Abstract
The opc gene from Neisseria meningitidis was cloned into the pRSETA vector, and recombinant protein was expressed at high levels in Escherichia coli. The protein was readily purified by affinity chromatography and used for immunization with conventional Al(OH)3 adjuvant or after incorporation into liposomes and Zwittergent micelles. The resulting sera were analyzed for their ability to recognize purified recombinant protein and “native” protein in an enzyme immunoassay with outer membranes and by whole-cell immunofluorescence. Immunization with Al(OH)3 induced high levels of antibodies which reacted with the purified protein but did not recognize whole cells. In contrast, liposomes and micelles induced antibodies which reacted with the native protein in whole cells. The addition of monophosphoryl lipid A (MPLA) to either liposomes or micelle preparations increased the magnitude of the immune response and induced a wider range of immunoglobulin subclasses. This was associated with the ability of the sera to induce complement-mediated killing of the homologous strain. The most effective bactericidal activity was observed with Opc protein incorporated into liposomes containing MPLA. The magnitude of the bactericidal effect was strongly influenced by the level of expression of the Opc protein and was abolished by limited variation in the sequence of the protein expressed by heterologous strains.
The development of an effective vaccine against serogroup B Neisseria meningitidis remains the most important problem for prevention of the potentially fatal consequences of meningococcal meningitis and septicemia. While recent vaccines based on conjugates of group-specific capsular polysaccharides are likely to provide effective immunity against serogroups A and C, this strategy is not applicable to serogroup B meningococci, which cause the majority of infections in most temperate countries. The serogroup B capsular polysaccharide is composed of N-acetylneuraminic acid residues similar to those found on developing fetal brain tissue and is therefore nonimmunogenic. In addition, attempts to increase the immunogenicity of the group B polysaccharide raise concerns over the consequences of inducing autoimmune antibodies (10).
An alternative approach is based on observations that antibodies raised against meningococcal outer membrane vesicles (OMV) promote complement-mediated killing of meningococci, the generally accepted correlate of protection against infection (11). Experimental OMV vaccines have been developed and undergone phase III trials in humans (2, 3, 9, 21). Although these vaccines induced limited protection in adults (1), the immune responses were of short duration (23) and did not protect children under 2 years of age, the group at greatest risk of infection (9, 17). In addition, because of the heterogeneous nature of OMV vaccines, the antibodies were directed against a number of different antigens, the relative response varied between individuals, and only a proportion of these antibodies were protective. Detailed analysis of individuals immunized with an OMV vaccine has revealed that the protective immune response was directed against two outer membrane proteins, the class 1 and Opc proteins (17, 23).
Studies on the ability of individual proteins to induce a protective immune response are facilitated by the availability of purified protein free of other outer membrane components. Such studies with the class 1 protein have been achieved following the cloning of the porA gene, which encodes the protein, and its expression in Escherichia coli using high-level expression vectors (5, 31). The class 1 protein is a member of the family of Neisseria porin proteins which adopt a β-sheet structure within the outer membrane, with eight surface-exposed loops (27). Purified or recombinant porin proteins can be refolded to a native conformation by incorporation into micelles using a suitable detergent (22) or by incorporation into artificial membranes (liposomes) (14, 32). In contrast to the denatured protein, immunization with recombinant class 1 protein after incorporation into liposomes induced high levels of antibodies which were bactericidal for the homologous strain (5, 20, 31). A potential problem for the use of class 1 protein as a vaccine is that it is subject to high levels of interstrain variation, and immunization with OMV or class 1 protein in liposomes generates bactericidal antibodies which are serosubtype specific, so that an effective vaccine would have to contain multiple proteins.
The Opc protein is the only other protein that has been identified as contributing to the protective effect of OMV vaccines (23). The Opc protein is believed to exhibit less sequence variability than class 1 protein (25), although the levels of expression are hypervariable. Regulation of expression at the transcriptional level, by variation in the length of a polycytidine stretch in the promoter region, results in isolates that may express the protein at high levels (Opc++), lower levels (Opc+), or not at all (Opc−) (24). Opc protein has been shown to play an important role in meningococcal adhesion and invasion of both epithelial and endothelial cells and perhaps represents a common virulence factor (29, 30). Although the protein is not a porin, it is also believed to adopt a β-sheet structure in the outer membrane, with six surface-exposed loops (16). In this paper, we report the cloning of the opc gene using the E. coli expression system that has been effective with the class 1 protein, immunization with renatured recombinant protein using adjuvant formulations compatible with human immunization, and the effect of both sequence variation and degree of expression on the potential protective effect.
MATERIALS AND METHODS
Bacterial strains, vectors, and growth conditions.
N. meningitidis strains MC58 (B:15:P1.7,16b), H44/76 (B:15:P1.7,16), and MC114 (B:2a:P1.2) have all been described previously (6, 7, 15). Strains MC114, MC119, MC122, MC131, and MC139 were isolated from cases and carriers by the Meningococcal Reference Laboratory, Glasgow, Scotland (4). All strains were grown on protease-peptone agar at 37°C for 18 h in an atmosphere of 5% (vol/vol) CO2. Outer membranes (OM) were prepared by extraction of whole cells by lithium acetate as previously described (26).
The pRSETA expression vector and M13/T7 bacteriophage were from Invitrogen, Groningen, The Netherlands. E. coli JM109(DE3) (Promega, Southampton, United Kingdom) was transformed with recombinant plasmids as described by Ward et al. (31), and the transformants were grown in Luria-Bertani (LB) medium (Difco, West Molesey, United Kingdom) containing 100 μg of ampicillin ml−1 and on LB-ampicillin agar. E. coli JM101 (Promega) was maintained on M9 minimal medium. M13/T7 phage, containing the gene for T7 RNA polymerase, was propagated by infecting a 100-ml fresh culture of E. coli JM109 cells with 100 μl of phage stock (1011 to 1012 PFU ml−1). The culture was incubated overnight at 37°C with shaking at 250 rpm. The supernatant solution was then recovered by centrifugation, heated at 70°C for 20 min to ensure that any remaining cells were killed, and then stored at 4°C. The phage concentration was determined by titration.
Sequencing of opc gene.
The sequences of the opc genes were determined following selective amplification of the gene by PCR using methods previously described (8). The purified PCR products were used in sequencing reactions with the Thermo Sequenase kit (Amersham Pharmacia, Little Chalfont, United Kingdom) according to the manufacturer's instructions, and the resulting products were separated and analyzed on an ABI 373 sequencer (Perkin Elmer ABI, Warrington, United Kingdom). Sequencing of both strands of the opc gene was accomplished with a set of custom-synthesized oligonucleotide primers. The sequences of the opc genes have been deposited in the EMBL/GenBank database (see below).
Cloning and expression of opc gene in E. coli.
The sequence of the opc gene from N. meningitidis strain Z3476 (EMBL accession number M80195) was used to design two primers to amplify the entire opc open reading frame and introduce BamHI and HindIII restriction sites for cloning into the pRSETA expression vector. The forward primer was 5′-GCCGGATCC443GCACAAGAGCTTCAAACC460-3′, and the reverse primer was 5′-GTCTGAAAGC1305TTCAGACGGCATCGGCT1288-3′; numbers refer to positions within the Z3476 sequence, and underlining indicates the additional bases introduced for cloning. A single colony of N. meningitidis strain MC58 was resuspended in 10 μl of water, and the bacteria were lysed by the addition of 10 μl of 0.25 M KOH followed by boiling for 5 min. The pH was adjusted by the addition of 10 μl of 0.5 M Tris-HCl (pH 7.5) buffer, and then the preparation was diluted to 300 μl with water and centrifuged briefly. A 100-μl PCR mixture contained 500 ng of each primer, 200 μM concentrations of each deoxynucleoside triphosphate, 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 4 mM MgSO4, and 0.1% (vol/vol) Triton X-100. The reaction mixture was incubated at 96°C for 2 min before the addition of 2 U of Vent DNA polymerase (New England Biolabs, Hitchin, United Kingdom), and then PCR amplification was performed using 30 cycles of denaturation at 96°C for 40 s and primer annealing and extension at 72°C for 65 s. The final cycle was followed by an additional extension step at 72°C for 10 min. The pRSETA plasmid and the amplified 880-bp PCR products were digested with BamHI and HindIII restriction endonucleases (New England Biolabs), the fragments were separated by electrophoresis, and DNA was recovered from agarose using Geneclean II (Stratech Scientific, Luton, United Kingdom). The opc-containing fragments were ligated into pRSETA using T4 DNA ligase (New England Biolabs), and the ligation mixture was used to transform competent E. coli JM109(DE3) cells. The presence and integrity of the resulting plasmid pKAJ101b were tested in transformants by restriction endonuclease digestion and by sequencing of the coding region of the opc gene.
For expression, plasmid pKAJ101b was transformed into E. coli JM101. Three overnight cultures (10 ml each) were used to inoculate 21 flasks containing 750 ml of 2YT medium plus 100 μg of ampicillin ml−1, which were incubated with vigorous shaking (250 rpm) at 37°C for 1 h until an A600 of ≈0.3 was reached. Isopropyl-β-d-thiogalactoside (IPTG) was then added to a final concentration of 1 mM, and the cultures were incubated for a further hour, then infected with M13/T7 phage at a multiplicity of infection of 5 PFU cell−1, and incubated for a further 5 h. After this time, the resulting phage lysate was centrifuged at 10,000 × g for 1 h at 4°C, and the insoluble material, which contained the recombinant protein, was stored at −20°C.
Purification of recombinant Opc protein under denaturing conditions.
Recombinant Opc protein was purified under denaturing conditions (5). The crude insoluble protein was dissolved in 10 mM Tris-HCl buffer (pH 8.0) containing 8 M urea, 100 mM NaH2PO4, and 20 mM imidazole (lysis buffer; 5 ml per g [wet weight] of cell debris) with stirring at room temperature for 1 h. The suspension was then subjected to sonication (MSE Soniprep 150 sonicator) on ice until the solution cleared. Insoluble material was removed by centrifugation at 10,000 × g for 30 min at 4°C; recombinant Opc protein was purified from the lysate by affinity chromatography on a nickel-nitrilotriacetic acid (Ni-NTA) gel matrix (Qiagen, Crawley, United Kingdom), under denaturing conditions. A column (0.5 by 15 cm) of Ni-NTA resin (5 ml) was equilibrated with lysis buffer, and the cell lysate was loaded at a flow rate of 15 ml h−1. The column was then washed with lysis buffer and eluted sequentially with 10 mM Tris-HCl buffer (pH 6.3) containing 100 mM NaH2PO4, 8 M urea, and 20 mM imidazole, followed by the same buffer without imidazole and with decreasing pHs of 5.9 and 4.5. Fractions (3 ml) were collected, and the presence of eluted recombinant Opc was determined by absorbance at 280 nm, bicinchoninic acid protein assay (Pierce, Chester, United Kingdom), and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Fractions from the pH 5.9 and 4.5 eluates that contained recombinant protein were pooled, the protein was precipitated by the addition of ethanol to a final concentration of 80% (vol/vol), and after 18 h at 4°C, the precipitate was collected by centrifugation at 13,000 × g for 10 min. The purified protein was washed with water, dried briefly under vacuum, and then stored at −20°C with desiccant until used.
Incorporation of recombinant Opc into liposomes with and without adjuvants.
Liposomes were prepared using a dialysis-sonication method as previously described (31). Briefly, l-α-phosphatidylcholine and cholesterol (7:2 molar ratio, 20 mg total; Sigma, Poole, United Kingdom) were dissolved in chloroform (at 10 mg ml−1) in a round-bottomed flask, and the solvent was removed under vacuum with rotation to produce an even lipid film. The recombinant Opc protein (1 mg) was dissolved in 1 ml of 10 mM HEPES buffer (pH 7.2) containing 0.6% (wt/vol) SDS, diluted by the addition of 4 ml of 10 mM HEPES buffer (pH 7.2) containing 100 mg of octyl-β-d-glucoside, and incubated at room temperature for 3 h. This detergent-protein solution was then used to solubilize the shell-dried lipid film, and unilamellar membrane vesicles were produced by dialysis against repeated changes of phosphate-buffered saline (PBS) for 72 h, followed by sonication. Liposomes were also prepared containing the adjuvant monophosphoryl lipid A (MPLA; Ribi Immunochem, Hamilton, Mont.), at an adjuvant-to-protein ratio of 1:1. Control liposomes with and without MPLA were also prepared. The presence of small unilamellar liposomes was determined by electron microscopy as previously described (31). All liposome preparations were stored in aliquots at −20°C until used.
Preparation of protein-detergent-adjuvant mixtures.
Recombinant Opc protein was solubilized in 50 mM Tris-HCl buffer (pH 8.0) containing 100 mM NaCl and 2% SDS to a stock concentration of between 7 and 8 mg ml−1. Protein-Zwittergent mixtures were prepared containing 1 mg of Opc protein ml−1, 0.8% (wt/vol) Zwittergent 3-14 (Calbiochem, Beeston, Nottingham, United Kingdom), and 0.2% SDS, with and without MPLA (1 mg ml−1). In addition, Opc protein (100 μg ml−1) was adsorbed to aluminum hydroxide gel (2% Alhydrogel; Superfos, Biosector a/s, Vedback, Denmark) by mixing with an equal volume of Al(OH)3 suspension overnight at 4°C.
Immunization of animals.
BALB/c (H-2d) mice at 6 to 7 weeks of age were used for immunizations, with blood samples taken before primary immunization. Individual mice within groups of five of approximately equal weight were immunized intraperitoneally with 20 μg of recombinant Opc protein in each of the above preparations on days 0, 14, 28, and 50. Mice were terminally bled on day 60, and sera were stored at −20°C.
SDS-PAGE and Western immunoblotting.
SDS-PAGE was performed using a 10 to 25% (wt/vol) linear gradient gel at 200 V for 18 h at 4°C. OM and purified recombinant protein were loaded at 10 μg per well. Separated proteins were transferred to nitrocellulose by semidry blotting at 100 mA for 1 h, and following incubation with murine sera, immunological reactivity was detected using anti-mouse immunoglobulin (Ig)-alkaline phosphatase conjugate (Bio-Rad, Hemel Hempstead, United Kingdom) as described previously (6).
Detection of immune response. (i) ELISA.
Flat-bottomed polystyrene microtiter plates were coated overnight at 37°C with either recombinant protein or OM in 0.05 M sodium carbonate buffer (pH 9.6) (1 μg of protein ml−1). Serial dilutions of murine sera were incubated in the plates for 1 h at room temperature, and antibody binding was detected using anti-mouse Ig-horseradish peroxidase (HRP) conjugate (1:2,000 dilution; Zymed, Cambridge, United Kingdom) with 3,3′,5,5′-tetramethylbenzidine and H2O2 as the enzyme substrate. Absorbance was measured at 450 nm, and the enzyme-linked immunosorbent assay (ELISA) titer, extrapolated from the linear portion of the serum titration curve, was taken as the dilution which gave an increase in absorbance of 0.1 U h−1 (6).
(ii) Subclass-specific ELISA.
OM ELISA using rat monoclonal anti-mouse IgG1, -2a, and -2b and IgM HRP conjugates (Zymed) was used to determine antibody subclass as previously described (5). For IgG3, a biotin-conjugated rat monoclonal anti-mouse IgG3 antibody (Pharmingen, Becton Dickinson, Crawley, United Kingdom) and HRP-streptavidin (Zymed) were used. All conjugates were used at a dilution of 1:1,000. No cross-reactivity was observed between conjugates of defined specificity and Ig of other subclasses.
(iii) Immunofluorescence.
N. meningitidis strains were suspended in PBS, placed on microscope slides, and allowed to air dry. The air-dried suspensions were then fixed in acetone (100%) for 10 min and blocked in PBS containing 1% bovine serum albumin for 15 min. Pooled murine antisera diluted 1:100 were reacted with the fixed organisms for 1 h at 25°C with gentle mixing. After washing with PBS, bound antibody was detected by reactivity with anti-mouse Ig-fluorescein isothiocyanate conjugate (1:100 in PBS; Dako, Ely, United Kingdom) for 1 h in the dark. After a washing with PBS, the organisms were counterstained with propidium iodide (25 μg ml−1; Sigma), washed again with PBS, and examined using a fluorescence microscope (Leitz).
Bactericidal assays.
The bactericidal activities of antisera were determined using guinea pig serum as a source of exogenous complement, as previously described (6). Murine antiserum raised to purified outer membranes (P1.16) was used as a positive control. Statistical analysis was performed as previously described (6).
Nucleotide sequence accession numbers.
The sequences of the opc genes have been deposited in the EMBL/GenBank database under accession numbers AJ296283 to AJ296286 and AJ311619.
RESULTS
Sequencing of opc gene.
The opc gene was sequenced from several strains isolated from cases of meningococcal infection and from carriers. The inferred amino acid sequences were compared with the previously published sequence of strain H44/76 (25). The amino acid sequences of strains MC58 and MC131 were identical to that of strain H44/76. Strain MC122 showed two amino acid changes in Opc compared to MC58, from S to G at position 37 and K to T at position 227, which corresponds to the apex of loop 5 in the predicted model of the Opc protein structure (16). Strain MC119 showed four amino acid changes, occurring within predicted loops 3, 4, and 5, while strain MC139 showed five amino acid changes compared to MC58, four of which occurred within predicted loops 3, 4, and 5. The amino acid sequence 71NKLGK75 in predicted loop 2, which constitutes the epitope recognized by the Opc-specific monoclonal antibody (MAb) B306 (16), was present in all strains.
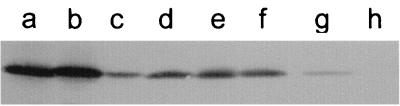
The levels of expression of Opc protein were determined for the sequenced strains by Western blotting with Opc-specific MAb B306 and with murine antisera raised against the purified recombinant protein (Fig. 1). Strain MC58 showed a high level of Opc expression, designated Opc++ (16), whereas the remaining strains, including strain H44/76, showed lower levels of expression (Opc+). One of the strains (MC139 Opc+) which showed the greatest sequence divergence from MC58 was selected for high-level expression (Opc++) by successive rounds of colony blotting and detection using the Opc-specific MAb B306.
FIG. 1.
Variation in expression of Opc protein. Meningococcal cell lysates were subjected to SDS-PAGE followed by Western blotting with antisera raised against recombinant Opc protein. Strains used were (a) MC58, (b) MC139 Opc++, (c) MC139 Opc+, (d) H44/76, (e) MC131, (f) MC119, (g) MC122, and (h) MC114.
Cloning of meningococcal opc gene in E. coli using pRSETA vector: expression and purification of recombinant Opc protein.
In order to study the vaccine potential of the Opc protein in isolation from other proteins present in the meningococcal OM, the pRSETA vector was used to express Opc protein in a heterologous host, E. coli. In this system, the gene of interest is cloned in frame with sequences which contain a bacteriophage T7 promoter and encode an N-terminal fusion peptide containing six histidine residues that function as a metal-binding domain in the translated protein. In previous studies with a similar plasmid carrying the meningococcal porA gene, expression was achieved by cloning the recombinant plasmid into E. coli JM109(DE3), which contains a T7 RNA polymerase gene under the control of the lacUV5 promoter (5). However, only low levels of expression were obtained using this system with the recombinant opc plasmid. Following expression trials with different media, strains, and time courses, optimal expression was obtained by cloning plasmid pKAJ101b into E. coli JM101 and inducing expression by infecting the culture with bacteriophage M13/T7 in the presence of IPTG. After solubilizing in 8 M urea, the recombinant Opc protein was readily purified by affinity chromatography on an Ni2+ column. From a typical batch culture (2.25 liters), the approximate yield of purified protein was 20 mg (from 15 g [wet weight] of E. coli cells). SDS-PAGE revealed a single homogeneous protein band of approximately 36 kDa that reacted with the Opc-specific MAb B306 on Western blots. In addition, SDS-PAGE and silver staining detected no lipopolysaccharide.
Humoral murine immune response to recombinant Opc protein.
The purified protein was used to immunize mice with adjuvant preparations that have the potential for use in humans. To attempt to present the protein in its native conformation for immunization, the Opc protein was incorporated into liposomes prepared by dialysis-sonication (Opc-liposomes). In addition, the recombinant protein was solubilized with the zwitterionic detergent Zwittergent 3–14 (Opc-Zwit), as an alternative means of refolding denatured proteins into native conformations (13, 18). In an attempt to increase the immunogenicity of the recombinant protein, the immunomodulator MPLA was incorporated into Opc-liposomes (Opc+MPLA-liposomes) and Opc-Zwittergent micelles (Opc+MPLA-Zwit). In addition, Opc-liposomes were mixed with liposomes incorporating MPLA (MPLA-liposomes). As a control, the protein was adsorbed to Al(OH)3, the standard adjuvant routinely licensed for human use.
The immune response to the purified Opc protein was studied initially by the reactivity of murine antisera in ELISA (Table 1). Immunization with Opc adsorbed to Al(OH)3 induced the highest immune response to the homologous protein, with a mean titer of approximately 4 × 106 (Table 1). In contrast, immunization with Opc protein incorporated into liposomes or Zwittergent induced immune responses that were approximately 7- to 10-fold lower. Mixing MPLA-liposomes with the Opc-liposomes increased the immune response twofold, while incorporation of MPLA into the Opc-liposomes produced a fivefold increase compared with Opc-liposomes alone. Similarly, addition of MPLA to the Zwittergent preparation produced a fivefold increase in response to the purified recombinant Opc protein.
TABLE 1.
ELISA reactivity of antisera raised against recombinant Opc protein preparationsa
| Formulation | Reciprocal geometric mean ELISA titer (10−3) measured against:
|
|||||
|---|---|---|---|---|---|---|
| Homologous recombinant Opc protein | OMs from strain:
|
|||||
| MC58 Opc++ | MC139 Opc++ | MC139 Opc+ | H44/76 Opc+ | MC114 Opc− | ||
| Opc+Al(OH)3 | 3,990 | 76 | 944 | <1 | <1 | <1 |
| Opc-liposomes | 344 | 10 | 27 | <1 | <1 | <1 |
| Opc+MPLA-liposomes | 1,837 | 93 | 291 | <1 | 3 | 1 |
| Opc-liposomes+MPLA-liposomes | 778 | 78 | 64 | <1 | 4 | 1 |
| Opc-Zwit | 650 | 10 | 78 | <1 | <1 | <1 |
| Opc+MPLA-Zwit | 3,026 | 49 | 287 | <1 | 2 | <1 |
Mice were immunized with recombinant Opc protein in the preparations described in Materials and Methods. Pre- and postimmunization sera were tested in ELISA against homologous protein and against OMs from the homologous and heterologous strains. Strains MC58 and H44/76 expressed the homologous Opc protein, strain MC139 expressed a heterologous protein; the levels of expression were high (Opc++), low (Opc+), and none (Opc−). Preimmune sera and antisera raised against control liposomes with and without MPLA showed no reactivity against antigens in the ELISA (<1).
Humoral murine immune response to Opc protein in OM.
Antisera raised against recombinant Opc was also tested in ELISA against the Opc protein present in the OM of the homologous strain MC58. The titers were significantly lower than observed against the recombinant Opc, and the relative patterns of reactivity were different, in that several of the preparations produced titers which were similar to that achieved with Al(OH)3. As with antibodies induced against the recombinant protein, the addition of MPLA significantly increased the immune response to the homologous OMs. The Opc+MPLA-liposomes, the liposome mixture, and the Opc+MPLA-Zwittergent all induced immune responses that were five- to ninefold greater than those observed with formulations without the immunomodulator (Table 1).
The antisera were also tested against strain H44/76, which expressed the same Opc as MC58, and against strain MC139, which contained significant amino acid changes compared to MC58. These strains were all Opc+, expressing lower levels of the protein than MC58, and showed little or no reactivity with any of the antisera. Similar results were obtained when the antisera were tested against the Opc− strain MC114. In contrast, when the antisera were tested against the Opc++ variant of MC139, the titers obtained were greater than even those seen with the homologous Opc++ MC58 (Table 1).
The immune response was also investigated by Western blotting using OM preparations as antigens. All of the antisera raised with Al(OH)3, liposome, and Zwittergent formulations, with or without MPLA, showed a single strong band of reactivity against denatured Opc from the homologous MC58. Weaker reactivity was seen with the Opc+ strains H44/76 and MC139, while reactivity with MC139 Opc++ was even greater than that observed with MC58. No reactivity was observed against the Opc− strain MC114.
Antibody subclass-specific response to Opc in OM.
The antibody subclass-specific response to Opc protein in the homologous (MC58) outer membranes was determined by ELISA using anti-mouse Ig isotype-specific conjugates. Immunization with Opc adsorbed to Al(OH)3 induced high levels of IgG1 subclass antibodies (92%), with some IgM (8%) (Table 2). Immunization with Opc-liposomes and Opc-Zwit induced lower levels of antibody of predominantly IgG1 subclass (75 to 96%). However, the addition of MPLA to the liposome and Zwittergent formulations induced a broader range of antibody subclasses, in particular, a significant increase in the proportion of IgG2 antibodies (33 to 50%). In contrast, the mixture of Opc-liposomes and MPLA-liposomes induced predominantly IgG1 antibodies (85%) and lower levels of IgG2 (11%).
TABLE 2.
Antibody subclass-specific response to homologous Opc protein following immunization of mice with recombinant Opc protein preparationsa
| Formulation | Mean conc of antibody isotype (μg ml−1)
|
||||
|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | IgM | |
| Opc+Al(OH)3 | 546 | <0.1 | <0.1 | <0.1 | 47 |
| Opc-liposomes | 33 | <0.1 | 4 | 1 | 6 |
| Opc+MPLA-liposomes | 49 | 32 | 9 | 1 | 7 |
| Opc-liposomes+MPLA-liposomes | 216 | 14 | 15 | 4 | 6 |
| Opc-Zwit | 114 | <0.1 | 2 | <0.1 | 3 |
| Opc+MPLA-Zwit | 82 | 32 | 12 | <0.1 | 7 |
| Liposomes (empty) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| MPLA-liposomes | <0.1 | <0.1 | <0.1 | <0.1 | 5 |
| Normal mouse serum | 0.2 | <0.1 | <0.1 | <0.1 | 2 |
ELISA with enzyme conjugates of rat MAbs specific for murine Ig subclasses was used to determine concentrations of antibody subclasses in murine antisera. Data are represented as the mean antibody isotype concentration in serum for groups of five individual mice. A concentration of <0.1 μg (ml of serum)−1 was below the minimum detection limit.
Antibody recognition of Opc protein on meningococcal cells by immunofluorescence.
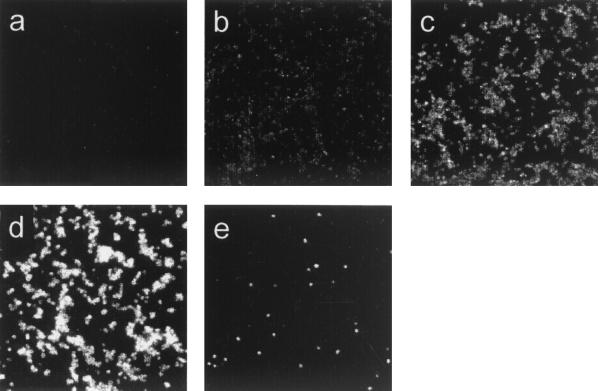
The ability of antisera to recognize Opc protein on meningococcal cells was determined (Fig. 2 and Table 3). No binding was observed to meningococcal cells of homologous or heterologous strains with antisera raised with recombinant Opc adsorbed to Al(OH)3. Antisera raised to Opc in liposomes and Zwittergent, with and without the addition of MPLA, showed strong binding to meningococcal cells of the homologous strain MC58. In contrast, little reactivity was seen with strain H44/76 Opc+, which expressed the homologous protein, although the population contained a few individual cells which showed strong reactivity (Fig. 2e). The antisera also showed little or no reactivity against heterologous Opc+ strain MC139 and the Opc− control MC114. In addition, only low reactivity was observed with strain MC139 Opc++, which expressed high levels of the heterologous protein.
FIG. 2.
Variation in antibody recognition of Opc protein on meningococcal cells by immunofluorescence. Sera raised against Opc preparations were tested for their ability to recognize meningococcal cells. Five patterns of reactivity were observed (Table 3): (a) nonreactivity (−), (b) weak reactivity (+/−), (c) strong reactivity (+), (d) very strong reactivity (++), and (e) majority of population failed to react but <1% of bacteria showed strong reactivity (−/+).
TABLE 3.
Reactivity of antisera with whole cells of meningococci determined by immunofluorescencea
| Formulation | Immunofluorescence reactivityb on whole cells
|
||||
|---|---|---|---|---|---|
| MC58 Opc++ | MC139 Opc++ | MC139 Opc+ | H44/76 Opc+ | MC114 Opc− | |
| Opc+Al(OH)3 | − | − | − | − | − |
| Opc-liposomes | + | − | − | −/+ | − |
| Opc+MPLA-liposomes | ++ | + | − | −/+ | − |
| Opc-liposomes+MPLA-liposomes | ++ | + | − | −/+ | − |
| Opc-Zwit | ++ | +/− | − | −/+ | − |
| Opc+MPLA-Zwit | ++ | + | − | −/+ | − |
| Liposomes (empty) | − | − | − | − | − |
| MPLA-liposomes (empty) | − | − | − | − | − |
| Normal mouse serum | − | − | − | − | − |
Antisera were reacted with whole cells of the homologous and heterologous strains. Strains MC58 and H44/76 expressed the homologous Opc protein, and strain MC139 expressed a heterologous protein. The five patterns of reactivity observed are illustrated in Fig. 2.
Symbols are defined in the legend to Fig. 2.
Bactericidal activity of antisera.
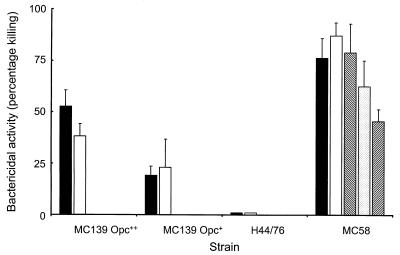
Antisera raised against the recombinant Opc protein were tested for their ability to promote in vitro complement-mediated killing of the homologous meningococcal strain MC58. While antisera raised against Opc plus Al(OH)3 showed no significant effect, all the antisera raised against liposome or Zwittergent preparations showed bactericidal activity. Antisera raised against Opc-Zwit produced 50% killing at a titer of 1:16, and this was increased to 1:32 with the incorporation of MPLA. Antisera raised against Opc-liposomes showed significant bactericidal activity, with a titer of 1:8 only, and a similar effect was seen with sera raised against the mixture of Opc-liposomes plus MPLA-liposomes. In contrast, incorporation of MPLA into the Opc-liposomes produced the most effective sera, with a bactericidal titer of 1:128 against the homologous strain. However, this antiserum showed very low levels of cross-reacting bactericidal activity against the Opc++ strain MC139 (titer, 1:4) and no detectable activity against Opc+ MC139 or the other Opc+ strains, including H44/76, which expressed the homologous protein (Fig. 3).
FIG. 3.
Bactericidal activity of antisera raised against recombinant Opc protein preparations. Pooled antisera raised against Opc+MPLA liposomes, which showed greatest activity against the homologous strain (MC58 Opc++), was also tested against strain H44/76, expressing the homologous Opc protein (Opc+), and strain MC139, expressing a heterologous protein with high (Opc++) and low (Opc+) levels of expression. Serum was tested at dilutions of 1:4, 1:8, 1:32, 1:128, and 1:256 (from left to right within each group). No activity was observed with the control Opc− strain MC114 (data not shown).
DISCUSSION
Human trials with OMV vaccines have demonstrated the potential protective immune response to meningococcal OM proteins. Although the immune response differed between individuals, detailed analysis of responses to the Norwegian vaccine revealed that antibodies to the class 1 and Opc proteins made the most important contribution to bactericidal activity against the vaccine strain (17, 23). In order to study further the vaccine potential of individual antigens, the expression of meningococcal proteins in a foreign host presents a convenient means of producing protein free from other meningococcal OM antigens.
The Opc protein is believed to be significantly less variable in sequence than class 1 protein, with a limited number of amino acid substitutions occurring among a large panel of strains of diverse origin (25), although levels of expression vary between Opc++, Opc+, and Opc− strains (24). The more conserved nature of Opc, combined with its important role in pathogenesis (29, 30) and as a target for bactericidal antibodies (23), suggests its potential for studies as a recombinant vaccine. In a previous study, Musacchio and colleagues (19) reported the expression of recombinant Opc protein in E. coli as a fusion protein with a fragment of another meningococcal protein. Following solubilization in the presence of urea and immunization using Freund's adjuvant, which is not suitable for human use, a bactericidal effect against the Opc++ homologous strain was reported. Reduced activity was seen with additional Opc+ strains, although the sequence of the protein in these heterologous strains was not reported (19).
The ability of liposomes to permit refolding of OM proteins combined with a natural adjuvant effect and suitability for human immunization make liposomes an attractive vehicle for delivery of vaccines based on recombinant meningococcal proteins. In the current study, we used the methods which have been successful with class 1 protein to produce recombinant Opc protein in formulations appropriate for human immunization. High levels of expression were achieved, and the purified recombinant protein was refolded by incorporation into liposomes and by detergent micelles containing Zwittergent. The immunomodulator MPLA was also used as an adjuvant with potential use for human immunization (28). Since previous studies with class 1 protein had shown that incorporation of MPLA into liposomes could increase immunogenicity but at the expense of protein refolding (5), an additional strategy was adopted by mixing Opc-liposomes with blank liposomes containing MPLA. Immunization with both liposome and Zwittergent preparations produced antibodies which reacted with the homologous protein, and addition of MPLA increased the immune response, but not to the level observed after immunization with denatured protein using Al(OH)3 as an adjuvant. The immune response to the homologous protein in OMs was considerably lower, but the presence of MPLA was again associated with an increased immune response, with the most effective response observed with Opc+MPLA-Zwittergent, Opc-liposomes mixed with MPLA liposomes, and Opc+MPLA-liposomes.
In order to examine the effect of variation in sequence and levels of expression of Opc on antigen recognition, the sera were reacted with a panel of strains of known Opc sequence and expression levels. The sera showed equal or greater reactivity with OMs of strain MC139 Opc++, which expressed high levels of an Opc protein with five amino acid substitutions compared with the homologous MC58 protein. In contrast, little or no reactivity was observed with Opc+ MC139 or with strain H44/76, which expressed the homologous protein at the Opc+ level. The greater reactivity with Opc++ MC139 than with MC58 appeared to correlate with a slightly higher level of expression of Opc revealed by Western blotting with Opc-specific antibodies.
Although OM preparations are likely to contain OM proteins in a “more native” conformation than purified recombinant proteins, previous studies have suggested that immunofluorescence of antibodies bound to meningococcal cells represents a more accurate measurement of antibodies directed against native epitopes than does reactivity with OMs in ELISA (5). In the current study, the greater relevance of the immunofluorescence assay was revealed by the observation that, in contrast to ELISA, antibodies raised against the denatured recombinant protein showed no reactivity with the homologous strain. However, high levels of reactivity were seen with the antisera to Zwittergent preparations and to both liposome formulations which contained MPLA, whereas lower reactivity was observed with antisera to liposomes lacking MPLA. In addition, immunofluorescence showed a significant difference from OM ELISA, in that reactivity with the homologous Opc++ MC58 was considerably greater than with the Opc++ MC139, suggesting that the native heterologous protein was recognized much less effectively than the homologous protein.
The generally accepted correlate of protection against meningococcal infection is the presence of antibodies with the ability to activate complement-mediated killing of meningococci (12). In the present study, immunization with the Opc liposomes containing MPLA produced the strongest bactericidal effect against the homologous strain, followed by the Zwittergent micelles incorporating MPLA, while the equivalent preparations lacking MPLA were much less effective. This difference in the functional immune response was despite the similar reactivity with native protein in immunofluorescence, indicating the importance of the ability of MPLA to induce a broader spectrum of antibody subclasses, including those most effective in complement activation (5). The improved response obtained by incorporating MPLA into the liposomes was in contrast to the previous studies with class 1 protein, where incorporation of the adjuvant into the liposome boosted the immune response to denatured protein at the expense of functional antibodies (5, 31). It may be that the different structure of Opc within the membrane permits the insertion of MPLA without a major effect on the conformation of the protein. Despite the significant bactericidal effect against the homologous Opc++ strain, the sera raised against the Opc liposomes containing MPLA showed barely detectable activity against the heterologous Opc++ MC139 strain. The two strains differ at only 5 amino acid positions out of a total of 253 in the mature protein. However, four of the substitutions are in regions which are predicted to form surface-exposed loops and thus to be exposed to immune surveillance. The level of expression of Opc also had a profound effect on the bactericidal activity of the serum, with the strain expressing the homologous protein at the Opc+ level being resistant to killing by the most effective serum. It is also interesting that the levels of bactericidal activity seen even with the Opc++ homologous strain (1:128) are considerably lower than previously obtained with recombinant class 1 protein in liposomes (1:1,024) (5). It is likely that the variations in either sequence or level of expression reduce antibody binding below the level required for significant bactericidal activity.
The ideal vaccine against meningococcal infection would induce high levels of bactericidal activity against a wide range of strains. A previous study concluded that Opc protein “appears to deserve more attention as a vaccine candidate” (19). While it should be recognized that experiments with laboratory animals may not reproduce precisely the human immune response, the lower immunogenicity of the protein, combined with the effects of variation in both sequence and levels of expression, suggests that it is unlikely to provide effective immunity alone. However, it may contribute a protective effect to multivalent vaccines that include other protective antigens, such as the class 1 protein.
ACKNOWLEDGMENTS
This work was supported by the National Meningitis Trust, Hope (the Wessex Medical Trust), and the University of Southampton Strategic Development Fund.
REFERENCES
- 1.Bjune G, Gronnesby J K H, Closs O, Nokleby H. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup b meningococcal disease in Norway. NIPH Ann. 1991;14:125–132. [PubMed] [Google Scholar]
- 2.Bjune G, Hoiby E A, Gronnesby J K, Arnesen O, Holstfredriksen J, Halstensen A, Holten E, Lindbak A K, Nokleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Froholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 3.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, Moran E, Hankins W, Gilly J, Mays J. Efficacy, safety, and immunogenicity of a meningococcal group B (15- P1.3) outer-membrane protein vaccine in Iquique, Chile. Vaccine. 1995;13:821–829. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 4.Brooks J L, Fallon R J, Heckels J E. Sequence variation in class 1 outer membrane protein in Neisseria meningitidis isolated from patients with meningococcal infection and close household contacts. FEMS Microbiol Lett. 1995;128:145–150. doi: 10.1111/j.1574-6968.1995.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 5.Christodoulides M, Brooks J L, Rattue E, Heckels J E. Immunisation with recombinant class 1 outer membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology. 1998;144:3027–3037. doi: 10.1099/00221287-144-11-3027. [DOI] [PubMed] [Google Scholar]
- 6.Christodoulides M, McGuinness B T, Heckels J E. Immunisation with synthetic peptides containing epitopes of the class 1 outer membrane protein of Neisseria meningitidis: production of bactericidal antibodies on immunisation with a cyclic peptide. J Gen Microbiol. 1993;139:1729–1738. doi: 10.1099/00221287-139-8-1729. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulides M, Rattue E, Heckels J E. Influence of adjuvants on the humoral immune response towards a synthetic peptide containing a B-cell epitope from meningococcal class 1 protein. Vaccine. 1999;18:131–139. doi: 10.1016/s0264-410x(99)00190-5. [DOI] [PubMed] [Google Scholar]
- 8.Cooke S J, Jolley K, Ison C A, Young H, Heckels J E. Naturally occurring isolates of Neisseria gonorrhoeae, which display anomalous serovar properties, express PIA/PIB hybrid porins, deletions in PIB or novel PIA molecules. FEMS Microbiol Lett. 1998;162:75–82. doi: 10.1111/j.1574-6968.1998.tb12981.x. [DOI] [PubMed] [Google Scholar]
- 9.Demoraes J C, Perkins B A, Camargo M C C, Hidalgo N T R, Barbosa H A, Sacchi C T, Gral I M L, Gattas V L, Vasconcelos H D, Plikaytis B D, Wenger J D, Broome C V. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 10.Finne J, Leinonen M, Makela P H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;ii:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 11.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969b;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Idanpaan-Heikkila I, Muttilainen S, Wahlstrom E, Saarinen L, Leinonen M, Sarvas M, Makela P H. The antibody response to a prototype liposome vaccine containing Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis. Vaccine. 1995;13:1501–1508. doi: 10.1016/0264-410x(95)00101-6. [DOI] [PubMed] [Google Scholar]
- 14.Jiskoot W, Teerlink T, Van Hoof M M M, Bartels K, Kanhai V, Crommelin D J, Beuvery E C. Immunogenic activity of gonococcal protein I in mice with three different lipoidal adjuvants delivered in liposomes and in complexes. Infect Immun. 1986;54:333–338. doi: 10.1128/iai.54.2.333-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuinness B T, Clarke I N, Lambden P R, Barlow A K, Poolman J T, Jones D M, Heckels J E. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet. 1991;337:514–517. doi: 10.1016/0140-6736(91)91297-8. [DOI] [PubMed] [Google Scholar]
- 16.Merker P, Tommassen J, Kusecek B, Virji M, Sesardic D, Achtman M. Two-dimensional structure of the Opc invasin from Neisseria meningitidis. Mol Microbiol. 1997;23:281–293. doi: 10.1046/j.1365-2958.1997.2051567.x. [DOI] [PubMed] [Google Scholar]
- 17.Milagres L G, Ramos S R, Sacchi C T, Melles C E A, Vieira V S D, Sato H, Brito G S, Moraes J C, Frasch C E. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun. 1994;62:4419–4424. doi: 10.1128/iai.62.10.4419-4424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minetti C A S A, Tai J Y, Blake M S, Pullen J K, Liang S M, Remeta D P. Structural and functional characterization of a recombinant PorB class 2 protein from Neisseria meningitidis—conformational stability and porin activity. J Biol Chem. 1997;272:10710–10720. doi: 10.1074/jbc.272.16.10710. [DOI] [PubMed] [Google Scholar]
- 19.Musacchio A, Carmenate T, Delgado M, Gonzalez S. Recombinant Opc meningococcal protein, folded in vitro, elicits bactericidal antibodies after immunization. Vaccine. 1997;15:751–758. doi: 10.1016/s0264-410x(96)00198-3. [DOI] [PubMed] [Google Scholar]
- 20.Muttilainen S, Idanpaan-Heikkila I, Wahlstrom E, Nurminen M, Makela P H, Sarvas M. The Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis and reconstituted into phospholipid vesicles elicits antibodies to native P1 epitopes. Microb Pathog. 1995;18:423–436. doi: 10.1006/mpat.1995.0038. [DOI] [PubMed] [Google Scholar]
- 21.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Hoiby E A, Rosenqvist E, Holst J, Nokleby H, Sotolongo F, Sierra G, Campa H C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 22.Qi H L, Tai J Y, Blake M S. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect Immun. 1994;62:2432–2439. doi: 10.1128/iai.62.6.2432-2439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenqvist E, Hoiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Ronnild E, Bjune G, Nokleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkari J, Pandit N, Moxon E R, Achtman M. Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Mol Microbiol. 1994;13:207–217. doi: 10.1111/j.1365-2958.1994.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 25.Seiler A, Reinhardt R, Sarkari J, Caugant D A, Achtman M. Allelic polymorphism and site-specific recombination in the opc locus of Neisseria meningitidis. Mol Microbiol. 1996;19:841–856. doi: 10.1046/j.1365-2958.1996.437970.x. [DOI] [PubMed] [Google Scholar]
- 26.Tinsley C R, Heckels J E. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J Gen Microbiol. 1986;132:2483–2490. doi: 10.1099/00221287-132-9-2483. [DOI] [PubMed] [Google Scholar]
- 27.van der Ley P, Heckels J E, Virji M, Hoogerhout P, Poolman J T. Topology of outer membrane porins in pathogenic Neisseria spp. Infect Immun. 1991;59:2963–2971. doi: 10.1128/iai.59.9.2963-2971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma J N, Rao M, Amselem S, Krzych U, Alving C R, Green S J, Wassef N M. Adjuvant effects of liposomes containing lipid A—enhancement of liposomal antigen presentation and recruitment of macrophages. Infect Immun. 1992;60:2438–2444. doi: 10.1128/iai.60.6.2438-2444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virji M, Makepeace K, Ferguson D J P, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 30.Virji M, Makepeace K, Ferguson D J P, Achtman M, Sarkari J, Moxon E R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–2795. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 31.Ward S J, Scopes D A, Christodoulides M, Clarke I N, Heckels J E. Expression of Neisseria meningitidis class 1 porin as a fusion protein in Escherichia coli: the influence of liposomes and adjuvants on the production of a bactericidal immune response. Microb Pathog. 1996;21:499–512. doi: 10.1006/mpat.1996.0079. [DOI] [PubMed] [Google Scholar]
- 32.Wetzler L M, Blake M S, Gotschlich E C. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J Exp Med. 1988;168:1883–1897. doi: 10.1084/jem.168.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]