Abstract
Background:
Adolescents with Down syndrome (DS) are 2–3 times more likely to be obese than their typically developing peers. When preventing or treating obesity, it is useful for clinicians to understand an individual’s energy intake needs. Predictive resting energy expenditure (REE) equations are often recommended for general use in energy intake recommendations; however, these predictive equations have not been validated in youth with DS. The aim of this study was to compare the accuracy of seven commonly used predictive equations for estimating REE in adolescents who are typically developing to REE measured by indirect calorimetry in adolescents with DS.
Methods:
Adolescents with DS participated in a 90-minute laboratory visit before 10 am after a 12-hour overnight fast and a 48-hour abstention from aerobic exercise. REE was measured via indirect calorimetry and estimated REE was derived using the Institute of Medicine, Molnar, Muller, and World Health Organization equations. Mean differences between the measured and predicted REE for each equation were evaluated with equivalency testing and p-values were adjusted for multiple comparisons using the Holm method.
Results:
Forty-six adolescents with DS (age: 15.5 ± 1.7 years, 47.8% female, 73.9% non-Hispanic white) completed the REE assessment. Average measured REE was 1,459.5 ± 267.8 kcals/day and the Institute of Medicine equations provided the most accurate prediction of REE with a 1.7 ± 11.2% (13.9 ± 170.3 kcals/day) overestimation. This prediction was not statistically different from the measured REE (p-value = 0.582; 95% CI: −64.5, 36.7) and the difference between the measured and predicted REE was statistically equivalent to zero (p-value = 0.024; 90% CI: −56.1, 28.3).
Conclusions:
The results suggest that the Institute of Medicine equation may be useful in predicting REE in adolescents with DS. Future research should confirm these results in a larger sample and determine the utility of the Institute of Medicine equation for energy intake recommendations during a weight management intervention.
Keywords: Down syndrome, Exercise, Diet, Obesity, Energy Expenditure
Background
Down syndrome (DS) is the most common genetic disorder in the United States and affects about 6,000 (1 in every 700) newborns each year (Presson et al., 2013). Adolescents with DS are 2–3 times more likely to be obese than youth who are typically developing (TD) and prevalence estimates for being overweight or obese varies between 23–70% (Bertapelli et al., 2016, Rimmer et al., 2010, Basil et al., 2016, Ptomey et al., 2020, O’Shea et al., 2018). The etiology of obesity is unclear in individuals with DS, but a recent review by Bertapelli et al. (2016) suggests increased leptin, co-occurring conditions, unhealthy diet, and low physical activity levels as likely determinants of obesity in youth with DS. It has also been suggested that lower resting energy expenditure (REE) relative to body size and composition, which can comprise 50–75% of an individual’s total daily energy expenditure (TDEE) (Molnár and Schutz, 1997, Wong et al., 1996, McDuffie et al., 2004), may also predispose individuals with DS to obesity by reducing their TDEE and causing excessive weight gain over time (Hill et al., 2013). However, research examining REE in adolescents with DS has been hindered by variations in protocols (e.g., time of day, length of fasting, abstention from exercise), equipment (e.g., ventilated hood, mouth piece, or face mask; metabolic cart or chamber), and problems occurring during the measurement (e.g., excessive subject movement) (Fernhall et al., 2005, Luke et al., 1994).
When preventing or treating obesity, clinicians must determine an individual’s energy needs to accurately prescribe an energy intake goal for either weight loss or maintenance. Predictive REE equations are often recommended for general use; however, there is uncertainty in the performance of these equations among populations, such as those with DS, not included in the validation studies. For example, Müller et al. (2004) found considerable variance between male (average underestimation of 253 kcal/day) and female (average difference of 0 kcal/day) adolescents aged 12–17 years using the World Health Organization (WHO) formulas. These researchers also observed significant deviations between underweight and normal weight subjects after generating and cross-validating new prediction equations which could suggest the need for sex-specific equations by weight category. Molnar et al. (1995) also noticed that equations were consistently overestimating REE by 7.5% to 18.1% in adolescents aged 10–16 years but reduced the error to < 5% in 78% (n = 110) of the subjects after developing new equations.
Given concerns that adolescents with DS may have a lower REE than TD adolescents, it is unknown if REE equations validated in children and adolescents who are TD provide reliable estimates of REE in youth with DS. For example, Luke et al. (1994) reported the REE of 13 prepubescent children with DS was 20.5 ± 10.4% lower than predicted using a WHO equation. Additionally, Hill et al. (2013) found absolute REE to be 107% higher than the WHO’s equation. The paucity of research in this area has left researchers and health care providers with uncertainty related to estimating energy requirements in adolescents with DS, which is needed for both weight loss and prevention of excessive weight gain. Therefore, the aim of our study was to compare the accuracy of seven commonly used predictive equations used for estimating REE in adolescents who are TD to REE measured by indirect calorimetry in adolescents with DS. Our hypothesis was that the predictive equations for typically developing adolescents would be statistically different from and not equivalent to measured REE in adolescents with DS.
Methods
Overview
The study took place from November 2020 to April 2021 in the greater Kansas City Metropolitan area. Participants were 13–18 years of age with Down syndrome, of sufficient cognitive ability to understand directions, and able to communicate through spoken language determined by parent report and speaking with each participant during their screening for inclusion. Participants were excluded if they had a known or newly diagnosed hypothyroid condition that was unable to be controlled by medication or were currently pregnant, pregnant during the previous 6 months, lactating, or planning pregnancy in the following 12 months. Participants were recruited from local clinics that serve adolescents with DS, Hospital and University list serves, and on-going studies by the research team (Donnelly et al., 2016, Ptomey et al., 2019). Interested families completed an online screener, which was reviewed by the study’s principal investigator. Parents of participants who met the inclusion/exclusion criteria were contacted by members of the study team and a remote consent/assent appointment was scheduled. During the consent/assent process, the study team used a social story in the form of pictures for each step of the visit with easy-to-understand text to explain the requirement of the study to parents and participants. The Children’s Mercy Hospital Institutional Review Board approved the study protocol and all participants provided written informed consent and adolescent assent.
Outcomes
Adolescents and a parent attended one 90-minute laboratory visit between the hours of 6 and 10 am after a 12-hour overnight fast and 48-hour abstention from aerobic exercise (Berke et al., 1992 , Haugen et al., 2003). Assessments included height, weight, body composition, and REE. Anthropometrics and the REE measurements were completed by two trained masters level research assistants. Inter-rater reliability coefficients for these measures were ≥ 0.95. Due to the COVID-19 pandemic, participants were required to wear a mask during all outcome procedures with the exception of the REE measurement.
Demographic Data and Health History.
Basic demographic information (age, race/ethnicity, sex) as well as information regarding any previous/current medications, medical conditions, surgeries/medical procedures, and changes in weight was collected by parent report.
Height, Weight, and Body Composition.
Anthropometric measurements were first taken for each participant. Height (to nearest 0.1 cm) and weight (to nearest 0.1 kg) were obtained using a wall-mounted stadiometer and electronic scale, respectively. Repeat measurements were performed to ensure accurate readings for height (both measurements within 4 mm) and weight (both measurements within 0.1 kg). Dual energy x-ray absorptiometry (DXA, Lunar iDXA, GE Healthcare, Madison, WI, USA) was used to determine fat-free mass and fat mass.
Resting Energy Expenditure.
REE was measured via indirect calorimetry using a Parvo Medics TrueOne 2400 ventilated hood system (TrueOne, ParvoMedics, Parvo, UT, USA). All participants were asked to refrain from eating or drinking (except for water) and exercising for 12 and 48 hours prior to their visit, respectively. The REE tests took place in the morning before 10 am with participants lying in a supine position on top of a hospital bed. A blanket and pillow were provided and the participants were asked to remain still and awake for the entirety of the test. The test exam room was kept at a consistent, comfortable temperature with quiet surroundings for all study visits. Prior to each REE assessment, the metabolic system was calibrated to manufacturer specifications using the gas and flowmeter modules in the system software. Each assessment lasted a total of 40 minutes, which involved an initial stabilization period (i.e., 10 minutes), followed by a 30-minute data collection period. Data were discarded from the stabilization period and the remaining data were reduced to 15-second averages for analysis. A rolling 10-minute window was applied to identify the period in which the 15-second averages had the lowest coefficient of variation. REE was then calculated as the average of the values collected during that period.
Resting Energy Expenditure Predictive Equations.
REE was predicted using seven equations (Table 1). These included the Institute of Medicine (IOM) general use (i.e., applied only to healthy weight participants) or overweight/obese specific equation (i.e., used for participants who were overweight or obese) (Institute of Medicine of the National Academies Food and Nutrition Board, 2005); the Molnar equations that are sex-stratified (Molnar 1) or use sex as a variable in the equation (Molnar 2) (Molnar et al., 1995); the Muller equations that use height and weight (Muller 1) or fat and fat-free mass (Muller 2) (Müller et al., 2004); and the World Health Organization sex-stratified equations that use weight only (WHO 1) or weight and height (WHO 2) (FAO/WHO/UNU, 1985). The Molnar and Muller equations were converted to kcals/day from kilojoules/day and megajoules/day, respectively. The rationale for choosing the selected predictive equations included a long history of use (e.g., Molnar and WHO equations), performance in pediatric populations (Fuentes-Servín et al., 2021), and diversity in the variables used to estimate REE in adolescents.
Table 1.
Predictive equations used to estimate resting energy expenditure in adolescents with Down syndrome
| Stratification | Predictive Equation | |
|---|---|---|
| IOM A | Healthy weight Male | 79 − 34.2 × age + 730 × height + 15.3 × weight |
| Healthy weight Female | 322 − 26 × age + 504 × height + 11.6 × weight | |
| Overweight / obese Male | 420 − 33.5 × age + 418.9 × height + 16.7 × weight | |
| Overweight / obese Female | 516 − 26.8 × age + 347 × height + 12.4 × weight | |
| Molnar 1 B | Male | 50.9 × weight + 25.3 × height − 50.3 × age + 26.9 |
| Female | 51.2 × weight + 24.5 × height − 207.5 × age + 1629.8 | |
| Molnar 2 B | 50.2 × weight + 29.6 × height − 144.5 × age − 550 × sex (female = 1) + 594.3 | |
| Muller 1 C | 0.02606 × weight + 0.04129 × height + 0.311 × sex (male = 1) − 0.08369 × age − 0.808 | |
| Muller 2 C | 0.07885 × FFM + 0.02132 × FM + 0.327 × sex (male = 1) + 2.694 | |
| WHO 1 D | Male | 17.5 × weight + 651 |
| Female | 12.2 × weight + 746 | |
| WHO 2 D | Male | 16.6 × weight + 77 × height + 572 |
| Female | 7.4 × weight + 482 × height + 217 |
IOM, Institute of Medicine equations: weight is measured in kilograms, height is measured in meters, and resting energy expenditure is measured in kcals/day(Institute of Medicine of the National Academies Food and Nutrition Board, 2005)
Molnar equations: weight is measured in kilograms, height is measured in centimeters, and resting energy expenditure is measured in kilojoules/day and converted to kcals/day by multiplying by 0.2390057361(Molnar et al., 1995)
Muller equations: weight, fat mass, and fat free mass are measured in kilograms, height is measured in centimeters, and resting energy expenditure is measured in megajoules/day and converted to kcals/day by multiplying by 238.90295761862(Müller et al., 2004)
WHO, World Health Organization equations: weight is measured in kilograms, height is measured in meters, and resting energy expenditure is measured in kcals/day(FAO/WHO/UNU, 1985)
Data analysis
The sample was stratified by body mass index (BMI) percentile categories (i.e., underweight [< 5.0 percentile], healthy [5.0–84.9 percentile], overweight [85.0–94.9 percentile], or obese [≥ 95.0 percentile]) based on recommendations used by the Centers for Disease Control and Prevention (Barlow, 2007). Mean ± standard deviation or frequency (percentage) are reported for participant demographics and anthropometrics. Mean differences between the measured REE and each predictive equation were calculated and described in kcals/day and as a percent difference. Equivalency testing using a two one-sided test was used to compare each predictive equation with the measured REE with equivalence bounds set at Cohen’s dz ± 0.5. These equivalence bounds were set to detect equivalency with 90% power and a type I error rate of 5% in a sample size of 45 adolescents with DS (Lakens, 2017). P-values were adjusted for multiple comparisons using the Holm method (Holm, 1979) to reduce the chance of type I error and statistical significance was set at p < 0.05. Bland-Altman plots are presented for all predictive equations and spearman correlations were used to examine bias between the measured and predicted REE differences and averages. All statistical analyses were performed in R version 4.0.5 (R Core Team).
Results
Forty-six adolescents with DS completed the REE assessment (age: 15.5 ± 1.7 years, 47.8% female, 73.9% non-Hispanic white) and were included in this analysis. Demographics and anthropometrics stratified by body weight status are reported in Table 2. Seventeen (37%) adolescents had a healthy weight, 14 (30%) had overweight, 15 (33%) had obesity, and no adolescents were classified as underweight. Mean BMI percentiles ranged from 60.8 ± 23.8 in those participants with a healthy weight to 97.7 ± 1.3 in those with obesity. Similarly, fat mass and body fat percentage were lowest in the healthy weight group (10.3 ± 2.4 kg; 22.5 ± 5.2%) when compared to those who were overweight (20.9 ± 3.6 kg; 36.4 ± 5.8%) or obese (27.3 ± 5.2 kg; 40.1 ± 5.7%). Fat free mass was similar across all body weight statuses, albeit slightly higher in those with obesity (healthy weight: 38.1 ± 7.1 kg; overweight: 39.2 ± 7.4 kg; obese: 43.8 ± 10.8 kg).
Table 2.
Demographic characteristics and anthropometrics by body weight status for adolescents with Down syndrome
| Healthy, n = 17 | Overweight, n = 14 | Obese, n = 15 | |
|---|---|---|---|
| Age | 15.1 ± 1.8 | 16.2 ± 2.0 | 15.3 ± 1.2 |
| Sex | |||
| Female | 6 (35) | 10 (71) | 6 (40) |
| Male | 11 (65) | 4 (29) | 9 (60) |
| Ethnicity | |||
| Hispanic | 0 (0) | 0 (0) | 3 (20) |
| Non-Hispanic | 17 (100) | 14 (100) | 12 (80) |
| Race | |||
| Asian/Pacific Islander | 1 (6) | 0 (0) | 0 (0) |
| African American | 0 (0) | 1 (7) | 0 (0) |
| Indian/Alaskan | 1 (6) | 0 (0) | 0 (0) |
| White | 13 (76) | 10 (71) | 14 (93) |
| Multi-Race | 2 (12) | 3 (21) | 1 (7) |
| Anthropometrics | |||
| Weight (kg) | 48.5 ± 7.4 | 60.1 ± 7.8 | 71.1 ± 13.7 |
| Fat Mass (kg) | 10.3 ± 2.4 | 20.9 ± 3.6 | 27.3 ± 5.2 |
| Fat Free Mass (kg) | 38.1 ± 7.1 | 39.2 ± 7.4 | 43.8 ± 10.8 |
| BMI | 20.9 ± 1.7 | 26.4 ± 2.0 | 31.3 ± 2.6 |
| BMI Z-Score | 0.3 ± 0.8 | 1.3 ± 0.2 | 2.1 ± 0.2 |
| BMI Percentile | 60.8 ± 23.8 | 90.6 ± 3.4 | 97.7 ± 1.3 |
| Body Fat % | 22.5 ± 5.2 | 36.4 ± 5.8 | 40.1 ± 5.7 |
| Android Fat % | 18.5 ± 5.9 | 39.5 ± 7.2 | 44.7 ± 6.6 |
| Gynoid Fat % | 24.7 ± 6.9 | 38.1 ± 7.2 | 40.7 ± 6.5 |
Mean ± standard deviation or frequency (percentage)
Average differences between measured and predicted REE that were aggregated by body weight status and sex are reported in Table 3. Average measured REE ranged from 1,172 ± 181 kcals/day in healthy weight females to 1,777 ± 302 kcals/day in obese males. The Molnar and Muller 1 equations generally underpredicted REE across the different body weight status groups, while the Muller 2 and WHO equations consistently overestimated REE. Additionally, the IOM equation underpredicted REE in healthy weight and overweight females and overestimated REE for obese females and in all male body weight status groups. Mean differences and percent differences between measured and predicted REE for the entire sample can be found in Table 4. Percent differences ranged from −8.6 ± 9.9% (Molnar 2) to 10.4 ± 12.7% (WHO 1). These percentages were equal to an absolute difference in REE of approximately 110–140 kcals/day. A boxplot of the measured and predicted REE values for all the participants can be found in Figure 1 with horizontal bars representing the median along with the 25th and 75th percentiles. Each point on the box plot represents an individual’s measured or predicted REE value.
Table 3.
Measured and predicted resting energy expenditure stratified by body mass index category and sex
| HealthyA | OverweightA | ObeseA | ||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| n = 6 | n = 11 | n = 10 | n = 4 | n = 6 | n = 9 | |
| Measured | 1,172 ± 181 | 1,455 ± 133 | 1,321 ± 144 | 1,613 ± 204 | 1,409 ± 167 | 1,777 ± 302 |
| Predicted | ||||||
| IOMB | 1,137 ± 94 | 1,491 ± 105 | 1,309 ± 54 | 1,666 ± 96 | 1,422 ± 78 | 1,807 ± 280 |
| Molnar 1 | 984 ± 103 | 1,394 ± 82 | 1,161 ± 76 | 1,597 ± 106 | 1,288 ± 75 | 1,676 ± 247 |
| Molnar 2 | 1,003 ± 104 | 1,344 ± 90 | 1,189 ± 64 | 1,512 ± 72 | 1,291 ± 86 | 1,605 ± 235 |
| Muller 1 | 1,180 ± 82 | 1,439 ± 66 | 1,300 ± 51 | 1,544 ± 64 | 1,325 ± 73 | 1,569 ± 187 |
| Muller 2 | 1,288 ± 97 | 1,562 ± 95 | 1,428 ± 69 | 1,721 ± 155 | 1,483 ± 107 | 1,768 ± 234 |
| WHO 1C | 1,264 ± 88 | 1,551 ± 93 | 1,444 ± 61 | 1,840 ± 152 | 1,541 ± 82 | 1,973 ± 278 |
| WHO 2C | 1,224 ± 78 | 1,546 ± 91 | 1,352 ± 49 | 1,822 ± 148 | 1,387 ± 74 | 1,945 ± 272 |
Mean kcals per day ± standard deviation
IOM, Institute of Medicine
WHO, World Health Organization
Table 4.
Differences between measured and predicted resting energy expenditure with paired t-tests and paired equivalency tests (N=46)
| REEA (kcals/day) |
DifferenceA (kcals) |
Percent DifferenceA | P-valueB | ||
|---|---|---|---|---|---|
| T-test | Equivalency | ||||
| Measured | 1,459.5 ± 267.8 | — | — | — | — |
| Predicted | |||||
| IOMC | 1,473.4 ± 257.5 | 13.9 ± 170.3 | 1.7 ± 11.2 | .582 | .024 |
| Molnar 1 | 1,348.7 ± 263.5 | −110.8 ± 178.7 | −7.1 ± 11.7 | < .001 | > .999 |
| Molnar 2 | 1,324.6 ± 227.7 | −134.8 ± 161.9 | −8.6 ± 9.9 | < .001 | > .999 |
| Muller 1 | 1,394.7 ± 163.4 | −64.8 ± 176.6 | −2.9 ± 11.2 | .033 | > .999 |
| Muller 2 | 1,540.9 ± 204.4 | 81.5 ± 180.5 | 7.1 ± 12.4 | .015 | > .999 |
| WHO 1D | 1,596.7 ± 272.0 | 137.2 ± 182.5 | 10.4 ± 12.7 | < .001 | > .999 |
| WHO 2D | 1,543.3 ± 287.0 | 83.8 ± 190.7 | 6.5 ± 13.1 | .015 | > .999 |
Mean ± SD
Paired t-test and paired equivalency test p-values adjusted for multiple comparisons using the Holm method
IOM, Institute of Medicine
WHO, World Health Organization
Figure 1.
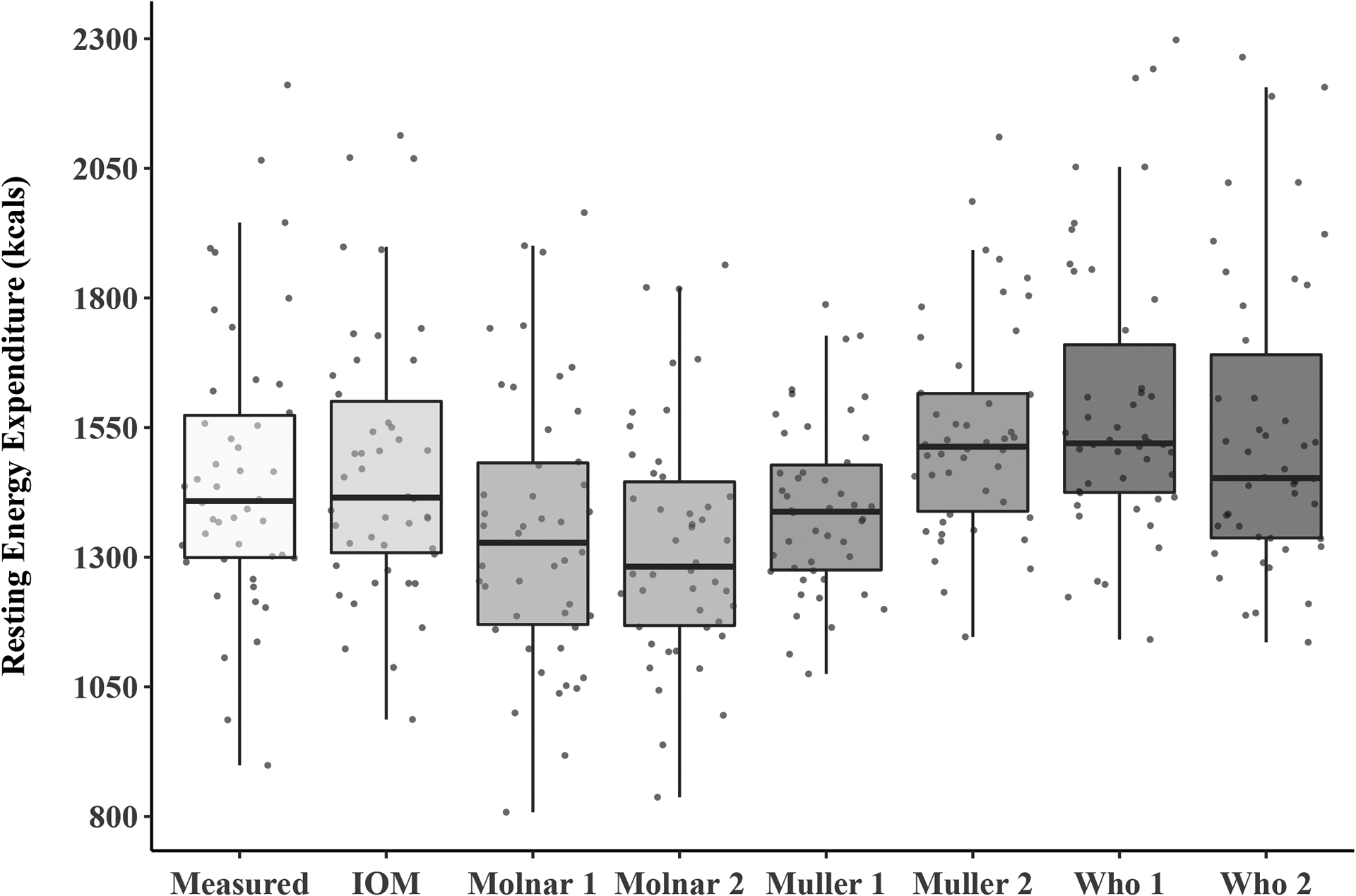
Measured and predicted resting energy Expenditures for adolescents with Down syndrome
The IOM estimates, which applied a general use or overweight/obese specific equation, predicted the measured REE most accurately when compared to the other equations. The IOM equation slightly overpredicted REE by 1.7 ± 11.2% or 13.9 ± 170.3 kcals/day, but this difference was statistically not different from (p-value = 0.582; 95% CI: −64.5, 36.7) and equivalent to (p-value = 0.024; 90% CI: −56.1, 28.3) zero after adjusting the p-values for multiple comparisons. Equivalence bounds were set using a Cohen’s dz ± 0.5 (equal to ± 85.1 kcals/day on a raw scale for the IOM equation). Other equations had more pronounced underestimations (Muller 1: −2.9 ± 11.2%; Molnar 1: −7.1 ±11.7%; and Molnar 2: −8.6 ± 9.9%) or overpredictions (Muller 2: 7.1 ± 12.4%; WHO 1: 10.4 ± 12.7%; and WHO 2: 6.5 ± 13.1%). The differences between the measured and predicted REE were statistically different from zero for the Molnar, Muller, and WHO equations (Table 4).
Bland-Altman plots for the measured and predicted REE are shown in Figure 2 for each equation with the difference in values on the y-axis and the average of the values on the x-axis. The 95% limits of agreement were between ± 500 kcals/day for all equations with the IOM (−319.8 to 347.6 kcals/day) and Molnar 2 (−452.1 to 182.4 kcals/day) equations providing the narrowest range. Spearman correlation estimates and p-values are reported under the x-axis of each Bland-Altman plot in Figure 2. We found that differences between measured and predicted REE change from positive to negative as the average REE rises for the Muller 1 (rho: −0.503; p-value: < 0.001) and Muller 2 (rho: −0.296; p-value: 0.046) equations.
Figure 2.
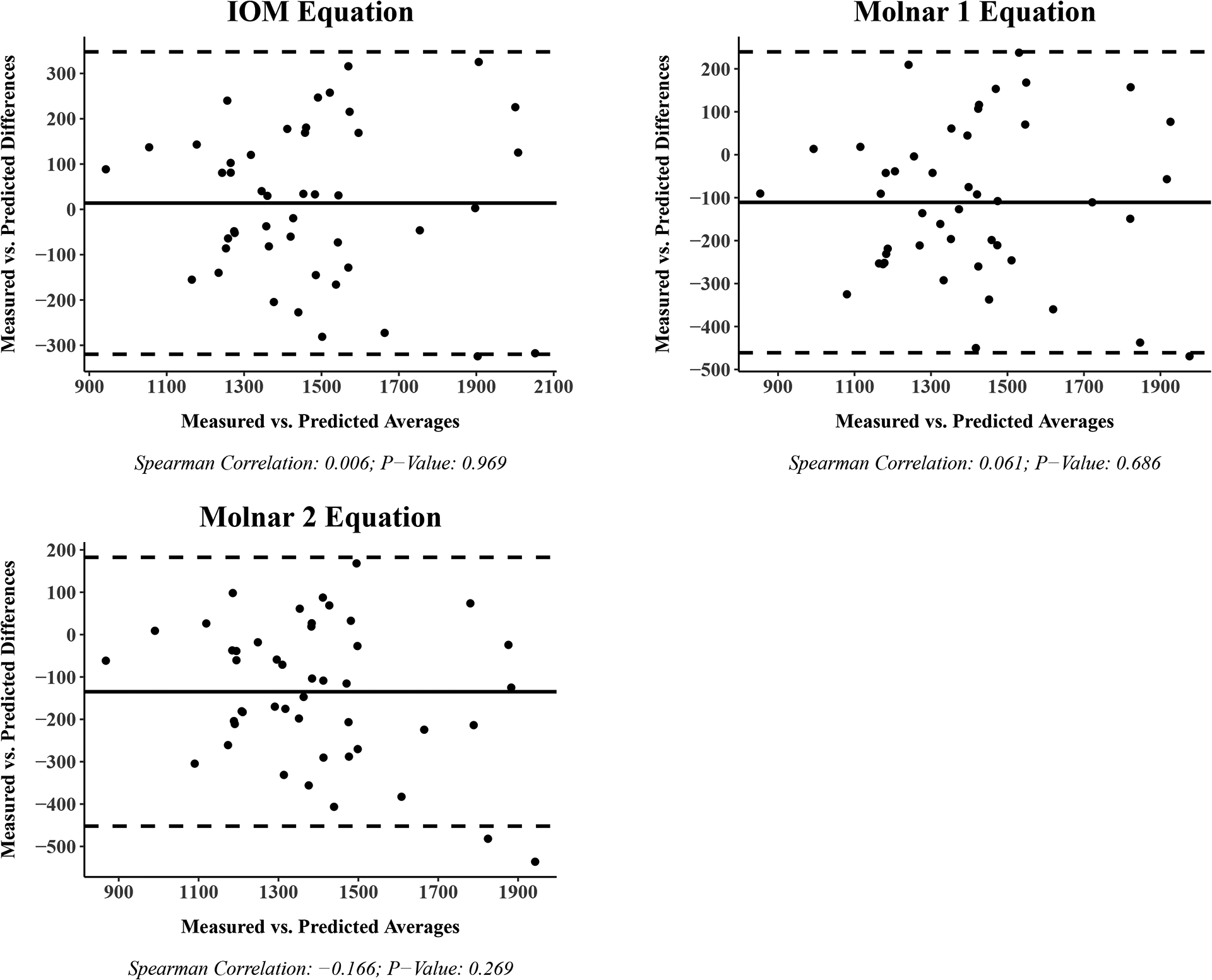
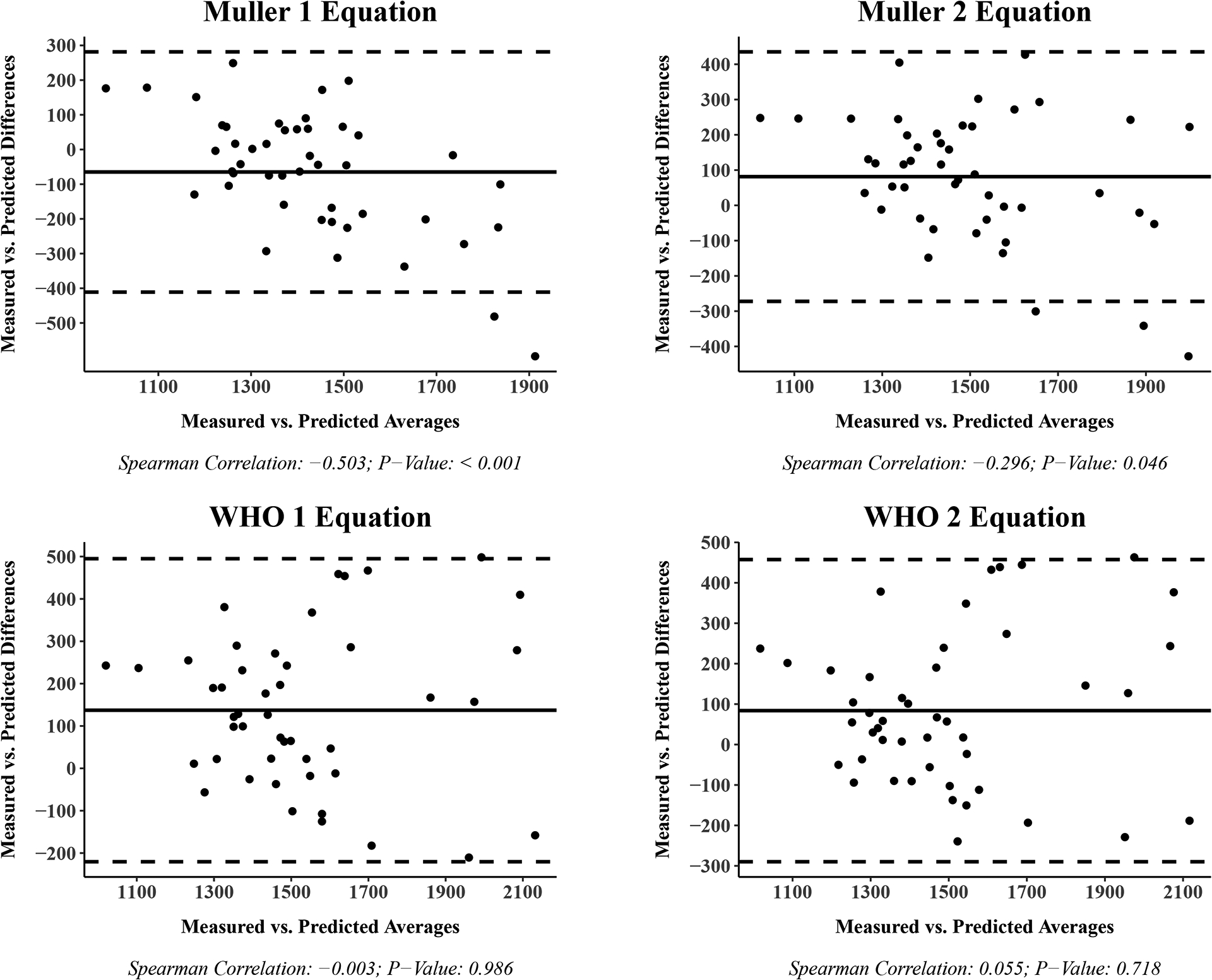
Bland-Altman plot: Measured vs. Predicted resting energy expenditure
Discussion
The objective of this study was to compare seven REE prediction equations validated in adolescents who were TD to measured REE in adolescents with DS. We found that of the seven equations tested, the Institute of Medicine’s (IOM) general use (i.e., applied to those who were a healthy weight) or overweight/obese specific equation was the only equation statistically equivalent to measured REE in adolescents with DS. The IOM equation produced estimates of REE that were within 2% of measured REE. Additionally, the 95% confidence intervals on the IOM Bland-Altman plots were nearly proportional (−319.8 to 347.6 kcals/day) and the mean difference was closer to zero, while the other predictive equations swayed more in the positive (i.e., Muller 2, WHO 1, and WHO 2) or negative (i.e., Molnar 1, Molnar 2, and Muller 1) directions. Together, these findings suggest that the IOM equation may provide the best estimation of REE in adolescents with DS compared to other predictive equations.
Equations used to estimate REE are frequently used and trusted, but limitations in their predictive accuracy in pediatrics may negatively affect nutritional care and patient outcomes (Carpenter et al., 2015). The equations are commonly derived using weight, weight and height, or fat and fat-free mass and can be stratified by sex or body weight status (Institute of Medicine of the National Academies Food and Nutrition Board, 2005, Molnar et al., 1995, Müller et al., 2004, FAO/WHO/UNU, 1985). A recent meta-analysis of 61 studies (N = 5,397 youth) suggested that fat free mass and BMI do not improve the amount of variance explained over age, height, and body mass. However, these authors recommend separate equations for each sex regardless of the variables used in the equation (Herrmann et al., 2017). Predictive equations for REE that target specific body mass index groups (e.g., IOM) have also been shown to reduce the deviations from measured REE values in healthy individuals across the lifespan (Müller et al., 2004).
We are unaware of previous studies which have examined the validity of the IOM equation in adolescents with DS. Two previous studies assessed the utility of the WHO equation in youth with DS with contradictory findings (Luke et al., 1994, Hill et al., 2013). For example, Hill et al. (2013) found that the WHO equation underestimated REE by 107% in individuals with DS aged 3–10 years. This was considerably greater than the overestimation of the WHO equations (20.5 ± 10.4%) by Luke et al. (1994) as well as our current study (WHO 1: 10.4 ± 12.7%; WHO 2: 6.5 ± 13.1%), which highlights the difficulties in using predictive equations in different samples.
Given that prior research suggests a lower REE for adolescents with DS (Bertapelli et al., 2016), applying predictive equations developed for adolescents without DS may not be advised. Our results insinuate that the IOM equation is statistically equivalent to measured REE, but more research is needed to determine whether the IOM equation could be used in place of an equation specific to adolescents with DS. One potential explanation for this finding is that the REE of adolescents with DS may not be much different than TD adolescents. For example, Chad et al. (1990) found that REE relative to body surface was only 10% lower in adolescents with DS compared to previously published data in children who were TD. This was similar to Hill et al. (2013) who found a 155 kcal/day (15.3%) difference between the mean REE for children with DS compared to healthy sibling controls. However, after adjusting for age, sex, race, and fat-free mass measured via DXA, Hill et al. (2013) found no statistically significant difference between children with DS and their healthy sibling controls (−17.0 kcal/day; 95% CI: −66.3 to 32.4; p=0.50). Additionally, previous studies have been limited by small sample sizes (n ≤ 28), inclusion of mostly prepubescent children, technical difficulties in REE measurements, and inclusion of mostly healthy weight participants. Future research is needed to examine if REE is significantly lower in adolescents with DS.
The results of the current study have many practical applications. When preventing or treating obesity, it is useful for a clinician to understand an individual’s energy needs to accurately prescribe an energy intake goal for either weight loss or maintenance. Many of the commonly used predictive equations only use age, sex, height, and weight to estimate REE making them easy-to-administer within clinical settings. However, differences in these equations can lead to a clinician recommending energy intake levels that can lead to weight gain. For example, the REE of a male adolescent with DS who is 14 years of age, 140 cm in height, and 70 kg in weight can be estimated using the (a) IOM, (b) Molnar 1, and (c) WHO 2 equations as the following:
| (a) |
| (b) |
| (c) |
Assuming this adolescent with DS has an actual REE within ± 2% of the IOM predicted value (1,673 – 1,741 kcals/day), a clinician could under- or over-predict REE by ~150–200 kcals/day which could lead to an additional pound of weight loss or gain every 2–3 weeks. It should also be noted that even the IOM equation had some variation at the individual level, with 24% of participants outside of the 200 kcal/day difference. This variation demonstrates that the IOM equation may not perform as well in some adolescents with DS and could lead to additional weight loss or gain over time. Thus, careful monitoring should be used with any of the REE equations to meet patient-centered goals for achieving a healthy weight.
To our knowledge, this research is the first to compare commonly used predictive equations and measured REE in adolescents with DS. Strengths of our study are the inclusion of a modest sample size of 46 adolescents with DS, the use of equations that have a diverse set of predictor variables (e.g., weight, weight and height, or fat and fat-free mass) and approaches (e.g., stratify by sex or body weight status), and a relatively equal distribution across body weight categories. Limitations include a lack of generalizability to all adolescents with DS (e.g., younger, different karyotypes) and the inability to determine how the estimations compare with age, sex, and weight-matched controls without DS. Future research should address these limitations and work toward determining the utility of the IOM equation for energy intake recommendations during a weight loss intervention. Recommendations for effective weight management interventions in adolescents with DS are needed (Curtin et al., 2013, Ulrich et al., 2011, Ordonez et al., 2006, Suarez-Villadat et al., 2020), but accurate predictions of REE to estimate energy intake needs will help in preventing excessive weight gain.
Conclusions
Accurate estimations of REE are necessary for prescribing diet recommendations to adolescents with DS. The results of this study suggest the IOM REE equation may be the most accurate REE equation for adolescents with DS. Our results suggest that the differences between measured REE and the IOM equation are statistically equivalent to zero. Future research should confirm these results in a larger sample of adolescents with DS and determine whether the IOM equation can accurately predict energy intake needs during a weight management trial.
Funding.
This project was supported by a grant from the Center for Children’s Healthy Lifestyles & Nutrition, a joint research center of Children’s Mercy Kansas City and The University of Kansas Medical Center. The first author of this manuscript was supported by a Clinical and Translational Science Award (CTSA) from National Center for Advancing Translational Sciences (NCATS) awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (Grant No. TL1TR002368).
Footnotes
Conflict of Interest Statement. The authors have no conflicts.
Ethics statement. This study involved human participants and was reviewed and approved by the Children’s Mercy Hospital Institutional Review Board.
Participant Consent. Written informed consent to participate in this study was provided by the participants’ parent or legal guardian and assent was provided by the participants.
Data Availability Statement.
The study protocol and deidentified participant data can be made available to researchers who submit a methodologically sound proposal to the principal investigator Dr. Robin Shook at rpshook@cmh.edu. As such requests for sharing will be subject to human subjects approvals from the respective institutions and researchers will be asked to specify the data, or variables, they would like to have. These data will be provided to the extent that subject confidentiality can be maintained.
References
- BARLOW SE 2007. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics, 120 Suppl 4, S164–92. [DOI] [PubMed] [Google Scholar]
- BASIL JS, SANTORO SL, MARTIN LJ, HEALY KW, CHINI BA & SAAL HM 2016. Retrospective study of obesity in children with Down syndrome. The Journal of pediatrics, 173, 143–148. [DOI] [PubMed] [Google Scholar]
- BERKE EM, GARDNER AW, GORAN MI & POEHLMAN ET 1992. Resting metabolic rate and the influence of pretesting environment. Am J Clin Nutr, 55, 626–629. [DOI] [PubMed] [Google Scholar]
- BERTAPELLI F, PITETTI K, AGIOVLASITIS S & GUERRA-JUNIOR G 2016. Overweight and obesity in children and adolescents with Down syndrome—prevalence, determinants, consequences, and interventions: A literature review. Research in developmental disabilities, 57, 181–192. [DOI] [PubMed] [Google Scholar]
- CARPENTER A, PENCHARZ P & MOUZAKI M 2015. Accurate estimation of energy requirements of young patients. J Pediatr Gastroenterol Nutr, 60, 4–10. [DOI] [PubMed] [Google Scholar]
- CHAD K, JOBLING A & FRAIL H 1990. Metabolic rate: a factor in developing obesity in children with Down syndrome? Am J Ment Retard, 95, 228–35. [PubMed] [Google Scholar]
- CURTIN C, BANDINI LG, MUST A, GLEASON J, LIVIDINI K, PHILLIPS S, ELIASZIW M, MASLIN M & FLEMING RK 2013. Parent support improves weight loss in adolescents and young adults with Down syndrome. J Pediatr, 163, 1402–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNELLY JE, PTOMEY LT, GOETZ JR, SULLIVAN DK, GIBSON CA, GREENE JL, LEE RH, MAYO MS, HONAS JJ & WASHBURN RA 2016. Weight management for adolescents with intellectual and developmental disabilities: Rationale and design for an 18month randomized trial. Contemp Clin Trials, 51, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO/UNU 1985. Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser, 724, 1–206. [PubMed] [Google Scholar]
- FERNHALL B, FIGUEROA A, COLLIER S, GOULOPOULOU S, GIANNOPOULOU I & BAYNARD T 2005. Resting metabolic rate is not reduced in obese adults with Down syndrome. Ment Retard, 43, 391–400. [DOI] [PubMed] [Google Scholar]
- FUENTES-SERVÍN J, AVILA-NAVA A, GONZÁLEZ-SALAZAR LE, PÉREZ-GONZÁLEZ OA, SERVÍN-RODAS MDC, SERRALDE-ZUÑIGA AE, MEDINA-VERA I & GUEVARA-CRUZ M 2021. Resting Energy Expenditure Prediction Equations in the Pediatric Population: A Systematic Review. Front Pediatr, 9, 795364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGEN HA, MELANSON EL, TRAN ZV, KEARNEY JT & HILL JO 2003. Variability of measured resting metabolic rate. Am J Clin Nutr, 78, 1141–1144. [DOI] [PubMed] [Google Scholar]
- HERRMANN SD, MCMURRAY RG, KIM Y, WILLIS EA, KANG M & MCCURDY T 2017. The influence of physical characteristics on the resting energy expenditure of youth: A meta-analysis. Am J Hum Biol, 29. [DOI] [PubMed] [Google Scholar]
- HILL DL, PARKS EP, ZEMEL BS, SHULTS J, STALLINGS VA & STETTLER N 2013. Resting energy expenditure and adiposity accretion among children with Down syndrome: a 3-year prospective study. European journal of clinical nutrition, 67, 1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLM S 1979. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics, 65–70. [Google Scholar]
- INSTITUTE OF MEDICINE OF THE NATIONAL ACADEMIES FOOD AND NUTRITION BOARD 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids, Washington, DC, The National Academies Press. [Google Scholar]
- LAKENS D 2017. Equivalence Tests: A Practical Primer for t Tests, Correlations, and Meta-Analyses. Soc Psychol Personal Sci, 8, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUKE A, ROIZEN NJ, SUTTON M & SCHOELLER DA 1994. Energy expenditure in children with Down syndrome: correcting metabolic rate for movement. The Journal of pediatrics, 125, 829–838. [DOI] [PubMed] [Google Scholar]
- MCDUFFIE JR, ADLER-WAILES DC, ELBERG J, STEINBERG EN, FALLON EM, TERSHAKOVEC AM, ARSLANIAN SA, DELANY JP, BRAY GA & YANOVSKI JA 2004. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am J Clin Nutr, 80, 365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLNAR D, JEGES S, ERHARDT E & SCHUTZ Y 1995. Measured and predicted resting metabolic rate in obese and nonobese adolescents. J Pediatr, 127, 571–7. [DOI] [PubMed] [Google Scholar]
- MOLNÁR D & SCHUTZ Y 1997. The effect of obesity, age, puberty and gender on resting metabolic rate in children and adolescents. Eur J Pediatr, 156, 376–81. [DOI] [PubMed] [Google Scholar]
- MÜLLER MJ, BOSY-WESTPHAL A, KLAUS S, KREYMANN G, LUHRMANN PM, NEUHAUSER-BERTHOLD M, NOACK R, PIRKE KM, PLATTE P, SELBERG O & STEINIGER J 2004. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr, 80, 1379–90. [DOI] [PubMed] [Google Scholar]
- O’SHEA M, O’SHEA C, GIBSON L, LEO J & CARTY C 2018. The prevalence of obesity in children and young people with Down syndrome. Journal of Applied Research in Intellectual Disabilities, 31, 1225–1229. [DOI] [PubMed] [Google Scholar]
- ORDONEZ FJ, ROSETY M & ROSETY-RODRIGUEZ M 2006. Influence of 12-week exercise training on fat mass percentage in adolescents with Down syndrome. Med Sci Monit, 12, Cr416–9. [PubMed] [Google Scholar]
- PRESSON AP, PARTYKA G, JENSEN KM, DEVINE OJ, RASMUSSEN SA, MCCABE LL & MCCABE ER 2013. Current estimate of Down syndrome population prevalence in the United States. The Journal of pediatrics, 163, 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PTOMEY L, WALPITAGE D, MOHSENI M, DREYER GILLETTE M, DAVIS AM, FORSETH B, DEAN E & WAITMAN L 2020. Weight status and associated comorbidities in children and adults with Down syndrome, autism spectrum disorder and intellectual and developmental disabilities. Journal of Intellectual Disability Research, 64, 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PTOMEY LT, WASHBURN RA, LEE J, GREENE JL, SZABO-REED AN, SHERMAN JR, DANON JC, OSBORNE LN, LITTLE TD & DONNELLY JE 2019. Individual and family-based approaches to increase physical activity in adolescents with intellectual and developmental disabilities: Rationale and design for an 18 month randomized trial. Contemp Clin Trials, 84, 105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R CORE TEAM. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing [Online]. Vienna, Austria. Available: https://www.R-project.org/ [Accessed]. [Google Scholar]
- RIMMER J, YAMAKI K, LOWRY BD, WANG E & VOGEL L 2010. Obesity and obesity‐related secondary conditions in adolescents with intellectual/developmental disabilities. Journal of Intellectual Disability Research, 54, 787–794. [DOI] [PubMed] [Google Scholar]
- SUAREZ-VILLADAT B, LUNA-OLIVA L, ACEBES C & VILLAGRA A 2020. The effect of swimming program on body composition levels in adolescents with Down syndrome. Res Dev Disabil, 102, 103643. [DOI] [PubMed] [Google Scholar]
- ULRICH DA, BURGHARDT AR, LLOYD M, TIERNAN C & HORNYAK JE 2011. Physical activity benefits of learning to ride a two-wheel bicycle for children with Down syndrome: A randomized trial. Physical therapy, 91, 1463–1477. [DOI] [PubMed] [Google Scholar]
- WONG WW, BUTTE NF, HERGENROEDER AC, HILL RB, STUFF JE & SMITH EO 1996. Are basal metabolic rate prediction equations appropriate for female children and adolescents? J Appl Physiol (1985), 81, 2407–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study protocol and deidentified participant data can be made available to researchers who submit a methodologically sound proposal to the principal investigator Dr. Robin Shook at rpshook@cmh.edu. As such requests for sharing will be subject to human subjects approvals from the respective institutions and researchers will be asked to specify the data, or variables, they would like to have. These data will be provided to the extent that subject confidentiality can be maintained.


