Abstract
Objective:
To analyze the association between maternal pesticide exposure and autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorders (ADHD) in offspring.
Method:
Five databases including PubMed, Embase, Web of Science, Medline, as well as PsycINFO were systematically retrieved for the records related to pesticide exposure during pregnancy and ASD and ADHD in offspring before August 30, 2022. The pesticide category, maternal age and window of exposure as the main subgroups were presented.
Results:
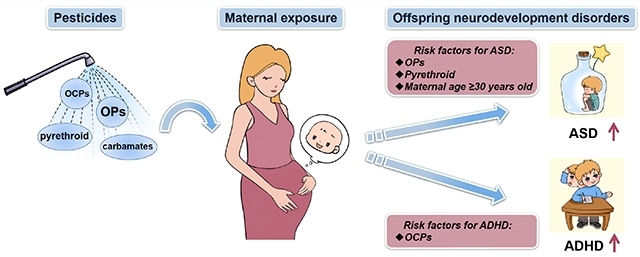
949 studies were initially identified, and 19 studies were eventually included. Eleven were on ASD, seven were on ADHD, and one was on both disorders. Maternal pesticide exposure was positively related to ASD (pooled OR = 1.19 (95%CI: 1.04 to 1.36)) and ADHD (pooled OR = 1.20 (95%CI: 1.04 to 1.38)) in offspring. In the subgroup analysis, organophosphorus pesticides (OPs) (pooled OR = 1.14 (95%CI: 1.04 to 1.24)), pyrethroid (pooled OR = 1.40 (95%CI: 1.09 to 1.80)), and maternal age ≥30 years old (pooled OR = 1.24 (95%CI: 1.10 to 1.40)) increased the risk of ASD in offspring. Maternal organochlorine pesticides (OCPs) exposure was a risk factor for ADHD in offspring (pooled OR = 1.22 (95%CI: 1.03 to 1.45)).
Conclusion:
Maternal pesticide exposure increased the risk of ASD and ADHD in offspring. Moreover, OPs, pyrethroid, and maternal age ≥30 years old were found to be risk factors affecting children’s ASD. Maternal exposure to OCPs increased the risk of ADHD in offspring. Our findings contribute to our understanding of health risks related to maternal pesticide exposure and indicate that the in utero developmental period is a vulnerable window-of-susceptibility for ASD and ADHD risk in offspring. These findings should guide policies that limit maternal exposure to pesticides, especially for pregnant women living in agricultural areas.
Keywords: Pesticides, Pregnancy, ASD, ADHD, Meta-analysis
Graphical Abstract
1. Introduction
Autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are two common neurodevelopmental disorders (NDDs) [1] characterized by learning challenges, as well as social and adaptive deficiencies that can persist throughout life. In recent years, the incidence of ASD and ADHD has been increasing globally. ASD has been demonstrated to affect roughly 3.14% of children and adolescents in the United States [2] and 2.64% of the general population [3], whereas ADHD has a significant influence on approximately 5.29% of school-age children [4] and 2.5% of adults [5]. It is difficult for children with both disorders in mastering adaptive skills necessary in adulthood [6], which places them at increased risk for employment difficulties and mental problems later in life [7].
Genetic [8, 9] and enviromnental [10, 11] factors are the key factors in the development of ASD and ADHD. Pesticides, a prevalent environmental contaminant, are applied to agricultural lands to protect crops from insects and weeds, boost crop yields, and protect forests [12]. A wide range of pesticides is used worldwide and some pesticides have cumulation toxicity and can cross the placental barrier [13, 14], posing a threat to the fetus’ health and potentially increasing the risk of ASD and ADHD in offspring [15, 16]. Rauh et al. [17] discovered that chlorpyrifos (CPF) exposure was negatively related to the risk of ADHD. Another study also found that children aged 4-5 years old exposed to pesticides during pregnancy were more likely to develop ASD [18]. However, on the contrary, other studies found that exposure to OCPs such as p,p′-DDE, and imidacloprid during pregnancy were not related to ASD and ADHD in children [19, 20]. Different types of pesticides can have different effects on offspring neurodevelopment including direct effects of pesticide exposure on fetal development and indirect effects through, for example, maternal factors which can act as catalysts for pesticide exposure effects on fetal neurodevelopment. There is an increasing trend in maternal age worldwide [21], and several population studies indicated that older maternal age increased the risk of ASD in offspring [22–24]. Overall, conclusions regarding the correlation between pesticides and ASD or ADHD were inconsistent, which might be influenced by the sample size, heterogeneous exposure, and outcome assessments. Considering the inconsistency and limitation of reported studies, in our study, a meta-analysis was conducted to systematically estimate whether maternal pesticide exposure was correlated with ASD and ADHD in offspring.
In this study, we comprehensively and systematically compiled epidemiology data presently accessible to examine the correlation between pesticide exposure during pregnancy and ASD and ADHD in children. Moreover, subgroup analyses were performed to explore impacts of pesticide category and potential confounding factors including maternal age, window of exposure as well as type of assessment on children’s ASD and ADHD.
2. Materials and methods
2.1. Search strategy
Our meta-analysis was refined under the guideline of PRISMA [25]. Five databases including PubMed, Web of Science, Embase, Medline, and PsycINFO were retrieved for all original studies published before August 30, 2022. The final search formula was as follows: (pesticides OR insecticides OR insect repellents OR organophosphorus pesticide OR organochlorine pesticide OR organophosphorus OR organochlorine OR chemical pest control OR fungicide OR herbicide OR insecticide OR molluscacide OR molluscicide OR rodenticide OR carbamate OR pyrethroid OR agricultural chemical) AND (autism spectrum disorder OR asperger syndrome OR autistic disorder OR autism OR ASD OR Attention Deficit Disorders with Hyperactivity OR attention deficit hyperactivity disorder OR attention deficit-hyperactivity disorder OR attention-deficit/hyperactivity disorder OR attention-deficit hyperactivity disorder OR ADHD).
2.2. Study selection and eligibility criteria
Two reviewers separately retrieved studies using the search formula and extracted the relevant data from the included articles. Disagreements were thoroughly explored until consensus was reached. The criteria included in our study were as follows: (1) High-quality population studies, such as cohort studies and case-control studies; (2) ASD or ADHD was treated as the outcome variable; (3) Outcome was diagnosed using clinical evaluation or self-report questionnaires. Specifically, ADHD was determined from the International Classification of Diseases (ICD)-9, 10 or Diagnostic and Statistical Manual of Mental Disorders (DSM)-III, III-R, IV, and ASD was derived from DSM-III, III-R, IV, Autism Diagnostic Observation Schedule (ADOS) or the Autism Diagnostic Interview-Revised (ADI-R) diagnosis; (4) Results were shown as Odds Ratio (OR), Risk Ratio (RR) or Hazard Ratio (HR). Exclusion criterion was as follows: (1) Irrelevant studies; (2) Meta-analysis, review, comments, protocol or case report or books; (3) Animal or cell studies; (4) About treatment and method; (5) About postpartum or adult exposure; (6) Low (<6) Newcastle-Ottawa Scale (NOS) score.
2.3. Data extraction
The following characteristics from studies were included (Table S1, Table S2): author, publication year, country, study design, number of participants, maternal age, children’s age, neurodevelopment assessment, sample assessment, pesticides name, adjusted OR with 95% confidence interval (CI), and NOS score. The results will be converted to OR based on the solution provided by Shor et al. [26] when the results were presented as HR values. RR value was approximately equal to the OR value because of the low prevalence of ASD and ADHD [27]. In addition, we standardized the logarithmic conversion to log10 according to Liu et al. [28].
2.4. Assessment of the risk of bias
NOS was used as the scoring system [29], which consists of three main parts: selection and comparability of study populations, a third component for determining outcomes in cohort studies, and exposure in case-control studies. In the NOS scoring system, 0-3 was considered as the low-level study, 4-6 as the medium level, and 7-9 as the high quality.
2.5. Meta-analysis
In the analysis of ASD and ADHD, heterogeneity was measured by I2 statistic. When I2 was greater than 50%, a random effects model was used, while a fixed effects model was used when it was less than 50% [30]. Since the heterogeneity of ASD was found to be high, a random effects model was chosen for future analysis of ASD. Subgroup analyses were carried out on the ASD-related data, with maternal age (30 years old as cut-off), types of pesticide, window of exposure and types of sample assessment as the main subgroups presented. A subgroup analysis based on type of pesticide was conducted on the ADHD-related studies. A sensitivity analysis was also completed to check whether each article had a significant impact on the final results. Publication bias in articles was detected by using Begg’s test [31], trim and fill method [32], and funnel plot. All analyses in tins study were performed using Stata version 15 (College Station, TX, USA) and R (version 4.2.2) in sensitivity analysis.
3. Results
3.1. Search results
949 articles were retrieved through the search strategy, and 339 records remained after removing the duplicates (Fig. 1). After screening the title, abstract, and the methodology, 277 records were excluded. Additional five studies were identified by hand-searching. Finally, 19 studies were included in our analysis, including seven on ADHD [17, 20, 33–37] and 11 on ASD [19, 38–47], as well as one study [48] describing both disorders.
Fig. 1.
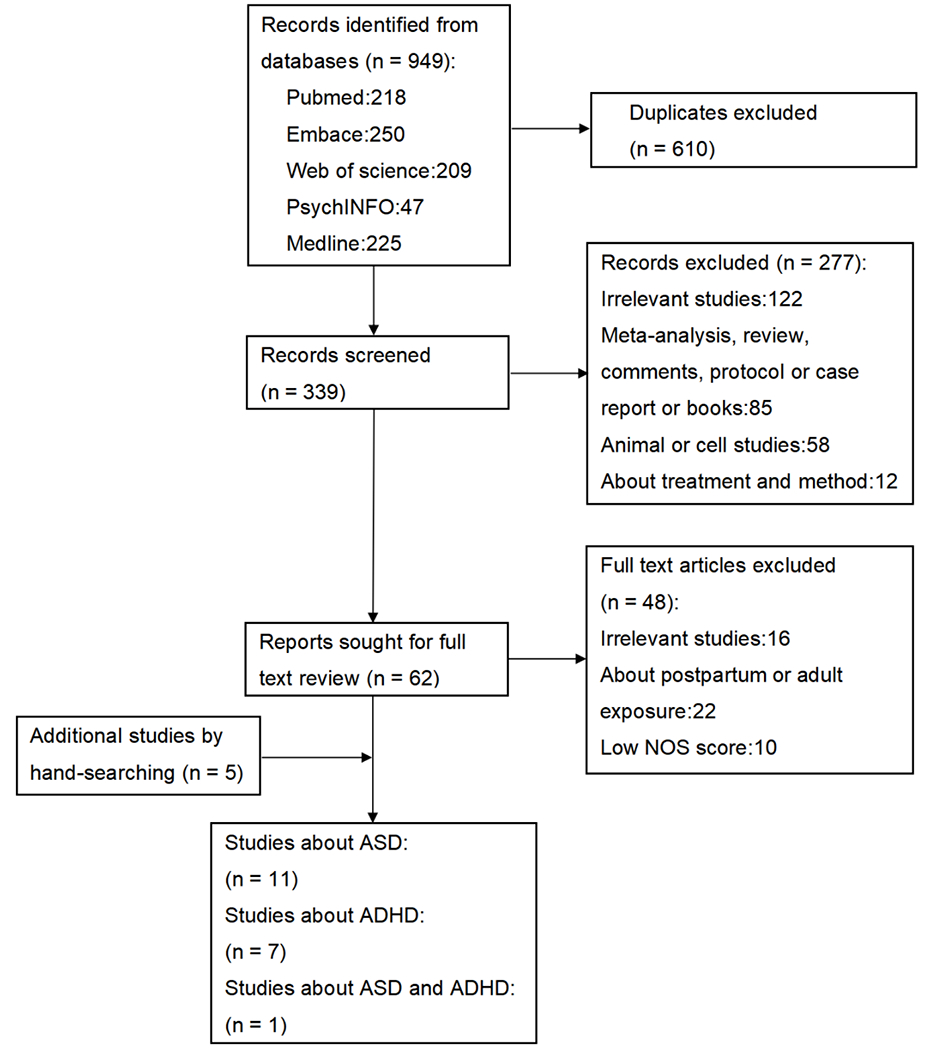
Flow diagram of the study selection process.
3.2. Study characteristics
From the 12 ASD-related studies, a total of 107,752 individuals were selected for our study (Table S1), with 24,761 of them diagnosed with ASD. Among included articles, seven [19, 39, 40, 42, 44, 46, 47] were case-control studies, and the remaining five studies [38, 41, 43, 45, 48] were cohort studies. These studies were conducted in four countries: USA (n=8), Finland (n=2), Jamaica (n=1), France (n=1). Seven [19, 38, 39, 43–45, 48] used quantifiable values as indications of exposure, including three maternal serum samples [19, 39, 44] and four maternal urine samples [38, 43, 45, 48]. The remaining five [40–42, 46, 47] used approximated exposure levels assessed using the pesticide use report (PUR), socioeconomic status (SES) questionnaire, environmental exposure questionnaire, and department of environmental conservation (DEC) database. In these included studies, we investigated four categories of pesticides including OPs (chlorpyrifos, glyphosate), OCPs (p,p’-DDE), pyrethroid, and carbamates (imidacloprid).
From the eight ADHD-related studies, a total of 5,029 individuals were selected for our study with 974 were diagnosed with ADHD (Table S2). Among included articles, six were cohort studies [17, 20, 33, 35, 37, 48] and two were case-control studies [34, 36]. The eight articles included were conducted in five countries: USA (n=3), Denmark (n=2), Finland (n=1), Norway (n=1), South Africa (n=1). Four of the included articles collected maternal serum samples [17, 20, 33, 34] while the other studies used maternal urine samples [35–37, 48]. The relevant studies included two categories of pesticides, three [20, 33, 34] focused on OCPs and five [17, 35–37, 48] on OPs. The NOS scores (Table S3, Table S4) of the included studies ranged from 6 (moderate) to 8 (high).
3.3. Study findings from systematic review: associations of maternal pesticide exposure with ASD and ADHD in offspring
ASD:
Of 12 studies that assessed pesticide exposure, four studies reported significant positive associations with ASD. Shelton et al. found that maternal exposure to OPs show a positive correlation with ASD in children (pooled OR = 1.60 (95%CI: 1.02 to 2.51)) [46]. Exposure to pyrethroids was more likely to have a higher prevalence of ASD in Hicks et al.’s study (pooled OR = 1.37 (95%CI: 1.06 to 1.78)) [41]. However, Lyall et al. demonstrated that maternal exposure to p,p’-DDE was not significantly associated with ASD in offspring (pooled OR = 0.90 (95%CI: 0.57 to 1.42)) [44].
ADHD:
Of eight studies that assessed pesticide exposure, two studies reported significant positive associations with ADHD. Rauh et al. found that maternal exposure to CPF was positively related to ADHD in children (pooled OR = 6.50 (95%CI: 1.09 to 38.69)) [17]. Dalsager et al. similarly concluded that maternal CPF exposure was significantly related to ADHD in children (pooled OR = 1.83 (95%CI: 1.13 to 2.96)) [35]. Whereas Strom et al. found that maternal exposure to p,p’-DDE did not correlated with ADHD in offspring (pooled OR = 0.91 (95%CI: 0.30 to 2.67)) [20].
3.3.1. Maternal pesticide exposure and children’s ASD
Twelve studies were included to determine whether maternal pesticide exposure was related to ASD in offspring. The pooled OR was 1.19 (95%CI: 1.04 to 1.36) using random effect model (I2 = 72.1%, p < 0.001) (Fig. 2a). The funnel-plot and Begg’s rank test indicated that the potential publication bias existed in included studies (p = 0.014, Fig. 2b). Then the trim-and-fill method was used (four studies filled), which showed that the significance of the association did not change indicating the reliability of our results (pooled OR = 3.10 (95%CI: 2.71 to 3.61) (Fig. S1). However, the high heterogeneity across the studies indicated the necessity for subgroup analysis. We used four subgroups as the main analysis including pesticides category, maternal age, window of exposure and method of exposure assessment.
Fig. 2.
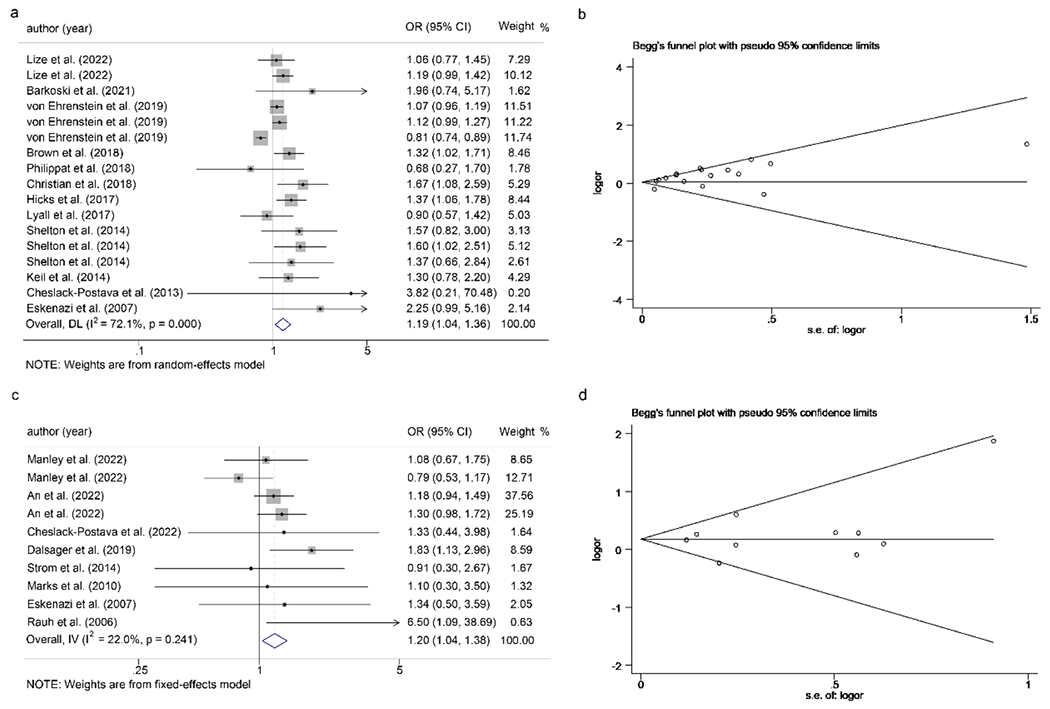
Forest plot of the association between maternal exposure to pesticides during pregnancy and risk of ASD (a) and ADHD (c) in offspring; Funnel plot for the association between maternal exposure to pesticides during pregnancy and risk of ASD (b) and ADHD (d) in offspring.
3.3.2. Maternal pesticide exposure and children’s ADHD
The Q-test results showed insignificant heterogeneity (p = 0.241 and I2 = 22.0%) across the included studies and then a fixed effects model was used. The pooled OR between maternal exposure to pesticides and children’s ADHD was 1.20 (95%CI: 1.04 to 1.38) (Fig. 2c). These results indicated that maternal pesticide exposure was positively related to ADHD. In addition, the Begg’s funnel-plot and Begg’s rank test (p = 0.511) indicated no publication bias (Fig. 2d).
3.3.3. Subgroup analysis for ASD and ADHD
Four subgroup analysis were conducted in ASD-related studies for: pesticides category, maternal age, window of exposure, and type of sample measurement. Subgroup analysis for pesticide category was performed in ADHD-related studies.
3.3.3.1. Pesticides category and children’s ASD
Eleven included studies were grouped by pesticides category to clarify whether pesticide exposure during pregnancy was related to ASD in children. One study did not specify the type of pesticide [40] and was excluded from subgroup analysis. A total of four major categories of pesticides were included in these studies: OPs [43, 45–48], OCPs [19, 39, 44], pyrethroid [38, 41] and carbamates [42, 46, 47]. OPs included CPF, glyphosate and DAP measured by serum and urine. OCPs included p,p’-DDE, and carbamates included imidacloprid. Maternal exposure to OPs (pooled OR =1.14 (95%CI: 1.04 to 1.24)) and pyrethroid (pooled OR = 1.40 (95%CI: 1.09 to 1.80)) were positively related to ASD in offspring (Fig. 3a). In contrast, the OCPs and carbamates pesticide categories revealed no significant associations.
Fig. 3.
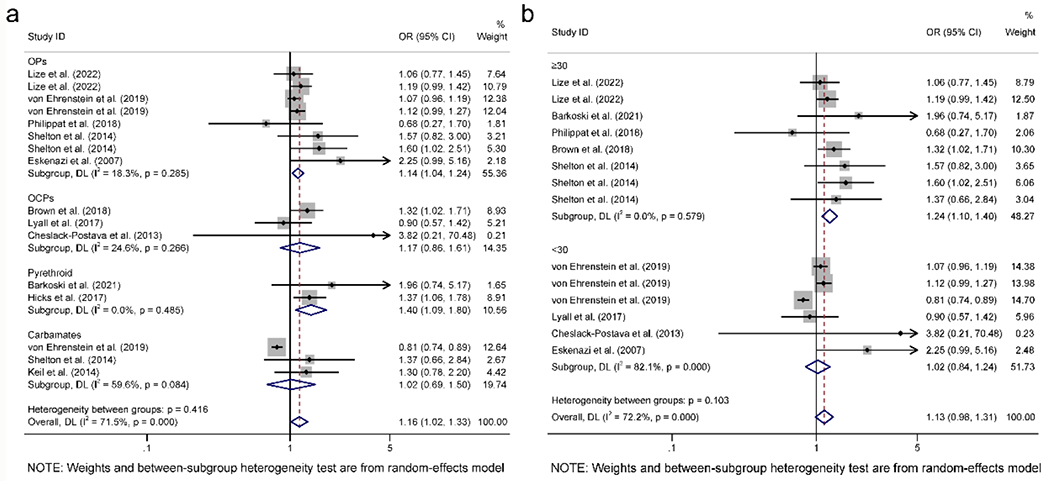
Subgroup analysis on studies for ASD adjusted for pesticide category (a) and maternal age (b).
3.3.3.2. Maternal age and children’s ASD
Nine studies were included to determine whether maternal age was correlated with ASD in offspring. Three articles were excluded since they did not include maternal age [41, 42], or maternal age could not precisely be determined [40]. Women in five studies [19, 38, 43, 45, 46] were no less than 30 years old, and the remaining four studies had maternal ages younger than 30 [39, 44, 47, 48]. Pesticide exposure in pregnant women no less than 30 years old was positively related to ASD in their children (pooled OR = 1.24 (95%CI: 1.10 to 1.40)) compared to those younger than 30 years old (Fig. 3b). The results suggested that maternal age >30 years old increased the risk of ASD in children after pesticide exposure during pregnancy.
3.3.3.3. Window of exposure and children’s ASD
Four studies conducted the association of pesticides exposure and ASD in offspring according to the window of exposure [46]. Second and third trimesters were considered in two studies [38, 45]. Lyall et al.’s study only included the second trimester [44]. Results indicated that maternal pesticide exposure during the third trimester was positively related to ASD in offspring (pooled OR = 1.56 (95%CI: 1.01 to 2.40) (Fig. S2).
3.3.3.4. Types of sample assessment and children’s ASD
Twelve studies were included in determining the association between the type of sample assessment and ASD in offspring. Five studies [40–42, 46, 47] used estimated exposure values, including PUR, SES questionnaire, environmental exposure questionnaire, and DEC database. Seven studies used GC/MS methods to detect levels of pesticides in serum [19, 39, 44] and urine [38, 43, 45, 48] samples to assess pesticide exposure. Estimated (pooled OR = 1.19 (95%CI: 1.00 to 1.42)) and measured (pooled OR = 1.19 (95%CI: 1.03 to 1.38)) values both revealed the effect of pesticide exposure in the development of ASD in children (Fig. S3).
3.3.3.5. Pesticides category and children’s ADHD
Eight studies were included in the subgroup analysis based on types of pesticides for children’s ADHD. Three studies explored the relationship between maternal OCPs exposure and ADHD in offspring [20, 33, 34], and five studies focused on maternal OPs exposure [17, 35–37, 48]. Maternal OCPs exposure were positively associated with ADHD in offspring (pooled OR = 1.22 (95%CI: 1.03 to 1.45) (Fig. 4).
Fig. 4.
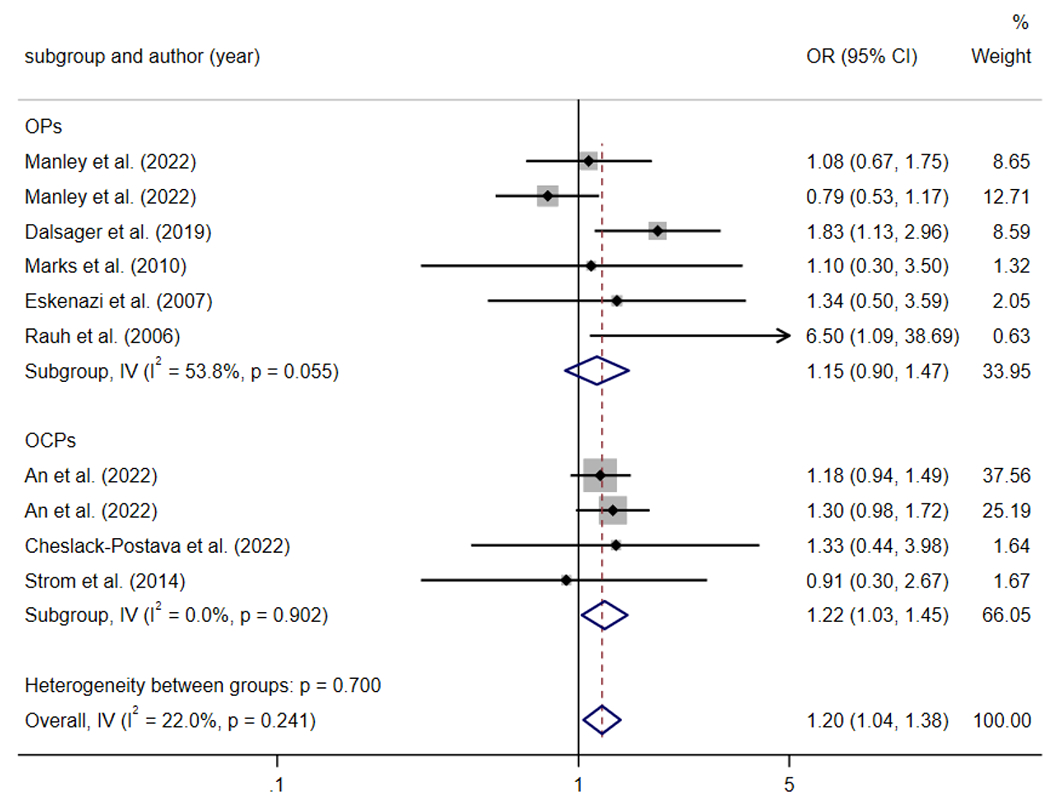
Subgroup analysis on studies for ADHD adjusted for pesticide category.
3.4. Sensitivity analysis
Sensitivity analyses were performed using the “leave-one-out ‘ approach. Omission of any study did not affect association between pesticide exposure and ASD (Fig. S4a). The result of sensitivity analysis showed that the overall estimate changed to nonsignificant when An et al.’s or Dalsager et al’s studies were excluded (Fig. S4b).
4. Discussion
In our meta-analysis, 19 studies were summarized to clarify the correlation of maternal pesticide exposure with ASD and ADHD in offspring. Random effects model in ASD and fixed effects model in ADHD were used to conduct the quantitative synthesis. The findings revealed that maternal pesticide exposure was positively related to ASD and ADHD in children. Subgroup analysis indicated that maternal OCPs exposure increased the risk of ADHD in children. Maternal OPs and pyrethroid exposure during pregnancy or pregnant aged >30 years old was risk factors for ASD in offspring.
These findings are consistent with studies that showed that maternal pesticide exposure leads to an increase in ASD-related symptoms [49–51]. It is known that pregnancy is a critical period for fetal neurodevelopment, which is also the susceptible time window of chemical exposure. During this period, certain pesticides such as OPs pass through the placental barrier and can directly affect fetal neurodevelopment [52, 53]. We found certain types of pesticide such as maternal OPs and pyrethroid exposure were positively associated with ASD in offspring. The potential mechanisms associated with maternal exposure to OPs and pyrethroids in the development of ASD in offspring were reported in previous animal and cellular studies. Maternal CPF exposure led to changes in the Prostaglandin E2 (PGE2) pathway inducing oxidative stress, which may result in the occurrence of ASD-like behaviors [54]. In addition, glyphosate also showed a significantly correlation with ASD in offspring potentially regulated through upregulating soluble epoxide hydrolase (sEH) expression [55] and alterations in subventricular zone (SVZ) neurogenesis [56]. Prenatal exposure of mice to pyrethroids inhibits angiogenesis and led to an increase in blood-brain barrier permeability [57]. These findings might help to explain the potential mechanisms by which maternal exposure to OPs and pyrethroids might increase the risk of ASD in offspring.
We found that maternal age had a great impact on the association of maternal pesticide exposure and ASD in children. The risk of ASD was higher in offspring whose mothers were older than 30 years of age at pregnancy. The potential reason is that older pregnancies may make the offspring more susceptible to hypoxia, which is a risk factor for ASD [58]. In addition, due to increased exposure time. higher levels of environmental toxins accumulated in older mother’s germ cells, resulting in an incidence in the levels of de novo mutations and epigenetic alterations [59], which was potentially detrimental to the fetus’s neurodevelopment. The heterogeneity was high in the subgroup of maternal age less than 30, which was derived from the study of von Ehrenstein et al [47]. The measurement of pesticide exposure in their study was based on CA-PUR, where dietary and occupational pesticide exposure were not included which may underestimate the total exposure dose. Other studies used serum and mine samples to evaluate the exposure level, which more accurately reflected internal pesticide levels.
The subgroup analysis revealed that maternal pesticide exposure during the third trimester increased the risk of ASD in children. The third trimester is a critical period for neurodevelopment such as synapses [60, 61], and exposure to pesticides during this period may lead to neuroinflammation, which impairs synapse formation and neurotransmitter receptor formation. It is worth noting that maternal pesticide exposures by trimesters are correlated. Specific types of pesticides including organochlorine pesticides can accumulate in human tissues, reaching their maximum concentration in late pregnancy, which may explain the increased risk of ASD development in the third trimester. In addition, the limited number of studies that included pesticide exposure for each trimester may reduce the statistical power to detect trimester-level differences in the development of ASD in offspring. Adequate studies of time-varying associations are necessary before conclusions can be drawn about the critical window of exposure associated with increased risk of ASD.
In addition to types of pesticides, maternal age and window of exposure that affected the development of ASD in offspring, we found that the methods for measuring pesticide exposure varied in the type of exposure assessment. The results measured by the biomarker method and estimated by PUR and questionnaire both suggested that maternal pesticide exposure positively correlated with ASD in offspring. Further studies with consistent high quality internal exposure assessment methods are warranted for the association.
The findings related to ADHD are consistent with previous studies that maternal exposure to pesticides is associated with the development of ADHD in offspring [62, 63]. Pesticide exposure-induced neuroinflammation with the reduced volume of cortical brain areas could be a risk factor in the development of ADHD [64–66]. Specific pesticides such as organochlorine could impair GABAergic neurodevelopment in mice, leading to deficits in neuronal differentiation and synaptic development [67]. However, the mechanisms associated with OCPs are not well investigated and more studies are needed for further exploration.
Our study has the following strengths. Firstly, we are the first to find that maternal age >30 years old and pyrethroids increased the risk of ASD in offspring. Secondly, we standardized the calibration methods and units, which guaranteed the accurate evaluation of the statistics. Thirdly, we performed a trim and fill approach in studies with publication bias to evaluate the effect of publication bias on the result, which ensured the accuracy of results. Finally, 107,752 participants were included in the ASD studies and 5,029 participants in ADHD studies, which ensured sufficient statistical power when conducting meta-analysis. However, some limitations still existed in our study. Firstly, pregnancy is a critical window period for neurodevelopmental abnormalities, but the effects of postnatal exposure cannot be ignored. Although many studies have controlled for postnatal variables, we could not rule out the possibility of postnatal bias in our study. Secondly, although we have included four major categories of pesticides, other types of pesticide such as acaricidethe, fungicides and rodenticide were not included in the studies used in our meta-analysis. We will conduct these analyses when adequate studies including these types of pesticides are available in the future.
5. Conclusion
In conclusion, our findings showed that pesticide exposure during pregnancy was positively related to ASD and ADHD. OPs, pyrethroid exposure, and maternal age >30 years old were risk factors for children’s ASD. In addition, maternal OCPs exposure increased the risk of ADHD in offspring. Further research should integrate epidemiological and experimental studies to clarify basic mechanisms in the development of ASD and ADHD. The findings indicate that maternal pesticide exposure should be avoided, especially for older pregnant women in agricultural areas, to protect early brain development in offspring.
Supplementary Material
Highlights:
Maternal pesticide exposure increased the risk of ASD and ADHD in offspring.
Maternal OPs, pyrethroid exposure and maternal age >30 years old increased the risk of ASD in offspring for pesticide exposure.
Maternal OCPs exposure were risk factors for ADHD in offspring.
Necessary measures should be taken to protect gravida from pesticide exposure.
Acknowledgements
This work was supported by grants from NSFC-NIH Biomedical collaborative research program (grant no. 81961128022), U.S.-China Program for Biomedical Collaborative Research NIEHS: R01ES031322 (A.M.S.), the fifth phase of “333 High-level Talent Training Project” of the Jiangsu Province (BRA2020070); the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ameis SH, et al. , A Diffusion Tensor Imaging Study in Children With ADHD, Autism Spectrum Disorder, OCD, and Matched Controls: Distinct and Non-Distinct White Matter Disruption and Dimensional Brain-Behavior Relationships. Am J Psychiatry, 2016. 173(12): p. 1213–1222. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, et al. , Prevalence of Autism Spectrum Disorder Among Children and Adolescents in the United States from 2019 to 2020. Jama Pediatrics, 2022. 176(9): p. 943–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YS, et al. , Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry, 2011. 168(9): p. 904–12. [DOI] [PubMed] [Google Scholar]
- 4.Polanczyk G, et al. , The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry, 2007. 164(6): p. 942–8. [DOI] [PubMed] [Google Scholar]
- 5.Simon V, et al. , Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry, 2009. 194(3): p. 204–11. [DOI] [PubMed] [Google Scholar]
- 6.Avni E, Ben-ltzchak E, and Zachor DA, The Presence of Comorbid ADHD and Anxiety Symptoms in Autism Spectrum Disorder: Clinical Presentation and Predictors. Front Psychiatry, 2018. 9: p. 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlin P and Magiati I, Autism spectrum disorder: outcomes in adulthood. Curr Opin Psychiatry, 2017. 30(2): p. 69–76. [DOI] [PubMed] [Google Scholar]
- 8.Gaugler T, et al. , Most genetic risk for autism resides with common variation. Nat Genet, 2014. 46(8): p. 881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraone SV and Larsson H, Genetics of attention deficit hyperactivity disorder. Mol Psychiatry, 2019. 24(4): p. 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegarty JP, 2nd, et al. , Genetic and environmental influences on structural brain measures in twins with autism spectrum disorder. Mol Psychiatry, 2020. 25(10): p. 2556–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan GH, et al. , Association between exposure to the natural environment, rurality, and attention-deficit hyperactivity disorder in children in New Zealand: a linkage study. Lancet Planet Health, 2019. 3(5): p. e226–e234. [DOI] [PubMed] [Google Scholar]
- 12.Ecobichon DJ, Pesticide use in developing countries. Toxicology, 2001.160(1-3): p. 27–33. [DOI] [PubMed] [Google Scholar]
- 13.Bradman A, et al. , Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect, 2003. 111(14): p. 1779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong Z, Günter M, and Randow FF, Meconium: a matrix reflecting potential fetal exposure to organochlorine pesticides and its metabolites. Ecotoxicol Environ Saf, 2002. 51(1): p. 60–4. [DOI] [PubMed] [Google Scholar]
- 15.Addissie YA, et al. , Prenatal exposure to pesticides and risk for holoprosencephaly: a case-control study. Environ Health, 2020. 19(1): p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy TS, Seidler FJ, and Slotkin TA, Morphologic effects of subtoxic neonatal chlorpyrifos exposure in developing rat brain: regionally selective alterations in neurons and glia. Brain Res Dev Brain Res, 2004. 148(2): p. 197–206. [DOI] [PubMed] [Google Scholar]
- 17.Rauh VA, et al. , Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics, 2006. 118(6): p. E1845–E1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun JM, et al. , Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect, 2014. 122(5): p. 513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown AS, et al. , Association of Maternal Insecticide Levels With Autism in Offspring From a National Birth Cohort. American Journal of Psychiatry, 2018. 175(11): p. 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strom M, et al. , Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes - A prospective study with long-term follow-up. Environment International, 2014. 68: p. 41–48. [DOI] [PubMed] [Google Scholar]
- 21.Cooke A, Mills TA, and Lavender T, Advanced maternal age: delayed childbearing is rarely a conscious choice a qualitative study of women’s views and experiences. Int J Nurs Stud, 2012. 49(1): p. 30–9. [DOI] [PubMed] [Google Scholar]
- 22.Grether JK, et al. , Risk of autism and increasing maternal and paternal age in a large north American population. Am J Epidemiol, 2009. 170(9): p. 1118–26. [DOI] [PubMed] [Google Scholar]
- 23.Lampi KM, et al. , Parental age and risk of autism spectrum disorders in a Finnish national birth cohort. J Autism Dev Disord, 2013. 43(11): p. 2526–35. [DOI] [PubMed] [Google Scholar]
- 24.Golding J, Steer C, and Pembrey M, Parental and grandparental ages in the autistic spectrum disorders: a birth cohort study. PLoS One, 2010. 5(4): p. e9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, et al. , Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj, 2009. 339: p. b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shor E, Roelfs D, and Vang ZM, The “Hispanic mortality paradox” revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants’ mortality. Soc Sci Med, 2017. 186: p. 20–33. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Quantitative methods in the review of epidemiologic literature. Epidemiol Rev, 1987. 9: p. 1–30. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, et al. , Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis, 2013. 12: p. 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margulis AV, et al. , Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol, 2014. 6: p. 359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan E, et al. , Comparison of Acetaminophen (Paracetamol) With Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years: A Systematic Review and Meta-analysis. JAMA Netw Open, 2020. 3(10): p. e2022398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begg CB and Mazumdar M, Operating characteristics of a rank correlation test for publication bias. Biometrics, 1994. 50(4): p. 1088–101. [PubMed] [Google Scholar]
- 32.Duval S and Tweedie R, Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 2000. 56(2): p. 455–63. [DOI] [PubMed] [Google Scholar]
- 33.An S, et al. , In-utero exposure to DDT and pyrethroids and child behavioral and emotional problems at 2 years of age in the VHEMBE cohort, South Africa. Chemosphere, 2022. 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheslack-Postava K, et al. , Maternal serum persistent organic pollutant exposure and offspring diagnosed ADHD in a national birth cohort. Environ Res, 2022. 212(Pt A): p. 113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalsager L, et al. , Maternal urinary concentrations of pyrethroid and chlorpyrifos metabolites and attention deficit hyperactivity disorder (ADHD) symptoms in 2-4-year old children from the Odense Child Cohort. Environmental Research, 2019. 176. [DOI] [PubMed] [Google Scholar]
- 36.Manley CK, et al. , Prenatal Exposure to Organophosphorus Pesticides and Preschool ADHD in the Norwegian Mother, Father and Child Cohort Study. International Journal of Environmental Research and Public Health, 2022. 19(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks AR, et al. , Organophosphate Pesticide Exposure and Attention in Young Mexican-American Children: The CHAMACOS Study. Environmental Health Perspectives, 2010. 118(12): p. 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkoski JM, et al. , In utero pyrethroid pesticide exposure in relation to autism spectrum disorder (ASD) and other neurodevelopmental outcomes at 3 years in the MARBLES longitudinal cohort. Environmental Research, 2021. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheslack-Postava K, et al. , Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicology and Teratology, 2013. 38: p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christian MA, et al. , Maternal Exposures Associated with Autism Spectrum Disorder in Jamaican Children. Journal of Autism and Developmental Disorders, 2018. 48(8): p. 2766–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hicks SD, et al. , Neurodevelopmental Delay Diagnosis Rates Are Increased in a Region with Aerial Pesticide Application. Frontiers in Pediatrics, 2017. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keil AP,Daniels JL,and Hertz-Picciotto I, Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Environmental Health, 2014. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lizé M, et al. , Prenatal exposure to organophosphate pesticides and autism spectrum disorders in 11-year-old children in the French PELAGIE cohort. Environ Res, 2022. 212(Pt C): p. 113348. [DOI] [PubMed] [Google Scholar]
- 44.Lyall K, et al. , Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environmental Health Perspectives, 2017. 125(3): p. 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philippat C, et al. , Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. International Journal of Hygiene and Environmental Health, 2018. 221(3): p. 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelton JF, et al. , Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The CHARGE Study. Environmental Health Perspectives, 2014. 122(10): p. 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Ehrenstein OS, et al. , Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. Bmj-British Medical Journal, 2019. 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eskenazi B, et al. , Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect, 2007. 115(5): p. 792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KS, et al. , The association of prenatal and childhood pyrethroid pesticide exposure with school-age ADHD traits. Environment International, 2022. 161. [DOI] [PubMed] [Google Scholar]
- 50.Ongono JS, et al. , Association between residential proximity to agricultural crops and adaptive behaviors in children with autism spectrum disorder from the French ELENA cohort. Journal of Psychiatric Research, 2022. 145: p. 197–204. [DOI] [PubMed] [Google Scholar]
- 51.Sagiv SK, et al. , Gestational Exposure to Organophosphate Pesticides and Longitudinally Assessed Behaviors Related to Attention-Deficit/Hyperactivity Disorder and Executive Function. American Journal of Epidemiology, 2021. 190(11): p. 2420–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C, Song G, and Lim W, A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere, 2019. 231: p. 326–336. [DOI] [PubMed] [Google Scholar]
- 53.Bouchard MF, et al. , Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect, 2011. 119(8): p. 1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Felice A, et al. , Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation, 2016. 13(1): p. 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pu Y, et al. , Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc Natl Acad Sci USA, 2020. 117(21): p. 11753–11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herzine A, et al. , Perinatal Exposure to Glufosinate Ammonium Herbicide Impairs Neurogenesis and Neuroblast Migration through Cytoskeleton Destabilization. Front Cell Neurosci, 2016. 10: p. 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiménez JA, et al. , Developmental pyrethroid exposure and age influence phenotypes in a Chd8 haploinsufficient autism mouse model. Sci Rep, 2022. 12(1): p. 5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maramara LA., W. He, and X. Ming, Pre-and perinatal risk factors for autism spectrum disorder in a New Jersey cohort. J Child Neurol, 2014. 29(12): p. 1645–51. [DOI] [PubMed] [Google Scholar]
- 59.Wu S, et al. , Advanced parental age and autism risk in children: a systematic review and metaanalysis. Acta Psychiatr Scand, 2017. 135(1): p. 29–41. [DOI] [PubMed] [Google Scholar]
- 60.Matta SM, Hill-Yardin EL, and Crack PJ, The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav Immun, 2019. 79: p. 75–90. [DOI] [PubMed] [Google Scholar]
- 61.Rice D and Barone S Jr., Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect, 2000. 108 Suppl 3(Suppl 3): p. 511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lenters V, et al. , Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environ Int, 2019. 125: p. 33–42. [DOI] [PubMed] [Google Scholar]
- 63.Rosenquist AH, et al. , Prenatal and Postnatal PCB-153 and ρ,ρ’-DDE Exposures and Behavior Scores at 5–9 Years of Age among Children in Greenland and Ukraine. Environ Health Perspect, 2017. 125(10): p. 107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunn GA, Nigg JT, and Sullivan EL, Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol Biochem Behav, 2019. 182: p. 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winstone JK, et al. , Glyphosate infiltrates the brain and increases pro-inflammatory cytokine TNFa: implications for neurodegenerative disorders. J Neuroinflammation, 2022. 19(1): p. 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weis GCC, et al. , Chlorpyrifos pesticide promotes oxidative stress and increases inflammatory states in BV-2 microglial cells: A role in neuroinflammation. Chemosphere, 2021. 278: p. 130417. [DOI] [PubMed] [Google Scholar]
- 67.Addae C, Cheng H, and Martinez-Ceballos E, Effect of the environmental pollutant hexachlorobenzene (HCB) on the neuronal differentiation of mouse embryonic stem cells. Int J Environ Res Public Health, 2013. 10(10): p. 5244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


