Abstract
To date, the highly adapted cave microbial communities are challenged by the expanding anthropization of these subterranean habitats. Although recent advances in characterizing show-caves microbiome composition and functionality, the anthropic effect on promoting the establishment, or reducing the presence of specific microbial guilds has never been studied in detail. This work aims to investigate the whole microbiome (Fungi, Algae, Bacteria and Archaea) of four Italian show-caves, displaying different environmental and geo-morphological conditions and one recently discovered natural cave to highlight potential human-induced microbial traits alterations. Results indicate how show-caves share common microbial traits in contrast to the natural one; the first are characterized by microorganisms related to outdoor environment and/or capable of exploiting extra inputs of organic matter eventually supplied by tourist flows (i.e. Chaetomium and Phoma for fungi and Pseudomonas for bacteria). Yet, variation in microalgae assemblage composition was reported in show-caves, probably related to the effect of the artificial lighting. This study provides insights into the potential microbiome cave contamination by human-related bacteria (e.g. Lactobacillus and Staphylococcus) and commensal/opportunistic human associated fungi (e.g. Candida) and dermatophytes. This work is critical to untangle caves microbiome towards management and conservation of these fragile ecosystems.
Subject terms: Ecology, Microbiology
Introduction
Caves are spatially confined, subsurface environments widespread on Earth, where peculiar abiotic factors (i.e. oligotrophy, total darkness and high mineral concentrations) and microclimatic conditions (i.e. scarcely fluctuating low temperature and very high humidity)1,2 impose special adaptations to microbial life-forms3. Microbial assemblages in caves include archaea, bacteria, fungi and other micro-eukaryotes4; these highly adapted microbial communities represent the living-backbone of cave ecosystems and play a key role in shaping structures (via direct and indirect metabolic activities) and sustaining trophic networks5–7. They live in constant balance between coping with unfavorable environmental conditions and maintaining microbe-microbe interactions, either competitive or cooperative1,8.
Yet, the growing speleological and touristic interest in these underground settings has led to a major anthropization of caves (thereafter show-caves), which have been turned into real attractions. To date, show-caves, with 1440 sites in 148 countries, among which 60 are in Italy (www.showcaves.com), represent an expanding worldwide scenario that results in a constant anthropization of a large number of natural hypogean settings. The presence of logistic interventions, such as the establishment of walkways, barriers and artificial lighting systems, coupled with tourist flows may alter the pristine local environmental conditions, by changing the local microclimate and introducing nutrients (i.e. soil, hair, skin, lint from clothing) and allochthonous microbial species9–13.
Recently, researchers started to characterize show-caves microbiome, analyzing different types of samples (sediments, speleothems, and biofilms) and comparing natural and show-caves to discern a potential human impact on microbial composition and functionality [e.g.13–17]. Potential bioindicators of altered conditions due to human activities have been identified in showcaves; for instance, the proliferation of lampenflora component, due to artificial light, is more abundant but generally less diverse than in natural caves18. Although some predominant microbial taxa reported in pristine settings have been detected in anthropized caves8,13, the effect of human impact on promoting the establishment or reducing the presence of specific microbial traits has never been investigated in detail. Therefore, the consequence of tourist flows and human activities in general on microbial assemblages composition remains largely unknown.
To address this knowledge gap, our study intends to provide novel comprehensive insights into the microbial diversity and composition of 5 caves that include 4 show-caves and 1 recently discovered natural cave, displaying different environmental and geo-morphological conditions across the Italian peninsula. Through taxonomic comparison, we have characterized the principal microbial traits found in anthropized and pristine habitats, starting to shed light on potential microbial taxa that may be considered as indicative of human impact.
Results
Microbial community composition in cave sediments
The ITS1 dataset generated 5,793,980 raw sequence reads, resulting in 5,458,895 gene quality-filtered reads, ranging from 1252 up to 540,803 per sample. After singletons and rare taxa (< 5 reads) removal (1108 out of 10,595 ASVs total), a total of 9176 high-quality ASVs were obtained (Table S1). A total of 5,453,881 raw reads were generated from 16S rDNA dataset and accounted for a total of 4,806,902, which were grouped into 31,878 ASVs (out of a total of 65,037 ASVs) after quality filtering, with sequencing depths between samples ranging from 2066 to 265,442 reads .
Subsequently, the total 16S dataset was splitted by grouping the bacterial (31,015 ASVs) and archaeal (863 ASVs) ASVs separately for the downstream analyses (Table S2; Table S3). The 18S dataset was processed through a specific evaluation, extracting and analyzing only the sequences related to the microalgal component. A total of 97 ASVs were mapped for a total of 10,084 quality filtered reads, spread over 4 different groups (Alveolata, Chloroplastida, Chromista and Stramenopiles), using the Class taxonomic rank as the threshold for taxonomic identification as a consequence of the weak accuracy in the assignment towards higher ranks (Table S4).
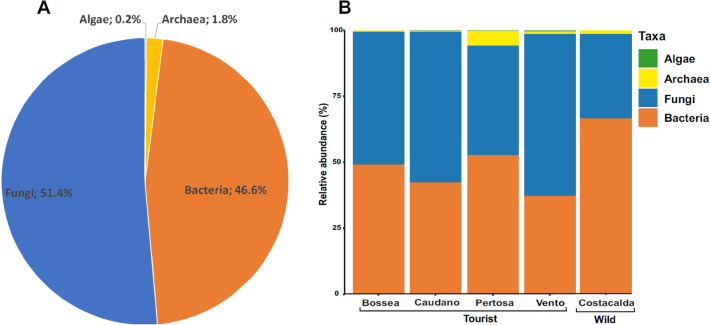
Considering the total composition of microbial communities in cave sediments as a unique dataset (Fig. 1A), fungi represented the most abundant group (51.4%), followed by bacteria (46.6%), while archaea (1.8%) and micro-algae (0.2%) constituted minor fractions.
Figure 1.
Total microbial cave community composition: as a unique dataset (A) and per single cave perspective (B).
When analyzing the assemblage composition of each cave individually (Fig. 1B), Bossea cave was characterized by an almost equal presence of fungi (50.22%) and bacteria (49.16%). Caudano and Vento caves were dominated by fungi (57.17% and 61.46%, respectively), while the bacterial community accounted for 42.37 and 37.22% of the total assemblage, respectively. Conversely, Costacalda and the Pertosa-Auletta caves showed a higher bacterial presence (66.66% and 52.70%, respectively) compared to fungi (32.05 and 41.48%, respectively). Concerning the archaea domain, Pertosa-Auletta cave was the most representative with 5.74% of the total microbial assemblage, followed by Costacalda (1.27%), Vento (0.85%), Bossea (0.52%) and Caudano (0.41%) caves. The algal component was scarcely represented along all caves, with a relative abundance ranging from 0.47 for Vento cave to 0.01% for Costacalda cave.
Fungal community composition
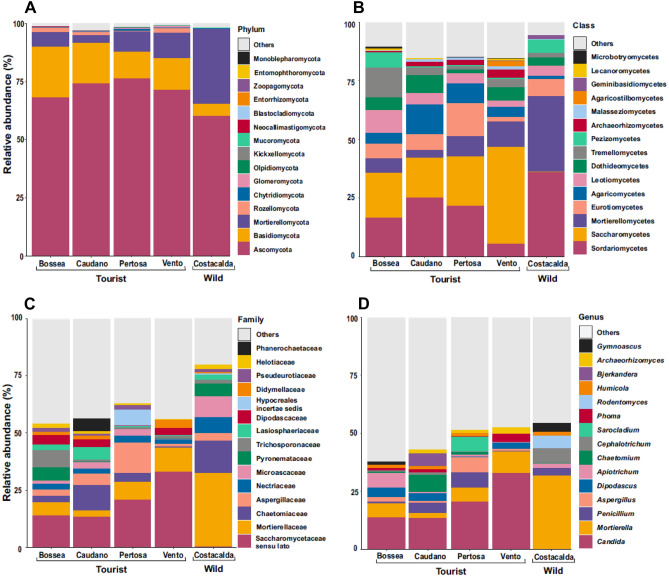
In all caves, the dominant phylum was Ascomycota (Fig. 2A) (ranging from 60% in Costacalda to 76.25% in Pertosa Auletta), followed by Basidiomycota and Mortierellomycota. A broad different distribution of fungal taxa pattern emerged between the four show -caves and the wild cave (Fig. 2A,B,C,D). Notably, the genus Mortierella (Fig. 2D) was particularly abundant in the Costacalda wild cave (up to 31.8%), while in the show- caves did not exceed 10% (from 2.31% in Caudano up to 9.30% in Vento). The opposite was observed for the genus Candida (class Saccharomycetes), which was more abundant in the tourist caves (ranging from 13.36% in Caudano to 37.32% in Vento). Also, the genus Dipodascus was mainly present in the anthropized settings, even if at much lower extent compared to Candida, ranging from 0.14% in Pertosa-Auletta to 4% in Bossea. The genus Archaeorhizomyces (class Archaeorhizomycetes) was reported in show- caves only, albeit with scarce relative abundance (e.g. 0.01% Bossea up to 2.70% Vento). A number of cave-specific genera in the class Sordariomycetes have been recorded: for instance, Chaetomium (7.50%) in Caudano, Sarocladium (6.4%) in Pertosa-Auletta, Cephalotrichum (6.30%) and Rodentomyces (5.60%) in Costacalda ; differently, the genus Humicola (average abundance 1.39%) was found in all caves (Fig. 2). The class Eurotiomycetes was mainly represented by the genera Aspergillus (ranging from 0.01% in Costacalda to 6.77% in Pertosa-Auletta) and Penicillium (ranging from 0.17% in Vento to 6.45% in Pertosa-Auletta), while the genus Gymnoascus was most abundant in Costacalda and Bossea caves, accounting for 3.72% and 1.50% respectively. Likewise, the genera Tetracladium and Pseudogymnoascus (not shown in the Top 15) were the dominant members of Leotiomycetes throughout the dataset, up to1.96% and 1.12% in Costacalda , respectively (Fig. 2D). Agaricomycetes class was particularly abundant in show-caves (Caudano 12.6%, Pertosa-Auletta 8.25%, Bossea 4.44%, Vento 4.30%, Costacalda 1.2%), with the genus Bjerkandera being the only one reported among the top 15 (Fig. 2D). Yet, despite a considerable proportion of classes Tremellomycetes (ranging from 1.41% at Pertosa-Auletta to 12.7% at Bossea) and Dothideomycetes (ranging from 1.64% at Pertosa-Auletta to 7.50% at Caudano) among caves, they were mainly represented by two genera, i.e. Apiotrichum (Trichosporonaceae) and Phoma (Didymellaceae). Apiotrichum was spotted across all sites investigated, while Phoma appeared more prevalent among tourist caves (Vento: 3.45%, Caudano: 1.51%, Bossea: 1.10%, Pertosa-Auletta: 0.07%, Costacalda: 0.004%). Some other fungal classes showed different abundance patterns between tourist and wild cave, such as: Malasseziomycetes, (over 1% in show-caves vs. 0.15% of Costacalda), Microbotryomycetes (Bossea: 0.93%, Vento: 0.3%, Caudano: 0.16%, Pertosa-Auletta: 0.13%, vs. Costacalda: < 0.001%) and Geminibasidiomycetes (Costacalda: 1.52%, vs. all tourist caves: < 0.2%). Finally, lichenized fungi class of Lecanoromycetes was especially reported in Bossea (0.85%) and Vento (0.52%) caves.
Figure 2.
Fungal community composition of 5 examined caves: Bossea, Caudano Pertosa-Auletta, Vento (show-caves) and Costacalda (wild cave). The top 15 most abundant taxa are shown for taxonomic ranks: phylum (A), class (B), family (C) and genus (D).
Bacterial community composition
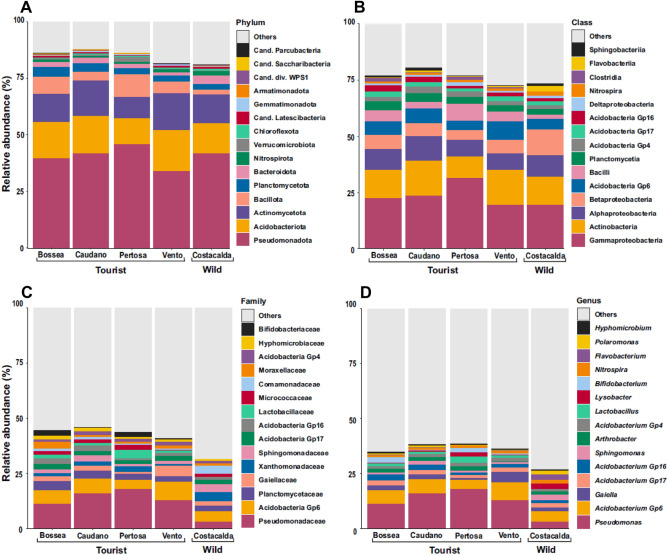
A different bacterial distribution was found in each cave (Fig. 3A–D). For instance, the phylum Pseudomonadota dominated throughout the dataset, with remarkable presence of its main classes (Gamma, Alpha, Beta and Delta-Proteobacteria) and by different genera reported in the top 15 (Fig. 3). The class Gamma-Proteobacteria was mainly represented by the genus Pseudomonas, particularly abundant in tourist caves (ranging from 11.1% in Bossea to 17.78% in Pertosa-Auletta, vs. Costacalda 2.95%). Conversely, the genera Sphingomonas (class Alpha-Proteobacteria, 2.46%), Lysobacter (class Gamma-Proteobacteria, 2.34%) and Polaromonas (class Beta-Proteobacteria, 1.8%) were prevalent in the pristine site, while Hyphomicrobium genus (class Alpha-Proteobacteria) was largely spread across all habitats (average of 0.6%). Three Acidobacteria sub-groups, i.e. Gp6 (mean 6.0%; highest in Bossea: 8.2%), Gp17 (mean 2. 0%), Gp16 (mean 1.74%), Gp4 (mean 1.35%) and the two main Actinobacteriota genera, i.e. Gaiella (with 0.78% in Pertosa-Auletta up to 4.51% in Vento caves) and Arthrobacter (ranging from 0.24% in Vento up to 2% in Pertosa-Auletta caves) showed a broad distribution across all caves. Yet, among Actinobacteriota, the genus Bifidobacterium was recorded only in show-caves (between 0.3% in Caudano and 2.5% in Pertosa-Bossea caves). However, similar abundance trends across all surveyed sites were also reported for the family Planctomycetaceae (ranging from 2.7% in Vento up to 4.27% in Bossea caves) and the genus Nitrospira (between 0.7% for Caudano and 1.75% of Costacalda caves). Other remarkable compositional spectra were represented by Bacillota with classes Clostridia (ranging from 0.48% in Caudano up to 1.62% in Bossea, vs. 0.07% in Costacalda) and Bacilli (from 3.15% in Caudano up to 7.66% in Pertosa-Auletta and 1.81% for Costacalda caves) with genus Lactobacillus (1.64% mean in show-caves, vs. 0.007% in Costacalda) all typically found in anthropized settings. Conversely, the Flavobacterium genus (Phylum Bacteroidota, 2.47% in Costacalda) was more than 8 times higher in the natural one than in show -caves.
Figure 3.
Bacterial community composition of 5 caves studied: Bossea, Caudano Pertosa-Auletta, Vento (show-caves) and Costacalda (wild cave). The top 15 most abundant taxa are shown for taxonomic ranks: phylum (A), class (B), family (C) and genus (D).
Archaeal community composition
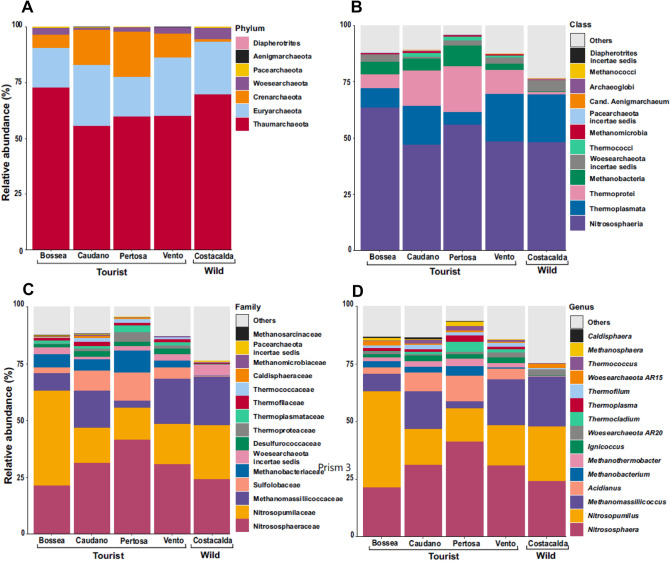
Archaea showed 2 main compositional trends describing the archaeome throughout the investigated caves (Fig. 4A,B,C,D). The first group is recurrent in all investigated caves and includes Thaumarchaeota and Euryarchaeota as the most abundant phyla, with the genera Nitrososphaera (ranging from 21.38% in Bossea to 41.35% in Pertosa-Auletta caves), Nitrosopumilus (between 14.23% and 41.76% in Bossea e Pertosa-Auletta) and Methanomassiliicoccus (ranging from 3% in Pertosa-Auletta to 21.35% in Costacalda). The same distribution across all caves was observed for taxa in the phylum Woesearchaeota, even if far less represented. The second group included taxa more frequent in show -caves as for the genera Acidianus (Phylum Chrenarcheota, class Thermoprotei, ranging from 2.62% Bossea to 11.34% in Pertosa-Auletta), Ignicoccus (average in show-caves 2.04%) and Thermocladium (4.14% highest value in Pertosa-Auletta); the genera Methanothermobacter and Methanobacterium (Phylum Euryarcheota, class Methanobacteria) were also found mainly in show -caves, the first with an average of 2.33% and the second with a frequency ranging between 0.55% and 3.9%. Other less abundant genera such as Methanosphaera and Thermococcus could be included in this second group.
Figure 4.
Archaeal community composition of 5 caves studied: Bossea, Caudano Pertosa-Auletta, Vento (show-caves) and Costacalda (wild cave). The top 15 most abundant taxa are shown for taxonomic ranks: phylum (A), class (B), family (C) and genus (D).
Micro-algal community composition
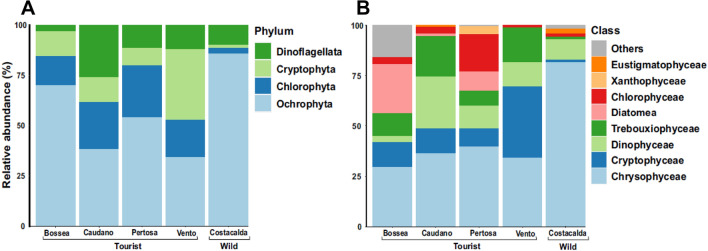
Algae is by definition a polyphyletic group; therefore, the micro-algal assemblage (Fig. 5A,B) was derived by clustering members belonging to different kingdoms in order to investigate the micro-photosynthetic component. The compositional scenario was characterized by the Class Chrysophyceae (phylum Ochrophyta) dominating in most of the caves, in particular in Costacalda (81.5%) and Pertosa-Auletta (39.76%) caves. Vento cave was dominated by Cryptophyceae (35.3%) members of the phylum Cryptophyta. Thereafter, community composition became patchy as no other widespread classes were found across the dataset. For instance, Dinophyceae showed a high abundance in the Caudano cave (25.86%), but it dropped in Vento (12.1%), Pertosa-Auletta and Costacalda (11.57%) caves; on the other hand, Trebouxiophyceae showed a decreasing trend along Caudano (20.10%) del Vento (17.37%), Bossea (11.47%) and Pertosa-Auletta (7.25%) caves. The class Diatomea was particularly present in Bossea cave instead (24.48%).
Figure 5.
Micro-algal assemblage composition of 5 caves studied: Bossea, Caudano Pertosa-Auletta, del Vento (show-caves) and Costacalda (wild cave). All taxa observed are shown for taxonomic ranks: phylum (A) and class (B).
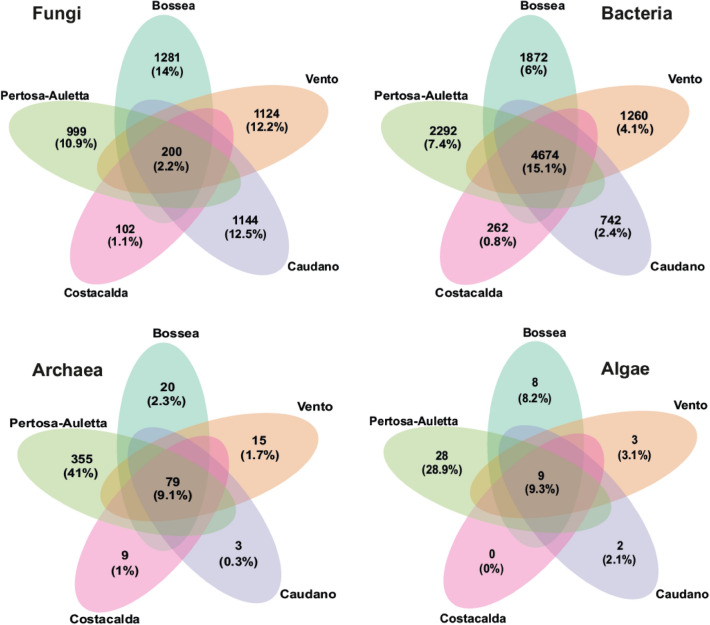
Shared and unique taxa
Venn diagrams (Fig. 6) displayed the ASVs that typically characterized each cave (Unique ASVs) and those that are representative of the community core, shared throughout the dataset. Regarding the fungal community, the core was scarcely populated (200 unique ASVs), accounting for only 2.2% of the total composition. The ASVs shared between all caves were represented by 54 genera; in particular Mortierella (13%) and Candida (10%). ollowed by Penicillium (5.4%), Humicola (3.8%), Cephalotrichum (3.1%), Geomyces (2.3%), Pseudogymnoascus (1.5%) and Aspergillus (1.5%) genera commonly found in cave environments. When considering the unique ASVs, Bossea cave had the highest number of unique ASVs (1281) representing 14% of the total composition, spread over 178 fungal genera. The other caves, showed a conspicuous number of unique ASVs, in particular the Caudano cave (1144 ASVs, 12.5% of the total), displayed the highest number of genera (192) identified among the typical ASVs, followed by Vento cave showed 1,124 unique ASVs (12.2%) and Pertosa-Auletta cave characterized by 999 unique ASVs (10.9%) with 171 identified genera. The wild Costacalda cave counted 102 unique ASVs (1.1%), with only 30 genera among the site-specific ASVs reported.
Figure 6.
Venn diagrams show shared and unique taxa distribution for each microbial component studied: Fungi, Bacteria, Archaea and Micro-Algae, among the 5 caves investigated: Bossea, Caudano Pertosa-Auletta, Vento (show -caves) and Costacalda (wild cave).
The bacterial community (Fig. 6) gravitated around a robust core of 4,674 ASVs (15.1%) and 283 genera, among which Acidobacteria Gp6 (15.8%), Gaiella (6%) and Nitrospira (1.9%) were the most abundant. However, the entire cluster of subgroups-Acidobacteria accounted for 37% of the shared ASVs. On the other hand, there were few unique ASVs for each cave, with Pertosa-Auletta cave showing 2,292 ASVs (7.4%), followed by Bossea cave (1,870 ASVs, 6%), Vento cave (1,260 ASVs, 4.1%), Caudano cave (742 ASVs, 2.4%) and Costacalda cave (262 ASVs, 0.8%). Moreover, some peculiar genera were uniquely reported for the different caves, such as Legionella (3.3%) in Bossea, Gemmatimonas (4.5%) in Caudano, Lactobacillus (9.5%) Desulfomicrobium (3.8%) and Clostridium sensu stricto (3.6%) in Pertosa-Auletta.
The distribution of ASVs belonging to the archaeal component among caves (Fig. 6) was sharply defined. Indeed, while the core group (79 ASVs, 9.1%) and unique ASVs of Bossea (20 ASVs, 2.3%), Vento (15 ASVs, 1.7%), Costacalda (9 ASVs, 1%) and Caudano (3 ASVs, 0.3%) caves were represented by a low number of ASVs, the Pertosa-Auletta cave was characterized by 355 unique ASVs (41%), taxonomically labeled by Acidianus (33%) and Thermoplasma (14%) genera. However, the genera of archaeal methanogens constituted a solid cluster either across the common core (24.3%) and unique ASVs for each cave.
The distribution of taxa detected for the phototrophic assemblage (Fig. 6) was extremely polarized between caves. One pole was represented by Costacalda Cave for which no unique ASVs were recorded. The other one was the cave of Pertosa-Auletta which accounted for 28 unique ASVs (28.9%), mostly belonging to the Chlorophyceae (39%), Chrysophyceae (32%) and Diatomea (18%) classes.
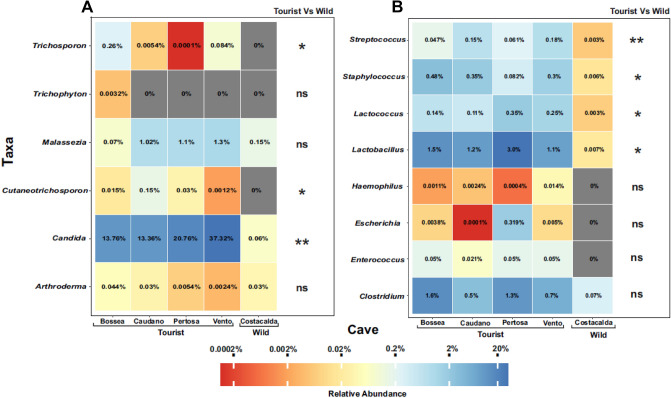
Human related microbial taxa
Due to the high abundance of some common human-related microbial taxa found in the show -caves investigated (i.e. Malasseziomycetes class and Candida genus for fungi, Lactobacillus and Bifidobacterium genera for bacteria), we decided to explore the occurrence of less abundant guilds too as potential proxies for direct human impact.
Throughout the fungal dataset (Fig. 7A) tourist caves were especially characterized by an higher significant presence of dermatophytes with the genus Trichosporon (Bossea 0.26%, Vento 0.084%, Caudano 0.0054% and Pertosa-Auletta 0.0001%, vs. 0% in Costacalda; p < 0.05) and Cutaneotrichosporon on average 0.05% for the show-caves, vs. 0% in Costacalda (p < 0.05). The genus Arthroderma, here recorded in all caves analyzed, is instead a geophilic dermatophyte mostly occurring in soil, but also frequent in cave environments, and it is rarely associated with human and animal cutaneous infections19. Moreover, Malassezia spp., here mainly found in human impacted caves (Bossea 0.07%, Caudano 1.02%, Pertosa-Auletta 1.10% and Vento 1.30%, vs. Costacalda 0.15%; p > 0.05), currently exist as a commensal fungi of the mammalian skin, and associated with atopic dermatitis in inflammatory skin disorders only.
Figure 7.
Heatmaps showing the relative abundances of fungal (A) and bacterial (B) taxa commonly associated to the human body, among the 5 caves investigated: Bossea, Caudano Pertosa-Auletta, Vento (show -caves) and Costacalda (wild cave). Kruskal–Wallis test results comparing tourist caves vs. wild cave are shown as follows: ***(p < 0.001); **(p < 0.01); *(p < 0.05); ns (p > 0.05).
Similarly, for bacterial assemblage (Fig. 7B), many widespread human tissues-inhabiting members of Bacilli class characterized the tourist caves, in contrast to Costacalda wild cave, where their relative abundance never exceeded the 0.007%; in particular Staphylococcus (ranging from 0.082% in Pertosa-Auletta to 0.48% in Bossea; p < 0.05), Streptococcus (from 0.061% in Pertosa-Auletta to 0.47% in Bossea; p < 0.01), Lactococcus (as a mean of 0.21% in the show -caves, vs. 0.003% in Costacalda; p < 0.05) and Lac tobacillus (with 1.7% on average in show -caves, vs. 0.007% in Costacalda; p < 0.05) showed a higher significant occurrence within show-caves.
Furthermore, even the Clostridium genus (from 0.5% in Caudano to 1.6% in Bossea, vs. 0.07% in Costacalda; p > 0.05) seemed to exhibit a higher occurrence across the anthropized settings.
Discussion
The effects of alterations occurring on structure, biodiversity, microclimate, energetic condition11,12 during the conversion of a natural cave into a show- cave are still almost unexplored. However, the increasing anthropization of new hypogean sites associated with human exploitation constantly impacts cave microbiomes, making their composition and functionalities unpredictable for future conservation and management9,10,13,20.
Herein, we deeply investigate, for the first time, microbiomes of four Italian show -caves using next generation sequencing approaches . We provided a first attempt to identify potential human-induced alterations by comparing show -caves with a recently discovered natural cave. The extensive sampling performed, together with the amplicon-sequencing analysis, led to a wide identification of microbial diversity, encompassing 17 phyla, 52 classes and 554 genera for the ITS dataset. On the other hand, the 16S barcode recorded 37 and 7 phyla, 73 and 8 classes, 1,339 and 49 genera for Bacteria and Archaea domains, respectively. For algae , a total of 4 phyla and 9 classes were identified; this low diversity of the phototropic counterpart is likely due to the condition of darkness characterizing the ground sampling area, although abundant algal growths were reported at the level of the rock formations in the examined show -caves21.
The mycobiome composition and the distribution of unique fungal ASVs across the 4 surveyed show -caves appeared more complex and diversified than those observed in the natural one. The predominance of Candida genus, which includes many species known as common colonizers of humans, in show -caves may reflect a relation with touristic flow. Candida has also been reported as an oligotrophic multi-stress tolerant fungus, skilled to colonize a wide range of natural, urbanized and human environmental contexts22,23. Cave visitors, as previously reported, may act as primary vector in the spreading of this genus, by bio-aerosols formation (breathing, sneezing or coughing), surface contact and shedding of skin scales, hair and lint from clothing24. Potential direct contamination by human-related species25 of cave mycobiome are further supported by the higher abundance of dermatophyte fungal genera within tourist caves (e.g. Cutaneotrichosporon and Trichosporon). Conversely, the mycobiome of the natural cave was dominated by taxa belonging to the genera Mortierella and Cephalotricum, which include psychrotolerant cellulose-degrading fungi26,27, and by the genera Rodentomyces and Gymnoascus, which include psychrotolerant, coprophilic and keratinolytic species28,29, that may be related to the main caves macro-fauna components, i.e. rodents and bats. Furthermore, fast-growing saprophytic fungal genera (Penicillium, Chaetomium and Humicola) were also reported, even if in lower relative abundance.
Such specific fungal traits have been reported as early wild cave colonizers30, able to cope with the constraining low-temperature and oligotrophic conditions in these habitats. On the other hand, within the 4 investigated show -caves, the notable abundance of Agaricomycetes (and generally Basidiomycota) may be the result of increasing ventilation during cave opening. In fact, airborne fungal spore contamination is facilitated by the atmospheric continuum between the outdoor and underground environment31,32. Moreover, the high occurrence of fast-growing and heavily-sporulating fungi (Aspergillus and Penicillium), or common competent degraders and plant pathogens (Chaetomium, Phoma, Sarocladium and Dipodascus) could be the result of combined effects of tourist flows, spreading fungal alien species and extra complex organic matter input, and the implement of nutrients due to the consumption of food and beverages during events hosted in caves33,34. Finally, artificial lighting may have promoted the growth of lichenized fungi (Lecanoromycetes) inside the caves, as reported in previous studies18,35. In particular, their colonization of carbonatic rock substrate and speleothems may result in severe aesthetic and structural damage, due to the large extent of thalli and the high lichen acids production. The genus Tetracladium was always present in all caves, even if at low frequency. It belongs to the group of “Ingoldian fungi” or aquatic hyphomycetes, phylogenetically diverse fungi growing on decaying leaves and plant litter in streams36; their presence in our environmental samples may be related to the presence of water flows present in all caves.
The compositional similarity found throughout the dataset for the Bacteria and Archaea domains, reflects their paramount role in biogeochemical cycles of caves biosphere: C, N, CH4-genesis and metabolism and S oxide-reduction, sustaining trophic networks and peculiar caves’ geochemical processes, even in these deeply impacted habitats. In fact, a wide range of polymers degraders particularly adapted to cope with low temperature and oligotrophic conditions were found. For instance, Acidobacteria-subgroups (37% of the shared ASVs among the caves), Sphingomonas, Lysobacter, Polaromonas and a few widespread Mn-Fe oxidizing bacteria, such as family Planctomycetaceae members, Hyphomicrobium (Bacteroidota), Flavobacterium and Pseudomonas (Pseudomonadota) genera. Yet, the Pseudomonas high abundance reported in show -caves might be due to the indirect effects of human activities in these habitats. Extra nutrient inputs by tourist flows might stimulate fungal metabolic activity37 and lead to high secretion of acids (i.e. oxalate and pyrophosphate), which in turn could trigger the metabolic activity and proliferation of Pseudomonas by chelating Mn38. Also related to the biogenic carbon cycle, the group of Methane-oxidizing bacteria (MOB) found along α,β,γ-Proetobacteria classes were widely present throughout the caves, underlining their key role in continuous CH4 consumption in these subterranean ecosystems39. In contrast, Methanobacterium and Methanothermobacter were more present in tourist caves among the most abundant genera of the Archaea-methanogens counterpart. This compositional alteration could be the result of the indirect effects of tourist flows, which may increase indoor CO2 levels up to 200%40, promoting the proliferation of these microorganisms, which use H2 to reduce carbon dioxide molecules into methane. Compositional variations for the archaeal community have already been reported in touristic caves, due to anthropic pressure and after simulated organic matter treatment on cave microcosms13,41. The similar compositional pattern throughout the dataset of the main bacterial and archaeal players involved in the N cycle, i.e. Nitrospira (nitrite-oxidant), Gaiella (nitrate-reducing), Nitrososphaera and Nitrosopumilus (ammonia-oxidant), underlines how human cave activities did not impact these microbial traits. Besides, some bacterial taxa could be also considered as bioindicators of human presence being a result of contamination from the entrance30,42. For instance, many human-related genera as, Lactobacillus (also as unique ASVs), Lactococcus, Legionella (only as unique ASVs), Staphylococcus and Streptococcus were mainly, or in some cases uniquely, found in show -caves. Similarly, the recurrence of opportunistic human pathogenic fungi such as Candida and dermatophytes (i.e. Trichosporon and Cutaneotrichosporon) throughout tourist caves, emphasize a probable direct mycobiome cave contamination as the result of the extensive human exploitation of these subterranean settings.
Concerning microalgae, except for a widespread presence among caves of Ochrophyta phylum and gold-brown algae Chrysophyceae related class, the eukaryotic photosynthetic abundance background appeared patchy. Some caves were largely characterized by presence of particular classes, e.g. Bossea cave by Diatomea, Caudano by Dinophyceae and Trebouxiophyceae, Pertosa-Auletta by Chlorophyceae and Vento cave with Cryptophyceae and Trebouxiophyceae. However, the only notable differences were related to the relative abundance values and lack of unique ASVs of microalgal components harbored by the wild Costacalda cave, dominated by the class Chrysophyceae. This class is composed of taxa that prefer oligotrophic conditions and most species in this group show a mixotrophic metabolism, being able to shift between photosynthesis and ingesting smaller organisms or particles for food. We may hypothesize that the micro-algal component in pristine caves was based on a poorly biodiverse common core18, populated by species well adapted to cope with the typical challenges of the cave environment, such as strict oligotrophy and darkness43. The subsequent anthropization (artificial lighting and tourist flows) of these settings would have had a key role, both in importing allochthonous species and spreading the local and alien microalgal component even in cave’s zones previously uncolonized18,44. Yet, the ASVs cores recorded in bacteria, archaea and algae indicate a high degree of adaptation and specialization for these microbial compartments in caves. Conversely, the narrow core observed for the fungal component may indicate a less strict adaptation to the cave environment and broad capacity to colonize different habitats, according to the high physiological, metabolic and stress-tolerance plasticity of these organisms.
In conclusion, this study provides for the first time a multi-spatial and extensive microbiota characterization of 5 Italian caves, among which 4 tourist and 1 pristine cave, shedding light on microbial diversity of different rapidly evolving subterranean environments. We highlighted how the 4 investigated show -caves share common microbial traits, by filtering microorganisms derived from the outside or human-related, multi-stress tolerant or capable of exploiting the extra supplies of organic matter or degradable compounds provided by tourist flows. Although human activities have been affecting these caves for a long time, the principal common microbial traits, related to the biogeochemical key processes, are still clearly detectable. Finally, we have provided some insights into the potential direct microbiome cave contamination by human-related microbial species. It is worth expanding this dataset by sampling additional underground environments, including both show-caves and wild caves, at local (Italian) geographic scales, to confirm the trends of microbial diversity pattern here found in anthropized caves with respect to the wild one. This study represents the groundwork for further microbiome cave analyses to shed light also on the functional guilds of each microbial component and their distribution and variation in both pristine and impacted settings.
Materials and methods
Study area
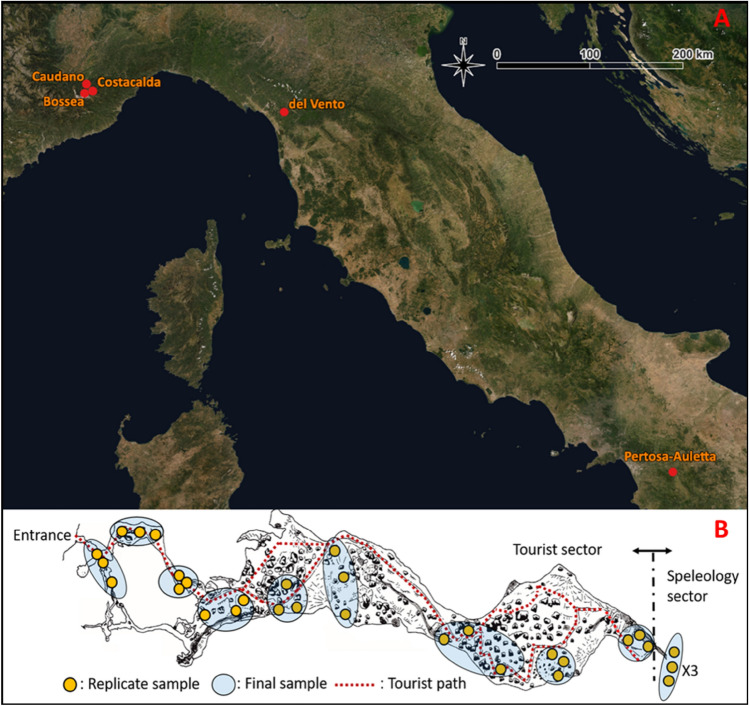
Five Italian karst caves were selected from north to south Italy (Fig. 8A): the Caudano (44°17′34.3"N; 7°47′25.7"E), Bossea (44°14′31.0"N; 7°50′24.0"E) and Costacalda (44°14′24.8"N; 7°50′54.9"E) caves, in the Maritime Alps complex (Piedmont region); Vento cave (44°02′02.0"N; 10°21′28.2"E) in the Apuan Alps (Tuscany) and the Pertosa-Auletta cave (40°32′14.7"N; 15°27′17.9"E) in the mountain region of the Campanian Apennines.
Figure 8.
Geographic map of Italy with locations of caves surveyed (A) and schematic overview of sampling method applied (B). Images were produced mapping the caves’ coordinates layer by qGIS 3.2245 on a WMS version of ESRI World Imagery map (https://server.arcgisonline.com/ArcGIS/rest/services/World_Imagery/MapServer/tile/{z}/{y}/{x}).
Besides sharing the same karstic origin, the caves investigated, with the exception of Costacalda, are characterized by a long human exploitation background. Bossea was the first Italian show cave established in 1875 (https://www.grottadibossea.com), while the Caudano cave was permanently opened to the public in 2002 (https://grottedelcaudano.com). Besides regular tourist flows, these caves have experienced exceptional human activity episodes over the last century, like the “700 h underground” experiment (Caudano cave in 1961), where 12 speleologists and some domestic animals lived inside the cave for 1 month, aiming to understand the effect of underground life on human and animals. In addition, Bossea cave has been the setting of many music and food-wine festivals. The Vento cave has been known since the seventeenth century and its cold air currents were used by local people as a natural cooler to preserve food b. The cave was later explored in the nineteenth century and opened to the public for the first time in 1967 (http://grottadelvento.com). The Pertosa-Auletta cave has been exploited by humans since the Neolithic, successively used during the Iron Age (the ruins of a stilt village were found), then converted into a Christian church during the twelfth century and into an air-raid shelter during WWII It was permanently turned into a tourist cave in the second half of the 1900s (https://fondazionemida.com/grotte-pertosa-auletta). Costacalda is a natural (wild; i.e. not impacted) cave discovered in the spring of 2018, when it appeared as a hole about 10 cm wide on the ground. To date, the cave is only partially explored and entirely preserves its pristine structural condition (http://www.speleologiassi.it/82-sommari).
Sampling design
We applied an extensive sampling of sediments along the whole extension of each cave (Fig. 8B) according to Piano et al. (2022)20, starting from the areas near the entrance, towards the deepest zones and at different distances from the tourist/speleological paths. A total of 12 in situ sampling sites were selected for the 4 show -caves, while 3 sites were selected in the Costacalda wild cave. For each sample, 9 technical sediment replicates subdivided into 3 triplets, up to 5 cm depth, were collected using sterile Falcon® tubes (50 ml), for a total of 459 samples. Samples were then stored in a cooler-bag until arrival at the laboratory, where the 3 replicates for each sample were pooled and homogenized. Twelve final samples were assembled for each show cave (Bossea, Caudano, del Vento and Pertosa-Auletta) and 3 for Costacalda, for a total of 51 samples. Sampling collection was carried out between June and September 2020.
Metagenomic DNA extraction and amplicon sequencing
Sediment samples were sieved, under sterile conditions, by removing coarse rock debris and metagenomic DNA was extracted from 0.5 g of sample using Qiagen DNeasy PowerSoil Pro Kit (Carlsbad, CA, USA). The Internal Transcribed Sequence 1 ribosomal region (ITS1), Eukaryotic SSU rRNA (18S) and hypervariable region V4 of 16S ribosomal gene were targeted to assess the fungal, general eukaryotic and prokaryotic community composition, respectively. The ITS1 region was amplified using barcoded primers ITS1F/ITS2, suitable for shorter read length46, the 18S region with the Euk_1391f./EukBr primers (http://www.earthmicrobiome.org), while for the V4 region of 16S, barcoded F515/R806 primer set was used according to Caporaso et al. (2012)47. PCRs was performed with 1 μL of each primer, 12.5 μL of Taq DNA Polymerase (Thermo Fisher Scientific Inc., Waltham, MA, USA), 9.5 μL of nuclease-free water (Sigma–Aldrich, St. Louis, MO, USA) and 5 ng of DNA template by an automated thermal cycler (BioRad, Hercules, CA, USA), for a total volume of 25 μL. The ITS1 locus, the eukaryotic SSU rRNA and 16S V4 region were amplified following the protocols and technical specifications according to Coleine et al. (2021)48.
Amplicons were quantified by a Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA) and then pooled. Paired-end sequencing (2 × 300 bp) was carried out on an Illumina MiSeq platform.
Bioinformatic processing data and downstream analysis
Demultiplexed ITS, 18S and 16S sequence datasets were processed using AMPtk49 v.1.5.4 software. Briefly, reads were merged using VSEARCH 2.21.150 and barcodes/indexes and primer sequences were removed from raw data; reads were then subjected to quality trimming to a maximum of 250 bp and discarding reads less than 100 bp in length, and sequencing artifacts were dropped by using USEARCH v.9.1.13 with default parameters51. Sequence quality filtering was performed with the expected error parameter of 0.952 and the cleaned reads were clustered at 99% similarity using DADA2 denoising algorithm53 that uses a statistical error model to correct sequencing errors to infer the Amplicon Sequence Variants (ASVs). Global singletons and rare taxa (< 5 reads) were skipped as likely false positives due to sequencing errors, following Lindahl et al. (2013)54. Finally, taxonomic identification was performed with a sequence identity of 97% as threshold, using hybrid database Global Alignment and SINTAX51 on reference databases UNITE 8.2.55 and RDP 1156.
All samples from each cave were analyzed as replicates to describe the global microbial diversity of each underground environment. The relative abundance data shown in this paper represent the average of the abundance values among all samples for each cave. Kruskal–Wallis test was used to assess significant abundance differences among tourist and natural settings, for the potential human-associated microbial taxa. All analyses were performed using the following R packages: “phyloseq”57, “microeco”58.
Supplementary Information
Acknowledgements
We are grateful to Benedetta Baroni for her help during the eld work. We also thank Cooperativa Alto Corsaglia (Grotta di Bossea), Mondole touristic oce (Grotta del Caudano), Vittorio Verole and Mario Verole (Grotta del Vento), and to the president, Dr. Francescantonio D’Orilia, and the scientific director, Prof. Mariana Amato, of MIdA Foundation (Grotte di Pertosa-Auletta) for endorsing our research and for their logistic support during the sampling activities.
Author contributions
F.B, C.C and L.S conceived and designed the study. Bioinformatic raw data and down-stream analyses were performed by F.B. Data interpretation were done by F.B, C.C, L.S and supported by all co-authors. The manuscript was written by F.B, C.C and L.S and all authors have read and agreed to the published version of the manuscript.
Funding
This work was realized within the framework of the PRIN SHOWCAVE “A multidisciplinary research project to study, classify and mitigate the environmental impact in tourist caves"—code 2017HTXT2R (PI: Marco Isaia), funded by the Italian Ministry of Education, University and Research. The SHOWCAVE project is endorsed by AGTI (Associazione Grotte Turistiche Italiane) and the SSI (Società Speleologica Italiana). The grant of EP is co-financed by the PON “Research and Innovation” Programme (Axis IV “Education and Research for recovery” – Action IV.6 “Research contracts on Green themes”). C.C. is supported by the European Union’s Horizon Europe research and innovation program under MarieSklodowska-Curie Grant Agreement 101062840.
Data availability
Raw sequences were deposited in Figshare’s repository and are openly available at 10.6084/m9.figshare.21354537.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-26511-5.
References
- 1.Gabriel CR, Northup DE. Microbial ecology: Caves as an extreme habitat. In: Gabriel CR, Northup DE, editors. Cave Microbiomes: A Novel Resource for Drug Discovery. Springer; 2013. pp. 85–108. [Google Scholar]
- 2.Zhang ZF, Zhao P, Cai L. Origin of cave fungi. Front. Microbiol. 2018;9:1407. doi: 10.3389/fmicb.2018.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culver DC, Pipan T. The Biology of Caves and Other Subterranean Habitats. Oxford University Press; 2019. [Google Scholar]
- 4.Romero A. Cave Biology: Life in Darkness. Cambridge University Press; 2019. [Google Scholar]
- 5.Engel AS. Chemoautotrophy. In: White WB, Culver DC, editors. Encyclopedia of Caves. Elsevier; 2012. pp. 125–134. [Google Scholar]
- 6.Miller, A. Z., Dionísio, A., Jurado, V., Cuezva, S., Sanchez-Moral, S., Cañaveras, J. C., Saiz-Jimenez, C. Biomineralization by cave dwelling microorganisms. Adv. Geochem. Res., 77–105 (2013).
- 7.Venarsky MP, Huntsman BM. Food webs in caves. In: Moldovan O, Kováč L, Halse S, editors. Cave Ecology. Springer; 2018. pp. 309–328. [Google Scholar]
- 8.Wiseschart, A., & Pootanakit, K. Metagenomic-based approach to a comprehensive understanding of cave microbial diversity. In Recent advancements in microbial diversity (eds Bhatt, P., de Mandal, S.) 561–586 (Academic Press, 2020).
- 9.Falasco E, Ector L, Isaia M, Wetzel CE, Hoffmann L, Bona F. Diatom flora in subterranean ecosystems: A review. Int. J. Speleol. 2014;43(3):1. doi: 10.5038/1827-806X.43.3.1. [DOI] [Google Scholar]
- 10.Mulec J. Human impact on underground cultural and natural heritage sites, biological parameters of monitoring and remediation actions for insensitive surfaces: Case of Slovenian show caves. J. Nat. Conserv. 2014;22(2):132–141. doi: 10.1016/j.jnc.2013.10.001. [DOI] [Google Scholar]
- 11.Cigna AA. Tourism and show caves. Z. Geomorphol. 2016;60(2):217–233. doi: 10.1127/zfg_suppl/2016/00305. [DOI] [Google Scholar]
- 12.Cigna AA. Show caves. In: White W, Culver D, Pipan T, editors. Encyclopedia of Caves. Academic Press; 2019. pp. 909–921. [Google Scholar]
- 13.Alonso L, Pommier T, Kaufmann B, Dubost A, Chapulliot D, Doré J, et al. Anthropization level of Lascaux cave microbiome shown by regional-scale comparisons of pristine and anthropized caves. Mol. Ecol. 2019;28(14):3383–3394. doi: 10.1111/mec.15144. [DOI] [PubMed] [Google Scholar]
- 14.Leuko S, Koskinen K, Sanna L, D’Angeli IM, De Waele J, Marcia P, et al. The influence of human exploration on the microbial community structure and ammonia oxidizing potential of the Su Bentu limestone cave in Sardiia Italy. PLoS ONE. 2017;12(7):e180700. doi: 10.1371/journal.pone.0180700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfendler S, Karimi B, Maron PA, Ciadamidaro L, Valot B, Bousta,, et al. Biofilm biodiversity in French and Swiss show caves using the metabarcoding approach: First data. Sci. Total Environ. 2018;615:1207–1217. doi: 10.1016/j.scitotenv.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Thompson, B., Richardson, D., Vangundy, R. D., Cahoon, A. B. Metabarcoding comparison of prokaryotic microbiomes from Appalachian karst caves to surface soils in southwest Virginia, USA. J. Cave Karst Stud.81(4), 244–253 (2019).
- 17.Bontemps Z, Alonso L, Pommier T, Hugoni M, Moënne-Loccoz Y. Microbial ecology of tourist Paleolithic caves. Sci. Total Environ. 2021;816:151492. doi: 10.1016/j.scitotenv.2021.151492. [DOI] [PubMed] [Google Scholar]
- 18.Burgoyne J, Crepeau R, Jensen J, Smith H, Baker G, Leavitt SD. Lampenflora in a show cave in the Great Basin is distinct from communities on naturally lit rock surfaces in nearby wild caves. Microorganisms. 2021;9(6):1188. doi: 10.3390/microorganisms9061188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hainsworth S, Kučerová I, Sharma R, Cañete-Gibas CF, Hubka V. Three-gene phylogeny of the genus Arthroderma: Basis for future taxonomic studies. Med. Mycol. 2021;59(4):355–365. doi: 10.1093/mmy/myaa057. [DOI] [PubMed] [Google Scholar]
- 20.Piano, E., Biagioli, F., Nicolosi, G., Coleine, C., Poli, A., Prigione, V., Isaia, M. et al. Human disturbance drives differential diversity patterns of microbial communities in hypogean habitats. Preprint at https://www.authorea.com/doi/full/10.22541/au.165538108.89649607 (2022).
- 21.Piano E, Bona F, Falasco E, La Morgia V, Badino G, Isaia M. Environmental drivers of phototrophic biofilms in an Alpine show cave (SW-Italian Alps) Sci. Total Environ. 2015;536:1007–1018. doi: 10.1016/j.scitotenv.2015.05.089. [DOI] [PubMed] [Google Scholar]
- 22.Kulesza K, Biedunkiewicz A, Nowacka K, Dynowska M, Urbaniak M, Stępień Ł. Dishwashers as an extreme environment of potentially pathogenic yeast species. Pathogens. 2021;10(4):446. doi: 10.3390/pathogens10040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and emerged pathogenic Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013;9(9):e1003550. doi: 10.1371/journal.ppat.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulec J, Oarga-Mulec A, Šturm S, Tomazin R, Matos T. Spacio-temporal distribution and tourist impact on airborne bacteria in a cave (Škocjan Caves, Slovenia) Diversity. 2017;9(3):28. doi: 10.3390/d9030028. [DOI] [Google Scholar]
- 25.Theelen B, Cafarchia C, Gaitanis G, Bassukas ID, Boekhout T, Dawson TL., Jr Malassezia ecology, pathophysiology and treatment. Med. Mycol. 2018;56(1):10–25. doi: 10.1093/mmy/myx134. [DOI] [PubMed] [Google Scholar]
- 26.Werner S, Peršoh D, Rambold G. New aspects of the biology of Mortierella alliacea. Mycol. Prog. 2016;15(12):1293–1301. doi: 10.1007/s11557-016-1243-3. [DOI] [Google Scholar]
- 27.Jiang JR, Cai L, Liu F. Oligotrophic fungi from a carbonate cave, with three new species of Cephalotrichum. Mycology. 2017;8(3):164–177. doi: 10.1080/21501203.2017.1366370. [DOI] [Google Scholar]
- 28.Voyron, S., Lazzari, A., Riccucci, M., Calvini, M., Varese, G. First mycological investigation on Italian bats (2011).
- 29.Gupta P, Vakhlu J, Sharma YP, Imchen M, Kumavath R. Metagenomic insights into the fungal assemblages of the northwest Himalayan cold desert. Extremophiles. 2020;24(5):749–758. doi: 10.1007/s00792-020-01191-z. [DOI] [PubMed] [Google Scholar]
- 30.Jones D, Lavoie K, Barton HA, Ortiz-Ortiz M, Neilson JW, Legatzki A, Sanchez-Moral S, et al. Microbial Life of Cave Systems. Walter de Gruyter GmbH & Co KG; 2015. [Google Scholar]
- 31.Dominguez-Moñino I, Jurado V, Rogerio-Candelera MA, Hermosin B, Saiz-Jimenez C. Airborne fungi in show caves from Southern Spain. Appl. Sci. 2021;11(11):5027. doi: 10.3390/app11115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Moral S, Jurado V, Fernandez-Cortes A, Cuezva S, Martin-Pozas T, Gonzalez-Pimentel JL, Saiz-Jimenez C, et al. Environment-driven control of fungi in subterranean ecosystems: The case of La Garma Cave (Northern Spain) Int. Microbiol. 2021;24(4):573–591. doi: 10.1007/s10123-021-00193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin DW, Gray MA, Lyles MB, Northup DE. The transport of nonindigenous microorganisms into caves by human visitation: A case study at Carlsbad Caverns National Park. Geomicrobiol. J. 2014;31(3):175–185. doi: 10.1080/01490451.2013.815294. [DOI] [Google Scholar]
- 34.Marques ELS, Dias JCT, Silva GS, Pirovani CP, Rezende RP. Effect of organic matter enrichment on the fungal community in limestone cave sediments. Genet. Mol. Res. 2016 doi: 10.4238/gmr.15038611. [DOI] [PubMed] [Google Scholar]
- 35.D'agostino D, Beccarisi L, Camassa M, Febbroriello P. Microclimate and microbial characterization in the zinzulusa show cave (south italy) after switching to led lighting. J. Cave Karst Stud. 2015;77(3):133–144. doi: 10.4311/2014EX0123. [DOI] [Google Scholar]
- 36.Kravetz S, Gonzalez B, Marano A, Giorgi A. The genus Tetracladium in Pampean streams (Buenos Aires, Argentina) Phytotaxa. 2018;338(3):276–284. doi: 10.11646/phytotaxa.338.3.5. [DOI] [Google Scholar]
- 37.Carmichael MJ, Bräuer SL, Engel AS. Microbial diversity and manganese cycling: A review of manganese oxidizing microbial cave communities. Microb. Life Cave Syst. Life Extreme Environ. 2015;3:137–160. [Google Scholar]
- 38.Carmichael SK, Zorn BT, Santelli CM, Roble LA, Carmichael MJ, Bräuer SL. Nutrient input influences fungal community composition and size and can stimulate manganese (II) oxidation in caves. Environ. Microbial. Rep. 2015;7(4):592–605. doi: 10.1111/1758-2229.12291. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Pozas T, Cuezva S, Fernandez-Cortes A, Cañaveras JC, Benavente D, Jurado V, Sanchez-Moral S, et al. Role of subterranean microbiota in the carbon cycle and greenhouse gas dynamics. Sci. Total Environ. 2022;831:154921. doi: 10.1016/j.scitotenv.2022.154921. [DOI] [PubMed] [Google Scholar]
- 40.Baker A, Genty D. Environmental pressures on conserving cave speleothems: Effects of changing surface land use and increased cave tourism. J. Environ. Manag. 1998;53(2):165–175. doi: 10.1006/jema.1998.0208. [DOI] [Google Scholar]
- 41.Marques ELS, Dias JCT, Silva GS, Pirovani CP, Rezende RP. Organic matter enrichment affects archaea community in limestone cave sediments. J. Cave Karst Stud. 2017;79(2):95–99. doi: 10.4311/2016MB0138. [DOI] [Google Scholar]
- 42.Bercea S, Năstase-Bucur R, Moldovan OT, Kenesz M, Constantin S. Yearly microbial cycle of human exposed surfaces in show caves. Subterr. Biol. 2019;31(1):1–14. doi: 10.3897/subtbiol.31.34490. [DOI] [Google Scholar]
- 43.Czerwik-Marcinkowska J, Wojciechowska A, Massalski A. Biodiversity of limestone caves: Aggregations of aerophytic algae and cyanobacteria in relation to site factors. Pol. J. Ecol. 2015;63(4):481–499. [Google Scholar]
- 44.Borderie F, Tête N, Cailhol D, Alaoui-Sehmer L, Bousta F, Rieffel D, Alaoui-Sossé B, et al. Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatments. Sci. Total Environ. 2014;484:43–52. doi: 10.1016/j.scitotenv.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 45.QGIS.org, %Y. QGIS 3.22 Geographic information system. QGIS Association. http://www.qgis.org.
- 46.Smith DP, Peay KG. Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLoS ONE. 2014;9(2):e90234. doi: 10.1371/journal.pone.0090234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Knight R, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coleine C, Biagioli F, de Vera JP, Onofri S, Selbmann L. Endolithic microbial composition in Helliwell Hills, a newly investigated Mars-like area in Antarctica. Environ. Microbiol. 2021;23(7):4002–4016. doi: 10.1111/1462-2920.15419. [DOI] [PubMed] [Google Scholar]
- 49.Palmer JM, Jusino MA, Banik MT, Lindner DL. Non-biological synthetic spike-in controls and the AMPtk software pipeline improve mycobiome data. PeerJ. 2018;6:e4925. doi: 10.7717/peerj.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: A versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 52.Edgar RC, Flyvbjerg H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics. 2015;31(21):3476–3482. doi: 10.1093/bioinformatics/btv401. [DOI] [PubMed] [Google Scholar]
- 53.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindahl BD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, Kauserud H, et al. Fungal community analysis by high-throughput sequencing of amplified markers—A user’s guide. New Phytol. 2013;199(1):288–299. doi: 10.1111/nph.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abarenkov K, Zirk A, Piirmann T, Pöhönen R, Ivanov F, Nilsson RH, Kõljalg U. UNITE top50 release. UNITE Community. 2020 doi: 10.15156/BIO/786374. [DOI] [Google Scholar]
- 56.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Tiedje JM, et al. Ribosomal database project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:633–642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMurdie PJ, Holmes S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C, Cui Y, Li X, Yao M. Microeco: An R package for data mining in microbial community ecology. FEMS Microbial. Ecol. 2021;97(2):fiaa255. doi: 10.1093/femsec/fiaa255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequences were deposited in Figshare’s repository and are openly available at 10.6084/m9.figshare.21354537.