Abstract
Background:
Opioid-related deaths continue to rise in the U.S. A shared decision-making (SDM) system to help primary care clinicians (PCCs) identify and treat patients with opioid use disorder (OUD) could help address this crisis.
Methods:
In this cluster-randomized trial, primary care clinics in three healthcare systems were randomized to receive or not receive access to an OUD-SDM system. The OUD-SDM system alerts PCCs and patients to elevated risk of OUD and supports OUD screening and treatment. It includes guidance on OUD screening and diagnosis, treatment selection, starting and maintaining patients on buprenorphine for waivered clinicians, and screening for common comorbid conditions. The primary study outcome is, of patients at high risk for OUD, the percentage receiving an OUD diagnosis within 30 days of index visit. Additional outcomes are, of patients at high risk for or with a diagnosis of OUD, (a) the percentage receiving a naloxone prescription, or (b) the percentage receiving a medication for OUD (MOUD) prescription or referral to specialty care within 30 days of an index visit, and (c) total days covered by a MOUD prescription within 90 days of an index visit.
Results:
The intervention started in April 2021 and continues through December 2023. PCCs and patients in 92 clinics are included; study results are expected in 2024.
Conclusion:
This protocol paper describes the design of a multi-site trial to help PCCs recognize and treat OUD. If effective, this OUD-SDM intervention could improve screening of at-risk patients and rates of OUD treatment for people with OUD.
Keywords: Opioid use disorder, opioid overdose, primary care, shared decision-making, pragmatic clinical trial
INTRODUCTION:
The opioid overdose epidemic in the United States continues to worsen.[1] From April 2020 to April 2021 there were 75,673 opioid-related overdose deaths, up from 56,064 the previous year, driven largely by a rise in synthetic opioid overdoses.[1] An estimated 10.1 million Americans misuse opioids, but only about 20% of Americans diagnosed with opioid use disorder (OUD) ultimately seek treatment, and of those, only 25% receive a medication to treat OUD (MOUD), the recommended treatment for OUD.[2, 3]
Primary care is the most common point of healthcare contact, making primary care clinicians (PCCs) well-positioned to reduce this OUD treatment gap. Similar to other chronic diseases, PCCs have a responsibility to diagnose and treat OUD, particularly given that the need for OUD treatment exceeds the capacity of specialty care clinicians.[4, 5] Additionally, PCCs are well-suited to identify and treat common comorbidities, including mental health conditions, chronic pain and infectious disease.[6–9] Buprenorphine is an effective OUD treatment that is available in primary care, but only PCCs with a Drug Addiction Treatment Act of 2000 waiver are authorized to prescribe buprenorphine for OUD.[10–16] Importantly, even when clinicians become waivered, only half ever actually prescribe buprenorphine.[17] Clinicians report barriers to prescribing buprenorphine including time constraints, poor access to clinical guidelines, stigma, and lack of institutional support, staff training and confidence.[18–22] There have been a handful of studies examining treatment of OUD in primary care, although most have had small sample sizes or utilize OUD care managers.[10, 14, 23–25] To our knowledge, there has not been a large-scale trial of a tool that systematically prompts the recognition and treatment of OUD in primary care.
In the pilot study[26] informing the multi-site trial described here, our team designed and implemented an OUD shared decision-making (OUD-SDM) system that operationalized the recommendations of a working group report sponsored by the National Institute on Drug Abuse’s (NIDA) Clinical Trials Network (CTN).[27] The OUD-SDM system uses web-based algorithms to identify patients with OUD and those at elevated risk for OUD or opioid overdose and provides an electronic health record (EHR)-integrated user interface that guides PCCs through OUD screening, diagnosis, management and/or referral. This paper describes the cluster-randomized pragmatic trial of the OUD-SDM tool that is now being tested in three large healthcare systems.
STUDY DESIGN:
Study Overview:
This 3-site, 2-arm, clinic-randomized pragmatic trial evaluates the effectiveness of the OUD-SDM intervention on the diagnosis and treatment of primary care patients at elevated risk of OUD. The OUD-SDM system provides tools to facilitate the screening, diagnosis and treatment of OUD. The OUD-SDM tool displays in intervention clinics (but not in control clinics) when patients have OUD or are at high risk for OUD or overdose.
Study Settings:
The study takes place in the primary care clinics of three large healthcare organizations (Essentia Health, Geisinger Clinic and HealthPartners) in Minnesota, Wisconsin, North Dakota and Pennsylvania.
Randomization:
Covariate-constrained randomization was used to balance treatment groups across the study and within healthcare systems on factors related to study outcomes, implementation of the intervention, and potential treatment modifiers. Specifically, variables used to constrain the randomization were the percentages of patients with diagnosed OUD, patients with diagnosed OUD prescribed a MOUD, and patients insured by Medicaid. Smaller clinics that share PCCs were combined into a single randomization unit to decrease contamination risk. The study statistician (ALC) wrote the randomization program and statisticians at the NIDA Clinical Trials Network Data and Statistics Center at Emmes reviewed it. SAS Version 9.4 and the CCR macro[28] were used to assign clinics 1:1 to two groups, and the study statistician randomly assigned one clinic group at each healthcare system to intervention, with the remaining clinic group assigned to control.
Enrollment and Eligibility:
Algorithms run by the OUD-SDM tool at every primary care visit in all randomized clinics identify eligible visits. The index date for each patient is the date when a patient first meets the following inclusion criteria: (1) aged 18-75 years, inclusive; (2) seen by a PCC in a randomized clinic, and (3) has a current diagnosis of OUD, a recent opioid overdose, a current prescription of a MOUD, or a risk score indicating high risk of OUD or opioid overdose. This approach assures complete and consistent evaluation of eligibility criteria for all patients seeking care in both intervention and control clinics. Patients are assigned to the treatment group of the primary care clinic where their index clinic visit takes place. Patients who reside in nursing homes, receive hospice care, or have active cancer diagnoses (except nonmelanoma skin cancer) are excluded from the intervention. Patients who have requested to be excluded from research studies receive care consistent with their clinic’s randomized treatment group but will be omitted from analyses.
Definition of High Risk:
Patients are considered high risk for OUD or opioid overdose if they have an elevated risk score using a risk equation released by Epic in 2019 (Verona, WI; unpublished). The risk equation aims to estimate a patient’s risk of having OUD or an opioid overdose in the next year. Patients with risk scores ≥55 on a 100-point scale, the cutoff established by Epic, are flagged as high risk. Data elements used to estimate this risk include patient demographics, social and family history, diagnoses, medications and utilization variables.
Because the Epic risk equation excludes patients with known OUD or an opioid overdose in the last 6 months, (1) we created algorithms to identify patients with an opioid overdose in the last six months using ICD-10 codes (T40.1X3S-T40.41X, T40.601-T40.604S, T40.691-T40.694S, T40.0X1-T40.494S) or an order for emergency department(ED)-administered naloxone on a day when there were no facility-administrated opioids (to avoid misclassifying naloxone administered following procedures involving facility-administered opioids as overdoses); and (2) we use algorithms to identify patients with OUD or on a MOUD, defined as buprenorphine, methadone, or intramuscular naltrexone; oral naltrexone is excluded because it lacks evidence as an effective MOUD and because most oral naltrexone used in participating health systems is prescribed for alcohol use disorder, not OUD.
Study Outcomes:
The primary study outcome is the percentage of patients at high risk for OUD (but without an OUD diagnosis) receiving an OUD diagnosis within 30 days of index visit. Additional outcomes are, among patients at high risk for or with a diagnosis of OUD, the percentage receiving (a) a naloxone prescription or (b) a medication for OUD (MOUD) prescription or referral to specialty care within 30 days of an index visit, and (c) the total days covered by a MOUD prescription within 90 days of an index visit. Days covered by a MOUD prescription was included to measure adherence to treatment over the first three months. Additionally, we will compare rates of ED visits and hospitalizations during the observation period, as well as healthcare costs during the observation period. Finally, we will compare healthcare costs during the observation period, and will use mixed methods to identify, describe and quantify barriers and facilitators to the OUD-SDM system implementation.
Healthcare System Engagement:
During the planning phase, study team members met with leaders and clinician champions from each healthcare system to obtain consensus regarding OUD-SDM content and intervention workflow. This included patient inclusion criteria, primary care clinic inclusion, PCC and rooming staff workflows, content of the OUD-SDM printouts, and clinic training procedures. During the intervention, clinic leaders receive monthly clinic- and PCC-level reports about print rates for OUD-SDM handouts, which clinic leaders use for quality improvement.
Clinician and Staff Training:
At Site A, training was conducted by two live webinars that were recorded for later viewing for PCCs unable to attend. At Site B, training was done via in-person presentations for PCCs and rooming staff at each clinic. Staff and PCCs were then provided with training materials, quick reference guides and video recordings. At Site C, a recorded webinar was assigned to intervention clinic leaders, PCCs and rooming staff using the health system’s standard training website that allows for tracking of training completion.
Description of the OUD-SDM Technology:
The OUD-SDM system is hosted on a secure web service that is linked with the EHR, allowing for maximum efficiency and versatility. The OUD-SDM system maintains an average run time of less than a second to call the web service, run the algorithms, and display to the end user. The OUD-SDM provides complete capture of most clinical data necessary for intervention, analysis and data and safety monitoring in both intervention and control clinics (while being invisible to staff in control clinics). In intervention clinics, the OUD-SDM tool displays within a web browser in the EHR and looks seamlessly integrated with the EHR to the front-end user. Hyperlinks in the OUD-SDM tool facilitate medication or referral orders in the EHR to review, edit, and sign.
Description of OUD-SDM Workflow:
The OUD-SDM tool is available at the point-of-care and identifies eligible primary care encounters by detecting patients who have an OUD diagnosis, elevated risk for OUD or overdose and meet study eligibility criteria. For patients who present for a clinic visit at any randomized clinic (intervention or control), when a blood pressure is entered into the EHR and the vitals section is closed, selected clinical data are sent from the EHR to the OUD-SDM website to assess intervention eligibility. For eligible patients, a best practice advisory (BPA) is displayed to clinic rooming staff in intervention clinics. For patients at risk for OUD or overdose, rooming staff at Sites A and C click on the BPA to open, print and distribute tailored patient and PCC printouts of the OUD-SDM information (Figure 1). At Site B, the BPA prompts rooming staff to screen at-risk patients using the opioid- and heroin-related questions from the Tobacco, Alcohol, Prescription medication and Substance use (TAPS) tool [29]. If the TAPS is negative, no further action is prompted. If the TAPS is positive (score > 0) or the patient is already known to have OUD or a prescription for a MOUD, rooming staff are prompted to print and distribute the patient and PCC printouts. Regardless of whether a visit is associated with a BPA, PCCs in intervention clinics can also access the OUD-SDM tool through the EHR.
Figue 1.
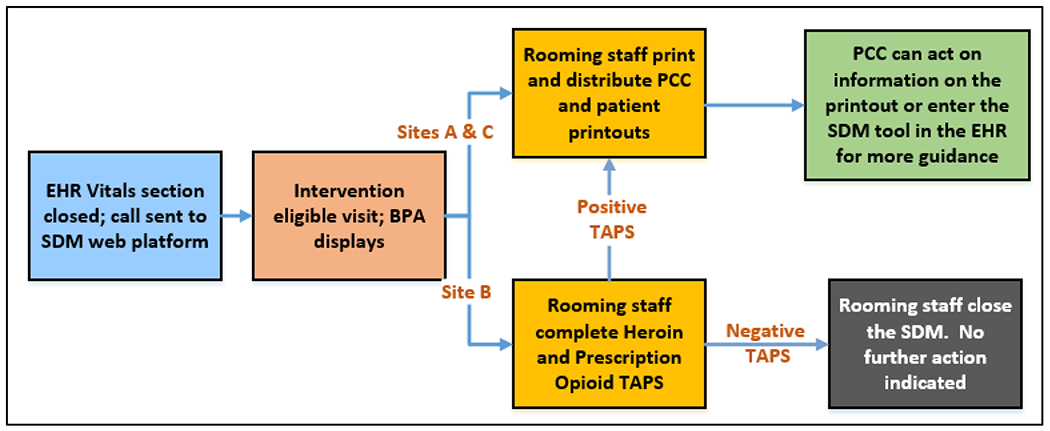
Opioid Use Disorder (OUD) Shared Decision Making (SDM) Workflow for At-Risk Patients.
In control clinics, the same data are sent from the EHR to the web service to evaluate eligibility, but no BPAs display for rooming staff, no printouts are provided to patients or PCCs, and the OUD-SDM tool is not available in the EHR. For all study-eligible patients in both intervention and control clinics, the web service assigns a random patient identification number and collects and retains relevant data needed for analysis on a secure server, including demographic data, vitals, current medications and comorbidities, allergies and laboratory data. The server also stores patient-specific treatment alerts and recommendations and, in intervention clinics, OUD-SDM tool usage.
Description of OUD-SDM Content:
At Sites A and C, opioid-related information is incorporated into a personalized and prioritized SDM system that also advises about diabetes and cardiovascular risk. At Site B, only opioid-related content is presented. Patient and PCC-facing language on the OUD-SDM printouts is summarized in Table 1.
Table 1.
Patient- and primary care clinician-facing language in the shared decision-making printouts by reason for display.
| Patient Status | Patient | PCC |
|---|---|---|
| Estimated high risk for OUD or overdose | 130 Americans die from preventable opioid overdoses every day. If you are concerned for you or your family or friends, talk to your clinician. | Risk algorithm detects high risk of problems with opioids. 1) screen for OUD, 2) if positive, consider MOUD, 3) prescribing naloxone |
| Opioid overdose in last 6 months identified | Our records suggest you were recently given naloxone, a medication used to treat opioid overdose or occasionally during a medical procedure requiring opioids. If you want help or support, please discuss with your clinician. | EHR indicates recent administration of naloxone or opioid overdose. No MOUD: Consider 1) prescribing naloxone, 2) screening for OUD, 3) if positive, consider MOUD MOUD: 1) assessing treatment adherence, 2) assessing need for naloxone, 3) offering additional resources for support |
| Diagnosis of OUD in Remission | If you are concerned about opioid use, treatments are available to help you and keep you and your friends and family safe from overdose. Talk to your clinician. | Previous diagnosis of opioid use disorder in remission detected. No MOUD: 1) screen for OUD, 2) if positive, consider MOUD, 3) prescribing naloxone MOUD: 1) assessing treatment adherence, 2) assessing need for naloxone, 3) offering additional resources for support |
| Diagnosis of OUD | Patient not prescribed a MOUD: If you are concerned about opioid use, treatments are available to help you and keep you and your friends and family safe from overdose. Talk to your clinician. Patient prescribed a MOUD: It appears you are taking positive steps to manage problems with opioids. Please continue to seek the support of your healthcare team. |
A previous encounter with a diagnosis of an opioid use problem was identified. no MOUD: 1) naloxone, 2) MOUD: 1) Assessing treatment adherence, 2) Assessing need for naloxone, 3) Offering additional resources for support. |
| Positive TAPS | If you are concerned about opioid use, treatments are available to help you and keep you and your friends and family safe from overdose. Talk to your clinician. | Positive screen for OUD detected. Consider 1) naloxone, 2) MOUD |
| Positive DSM | If you are concerned about opioid use, treatments are available to help you and keep you and your friends and family safe from overdose. Talk to your clinician. | Patient met criteria for OUD in the past year. 1) Add OUD to problem list, 2) Naloxone, 3) MOUD |
When PCCs click into the OUD-SDM tool in the EHR, there are four modules available. When PCCs first open the OUD-SDM tool, they are brought to the most clinically relevant module for that patient at that visit. All modules contain hyperlinks for quick orders to increase the efficiency of ordering recommended labs, medications, and referrals. The orders are based on preference lists within the care system and can be reviewed, edited, and signed within the EHR. The information on the top of the OUD-SDM tool (Figure 2) remains visible as the user clicks through the modules. Content presented includes the reason the OUD-SDM displayed, relevant conditions and medications, recent urine drug screen results, “Quick Actions” for easy ordering of naloxone or referrals, and a link to the Prescription Drug Monitoring Program databases for Minnesota, Wisconsin, North Dakota and Pennsylvania (depending on study site) with automatic log-in using PCC information from the EHR (except at one site where PCCs need to manually log into the Wisconsin database). All actions taken in the OUD-SDM tool are saved for the clinician to copy and paste into a progress note in the EHR.
Figure 2.
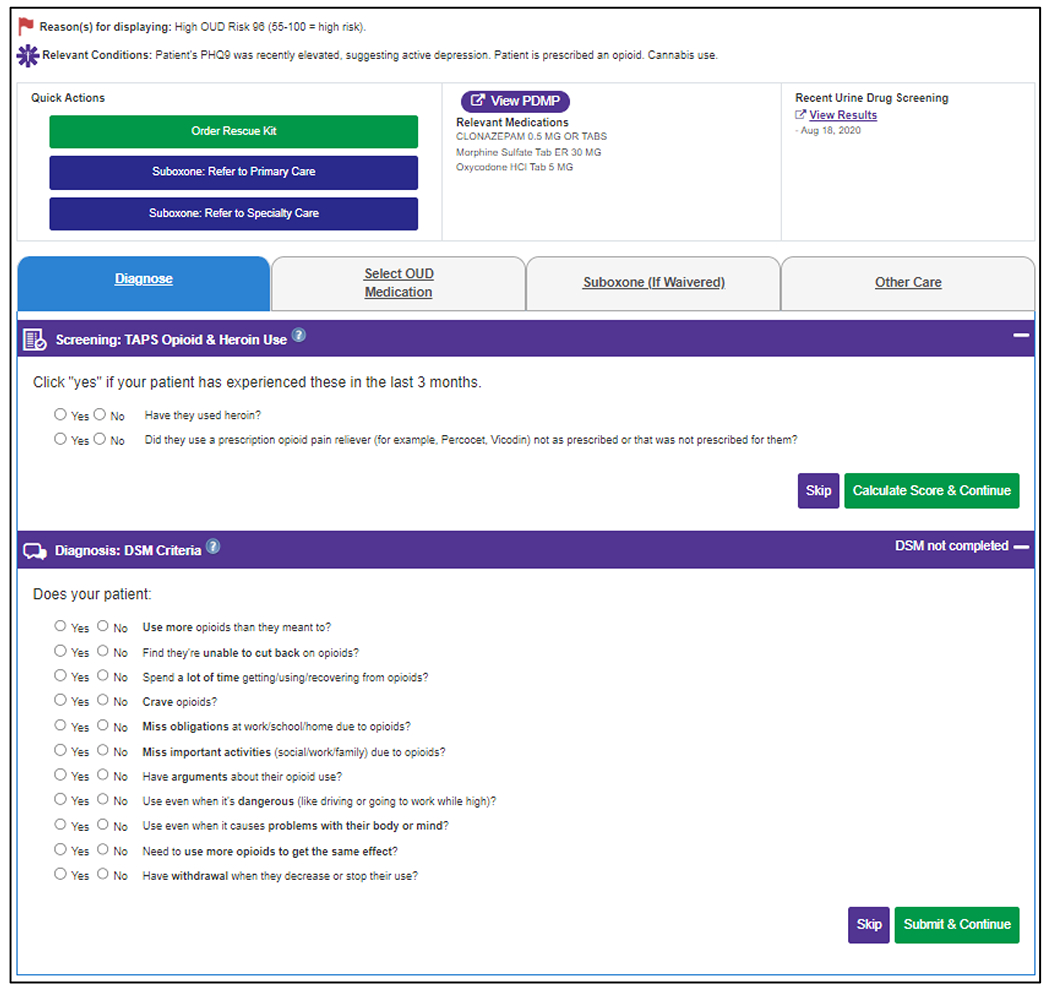
Top of Shared Decision-Making Tool (Visible Across All Modules) and Screening and Diagnosis Module for Example Patient with Elevated Risk of Opioid Use Disorder.
The first module is Screening and Diagnosis (Figure 2), which prompts PCCs (Sites A and C) or rooming staff (Site B) to complete the prescription opioid and heroin components of the TAPS to screen patients for OUD. If either component is positive, PCCs are prompted to assess patients using the OUD diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [30].
The second module, Treatment Selection (Figure 3), displays for patients with OUD. It alerts PCCs to relative contraindications to MOUDs and allows PCCs to change these should they have updated information not yet recorded in the EHR. Relative contraindications to OUD treatment in primary care may include pregnancy, comorbid substance use disorders, or active liver or pulmonary disease. The module provides printable patient educational materials, including a one-page summary of MOUDs and detailed MOUD-specific handouts.
Figure 3.
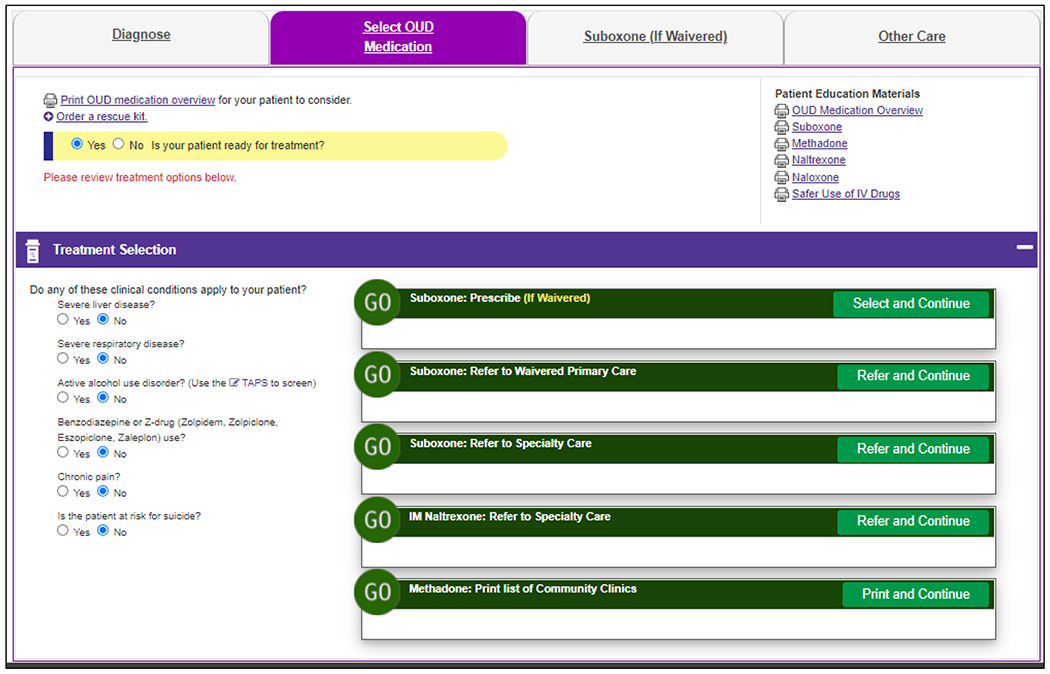
Treatment Selection Module for Example Patient with a New Diagnosis of Opioid Use Disorder.
The third module is Suboxone (Figure 4), which gives waivered PCCs guidance on both inclinic and at-home buprenorphine/naloxone induction. It offers the Clinical Opiate Withdrawal Scale (COWS)[31] and the Subjective Opiate Withdrawal Scale (SOWS)[32] to help assess opioid withdrawal, orders for three starting doses of buprenorphine/naloxone depending on the patient’s tolerance level (along with three different detailed printable patient handouts), and orders for naloxone, clonidine, ondansetron, pregnancy and liver function tests, and behavioral health referrals. The Suboxone module also provides guidance for subsequent visits, including assessing symptoms and adherence and adjusting the buprenorphine/naloxone dose as needed.
Figure 4.
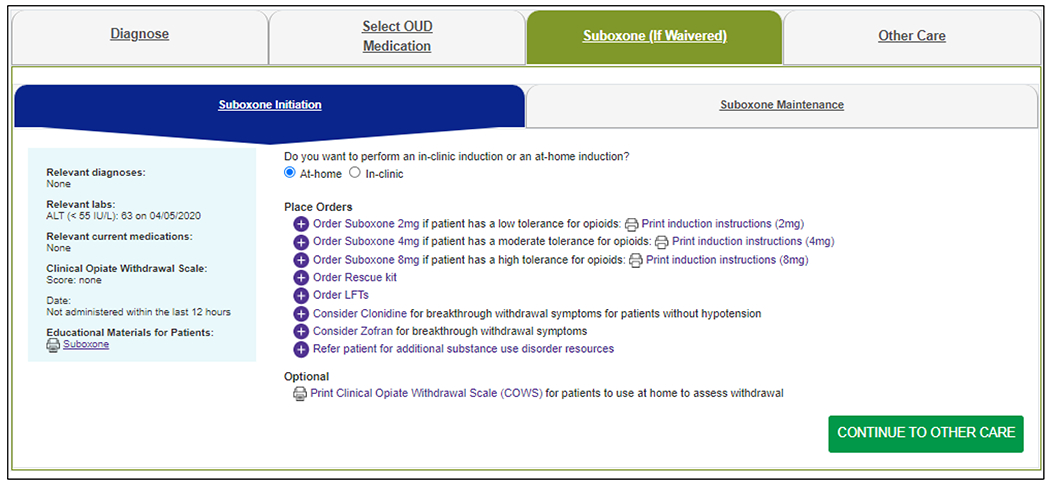
Suboxone Module for Example Patient Starting Buprenorphine via an At-Home Induction.
The fourth and final module is Other Care (Figure 5), which prompts screening for common comorbidities, including infectious diseases (e.g., HIV and viral hepatitis) and mental health conditions (e.g., depression and anxiety), and suggests administration of recommended vaccinations. For injection drug users, the module offers a patient handout on safer injection drug use and a recommendation to consider pre-exposure prophylaxis with emtricitabine/ tenofovir for patients at risk for HIV.
Figure 5.
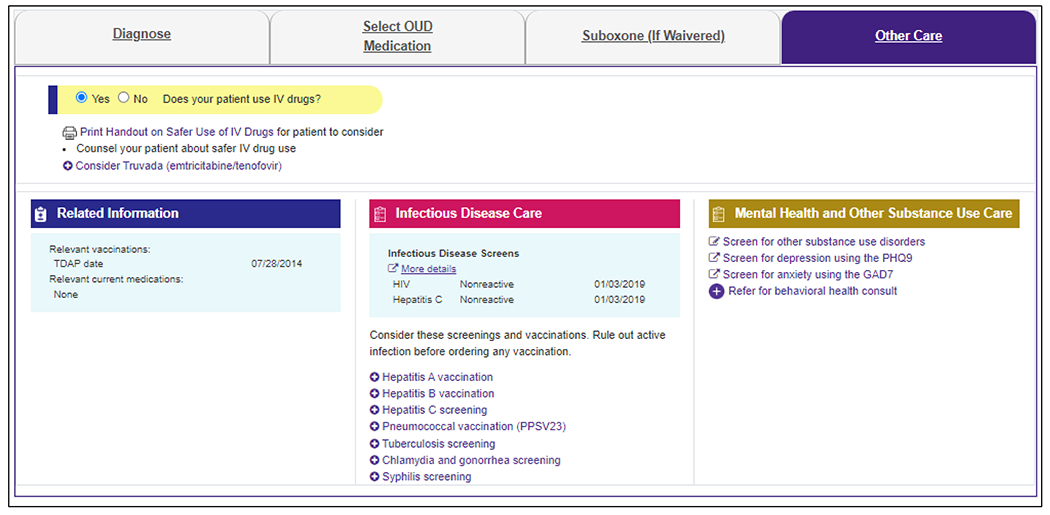
Other Care Module for an Example Patient who Uses Intravenous Drugs.
Data Sources:
The automated EHR data collection and calculations performed by the OUD-SDM tool at each primary care visit in all randomized clinics are saved in a repository for analysis. These data include medications, diagnoses, lab values and orders for up to two years before each visit. Outcomes will be calculated from medications, diagnoses, lab values, orders and encounters documented in the EHR in the 30- and 90-days post-index, while safety events (opioid overdoses, hospitalizations, ED visits) will be monitored through the end of the study period. We will extract claims data to assess ED visits and hospitalizations for the 40% of patients for whom this is available at two sites; for the third site, no claims data are available. It is assumed that undocumented care in these health systems is extremely rare.
Prior to implementation at each health system, PCCs completed surveys about their perceptions of OUD and appropriateness of OUD care in primary care settings. Nine months after go-live, PCCs will complete a second survey with similar content to the baseline survey, and intervention PCCs will also report their impressions of the OUD-SDM system. A sample of patients at each health system will be surveyed about their recent primary care visits where the OUD-SDM handouts were printed. The surveys include questions about the OUD-SDM patient handouts and conversations patients may have had with their PCCs about opioids or OUD. A sample of patients, PCCs, rooming staff, and healthcare system leaders at each health system will be interviewed about their experiences with the OUD-SDM tool and their impressions of the suitability of primary care settings to address OUD.
Fidelity to the Intervention:
Use of the OUD-SDM tool is monitored via two measures: how often the tool is printed by rooming staff (“print rates”) and how often PCCs click into and use the tool (“click rates”). Monthly print rate reports are provided to clinic leadership to promote high print and distribution rates. Reports compare print rates across clinics in each healthcare system, as well as PCC-level print rates at each clinic. In addition, study staff contact clinic leads at clinics with low print rates to troubleshoot any issues. As clicking into the OUD-SDM tool is up to PCC discretion, click rate reports at the PCC level are not reported to clinic leadership. However, PCC click rates are monitored and are used to identify PCCs who do and do not routinely use the OUD-SDM tool for qualitative interviews.
Analysis Plan:
The analyses of OUD diagnosis and naloxone prescription rates, as well as analyses of MOUD orders/referrals and days covered by MOUD prescriptions, will use generalized linear mixed models. This allows for accurate estimations of the likelihood or counts of outcomes among intervention relative to control clinics, accounting for the clustering of patients within randomized clinics. Binary and count outcomes will be normalized using appropriate link functions (e.g., logit, log). The models will estimate fixed effects of the OUD-SDM tool, care system and balancing or other covariates on outcomes, and random clinic intercepts. Patient-year rates of ED visits and hospitalizations will be analyzed by clinic using generalized estimating equations that apply appropriate link functions to normalize these outcomes (e.g., log) and account for varying patient-years across clinics. The models will estimate fixed effects of the OUD-SDM tool, care system and balancing and other covariates on the rate of each outcome per patient-year.
Analysis of healthcare costs will include delivery costs of the OUD-SDM tool and incremental medical care costs attributable to the intervention, defined from the health system perspective. Costs of delivering the OUD-SDM tool—which may include programming, training, and ongoing algorithm maintenance but exclude research costs—will be estimated using micro-cost accounting methods and nationally-representative pricing of inputs. Medical care costs will include all prescribed medications and clinical inpatient, ED and outpatient services incurred in the pre- and post-index observation periods as indicated by insurance claims and clinical encounter data. Medical care costs will be standardized using Total Care Relative Resource Values (TCRRVs), a nationally representative and standardized set of pricing measures derived from Centers for Medicare and Medicaid Services relative value units.[33] The study sample for the cost analysis will be limited to patients who have continuous insurance coverage from a participating insurer, including pharmacy benefits, during the pre- and post-index observation periods. This sample will be compared to patients without continuous insurance coverage to assess representativeness and comparative completeness of utilization measurement in comparison to all study patients. Standard health econometric and specification testing methods will be used to assess the incremental differences in healthcare costs between study groups.[34–36]
Mixed method analysis occurs rapidly as data are collected to support possible revisions in implementation as the study proceeds. After intervention completion, monitoring data, survey data, and qualitative data will be triangulated using the RE-AIM framework[37] to identify barriers, facilitators, adaptations, and recommendations for future dissemination of the OUD-SDM tool.
Sample Size:
A priori power estimates were based on a plan to recruit and randomly assign 1:1 at least 30 primary care clinics (at least 10 per health system). Clinics need at least 50 patients with or at high risk for OUD or opioid overdose to be included in analyses. A preliminary power analysis was conducted to estimate the minimum detectable difference in the likelihood of an OUD diagnosis within 30 days of the index visit. The power analysis relied on conservative assumptions regarding the numbers of randomized clinics (10, 30, 50) and patients (50 or 100 per clinic), of whom 50% would be at high risk for OUD or opioid overdose (i.e., not diagnosed with OUD); a range of clinic intraclass correlations consistent with those observed for other process measures (i.e., ICCclin=0.01, 0.03, 0.05); and control group OUD diagnosis rates (2%, 5%, 10% and 20%). Effect size estimates were calculated using the NIH group-randomized trial sample size calculator.[38]
The preliminary power calculations estimated that the primary analysis would be powered (power=0.80, α2=0.05) to detect a difference in OUD diagnoses if ≥11.4% (odds ratio [OR]=2.44) of intervention patients had a diagnosis within 30 days post-index, assuming 30 clinics with 25 high risk patients each, that 5% of control patients would have an OUD diagnosis within 30 post-index, and that ICCclin=0.01. Increasing the control group diagnosis rate to 10% resulted in power to detect a difference if it occurred among ≥ 18.1% (OR=1.99) of intervention patients. More patients per clinic (e.g., 30 clinics*50 patients=1500 patients) resulted in a smaller detectable effect (i.e., 16.2% intervention vs. 10% control; OR=1.74) although randomizing more clinics (e.g., 50 clinics*25 patients=1250 patients) would reduce the minimum detectable effect further (i.e., 16.0% intervention vs. 10% control; OR=1.72) with fewer patients.
We have since updated the power calculations with data-informed assumptions after clinics at all three sites were randomized. The updated assumptions included updating the number of clinics from 30 to the 92 randomized primary care clinics participating in the study, and assuming that 40% of intervention-eligible patients per clinic would be at high risk but not diagnosed with OUD. We also updated the method for estimating the design effect used in calculation of the effective sample size. The updated design effect (1 + [(CV2 + 1)*m − 1]*ICCclin) incorporates the variability in clinic size (coefficient of variation, CV=SDm/Mm), and we assumed CV=0.7.[39] Assumptions about the 30-day outcome rates among patients in control clinics and variability in outcomes across clinics (ICCclin=0.03) could not be updated without having primary outcome data available. With these updated assumptions, the analysis of OUD diagnoses is powered to detect a significant difference if ≥9.6% (OR=2.02) of intervention patients have an OUD diagnosis within 30 days of their index visit relative to 5% of control patients. All intervention-eligible patients will be included in the analysis of other outcomes (e.g., naloxone prescription, specialty care referral). Should these events occur among 5% of control patients, analyses will be powered to detect a difference if an event occurs among ≥8.7% (OR=1.82) of intervention patients; or 14.9% intervention compared to 10% control (OR=1.57).
Alternative Designs Considered:
In designing this trial, while we considered a patient- or PCC-randomized clinical trial, we instead opted for a clinic-randomized design to minimize risk of contamination and allow the alert to display to rooming staff. Importantly, across the health systems involved, rooming staff can be reliably attributed to a particular clinic but not to a particular PCC or patient encounter. We opted to have the EHR alert display to rooming staff, as they follow uniform rooming procedures to trigger (via closing of the vitals section in the EHR) and print (via clicking on the hyperlink in the alert) the handouts. In the small pilot study of 55 PCCs that informed this full-scale trial[26], PCCs were volunteers scattered across clinics, necessitating that the alert for the OUD-SDM faced the PCC in the EHR. This led to low use rates (about 5% of eligible encounters), with PCCs reporting that they never saw the EHR alerts. This experience solidified our decision to use a cluster-randomized trial design with rooming staff triggering, printing and distributing the handouts.
We opted to have the interface be a web-based tool hosted by a web browser in the EHR and not hosted by the EHR itself. This allows for much greater flexibility in the design, appearance and function of the tool. Additionally, we opted against the interface being an online-only tool, as patients and PCCs have told us they like being able to review the printout together, and patients like the ability to bring it home to further consider the information presented.
Ethical and Regulatory Approval:
Study design and procedures were reviewed and approved by Institutional Review Boards (IRBs) at all three health systems. IRBs at Sites B and C then ceded oversight of the study to the IRB at Site A.
Informed Consent:
As the care recommendations in the OUD-SDM tool are limited to those recommended by current evidence-based national clinical guidelines, the IRB granted waivers of consent for use of the OUD-SDM tool in clinical care. Patients, PCCs and clinic leaders are consented for study-related surveys and interviews.
Data and Safety Monitoring:
A Data and Safety Monitoring Board (DSMB) appointed by the NIDA CTN is examining accumulating data to assure the protection of participants’ safety and that the study’s scientific goals are being met. As this study is deemed minimal risk, adverse events and serious adverse events will not be collected in the context of this trial. However, ED visits, hospitalizations, overdose events and all-cause mortality will be captured through EHR and claims data and reported to the DSMB. OUD-SDM suggestions are based on electronically available data and are not intended to substitute for clinical judgement. PCCs are asked to notify study staff of any clinical situations where their clinical judgment differs from the OUD-SDM tool’s suggestions. There is a “Suggestions” button on the OUD-SDM tool’s online interface to encourage reporting of discrepancies, which allows for easy troubleshooting of specific content. Study staff will take any necessary actions to correct the OUD-SDM tool and will provide all PCC feedback to the DSMB.
Trial Status:
The trial was funded through a cooperative agreement with the NIDA CTN. The study began enrolling patients on 04/13/2021 at Site A, 02/7/2022 at Site B, and 04/21/2022 at Site C. Study enrollment at all sites is planned to end on 12/31/2022, with the intervention continuing through 12/31/2023, allowing for at least one year of follow-up for all participants.
Conclusions:
Timely interventions in primary care are important to address the continued opioid overdose crisis. An SDM tool that facilitates the diagnosis and treatment of OUD in primary care could significantly improve patient access to effective OUD care. Results of this pragmatic cluster-randomized clinical trial are expected in 2024, and if effective, could be quickly and efficiently scaled for implementation in primary care clinics across the country.
Funding:
This work was supported by the National Institutes of Health through the NIH HEAL Initiative under award number (UG1DA040316). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the NIH Helping to End Addiction Long-term (HEAL) Initiative. This research is also supported by the Department of Health and Human Services, National Institute on Drug Abuse, Center for the Clinical Trials Network, under contract numbers 75N95019D00013 (Data and Statistics Center, the Emmes Company).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: ClinicalTrials.gov Identifier: NCT04198428
Declarations
Dr. Huntley’s spouse is eligible for a defined benefit plan through Pfizer from previous employment. The remaining authors have no competing interests to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Drug Overdose Deaths in the U.S. Top 100,000 Annually. 2021. [cited 2022 May 22]; Available from: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm.
- 2.Treatment episode data set - admissions (TEDS-A) 2012. [cited 2018 May 15]; Available from: http://wwwdasis.samhsa.gov/webt/quicklink/US09.htm.
- 3.Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. 2020, Substance Abuse and Mental Health Services Administration: Rockville, MD. [Google Scholar]
- 4.Jones CM, et al. , National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health, 2015. 105(8): p. e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration, Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) Series 63 Publication No. PEP21-02-01-002. 2021, Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 6.Marks LR, et al. , Infectious Complications of Injection Drug Use. Med Clin North Am, 2022. 106(1): p. 187–200. [DOI] [PubMed] [Google Scholar]
- 7.Rich KM, et al. , Integrated Models of Care for Individuals with Opioid Use Disorder: How Do We Prevent HIV and HCV? Curr HIV/AIDS Rep, 2018. 15(3): p. 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gros DF, et al. , Frequency and severity of comorbid mood and anxiety disorders in prescription opioid dependence. Am J Addict, 2013. 22(3): p. 261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebschutz J, Beers D, and Lange A, Managing Chronic Pain in Patients with Opioid Dependence. Curr Treat Options Psychiatry, 2014. 1(2): p. 204–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alford DP, et al. , Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med, 2011. 171(5): p. 425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebschutz JM, et al. , Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med, 2014. 174(8): p. 1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore BA, et al. , Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med, 2007. 22(4): p. 527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schackman BR, et al. , Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med, 2012. 27(6): p. 669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soeffing JM, et al. , Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat, 2009. 37(4): p. 426–30. [DOI] [PubMed] [Google Scholar]
- 15.Weiss L, et al. , Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr, 2011. 56 Suppl 1: p. S68–75. [DOI] [PubMed] [Google Scholar]
- 16.Coviello DM, et al. , A multisite pilot study of extended-release injectable naltrexone treatment for previously opioid-dependent parolees and probationers. Subst Abus, 2012. 33(1): p. 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan A, et al. , Monthly Patient Volumes of Buprenorphine-Waivered Clinicians in the US. JAMA Network Open, 2020. 3(8): p. e2014045–e2014045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson E, et al. , Barriers to primary care physicians prescribing buprenorphine. Ann Fam Med, 2014. 12(2): p. 128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry DT, et al. , Integrating buprenorphine treatment into office-based practice: a qualitative study. J Gen Intern Med, 2009. 24(2): p. 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netherland J, et al. , Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treat, 2009. 36(3): p. 244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walley AY, et al. , Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Intern Med, 2008. 23(9): p. 1393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madden EF, et al. , Intervention Stigma toward Medications for Opioid Use Disorder: A Systematic Review. Subst Use Misuse, 2021. 56(14): p. 2181–2201. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YJ, et al. , Integration of Buprenorphine Treatment with Primary Care: Comparative Effectiveness on Retention, Utilization, and Cost. Popul Health Manag, 2019. 22(4): p. 292–299. [DOI] [PubMed] [Google Scholar]
- 24.Fiellin DA, et al. , Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5years. Am J Addict, 2008. 17(2): p. 116–20. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki J, et al. , Implementation of a collaborative care management program with buprenorphine in primary care: a comparison between opioid-dependent patients and patients with chronic pain using opioids nonmedically. J Opioid Manag, 2014. 10(3): p. 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossom RC, et al. , A pilot study of the functionality and clinician acceptance of a clinical decision support tool to improve primary care of opioid use disorder. Addict Sci Clin Pract, 2021. 16(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bart GB, et al. , Developing a clinical decision support for opioid use disorders: a NIDA center for the clinical trials network working group report. Addict Sci Clin Pract, 2020. 15(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene EJ, A SAS Macro for Covariate-Constrained Randomization of General Cluster-Randomized and Unstratified Designs. Journal of Statistical Software, Code Snippets, 2017. 77(1): p. 1 – 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeely J, et al. , Performance of the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) Tool for Substance Use Screening in Primary Care Patients. Ann Intern Med, 2016. 165(10): p. 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013, Washington DC. [Google Scholar]
- 31.Wesson DR and Ling W, The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs, 2003. 35(2): p. 253–9. [DOI] [PubMed] [Google Scholar]
- 32.Handelsman L, et al. , Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse, 1987. 13(3): p. 293–308. [DOI] [PubMed] [Google Scholar]
- 33.HealthPartners, Total Care Relative Resource Value (TCRRVTM): A Measurement Approach to Achieve the Triple Aim. HealthPartners White Paper, https://www.healthpartners.com/ucm/groups/public/@hp/@public/documents/documents/cntrb_039627.pdf. Sep 21, 2017, HealthPartners: Bloomington, MN. [Google Scholar]
- 34.Blough DK, Madden CW, and Hornbrook MC, Modeling risk using generalized linear models. J Health Econ, 1999. 18(2): p. 153–71. [DOI] [PubMed] [Google Scholar]
- 35.Manning WG and Mullahy J, Estimating log models: to transform or not to transform? J Health Econ, 2001. 20(4): p. 461–94. [DOI] [PubMed] [Google Scholar]
- 36.Mullahy J, Much ado about two: reconsidering retransformation and the two-part model in health econometrics. J Health Econ, 1998. 17(3): p. 247–81. [DOI] [PubMed] [Google Scholar]
- 37.Glasgow RE, et al. , RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Front Public Health, 2019. 7: p. 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sample Size Calculator for GRTs. [cited 2019 May 3]; Available from: https://researchmethodsresources.nih.gov/SampleSizeCalculator.aspx.
- 39.Eldridge SM, Ashby D, and Kerry S, Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol, 2006. 35(5): p. 1292–300. [DOI] [PubMed] [Google Scholar]


