Abstract
Apolipoprotein E (ApoE) is the main cholesterol carrier of the brain and the ε4 gene variant (APOE4) is the most prevalent genetic risk factor for Alzheimer’s disease (AD), increasing risk up 15-fold. Several studies indicate that APOE4 modulates critical factors for neuronal function, including brain-derived neurotrophic factor (BDNF) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α). Both proteins show exercise-induced upregulation, which is presumed to mediate many of the beneficial effects of physical activity including improved cognition; however, there is variability in results between individuals potentially in-part due to genetic variations including APOE isoform. This study aimed to determine if the two most prevalent human APOE isoforms influence adaptive responses to exercise-training. Targeted replacement mice, homozygous for either APOE3 or APOE4 were randomized into exercised and sedentary groups. Baseline locomotor function and voluntary wheel-running behavior was reduced in APOE4 mice. Exercised groups were subjected to daily treadmill running for 8 weeks. ApoE protein in brain cortex was significantly increased by exercise in both genotypes. PGC-1α mRNA levels in brain cortex were significantly lower in APOE4 mice, and only tended to increase with exercise in both genotypes. Hippocampal BDNF protein were similar between genotypes and was not significantly modulated by treadmill running. Behavioral and biochemical variations between APOE3 and APOE4 mice likely contribute to the differential risk for neurological and vascular diseases and the exercise-induced increase in ApoE levels suggests an added feature of the potential efficacy of physical activity as a preventative and therapeutic strategy for neurogenerative processes in both genotypes.
Keywords: apolipoprotein E (APOE), exercise, brain cortex, PGC-1α, BDNF
Introduction
Apolipoprotein E (ApoE) is a glycoprotein and ligand for low density lipoprotein receptors that functions as a trafficking protein for cholesterol, lipoproteins and fat-soluble vitamins (Mahley 1988; Mahley et al. 2009). ApoE is the principal cholesterol trafficking agent of the brain where it is produced by astrocytes and expressed to a lesser extent by microglia and neurons. The ApoE gene (APOE) is located on chromosome 19q13 and has three human variants, APOEε2 (APOE2), APOEε3 (APOE3) and APOEε4 (APOE4). Relative to APOE3, which is the most common allele, carried by (1 or both alleles) approximately 70% of people, the APOE4 allele, carried by approximately 25% of people, is associated with substantially elevated risk and early onset for the development of both dementia and cardiovascular disease (Liu et al. 2019). Sedentary lifestyles are reported to add additional risk for both diseases (Yan et al. 2020, Lavie et al. 2019); as such, daily engagement in physical activity is highly regarded as preventative and therapeutic for those at risk for cardiovascular and/or neurodegenerative diseases.
Health promoting, molecular adaptations reported to be induced through exercise training include modulation of blood lipids (Muscella 2020, Costa et al. 2019); increased biosynthesis of the transcriptional co-activator and master regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) (Babaei et al. 2021; Steiner et al, 2011); and upregulation of brain-derived neurotrophic factor (BDNF) (de Sousa Fernandes et al. 2020; Malczynska-sims et al., 2020), a critical promoter of synaptic plasticity, neurogenesis and neuron survival (De Assis et al, 2018; Dinoff et al. 2017, Garza et al. 2004). There are however, inconsistencies within studies, with some subjects showing a lack of response to exercise interventions in one or more of these factors. A few studies have even reported exercise-induced decreases in BDNF expression (Babaei et al. 2014; Swift et al 2012; Goda et al 2013). APOE genetic variance is among the copious, potential confounders which may induce differences in individual exercised-induced adaptivity.
The APOE4 variant has been associated with down-regulation of brain expression of both BDNF (Liu et al. 2015; Sen et al. 2017) and PGC-1α (Yin eta l, 2020; Wu et al. 2018) and it has been demonstrated that APOE isoforms have differential impacts on BDNF secretion, metabolism and neuronal survival with APOE4 treated astrocytes showing reduced levels of BDNF (Sen et al. 2017; Iannaucci et al. 2021). There is also evidence that APOE4-associated reductions in BDNF is more pronounced in female APOE4 carriers (Alvarez et al. 2014) and females are more susceptible to mitochondrial dysfunction (Arnold et al. 2020). It is well-established that second to age, female sex is the major risk factor for Alzheimer’s disease (AD). In our previous study, we found a depressed response in exercise-induced BDNF upregulation, in a cohort of elderly African Americans carrying the APOE4 variant (most of whom were female) compared to non-APOE4 carriers (Allard et al., 2017). Additionally, a large population-based study revealed that only non-APOE4 carriers showed a cognitive benefit to engagement in non-exercise physical activity (Krell-Roesch et al. 2019). Both studies implicate APOE genotype as a critical determinant for the efficacy of exercise as a neuroprotective, therapeutic strategy. In contrast to our previous study, various exercise studies have produced promising, alternate results showing increased or at least comparable responses to exercise interventions in APOE4 carriers relative to APOE3 carriers, including higher exercise-induced increases in HDL cholesterol (Bernstein et al 2002), enhanced memory function (Deeny et al 2008, Erickson et al 2011, Coelho et al 2014), and brain wave patterns (de Frutos-Lucas et al. 2020). The goal of this study was to identify any differential impact of the two predominant human APOE isoforms on the regulation of biological adaptations to physical exercise in female, humanized APOE targeted replacement mouse models.
Materials and Methods
Animals and Housing
Thirty-two female mice homozygous for the human APOE3 or APOE4 gene, through targeted replacement of the mouse APOE gene, were purchased from Taconic Biosciences (Rensselaer, NY, USA). Mice were generated on the C57BL/6 genetic background, with the mouse APOE gene replaced with the human APOE genes under the control of murine APOE regulatory sequences. All mice were 6-months-old at baseline and 9-months-old at the completion of experimental protocols. Mice in this age range are adult but not yet categorized as aged, thereby allowing us to evaluate general differences in running activity prior to drastic age-related declines in natural running activity and motor function typically seen in aged mice (Manzanares et al. 2019).
Mouse housing conditions consisted of a 12-h light/dark cycle (lights on from 7am to 7pm) with controlled temperature (22±2°C) and humidity (50± 10 %). Mice were caged in groups of 3–4 except during the 7-day in-cage, voluntary, wheel-running assessment, during which mice were single housed in order to quantify individual running activity. Mice received ad libitum access to water and standard rodent chow (Diet no. D11112201, Research Diets, New Brunswick, NJ, USA). Body weight was measured every 2 weeks. This research was carried out with approval from the Institutional Animal Care and Use Committee (IACUC) of Howard University, and in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the NIH (8th edition, revised 2011).
Rotarod Motor Function
In order to determine any differences in motor behavior and function between the two genotypes, running activity and performance was assessed prior to the treadmill running intervention. Motor function, balance and coordination was tested using the Rotamex-5, an automated, motorized, rotating rod instrument (Columbus Instruments, Columbus, OH USA). The rotating drum (3cm in diameter) is divided into test zones, by round divider plates, allowing for simultaneous testing of 4 mice. Mice were habituated to the rotarod one day prior to testing by first being placed on the non-rotating drum for 10 seconds immediately followed by a 120-second period with the drum rotating at a constant 4 rpm. During testing, mice were placed on the rotating drum which was set to gradually accelerate from 4–40 rpm in 300 seconds. Mice were motivated to move at increasing speeds to avoid the 40 cm fall to the base of the instrument which was cushioned to prevent injury after falls. Each mouse performed 3 trials, with a 30-minute inter-trial rest interval. All mice were tested under dimly lit conditions, and at the same time of the day, limited to a 2-hr time window.
Voluntary Wheel-Running Behavior
After rotarod testing and before the 8 weeks of daily treadmill running, voluntary running behavior was evaluated for 1 week. Only mice designated for the exercised group, were evaluated for voluntary running behavior. This was to avoid any potential confounding results, as a result of 7 days of voluntary running, in mice assigned to the sedentary condition. Mice were temporarily separated out of their group housing and individually housed in cages outfitted with free spinning run-wheels, connected to a computerized automated monitoring system (Columbus Instruments; Columbus, OH, USA). The system was set to keep continuous record of the number of wheel rotations in 6-hour intervals. The 6-hour intervals were meant to coincide with the lights-on-off sequence within the vivarium; however, due to a 2hr difference on the computer time, the hourly intervals were inadvertently started at 9am instead of 7am as intended. Voluntary running behavior was recorded for 7 days; after which, mice were returned to their group housing conditions and subsequently subjected to 8 weeks of daily treadmill running.
Daily Treadmill Running
The 8-week treadmill exercise protocol was implemented after both rotarod and voluntary running assessments were completed. The use of voluntary, in cage wheel-running as the exercise intervention was initially considered; however, since running activity was significantly different between genotypes, in order to ensure equivalent levels or running activity between genotypes, the treadmill running protocol was implemented. Prior to initiation of the treadmill training protocol, mice were acclimatized to the treadmill (Columbus Instruments; Columbus, OH, USA) 10 min/day for 5 days at a speed of 3 meters(m)/minute(min). The exercise intervention consisted of 30-minutes of daily treadmill running during which the speed was increased at 1 m/minute increments, every 2 minutes to a maximum of 15 m/min. Treadmill training occurred between 7pm and 10pm, under dim lighting, for 8 weeks. To ensure that both groups were exposed to the same environments, mice in the sedentary group were also exposed to the treadmill environment daily, during the training period, but without activation of the treadmill running belt. The shock feature of the treadmill was not used. Mice that began to slow down and run along the posterior third of the running belt were encouraged to speed up toward the top of the running belt by a gentle tap of the hindquarter using a wooden tongue depressor. This modified technique has been documented to significantly reduce the stress induced by forced treadmill running (Khataei et al. 2021). See Figure 2A for a timeline illustration of the sequence of all locomotor behavior and testing procedures.
Figure 2. Motor Activity Female APOE3 and APOE4 Mice.
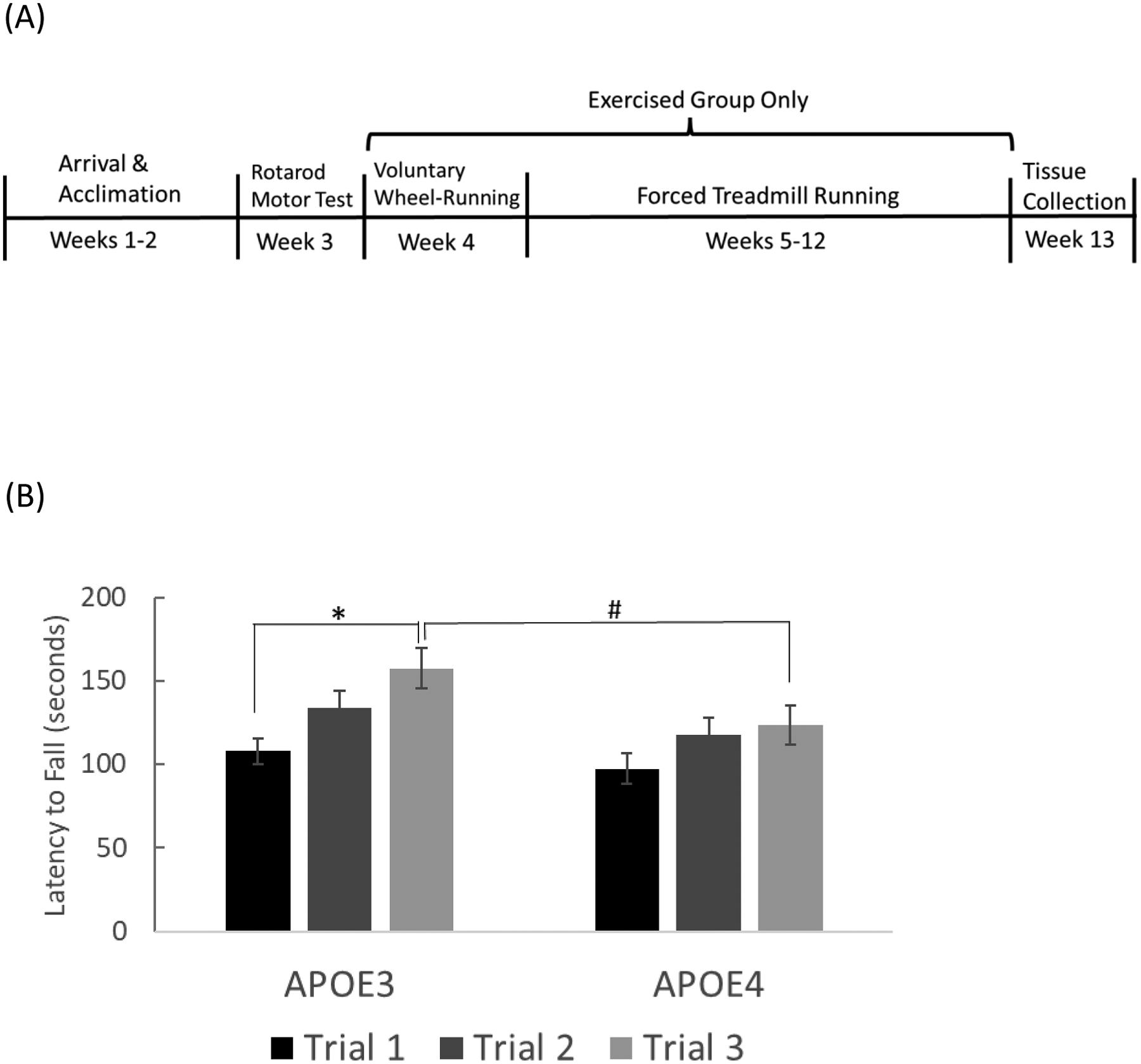

(A) Timeline of locomotor procedures (B) Locomotor performance on rotarod apparatus as denoted by latency to fall. (* p= 0.001 vs. APOE3 trial 1; # p=0.027 vs. APOE3 trial 3; n=14 for APOE3, n=16 for APOE4) (C) Averaged daily voluntary wheel-running activity in APOE3 and APOE4 mice during the 7-day period, shown in 6-hr intervals. Inserted graph shows averaged total wheel turns during the 7-day period (*p= 0.037). Error bars = ± SEM.
Sacrifice, Fasted Blood Glucose and Tissue Collection
At the completion of the 8-week treadmill running intervention mice were euthanized with Carbon dioxide CO2. Prior to sacrifice, mice were fasted for 6 hrs. Just prior to euthanasia, blood glucose was measured from a single drop extracted from a tail snip, applied to a glucose strip, and measured using a glucose meter (CVS Corp., Woonsocket, RI). Immediately, following euthanasia, blood was drawn from the inferior vena cava and allowed to clot for 60 minutes at room temperature after which serum was separated by centrifugation at 4,000 rpm at 4°C for 20 minutes. Serum was then aliquoted and stored at −80°C until use. Brain and other tissues were excised, micro dissected, flash frozen in liquid nitrogen and stored at −80°C until used.
Blood Glucose and Serum Lipid Analyses
Measurements of high-density lipoprotein (HDL), Low-density lipoprotein (LDL), total cholesterol and triglycerides were performed using a clinical biochemistry analyzer (JEOL USA Inc., Peabody, MA, USA) in accordance with the manufacturer’s protocols. Unfortunately, measurements of LDL and total cholesterol levels were not reliably detected in several samples, which prevented valid analyses.
Enzyme-Linked Immunosorbent Assay (ELISA)
Protein levels of APOE and BDNF in brain cortex and hippocampus respectively were determined by enzyme-linked immunosorbent assays (ELISAs). Hippocampi and whole cortices which were previously rapid-frozen and stored at −80°C were pulverized on dry ice and homogenized in tissue lysis buffer containing 150mM NaCl, 50mM Tris-HCl, 1% Nonidet P-40, 0.5% Sodium deoxycholate, 0.1% SDS and protease inhibitors. Homogenates were subsequently centrifuged, and the supernatant was used for ELISA detection of ApoE and BDNF protein in accordance with the manufacturer’s instruction (ABCAM, Cambridge, MA USA; Cat# ab108813 for APOE and Cat# ab212166 for BDNF). For the APOE ELISA, cortex supernatant was diluted 1:2000 in diluent buffer provided by the kit manufacturer. All samples were run in triplicates. BDNF and APOE levels were normalized to total protein concentration in each sample as determined by standard bicinchoninic acid (BCA) assay. ELISA plates were quantified using the Multiskan Sky spectrophotometer system (Thermo Fisher Scientific, Waltham, MA).
Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
To determine gene expression for BDNF and PGC-1α, total RNA from hemisected brain cortex tissue were extracted using Qiagen Universal Kit (Qiagen) in accordance with the manufacturer’s instructions. RNA quantity and quality was quantified using the Multiskan Sky μDrop plate system (Thermo Fisher Scientific, Waltham MA, USA). Reverse transcriptase (RT) was performed using 2 ug of total RNA with a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific) and qPCR utilized TaqMan Gene Expression Assay kits (Thermo Fisher Scientific) for BDNF (Mm04230607_s1), PGC-1α (Mm01208835_m1) and GAPDH (Mm99999915_g1). Each PCR reaction contained 10 μL of PCR master mix, 9 μL of diluted cDNA (diluted1:16 in Nuclease free water) and 1 μL of gene expression assay. cDNA samples were processed in triplicates for each gene. The thermal cycle protocol consisted of 2 minutes at 50°C, then 2 minutes at 95°C, followed by 40 cycles at 95°C for 15 seconds and 60 °C for 60 seconds each cycle. Real-time data were analyzed using the QuantStudio3 Real Time qPCR system software (Thermo Fisher Scientific, Waltham MA, USA). A standard curve was created using a calibrator cDNA sample, and gene expression was calculated based on threshold cycles that were converted to quantities by interpolation from the standard curves. BDNF and PGC-1α data were normalized to values obtained for the reference gene, GAPDH.
Statistical Analysis
All data were analyzed using the SigmaStat 4.0 statistical software (Systat Software Inc., San Jose, CA, USA). The number of mice used was determined using the resource equation method (Festing 2018; Arifin and Zahiruddin 2017) with the E (error degrees of freedom) value set at 20 and the number of groups equal to 4; this resulted in n=6 for each group. We added an additional 2 animals per group to account for an estimated loss of 1–2 samples in any given group. Two-way analysis of variance for genotype (APOE3 and APOE4) and activity (EX and SED) followed by the Holm-Sidak method was used to perform pairwise multiple comparisons. For comparisons of body weight and rotarod analyses, a two-way repeated measures ANOVA was used with Bonferroni post-hoc analysis. Results are presented as mean ± standard error of the mean (SEM). Differences of P<0.05 were considered significant.
Results
Body Weight
At baseline APOE4 mice weighed, on average, 10% less than APOE3 mice (t= −4.120, df= 29, P= 0.0003) with mean weights in grams (g) at baseline at 24.787 ± 0.440 and 22.313± 0.409 for APOE3 and APOE4 mice respectively (Fig. 1A). A significant bodyweight difference between the two genotypes was maintained (t=2.746, P= 0.011) after the 8-week treadmill running intervention (Fig. 1B). Exercised APOE3 mice weighed 1.89g more than sedentary APOE3, and exercised APOE4 mice weighed 0.86g less than sedentary APOE4 mice; however, activity had no statistically significant effect on bodyweight within APOE3 mice (t=1.356, p=0.186) or APOE4 mice (t=0.638, p=0.529). Activity (exercised versus sedentary) had no significant impact on final body weight (F1,27= 0.284, p= 0.599) and there was not a statistically significant interaction between activity and genotype on final body weight (F1,27 = 2.012, p =0.167).
Figure 1. Body Weight Female APOE3 and APOE4 Mice.
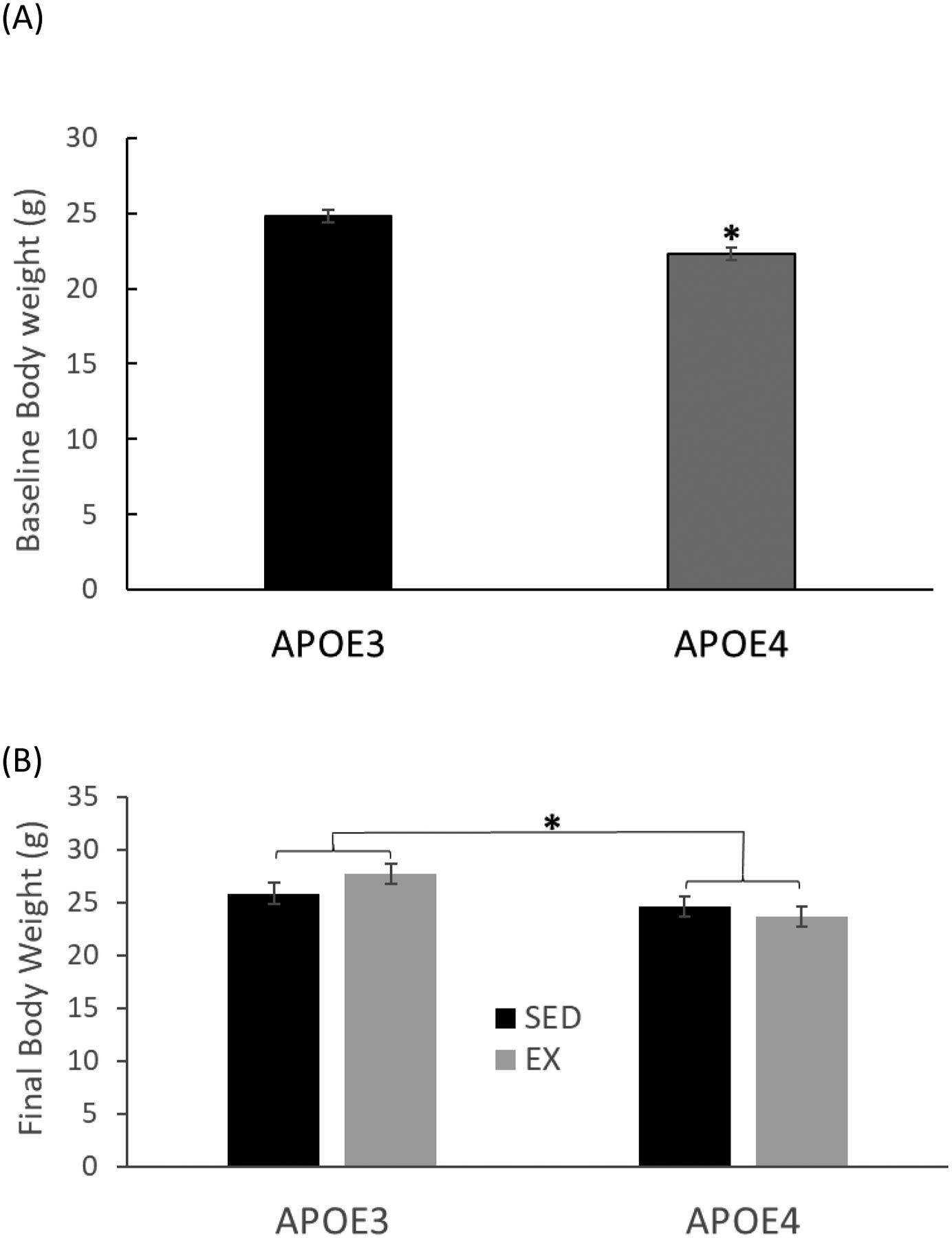
(A) Baseline body weight of 6-month-old APOE3 and APOE4 female mice (n=14 for APOE3, n=16 for APOE4, *p=0.0003). (B) Final body weight, of mice measured after the 8-week treadmill running regimen (n=7–8 per group, *p = 0.011, APOE3 vs. APOE4). Error bar = ± standard error of mean (SEM), SED = sedentary group, EX = exercised group
Locomotor Performance and Activity
To clarify the sequence and duration of motor behavior assessments, a timeline of events is shown in figure 1A. Rotarod performance significantly differed (t= 2.021, df=28, p= 0.027) between APOE3 and APOE4 mice during the third trial, with latency to fall measuring 157 ± 11.9 seconds for APOE3 mice and 123.6 ± 11.6 seconds for APOE4 mice (Fig. 2B). A repeated measures 2-Way ANOVA revealed a significant difference between trials (F2,28=10.289, P< 0.001) with APOE3 mice demonstrating significantly improved performance times on the 3rd trial compared to the first trial (t=4.485, p<0.001), but no significant differences between trials 1 and 2 (t=2.347, p=0.068) or trials 2 and 3 (t=2.138, p<0.112). APOE4 mice showed no statistically significant improvement between any trials (trial 1 vs. 3, t=1.792, p= 0.237; trial 1 vs. 2, t=1.985, p=0.157; trial 2 vs. 3, t=0.0168, p=1.000).
Voluntary running activity was measured from in-cage running wheels and logged by a computerized system, as number of wheel-turns in 6-hour intervals (Fig. 2C). Both genotypes showed similar circadian patterns of running activity, with the vast majority of running activity occurring during the lights out period, for the 7- day voluntary running trial. APOE4 mice covered, on average 75,042± 15,494 wheel-turns, which was 36% less than APOE3 mice (117,239± 14,584-wheel turns) (t= 1.98, df= 11, P = 0.037) during the 7 days.
ApoE Levels in Brain Cortex
Analysis of ELISA results identified a significant effect of activity (F1,26= 9.596, p= 0.005) and a strong trend toward a difference between genotypes (F1,26= 3.962, p=0.057) on levels of ApoE protein in brain cortex. Exercise-induced, 38.1% (p= 0.029) and 38.7% (p= 0.050) increase in cortex ApoE levels for APOE3 and APOE4 mice, respectively (Fig. 3A). Levels of cortex ApoE were lower in SED APOE4 mice compared to SED APOE3 mice; however, that difference did not reach statistical significance.
Figure 3. Gene Expression of APOE, BDNF and PGC-1α in Brain Cortical Tissue of Female APOE3 and APOE4 mice.


(A) APOE protein levels in whole cortex brain tissue (*= significantly different from SED, p= 0.005) (B) BDNF protein in hippocampal tissue (C) PGC-1α mRNA in brain cortex (*= Significantly different by genotype, p = 0.002). (n=5–8 per group)
BDNF Levels in Hippocampus
Hippocampal BDNF protein as measured via ELISA showed no significant differences between genotypes (F1,26 = 1.094, p= 0.31) or activity (F1,26 = 0.57, p= 0.46) groups (Fig 3B) and there was no significant interaction between genotype and activity (F1,26 = 0.61, p= 0.44). BDNF levels (ng/μg total protein) in APOE3 groups averaged 739.29± 59.2 and 996.43± 271.2 for SED and EX mice respectively. BDNF levels in APOE4 groups averaged 694.93± 61.7 and 690.59± 59.3 for mice in SED and EX groups respectively.
PGC-1α Gene Expression in Cortex
Two-way ANOVA analysis of the impact of activity and genotype on PGC-1α mRNA levels in brain cortex revealed significant differences between genotypes (F1,24= 12.119 p = 0.002) with lower levels in APOE4 mice (0.697 ± 0.0493) compared to APOE3 mice (0.948 ± 0.0526). Exercise only tended to increase PGC-1α levels (F1,24= 3.08 p = 0.093), and there was no significant interaction between genotype and activity (F1,24= 0.53, p = 0.474).
Blood Glucose and Serum Lipids
Blood glucose and serum lipids data are shown in table 1. Fasted blood glucose levels did not significantly differ by genotype (F1,26 = 1.126, p= 0.298) or activity (F1,26 = 0.889, p= 0.355). Serum HDL also did not differ by genotype (F1,23 = 0.153, p= 0.699) or activity (F1,23 = 0.154, p= 0.699). Likewise, serum triglycerides levels were similar between genotypes (F1,22 = 0.518, p= 0.479) and activity groups (F1,22 = 0.569, p= 0.459).
Table 1.
Fasted Blood Glucose and Serum Lipids Female APOE3 and APOE4 mice.
| Parameters (mg/dl) | APOE3 SED (n=5) | APOE3 EX (n=8) | APOE4 SED (n=7) | APOE4 EX (n=6) | P-value Genotype | P-value Activity |
|---|---|---|---|---|---|---|
| Blood Glucose | 153.7±9.2 | 162.7±11.3 | 140.9±10.1 | 152.4±9.0 | 0.298 | 0.355 |
| Serum HdL | 46.7±3.4 | 49.3±5.5 | 45.9±1.5 | 46.7±4.7 | 0.699 | 0.699 |
| Serum TG | 72.3±6.4 | 62.8±4.7 | 63.1±9.5 | 60.6±9.7 | 0.479 | 0.459 |
Values are mean± standard error. TG: triglycerides, HdL: High density lipoprotein, SED: sedentary group, EX: exercised group (n=5–8 per group)
Discussion
In this study, we demonstrated that a 30-minute daily treadmill running regimen increased ApoE levels in brain cortex of humanized, female APOE targeted replacement mice. To our knowledge, this is the first report to show an exercise-induced modulation of ApoE protein in brain or any tissue. Other studies have demonstrated that various mechanical and chemical stressors lead to increased synthesis of ApoE in brain and particularly in neurons (Aoki et al. 2003; Boschert et al. 1999, Yin et al. 2012). Our use of forced treadmill exercise, despite not utilizing the shock feature of the treadmill, may have induced some level of stress in the animals which may have also attributed to the increased ApoE levels. Lower ApoE levels are typically reported in Alzheimer’s patients and therefore has been associated with increased risk for the disease (Wang et al. 2014). A recent study however, revealed decreased ApoE levels in AD patients relative to those with mild cognitive impairment (MCI). The authors suggest that the higher levels of APOE in MCI patients is indicative of cells programmed for death, while the lowered levels in AD is indicative of cell loss (Zalocusky et al 2021). There are conflicting reports on the impact of APOE upregulation. Whether increased ApoE is indicative of beneficial functional outcomes likely depends not only on the APOE variant but also on the cellular source of ApoE protein expression. Although ApoE protein, mainly synthesized by astrocytes and microglia, has been shown in some studies to have neuroprotective functions including providing an avenue for clearance of β-Amyloid (Castellano et al., 2011; Chai et al. 2021); neuron-derived ApoE (most especially ApoE4) is subject to high rates of proteolytic cleavage resulting in neurotoxic fragments which activate neurodegenerative pathways (Brecht et al. 2004; Tamboli et al. 2014, Liang et al. 2019, Mahley and Huang 2012). In addition, ApoE4 has been shown to impair hippocampal neurogenesis (Tensaouti et al. 2018) and exacerbate tau pathology in mouse models (Shi et al. 2017). Alternatively, a recent study suggests that APOE4 derived from microglia is potentially neuroprotective provided that there is a deficit of astrocyte-derived ApoE4 (Wang et al. 2021). Another recent study, reported that neuroprotective effects of an I2-Imidazoline ligand was associated with increased astrocytic ApoE expression in mice (Mota et al. 2022). ApoE expression may have different functional consequences governed by the specific gene variant, cell-type expression, susceptibility to proteolytic cleavage and other variables. Additional investigation is required to determine the cellular source of exercise-induced ApoE synthesis and whether or not exercise affects cleavage of ApoE and the generation of toxic ApoE fragments.
Our result of lower baseline levels of cortical ApoE levels in APOE4 mice relative to APOE3 mice did not reach statistical significance, however; numerous studies have determined that APOE4 carriers have lower ApoE protein levels in hippocampus, neocortex, cerebrospinal fluid (CSF), serum and plasma (Ramaswamy et al. 2005; Bertand et al. 1995; Riddell et al. 2008; Sullivan et al. 2011; Cruchaga et al. 2012; Martinez-Morillo et al. 2014).
Locomotor rotarod performance and voluntary wheel running behavior differed significantly between APOE3 and APOE4 mice at baseline. This implicates APOE isoform as a determinant factor in motor speed and/or endurance capacity. Our finding of decreased locomotor behavior in APOE4 mice is supported by studies which have reported a generalized decrease in motor function in transgenic, humanized APOE4 mice (Kornecook et al. 2010; Huber et al. 2000, Chaudhari 2020). Furthermore, a related study identified reduced gait speed in APOE4 patients with mild cognitive impairment (Doi et al. 2015). In contrast, one study reported similar levels of voluntary running activity in older (10–12month old) APOE4 transgenic mice relative to APOE3 mice (Nichol et al. 2009). Interestingly, a genome-wide association study of habitual activity identified the APOE gene as a genetic factor influencing physical activity behavior (Klimentidis et al. 2018). Overall, voluntary running behavior in rodents is connected to both energy balance and reward circuitry of the brain, which in turn likely influences motor function and behavior. Our finding of lowered PGC-1 α levels in APOE4 brain cortex may be related to an overall altered energy balance state influencing the reward circuitry and thus voluntary running behavior in APOE4 mice.
To date, our study is one of two studies to report reduced gene expression for PGC-1α, an essential transcription factor for mitochondrial biogenesis levels, in brain tissue of APOE4 mice relative to APOE3 mice. The previous report (Yin et al 2019) showed lowered PGC-1α protein levels in brain tissue and cultured neurons from APOE4 transgenic mice relative to APOE3 mice. The same group also examined post-mortem brains and found that mitochondrial biogenesis including PGC-1alpha protein levels were reduced in APOE4 carriers relative to non-APOE4 carriers (Yin et al, 2020). Wu et al. 2018, used ingenuity pathway analysis of brain tissue from female transgenic APOE mice to surmise an inhibitory effect of the APOE4 variant on the PGC-1α signaling pathway. Our result of lowered PGC-1α mRNA levels in the brains of APOE4 mice relative to APOE3 mice demonstrates and confirms that interconnection. PGC-1α levels in skeletal muscle were not measured in this study and would indicate a more direct connection to the reduced motor function in APOE4 mice. Future measures of PGC-1α in skeletal muscle of APOE3 and APOE4 mice are necessary. Low levels of PGC-1α have been reported in patients with Alzheimer’s disease (Sweeney & Song 2016; Win et al. 2009) and another study has shown decreased protein and mRNA levels of PGC-1α in the brains of aged mice and humans (Reutzel et al. 2020). Interestingly, Alzheimer’s disease has been associated with decreased gait speed and motor dysfunction (Dyer et al. 2020) which has also been demonstrated in mouse models of the disease (O’Leary et al. 2018). Although we did not find a statistically significant exercise-induced change in PGC-1α transcription; the exercised group of both genotypes did trend toward increased levels and supporting studies have shown exercise-induced increases in brain levels of PGC-1α (Babaei et al. 2021; Azimi et al. 2018; Steiner et al. 2011). All together support is strong for a significant role of ApoE in mitochondrial regulation, implicating APOE4 in reduced mitochondrial function and providing a plausible explanation for the reduced motor function and running activity we report in APOE4 mice.
Exercise is considered a highly cardio-protective behavior known to attenuate elevated triglycerides and LDL/HDL ratios, thereby reducing cardiovascular disease risk. Our results show no impact of exercise or APOE variance on serum HdL or triglycerides which are inconsistent with several human studies that have reported lowered levels of HdL and/or triglycerides in carriers of APOE4 (Li et al. 2019; Taimela et al. 1996; Hopkins et al. 2002). There is a deficit of reports comparing the impact of APOE variances on the efficacy of exercise in altering blood lipid levels in transgenic animal models with at least one that also failed to find any association between APOE genotype and HDL (Rahilly-Tierney et al. 2011).
A small but statistically significant difference in bodyweight between APOE3 and APOE4 mice implicates metabolic differences between the two genotypes as previously reported (Arbones-Mainar et al. 2016). Arbones-Mainar demonstrated resistance to western diet-induced weight gain in the APOE4 due to increased lipid oxidation and thermogenesis. In our study, metabolic divergence seems to be enhanced by increased activity as exercised APOE3 mice trended towards increased bodyweight, while exercised APOE4 mice trended towards lower body weight.
BDNF is a critical factor for memory and other cognitive functions and it is well-documented that aerobic activity particularly running, induces an upregulation of BDNF (Erickson et al. 2014; Coelho et al. 2014). Several studies have reported lower BDNF levels associated with APOE4 (Chhibber & Zhao 2017; Liu et al. 2015; Sen et al. 2017). Although this study found no significant differences in hippocampal BDNF protein between genotypes or activity groups, APOE4 mice tended to have lower BDNF levels relative to APOE3 mice and exercised APOE3 mice trended toward increased BDNF levels while exercised APOE4 mice showed no trend. A similar study (Nichol K et al. 2009) reported that APOE4 and APOE3 mice showed comparative increases in BDNF protein in response to 6-weeks of voluntary run-wheel activity. There is important and considerable difference in the amount of running activity mice attain via voluntary running and that of the forced treadmill running paradigm implemented in our study, which may not have been adequate to induce significant increases in BDNF. Additional studies on the influence of exercise duration and intensity on BDNF regulation is needed.
Conclusion
In summary, apoe3 and apoe4 mice showed differences in baseline molecular and motor function measures, but a similar exercise-induced upregulation of cortical ApoE levels. Recent studies have indicated an exercise-induced neuroprotective effect for both APOE3 and APOE4 genotypes and a recent review on the effects of physical activity on cognitive decline in APOE4 carriers concludes with the view of an overall neuroprotective effect of physical activity for APOE4 carriers (Perez-Lasierra et al. 2021). Still the functional consequences of increased brain levels of APOE remains unsettled. Our finding of increased APOE brain cortex levels in response to exercise in both APOE4 and APOE3 mice may be associated with differential neuroprotective effects based on genotype and requires additional investigation.
Acknowledgements
We thank and are grateful to Dr. David Baer and Ms. Theresa Henderson of the Food Component & Health Laboratory at the USDA for providing serum biochemical analysis services.
This research was supported by NIH grants R25AG047843, 5R03AG049288 and 2U54MD007597-31 The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Allard JS, Ntekim O, Johnson SP, Ngwa JS, Bond V, Pinder D, Gillum RF, Fungwe TV, Kwagyan J, Obisesan TO. (2017) APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: A pilot study. Exp Gerontol; 87(Pt A),129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A, Aleixandre M, Linares C, Masliah E, Moessler H. (2014) Apathy and APOE4 are associated with reduced BDNF levels in Alzheimer’s disease. J Alzheimers Dis;42(4):1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbones-Mainar JM, Johnson LA, Torres-Perez E, Garcia AE, Perez-Diaz S, Raber J, Maeda N. (2016) Metabolic shifts toward fatty-acid usage and increased thermogenesis are associated with impaired adipogenesis in mice expressing human APOE4. International Journal of Obesity; 40, 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arifin WN, Zahiruddin WM. (2017) Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 24(5):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi M, Gharakhanlou R, Naghdi N, Khodadadi D, Heysieattalab S. (2018) Moderate treadmill exercise ameliorates amyloid-beta-induced learning and memory impairment, possibly via increasing AMPK activity and up-regulation of the PGC-1alpha/FNDC5/BDNF pathway. Peptides. Apr;102:78–88. [DOI] [PubMed] [Google Scholar]
- Aoki K, Uchihara T, Sanjo N, Nakamura A, Ikeda K, Tsuchiya K, Wakayama Y. (2003) Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke Apr;34(4):875–80. [DOI] [PubMed] [Google Scholar]
- Babaei P, Damirchi A, Mehdipoor M, Tehrani BS. (2014) Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci Lett. Apr 30;566:304–8. [DOI] [PubMed] [Google Scholar]
- Babaei A, Nourshahi M, Fani M, Entezari Z, Jameie SB, Haghparast A. (2021) The effectiveness of continuous and interval exercise preconditioning against chronic unpredictable stress: Involvement of hippocampal PGC-1alpha/FNDC5/BDNF pathway. J Psychiatr Res. Apr;136:173–183. [DOI] [PubMed] [Google Scholar]
- Bernstein MS, Costanza MC, James RW, Morris MA, Cambien F, Raoux S, Morabia A. (2002) Physical activity may modulate effects of ApoE genotype on lipid profile. Arterioscler Thromb Vasc Biol. Jan;22(1):133–40. [DOI] [PubMed] [Google Scholar]
- Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. (1995) Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res Mol Brain Res. Oct;33(1):174–8. [DOI] [PubMed] [Google Scholar]
- Boer JM, Ehnholm C, Menzel HJ, Havekes LM, Rosseneu M, O’Reilly DS, et al. (1997) Interactions between lifestyle-related factors and the ApoE polymorphism on plasma lipids and apolipoproteins. The EARS Study. European Atherosclerosis Research Study. Arterioscler Thromb Vasc Biol;17(9):1675–81. [DOI] [PubMed] [Google Scholar]
- Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. (1999) Apolipoprotein E expression by neurons surviving excitotoxic stress Neurobiol Dis. Dec;6(6):508–14. [DOI] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu G, Xu Q, Fish JD, Wyss-Coray T, Manuel M, Mucke L, Mahley RW, Huang Y. (2004) Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. Mar 10;24(10):2527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM. (2011) Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. Jun 29;3(89):89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai AB, Lam HHJ, Kockx M, Gelissen IC. (2021) Apolipoprotein E isoform-dependent effects on the processing of Alzheimer’s amyloid-β. Biochim Biophys Acta Mol Cell Biol Lipids. Sep;1866(9):158980. [DOI] [PubMed] [Google Scholar]
- Chaudhari K, Wong JM, Vann PH, Como T, et al. (2020) ApoE Genotype-Dependent Response to Antioxidant and Exercise Interventions on Brain Function. [DOI] [PMC free article] [PubMed]
- Chhibber A, Zhao L. ERβ and ApoE isoforms interact to regulate BDNF-5-HT2A signaling and synaptic function in the female brain. Alzheimers Res Ther. 2017. Sep 21;9(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodorov E, Santos-Galduróz RF. (2014) Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer’s disease. J Alzheimers Dis;39(2):401–8. [DOI] [PubMed] [Google Scholar]
- Costa RR, Buttelli ACK, Vieira AF, Coconcelli L, Magalhães RL, Delevatti RS, Kruel LFM. (2019) Effect of Strength Training on Lipid and Inflammatory Outcomes: Systematic Review with Meta-Analysis and Meta-Regression. J Phys Act Health. Jun 1;16(6):477–491. [DOI] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JS, Nowotny P, Bales K, Pickering EH, Mayo K, Bertelsen S, Hinrichs A; (2012) Alzheimer’s Disease Neuroimaging Initiative, Fagan AM, Holtzman DM, Morris JC, Goate AM. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Hum Mol Genet. Oct 15;21(20):4558–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Assis GG, Gasanov EV, de Sousa MBC, Kozacz A, Murawska-Cialowicz E. (2018) Brain derived neutrophic factor, a link of aerobic metabolism to neuroplasticity. J Physiol Pharmacol. Jun;69(3). [DOI] [PubMed] [Google Scholar]
- Deeny SP, Poeppel D, Zimmerman JB, Roth SM, Brandauer J, Witkowski S, Hearn JW, Ludlow AT, Contreras-Vidal JL, Brandt J, Hatfield BD. (2008) Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. May;78(2):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frutos-Lucas J, Cuesta P, Ramirex-Toraño F, Nebreda A, Cuadrado-Soto E, Peral-Suárez Á, Lopez-Sanz D, Bruña R, Marcos-de Pedro S, Delgado-Losada ML, López-Sobaler AM, Concepción Rodríguez-Rojo I, Barabash A, Serrano Rodriguez JM, Laws SM, Dolado AM, López-Higes R, Brown BM, Maestú F. (2020) Age and APOE genotype affect the relationship between objectively measured physical activity and power in the alpha band, a maker of brain disease. Alzheimers Res Ther. Sp 22; 12(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Fernandes MS, Ordônio TF, Santos GCJ, Santos LER, Calazans CT, Gomes DA, Santos TM. (2020) Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. Dec 14;2020:8856621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinoff A, Herrmann N, Swardfager W, Lanctôt KL (2017) The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. Jul;46(1):1635–1646. [DOI] [PubMed] [Google Scholar]
- Doi T, Shimada H, Makizako H, Tsutsumimoto K, Uemura K, Suzuki T. (2015) Apolipoprotein E genotype and physical function among older people with mild cognitive impairment. Geriatr Gerontol Int. Apr;15(4):422–7. [DOI] [PubMed] [Google Scholar]
- Dyer AH, Lawlor B, Kennelly SP; NILVAD Study Group. (2020) Gait speed, cognition and falls in people living with mild-to-moderate Alzheimer disease: data from NILVAD. BMC Geriatr. Mar 30;20(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ. (2010) Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. May 15; 588(Pt 10):1779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA. (2012) The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist; 18:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A; 108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festing MFW. (2018) On determining sample size in experiments involving laboratory animals. Laboratory Animals;52(4): 341–350. [DOI] [PubMed] [Google Scholar]
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. (2004) Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. Feb;77(2):209–20. [DOI] [PubMed] [Google Scholar]
- Goda A, Ohgi S, Kinpara K, Shigemori K, Fukuda K, Schneider EB. (2013) Changes in serum BDNF levels associated with moderate-intensity exercise in healthy young Japanese men. Springerplus. Dec 18;2:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. (2011) Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav; 104:934–941. [DOI] [PubMed] [Google Scholar]
- Hopkins PC, Huang Y, McGuire JG, Pitas RE. (2002) Evidence for differential effects of apoE3 and apoE4 on HDL metabolism. Evidence for differential effects of apoE3 and apoE4 on HDL metabolism. J Lipid Res. Nov;43(11):1881–9. [DOI] [PubMed] [Google Scholar]
- Huber G, März W, Martin JR, Malherbe P, Richards JG, Sueoka N, Ohm T, Hoffmann MM. (2000) Characterization of transgenic mice expressing apolipoprotein E4(C112R) and apolipoprotein E4(L28P; C112R). .Neuroscience.;101(1):211–8. [DOI] [PubMed] [Google Scholar]
- Iannucci J, Sen A, Grammas P. (2021) Isoform-Specific Effects of Apolipoprotein E on Markers of Inflammation and Toxicity in Brain Glia and Neuronal Cells in Vitro. Curr Issues Mol Biol May 27;43(1)215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khataei T, Romig-Martin SA, Harding AMS, Radley JJ, Benson CJ. (2021) Comparison of murine behavioural and physiological responses after forced exercise by electrical shock versus manual prodding. Experimental Phsiology; 106(4);812–819. [DOI] [PubMed] [Google Scholar]
- Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. (2018) Genome-wide association study of habitual physical activity in over 377,000 UK biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes;42(6):1161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornecook TJ, McKinney AP, Ferguson MT, Dodart J-C. (2010) Isoform-specific effects of apolipoprotein E on cognitive performance in targeted-replacement mice overexpressing human APP. Genes Brain Behav.; Mar 1;9(2):182–92. [DOI] [PubMed] [Google Scholar]
- Krell-Roesch J, Syrjanen JA, Vassilaki M, Barisch-Fritz B, et al. (2019) Association of non-exercise physical activity in mid-and late-life with cognitive trajectories and the impact of APOEe4 genotype status: the Mayo Clinic Study of Aging. Eur J Ageing.;Apr 12;16(4):491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. (2019) Sedentary Behavior, Exercise, and Cardiovascular Health. Circ Res. Mar;124(5):799–815. [DOI] [PubMed] [Google Scholar]
- Li W, Li Y, Qiu Q, Sun L, Yue L, Li X and Xiao S. (2019) Associations Between the Apolipoprotein E ε4 Allele and Reduced Serum Levels of High Density Lipoprotein a Cognitively Normal Aging Han Chinese Population. Front. Endocrinol; 10:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Xue F, Hang W, Wen B, Zhang Q, Chen J, Liu X, Chen J (2019).. Neuron-Specific Apolipoprotein E4 (1–272) Fragment Induces Tau Hyperphosphorylation and Axonopathy via Triggering Endoplasmic Reticulum Stress. J Alzheimers Dis.;71(2):597–611. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu J, Weng R, Gu X, Zhong Z. (2019) Apolipoprotein E gene polymorphism and the risk of cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord. Sep 14;19(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Jiao SS, Wang YR, Bu XL, Yao XQ, Xiang Y, Wang QH, Wang L, Deng J, Li J, Zhou XF, Zhou HD, Wang YJ. (2015) Associations Between ApoEepsilon4 Carrier Status and Serum BDNF Levels-New Insights into the Molecular Mechanism of ApoEepsilon4 Actions in Alzheimer’s Disease. Mol Neurobiol.;51(3):1271–7. [DOI] [PubMed] [Google Scholar]
- Mahley RW. (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. Apr 29;240(4852):622–30. [DOI] [PubMed] [Google Scholar]
- Mahley RW and Huang Y. (2012) Apolipoprotein E set the Stage: Response to Injury Triggers Neuropathology, Including Alzheimer’s Disease. Neuron. December 6; 76(5): 871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. (2009) Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. Apr;50 (Suppl):S183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares G, Brito-da-Silva G, Gandra PG. (2019) Voluntary wheel running: patterns and physiological effects in mice. Bra j Med biol Res: 52(1):e7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małczyńska-Sims P, Chalimoniuk M, Sułek A. (2020) The Effect of Endurance Training on Brain-Derived Neurotrophic Factor and Inflammatory Markers in Healthy People and Parkinson’s Disease. A Narrative Review Front Physiol Nov 19;11:578981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Morillo E, Hansson O, Atagi Y, Bu G, Minthon L, Diamandis EP, Nielsen HM. (2014) Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls. Acta Neuropathol. May;127(5):633–43. [DOI] [PubMed] [Google Scholar]
- Muscella A, Stefàno E, Marsigliante S. (2020) The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Heart Circ Physiol. Jul 1;319(1):H76–H88. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. (1996) Physical activity increases mRNA for brain derived neurotrophic factor and nerve growth factor in rat brain. Brain Res; 726:49–56. [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. (2009) Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. Jul;5(4):287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg NB, Lundby C, Leick L, Pilegaard H. (2010) Relative workload determines exercise-induced increases in PGC-1alpha mRNA. Med Sci Sports Exerc. Aug; 42(8):1477–84. [DOI] [PubMed] [Google Scholar]
- O’Leary TP, Robertson A, Chipman PH, Rafuse VF, Brown RE (2018) Motor function deficit in the 12 month-old female 5xFAD mouse model of Alzheimer’s disease. Behav Brain Res. Jan 30;337:256–263. [DOI] [PubMed] [Google Scholar]
- Perez-Lasierra JL, Casajús JA, Casasnovas JA, Arbones-Mainar JM, Lobo A, Lobo E, Moreno-Franco B, Gonzalez-Agüero A. (2021) Can Physical Activity Reduce the Risk of Cognitive Decline in Apolipoprotein e4 Carriers? A Systematic Review. Int J Environ Res Public Health. Jul 6;18(14):7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. (2009) PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. Mar;66(3):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Darweesh SKL, Savica R. (2021) Physical Activity May Reduce Apolipoprotein E4-Associated Cognitive Decline in Parkinson Disease. May 11;96(19):877–878. [DOI] [PubMed] [Google Scholar]
- Rahilly-Tierney CR, Arnett DK, North KE, Pankow JS, Hunt SC, Ellison RC, Gaziano JM, Djoussé L. (2011) Apolipoprotein ε4 polymorphism does not modify the association between body mass index and high-density lipoprotein cholesterol: a cross-sectional cohort study. Lipids Health Dis. Sep 23;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. (2005) Effect of domain interaction on apolipoprotein E levels in mouse brain. J. Neurosci; 25:10658–10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. (2009) Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. Oct;94(10):1062–9. [DOI] [PubMed] [Google Scholar]
- Reutzel M, Grewal R, Dilberger B, Silaidos C, Joppe A, Eckert GP. (2020) Cerebral Mitochondrial Function and Cognitive Performance during Aging: A Longitudinal Study in NMRI Mice. Oxid Med Cell Longev. Apr 13;2020:4060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. (2008) Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. Nov 5;28(45):11445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifer T, Schulte S, Hollmann W, Bloch W, Struder HK. (2009) Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res; 41, 250–254. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Webb DL & Hansen RA (2013) The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J Sports Sci Med; 12, 502–511. [PMC free article] [PubMed] [Google Scholar]
- Sen A, Nelson TJ, Alkon DL. (2017) ApoE isoforms differentially regulates cleavage and secretion of BDNF. Mol Brain. Jun 1;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, et al. (2017). Alzheimer’s Disease Neuroimaging Initiative: ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. (2011) Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol (1985). Oct;111(4):1066–71. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Han B, Liu F, Mace BE, Ervin JF, Wu S, Koger D, Paul S, Bales KR. (2011) Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging. May;32(5):791–801. [DOI] [PubMed] [Google Scholar]
- Sweeney G, Song J. (2016) The association between PGC-1α and Alzheimer’s disease. Anat Cell Biol. Mar;49(1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, Church TS. (2012) The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes PLoS One;7(8):e42785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadaishi M, Miura S, Kai Y, Kawasaki E, Koshinaka K, Kawanaka K, Nagata J, Oishi Y, Ezaki O. (2011) Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1α mRNA: a role of β2-adrenergic receptor activation. Am J Physiol Endocrinol Metab. Feb; 300(2):E341–9. [DOI] [PubMed] [Google Scholar]
- Taimela S, Lehtimaki T, Porkka KV, Rasanen et al. (1996) The effect of physical activity on serum total and low-density lipoprotein cholesterol concentrations varies with apolipoprotein E phenotype in male children and young adults: The Cardiovascular Risk in Young Finns Study. Metabolism. Jul;45(7):797–803. [DOI] [PubMed] [Google Scholar]
- Tamboli IY, Heo D, Rebeck GW. (2014) Extracellular proteolysis of apolipoprotein E (apoE) by secreted serine neuronal protease PLoS One. Mar 27;9(3):e93120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tensaouti Y, Stephanz EP, Yu TS, Kernie SG. (2018) ApoE Regulates the Development of Adult Newborn Hippocampal Neurons. eNeuro Aug 2;5(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xiong M, Gratuze M, Bao X, Shi Y, Andhey PS, Manis M, Schroeder C, Yin Z, Madore C, et al. (2021). Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron 109, 1657–1674.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yu JT, Wang HF, Jiang T, Tan CC, Meng XF, Soares HD, Tan L. (2014) Meta-analysis of peripheral blood apolipoprotein E levels in Alzheimer’s disease. PLoS One. Feb 18;9(2):e89041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE & Shannon Weickert C (2006) BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns; 6, 941–951. [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang X, Zhao L. (2018) Human ApoE Isoforms Differentially Modulate Brain Glucose and Ketone Body Metabolism: Implications for Alzheimer’s Disease Risk Reduction and Early Intervention. alvarezJ Neurosci. Jul 25;38(30):6665–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Fu W, Wang C, Mao J, Liu B, Zou L, Lv C. (2020) Association between sedentary behavior and the risk of dementia: a systematic review and meta-analysis. Transl Psychiatry. Apr 21;10(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Zhou S, Jiang L, Sun X. (2012) Mechanical injured neurons stimulate astrocytes to express apolipoprotein E through ERK pathway Neurosci Lett. Apr 25;515(1):77–81. [DOI] [PubMed] [Google Scholar]
- Yin J, Nielsen M, Carcione T, Li S, Shi J. (2019) Apolipoprotein E regulates mitochondrial function through the PGC-1alpha-sirtuin 3 pathway. Aging (Albany NY). Dec 6;11(23):11148–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Reiman EM, Beach TG, Serrano GE, Sabbagh MN, Nielsen M, Caselli RJ, Shi J. (2020) Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology. Jun 9;94(23):e2404–e2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalocusky KA, Najm R, Taubes AL, Hao Y, et al. (2021) Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease. Nat Neurosci. Jun;24(6):j786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


