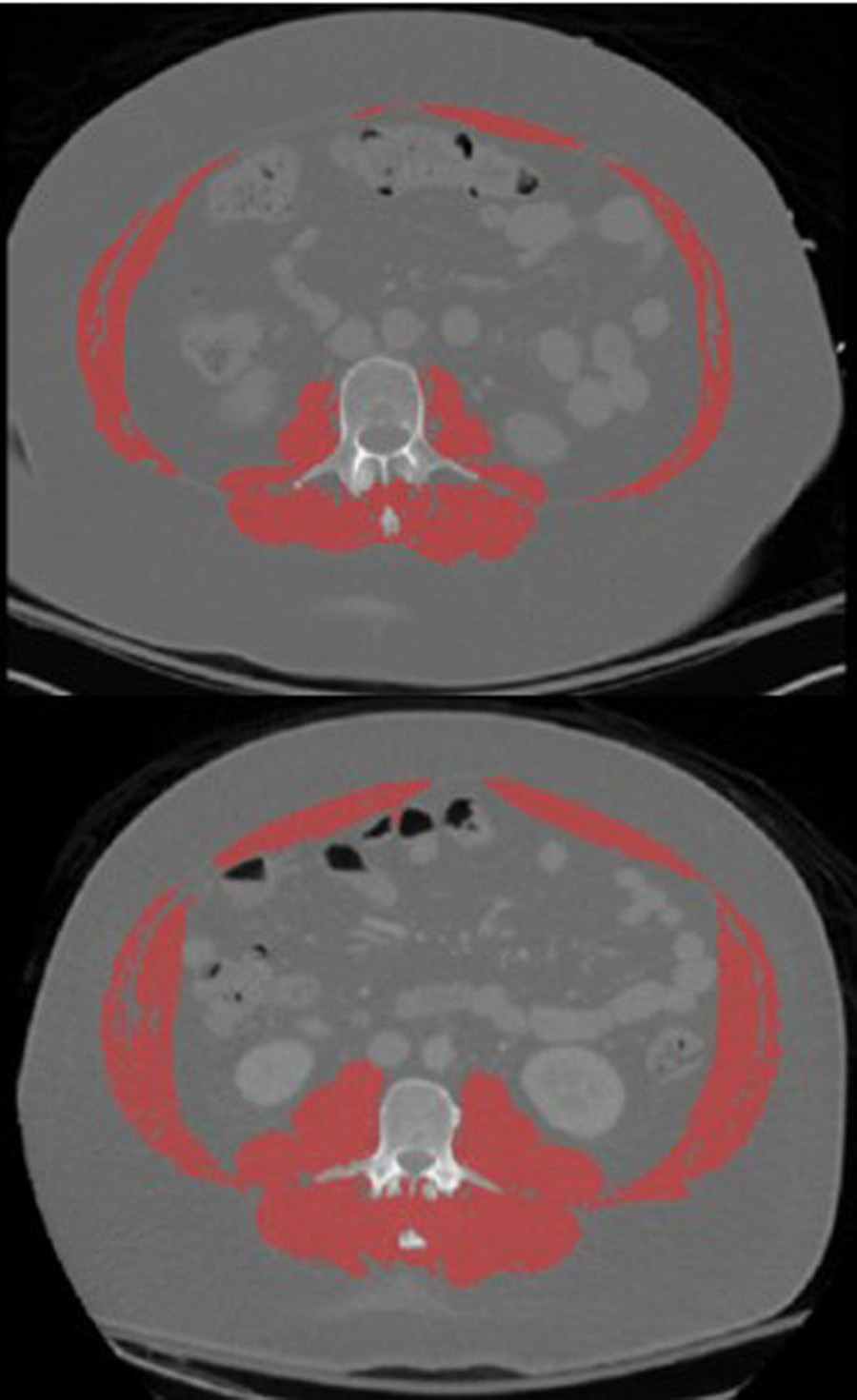
CT segmentation of all visualized muscles (red) at the L3 vertebral level for individuals with low muscle mass (top) and normal muscle mass (bottom).
| (1) |
BMD was obtained for this population in a previous study using a validated, phantomless CT calibration method to calibrate volumetric BMD in the L1-L5 vertebral body trabeculae (Saffarzadeh, et al., 2016). Briefly, mean HU measurements collected from regions of interest in the right psoas muscle and anterior subcutaneous fat were linearly regressed against ground-truth values for muscle (77 mg/cm3) and fat (−69 mg/cm3) to establish a conversion from HU to mg/cm3 BMD for L1-L5. This technique was validated in a sample of 50 older adults subjects to establish an osteopenia threshold of <145 mg/cm3 (Weaver, et al., 2015).
Statistical Data Analysis
All statistical analyses implemented either negative binomial regression for score outcomes or multiple logistic regression for binary outcomes with sarcopenia, osteopenia, and osteosarcopenia categories being used as predictor variables of interest, and occupant and crash characteristics used as covariates. An alpha level of 0.05 was used to determine statistical significance.
Injury Outcomes:
The relationships between predictor variables and injuries were determined for whole-body ISS, maximum AIS (MAIS), and the presence of fracture, as well as MAIS and fracture incidence by body region. Body regions were divided into head, face, neck, thorax, abdomen, spine, upper extremity (UEX), and pelvis/lower extremity (PLEX) based on AIS coding rules. All non-fracture AIS 1 (minor) injuries were excluded because they were generally skin abrasions, contusions, and/or minor lacerations. Logistic regression was conducted for MAIS (MAIS 1+, 2+, 3+ or 4+), ISS (severe: >15 vs not severe: <=15), and likelihood of experiencing fracture.
In-Hospital Outcomes:
In-hospital outcome variables included total number of days in the hospital, or length of stay (LOS), number of days in the ICU, number of days on a ventilator, number and nature of complications, and discharge destination. Same covariate adjustment as specified previously. Zero inflated negative binomial regression was used to assess number of days in the ICU for occupants admitted to the ICU, and number of days on a ventilator for occupants that received ventilator support because of excessive zeroes. Linear regression was used to assess log transformed LOS. LOS was log transformed to satisfy the conditional normality assumption. Logistic regression was used to determine the likelihood of being sent to the ICU, being placed on a ventilator, experiencing a complication, and discharge destination. Complications were assessed for presence/absence of any type of complication and by complication nature (e.g., pulmonary, infection, cardiac, etc.). Discharge destinations were categorized into home, skilled nursing facility (SNF), and rehabilitation, with home used as the reference value, and were analyzed using multinomial logistic regression to determine the likelihood of SNF or rehabilitation facility over home.
Covariates:
Age (in years), sex, BMI (in kg/m2), and delta-v (in kph) were included in all statistical models as covariates. Crash mode, seatbelt status (1 = belt worn; 0 = belt not worn), and airbag status (1 = airbags deployed; 0 = airbags did not deploy) were also included in the injury outcomes model, and ISS were included in models assessing in-hospital outcomes. Crash mode was defined as frontal, near-side, far-side, rear, or other according to plane of the vehicle impacted and principal direction of force (Stitzel, et al., 2016). Airbag status also accounts for crash mode, with airbags only considered deployed if they corresponded with the respective crash mode. For a frontal collision, airbags were considered deployed if there was a deployment in the respective side of the occupant (e.g., steering wheel airbag deployed for the driver; right instrument panel airbag deployed for the right front passenger). For near side collisions: side, curtain, or front airbags had to have deployed for the airbag variable to have been coded as deployed. Far side impacts were required to have front or curtain airbags to have deployed in order to be coded as deployed. The airbag for a rear collision (n = 2) was considered deployed if the front airbags activated on the respective side of the occupant in order to lessen any rebounding that may occur. Non-horizontal collisions had an airbag status of deployed if there was a seat cushion airbag present and activated. For multi-event crashes, the highest severity event was used to determine crash mode and delta-v. Unless otherwise specified, airbags were assumed to have deployed in the highest severity event.
RESULTS
336 occupants had CT scans that could be used to measure skeletal muscle area and SMI. Of the 336 occupants, 210 (62.5%) were female and 126 (37.5%) were male (Table 1), with an average±SD occupant age of 66.3±10.6 years. SMI was 41.7 ± 8.0 cm2/m2 in females and 51.2±10.8 cm2/m2 in males. Sarcopenia based on SMI thresholds was found in 85 females (40.5%; SMI <38.5 cm2/m2; Figure 2) and 69 males (54.8%; SMI <52.4 cm2/m2; Figure 3). BMD was 163.2±38.3 mg/cm3 in females and 164.1±35.4 mg/cm3 in males, with 75 females (35.7%) and 37 males (29.4%) having BMD <145 mg/cm3 indicating osteopenia/osteoporosis. Similar prevalence of osteosarcopenia were seen between males (n=28, 22.2%) and females (n=44, 21.0%). The incidence of low SMI and BMD increased with age in females (Figure 2) and males (Figure 3).
Table 1. Occupant characteristics by sex.
Numerical variables are given as Mean (SD) and categorical variables are given as N (proportion of sex).
| Female (n = 210) | Male (n =126) | Total (n = 336) | |
|---|---|---|---|
| Age (years) | 66.0 (10.9) | 66.9 (10.0) | 66.3 (10.6) |
| Height (cm) | 162.7 (7.0) | 179.0 (6.4) | 168.8 (10.4) |
| Mass (kg) | 75.5 (19.5) | 90.8 (18.6) | 81.2 (20.5) |
| Body Mass Index (kg/m2) | 28.5 (7.4) | 28.3 (5.1) | 28.4 (6.6%) |
| Skeletal Muscle Index (cm2/m2) | 41.7 (8.0) | 51.2 (10.8) | 45.2 (10.2) |
| Bone Mineral Density (mg/cm3) | 163.2 (38.3) | 164.1 (35.4) | 163.5 (37.2) |
| Classification - Osteopenia | 42 (20.0%) | 14 (11.1%) | 56 (16.7%) |
| Classification - Sarcopenia | 41 (19.5%) | 41 (32.5%) | 82 (24.4%) |
| Classification - Osteosarcopenia | 44 (21.0%) | 28 (22.2%) | 72 (21.4%) |
Figure 2.
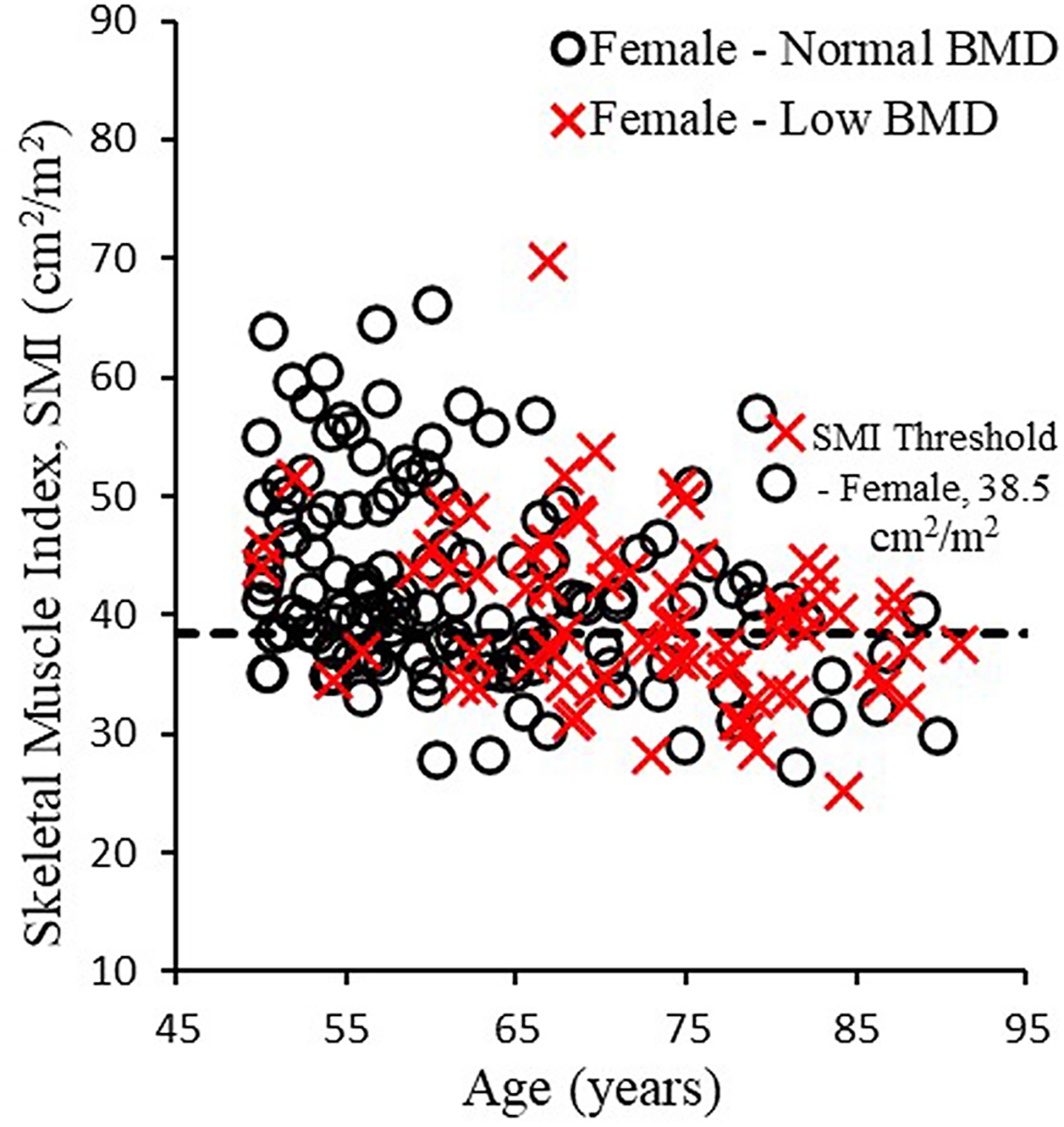
Skeletal muscle index versus age in females with normal BMD (≥ 145 mg/cm3) and low BMD (< 145 mg/cm3).
Figure 3.
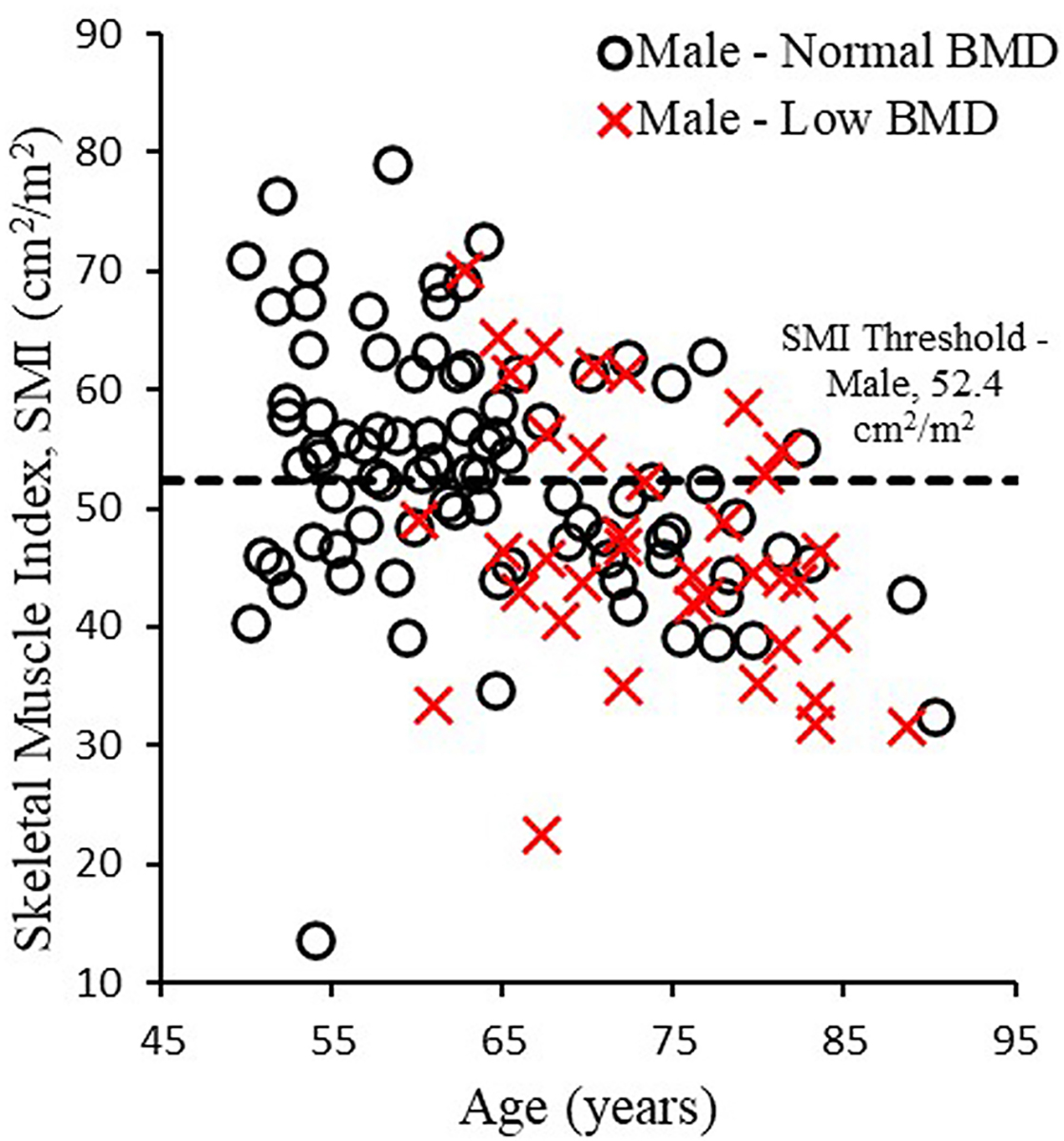
Skeletal muscle index versus age in males with normal bone mineral density (BMD ≥ 145 mg/cm3) and low bone mineral density (BMD < 145 mg/cm3).
The majority of occupants experienced a MAIS 3 (Table B1, Appendix B), most commonly in the thorax (44.6%) and pelvis/lower extremities (30.7%), which is common in frontal crashes (Cavanaugh, et al., 2015). MAIS incidence per body region is reported by sarcopenia/BMD classification in Tables B2–B5. Frontal crashes were the most common (60.4%), and rear crashes were least common (0.6%; Table B6). Nearly 20% of occupants were placed on ventilator support in the hospital and over 50% were admitted to the ICU (Table 2). 38.7% of occupants experienced complications in the hospital, most of which were pulmonary in nature (Table B7). Only 11 (3.3%) of the crashes were fatal.
Table 2. Distribution of hospital outcomes.
Numerical variables are given as Mean (SD) and categorical variables are given as N (proportion of column).
| Normal SMI/BMD | Osteopenic | Sarcopenic | Osteosarcopenia | Aggregate | |
|---|---|---|---|---|---|
| Hospital stay (days) | 13.5 (8.9) | 8.9 (8.2) | 11.0 (11.3) | 8.5 (7.4) | 11.1 (12.2) |
| Ventilator support | 38 (30.1%) | 5 (8.9%) | 10 (12.2%) | 14 (19.4%) | 67 (19.9%) |
| Length of ventilator support (days) | 3.3 (8.5) | 1.2 (5.9) | 1.8 (6.4) | 3.1 (9.2) | 2.6 (7.9) |
| ICU admission | 73 (57.9%) | 30 (53.6%) | 37 (45.1%) | 31 (43.1%) | 171 (50.9%) |
| Length of ICU stay (days) | 5.4 (10.5) | 2.8 (7.0) | 4.7 (9.9) | 3.4 (7.5) | 4.4 (9.3) |
| Discharge - Home | 55 (43.7%) | 29 (51.8%) | 40 (48.8%) | 30 (41.7%) | 154 (45.8%) |
| Discharge - Rehabilitation | 40 (31.7%) | 8 (14.3%) | 23 (28.0%) | 26 (36.1%) | 97 (28.9%) |
| Discharge - Skilled Nursing Facility | 27 (21.4%) | 18 (32.1%) | 15 (18.3%) | 10 (13.9%) | 70 (20.8%) |
| Discharge - Other | 5 (4.0%) | 3 (5.4%) | 4 (4.9%) | 3 (4.2%) | 15 (4.5%) |
Occupants with AIS 2+ abdomen, spine, or extremity injuries had longer hospital and ICU stays compared to their AIS <2 counterparts; hospital stays were 4–6 days longer and ICU stays were 2–5 days longer on average (all p<0.01). Occupants were more likely to be admitted to the ICU if they had AIS 2+ injury to the head (68% vs. 47%; p=0.002), thorax (55% vs. 39%; p=0.01), or abdomen (70% vs. 43%; p<0.001). Occupants were more likely to require ventilation if they had AIS 2+ injury to the head (32% vs. 17%; p=0.007), abdomen (33% vs. 14%; p<0.001), and upper extremity (31% vs. 15%; p=0.001) injury were more likely to require ventilation. Occupants with AIS 2+ abdomen, spine, and pelvis/lower extremity injuries had 2–3 more ventilator days on average (all p<0.01). Complications were more likely in occupants with AIS 2+ injury to the thorax, abdomen, and upper extremity (all p<0.05). Gastrointestinal complications were more likely in occupants with AIS 2+ abdomen or upper extremity injury, and pulmonary complications were more likely in occupants with AIS 2+ thorax, abdomen, spine, or pelvis/lower extremity injury (all p<0.05). All other AIS 2+ vs AIS <2 comparisons were not statistically significant (Table B8, Appendix B).
Injury Outcomes
The odds ratios (OR) with respective 95% confidence intervals for each injury model are given in Tables B9–B11 (Appendix B). An OR less than one indicates a negative association and that the likelihood of experiencing the given injury decreases. Sarcopenic individuals were less likely to experience a MAIS 2+ injury to the thorax (OR: 0.460; p=0.030; Table B9, Appendix B) than healthy individual. However, osteopenic individuals were more likely to have upper extremity injuries (OR: 2.319; p=0.034; Table B10, Appendix B) and upper extremity fractures (OR: 2.568; p=0.017; Table B12, Appendix B). Osteosarcopenic individuals had no significant associations to injury metrics.
In-Hospital Outcomes
Osteosarcopenic individuals were less likely to be ventilated (OR: 0.281; p=0.50; however, those that were spent significantly more time on the ventilator (OR: 4.291; p=0.043; Table 3). Additionally, individuals with osteosarcopenia were less likely to be admitted to the ICU (OR: 0.389; p=0.031. Individuals with osteopenia alone had a decreased likelihood of being discharged to a rehabilitation center (OR: 0.344; p=0.023; Table B13, Appendix B). Sarcopenic individuals had longer ICU stays than non-affected individuals (Ratio of counts: 1.928; p=0.030; Table B15, Appendix B).
Table 3. In-hospital outcome regression models for osteosarcopenic versus normal SMI/BMD individuals.
| Osteosarcopenic vs Normal SMI/BMD | Regression Coefficient Estimate | 95% CI | p-value |
|---|---|---|---|
| Hospital stay (log transformed) | −0.172 | −0.418 – 0.074 | 0.169 |
| Ventilator support | 0.281 | 0.079 – 0.999 | 0.050 |
| Length Ventilated | 4.291 | 1.047 – 17.591 | 0.043 |
| ICU admission | 0.389 | 0.165 – 0.915 | 0.031 |
| Length in ICU | 0.788 | 0.393 – 1.580 | 0.503 |
| Complications | 0.989 | 0.453 – 2.161 | 0.978 |
| Discharge destination | |||
| Rehabilitation | 1.194 | 0.542 – 2.628 | 0.660 |
| Skilled Nursing Facility | 0.421 | 0.156 – 1.133 | 0.087 |
p-value obtained through logistic regression after adjusting for age, sex, body mass index (BMI), delta-v, and Injury Severity Score (ISS) except the following: for hospital stay (log transformed), a linear regression model was used. Regression coefficient estimates were reported. For length ventilated, the logistic regression model, part of the zero-inflated negative binomial model was used and odds ratios were reported. For length in ICU, the negative binomial model, part of the zero-inflated negative binomial model was used and ratio of counts were reported.
DISCUSSION
Prevalence of sarcopenia was higher compared to the general population of adults ages 50+ (55% compared to 22% in males and 40% compared to 18% in females respectively; Liu, et al., 2020). Since older adults are more likely to be seriously injured in MVCs and sarcopenia prevalence increases with age (see additional references in Appendix A), this may lead to overrepresentation of sarcopenia within CIREN compared to the general population. For BMD, the prevalence of osteopenia observed in this study (38%) was lower than the prevalence observed in the general population for ages 50+ (54%, Wright, et al., 2014). With previous work showing sarcopenia alone is associated with fewer ventilator-free and ICU-free days (see additional references in Appendix A), it is expected that similar observations would occur in osteosarcopenic individuals. With results indicating that osteosarcopenia is associated with a decrease in likelihood for ICU admission or being ventilated, rather than the expected increase, this result is likely attributable by random chance. The increase in likelihood for upper extremity injury and fracture within the osteopenic group may be attributable, in-part, to the sex-specific disparities of osteopenia itself. Previous literature has shown older females to have significantly more upper extremity injuries than males, and with females being at a much higher risk for osteoporosis (see additional references in Appendix A), these factors may coincide and explain the link between osteopenic individuals and upper extremity injuries.
Previous studies have investigated the effects of SMI in trauma patients, but have lumped together all types of blunt trauma, limiting control of the details of the causative traumatic event (see additional references in Appendix A). The combined effects of SMI and BMD have been studied in trauma patients even less. There is also a need for further clarification regarding the effects of SMI on the likelihood of injuries and hospital outcomes because discrepancies exist in previous studies. A meta-analysis of nine studies determined that low skeletal muscle mass is associated with greater fracture risk in male, community-dwelling older adults (Zhang, et al., 2017), but when looking at the studies individually, only two found a statistically significant association between low skeletal muscle mass and fracture risk (Hars, et al., 2016; see additional references in Appendix A). Our study of MVC occupants found no significant association between low SMI and fracture incidence. Across six studies investigating the effects of skeletal muscle mass on in-hospital outcomes in trauma patients, five found the effect of CT-diagnosed low skeletal muscle mass to be associated with an increased hospital length of stay, increased ICU admission and length of stay, more in-hospital complications, and/or discharge destination (Akahoshi, et al., 2016; Malekpour, et al., 2017; see additional references in Appendix A).
This study characterized the individual and combined effects of SMI and BMD on injuries and hospital outcomes in MVC occupants while adjusting for trauma severity. While there were no statistically significant findings relating osteosarcopenia to injury outcomes, injury outcomes were associated with sarcopenia and osteopenia individually. Sarcopenia, osteopenia and osteosarcopenia were associated with in-hospital outcomes. As such, the potential relationship between sarcopenia, osteopenia, or osteosarcopenia, and clinical outcomes may be useful to patients and medical professionals alike to understand what MVC occupants are predisposed to, and further demonstrate the need for careful consideration when dealing with high-risk patients if they are identified prior to injury.
Limitations
Though the CIREN study is one of the only databases suitable for the given study since it contains both CT scans and detailed data on crash, occupant, injury, and hospital outcome characteristics, it has some limitations. The focus of the CIREN study is the investigation of injury causation and mechanism in severely injured MVC occupants. As a result, case selection is not random or representative of the general population and, therefore, generalizability of the results are limited to epidemiological studies of severely injured MVC occupants as defined by the CIREN inclusion criteria. Also, among CIREN subjects ages 50+ enrolled between 2005–2015, those with an abdominal CT scan available were more likely to be female, unbelted, in non-frontal crashes, and have a lower ISS (Table B16, Appendix B) which may introduce some bias. Additionally, the semi-automated approach to muscle segmentation for quantification of muscle area leads to the possibility of being subject to human error during the manual segmenting of non-muscle tissues, and the image resolution of the CT scan (1 mm in-plane resolution) may limit precision.
CONCLUSION
Individuals with sarcopenia were less likely to have a thoracic MAIS of 2+, whereas osteopenic individuals were more likely to sustain an upper extremity injury. Osteosarcopenia was not associated with any injury outcomes. Individuals with lower than normal BMD were less likely be discharged to a rehabilitation facility. Sarcopenic individuals spent significantly longer time in the ICU than the normal group. Osteosarcopenia was associated with a decreased likelihood of being ventilated or admitted to the ICU; however, the osteosarcopenic individuals that were ventilated tended to spend more time on the ventilator. These findings have the potential to improve the management of MVC occupants that receive abdominal CT upon admission as muscle quantity (SMI) and BMD can both be derived and used for opportunistic screening and musculoskeletal health characterization.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the National Highway Traffic Safety Administration for providing funding for this study under the Crash Injury Research and Engineering Network study (DTNH2217D00069). Additionally, the authors would like to thank the Wake Forest School of Medicine’s Medical Student Research Program (MSRP) for funding. No conflicts of interest were reported by authors.
REFERENCES
- 1.Akahoshi T, Yasuda M, Momii K, et al. Sarcopenia is a predictive factor for prolonged intensive care unit stays in high-energy blunt trauma patients. Acute Med Surg. Oct 2016;3(4):326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. Apr 15 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 3.Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. Jun 2011;22(6):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanaugh JM, Yoganandan N. Thorax Injury Biomechanics. In: Yoganandan N, Nahum AM, Melvin JW, eds. Accidental Injury (3E). New York: Springer; 2015. [Google Scholar]
- 5.Cawthon PM, Blackwell TL, Cauley J, et al. An evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the observational Osteoporotic Fractures in Men (MrOS) cohort study. Journal of the American Geriatrics Society. 2015;63(11):2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and frailty fractures. European Journal of Physical and Rehabilitation Medicine. 2013;49(1):111–117. [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisoli A, Chaves P, Inghan S, Carvalho A. Osteposarcopenia has stronger association with impaired physical function than sarcopenia only. Innov Aging. 2018;2(Supplement 1):304. [Google Scholar]
- 10.Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic Impact of Hospitalizations in US Adults with Sarcopenia. J Frailty Aging. 2019;8(2):93–99. [DOI] [PubMed] [Google Scholar]
- 11.Hars M, Biver E, Chevalley T, et al. Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res. 2016;31(11):2048–2056. [DOI] [PubMed] [Google Scholar]
- 12.Huo YR, Suriyaarachchi P, Gomez F, et al. Comprehensive nutritional status in sarco-osteoporotic older fallers. J Nutr Health Aging. 2015;19(4):474–480. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Hou L, Xia X, et al. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: findings from West-China health and aging trend study. BMC Geriatr. Feb 17 2020;20(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looijaard WGPM Dekker IM, Stapel SN, et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Critical Care. 2016;20(386). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malekpour M, Bridgham K, Jaap K, et al. The Effect of Sarcopenia on Outcomes in Geriatric Blunt Trauma. Am Surg. Nov 1 2017;83(11):1203–1208. [PubMed] [Google Scholar]
- 16.Office of Highway Policy Information. Highway Statistics; 2016. 2018. [Google Scholar]
- 17.Riggs BL, Melton LJ. The worldwide problem of osteoporosis: Insights afforded by epidemiology. Bone. 1995;17(Issue 5, Supplement 1):S505–S511. [DOI] [PubMed] [Google Scholar]
- 18.Saffarzadeh M, Hightower RC, Talton JW, Miller AN, Stitzel JD, Weaver AA. Multicenter analysis of CIREN occupant lumbar bone mineral density and correlation with age and fracture incidence. Traffic Inj Prev. Sep 2016;17 Suppl 1:34–41. [DOI] [PubMed] [Google Scholar]
- 19.Schneider LW, Rupp JD, Scarboro M, et al. Biotab - A New Method for Analyzing and Documenting Injury Causation in Motor Vehicle Crashes. Traffic Injury Prevention. 2011;12(3):256–265. [DOI] [PubMed] [Google Scholar]
- 20.Stitzel JD, Weaver AA, Talton JW, et al. An Injury Severity-, Time Sensitivity-, and Predictability-Based Advanced Automatic Crash Notification Algorithm Improves Motor Vehicle Crash Occupant Triage. J Am Coll Surg. Jun 2016;222(6):1211–1219 e1216. [DOI] [PubMed] [Google Scholar]
- 21.Weaver AA, Beavers AM, Hightower RC, Lynch SK, Miller AN, Stitzel JD. Lumbar bone mineral density phantomless computed tomography measurements and correlation with age and fracture incidence. Traffic Inj Prev. 2015;16(Suppl 2):S153–S160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver AA, Costa C, Ambrosini A, et al. Sarcopenia and osteosarcopenia in seriously injured motor vehicle crash occupants. Traffic Inj Prev. 2019;20(sup2):S195–S197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. Nov 2014;29(11):2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo JI, Kim H, Ha YC, Kwon HB, Koo KH. Osteosarcopenia in patients with hip fracture Is related with high mortality. J Koren Med Sci. 2018;33(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Hao Q, Ge M, Dong B. Association of sarcopenia and fractures in community-dwelling older adults: a systematic review and meta-analysis of cohort studies. Osteoporosis International. 2017;29:1253–1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


