Abstract
Background:
Steps per day (steps/d) less than the widely-promoted 10,000 steps has recently been associated with lower risk of all-cause mortality. The relationship of steps and cardiovascular disease (CVD) risk remains poorly described. A meta-analysis examining the dose-response relationship between steps/d with CVD can help inform clinical and public health guidelines.
Methods:
Eight prospective studies (20,152 adults, ≥18 years of age) were included with device-measured steps and participants followed for CVD events. Studies quantified steps/d and CVD events were defined as fatal and non-fatal coronary heart disease, stroke, and heart failure. Cox proportional hazards regression analyses were completed using study-specific quartiles, and hazard ratios (HR) and 95% confidence intervals (CI) were meta-analyzed with inverse-variance weighted random effects models.
Results:
Mean age of participants was 63.2 (12.4) years and 52% women. Mean follow-up was 6.2 years (123,209 person-years), with 1,523 CVD events (12.4 per 1,000 participant-years). There was a significant difference in the association of steps/d and CVD between older (≥60 years) and younger adults (<60 years). For older adults, the HR was 0.80 [quartile 2 (Q2), 95% CI, 0.69, 0.93], 0.62 [Q3, 95% CI, 0.52, 0.74], and 0.51 [Q4, 95% CI, 0.41, 0.63] compared with the lowest quartile. For younger adults, the HR was 0.79 [Q2, 95% CI, 0.46–1.35], 0.90 [Q3, 95% CI, 0.64–1.25], and 0.95 [Q4 95% CI, 0.61–1.48] compared with the lowest quartile. Restricted cubic splines demonstrated a non-linear association whereby higher steps were associated with lower risk of CVD among older adults.
Conclusion:
For older adults, taking more daily steps was associated with a progressively lower risk of CVD. Monitoring and promoting steps/d is a simple metric for clinician-patient communication and population health to reduce the risk of CVD.
Keywords: Steps per day, Physical activity, Cardiovascular Disease
Introduction
Greater amounts of physical activity are associated with decreased risk of cardiovascular disease (CVD), including coronary heart disease, stroke, and heart failure.1–3 The 2018 U.S. federal guidelines4 and the 2019 ACC/AHA Guideline on the Primary Prevention of CVD5 recommend at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity or an equivalent combination of aerobic activity per week. Despite the evidence, many adults do not engage in recommended amounts of physical activity.6, 7
Cardiovascular risk reduction interventions using devices, often monitoring steps per day (steps/d), are effective strategies to increase physical activity.8 A standard goal is often 10,000 steps/d, although this goal is not evidence based, having originated from a marketing campaign in Japan.9 A recent meta-analysis on steps and all-cause mortality demonstrated reductions in risk occur at fewer than 10,000 steps/d.10 A previous meta-analysis of four published studies demonstrated a nonlinear association of daily steps and CVD risk.11 However, this meta-analysis included studies with large heterogeneity in CVD definition and analytic approach and was unable to investigate associations by age or sex.
A harmonized meta-analysis of prospective studies examining steps/d would be useful for providing health care professionals with a precise estimate of steps/d needed for CVD benefit informing provider-patient interactions and population health guidelines. Thus, the primary objective of the present analysis is to test whether steps/d is associated with risk for CVD. Given the known age and sex differences in risk of CVD,2, 12, 13 all associations were tested among females and males, and among younger and older adults. It was hypothesized there would be a dose-response association of steps/d and stepping rate with CVD.
Methods
Data, Methods, and Materials Disclosure Statement
The data, methods used in the analyses, and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Study population
The Steps for Health Collaborative is a consortium formed to investigate the associations of device-measured step volume and rate with prospective health outcomes among adults. The Collaborative identified studies through a 2019 systematic review.14 Three of four studies from this review agreed to participate but were too few for a meta-analysis. An additional five studies were identified through awareness of studies measuring steps and CVD, culminating in eight studies meeting inclusion criteria of device-measured steps and prospective follow-up for CVD events in adult populations (≥18 years).
The Newcastle Ottawa quality assessment scale was used to assess the methodological quality of each study.15 Assessments were performed independently by two reviewers (AP and SB), and disagreements were resolved by consensus.
Individual Study Data Processing and Analyses
Investigators from each study processed their participant-level data using a standardized protocol to limit heterogeneity in analyses across studies developed by the Steps for Health Collaborative. Studies quantified step volume as steps/d, averaged over three to seven days where step data were collected. Baseline was designated as the time point when steps data were collected. Participant’s first subsequent fatal or nonfatal CVD event was considered the primary outcome. Each study defined CVD as adjudicated stroke, coronary heart disease, or heart failure (Table S1). All studies were approved by an institutional review committee and the subjects gave informed consent.
Study level analyses
All studies followed a standardized analytic plan developed by the collaborative. Studies categorized steps/d into study-specific quartiles and examined associations with CVD events (reference: lowest quartile) using Cox proportional hazards regression (satisfying proportional hazards assumptions) producing hazard ratios [HR] and 95% confidence intervals [95%CI]. Models were completed for each study’s overall sample, by age group and by sex, where applicable. Age was grouped at < or ≥ 60 years based on the World Health Organization’s definition of older persons from the 2020 Decade of Healthy Ageing Baseline Report.16, 17 Model 1 adjusted for age and sex (when studies had both sexes). Model 2 adjusted for age, sex, race/ethnicity, education or income, body mass index (BMI), device wear time, lifestyle factors (e.g. smoking, alcohol), and study-specific variables representing diabetes, hypertension, high cholesterol, other chronic conditions, and self-rated health or functional status (Table S1). For the four studies with step rate, the same analytic approach was followed and an additional model (model 3) adjusted for steps/d using the residual method where step rate was regressed on steps/d and the resulting step rate residuals and steps/d were independent variables in the model.18, 19
Meta-level analysis
The total number of participants, CVD events, and person-years of follow-up were summed across all studies. For the total sample, median steps/d by quartile were calculated from the medians of the individual studies. Pooled HRs and 95% CIs were computed using inverse-variance weighted random effects models. The final adjusted model (model 2) was the primary model. Because of the known associations of age and sex with CVD13, a priori stratified analyses were conducted by age and sex for the associations of CVD with steps/d. Heterogeneity across studies was determined by I2 statistics,20 representing the proportion of total variation attributable to systematic differences between studies rather than chance. I2 values were considered low (<25%), moderate (25%-75%), or high (>75%).20 Funnel plots were used to assess study bias by comparing study hazard ratios against standard errors and Egger’s test for funnel plot symmetry.21
Restricted cubic spline models were used to generate log-transformed hazard ratios from model 2 with knots at 10th, 50th, and 90th percentiles of steps/d for the total sample, by age and sex.22 References were set at the median of the study-level medians in the lowest quartile group. Multiplicative interaction terms were used to test for differences by age and sex. The Wald test was used to evaluate non-linearity.23, 24
To evaluate the robustness of findings, the following series of sensitivity analyses was conducted: 1) participants with CVD at baseline were excluded to investigate incident CVD; 2) findings were stratified by publication status to test for publication bias (3 published, 5 unpublished); 3) a “leave-one-out analysis” to exclude one study at a time to determine the influence of any single study with an extreme result; 4) stratification by device type (i.e., pedometer vs. accelerometer); and, 5) analysis of stepping rate using several different thresholds—peak 30-minute stepping rate; peak 60-minute stepping rate; minutes per day at≥ 40 steps/min stepping rate (intentional walking) and minutes per day at ≥100 steps/min stepping rate (moderate intensity walking pace).25 Peak 30- and 60- minute stepping rates were calculated by selecting the 30 or 60 minutes (not necessarily consecutive) throughout each day with the highest number of steps/min. Stepping rate variable were calculated per day and averaged across all days.25 Meta-analyses were performed using Rv4.1 and SAS v9.4 (Cary, NC).
Results
The total sample included 20,152 participants (mean age 63.2 (12.4) years, 52% women, >70% non-Hispanic White race) with a mean study follow-up time of 6.2 years (range 2.8 to 12.6 years, 123,209 person-years) (Table 1). The overall median of the median steps/d was 4323 [IQR 2760–6924] for older adults and 6911 [IQR 4783–9794] for younger adults. A total of 1,523 events were reported (12.4 per 1,000 person-years). The Newcastle Ottawa quality scores were high, ranging from 7 to 9 out of a possible 9 points (Table S2).
Table 1.
Selected Characteristics of Studies
| Country | Study Entry | Step Device | No. of Participants | Mean Age, y (S.D.) | Females (%) | Steps/d, Median [IQR] | Mean Years of follow-up | No. of CVD Events | |
|---|---|---|---|---|---|---|---|---|---|
| Published Studies | |||||||||
| British Regional Heart Study (BRHS)41 | United Kingdom | 2010–2012 | ActiGraph GT3X | 1172 | 78.4 (4.6) | 0% | 4572 [2848, 6296] | 4.6 | 122 |
| Lifestyle Interventions and Independence For Elders (LIFE)42 | U.S. | 2010–2013 | ActiGraph GT3X | 1341 | 78.7 (5.2) | 67% | 2415 [1627, 3353] | 3.1 | 202 |
| Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR)43 | 40 Countries | 2002–2004 | Accusplit AE120 | 7271 | 63.7 (6.9) | 51% | 5662 [3435, 8563] | 6.3 | 730 |
| Unpublished Studies | |||||||||
| Atherosclerosis Risk in Communities Study (ARIC) | U.S. | 2016–2017 | ActiGraph GT3X | 452 | 78.4 (4.7) | 59% | 3065 [2083, 4454] | 2.8 | 34 |
| Coronary Artery Risk Development in Young Adults (CARDIA) | U.S. | 2005–2006 | ActiGraph 7164 | 2085 | 45.2 (3.6) | 57% | 9164 [7324, 11163] | 10.7 | 71 |
| Framingham Heart Study (FHS) | U.S. | 2008–2014 | Actical | 4223 | 55.3 (13.9) | 54% | 6906 [4809, 9419] | 7.0 | 151 |
| Healthy Ageing Initiative (HAI) | Sweden | 2012–2018 | ActiGraph GT3X | 3207 | 70.4 (0.1) | 51% | 6967 [5032, 8991] | 3.2 | 139 |
| Jackson Heart Study (JHS) | U.S. | 2000 | Yamax SW200 | 401 | 60.2 (9.8) | 61% | 4748 [2847, 7284] | 12.6 | 74 |
| SUMMARY | Range 2000–2018 | 5 devices (all Waist-Worn) | 20152 | 63.2 (12.4) | 52% | 5459 [3353, 8029] | 6.2 | 1523 |
Summary age, % female, and years of follow-up are calculated as means at the individual-level.
Summary steps/d is calculated as the median at the study-level.
CVD events defined as fatal or non-fatal, and including coronary heart disease, stroke, and heart failure.
There were significant subgroup differences by age in the association of steps/d with CVD events in third (p-value=0.048) and fourth quartile comparisons (p-value=0.014) compared to the first quartile (Figure 1). Among seven studies of older adults ≥60 years there were 1210 events among 12,741 individuals (19.3 events per 1,000 person-years). There was a significant association in the age and sex adjusted model 1 and the results remained significant in the final adjusted model. In the final adjusted (model 2), compared to the lowest quartile, HR for risk of CVD were 0.80 [0.69–0.93] in the second quartile, 0.62 [0.52–0.74] in the third quartile, and 0.51 [0.41–0.63] in the fourth quartile (Figure 1). In the spline model, there was a significant curvilinear association among older adults ≥60 years (p-value for nonlinearity <0.0001, Figure 2).
Figure 1. Association of Steps per Day and CVD Events Stratified by Age and Sex.
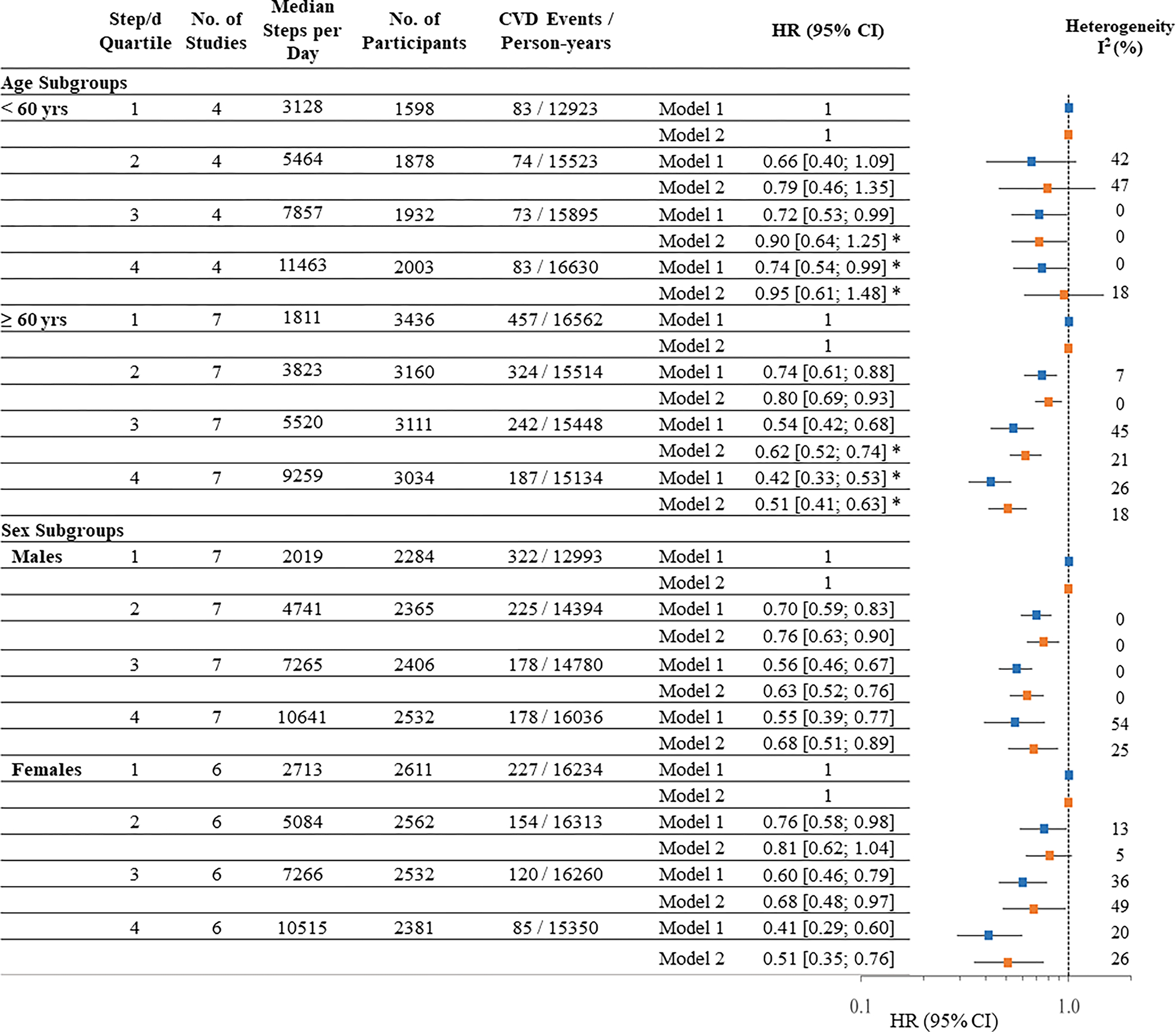
Hazard Ratio and 95% Confidence Intervals [HR (95%CI)]. Model 1 is adjusted for age and sex (if applicable). Model 2: Model 1 + device wear time, race/ethnicity (if applicable), education or income, body mass index, and plus study-specific variables for lifestyle (smoking, alcohol), hypertension, diabetes, dyslipidemia, chronic conditions, and general health status. I2 values were considered low (<25%), moderate (25%-75%), or high (>75%). The x-axis is a log scale. *p<0.05 for subgroup difference
Figure 2. Association of Steps per Day with CVD events for (a) older adults ≥ 60 years and (b) younger adults < 60 year.
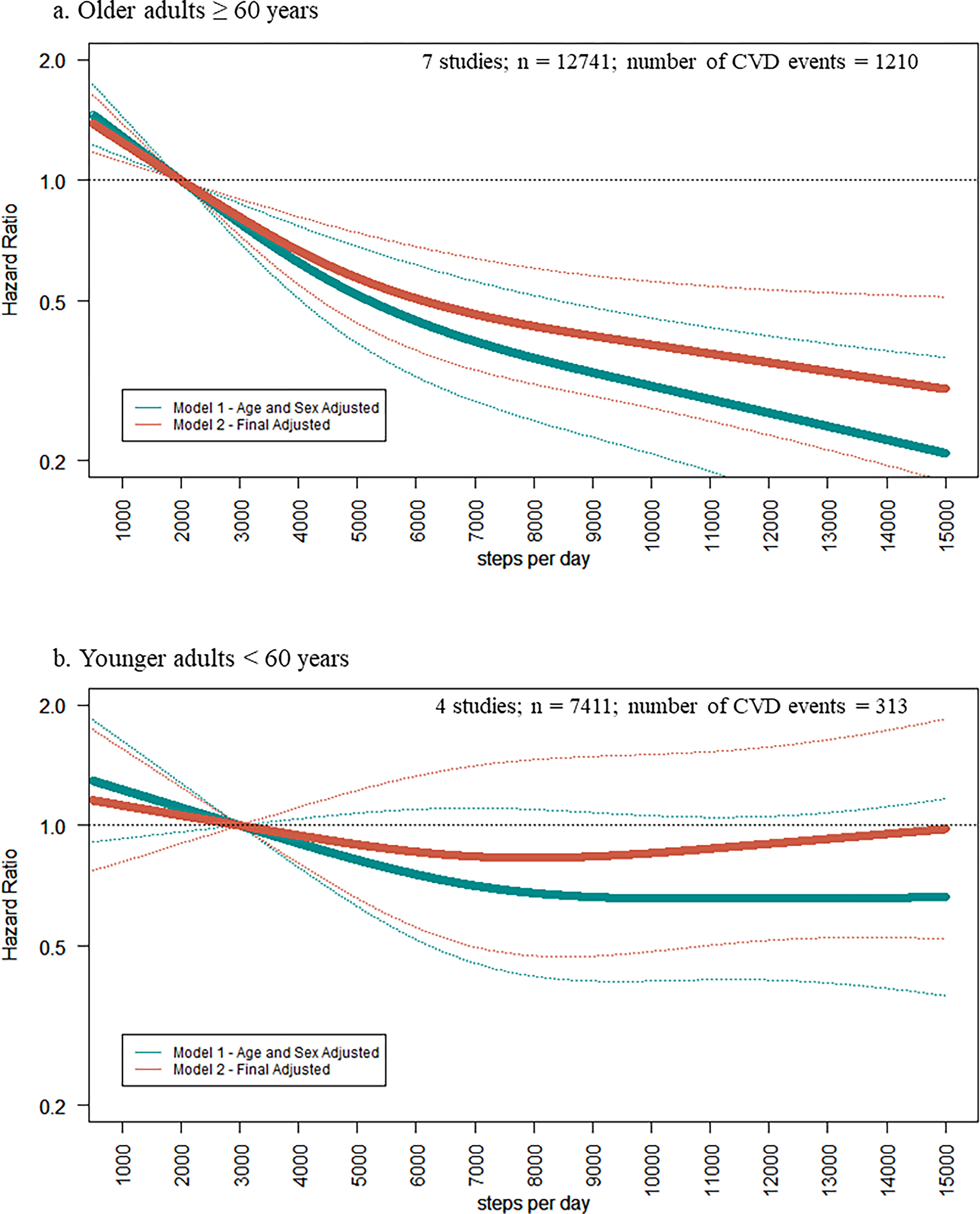
Restricted cubic splines of hazard ratios of steps/d with CVD events. Knots set at 10th, 50th, and 90th, percentile of steps/d. Reference set at median of lowest quartile (2,000 for older adults, 3,000 for younger adults).Hazard Ratios are indicated by solid lines and 95% Confidence Intervals are indicated by dotted lines. Model 1 adjusted for age and sex (if applicable). Model 2: Model 1 + device wear time, race/ethnicity (if applicable), education or income, body mass index, lifestyle (smoking, alcohol), hypertension, diabetes, dyslipidemia, and self-rated health. The y-axis is a log scale.
Among four studies of younger adults <60 years there were 313 events among 7411 individuals (5.1 events per 1,000 person years). Compared to the first quartile, there was a significant association in the age and sex adjusted model 1 in the third (0.72 [0.53–0.99]) and fourth (0.74 [0.54–0.99]) quartiles. Results were no longer significant in the final adjusted model. Compared to the lowest quartile, HRs for risk of CVD were 0.79 [0.46–1.35] in the second quartile, 0.90 [0.64–1.25] in the third quartile, and 0.95 [0.61–1.48] in the fourth quartile in the final adjusted model 2 (Figure 1). There was no significant association of steps/d and CVD events in the spline model for younger adults (Figure 2).
The HR in the final adjusted model in females was 0.81 [0.62–1.04] in the second quartile, 0.68 [0.48–0.97] in the third quartile, and 0.51 [0.35–0.76] in the fourth quartile (Figure 1), compared to the lowest quartile. The HR for males was 0.76 [0.63–0.90] in the second quartile, 0.63 [0.52–0.76] in the third quartile, and 0.68 [0.51–0.89] in the fourth quartile (Figure 1), compared to the lowest quartile. There were no significant subgroup differences by sex in quartile comparison or spline models. The spline models demonstrated a non-linear (p-value=0.001 for males and p-value =0.012 for females for non-linearity) dose response association with the leveling of the curve observed at approximately 8,000 steps/d for males and females (Figure S3).
Restricting the analysis to individuals without known CVD at baseline showed similar results. Among six studies excluding participants with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 [0.60–0.91] in the second quartile, 0.60 [0.47–0.77] in the third quartile, and 0.55 [0.40, 0.76] in the fourth quartile (Table 2).
Table 2.
Associations of Steps per Day with Overall CVD Events and Incidence CVD Events
| no of studies | Median Steps/d | n | events | HR (95% CI) | |
|---|---|---|---|---|---|
| Q1 | |||||
| Overall CVD Events | 8 | 1985 | 5034 | 551 | 1 |
| Incidence CVD Eventsa | 6 | 2778 | 3005 | 264 | 1 |
| Q2 | |||||
| Overall CVD Events | 8 | 4178 | 5038 | 396 | 0.81 [0.71; 0.93] |
| Incidence CVD Eventsa | 6 | 4831 | 3008 | 160 | 0.74 [0.60; 0.91] |
| Q3 vs Q1 | |||||
| Overall CVD Events | 8 | 6327 | 5043 | 312 | 0.67 [0.58; 0.78] |
| Incidence CVD Eventsa | 6 | 6794 | 3013 | 127 | 0.60 [0.47; 0.77] |
| Q4 vs Q1 | |||||
| Overall CVD Events | 8 | 10090 | 5037 | 264 | 0.57 [0.45; 0.74] |
| Incidence CVD Eventsa | 6 | 10105 | 3007 | 107 | 0.55 [0.40; 0.76] |
For incidence CVD: NAVIGATOR and ARIC removed, and subsample of LIFE study participants (sample size reduced from n= 1341 to 945 participants without previous CVD history at baseline for LIFE study). Hazard Ratio and 95% Confidence Intervals [HR (95% CI)] is adjusted for age, device wear time, race/Ethnicity (if applicable), sex (if applicable), education or income, body mass index, and study-specific variables for lifestyle, chronic conditions or risk factors, and general health status.
In sensitivity analyses, there were no subgroup differences by publication status (3 published vs 5 unpublished, Figure S4) or by device type (6 accelerometer vs 2 pedometer, Figure S5). There was no significant effect modification by device type influencing the studies’ effect sizes when including device type as a covariate in the meta-regression model (p-values for test of interaction > 0.05). The magnitude or direction of association between steps/d and CVD did not change when excluding any one study (Table S3). We re-analyzed data using fixed effects inverse variance method, and the main findings were unchanged (Table S4). Heterogeneity (I2) was low to moderate, ranging from 0 to 54% across quartiles (Figure 1). Funnel plots had minor asymmetry among lower weighted studies with visual inspection (Figure S2b). Egger’s test for symmetry suggested no evidence of study selection bias. There was no association between any threshold of stepping rate (30-minute, 60-minute or time spent at ≥40 steps/min or ≥100 steps per min) and CVD events before or after adjusting for steps/d (Table S5, Figures S5–S9).
Discussion
In the present meta-analysis of eight prospective studies, taking more steps/d was associated with lower CVD in older adults ≥60 years. Taking 6,000 to 9,000 steps/day was associated with 40% to 50% lower risk of CVD, compared to taking 2,000 steps/day. Findings from this meta-analysis can be used to generate evidence-based guidelines for cardiovascular benefit.
The curvilinear pattern observed in the steps and CVD dose-response curves are similar to a recent meta-analysis on steps and all-cause mortality in which there was an incremental lower risk of mortality until leveling around 6,000–8,000 in older adults.10 These recent results on steps and mortality included 15 studies, seven of which are included in the present meta-analysis on CVD. The steep early slope suggests taking more steps is better, particularly among individuals at lower steps/d. Additionally, although the slope is not as steep above 6,000, higher step counts appear to be associated with a continuing lower risk of CVD in older adults. This curvilinear relationship is consistent with meta-analyses on self-reported physical activity and coronary heart disease and stroke.1, 26 Conversely, a meta-analysis on heart failure risk reported a linear dose-response relationship with self-reported physical activity.27 The present study was unable to examine associations of steps with subtypes of CVD (e.g. heart failure, stroke) representing an area for future investigation.
Older adults who achieve higher thresholds of steps/d demonstrate a 40–50% lower risk for CVD, a magnitude that is similar to previous studies using accelerometer-measured total minutes per day of physical activity.28, 29 This magnitude of association is stronger compared with studies using self-reported physical activity, which report a 20–30% lower risk of CVD.1, 26, 27 For example, adults reporting high levels of physical activity of at least 300 minutes per week of moderate-intensity had a 20% (0.74 to 0.88) lower risk of coronary heart disease compared to adults reporting no leisure-time physical activity.1 The stronger associations may be due to the improved precision and lower bias seen with device-measured activity compared to self-reported questionnaires.30
In a prior meta-analysis, including only four studies demonstrated a non-linear association with CVD; however, that meta-analysis reported a high degree of heterogeneity (I2=80%).11 Heterogeneity in the present study was lower because the analytical approaches were uniform and events were similarly defined and adjudicated. The present study was additionally sufficiently large enough to conduct subgroup analyses by age and sex.
Despite an inverse association of steps with CVD in older adults, there was no association in younger adults. CVD is a disease of aging and often does not present itself as a diagnosed condition until years of progression. Therefore, the follow-up period may not be long enough to capture incidence of CVD for younger adults. Only 4.2% of younger adults (5.1 per 1,000 person years) versus 9.5% of older adults (19.3 per 1,000 person years) in the present study had a subsequent CVD event. These findings are consistent with a nationally-representative sample of U.S. adults, showing the percentage of deaths attributed to inadequate physical activity levels was only significant among older adults.31 The association of steps/d with intermediate CVD risk factors such as hypertension, high cholesterol, and diabetes may be the most appropriate outcome in young to middle aged adults.
Stepping rate (i.e., pace or cadence) was not associated with CVD risk beyond that of total steps/d. The absence of an association of stepping rate is consistent with prior research evaluating device measured stepping rate with mortality risk.10, 32 However, this finding is converse to a previous meta-analysis of self-reported walking which demonstrated walking pace was a stronger independent predictor of CVD risk compared with walking volume.33 The present findings should be viewed as preliminary, given only four studies reported data on stepping rate.
Implications of the present results for clinical care and public health guidelines reporting are multifold. Steps/d is a simple metric health care professionals can use during patient encounters to help monitor and promote physical activity. Over the past decade, there has been a rapid rise in the adoption of fitness trackers and smartphones monitoring steps and is expected to continue to grow. Steps/d estimates from waist-worn devices used in research studies may not precisely match consumer devices, which are often worn on the wrist. However, steps/d measured by research and consumer devices are highly correlated.34 Additionally, some step counting devices are less accurate at very slow walking speeds typical of many patient populations.35 Given low levels of activity in older adults,36, 37 empirical findings from the present study suggest that interventions may consider setting attainable step goals for cardiovascular health in older adults that fall below 10,000 steps/d.
Our study has several limitations. Despite adjusting for sociodemographic, lifestyle, and health status characteristics, the potential for residual confounding and reverse causality remains. The study level analyses did not account for competing risk of non-CVD related death, and therefore may overestimate CVD events and predicted risk. Although the present meta-analysis used study-level data and standardized analyses across studies, the heterogeneity in participants between studies (e.g. demographics, health status) and design (e.g. step device, covariates) may not be fully accounted for compared to individual-level pooled meta-analysis. Because this study did not have access to individual-level data we were limited to study-specific quartiles and unable to investigate differential effects across individuals or distinct subgroups. For example, further stratification by age-sex subgroups was not possible due to sample size limitations within each study. Additionally, this study was unable to investigate associations in patients with CVD at baseline and risk of secondary CVD events. Conclusions in the present study are generalizable only to the range of step counts observed in those samples—thus the very highest levels are activity are not represented (e.g., ≥15,000 steps/d). Participants were primarily among non-Hispanic White adults, which limits generalizability to other race-ethnic groups even though there is no a priori hypothesis to suggest a differential association of activity with CVD by race or ethnicity. The subset of studies included in older versus younger adult comparisons were not identical, which limits the ability to directly compare age groups. As all studies did not have longitudinal measurement of steps, this study only evaluated steps at a single time point and did not investigate the influence of changes in steps/d over time. Other studies, however, have demonstrated three to seven days of device measurement is representative of usual physical activity.38, 39 This study represents associations assuming an unchanging level of steps per day with CVD risk. Conclusions on causality require a prospective trial demonstrating increases in steps leads to a reduction in CVD risk. The majority of the data was obtained from unpublished studies, allowing for a harmonized approach where all studies used a standardized analytic approach to reduce study heterogeneity. Additionally, unpublished studies were invited to participate to reduce publication bias. Positive findings tend to be published earlier and more often compared to negative or null findings. When only relying on published evidence the pooled effect size can be overestimated.40 Our meta-analysis demonstrated associations in both published and unpublished providing robust evidence of the association of steps with risk of CVD.
Conclusion
Step goals based on empirical evidence are needed to guide technology-based monitoring and promotion of physical activity. The present meta-analysis is responsive to this gap in the literature since pedometers and accelerometers are more accurate for measuring ambulatory physical activity than self-report methods.30 Among older adults, taking 6,000 to 9,000 steps/day was associated with 40% to 50% lower risk of CVD. Findings from this meta-analysis can inform step guidelines for promotion of physical activity for cardiovascular health.
Supplementary Material
Clinical Perspective.
What is new?
In this meta-analysis of eight studies, taking more daily steps was associated with a progressively lower risk of cardiovascular disease (CVD) among older adults ≥60 years of age.
Among older adults, taking about 6,000 to 9,000 steps/d was associated with 40% to 50% lower risk of CVD, compared to taking about 2,000 steps/d.
What are the clinical implications?
Monitoring and promoting steps/d can be a simple, easy to interpret metric used for clinician-patient communication and population health to reduce the risk of CVD events.
Findings from the present study suggest that interventions may consider setting attainable step goals for cardiovascular health in older adults that fall below 10,000 steps/d.
ACKNOWLEDGEMENTS
We acknowledge and express sincere appreciation to all research staff for data collection and participants of all studies for their important contributions.
SOURCES OF FUNDING
This project was supported by an Intergovernmental Personnel Act (IPA) Agreement through the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health,
Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Accelerometer data were collected under R56AG049886.
British Regional Heart Study was supported by grants from the British Heart Foundation [PG/13/86/30546 and RG/13/16/30528 and RG/19/4/34452];
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by the National Institutes of Health (NHLBI and NIA), (HHSN268201800005I & HHSN268201800007I), (HHSN268201800003I),(HHSN268201800006I), (HHSN268201800004I), (AG0005), (Year 20 accelerometer data funded by R01 HL078972);
The Framingham Heart Study’s data collection and analysis was funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC25195 HHSN268201500001I and 75N92019D00031; Health and Human Services (N268201500001I; R01-AG047645; R01-HL131029); and American Heart Association (15GPSGC24800006); Dr. Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. Dr. Spartano received funding from Novo Nordisk for an investigator-initiated research grant unrelated to the current paper.
The Healthy Ageing Initiative’s data collection and analysis was funded by the Swedish Research Council (grant 2016–02589 to P. Nordström)
The Jackson Heart Study was funded by NIH contracts N01-HC95170, N01HC95171, and N01HC95172 that were provided by the National Heart, Lung, and Blood Institute and the National Center for Minority Health and Health Disparities.
The Lifestyle Interventions and Independence for Elders (LIFE) Study was funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #U01 AG22376 and a supplement from the National Heart, Lung, and Blood Institute 3U01AG022376–05A2S, and it was sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The study specific funders had no role in the design and conduct of the study; collection, management, analysis, interpretation of the data; preparation, review, approval of or decision to publish the manuscript. The views expressed in this publication are those of the author(s) and not necessarily those of the study specific funders.
Non-standard Abbreviations and Acronyms
- BMI
Body Mass Index
- DBP
Diastolic blood pressure
- CVD
Cardiovascular Disease
- CI
Confidence Interval
- HR
Hazard Ratio
- Steps/d
Steps per day
- SBP
Systolic blood pressure
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None.
Disclaimer:
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the CDC or the NIH.
Contributor Information
Amanda E. Paluch, Department of Kinesiology, Institute for Applied Life Sciences, University of Massachusetts Amherst, Amherst, MA.
Shivangi Bajpai, Department of Kinesiology, University of Massachusetts Amherst, Amherst, MA.
Marcel Ballin, Department of Community Medicine and Rehabilitation, Unit of Geriatric Medicine, Umeå University, Umeå, Sweden.
David R. Bassett, Department of Kinesiology, Recreation, and Sport Studies, University of Tennessee, Knoxville, TN.
Thomas W. Buford, Department of Medicine, Division of Gerontology/Geriatrics/Palliative Care, University of Alabama At Birmingham, Birmingham, AL; Birmingham/Atlanta Geriatric Research, Education, and Clinical Center, Birmingham VA Medical Center, Birmingham, AL.
Mercedes R. Carnethon, Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Ariel Chernofsky, Department of Biostatistics, Boston University, Boston, MA.
Erin E. Dooley, Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL.
Ulf Ekelund, Department of Sport Medicine, Norwegian School of Sport Sciences, and Department of Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway.
Kelly R. Evenson, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina Chapel Hill, Chapel Hill, NC.
Deborah A. Galuska, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA.
Barbara J. Jefferis, Department of Primary Care and Population Health, UCL Medical School, London, UK.
Lingsong Kong, Department of Kinesiology, University of Massachusetts Amherst, Amherst, MA.
William E. Kraus, Duke Molecular Physiology Institute, and the Department of Medicine, Duke University, Durham, NC.
Martin G. Larson, Department of Biostatistics, Boston University, Boston, MA.
I-Min Lee, Brigham and Women’s Hospital, Harvard Medical School and Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA.
Charles E. Matthews, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD.
Robert L. Newton, Jr., Pennington Biomedical Research Center, Baton Rouge, LA.
Anna Nordström, Department of Community Medicine and Rehabilitation, Unit of Geriatric Medicine, Umeå University, Umeå, Sweden, Department of Public Health and Clinical Medicine, Section of Sustainable Health, Umeå University, Umeå, Sweden, Sweden and School of Sport Sciences, UiT The Arctic University of Norway, Tromsø, Norway.
Peter Nordström, Department of Community Medicine and Rehabilitation, Unit of Geriatric Medicine, Umeå University, Umeå, Sweden.
Priya Palta, Department of Medicine, Columbia, University, New York, NY.
Alpa V. Patel, American Cancer Society, Population Science Department, Atlanta, GA.
Kelley Pettee Gabriel, Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL.
Carl F. Pieper, Department of Medicine, Duke University, Durham, NC.
Lisa Pompeii, Department of Pediatrics, Center for Epidemiology & Population Health, Baylor College of Medicine, Houston, TX.
Erika Rees-Punia, American Cancer Society, Population Science Department, Atlanta, GA.
Nicole L. Spartano, Department of Endocrinology, Diabetes, Nutrition and Weight Management, Boston University School of Medicine, Boston, MA.
Ramachandran S. Vasan, Departments of Medicine and Epidemiology, Boston University School of Medicine and Boston University School of Public Health, Boston, MA.
Peter H. Whincup, Population Health Research Institute, St George’s, University of London, London, U.K..
Shengping Yang, Pennington Biomedical Research Center, Baton Rouge, LA.
Janet E. Fulton, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA.
References
- 1.Sattelmair J, Pertman J, Ding EL, Kohl HW 3rd, Haskell W and Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY and Tsao CW. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 3.2018 Physical Activity Guidelines Advisory Committee. 2018 physical activity guidelines advisory committee scientific report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 4.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM and Olson RD. The physical activity guidelines for americans. JAMA. 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr., Virani SS, Williams KA, Yeboah J and Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omura JD, Hyde ET, Imperatore G, Loustalot F, Murphy L, Puckett M, Watson KB and Carlson SA. Trends in meeting the aerobic physical activity guideline among adults with and without select chronic health conditions, United States, 1998–2018. J Phys Act Health. 2021;18:S53–s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitfield GP, Hyde ET and Carlson SA. Participation in leisure-time aerobic physical activity among adults, National Health Interview Survey, 1998–2018. J Phys Act Health. 2021;18:S25–s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MS, Bachireddy C, Small DS, Harrison JD, Harrington TO, Oon AL, Rareshide CAL, Snider CK and Volpp KG. Effect of goal-setting approaches within a gamification intervention to increase physical activity among economically disadvantaged adults at elevated risk for major adverse cardiovascular events: the ENGAGE randomized clinical trial. JAMA Cardiology. 2021;6:1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassett DR Jr., Toth LP, LaMunion SR and Crouter SE. Step Counting: A review of measurement considerations and health-related applications. Sports Med. 2017;47:1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paluch AE, Bajpai S, Bassett DR, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, Jefferis BJ, Kraus WE, Matthews CE, Omura JC, Patel AV, Pieper CF, Rees-Punia E, Dallmeier D, Klenk J, Whincup PH, Dooley EE, Pettee Gabriel K, Palta P, Pompeii L, Chernofsky A, Larson MG, Vasan RS, Spartano N, Ballin M, Nordström P, Nordström A, Anderssen SA, Hansen BH, Cochrane JA, Dwyer T, Wang J, Ferrucci L, Liu F, Schrack J, Urbanek J, Saint-Maurice PF, Yamamoto N, Yoshitake Y, Newton RL Jr., Yang S, Shiroma EJ and Fulton JE. Daily steps and mortality: a meta-analysis of 15 international cohorts. The Lancet Public Health. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng M, Yang J, Bao M, Chen T, Cai R, Zhang N, Chen H, Liu M, Wu X, Zhang B, Liu Y and Chao J. The relationships between step count and all-cause mortality and cardiovascular events: A dose-response meta-analysis. J Sport Health Sci. 2021;10:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leening MJG, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, Heeringa J, Portegies MLP, Hofman A, Ikram MA, Hunink MGM, Franco OH, Stricker BH, Witteman JCM and Roos-Hesselink JW. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ : British Medical Journal. 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merz AA and Cheng S. Sex differences in cardiovascular ageing. Heart. 2016;102:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall KS, Hyde ET, Bassett DR, Carlson SA, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, Kraus WE, Lee IM, Matthews CE, Omura JD, Paluch AE, Thomas WI and Fulton JE. Systematic review of the prospective association of daily step counts with risk of mortality, cardiovascular disease, and dysglycemia. International Journal of Behavioral Nutrition and Physical Activity. 2020;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M and Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. [Google Scholar]
- 16.Michel J-P, Leonardi M, Martin M and Prina M. WHO’s report for the decade of healthy ageing 2021–30 sets the stage for globally comparable data on healthy ageing. The Lancet Healthy Longevity. 2021;2:e121–e122. [DOI] [PubMed] [Google Scholar]
- 17.Decade of healthy ageing: baseline report. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 18.Lee I-M, Shiroma EJ, Kamada M, Bassett DR, Matthews CE and Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Internal Medicine. 2019;179:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett WC, Howe GR and Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S; discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ and Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Smith GD, Schneider M and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn I-M, Hagströmer M, Edwardson C, Yates T, Shiroma E, Anderssen SA and Lee I-M. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ. 2019;366:l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crippa A, Discacciati A, Bottai M, Spiegelman D and Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–1596. [DOI] [PubMed] [Google Scholar]
- 24.Orsini N, Li R, Wolk A, Khudyakov P and Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tudor-Locke C, Han H, Aguiar EJ, Barreira TV, Schuna JM Jr., Kang M and Rowe DA. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: a narrative review. Br J Sports Med. 2018;52:776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJL and Forouzanfar MH. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA and Berry JD. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132:1786–94. [DOI] [PubMed] [Google Scholar]
- 28.Evenson KR, Wen F and Herring AH. Associations of accelerometry-assessed and self-reported physical activity and sedentary behavior with all-cause and cardiovascular mortality among US adults. Am J Epidemiol. 2016;184:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramakrishnan R, Doherty A, Smith-Byrne K, Rahimi K, Bennett D, Woodward M, Walmsley R and Dwyer T. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank cohort study. PLoS Med. 2021;18:e1003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S and Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson SA, Adams EK, Yang Z and Fulton JE. Percentage of deaths associated with inadequate physical activity in the United States. Prev Chronic Dis. 2018;15:E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saint-Maurice PF, Troiano RP, Bassett DR Jr, Graubard BI, Carlson SA, Shiroma EJ, Fulton JE and Matthews CE. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamer M and Chida Y. Walking and primary prevention: a meta-analysis of prospective cohort studies. Br J Sports Med. 2008;42:238–43. [DOI] [PubMed] [Google Scholar]
- 34.Toth LP, Park S, Springer CM, Feyerabend MD, Steeves JA and Bassett DR. Video-recorded validation of wearable step counters under free-living conditions. Med Sci Sports Exerc. 2018;50:1315–1322. [DOI] [PubMed] [Google Scholar]
- 35.Thorup CB, Andreasen JJ, Sørensen EE, Grønkjær M, Dinesen BI and Hansen J. Accuracy of a step counter during treadmill and daily life walking by healthy adults and patients with cardiac disease. BMJ Open. 2017;7:e011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conger SA, Toth LP, Cretsinger C, Raustorp A, Mitáš J, Inoue S and Bassett DR. Time trends in physical activity using wearable devices: a systematic review and meta-analysis of studies from 1995 to 2017. Med Sci Sports Exerc. 2022;54:288–298. [DOI] [PubMed] [Google Scholar]
- 37.Watson KB, Carlson SA, Gunn JP, Galuska DA, O’Connor A, Greenlund KJ and Fulton JE. Physical inactivity among adults aged 50 years and older - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954–8. [DOI] [PubMed] [Google Scholar]
- 38.Keadle SK, Shiroma EJ, Kamada M, Matthews CE, Harris TB and Lee IM. Reproducibility of accelerometer-assessed physical activity and sedentary time. Am J Prev Med. 2017;52:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao J, Tan CS, Lim N, Tan J, Chen C and Müller-Riemenschneider F. Number of daily measurements needed to estimate habitual step count levels using wrist-worn trackers and smartphones in 212,048 adults. Sci Rep. 2021;11:9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murad MH, Chu H, Lin L and Wang Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid Based Med. 2018;23:84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jefferis BJ, Parsons TJ, Sartini C, Ash S, Lennon LT, Papacosta O, Morris RW, Wannamethee SG, Lee IM and Whincup PH. Does total volume of physical activity matter more than pattern for onset of CVD? A prospective cohort study of older British men. Int J Cardiol. 2019;278:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cochrane SK, Chen SH, Fitzgerald JD, Dodson JA, Fielding RA, King AC, McDermott MM, Manini TM, Marsh AP, Newman AB, Pahor M, Tudor-Locke C, Ambrosius WT and Buford TW. Association of accelerometry-measured physical activity and cardiovascular events in mobility-limited older adults: the LIFE (Lifestyle Interventions and Independence for Elders) Study. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, Califf RM, Holman RR, McMurray JJ, Bethel MA, Tuomilehto J, Davies MJ and Kraus WE. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


